User login
Home ventilation consult
Specialist input on a sudden shift in device availability
Philips Respironics released a public statement on January 25, 2024, that would dramatically change the landscape of home mechanical ventilation and sleep-disordered breathing management in the United States. The company announced that, effective immediately in the US and US territories, Philips Respironics would stop production and sale of all hospital and home mechanical ventilation products, home and hospital ventilation devices, and oxygen concentrators.
There are many unknowns and uncertainties about how to proceed with care for patients requiring these devices. So we gathered an expert panel of clinicians from CHEST’s Home-Based Mechanical Ventilation and Neuromuscular Section within the Sleep Medicine Network to explain the current situation and offer suggestions on moving forward in caring for these patients.
Why is this happening?
John M. Coleman III, MD, FCCP: To understand the current Philips Respironics announcement, we must go back to June 2021. At that time, Philips recalled certain home mechanical ventilators, CPAP machines, and BiPAP machines due to potential health risks related to breakdown of the polyester-based polyurethane (PE-PUR) foam placed in these devices for noise reduction. Small and microscopic particles of this foam were at risk for being inhaled or ingested by patients using these devices. It was suspected that inhalation of these particles could potentially result in temporary or permanent injury. Machines in hot temperatures or using ozone cleaning were at increased risk. The US Food and Drug Administration (FDA) issued a class 1 recall, defined as “a situation in which there is reasonable probability that the use of or exposure to a violative product will cause serious adverse health consequences or death.”
In the months following the initial recall, there were additional recalls of both in-hospital and home ventilators related to the potential of these foam particles to move and block the air path, reducing airflow and causing the device to alarm.
Over the next few years, tens of thousands of medical device reports were filed about PE-PUR foam-related injuries, with some cases resulting in death. At this time, the Department of Justice began collaborating with the FDA on a consent decree. There were ongoing recalls of the CoughAssist T70 device, as well as the newest generation of Philips Respironics home ventilators, the Trilogy EVO.
Ultimately, after years of ongoing recalls and reports of numerous deaths and injuries, with multiple class action lawsuits, the consent decree was finalized. Philips Respironics agreed to stop production of all respiratory-related products in the US and US territories.
What devices does this apply to?
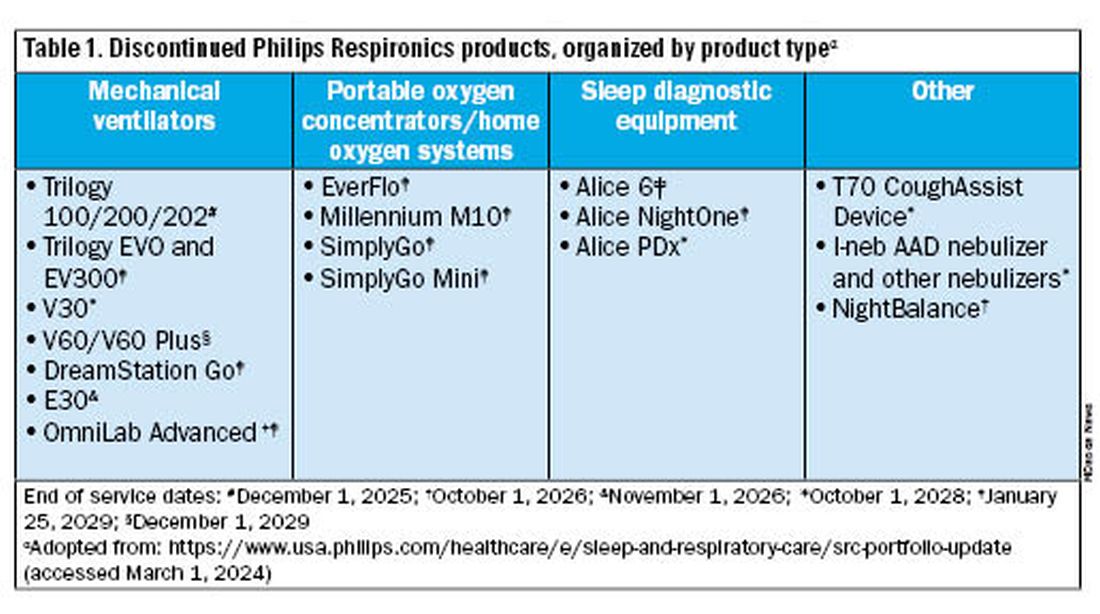
Jason Ackrivo, MD: This notice affects the devices shown in Table 1. All sales and device shipments have been discontinued as of January 25, 2024. Philips Respironics will continue to service the devices, subject to part availability, up to 5 years after sales discontinuation. However, Philips Respironics will continue to sell consumables and accessories, including masks.
What are my options for home mechanical ventilators?
Bethany L. Lussier, MD, FCCP: In the US, alternative approved home mechanical ventilator (HMV) devices include Astral by ResMed, Vivo 45 and Vivo 65 by Breas, and VOCSN by Ventec. Additional options made available through emergency use authorization by the FDA between 2020 and 2022 included Luisa by Löwenstein Medical, the V+ by Ventec, and Life2000 by Baxter. Many of us expedite disposition from the hospital by prescribing HMVs rather than respiratory assist devices (RADs) because it is easier to meet qualifying criteria for insurance. In efforts to promote just allocation of resources, now might be the ideal time to reconsider higher utilization of RADs over HMVs. Reasonable RAD candidates are those who do not need autotitration of EPAP, dual mode therapy, or invasive ventilation. In these cases, the qualifying criteria and patient needs may be met with a RAD capable of VAPS or BPAP-ST mode.
How are these alternative devices similar to and different from the Trilogy EVO?
Dr. Ackrivo:All these devices are portable ventilators that can deliver noninvasive or invasive ventilation. They have internal batteries for enabling portability. They offer multiple programmable presets and mouthpiece ventilation, and some offer both oxygenation and CO2 monitoring (both TcCO2 and EtCO2).
All alternative portable ventilators include a proprietary ventilation mode analogous to the Trilogy AVAPS algorithm (Table 2). The ResMed Astral has a safety tidal volume feature that targets a minimum tidal volume in PS, S/T, or P(A)C modes. The ResMed iVAPS algorithm adjusts inspiratory pressure and respiratory rate to target an alveolar ventilation based on patient-entered height. The Breas Vivo can target a tidal volume (TgV) in either PSV or PCV mode.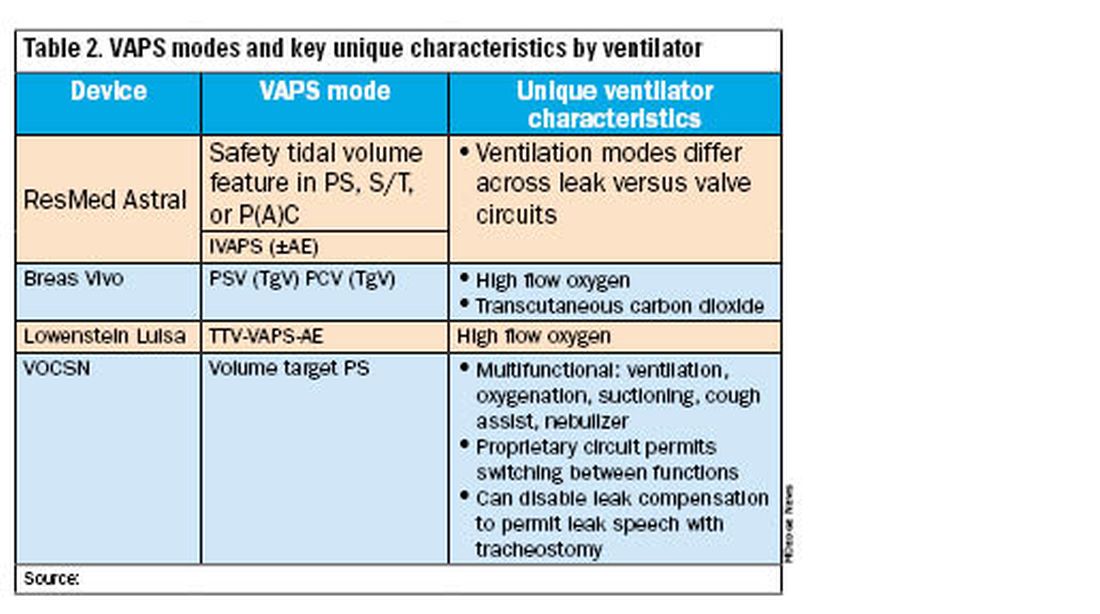
Unique ventilator characteristics are shown in Table 2. ResMed Astral mode options will differ between leak (passive) or valve (active) circuits. Both the Breas Vivo and Löwenstein Luisa enable high-flow oxygen delivery. Only the Breas Vivo enables connecting to a transcutaneous carbon dioxide monitor. The VOCSN name is an acronym for its multifunctional capabilities: ventilation, oxygenation, cough assist, suction, and nebulizer treatments. Lastly, the VOCSN can disable leak compensation, which may be advantageous for enabling leak speech with a tracheostomy.
I just provided my patient with a Trilogy EVO. Do I need to change this immediately?
Dr. Coleman: No, but you should start conversations with your patient/caregiving support and with your durable medical equipment (DME) provider about alternative options. The ripple effects of the Philips Respironics recall will be ongoing for years. The silver lining of this situation is that there are numerous HMV options on the market currently. It is important to review the differences between these new devices and consider what will work best for your patient and your practice. In addition, it is critical that your DME provider is familiar with these new devices, both for support and education, and is taking steps to make alternate devices available. We anticipate a push in coming months to switch patients off Trilogy EVO, so it important to get this process started.
For patients not interested in switching just yet, Philips Respironics will continue to service and offer supplies for these devices for up to 5 years, depending on part availability (Table 1). Refer to the Philips Respironics Sleep & Respiratory Product Portfolio Changes website for the most up-to-date information.
I have a patient on AVAPS, and I must change to iVAPS. What now?
Dr. Lussier: As mentioned previously by Dr. Ackrivo, the ResMed iVAPS algorithm adjusts inspiratory pressure and respiratory rate to target an alveolar ventilation based on patient-entered height. A download from a current VAPS setting can be helpful in defining target ventilation and pressure ranges for a tailored prescription. ResMed has an online iVAPS calculator (resmed.com) to assist in making this switch. Close clinical monitoring with data downloads is recommended to assure desired targets are still achieved.
What will happen to Philips Respironics’ cloud patient data?
Dr. Lussier: Representatives have reported that both providers and DME companies will have continued access to Care Orchestrator going forward. Currently, the logistics of data maintenance and ownership remain unclear, which poses additional questions about global access to patients’ data downloads.
----------
The recent discontinuation of Philips Respironics ventilation devices will induce a dramatic shift in home ventilation options in the US. Clinicians and DME companies should begin familiarizing themselves with alternative ventilators and their unique features. While significant uncertainty exists, we encourage a proactive approach to education and communication to ensure a smooth transition for patients on home ventilation.
John M. Coleman III, MD, FCCP, is Associate Professor, Division of Pulmonary & Critical Care Medicine, Department of Neurology, Northwestern University Feinberg School of Medicine. Bethany L. Lussier, MD, FCCP, is in the Department of Internal Medicine, Division of Pulmonary & Critical Care Medicine, Department of Neurology, Division of Neurocritical Care, UT Southwestern Medical Center. Jason Ackrivo, MD, is Assistant Professor of Medicine and Neurology, and Associate Director, Jay and Randy Fishman Program for Home Assisted Ventilation, Pulmonary, Allergy, and Critical Care Division, Perelman School of Medicine, University of Pennsylvania.
Specialist input on a sudden shift in device availability
Specialist input on a sudden shift in device availability
Philips Respironics released a public statement on January 25, 2024, that would dramatically change the landscape of home mechanical ventilation and sleep-disordered breathing management in the United States. The company announced that, effective immediately in the US and US territories, Philips Respironics would stop production and sale of all hospital and home mechanical ventilation products, home and hospital ventilation devices, and oxygen concentrators.
There are many unknowns and uncertainties about how to proceed with care for patients requiring these devices. So we gathered an expert panel of clinicians from CHEST’s Home-Based Mechanical Ventilation and Neuromuscular Section within the Sleep Medicine Network to explain the current situation and offer suggestions on moving forward in caring for these patients.
Why is this happening?
John M. Coleman III, MD, FCCP: To understand the current Philips Respironics announcement, we must go back to June 2021. At that time, Philips recalled certain home mechanical ventilators, CPAP machines, and BiPAP machines due to potential health risks related to breakdown of the polyester-based polyurethane (PE-PUR) foam placed in these devices for noise reduction. Small and microscopic particles of this foam were at risk for being inhaled or ingested by patients using these devices. It was suspected that inhalation of these particles could potentially result in temporary or permanent injury. Machines in hot temperatures or using ozone cleaning were at increased risk. The US Food and Drug Administration (FDA) issued a class 1 recall, defined as “a situation in which there is reasonable probability that the use of or exposure to a violative product will cause serious adverse health consequences or death.”
In the months following the initial recall, there were additional recalls of both in-hospital and home ventilators related to the potential of these foam particles to move and block the air path, reducing airflow and causing the device to alarm.
Over the next few years, tens of thousands of medical device reports were filed about PE-PUR foam-related injuries, with some cases resulting in death. At this time, the Department of Justice began collaborating with the FDA on a consent decree. There were ongoing recalls of the CoughAssist T70 device, as well as the newest generation of Philips Respironics home ventilators, the Trilogy EVO.
Ultimately, after years of ongoing recalls and reports of numerous deaths and injuries, with multiple class action lawsuits, the consent decree was finalized. Philips Respironics agreed to stop production of all respiratory-related products in the US and US territories.
What devices does this apply to?
Jason Ackrivo, MD: This notice affects the devices shown in Table 1. All sales and device shipments have been discontinued as of January 25, 2024. Philips Respironics will continue to service the devices, subject to part availability, up to 5 years after sales discontinuation. However, Philips Respironics will continue to sell consumables and accessories, including masks.

What are my options for home mechanical ventilators?
Bethany L. Lussier, MD, FCCP: In the US, alternative approved home mechanical ventilator (HMV) devices include Astral by ResMed, Vivo 45 and Vivo 65 by Breas, and VOCSN by Ventec. Additional options made available through emergency use authorization by the FDA between 2020 and 2022 included Luisa by Löwenstein Medical, the V+ by Ventec, and Life2000 by Baxter. Many of us expedite disposition from the hospital by prescribing HMVs rather than respiratory assist devices (RADs) because it is easier to meet qualifying criteria for insurance. In efforts to promote just allocation of resources, now might be the ideal time to reconsider higher utilization of RADs over HMVs. Reasonable RAD candidates are those who do not need autotitration of EPAP, dual mode therapy, or invasive ventilation. In these cases, the qualifying criteria and patient needs may be met with a RAD capable of VAPS or BPAP-ST mode.
How are these alternative devices similar to and different from the Trilogy EVO?
Dr. Ackrivo:All these devices are portable ventilators that can deliver noninvasive or invasive ventilation. They have internal batteries for enabling portability. They offer multiple programmable presets and mouthpiece ventilation, and some offer both oxygenation and CO2 monitoring (both TcCO2 and EtCO2).
All alternative portable ventilators include a proprietary ventilation mode analogous to the Trilogy AVAPS algorithm (Table 2). The ResMed Astral has a safety tidal volume feature that targets a minimum tidal volume in PS, S/T, or P(A)C modes. The ResMed iVAPS algorithm adjusts inspiratory pressure and respiratory rate to target an alveolar ventilation based on patient-entered height. The Breas Vivo can target a tidal volume (TgV) in either PSV or PCV mode.
Unique ventilator characteristics are shown in Table 2. ResMed Astral mode options will differ between leak (passive) or valve (active) circuits. Both the Breas Vivo and Löwenstein Luisa enable high-flow oxygen delivery. Only the Breas Vivo enables connecting to a transcutaneous carbon dioxide monitor. The VOCSN name is an acronym for its multifunctional capabilities: ventilation, oxygenation, cough assist, suction, and nebulizer treatments. Lastly, the VOCSN can disable leak compensation, which may be advantageous for enabling leak speech with a tracheostomy.
I just provided my patient with a Trilogy EVO. Do I need to change this immediately?
Dr. Coleman: No, but you should start conversations with your patient/caregiving support and with your durable medical equipment (DME) provider about alternative options. The ripple effects of the Philips Respironics recall will be ongoing for years. The silver lining of this situation is that there are numerous HMV options on the market currently. It is important to review the differences between these new devices and consider what will work best for your patient and your practice. In addition, it is critical that your DME provider is familiar with these new devices, both for support and education, and is taking steps to make alternate devices available. We anticipate a push in coming months to switch patients off Trilogy EVO, so it important to get this process started.
For patients not interested in switching just yet, Philips Respironics will continue to service and offer supplies for these devices for up to 5 years, depending on part availability (Table 1). Refer to the Philips Respironics Sleep & Respiratory Product Portfolio Changes website for the most up-to-date information.
I have a patient on AVAPS, and I must change to iVAPS. What now?
Dr. Lussier: As mentioned previously by Dr. Ackrivo, the ResMed iVAPS algorithm adjusts inspiratory pressure and respiratory rate to target an alveolar ventilation based on patient-entered height. A download from a current VAPS setting can be helpful in defining target ventilation and pressure ranges for a tailored prescription. ResMed has an online iVAPS calculator (resmed.com) to assist in making this switch. Close clinical monitoring with data downloads is recommended to assure desired targets are still achieved.
What will happen to Philips Respironics’ cloud patient data?
Dr. Lussier: Representatives have reported that both providers and DME companies will have continued access to Care Orchestrator going forward. Currently, the logistics of data maintenance and ownership remain unclear, which poses additional questions about global access to patients’ data downloads.
----------
The recent discontinuation of Philips Respironics ventilation devices will induce a dramatic shift in home ventilation options in the US. Clinicians and DME companies should begin familiarizing themselves with alternative ventilators and their unique features. While significant uncertainty exists, we encourage a proactive approach to education and communication to ensure a smooth transition for patients on home ventilation.
John M. Coleman III, MD, FCCP, is Associate Professor, Division of Pulmonary & Critical Care Medicine, Department of Neurology, Northwestern University Feinberg School of Medicine. Bethany L. Lussier, MD, FCCP, is in the Department of Internal Medicine, Division of Pulmonary & Critical Care Medicine, Department of Neurology, Division of Neurocritical Care, UT Southwestern Medical Center. Jason Ackrivo, MD, is Assistant Professor of Medicine and Neurology, and Associate Director, Jay and Randy Fishman Program for Home Assisted Ventilation, Pulmonary, Allergy, and Critical Care Division, Perelman School of Medicine, University of Pennsylvania.
Philips Respironics released a public statement on January 25, 2024, that would dramatically change the landscape of home mechanical ventilation and sleep-disordered breathing management in the United States. The company announced that, effective immediately in the US and US territories, Philips Respironics would stop production and sale of all hospital and home mechanical ventilation products, home and hospital ventilation devices, and oxygen concentrators.
There are many unknowns and uncertainties about how to proceed with care for patients requiring these devices. So we gathered an expert panel of clinicians from CHEST’s Home-Based Mechanical Ventilation and Neuromuscular Section within the Sleep Medicine Network to explain the current situation and offer suggestions on moving forward in caring for these patients.
Why is this happening?
John M. Coleman III, MD, FCCP: To understand the current Philips Respironics announcement, we must go back to June 2021. At that time, Philips recalled certain home mechanical ventilators, CPAP machines, and BiPAP machines due to potential health risks related to breakdown of the polyester-based polyurethane (PE-PUR) foam placed in these devices for noise reduction. Small and microscopic particles of this foam were at risk for being inhaled or ingested by patients using these devices. It was suspected that inhalation of these particles could potentially result in temporary or permanent injury. Machines in hot temperatures or using ozone cleaning were at increased risk. The US Food and Drug Administration (FDA) issued a class 1 recall, defined as “a situation in which there is reasonable probability that the use of or exposure to a violative product will cause serious adverse health consequences or death.”
In the months following the initial recall, there were additional recalls of both in-hospital and home ventilators related to the potential of these foam particles to move and block the air path, reducing airflow and causing the device to alarm.
Over the next few years, tens of thousands of medical device reports were filed about PE-PUR foam-related injuries, with some cases resulting in death. At this time, the Department of Justice began collaborating with the FDA on a consent decree. There were ongoing recalls of the CoughAssist T70 device, as well as the newest generation of Philips Respironics home ventilators, the Trilogy EVO.
Ultimately, after years of ongoing recalls and reports of numerous deaths and injuries, with multiple class action lawsuits, the consent decree was finalized. Philips Respironics agreed to stop production of all respiratory-related products in the US and US territories.
What devices does this apply to?
Jason Ackrivo, MD: This notice affects the devices shown in Table 1. All sales and device shipments have been discontinued as of January 25, 2024. Philips Respironics will continue to service the devices, subject to part availability, up to 5 years after sales discontinuation. However, Philips Respironics will continue to sell consumables and accessories, including masks.

What are my options for home mechanical ventilators?
Bethany L. Lussier, MD, FCCP: In the US, alternative approved home mechanical ventilator (HMV) devices include Astral by ResMed, Vivo 45 and Vivo 65 by Breas, and VOCSN by Ventec. Additional options made available through emergency use authorization by the FDA between 2020 and 2022 included Luisa by Löwenstein Medical, the V+ by Ventec, and Life2000 by Baxter. Many of us expedite disposition from the hospital by prescribing HMVs rather than respiratory assist devices (RADs) because it is easier to meet qualifying criteria for insurance. In efforts to promote just allocation of resources, now might be the ideal time to reconsider higher utilization of RADs over HMVs. Reasonable RAD candidates are those who do not need autotitration of EPAP, dual mode therapy, or invasive ventilation. In these cases, the qualifying criteria and patient needs may be met with a RAD capable of VAPS or BPAP-ST mode.
How are these alternative devices similar to and different from the Trilogy EVO?
Dr. Ackrivo:All these devices are portable ventilators that can deliver noninvasive or invasive ventilation. They have internal batteries for enabling portability. They offer multiple programmable presets and mouthpiece ventilation, and some offer both oxygenation and CO2 monitoring (both TcCO2 and EtCO2).
All alternative portable ventilators include a proprietary ventilation mode analogous to the Trilogy AVAPS algorithm (Table 2). The ResMed Astral has a safety tidal volume feature that targets a minimum tidal volume in PS, S/T, or P(A)C modes. The ResMed iVAPS algorithm adjusts inspiratory pressure and respiratory rate to target an alveolar ventilation based on patient-entered height. The Breas Vivo can target a tidal volume (TgV) in either PSV or PCV mode.
Unique ventilator characteristics are shown in Table 2. ResMed Astral mode options will differ between leak (passive) or valve (active) circuits. Both the Breas Vivo and Löwenstein Luisa enable high-flow oxygen delivery. Only the Breas Vivo enables connecting to a transcutaneous carbon dioxide monitor. The VOCSN name is an acronym for its multifunctional capabilities: ventilation, oxygenation, cough assist, suction, and nebulizer treatments. Lastly, the VOCSN can disable leak compensation, which may be advantageous for enabling leak speech with a tracheostomy.
I just provided my patient with a Trilogy EVO. Do I need to change this immediately?
Dr. Coleman: No, but you should start conversations with your patient/caregiving support and with your durable medical equipment (DME) provider about alternative options. The ripple effects of the Philips Respironics recall will be ongoing for years. The silver lining of this situation is that there are numerous HMV options on the market currently. It is important to review the differences between these new devices and consider what will work best for your patient and your practice. In addition, it is critical that your DME provider is familiar with these new devices, both for support and education, and is taking steps to make alternate devices available. We anticipate a push in coming months to switch patients off Trilogy EVO, so it important to get this process started.
For patients not interested in switching just yet, Philips Respironics will continue to service and offer supplies for these devices for up to 5 years, depending on part availability (Table 1). Refer to the Philips Respironics Sleep & Respiratory Product Portfolio Changes website for the most up-to-date information.
I have a patient on AVAPS, and I must change to iVAPS. What now?
Dr. Lussier: As mentioned previously by Dr. Ackrivo, the ResMed iVAPS algorithm adjusts inspiratory pressure and respiratory rate to target an alveolar ventilation based on patient-entered height. A download from a current VAPS setting can be helpful in defining target ventilation and pressure ranges for a tailored prescription. ResMed has an online iVAPS calculator (resmed.com) to assist in making this switch. Close clinical monitoring with data downloads is recommended to assure desired targets are still achieved.
What will happen to Philips Respironics’ cloud patient data?
Dr. Lussier: Representatives have reported that both providers and DME companies will have continued access to Care Orchestrator going forward. Currently, the logistics of data maintenance and ownership remain unclear, which poses additional questions about global access to patients’ data downloads.
----------
The recent discontinuation of Philips Respironics ventilation devices will induce a dramatic shift in home ventilation options in the US. Clinicians and DME companies should begin familiarizing themselves with alternative ventilators and their unique features. While significant uncertainty exists, we encourage a proactive approach to education and communication to ensure a smooth transition for patients on home ventilation.
John M. Coleman III, MD, FCCP, is Associate Professor, Division of Pulmonary & Critical Care Medicine, Department of Neurology, Northwestern University Feinberg School of Medicine. Bethany L. Lussier, MD, FCCP, is in the Department of Internal Medicine, Division of Pulmonary & Critical Care Medicine, Department of Neurology, Division of Neurocritical Care, UT Southwestern Medical Center. Jason Ackrivo, MD, is Assistant Professor of Medicine and Neurology, and Associate Director, Jay and Randy Fishman Program for Home Assisted Ventilation, Pulmonary, Allergy, and Critical Care Division, Perelman School of Medicine, University of Pennsylvania.



