User login
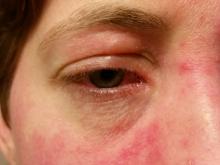
Retinal Perfusion Is Reduced During Migraine Attacks
Together, these changes could one day represent migraine biomarkers, authors say. The study was published online in Headache.
“We’re always looking for a biological marker for migraine,” said Alan M. Rapoport, MD, a clinical professor of neurology in the David Geffen School of Medicine at the University of California Los Angeles and past president of the International Headache Society. Researchers have identified many parameters that make people more likely to experience migraine, he said, but there remains no smoking gun. “We do not yet have a diagnostic test.”
Investigators have long been examining ocular vascular supply, added Dr. Rapoport, who was not involved with the study, because the eyes, visual system of the brain, and migraine are closely related. “But no one has ever figured out that one could use anything related to the eye as a definitive diagnostic test. This study was interesting because researchers used a very advanced technique to see if there are changes in the vascular supply to the eyeball during migraine.”
During Attacks
Study investigators prospectively enrolled 37 patients diagnosed with migraine with aura (MA), 30 with migraine without aura (MO), and 20 healthy controls. All subjects underwent macular OCTA for interictal analysis. A total of 20 patients with migraine (12 with MA and 8 with MO) underwent repeat scans during migraine attacks, and 5 control patients had repeat scans.
Compared with interictal measurements, significant parafoveal reductions in vessel flux index, an indicator of retinal perfusion, occurred in both the MA and MO groups during migraine attacks: –7% (95% CI, –10% to –4%; P = .006) and –7% (95% CI, –10% to –3%; P = .016), respectively, versus controls (2%, 95% CI, –3% to 7%).
The fact that migraine attacks resulted in reduced blood supply to the retinal region responsible for central vision is intriguing, said Dr. Rapoport, because sufficient reductions in blood supply there could result in blurred vision or other visual difficulties that might be mistaken for a true aura. “Many patients describe blurred vision related to their migraine headaches which do not usually qualify for an aura diagnosis,” he said.
Diagnostic criteria for MA, which afflicts around one third of people with migraine, include visual aberrations lasting at least 5 minutes and no more than 60 minutes. Visual aberrations average about 20-25 minutes, said Dr. Rapoport. “And we don’t usually accept blurred vision.” For most people who experience ictal blurred vision, he added, the phenomenon only lasts a short time and is not considered an aura.
More typical visual manifestations of MA include zigzag lines in an overall crescent shape that may blink, have bright edges, grow and shrink in size, and/or move across the visual field; patients also may have blind spots or distortions (e.g. far away vision, smaller or larger vision, or kaleidoscopic fractured vision). Nevertheless, said Dr. Rapoport, the study may shed light on why some people experiencing a migraine attack may suffer a brief bout of blurred vision and mistakenly report experiencing an aura.
Between Attacks
Comparing the two migraine groups interictally showed statistically significant differences in macular structure and function. Compared with the MO cohort, the MA cohort had higher circularity (mean [SD] 0.686 [0.088] vs. 0.629 [0.120] MO, P = .004), as well as a 13% (SD ± 10%, P = .003) lower foveal vessel flux index. “Not only is perfusion lower in both types of migraine during the attack,” said Dr. Rapoport, “but between attacks, people with MA had a lower blood supply to the retina than those who had MO.”
Unilateral Migraine
In a subset of patients (14 with MA and 12 with MO) whose headaches occurred unilaterally, investigators found retinal vascular parameters consistent with greater perfusion in the ipsilateral eye versus the contralateral eye. The significance of these findings remains unclear, Dr. Rapoport said, because circulatory findings revealed by CAT or MRI scans of patients with unilateral headaches are often normal or involve complex changes or mild edema on the side of the headache. The visual cortex on either side receives input from both eyes, he added.
Study Limitations
Authors acknowledged several study shortcomings. Most notably, COVID-19 restrictions resulted in a small sample size, and several patients (excluded from analysis) failed to return for repeat scans during migraine attacks. The study included patients with migraine attacks of varying frequency, and a handful of patients used acute rescue medications before undergoing ictal scans.
“If a future study corrected all these shortcomings,” Dr. Rapoport said, “the results might be more impressive and even more significant.” Based on these results alone, he said, it would be premature to pronounce OCTA-derived measurements of retinal perfusion and related parameters as future migraine biomarkers.
“But it’s a good start. If this hasn’t been done before, in quite this way, this is a very interesting study which, when repeated, should lead to even more significant findings.”
For now, the paper should remind practicing neurologists to dig deeper when patients complain of visual problems during migraine attacks. “It might be blurred vision for just 3 minutes,” he said. “Some patients may be calling it an aura, or the doctor may be thinking it is an aura because they’re not digging for further information in the history. We may now have a window into decreased retinal perfusion during a migraine attack and why some patients have blurred vision.”
The study was funded by the Amgen and the Baldwin Foundation. Dr. Rapoport is editor-in-chief of Neurology Reviews but reports no relevant relationships with the funders of this research.
Together, these changes could one day represent migraine biomarkers, authors say. The study was published online in Headache.
“We’re always looking for a biological marker for migraine,” said Alan M. Rapoport, MD, a clinical professor of neurology in the David Geffen School of Medicine at the University of California Los Angeles and past president of the International Headache Society. Researchers have identified many parameters that make people more likely to experience migraine, he said, but there remains no smoking gun. “We do not yet have a diagnostic test.”
Investigators have long been examining ocular vascular supply, added Dr. Rapoport, who was not involved with the study, because the eyes, visual system of the brain, and migraine are closely related. “But no one has ever figured out that one could use anything related to the eye as a definitive diagnostic test. This study was interesting because researchers used a very advanced technique to see if there are changes in the vascular supply to the eyeball during migraine.”
During Attacks
Study investigators prospectively enrolled 37 patients diagnosed with migraine with aura (MA), 30 with migraine without aura (MO), and 20 healthy controls. All subjects underwent macular OCTA for interictal analysis. A total of 20 patients with migraine (12 with MA and 8 with MO) underwent repeat scans during migraine attacks, and 5 control patients had repeat scans.
Compared with interictal measurements, significant parafoveal reductions in vessel flux index, an indicator of retinal perfusion, occurred in both the MA and MO groups during migraine attacks: –7% (95% CI, –10% to –4%; P = .006) and –7% (95% CI, –10% to –3%; P = .016), respectively, versus controls (2%, 95% CI, –3% to 7%).
The fact that migraine attacks resulted in reduced blood supply to the retinal region responsible for central vision is intriguing, said Dr. Rapoport, because sufficient reductions in blood supply there could result in blurred vision or other visual difficulties that might be mistaken for a true aura. “Many patients describe blurred vision related to their migraine headaches which do not usually qualify for an aura diagnosis,” he said.
Diagnostic criteria for MA, which afflicts around one third of people with migraine, include visual aberrations lasting at least 5 minutes and no more than 60 minutes. Visual aberrations average about 20-25 minutes, said Dr. Rapoport. “And we don’t usually accept blurred vision.” For most people who experience ictal blurred vision, he added, the phenomenon only lasts a short time and is not considered an aura.
More typical visual manifestations of MA include zigzag lines in an overall crescent shape that may blink, have bright edges, grow and shrink in size, and/or move across the visual field; patients also may have blind spots or distortions (e.g. far away vision, smaller or larger vision, or kaleidoscopic fractured vision). Nevertheless, said Dr. Rapoport, the study may shed light on why some people experiencing a migraine attack may suffer a brief bout of blurred vision and mistakenly report experiencing an aura.
Between Attacks
Comparing the two migraine groups interictally showed statistically significant differences in macular structure and function. Compared with the MO cohort, the MA cohort had higher circularity (mean [SD] 0.686 [0.088] vs. 0.629 [0.120] MO, P = .004), as well as a 13% (SD ± 10%, P = .003) lower foveal vessel flux index. “Not only is perfusion lower in both types of migraine during the attack,” said Dr. Rapoport, “but between attacks, people with MA had a lower blood supply to the retina than those who had MO.”
Unilateral Migraine
In a subset of patients (14 with MA and 12 with MO) whose headaches occurred unilaterally, investigators found retinal vascular parameters consistent with greater perfusion in the ipsilateral eye versus the contralateral eye. The significance of these findings remains unclear, Dr. Rapoport said, because circulatory findings revealed by CAT or MRI scans of patients with unilateral headaches are often normal or involve complex changes or mild edema on the side of the headache. The visual cortex on either side receives input from both eyes, he added.
Study Limitations
Authors acknowledged several study shortcomings. Most notably, COVID-19 restrictions resulted in a small sample size, and several patients (excluded from analysis) failed to return for repeat scans during migraine attacks. The study included patients with migraine attacks of varying frequency, and a handful of patients used acute rescue medications before undergoing ictal scans.
“If a future study corrected all these shortcomings,” Dr. Rapoport said, “the results might be more impressive and even more significant.” Based on these results alone, he said, it would be premature to pronounce OCTA-derived measurements of retinal perfusion and related parameters as future migraine biomarkers.
“But it’s a good start. If this hasn’t been done before, in quite this way, this is a very interesting study which, when repeated, should lead to even more significant findings.”
For now, the paper should remind practicing neurologists to dig deeper when patients complain of visual problems during migraine attacks. “It might be blurred vision for just 3 minutes,” he said. “Some patients may be calling it an aura, or the doctor may be thinking it is an aura because they’re not digging for further information in the history. We may now have a window into decreased retinal perfusion during a migraine attack and why some patients have blurred vision.”
The study was funded by the Amgen and the Baldwin Foundation. Dr. Rapoport is editor-in-chief of Neurology Reviews but reports no relevant relationships with the funders of this research.
Together, these changes could one day represent migraine biomarkers, authors say. The study was published online in Headache.
“We’re always looking for a biological marker for migraine,” said Alan M. Rapoport, MD, a clinical professor of neurology in the David Geffen School of Medicine at the University of California Los Angeles and past president of the International Headache Society. Researchers have identified many parameters that make people more likely to experience migraine, he said, but there remains no smoking gun. “We do not yet have a diagnostic test.”
Investigators have long been examining ocular vascular supply, added Dr. Rapoport, who was not involved with the study, because the eyes, visual system of the brain, and migraine are closely related. “But no one has ever figured out that one could use anything related to the eye as a definitive diagnostic test. This study was interesting because researchers used a very advanced technique to see if there are changes in the vascular supply to the eyeball during migraine.”
During Attacks
Study investigators prospectively enrolled 37 patients diagnosed with migraine with aura (MA), 30 with migraine without aura (MO), and 20 healthy controls. All subjects underwent macular OCTA for interictal analysis. A total of 20 patients with migraine (12 with MA and 8 with MO) underwent repeat scans during migraine attacks, and 5 control patients had repeat scans.
Compared with interictal measurements, significant parafoveal reductions in vessel flux index, an indicator of retinal perfusion, occurred in both the MA and MO groups during migraine attacks: –7% (95% CI, –10% to –4%; P = .006) and –7% (95% CI, –10% to –3%; P = .016), respectively, versus controls (2%, 95% CI, –3% to 7%).
The fact that migraine attacks resulted in reduced blood supply to the retinal region responsible for central vision is intriguing, said Dr. Rapoport, because sufficient reductions in blood supply there could result in blurred vision or other visual difficulties that might be mistaken for a true aura. “Many patients describe blurred vision related to their migraine headaches which do not usually qualify for an aura diagnosis,” he said.
Diagnostic criteria for MA, which afflicts around one third of people with migraine, include visual aberrations lasting at least 5 minutes and no more than 60 minutes. Visual aberrations average about 20-25 minutes, said Dr. Rapoport. “And we don’t usually accept blurred vision.” For most people who experience ictal blurred vision, he added, the phenomenon only lasts a short time and is not considered an aura.
More typical visual manifestations of MA include zigzag lines in an overall crescent shape that may blink, have bright edges, grow and shrink in size, and/or move across the visual field; patients also may have blind spots or distortions (e.g. far away vision, smaller or larger vision, or kaleidoscopic fractured vision). Nevertheless, said Dr. Rapoport, the study may shed light on why some people experiencing a migraine attack may suffer a brief bout of blurred vision and mistakenly report experiencing an aura.
Between Attacks
Comparing the two migraine groups interictally showed statistically significant differences in macular structure and function. Compared with the MO cohort, the MA cohort had higher circularity (mean [SD] 0.686 [0.088] vs. 0.629 [0.120] MO, P = .004), as well as a 13% (SD ± 10%, P = .003) lower foveal vessel flux index. “Not only is perfusion lower in both types of migraine during the attack,” said Dr. Rapoport, “but between attacks, people with MA had a lower blood supply to the retina than those who had MO.”
Unilateral Migraine
In a subset of patients (14 with MA and 12 with MO) whose headaches occurred unilaterally, investigators found retinal vascular parameters consistent with greater perfusion in the ipsilateral eye versus the contralateral eye. The significance of these findings remains unclear, Dr. Rapoport said, because circulatory findings revealed by CAT or MRI scans of patients with unilateral headaches are often normal or involve complex changes or mild edema on the side of the headache. The visual cortex on either side receives input from both eyes, he added.
Study Limitations
Authors acknowledged several study shortcomings. Most notably, COVID-19 restrictions resulted in a small sample size, and several patients (excluded from analysis) failed to return for repeat scans during migraine attacks. The study included patients with migraine attacks of varying frequency, and a handful of patients used acute rescue medications before undergoing ictal scans.
“If a future study corrected all these shortcomings,” Dr. Rapoport said, “the results might be more impressive and even more significant.” Based on these results alone, he said, it would be premature to pronounce OCTA-derived measurements of retinal perfusion and related parameters as future migraine biomarkers.
“But it’s a good start. If this hasn’t been done before, in quite this way, this is a very interesting study which, when repeated, should lead to even more significant findings.”
For now, the paper should remind practicing neurologists to dig deeper when patients complain of visual problems during migraine attacks. “It might be blurred vision for just 3 minutes,” he said. “Some patients may be calling it an aura, or the doctor may be thinking it is an aura because they’re not digging for further information in the history. We may now have a window into decreased retinal perfusion during a migraine attack and why some patients have blurred vision.”
The study was funded by the Amgen and the Baldwin Foundation. Dr. Rapoport is editor-in-chief of Neurology Reviews but reports no relevant relationships with the funders of this research.
FROM HEADACHE
GVHD raises vitiligo risk in transplant recipients
published online in JAMA Dermatology December 13.
In the cohort study, the greatest risk occurred with hematopoietic stem cell transplants (HSCTs) and in cases involving GVHD. Kidney and liver transplants carried slight increases in risk.
“The findings suggest that early detection and management of vitiligo lesions can be improved by estimating the likelihood of its development in transplant recipients and implementing a multidisciplinary approach for monitoring,” wrote the authors, from the departments of dermatology and biostatistics, at the Catholic University of Korea, Seoul.
Using claims data from South Korea’s National Health Insurance Service database, the investigators compared vitiligo incidence among 23,829 patients who had undergone solid organ transplantation (SOT) or HSCT between 2010 and 2017 versus that of 119,145 age- and sex-matched controls. At a mean observation time of 4.79 years in the transplant group (and 5.12 years for controls), the adjusted hazard ratio (AHR) for vitiligo among patients who had undergone any transplant was 1.73. AHRs for HSCT, liver transplants, and kidney transplants were 12.69, 1.63, and 1.50, respectively.
Patients who had undergone allogeneic HSCT (AHR, 14.43) or autologous transplants (AHR, 5.71), as well as those with and without GVHD (24.09 and 8.21, respectively) had significantly higher vitiligo risk than the control group.
Among those with GVHD, HSCT recipients (AHR, 16.42) and those with allogeneic grafts (AHR, 16.81) had a higher vitiligo risk than that of control patients.
In a subgroup that included 10,355 transplant recipients who underwent posttransplant health checkups, investigators found the highest vitiligo risk — AHR, 25.09 versus controls — among HSCT recipients with comorbid GVHD. However, patients who underwent SOT, autologous HSCT, or HSCT without GVHD showed no increased vitiligo risk in this analysis. “The results of health checkup data analysis may differ from the initial analysis due to additional adjustments for lifestyle factors and inclusion of only patients who underwent a health checkup,” the authors wrote.
Asked to comment on the results, George Han, MD, PhD, who was not involved with the study, told this news organization, “this is an interesting paper where the primary difference from previous studies is the new association between GVHD in hematopoietic stem cell transplant recipients and vitiligo.” Prior research had shown higher rates of vitiligo in HSCT recipients without making the GVHD distinction. Dr. Han is associate professor of dermatology in the Hofstra/Northwell Department of Dermatology, Hyde Park, New York.
Although GVHD may not be top-of-mind for dermatologists in daily practice, he said, the study enhances their understanding of vitiligo risk in HSCT recipients. “In some ways,” Dr. Han added, “the association makes sense, as the activated T cells from the graft attacking the skin in the HSCT recipient follow many of the mechanisms of vitiligo, including upregulating interferon gamma and the CXCR3/CXCL10 axis.”
Presently, he said, dermatologists worry more about solid organ recipients than about HSCT recipients because the long-term immunosuppression required by SOT increases the risk of squamous cell carcinoma (SCC). “However, the risk of skin cancers also seems to be elevated in HSCT recipients, and in this case the basal cell carcinoma (BCC):SCC ratio is not necessarily reversed as we see in solid organ transplant recipients. So the mechanisms are a bit less clear. Interestingly, acute and chronic GVHD have both been associated with increased risks of BCC and SCC/BCC, respectively.”
Overall, Dr. Han said, any transplant recipient should undergo yearly skin checks not only for skin cancers, but also for other skin conditions such as vitiligo. “It would be nice to see this codified into official guidelines, which can vary considerably but are overall more consistent in solid organ transplant recipients than in HSCT recipients. No such guidelines seem to be available for HSCTs.”
The study was funded by the Basic Research in Science & Engineering program through the National Research Foundation of Korea, which is funded by the country’s Ministry of Education. The study authors had no disclosures. Dr. Han reports no relevant financial interests.
published online in JAMA Dermatology December 13.
In the cohort study, the greatest risk occurred with hematopoietic stem cell transplants (HSCTs) and in cases involving GVHD. Kidney and liver transplants carried slight increases in risk.
“The findings suggest that early detection and management of vitiligo lesions can be improved by estimating the likelihood of its development in transplant recipients and implementing a multidisciplinary approach for monitoring,” wrote the authors, from the departments of dermatology and biostatistics, at the Catholic University of Korea, Seoul.
Using claims data from South Korea’s National Health Insurance Service database, the investigators compared vitiligo incidence among 23,829 patients who had undergone solid organ transplantation (SOT) or HSCT between 2010 and 2017 versus that of 119,145 age- and sex-matched controls. At a mean observation time of 4.79 years in the transplant group (and 5.12 years for controls), the adjusted hazard ratio (AHR) for vitiligo among patients who had undergone any transplant was 1.73. AHRs for HSCT, liver transplants, and kidney transplants were 12.69, 1.63, and 1.50, respectively.
Patients who had undergone allogeneic HSCT (AHR, 14.43) or autologous transplants (AHR, 5.71), as well as those with and without GVHD (24.09 and 8.21, respectively) had significantly higher vitiligo risk than the control group.
Among those with GVHD, HSCT recipients (AHR, 16.42) and those with allogeneic grafts (AHR, 16.81) had a higher vitiligo risk than that of control patients.
In a subgroup that included 10,355 transplant recipients who underwent posttransplant health checkups, investigators found the highest vitiligo risk — AHR, 25.09 versus controls — among HSCT recipients with comorbid GVHD. However, patients who underwent SOT, autologous HSCT, or HSCT without GVHD showed no increased vitiligo risk in this analysis. “The results of health checkup data analysis may differ from the initial analysis due to additional adjustments for lifestyle factors and inclusion of only patients who underwent a health checkup,” the authors wrote.
Asked to comment on the results, George Han, MD, PhD, who was not involved with the study, told this news organization, “this is an interesting paper where the primary difference from previous studies is the new association between GVHD in hematopoietic stem cell transplant recipients and vitiligo.” Prior research had shown higher rates of vitiligo in HSCT recipients without making the GVHD distinction. Dr. Han is associate professor of dermatology in the Hofstra/Northwell Department of Dermatology, Hyde Park, New York.
Although GVHD may not be top-of-mind for dermatologists in daily practice, he said, the study enhances their understanding of vitiligo risk in HSCT recipients. “In some ways,” Dr. Han added, “the association makes sense, as the activated T cells from the graft attacking the skin in the HSCT recipient follow many of the mechanisms of vitiligo, including upregulating interferon gamma and the CXCR3/CXCL10 axis.”
Presently, he said, dermatologists worry more about solid organ recipients than about HSCT recipients because the long-term immunosuppression required by SOT increases the risk of squamous cell carcinoma (SCC). “However, the risk of skin cancers also seems to be elevated in HSCT recipients, and in this case the basal cell carcinoma (BCC):SCC ratio is not necessarily reversed as we see in solid organ transplant recipients. So the mechanisms are a bit less clear. Interestingly, acute and chronic GVHD have both been associated with increased risks of BCC and SCC/BCC, respectively.”
Overall, Dr. Han said, any transplant recipient should undergo yearly skin checks not only for skin cancers, but also for other skin conditions such as vitiligo. “It would be nice to see this codified into official guidelines, which can vary considerably but are overall more consistent in solid organ transplant recipients than in HSCT recipients. No such guidelines seem to be available for HSCTs.”
The study was funded by the Basic Research in Science & Engineering program through the National Research Foundation of Korea, which is funded by the country’s Ministry of Education. The study authors had no disclosures. Dr. Han reports no relevant financial interests.
published online in JAMA Dermatology December 13.
In the cohort study, the greatest risk occurred with hematopoietic stem cell transplants (HSCTs) and in cases involving GVHD. Kidney and liver transplants carried slight increases in risk.
“The findings suggest that early detection and management of vitiligo lesions can be improved by estimating the likelihood of its development in transplant recipients and implementing a multidisciplinary approach for monitoring,” wrote the authors, from the departments of dermatology and biostatistics, at the Catholic University of Korea, Seoul.
Using claims data from South Korea’s National Health Insurance Service database, the investigators compared vitiligo incidence among 23,829 patients who had undergone solid organ transplantation (SOT) or HSCT between 2010 and 2017 versus that of 119,145 age- and sex-matched controls. At a mean observation time of 4.79 years in the transplant group (and 5.12 years for controls), the adjusted hazard ratio (AHR) for vitiligo among patients who had undergone any transplant was 1.73. AHRs for HSCT, liver transplants, and kidney transplants were 12.69, 1.63, and 1.50, respectively.
Patients who had undergone allogeneic HSCT (AHR, 14.43) or autologous transplants (AHR, 5.71), as well as those with and without GVHD (24.09 and 8.21, respectively) had significantly higher vitiligo risk than the control group.
Among those with GVHD, HSCT recipients (AHR, 16.42) and those with allogeneic grafts (AHR, 16.81) had a higher vitiligo risk than that of control patients.
In a subgroup that included 10,355 transplant recipients who underwent posttransplant health checkups, investigators found the highest vitiligo risk — AHR, 25.09 versus controls — among HSCT recipients with comorbid GVHD. However, patients who underwent SOT, autologous HSCT, or HSCT without GVHD showed no increased vitiligo risk in this analysis. “The results of health checkup data analysis may differ from the initial analysis due to additional adjustments for lifestyle factors and inclusion of only patients who underwent a health checkup,” the authors wrote.
Asked to comment on the results, George Han, MD, PhD, who was not involved with the study, told this news organization, “this is an interesting paper where the primary difference from previous studies is the new association between GVHD in hematopoietic stem cell transplant recipients and vitiligo.” Prior research had shown higher rates of vitiligo in HSCT recipients without making the GVHD distinction. Dr. Han is associate professor of dermatology in the Hofstra/Northwell Department of Dermatology, Hyde Park, New York.
Although GVHD may not be top-of-mind for dermatologists in daily practice, he said, the study enhances their understanding of vitiligo risk in HSCT recipients. “In some ways,” Dr. Han added, “the association makes sense, as the activated T cells from the graft attacking the skin in the HSCT recipient follow many of the mechanisms of vitiligo, including upregulating interferon gamma and the CXCR3/CXCL10 axis.”
Presently, he said, dermatologists worry more about solid organ recipients than about HSCT recipients because the long-term immunosuppression required by SOT increases the risk of squamous cell carcinoma (SCC). “However, the risk of skin cancers also seems to be elevated in HSCT recipients, and in this case the basal cell carcinoma (BCC):SCC ratio is not necessarily reversed as we see in solid organ transplant recipients. So the mechanisms are a bit less clear. Interestingly, acute and chronic GVHD have both been associated with increased risks of BCC and SCC/BCC, respectively.”
Overall, Dr. Han said, any transplant recipient should undergo yearly skin checks not only for skin cancers, but also for other skin conditions such as vitiligo. “It would be nice to see this codified into official guidelines, which can vary considerably but are overall more consistent in solid organ transplant recipients than in HSCT recipients. No such guidelines seem to be available for HSCTs.”
The study was funded by the Basic Research in Science & Engineering program through the National Research Foundation of Korea, which is funded by the country’s Ministry of Education. The study authors had no disclosures. Dr. Han reports no relevant financial interests.
FROM JAMA DERMATOLOGY
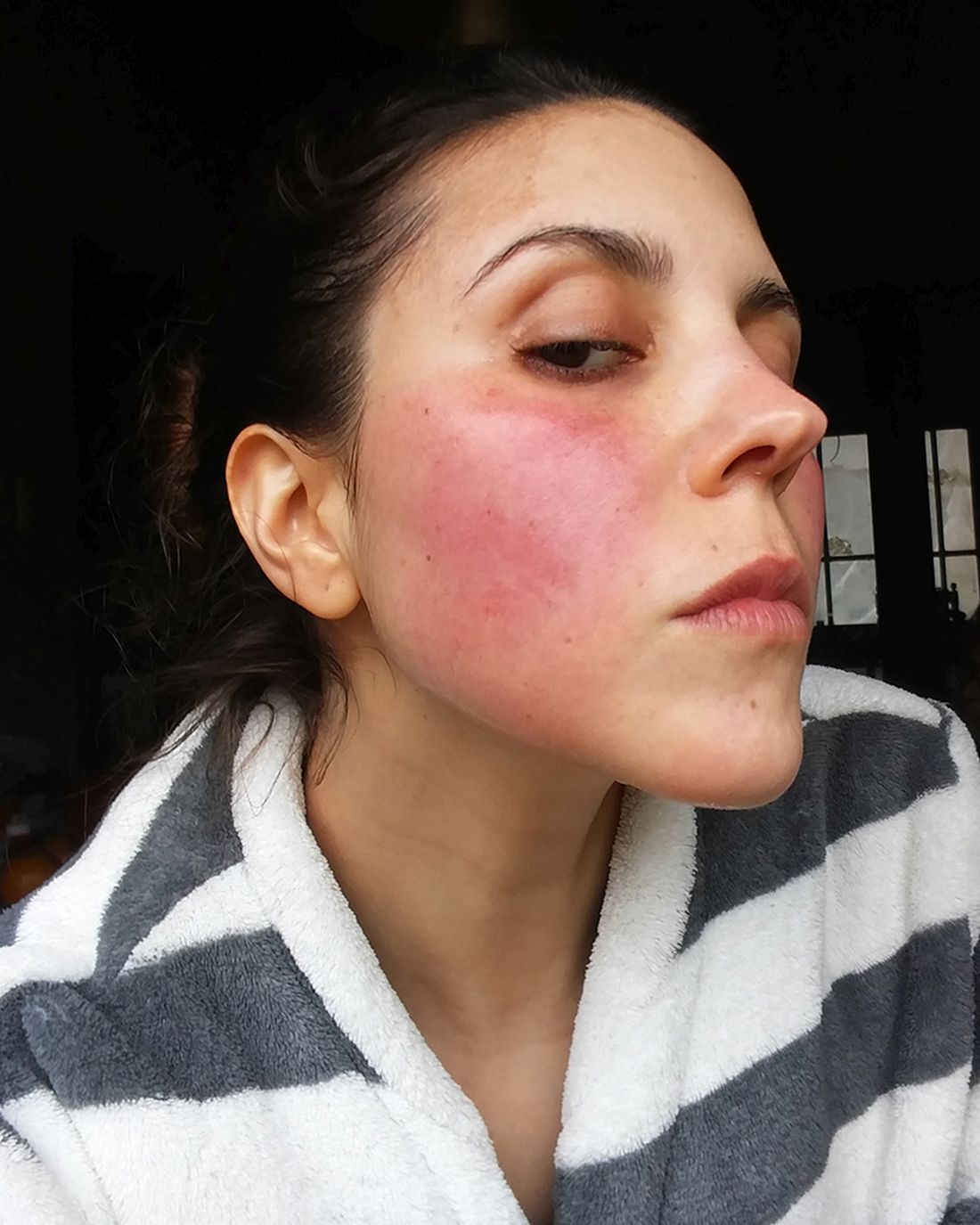
Dupilumab-associated lymphoid reactions require caution
, according to a study published in JAMA Dermatology
The potential for such reactions requires diagnosing AD carefully, monitoring patients on dupilumab for new and unusual symptoms, and thoroughly working up suspicious LRs, according to an accompanying editorial and experts interviewed for this article.
“Dupilumab has become such an important first-line systemic medication for our patients with moderate to severe atopic dermatitis. It’s important for us to understand everything we can about its use in the real world – both good and bad,”Raj Chovatiya, MD, PhD, MSCI, assistant professor of dermatology at Northwestern University, Chicago, said in an interview. He was uninvolved with either publication.
Robert Sidbury, MD, MPH, added that, although the affected patient group was small, studying lymphoid reactions associated with dupilumab is important because of the risk for diagnostic misadventure that these reactions carry. He is a professor of pediatrics and division head of dermatology at Seattle Children’s Hospital and the University of Washington, Seattle.
“AD and MF are easily confused for one another at baseline,” explained Dr. Sidbury, who was not involved with the study or editorial. “Dupilumab is known to make AD better and theoretically could help MF via its effect on interleukin (IL)–13, yet case reports of exacerbation and/or unmasking of MF are out there.”
For the study, researchers retrospectively examined records of 530 patients with AD treated with dupilumab at the University Medical Center Utrecht (the Netherlands). Reviewing pretreatment biopsies revealed that among 14 (2.6%) patients who developed clinical suspicion of cutaneous T-cell lymphoma (CTCL) while on treatment, three actually had preexisting MF.
All 14 patients with LR initially responded to dupilumab then developed worsening symptoms at a median of 4 months. Patients reported that the worsening lesions looked and felt different than did previous lesions, with symptoms including burning/pain and an appearance of generalized erythematous maculopapular plaques, sometimes with severe lichenification, on the lower trunk and upper thighs.
The 14 patients’ posttreatment biopsies showed an atypical lymphoid infiltrate with lichenoid or perivascular distribution and intraepithelial T-cell lymphocytes. Whereas patients with MF had hyperconvoluted cerebriform lymphocytes aligned in the epidermal basal layer at the dermoepidermal junction, the 11 with LR had similar-looking lesions dispersed throughout the upper epidermis.
Immunohistochemically, both groups had a dysregulated (mostly increased) CD4:CD8 ratio. CD30 overexpression, usually absent in early-stage MF, affected only patients with LR and one patient with advanced MF. In addition, patients with LR maintained pan–T-cell antigens (CD2, CD3, and CD5), whereas those with MF did not. The 11 patients with LR experienced biopsy-confirmed resolution once they discontinued dupilumab.
It is reassuring that the LRs resolved after dupilumab discontinuation, writes the author of the accompanying editorial, Joan Guitart, MD, chief of dermatopathology at Northwestern University. Nevertheless, he added, such patients deserve “a comprehensive workup including skin biopsy with T-cell receptor clonality assay, blood cell counts with flow cytometry analysis, serum lactate dehydrogenase, and documentation of possible adenopathy, followed with imaging studies and/or local biopsies in cases with abnormal results.”
The possibility that these LRs may represent a first step toward lymphoma requires dermatologists to remain vigilant in ruling out MF, Dr. Guitart wrote, particularly in atypical presentations such as adult-onset AD, cases lacking a history of AD, and cases involving erythrodermic and other uncharacteristic presentations such as plaques, nodules, or spared flexural sites.
For dermatopathologists, Dr. Guitart recommended a cautious approach that resists overdiagnosing MF and acknowledging that insufficient evidence exists to report such reactions as benign. The fact that one study patient had both MF and LR raises concerns that the LR may not always be reversible, Dr. Guitart added.
Clinicians and patients must consider the possibility of dupilumab-induced LR as part of the shared decision-making process and risk-benefit calculus, Dr. Sidbury said. In cases involving unexpected responses or atypical presentations, he added, clinicians must have a low threshold for stopping dupilumab.
For patients who must discontinue dupilumab because of LR, the list of treatment options is growing. “While more investigation is required to understand the role of newer IL-13–blocking biologics and JAK inhibitors among patients experiencing lymphoid reactions,” said Dr. Chovatiya, “traditional atopic dermatitis therapies like narrowband UVB phototherapy and the oral immunosuppressant methotrexate may be reassuring in this population.” Conversely, cyclosporine has been associated with progression of MF.
Also reassuring, said Dr. Sidbury and Dr. Chovatiya, is the rarity of LR overall. Dr. Sidbury said, “The numbers of patients in whom LR or onset/exacerbation of MF occurs is extraordinarily low when compared to those helped immeasurably by dupilumab.”
Dr. Sidbury added that the study and accompanying editorial also will alert clinicians to the potential for newer AD biologics that target solely IL-13 and not IL-4/13, as dupilumab does. “If the deregulated response leading to LR and potentially MF in the affected few is driven by IL-4 inhibition,” he said, “drugs such as tralokinumab (Adbry), lebrikizumab (once approved), and perhaps other newer options might calm AD without causing LRs.”
(Lebrikizumab is not yet approved. In an Oct. 2 press release, Eli Lilly and Company, developer of lebrikizumab, said that it would address issues the U.S. Food and Drug Administration had raised about a third-party manufacturing facility that arose during evaluation of the lebrikizumab biologic license application.)
Study limitations include the fact that most patients who experienced LR had already undergone skin biopsies before dupilumab treatment, which suggests that they had a more atypical AD presentation from the start. The authors add that their having treated all study patients in a tertiary referral hospital indicates a hard-to-treat AD subpopulation.
Study authors reported relationships with several biologic drug manufacturers including Sanofi and Regeneron (dupilumab), LEO Pharma (tralokinumab), and Eli Lilly (lebrikizumab). However, none of these companies provided support for the study.
Dr. Sidbury has been an investigator for Regeneron, Pfizer, and Galderma and a consultant for LEO Pharma and Eli Lilly. Dr. Chovatiya has served as an advisor, consultant, speaker, and investigator for Sanofi and Regeneron. Dr. Guitart reported no conflicts of interest.
A version of this article appeared on Medscape.com.
, according to a study published in JAMA Dermatology
The potential for such reactions requires diagnosing AD carefully, monitoring patients on dupilumab for new and unusual symptoms, and thoroughly working up suspicious LRs, according to an accompanying editorial and experts interviewed for this article.
“Dupilumab has become such an important first-line systemic medication for our patients with moderate to severe atopic dermatitis. It’s important for us to understand everything we can about its use in the real world – both good and bad,”Raj Chovatiya, MD, PhD, MSCI, assistant professor of dermatology at Northwestern University, Chicago, said in an interview. He was uninvolved with either publication.
Robert Sidbury, MD, MPH, added that, although the affected patient group was small, studying lymphoid reactions associated with dupilumab is important because of the risk for diagnostic misadventure that these reactions carry. He is a professor of pediatrics and division head of dermatology at Seattle Children’s Hospital and the University of Washington, Seattle.
“AD and MF are easily confused for one another at baseline,” explained Dr. Sidbury, who was not involved with the study or editorial. “Dupilumab is known to make AD better and theoretically could help MF via its effect on interleukin (IL)–13, yet case reports of exacerbation and/or unmasking of MF are out there.”
For the study, researchers retrospectively examined records of 530 patients with AD treated with dupilumab at the University Medical Center Utrecht (the Netherlands). Reviewing pretreatment biopsies revealed that among 14 (2.6%) patients who developed clinical suspicion of cutaneous T-cell lymphoma (CTCL) while on treatment, three actually had preexisting MF.
All 14 patients with LR initially responded to dupilumab then developed worsening symptoms at a median of 4 months. Patients reported that the worsening lesions looked and felt different than did previous lesions, with symptoms including burning/pain and an appearance of generalized erythematous maculopapular plaques, sometimes with severe lichenification, on the lower trunk and upper thighs.
The 14 patients’ posttreatment biopsies showed an atypical lymphoid infiltrate with lichenoid or perivascular distribution and intraepithelial T-cell lymphocytes. Whereas patients with MF had hyperconvoluted cerebriform lymphocytes aligned in the epidermal basal layer at the dermoepidermal junction, the 11 with LR had similar-looking lesions dispersed throughout the upper epidermis.
Immunohistochemically, both groups had a dysregulated (mostly increased) CD4:CD8 ratio. CD30 overexpression, usually absent in early-stage MF, affected only patients with LR and one patient with advanced MF. In addition, patients with LR maintained pan–T-cell antigens (CD2, CD3, and CD5), whereas those with MF did not. The 11 patients with LR experienced biopsy-confirmed resolution once they discontinued dupilumab.
It is reassuring that the LRs resolved after dupilumab discontinuation, writes the author of the accompanying editorial, Joan Guitart, MD, chief of dermatopathology at Northwestern University. Nevertheless, he added, such patients deserve “a comprehensive workup including skin biopsy with T-cell receptor clonality assay, blood cell counts with flow cytometry analysis, serum lactate dehydrogenase, and documentation of possible adenopathy, followed with imaging studies and/or local biopsies in cases with abnormal results.”
The possibility that these LRs may represent a first step toward lymphoma requires dermatologists to remain vigilant in ruling out MF, Dr. Guitart wrote, particularly in atypical presentations such as adult-onset AD, cases lacking a history of AD, and cases involving erythrodermic and other uncharacteristic presentations such as plaques, nodules, or spared flexural sites.
For dermatopathologists, Dr. Guitart recommended a cautious approach that resists overdiagnosing MF and acknowledging that insufficient evidence exists to report such reactions as benign. The fact that one study patient had both MF and LR raises concerns that the LR may not always be reversible, Dr. Guitart added.
Clinicians and patients must consider the possibility of dupilumab-induced LR as part of the shared decision-making process and risk-benefit calculus, Dr. Sidbury said. In cases involving unexpected responses or atypical presentations, he added, clinicians must have a low threshold for stopping dupilumab.
For patients who must discontinue dupilumab because of LR, the list of treatment options is growing. “While more investigation is required to understand the role of newer IL-13–blocking biologics and JAK inhibitors among patients experiencing lymphoid reactions,” said Dr. Chovatiya, “traditional atopic dermatitis therapies like narrowband UVB phototherapy and the oral immunosuppressant methotrexate may be reassuring in this population.” Conversely, cyclosporine has been associated with progression of MF.
Also reassuring, said Dr. Sidbury and Dr. Chovatiya, is the rarity of LR overall. Dr. Sidbury said, “The numbers of patients in whom LR or onset/exacerbation of MF occurs is extraordinarily low when compared to those helped immeasurably by dupilumab.”
Dr. Sidbury added that the study and accompanying editorial also will alert clinicians to the potential for newer AD biologics that target solely IL-13 and not IL-4/13, as dupilumab does. “If the deregulated response leading to LR and potentially MF in the affected few is driven by IL-4 inhibition,” he said, “drugs such as tralokinumab (Adbry), lebrikizumab (once approved), and perhaps other newer options might calm AD without causing LRs.”
(Lebrikizumab is not yet approved. In an Oct. 2 press release, Eli Lilly and Company, developer of lebrikizumab, said that it would address issues the U.S. Food and Drug Administration had raised about a third-party manufacturing facility that arose during evaluation of the lebrikizumab biologic license application.)
Study limitations include the fact that most patients who experienced LR had already undergone skin biopsies before dupilumab treatment, which suggests that they had a more atypical AD presentation from the start. The authors add that their having treated all study patients in a tertiary referral hospital indicates a hard-to-treat AD subpopulation.
Study authors reported relationships with several biologic drug manufacturers including Sanofi and Regeneron (dupilumab), LEO Pharma (tralokinumab), and Eli Lilly (lebrikizumab). However, none of these companies provided support for the study.
Dr. Sidbury has been an investigator for Regeneron, Pfizer, and Galderma and a consultant for LEO Pharma and Eli Lilly. Dr. Chovatiya has served as an advisor, consultant, speaker, and investigator for Sanofi and Regeneron. Dr. Guitart reported no conflicts of interest.
A version of this article appeared on Medscape.com.
, according to a study published in JAMA Dermatology
The potential for such reactions requires diagnosing AD carefully, monitoring patients on dupilumab for new and unusual symptoms, and thoroughly working up suspicious LRs, according to an accompanying editorial and experts interviewed for this article.
“Dupilumab has become such an important first-line systemic medication for our patients with moderate to severe atopic dermatitis. It’s important for us to understand everything we can about its use in the real world – both good and bad,”Raj Chovatiya, MD, PhD, MSCI, assistant professor of dermatology at Northwestern University, Chicago, said in an interview. He was uninvolved with either publication.
Robert Sidbury, MD, MPH, added that, although the affected patient group was small, studying lymphoid reactions associated with dupilumab is important because of the risk for diagnostic misadventure that these reactions carry. He is a professor of pediatrics and division head of dermatology at Seattle Children’s Hospital and the University of Washington, Seattle.
“AD and MF are easily confused for one another at baseline,” explained Dr. Sidbury, who was not involved with the study or editorial. “Dupilumab is known to make AD better and theoretically could help MF via its effect on interleukin (IL)–13, yet case reports of exacerbation and/or unmasking of MF are out there.”
For the study, researchers retrospectively examined records of 530 patients with AD treated with dupilumab at the University Medical Center Utrecht (the Netherlands). Reviewing pretreatment biopsies revealed that among 14 (2.6%) patients who developed clinical suspicion of cutaneous T-cell lymphoma (CTCL) while on treatment, three actually had preexisting MF.
All 14 patients with LR initially responded to dupilumab then developed worsening symptoms at a median of 4 months. Patients reported that the worsening lesions looked and felt different than did previous lesions, with symptoms including burning/pain and an appearance of generalized erythematous maculopapular plaques, sometimes with severe lichenification, on the lower trunk and upper thighs.
The 14 patients’ posttreatment biopsies showed an atypical lymphoid infiltrate with lichenoid or perivascular distribution and intraepithelial T-cell lymphocytes. Whereas patients with MF had hyperconvoluted cerebriform lymphocytes aligned in the epidermal basal layer at the dermoepidermal junction, the 11 with LR had similar-looking lesions dispersed throughout the upper epidermis.
Immunohistochemically, both groups had a dysregulated (mostly increased) CD4:CD8 ratio. CD30 overexpression, usually absent in early-stage MF, affected only patients with LR and one patient with advanced MF. In addition, patients with LR maintained pan–T-cell antigens (CD2, CD3, and CD5), whereas those with MF did not. The 11 patients with LR experienced biopsy-confirmed resolution once they discontinued dupilumab.
It is reassuring that the LRs resolved after dupilumab discontinuation, writes the author of the accompanying editorial, Joan Guitart, MD, chief of dermatopathology at Northwestern University. Nevertheless, he added, such patients deserve “a comprehensive workup including skin biopsy with T-cell receptor clonality assay, blood cell counts with flow cytometry analysis, serum lactate dehydrogenase, and documentation of possible adenopathy, followed with imaging studies and/or local biopsies in cases with abnormal results.”
The possibility that these LRs may represent a first step toward lymphoma requires dermatologists to remain vigilant in ruling out MF, Dr. Guitart wrote, particularly in atypical presentations such as adult-onset AD, cases lacking a history of AD, and cases involving erythrodermic and other uncharacteristic presentations such as plaques, nodules, or spared flexural sites.
For dermatopathologists, Dr. Guitart recommended a cautious approach that resists overdiagnosing MF and acknowledging that insufficient evidence exists to report such reactions as benign. The fact that one study patient had both MF and LR raises concerns that the LR may not always be reversible, Dr. Guitart added.
Clinicians and patients must consider the possibility of dupilumab-induced LR as part of the shared decision-making process and risk-benefit calculus, Dr. Sidbury said. In cases involving unexpected responses or atypical presentations, he added, clinicians must have a low threshold for stopping dupilumab.
For patients who must discontinue dupilumab because of LR, the list of treatment options is growing. “While more investigation is required to understand the role of newer IL-13–blocking biologics and JAK inhibitors among patients experiencing lymphoid reactions,” said Dr. Chovatiya, “traditional atopic dermatitis therapies like narrowband UVB phototherapy and the oral immunosuppressant methotrexate may be reassuring in this population.” Conversely, cyclosporine has been associated with progression of MF.
Also reassuring, said Dr. Sidbury and Dr. Chovatiya, is the rarity of LR overall. Dr. Sidbury said, “The numbers of patients in whom LR or onset/exacerbation of MF occurs is extraordinarily low when compared to those helped immeasurably by dupilumab.”
Dr. Sidbury added that the study and accompanying editorial also will alert clinicians to the potential for newer AD biologics that target solely IL-13 and not IL-4/13, as dupilumab does. “If the deregulated response leading to LR and potentially MF in the affected few is driven by IL-4 inhibition,” he said, “drugs such as tralokinumab (Adbry), lebrikizumab (once approved), and perhaps other newer options might calm AD without causing LRs.”
(Lebrikizumab is not yet approved. In an Oct. 2 press release, Eli Lilly and Company, developer of lebrikizumab, said that it would address issues the U.S. Food and Drug Administration had raised about a third-party manufacturing facility that arose during evaluation of the lebrikizumab biologic license application.)
Study limitations include the fact that most patients who experienced LR had already undergone skin biopsies before dupilumab treatment, which suggests that they had a more atypical AD presentation from the start. The authors add that their having treated all study patients in a tertiary referral hospital indicates a hard-to-treat AD subpopulation.
Study authors reported relationships with several biologic drug manufacturers including Sanofi and Regeneron (dupilumab), LEO Pharma (tralokinumab), and Eli Lilly (lebrikizumab). However, none of these companies provided support for the study.
Dr. Sidbury has been an investigator for Regeneron, Pfizer, and Galderma and a consultant for LEO Pharma and Eli Lilly. Dr. Chovatiya has served as an advisor, consultant, speaker, and investigator for Sanofi and Regeneron. Dr. Guitart reported no conflicts of interest.
A version of this article appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Guillain-Barré syndrome: Honing treatment strategies
Recent insights into the pathophysiology of Guillain-Barré syndrome (GBS) – which affects 1 or 2 persons for every 100,000 people annually, usually post infection – indicate that classic subtypes represent varying manifestations of a shared disease process. This knowledge is yielding new treatment strategies aimed at halting the illness in its tracks. Promising therapies include inhibitors of complement and, perhaps one day, the calcium-activated protease calpain.
Meanwhile, an association between COVID-19 and GBS has been debunked, whereas a small risk of GBS following adenovirus-vectored COVID vaccination is now accepted and quantified. Regardless of cause, the potential severity of GBS and variability in its presentation demand constant vigilance.
Shutting down the disease process
When patients present to an emergency department with sensory symptoms and increasing muscle weakness, “most of the damage has been or is being done,” said Michael P. Lunn, MBBS, MRCP, PhD, professor of clinical neurology, consultant neurologist, and clinical lead in neuroimmunology at University College London Queen Square Institute of Neurology, who spoke at length about GBS with Neurology Reviews 2023 Rare Neurological Disease Special Report. “The crucial reason that GBS treatment has not advanced significantly – and why we’re still slightly stuck where we are in terms of helping people get better more quickly – is that we need something that absolutely turns the disease off as patients come through the door.”
GBS is probably the best-understood autoimmune-mediated neurological disease, in some respects surpassing myasthenia gravis, Dr. Lunn said. “We know very frequently the organisms and stimuli that set off Guillain-Barré syndrome. We understand, to an extent, the immunology and how you break tolerance of the immune system so that an invading organism can provoke an immune response that damages peripheral nerves.”
Compared to what was known about GBS in decades past, neurologists now better understand how and where antibodies attack the nerve; how complement then damages the nodes of Ranvier and paranodes; and how an external attack results in sometimes irreparable internal nerve damage. “We’ve got a string, beginning to end, of understanding the disease,” declared Dr. Lunn.
Understanding of differences in the spectrum of pathology of GBS has led to additional diagnostic categories, said Dr. Lunn. Acute inflammatory demyelinating polyradiculoneuropathy, or typical GBS, represents the most common form in affluent Western nations. A motor variant was recognized in the 1980s; in the mid-1990s, Ho and colleagues described a cohort of patients in China who had acute motor axonal neuropathy and acute motor sensory axonal neuropathy1 – two forms that are particularly common throughout Asia and South America.
Shared mechanism
Based on the findings of electrophysiologic studies, Dr. Lunn said, experts traditionally believed that GBS attacked either axons themselves or their myelin sheaths. “That’s where the anti-ganglioside antibodies come in, providing targeting to nerve structures.” The dichotomous classification system, he added, was partially correct.
Then, through the 2010s and 2020s, neurophysiologist Antonio Uncini, MD, recognized, based partly on histologic studies by Ho and colleagues, that the myelin and axonal subtypes are both likely to stem from the same mechanism.2 When antibodies and complement damage the node of Ranvier, Dr. Lunn said, “the myelin gets stripped off and the conduction becomes slow. But then the myelin can return, and patients get better.” But if damage is severe, it severs the axon, resulting in unrecoverable motor axonal neuropathy. “It’s basically all the same spectrum of disease,” Dr. Lunn said. “Anti-ganglioside antibodies may account for different GBS ‘flavors,’ but the immunological attack all occurs at the node of Ranvier in one way or another.”
The foregoing insight has focused development efforts on the shared seminal pathway of all GBS subtypes and given rise to the concept of nodo-paranodopathy, which incorporates damage at either the node of Ranvier or nearby paranodes.3
Simultaneously, Spanish and French researchers began elucidating new antibodies responsible for neuropathology at the node of Ranvier.4 Anti-ganglioside antibodies have long been loosely associated with acute motor axonal neuropathy and poor outcomes, although, Dr. Lunn said, they fail to tell the full story. Anti-GQ1b antibodies are associated with the Miller-Fisher syndrome subtype, well recognized for its medical features: double vision, loss of tendon reflexes, and arm and leg weakness.
However, Dr. Lunn said, most GBS cases lack anti-ganglioside antibodies. In some GBS cases, antibodies attack neurofascin, contactins, and gliomedin, which are mainly adhesion proteins at nodes of Ranvier.
“Therefore,” Dr. Lunn said, “there must be an antibody-mediated attack of the node of Ranvier or the paranode. That’s an important series of discoveries, primarily because it helps us understand the immunological attack at the node of Ranvier, which goes along with what Dr. Uncini was saying. But it also divides off a group of chronic inflammatory demyelinating polyradiculoneuropathies (CIDP) that present acutely and look initially, for all purposes, like GBS.”
Recognizing acute CIDP (A-CIDP) is critically important for clinicians, Dr. Lunn stressed, because it requires treatment with rituximab (the most commonly used option), steroids, or plasma exchange.
Key clues that distinguish A-CIDP from GBS include:
• A high level of cerebrospinal fluid protein.
• Very slow nerve conduction.
• Early muscle wasting (rare in GBS).
Recognizing CIDP and A-CIDP is crucial, said Dr. Lunn, because it begins to bring all the pathology back together to make sense of GBS. Neurologists have known for decades that, if one damages a nerve with antibodies, then binds complement to those antibodies, the complement punches holes in the affected cells, resulting in death. “But it wasn’t quite clear how those cells might die,” Dr. Lunn said.
After complement-induced injury, calcium-activated calpain permanently damages the entire internal axonal structure.5 Perhaps more important, a 2022 mouse study showed that complement-mediated damage could be directed to myelin or axons using the genetically programmed presence or absence of gangliosides to understand subsequent calpain-induced destruction in either axons or myelin.6
Some of the engineered mouse cells included ganglioside; others did not. “So you can have anti-ganglioside antibodies directed at one cell type or the other, which would, or would not, have calpain within them,” Dr. Lunn said. Investigators also showed that a calpain inhibitor (AK295) or overproduction of an endogenous inhibitor, calpastatin, prevented damage to both cell types.6All existing calpain inhibitors are unsuitable for clinical use because they are highly toxic. “But if you could inhibit calpain and stop it from being activated by calcium,” Dr. Lunn explained, “you would have a mechanism for stopping cell degradation during GBS. That would be an important future target for pharmacotherapy. That whole story – from the beginning to the end of GBS – has opened up options for treatment.”
Because complement bound to antibodies, set up by infection, plays a pivotal role, complement inhibitors have become an exciting area of research over the past decade. The 36-patient Japanese Eculizumab Trial for GBS (JET-GBS) trial showed that, after 6 months, significantly more eculizumab-treated patients could run, compared with placebo-treated patients.7
“No other trials of complement inhibitors have yet been completed,” Dr. Lunn said. “But several different complement inhibitors work at different places, in a very complicated immune process. One of the complement inhibitors will become transformative in treating GBS – preventing disability and improving recovery – in the not-very-distant future.”
Additional investigational treatments that have demonstrated early promise in eliminating problem antibodies faster include imlifidase (Idefirix [Hansa Biopharma]), which destroys antibodies, and Fc receptor inhibitors such as efgartigimod alfa-fcab (Vyvgart [argenx]), which push antibodies into the natural catabolic pathway.
“We’ve been stuck with plasma exchange and intravenous immunoglobulin (IVIg) for three or four decades,” Dr. Lunn said. “We now have a series of strategies by which we can completely turn off complement and resulting nerve damage. If we can find a calpain inhibitor that turns off the end of that pathway, we will make dramatic improvements. Our understanding of the immunopathology has changed enormously and influences pharmacotherapy going forward.”
Recap of diagnosis and treatment
For decades, the diagnosis of GBS has relied on the presence of symptoms, including progressive weakness and loss of reflexes and sensations. Nerve-conduction studies and cerebrospinal fluid evaluation can help confirm the diagnosis.
IVIg shortens recovery, said Dr. Lunn, although nothing cures GBS. “And that’s a common problem: Clinicians think that they’re going to give somebody IVIg, and the patient’s going to get better immediately.” When that doesn’t happen, he said, physicians are tempted to give a second immunoglobulin dose.
However, a study published in 2021 shows that a second IVIg dose does not result in faster or better improvement – only in a significant risk of cardiovascular, cerebrovascular, and other thrombotic events 3 weeks later.8 Dr. Lunn noted that, although adverse-event data were “buried” in the supplemental materials of that study, the high cost of IVIg (approximately $12,500 per dose) means that the study has changed practice for the benefit of patients, providers, and health care systems.
COVID-19 and GBS triggers
Campylobacter jejuni infection still accounts for 30% to 40% of GBS cases, followed by other bacteria, including Mycoplasma pneumoniae and Haemophilus influenzae, and then by viruses, including cytomegalovirus and, rarely, human immunodeficiency virus. In recent years, severe acute respiratory syndrome coronavirus (SARS-CoV-2) infection – COVID-19 – and vaccines against the viral infection have captured headlines for purportedly being a cause of GBS.
The Zika virus epidemic of 2015-2016 has been linked to GBS-like illness. The 2003 severe acute respiratory syndrome (SARS) pandemic and the Middle East respiratory syndrome coronavirus (MERS-CoV) epidemic were associated with GBS – although, taken together, SARS and MERS-CoV produced fewer than 10 cases of GBS, Dr. Lunn noted. Nevertheless, heightened awareness of these viruses fueled hypervigilance regarding the prospect that COVID-19 could cause GBS. Following reports of a single such case in Wuhan and hundreds in Italy, worry over pandemic GBS grew worldwide.
Dr. Lunn and colleagues addressed the COVID-19–GBS question in a 2023 publication.9 “Because GBS is largely treated only with IVIg, and IVIg costs a lot of money, and the U.K. government insists on every dose of IVIg being logged in a government database, we were able to identify virtually every case of GBS,” he said.
GBS diagnoses were reliable, he added, because each case was confirmed by physicians outside the emergency department. Analysis revealed that, in 2020, U.K. GBS cases actually declined by around one-third. “And even when there was a second wave of COVID-19 at the end of 2020, partly caused by better counting,” Dr. Lunn said, “there was no further increase in GBS cases. We concluded that there was no link between GBS and COVID-19, as the cases simply didn’t appear.”
The foregoing findings have since been corroborated by studies in Singapore, the United States, and South America, he pointed out. Earlier case series suggesting a link between COVID-19 and GBS were selective, Dr. Lunn added, with numbers too small to support robust conclusions.
The lack of a causal link between COVID-19 and GBS suggested to Dr. Lunn that there was no reason COVID-19 vaccination should cause GBS. All COVID-19 vaccines were designed to provoke an immune response either (1) by producing the SARS-CoV-2 spike protein on the surface of virus (through a replication-incompetent adenoviral vector) or (2) through DNA or mRNA transcription, he explained. “The spike protein is only a small part of COVID-19.”
GBS: ‘Adverse event of interest’
A link between modern vaccines and GBS first appeared in the 1970s with the hastily developed swine flu vaccine. “In late 1976,” Dr. Lunn explained, “it was identified that patients who were given that vaccine seemed to be developing illnesses consistent with GBS.” By 1980, Dr. Lunn said, the risk level was determined to be only five or six cases for every 1 million doses of vaccine administered. “But the vaccine program was aborted, and swine flu never really happened.” Every year since, “there has been a surveillance program looking at the occurrence of an association of GBS with influenza vaccine.”
Minor fluctuations aside, he said, the overall incidence of GBS with influenza vaccination – 1 GBS case for every 1 million vaccine doses given – has remained consistent over several decades. “Nevertheless, GBS became an adverse event of special interest for any vaccination campaign.”
COVID-19 vaccination. Dr. Lunn and colleagues used the United Kingdom National Health Service (NHS) National Immunoglobulin Database, and other databases, to pinpoint the risk of GBS presented by the first dose of the AstraZeneca ChAdOx1 nCoV-19 adenoviral vaccine.10 As with U.K. GBS cases, every COVID-19 vaccination is linked to an NHS number. “We identified all the cases of GBS, found their NHS numbers, and went back and found the exact dates they’d been vaccinated, and with which vaccine.” Only the adenoviral-vector vaccine carried an excess risk of GBS – 5.8 cases for every 1 million doses, associated only with the first dose and peaking at approximately 25 days post vaccination – compared with other vaccines used in the United Kingdom.
Researchers looked at data from the Vaccine Adverse Event Reporting System (VAERS), a program of the Centers for Disease Control and Prevention and the Food and Drug Administration, encompassing nearly 500 million COVID-19 vaccine doses given between December 2020 and January 2022. They found that patients who received the Ad26.COV2.S vaccine (Janssen/Johnson & Johnson) had a rate of GBS (within 21 and 41 days post vaccination) that was 9 and 12 times higher, respectively, than corresponding rates for the mRNA-1273 (Moderna) and BNT162b2 (Pfizer BioNTech) COVID-19 vaccines.11 Risk was distributed relatively evenly by gender and age. Also at day 21 and day 41, observed event ratios with the adenoviral-vector vaccine (use of which has been suspended in the United States) were 3.79 and 2.34, respectively. Observed-event ratios with the other vaccines mirrored expected background rates.
The VAERS analysis confirms earlier data from the CDC’s Vaccine Safety Datalink, which showed that, among approximately 15 million U.S. vaccine doses given between mid-December 2020 and mid-November 2021, the unadjusted GBS incidence rate for every 100,000 person-years for the adenoviral vaccine, 21 days post exposure, was 32.4, compared with 1.3 for the mRNA vaccine. The adjusted relative risk with the adenoviral vaccine in the first 3 weeks post vaccination, compared to the 3- to 6-week interval post vaccination, was 6.03.12 In addition, a head-to-head comparison of adenoviral versus mRNA vaccines at 21 days revealed an adjusted rate ratio of 20.56. Mechanistically, some experts theorize that antibodies induced by the Janssen vaccine might cross-react with glycoproteins on the myelin sheath of peripheral nerve axons to cause GBS, but this remains unproven.11
The AstraZeneca vaccine uses a chimpanzee adenovirus; the Janssen vaccine uses a human adenoviral carrier. “The only commonality between the Janssen/Johnson & Johnson and AstraZeneca vaccines, and the only thing that’s different from the other vaccines, is the adenoviral vector packaging,” Dr. Lunn emphasized. “I believe it’s what generates GBS after COVID-19 vaccination. It has nothing to do with the COVID-19 vaccination, the spike protein, the nucleic acid, the DNA, or anything else.”
The adenoviral vector probably also explains why GBS peaks during winter, said Dr. Lunn. “That’s when adenovirus is circulating.” When people contract the common cold, he explained, they don’t visit their family physician and request a swab to isolate the adenovirus. “By the time you get GBS, the adenovirus has been cleared. We’ve all got antibodies to adenovirus all over the place, anyway, because we get it so often.”
It would be difficult to prove conclusively that adenovirus belongs on the list of GBS causes, Dr. Lunn allowed. “But I have a strong suspicion that it does. COVID-19 and COVID-19 vaccination have given us some new avenues into identifying GBS causation potentially in the near future.” More research is needed in this area, he said.
Dr. Lunn has been a principal investigator for argenx (efgartigimod) and an adviser to AstraZeneca (ChAdOx1 nCoV-19). He has received travel grants from CSL Behring.
References
1. Ho TW et al. Guillain-Barré syndrome in northern China. Relationship to Campylobacter jejuni infection and anti-glycolipid antibodies. Brain. 1995;118(Pt 3):597-605. doi: 10.1093/brain/118.3.597.
2. Uncini A. A common mechanism and a new categorization for anti-ganglioside antibody-mediated neuropathies. Exp Neurol. 2012;235(2):513-6. doi: 10.1016/j.expneurol.2012.03.023.
3. Uncini A and Kuwabara S. The electrodiagnosis of Guillain-Barré syndrome subtypes: where do we stand? Clin Neurophysiol. 2018;129(12):2586-93. doi: 10.1016/j.clinph.2018.09.025.
4. Delmont E et al. Autoantibodies to nodal isoforms of neurofascin in chronic inflammatory demyelinating polyneuropathy. Brain. 2017;140(7):1851-8. doi: 10.1093/brain/awx124.
5. McGonigal R et al. Anti-GD1a antibodies activate complement and calpain to injure distal motor nodes of Ranvier in mice. Brain. 2010;133(Pt 7):1944-60. doi: 10.1093/brain/awq119.
6. Cunningham ME et al. Real time imaging of intra-axonal calcium flux in an explant mouse model of axonal Guillain-Barré syndrome. Exp Neurol. 2022 Sep;355:114127. doi: 10.1016/j.expneurol.2022.114127.
7. Misawa S et al; Japanese Eculizumab Trial for GBS (JET-GBS) Study Group. Safety and efficacy of eculizumab in Guillain-Barré syndrome: a multicentre, double-blind, randomised phase 2 trial. Lancet Neurol. 2018;17(6):519-29. doi: 10.1016/S1474-4422(18)30114-5.
8. Walgaard C et al; Dutch GBS Study Group. Second intravenous immunoglobulin dose in patients with Guillain-Barré syndrome with poor prognosis (SID-GBS): a double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2021;20(4):275-83. doi: 10.1016/S1474-4422(20)30494-4.
9. Keddie S et al. Epidemiological and cohort study finds no association between COVID-19 and Guillain-Barré syndrome. Brain. 2021;144(2):682-93. doi: 10.1093/brain/awaa433.
10. Keh RYS et al; BPNS/ABN COVID-19 Vaccine GBS Study Group. COVID-19 vaccination and Guillain-Barré syndrome: Analyses using the National Immunoglobulin Database. Brain. 2023;146(2):739-48. doi: 10.1093/brain/awac067.
11. Abara WE et al. Reports of Guillain-Barré syndrome after COVID-19 vaccination in the United States. JAMA Netw Open. 2023;6(2):e2253845. doi: 10.1001/jamanetworkopen.2022.53845.
12. Hanson KE et al. Incidence of Guillain-Barré syndrome after COVID-19 vaccination in the Vaccine Safety Datalink. JAMA Netw Open. 2022;5(4):e228879. doi: 10.1001/jamanetworkopen.2022.8879.
Recent insights into the pathophysiology of Guillain-Barré syndrome (GBS) – which affects 1 or 2 persons for every 100,000 people annually, usually post infection – indicate that classic subtypes represent varying manifestations of a shared disease process. This knowledge is yielding new treatment strategies aimed at halting the illness in its tracks. Promising therapies include inhibitors of complement and, perhaps one day, the calcium-activated protease calpain.
Meanwhile, an association between COVID-19 and GBS has been debunked, whereas a small risk of GBS following adenovirus-vectored COVID vaccination is now accepted and quantified. Regardless of cause, the potential severity of GBS and variability in its presentation demand constant vigilance.
Shutting down the disease process
When patients present to an emergency department with sensory symptoms and increasing muscle weakness, “most of the damage has been or is being done,” said Michael P. Lunn, MBBS, MRCP, PhD, professor of clinical neurology, consultant neurologist, and clinical lead in neuroimmunology at University College London Queen Square Institute of Neurology, who spoke at length about GBS with Neurology Reviews 2023 Rare Neurological Disease Special Report. “The crucial reason that GBS treatment has not advanced significantly – and why we’re still slightly stuck where we are in terms of helping people get better more quickly – is that we need something that absolutely turns the disease off as patients come through the door.”
GBS is probably the best-understood autoimmune-mediated neurological disease, in some respects surpassing myasthenia gravis, Dr. Lunn said. “We know very frequently the organisms and stimuli that set off Guillain-Barré syndrome. We understand, to an extent, the immunology and how you break tolerance of the immune system so that an invading organism can provoke an immune response that damages peripheral nerves.”
Compared to what was known about GBS in decades past, neurologists now better understand how and where antibodies attack the nerve; how complement then damages the nodes of Ranvier and paranodes; and how an external attack results in sometimes irreparable internal nerve damage. “We’ve got a string, beginning to end, of understanding the disease,” declared Dr. Lunn.
Understanding of differences in the spectrum of pathology of GBS has led to additional diagnostic categories, said Dr. Lunn. Acute inflammatory demyelinating polyradiculoneuropathy, or typical GBS, represents the most common form in affluent Western nations. A motor variant was recognized in the 1980s; in the mid-1990s, Ho and colleagues described a cohort of patients in China who had acute motor axonal neuropathy and acute motor sensory axonal neuropathy1 – two forms that are particularly common throughout Asia and South America.
Shared mechanism
Based on the findings of electrophysiologic studies, Dr. Lunn said, experts traditionally believed that GBS attacked either axons themselves or their myelin sheaths. “That’s where the anti-ganglioside antibodies come in, providing targeting to nerve structures.” The dichotomous classification system, he added, was partially correct.
Then, through the 2010s and 2020s, neurophysiologist Antonio Uncini, MD, recognized, based partly on histologic studies by Ho and colleagues, that the myelin and axonal subtypes are both likely to stem from the same mechanism.2 When antibodies and complement damage the node of Ranvier, Dr. Lunn said, “the myelin gets stripped off and the conduction becomes slow. But then the myelin can return, and patients get better.” But if damage is severe, it severs the axon, resulting in unrecoverable motor axonal neuropathy. “It’s basically all the same spectrum of disease,” Dr. Lunn said. “Anti-ganglioside antibodies may account for different GBS ‘flavors,’ but the immunological attack all occurs at the node of Ranvier in one way or another.”
The foregoing insight has focused development efforts on the shared seminal pathway of all GBS subtypes and given rise to the concept of nodo-paranodopathy, which incorporates damage at either the node of Ranvier or nearby paranodes.3
Simultaneously, Spanish and French researchers began elucidating new antibodies responsible for neuropathology at the node of Ranvier.4 Anti-ganglioside antibodies have long been loosely associated with acute motor axonal neuropathy and poor outcomes, although, Dr. Lunn said, they fail to tell the full story. Anti-GQ1b antibodies are associated with the Miller-Fisher syndrome subtype, well recognized for its medical features: double vision, loss of tendon reflexes, and arm and leg weakness.
However, Dr. Lunn said, most GBS cases lack anti-ganglioside antibodies. In some GBS cases, antibodies attack neurofascin, contactins, and gliomedin, which are mainly adhesion proteins at nodes of Ranvier.
“Therefore,” Dr. Lunn said, “there must be an antibody-mediated attack of the node of Ranvier or the paranode. That’s an important series of discoveries, primarily because it helps us understand the immunological attack at the node of Ranvier, which goes along with what Dr. Uncini was saying. But it also divides off a group of chronic inflammatory demyelinating polyradiculoneuropathies (CIDP) that present acutely and look initially, for all purposes, like GBS.”
Recognizing acute CIDP (A-CIDP) is critically important for clinicians, Dr. Lunn stressed, because it requires treatment with rituximab (the most commonly used option), steroids, or plasma exchange.
Key clues that distinguish A-CIDP from GBS include:
• A high level of cerebrospinal fluid protein.
• Very slow nerve conduction.
• Early muscle wasting (rare in GBS).
Recognizing CIDP and A-CIDP is crucial, said Dr. Lunn, because it begins to bring all the pathology back together to make sense of GBS. Neurologists have known for decades that, if one damages a nerve with antibodies, then binds complement to those antibodies, the complement punches holes in the affected cells, resulting in death. “But it wasn’t quite clear how those cells might die,” Dr. Lunn said.
After complement-induced injury, calcium-activated calpain permanently damages the entire internal axonal structure.5 Perhaps more important, a 2022 mouse study showed that complement-mediated damage could be directed to myelin or axons using the genetically programmed presence or absence of gangliosides to understand subsequent calpain-induced destruction in either axons or myelin.6
Some of the engineered mouse cells included ganglioside; others did not. “So you can have anti-ganglioside antibodies directed at one cell type or the other, which would, or would not, have calpain within them,” Dr. Lunn said. Investigators also showed that a calpain inhibitor (AK295) or overproduction of an endogenous inhibitor, calpastatin, prevented damage to both cell types.6All existing calpain inhibitors are unsuitable for clinical use because they are highly toxic. “But if you could inhibit calpain and stop it from being activated by calcium,” Dr. Lunn explained, “you would have a mechanism for stopping cell degradation during GBS. That would be an important future target for pharmacotherapy. That whole story – from the beginning to the end of GBS – has opened up options for treatment.”
Because complement bound to antibodies, set up by infection, plays a pivotal role, complement inhibitors have become an exciting area of research over the past decade. The 36-patient Japanese Eculizumab Trial for GBS (JET-GBS) trial showed that, after 6 months, significantly more eculizumab-treated patients could run, compared with placebo-treated patients.7
“No other trials of complement inhibitors have yet been completed,” Dr. Lunn said. “But several different complement inhibitors work at different places, in a very complicated immune process. One of the complement inhibitors will become transformative in treating GBS – preventing disability and improving recovery – in the not-very-distant future.”
Additional investigational treatments that have demonstrated early promise in eliminating problem antibodies faster include imlifidase (Idefirix [Hansa Biopharma]), which destroys antibodies, and Fc receptor inhibitors such as efgartigimod alfa-fcab (Vyvgart [argenx]), which push antibodies into the natural catabolic pathway.
“We’ve been stuck with plasma exchange and intravenous immunoglobulin (IVIg) for three or four decades,” Dr. Lunn said. “We now have a series of strategies by which we can completely turn off complement and resulting nerve damage. If we can find a calpain inhibitor that turns off the end of that pathway, we will make dramatic improvements. Our understanding of the immunopathology has changed enormously and influences pharmacotherapy going forward.”
Recap of diagnosis and treatment
For decades, the diagnosis of GBS has relied on the presence of symptoms, including progressive weakness and loss of reflexes and sensations. Nerve-conduction studies and cerebrospinal fluid evaluation can help confirm the diagnosis.
IVIg shortens recovery, said Dr. Lunn, although nothing cures GBS. “And that’s a common problem: Clinicians think that they’re going to give somebody IVIg, and the patient’s going to get better immediately.” When that doesn’t happen, he said, physicians are tempted to give a second immunoglobulin dose.
However, a study published in 2021 shows that a second IVIg dose does not result in faster or better improvement – only in a significant risk of cardiovascular, cerebrovascular, and other thrombotic events 3 weeks later.8 Dr. Lunn noted that, although adverse-event data were “buried” in the supplemental materials of that study, the high cost of IVIg (approximately $12,500 per dose) means that the study has changed practice for the benefit of patients, providers, and health care systems.
COVID-19 and GBS triggers
Campylobacter jejuni infection still accounts for 30% to 40% of GBS cases, followed by other bacteria, including Mycoplasma pneumoniae and Haemophilus influenzae, and then by viruses, including cytomegalovirus and, rarely, human immunodeficiency virus. In recent years, severe acute respiratory syndrome coronavirus (SARS-CoV-2) infection – COVID-19 – and vaccines against the viral infection have captured headlines for purportedly being a cause of GBS.
The Zika virus epidemic of 2015-2016 has been linked to GBS-like illness. The 2003 severe acute respiratory syndrome (SARS) pandemic and the Middle East respiratory syndrome coronavirus (MERS-CoV) epidemic were associated with GBS – although, taken together, SARS and MERS-CoV produced fewer than 10 cases of GBS, Dr. Lunn noted. Nevertheless, heightened awareness of these viruses fueled hypervigilance regarding the prospect that COVID-19 could cause GBS. Following reports of a single such case in Wuhan and hundreds in Italy, worry over pandemic GBS grew worldwide.
Dr. Lunn and colleagues addressed the COVID-19–GBS question in a 2023 publication.9 “Because GBS is largely treated only with IVIg, and IVIg costs a lot of money, and the U.K. government insists on every dose of IVIg being logged in a government database, we were able to identify virtually every case of GBS,” he said.
GBS diagnoses were reliable, he added, because each case was confirmed by physicians outside the emergency department. Analysis revealed that, in 2020, U.K. GBS cases actually declined by around one-third. “And even when there was a second wave of COVID-19 at the end of 2020, partly caused by better counting,” Dr. Lunn said, “there was no further increase in GBS cases. We concluded that there was no link between GBS and COVID-19, as the cases simply didn’t appear.”
The foregoing findings have since been corroborated by studies in Singapore, the United States, and South America, he pointed out. Earlier case series suggesting a link between COVID-19 and GBS were selective, Dr. Lunn added, with numbers too small to support robust conclusions.
The lack of a causal link between COVID-19 and GBS suggested to Dr. Lunn that there was no reason COVID-19 vaccination should cause GBS. All COVID-19 vaccines were designed to provoke an immune response either (1) by producing the SARS-CoV-2 spike protein on the surface of virus (through a replication-incompetent adenoviral vector) or (2) through DNA or mRNA transcription, he explained. “The spike protein is only a small part of COVID-19.”
GBS: ‘Adverse event of interest’
A link between modern vaccines and GBS first appeared in the 1970s with the hastily developed swine flu vaccine. “In late 1976,” Dr. Lunn explained, “it was identified that patients who were given that vaccine seemed to be developing illnesses consistent with GBS.” By 1980, Dr. Lunn said, the risk level was determined to be only five or six cases for every 1 million doses of vaccine administered. “But the vaccine program was aborted, and swine flu never really happened.” Every year since, “there has been a surveillance program looking at the occurrence of an association of GBS with influenza vaccine.”
Minor fluctuations aside, he said, the overall incidence of GBS with influenza vaccination – 1 GBS case for every 1 million vaccine doses given – has remained consistent over several decades. “Nevertheless, GBS became an adverse event of special interest for any vaccination campaign.”
COVID-19 vaccination. Dr. Lunn and colleagues used the United Kingdom National Health Service (NHS) National Immunoglobulin Database, and other databases, to pinpoint the risk of GBS presented by the first dose of the AstraZeneca ChAdOx1 nCoV-19 adenoviral vaccine.10 As with U.K. GBS cases, every COVID-19 vaccination is linked to an NHS number. “We identified all the cases of GBS, found their NHS numbers, and went back and found the exact dates they’d been vaccinated, and with which vaccine.” Only the adenoviral-vector vaccine carried an excess risk of GBS – 5.8 cases for every 1 million doses, associated only with the first dose and peaking at approximately 25 days post vaccination – compared with other vaccines used in the United Kingdom.
Researchers looked at data from the Vaccine Adverse Event Reporting System (VAERS), a program of the Centers for Disease Control and Prevention and the Food and Drug Administration, encompassing nearly 500 million COVID-19 vaccine doses given between December 2020 and January 2022. They found that patients who received the Ad26.COV2.S vaccine (Janssen/Johnson & Johnson) had a rate of GBS (within 21 and 41 days post vaccination) that was 9 and 12 times higher, respectively, than corresponding rates for the mRNA-1273 (Moderna) and BNT162b2 (Pfizer BioNTech) COVID-19 vaccines.11 Risk was distributed relatively evenly by gender and age. Also at day 21 and day 41, observed event ratios with the adenoviral-vector vaccine (use of which has been suspended in the United States) were 3.79 and 2.34, respectively. Observed-event ratios with the other vaccines mirrored expected background rates.
The VAERS analysis confirms earlier data from the CDC’s Vaccine Safety Datalink, which showed that, among approximately 15 million U.S. vaccine doses given between mid-December 2020 and mid-November 2021, the unadjusted GBS incidence rate for every 100,000 person-years for the adenoviral vaccine, 21 days post exposure, was 32.4, compared with 1.3 for the mRNA vaccine. The adjusted relative risk with the adenoviral vaccine in the first 3 weeks post vaccination, compared to the 3- to 6-week interval post vaccination, was 6.03.12 In addition, a head-to-head comparison of adenoviral versus mRNA vaccines at 21 days revealed an adjusted rate ratio of 20.56. Mechanistically, some experts theorize that antibodies induced by the Janssen vaccine might cross-react with glycoproteins on the myelin sheath of peripheral nerve axons to cause GBS, but this remains unproven.11
The AstraZeneca vaccine uses a chimpanzee adenovirus; the Janssen vaccine uses a human adenoviral carrier. “The only commonality between the Janssen/Johnson & Johnson and AstraZeneca vaccines, and the only thing that’s different from the other vaccines, is the adenoviral vector packaging,” Dr. Lunn emphasized. “I believe it’s what generates GBS after COVID-19 vaccination. It has nothing to do with the COVID-19 vaccination, the spike protein, the nucleic acid, the DNA, or anything else.”
The adenoviral vector probably also explains why GBS peaks during winter, said Dr. Lunn. “That’s when adenovirus is circulating.” When people contract the common cold, he explained, they don’t visit their family physician and request a swab to isolate the adenovirus. “By the time you get GBS, the adenovirus has been cleared. We’ve all got antibodies to adenovirus all over the place, anyway, because we get it so often.”
It would be difficult to prove conclusively that adenovirus belongs on the list of GBS causes, Dr. Lunn allowed. “But I have a strong suspicion that it does. COVID-19 and COVID-19 vaccination have given us some new avenues into identifying GBS causation potentially in the near future.” More research is needed in this area, he said.
Dr. Lunn has been a principal investigator for argenx (efgartigimod) and an adviser to AstraZeneca (ChAdOx1 nCoV-19). He has received travel grants from CSL Behring.
References
1. Ho TW et al. Guillain-Barré syndrome in northern China. Relationship to Campylobacter jejuni infection and anti-glycolipid antibodies. Brain. 1995;118(Pt 3):597-605. doi: 10.1093/brain/118.3.597.
2. Uncini A. A common mechanism and a new categorization for anti-ganglioside antibody-mediated neuropathies. Exp Neurol. 2012;235(2):513-6. doi: 10.1016/j.expneurol.2012.03.023.
3. Uncini A and Kuwabara S. The electrodiagnosis of Guillain-Barré syndrome subtypes: where do we stand? Clin Neurophysiol. 2018;129(12):2586-93. doi: 10.1016/j.clinph.2018.09.025.
4. Delmont E et al. Autoantibodies to nodal isoforms of neurofascin in chronic inflammatory demyelinating polyneuropathy. Brain. 2017;140(7):1851-8. doi: 10.1093/brain/awx124.
5. McGonigal R et al. Anti-GD1a antibodies activate complement and calpain to injure distal motor nodes of Ranvier in mice. Brain. 2010;133(Pt 7):1944-60. doi: 10.1093/brain/awq119.
6. Cunningham ME et al. Real time imaging of intra-axonal calcium flux in an explant mouse model of axonal Guillain-Barré syndrome. Exp Neurol. 2022 Sep;355:114127. doi: 10.1016/j.expneurol.2022.114127.
7. Misawa S et al; Japanese Eculizumab Trial for GBS (JET-GBS) Study Group. Safety and efficacy of eculizumab in Guillain-Barré syndrome: a multicentre, double-blind, randomised phase 2 trial. Lancet Neurol. 2018;17(6):519-29. doi: 10.1016/S1474-4422(18)30114-5.
8. Walgaard C et al; Dutch GBS Study Group. Second intravenous immunoglobulin dose in patients with Guillain-Barré syndrome with poor prognosis (SID-GBS): a double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2021;20(4):275-83. doi: 10.1016/S1474-4422(20)30494-4.
9. Keddie S et al. Epidemiological and cohort study finds no association between COVID-19 and Guillain-Barré syndrome. Brain. 2021;144(2):682-93. doi: 10.1093/brain/awaa433.
10. Keh RYS et al; BPNS/ABN COVID-19 Vaccine GBS Study Group. COVID-19 vaccination and Guillain-Barré syndrome: Analyses using the National Immunoglobulin Database. Brain. 2023;146(2):739-48. doi: 10.1093/brain/awac067.
11. Abara WE et al. Reports of Guillain-Barré syndrome after COVID-19 vaccination in the United States. JAMA Netw Open. 2023;6(2):e2253845. doi: 10.1001/jamanetworkopen.2022.53845.
12. Hanson KE et al. Incidence of Guillain-Barré syndrome after COVID-19 vaccination in the Vaccine Safety Datalink. JAMA Netw Open. 2022;5(4):e228879. doi: 10.1001/jamanetworkopen.2022.8879.
Recent insights into the pathophysiology of Guillain-Barré syndrome (GBS) – which affects 1 or 2 persons for every 100,000 people annually, usually post infection – indicate that classic subtypes represent varying manifestations of a shared disease process. This knowledge is yielding new treatment strategies aimed at halting the illness in its tracks. Promising therapies include inhibitors of complement and, perhaps one day, the calcium-activated protease calpain.
Meanwhile, an association between COVID-19 and GBS has been debunked, whereas a small risk of GBS following adenovirus-vectored COVID vaccination is now accepted and quantified. Regardless of cause, the potential severity of GBS and variability in its presentation demand constant vigilance.
Shutting down the disease process
When patients present to an emergency department with sensory symptoms and increasing muscle weakness, “most of the damage has been or is being done,” said Michael P. Lunn, MBBS, MRCP, PhD, professor of clinical neurology, consultant neurologist, and clinical lead in neuroimmunology at University College London Queen Square Institute of Neurology, who spoke at length about GBS with Neurology Reviews 2023 Rare Neurological Disease Special Report. “The crucial reason that GBS treatment has not advanced significantly – and why we’re still slightly stuck where we are in terms of helping people get better more quickly – is that we need something that absolutely turns the disease off as patients come through the door.”
GBS is probably the best-understood autoimmune-mediated neurological disease, in some respects surpassing myasthenia gravis, Dr. Lunn said. “We know very frequently the organisms and stimuli that set off Guillain-Barré syndrome. We understand, to an extent, the immunology and how you break tolerance of the immune system so that an invading organism can provoke an immune response that damages peripheral nerves.”
Compared to what was known about GBS in decades past, neurologists now better understand how and where antibodies attack the nerve; how complement then damages the nodes of Ranvier and paranodes; and how an external attack results in sometimes irreparable internal nerve damage. “We’ve got a string, beginning to end, of understanding the disease,” declared Dr. Lunn.
Understanding of differences in the spectrum of pathology of GBS has led to additional diagnostic categories, said Dr. Lunn. Acute inflammatory demyelinating polyradiculoneuropathy, or typical GBS, represents the most common form in affluent Western nations. A motor variant was recognized in the 1980s; in the mid-1990s, Ho and colleagues described a cohort of patients in China who had acute motor axonal neuropathy and acute motor sensory axonal neuropathy1 – two forms that are particularly common throughout Asia and South America.
Shared mechanism
Based on the findings of electrophysiologic studies, Dr. Lunn said, experts traditionally believed that GBS attacked either axons themselves or their myelin sheaths. “That’s where the anti-ganglioside antibodies come in, providing targeting to nerve structures.” The dichotomous classification system, he added, was partially correct.
Then, through the 2010s and 2020s, neurophysiologist Antonio Uncini, MD, recognized, based partly on histologic studies by Ho and colleagues, that the myelin and axonal subtypes are both likely to stem from the same mechanism.2 When antibodies and complement damage the node of Ranvier, Dr. Lunn said, “the myelin gets stripped off and the conduction becomes slow. But then the myelin can return, and patients get better.” But if damage is severe, it severs the axon, resulting in unrecoverable motor axonal neuropathy. “It’s basically all the same spectrum of disease,” Dr. Lunn said. “Anti-ganglioside antibodies may account for different GBS ‘flavors,’ but the immunological attack all occurs at the node of Ranvier in one way or another.”
The foregoing insight has focused development efforts on the shared seminal pathway of all GBS subtypes and given rise to the concept of nodo-paranodopathy, which incorporates damage at either the node of Ranvier or nearby paranodes.3
Simultaneously, Spanish and French researchers began elucidating new antibodies responsible for neuropathology at the node of Ranvier.4 Anti-ganglioside antibodies have long been loosely associated with acute motor axonal neuropathy and poor outcomes, although, Dr. Lunn said, they fail to tell the full story. Anti-GQ1b antibodies are associated with the Miller-Fisher syndrome subtype, well recognized for its medical features: double vision, loss of tendon reflexes, and arm and leg weakness.
However, Dr. Lunn said, most GBS cases lack anti-ganglioside antibodies. In some GBS cases, antibodies attack neurofascin, contactins, and gliomedin, which are mainly adhesion proteins at nodes of Ranvier.
“Therefore,” Dr. Lunn said, “there must be an antibody-mediated attack of the node of Ranvier or the paranode. That’s an important series of discoveries, primarily because it helps us understand the immunological attack at the node of Ranvier, which goes along with what Dr. Uncini was saying. But it also divides off a group of chronic inflammatory demyelinating polyradiculoneuropathies (CIDP) that present acutely and look initially, for all purposes, like GBS.”
Recognizing acute CIDP (A-CIDP) is critically important for clinicians, Dr. Lunn stressed, because it requires treatment with rituximab (the most commonly used option), steroids, or plasma exchange.
Key clues that distinguish A-CIDP from GBS include:
• A high level of cerebrospinal fluid protein.
• Very slow nerve conduction.
• Early muscle wasting (rare in GBS).
Recognizing CIDP and A-CIDP is crucial, said Dr. Lunn, because it begins to bring all the pathology back together to make sense of GBS. Neurologists have known for decades that, if one damages a nerve with antibodies, then binds complement to those antibodies, the complement punches holes in the affected cells, resulting in death. “But it wasn’t quite clear how those cells might die,” Dr. Lunn said.
After complement-induced injury, calcium-activated calpain permanently damages the entire internal axonal structure.5 Perhaps more important, a 2022 mouse study showed that complement-mediated damage could be directed to myelin or axons using the genetically programmed presence or absence of gangliosides to understand subsequent calpain-induced destruction in either axons or myelin.6
Some of the engineered mouse cells included ganglioside; others did not. “So you can have anti-ganglioside antibodies directed at one cell type or the other, which would, or would not, have calpain within them,” Dr. Lunn said. Investigators also showed that a calpain inhibitor (AK295) or overproduction of an endogenous inhibitor, calpastatin, prevented damage to both cell types.6All existing calpain inhibitors are unsuitable for clinical use because they are highly toxic. “But if you could inhibit calpain and stop it from being activated by calcium,” Dr. Lunn explained, “you would have a mechanism for stopping cell degradation during GBS. That would be an important future target for pharmacotherapy. That whole story – from the beginning to the end of GBS – has opened up options for treatment.”
Because complement bound to antibodies, set up by infection, plays a pivotal role, complement inhibitors have become an exciting area of research over the past decade. The 36-patient Japanese Eculizumab Trial for GBS (JET-GBS) trial showed that, after 6 months, significantly more eculizumab-treated patients could run, compared with placebo-treated patients.7
“No other trials of complement inhibitors have yet been completed,” Dr. Lunn said. “But several different complement inhibitors work at different places, in a very complicated immune process. One of the complement inhibitors will become transformative in treating GBS – preventing disability and improving recovery – in the not-very-distant future.”
Additional investigational treatments that have demonstrated early promise in eliminating problem antibodies faster include imlifidase (Idefirix [Hansa Biopharma]), which destroys antibodies, and Fc receptor inhibitors such as efgartigimod alfa-fcab (Vyvgart [argenx]), which push antibodies into the natural catabolic pathway.
“We’ve been stuck with plasma exchange and intravenous immunoglobulin (IVIg) for three or four decades,” Dr. Lunn said. “We now have a series of strategies by which we can completely turn off complement and resulting nerve damage. If we can find a calpain inhibitor that turns off the end of that pathway, we will make dramatic improvements. Our understanding of the immunopathology has changed enormously and influences pharmacotherapy going forward.”
Recap of diagnosis and treatment
For decades, the diagnosis of GBS has relied on the presence of symptoms, including progressive weakness and loss of reflexes and sensations. Nerve-conduction studies and cerebrospinal fluid evaluation can help confirm the diagnosis.
IVIg shortens recovery, said Dr. Lunn, although nothing cures GBS. “And that’s a common problem: Clinicians think that they’re going to give somebody IVIg, and the patient’s going to get better immediately.” When that doesn’t happen, he said, physicians are tempted to give a second immunoglobulin dose.
However, a study published in 2021 shows that a second IVIg dose does not result in faster or better improvement – only in a significant risk of cardiovascular, cerebrovascular, and other thrombotic events 3 weeks later.8 Dr. Lunn noted that, although adverse-event data were “buried” in the supplemental materials of that study, the high cost of IVIg (approximately $12,500 per dose) means that the study has changed practice for the benefit of patients, providers, and health care systems.
COVID-19 and GBS triggers
Campylobacter jejuni infection still accounts for 30% to 40% of GBS cases, followed by other bacteria, including Mycoplasma pneumoniae and Haemophilus influenzae, and then by viruses, including cytomegalovirus and, rarely, human immunodeficiency virus. In recent years, severe acute respiratory syndrome coronavirus (SARS-CoV-2) infection – COVID-19 – and vaccines against the viral infection have captured headlines for purportedly being a cause of GBS.
The Zika virus epidemic of 2015-2016 has been linked to GBS-like illness. The 2003 severe acute respiratory syndrome (SARS) pandemic and the Middle East respiratory syndrome coronavirus (MERS-CoV) epidemic were associated with GBS – although, taken together, SARS and MERS-CoV produced fewer than 10 cases of GBS, Dr. Lunn noted. Nevertheless, heightened awareness of these viruses fueled hypervigilance regarding the prospect that COVID-19 could cause GBS. Following reports of a single such case in Wuhan and hundreds in Italy, worry over pandemic GBS grew worldwide.
Dr. Lunn and colleagues addressed the COVID-19–GBS question in a 2023 publication.9 “Because GBS is largely treated only with IVIg, and IVIg costs a lot of money, and the U.K. government insists on every dose of IVIg being logged in a government database, we were able to identify virtually every case of GBS,” he said.
GBS diagnoses were reliable, he added, because each case was confirmed by physicians outside the emergency department. Analysis revealed that, in 2020, U.K. GBS cases actually declined by around one-third. “And even when there was a second wave of COVID-19 at the end of 2020, partly caused by better counting,” Dr. Lunn said, “there was no further increase in GBS cases. We concluded that there was no link between GBS and COVID-19, as the cases simply didn’t appear.”
The foregoing findings have since been corroborated by studies in Singapore, the United States, and South America, he pointed out. Earlier case series suggesting a link between COVID-19 and GBS were selective, Dr. Lunn added, with numbers too small to support robust conclusions.
The lack of a causal link between COVID-19 and GBS suggested to Dr. Lunn that there was no reason COVID-19 vaccination should cause GBS. All COVID-19 vaccines were designed to provoke an immune response either (1) by producing the SARS-CoV-2 spike protein on the surface of virus (through a replication-incompetent adenoviral vector) or (2) through DNA or mRNA transcription, he explained. “The spike protein is only a small part of COVID-19.”
GBS: ‘Adverse event of interest’
A link between modern vaccines and GBS first appeared in the 1970s with the hastily developed swine flu vaccine. “In late 1976,” Dr. Lunn explained, “it was identified that patients who were given that vaccine seemed to be developing illnesses consistent with GBS.” By 1980, Dr. Lunn said, the risk level was determined to be only five or six cases for every 1 million doses of vaccine administered. “But the vaccine program was aborted, and swine flu never really happened.” Every year since, “there has been a surveillance program looking at the occurrence of an association of GBS with influenza vaccine.”
Minor fluctuations aside, he said, the overall incidence of GBS with influenza vaccination – 1 GBS case for every 1 million vaccine doses given – has remained consistent over several decades. “Nevertheless, GBS became an adverse event of special interest for any vaccination campaign.”
COVID-19 vaccination. Dr. Lunn and colleagues used the United Kingdom National Health Service (NHS) National Immunoglobulin Database, and other databases, to pinpoint the risk of GBS presented by the first dose of the AstraZeneca ChAdOx1 nCoV-19 adenoviral vaccine.10 As with U.K. GBS cases, every COVID-19 vaccination is linked to an NHS number. “We identified all the cases of GBS, found their NHS numbers, and went back and found the exact dates they’d been vaccinated, and with which vaccine.” Only the adenoviral-vector vaccine carried an excess risk of GBS – 5.8 cases for every 1 million doses, associated only with the first dose and peaking at approximately 25 days post vaccination – compared with other vaccines used in the United Kingdom.
Researchers looked at data from the Vaccine Adverse Event Reporting System (VAERS), a program of the Centers for Disease Control and Prevention and the Food and Drug Administration, encompassing nearly 500 million COVID-19 vaccine doses given between December 2020 and January 2022. They found that patients who received the Ad26.COV2.S vaccine (Janssen/Johnson & Johnson) had a rate of GBS (within 21 and 41 days post vaccination) that was 9 and 12 times higher, respectively, than corresponding rates for the mRNA-1273 (Moderna) and BNT162b2 (Pfizer BioNTech) COVID-19 vaccines.11 Risk was distributed relatively evenly by gender and age. Also at day 21 and day 41, observed event ratios with the adenoviral-vector vaccine (use of which has been suspended in the United States) were 3.79 and 2.34, respectively. Observed-event ratios with the other vaccines mirrored expected background rates.
The VAERS analysis confirms earlier data from the CDC’s Vaccine Safety Datalink, which showed that, among approximately 15 million U.S. vaccine doses given between mid-December 2020 and mid-November 2021, the unadjusted GBS incidence rate for every 100,000 person-years for the adenoviral vaccine, 21 days post exposure, was 32.4, compared with 1.3 for the mRNA vaccine. The adjusted relative risk with the adenoviral vaccine in the first 3 weeks post vaccination, compared to the 3- to 6-week interval post vaccination, was 6.03.12 In addition, a head-to-head comparison of adenoviral versus mRNA vaccines at 21 days revealed an adjusted rate ratio of 20.56. Mechanistically, some experts theorize that antibodies induced by the Janssen vaccine might cross-react with glycoproteins on the myelin sheath of peripheral nerve axons to cause GBS, but this remains unproven.11
The AstraZeneca vaccine uses a chimpanzee adenovirus; the Janssen vaccine uses a human adenoviral carrier. “The only commonality between the Janssen/Johnson & Johnson and AstraZeneca vaccines, and the only thing that’s different from the other vaccines, is the adenoviral vector packaging,” Dr. Lunn emphasized. “I believe it’s what generates GBS after COVID-19 vaccination. It has nothing to do with the COVID-19 vaccination, the spike protein, the nucleic acid, the DNA, or anything else.”
The adenoviral vector probably also explains why GBS peaks during winter, said Dr. Lunn. “That’s when adenovirus is circulating.” When people contract the common cold, he explained, they don’t visit their family physician and request a swab to isolate the adenovirus. “By the time you get GBS, the adenovirus has been cleared. We’ve all got antibodies to adenovirus all over the place, anyway, because we get it so often.”
It would be difficult to prove conclusively that adenovirus belongs on the list of GBS causes, Dr. Lunn allowed. “But I have a strong suspicion that it does. COVID-19 and COVID-19 vaccination have given us some new avenues into identifying GBS causation potentially in the near future.” More research is needed in this area, he said.
Dr. Lunn has been a principal investigator for argenx (efgartigimod) and an adviser to AstraZeneca (ChAdOx1 nCoV-19). He has received travel grants from CSL Behring.
References
1. Ho TW et al. Guillain-Barré syndrome in northern China. Relationship to Campylobacter jejuni infection and anti-glycolipid antibodies. Brain. 1995;118(Pt 3):597-605. doi: 10.1093/brain/118.3.597.
2. Uncini A. A common mechanism and a new categorization for anti-ganglioside antibody-mediated neuropathies. Exp Neurol. 2012;235(2):513-6. doi: 10.1016/j.expneurol.2012.03.023.
3. Uncini A and Kuwabara S. The electrodiagnosis of Guillain-Barré syndrome subtypes: where do we stand? Clin Neurophysiol. 2018;129(12):2586-93. doi: 10.1016/j.clinph.2018.09.025.
4. Delmont E et al. Autoantibodies to nodal isoforms of neurofascin in chronic inflammatory demyelinating polyneuropathy. Brain. 2017;140(7):1851-8. doi: 10.1093/brain/awx124.
5. McGonigal R et al. Anti-GD1a antibodies activate complement and calpain to injure distal motor nodes of Ranvier in mice. Brain. 2010;133(Pt 7):1944-60. doi: 10.1093/brain/awq119.
6. Cunningham ME et al. Real time imaging of intra-axonal calcium flux in an explant mouse model of axonal Guillain-Barré syndrome. Exp Neurol. 2022 Sep;355:114127. doi: 10.1016/j.expneurol.2022.114127.
7. Misawa S et al; Japanese Eculizumab Trial for GBS (JET-GBS) Study Group. Safety and efficacy of eculizumab in Guillain-Barré syndrome: a multicentre, double-blind, randomised phase 2 trial. Lancet Neurol. 2018;17(6):519-29. doi: 10.1016/S1474-4422(18)30114-5.
8. Walgaard C et al; Dutch GBS Study Group. Second intravenous immunoglobulin dose in patients with Guillain-Barré syndrome with poor prognosis (SID-GBS): a double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2021;20(4):275-83. doi: 10.1016/S1474-4422(20)30494-4.
9. Keddie S et al. Epidemiological and cohort study finds no association between COVID-19 and Guillain-Barré syndrome. Brain. 2021;144(2):682-93. doi: 10.1093/brain/awaa433.
10. Keh RYS et al; BPNS/ABN COVID-19 Vaccine GBS Study Group. COVID-19 vaccination and Guillain-Barré syndrome: Analyses using the National Immunoglobulin Database. Brain. 2023;146(2):739-48. doi: 10.1093/brain/awac067.
11. Abara WE et al. Reports of Guillain-Barré syndrome after COVID-19 vaccination in the United States. JAMA Netw Open. 2023;6(2):e2253845. doi: 10.1001/jamanetworkopen.2022.53845.
12. Hanson KE et al. Incidence of Guillain-Barré syndrome after COVID-19 vaccination in the Vaccine Safety Datalink. JAMA Netw Open. 2022;5(4):e228879. doi: 10.1001/jamanetworkopen.2022.8879.
NPF provides guidance for virtual psoriasis visits
.
The success of telemedicine in managing chronic inflammatory skin conditions including psoriasis during the COVID-19 pandemic “highlighted that teledermatology can be used beyond the context of a global health crisis to provide continuity of care and improve access to health care more broadly,” the task force wrote in a paper published online in JAAD International.
Co–senior author George Han, MD, PhD, said in an interview that the impetus for the guidelines came from NPF patient advocates, who realized that the organization needed something to take to payers and governmental agencies to advocate for better access to dermatologic care. He is associate professor of dermatology and director of teledermatology at the Hofstra/Northwell department of dermatology, Hyde Park, New York.
“We realized that, in many places around the country, people don’t have access to dermatology.” In upstate New York, said Dr. Han, his anecdotal research has revealed wait times of 6 months or more.
As a guiding principle, the authors pronounce teledermatology “a reasonable alternative for providing long-term management of patients with psoriasis.” Research shows that nearly all dermatologists used teledermatology during the pandemic, the authors noted, and that well-run programs improve Psoriasis Area and Severity Index (PASI) scores and other measures on par with in-person care. Telemedicine may be especially useful for initial visits, they added, particularly when distance, patient incapacity, and circumstances prevent face-to-face evaluation.
Additional position statements emphasize that teledermatology should support rather than supplant in-person visits, and that this balance may be particularly important in cases involving psoriatic arthritis (PsA). “Even though we can’t do a physical exam and palpate some of those joints in person,” said Dr. Han, “tools have been developed that, through a series of questions the patient can answer, can guide you towards whether there is a high index of suspicion for psoriatic arthritis.” Such patients require in-person evaluation with urgency, he said, because delays in PsA diagnosis and treatment can lead to irreversible joint damage and significant functional impairment.
Another motivation for producing the guidelines, said Dr. Han, was that, even when underserved patients get a dermatology appointment, some providers may not have all the latest tools or medicines available for treating psoriasis. In such cases, telemedicine may allow dermatologists specializing in psoriasis care to extend their reach in comanaging patients with primary care physicians and community dermatologists.
Before the appointment, guidelines suggest determining what form of teledermatology will best suit each patient. Authors recommended gauging patients’ savviness with computers and cameras, and counseling patients regarding available virtual evaluation tools – such as live video visits, store-and-forward photo strategies, and assessment-tool training videos.
A subsequent guideline underscores the importance of continuously improving technology to support expeditious image capture and workflows that emulate in-person practice. Dr. Han explained, “we wanted to make sure that on the back end there’s adequate support such that – if through teledermatology, we determine that the patient should get, say, a systemic treatment – the patient is able to get the appropriate lab tests, get the medicine, and know how to inject it.”
Regarding reimbursement, Dr. Han said that policies varied prepandemic, but many commercial insurers covered telemedicine at a rate 20% lower than the in-person rate. During the pandemic, he said, insurers shifted to provide the higher rate for telemedicine, consistent with policies adopted by the Centers for Medicare & Medicaid Services.
“There are differences in coverage and reimbursement from plan to plan,” Dr. Han added. “And even within the same plan, there are carve-outs so that some plans don’t allow certain services. The big picture is that for the most part these services are covered at a level comparable to an in-person visit at present.”
With the Department of Health & Human Services’ public health emergency declaration expiring in May, he said, physicians have worried that some of the allowances made by CMS – such as lifting requirements that Medicare patients in rural areas be seen at care sites – will expire. “It seems that some of those limitations have been addressed, and those allowances are going to be extended until Congress is able to pass something that gives us durable access to telemedicine care. We think that based on the current environment telemedicine is here to stay.”
The study was funded by the NPF. Dr. Han has been an investigator, adviser, speaker, or researcher for AbbVie, Amgen, Apogee Therapeutics, Arcutis, Athenex, Bausch Health, Beiersdorf, Boehringer Ingelheim, Bond Avillion, Bristol Myers Squibb, Celgene, CeraVe, Dermavant, DermTech, Eli Lilly, EPI Health, Janssen Pharmaceuticals, LEO Pharma, L’Oreal, MC2 Therapeutics, Novartis, Ortho Dermatologics, PellePharm, Pfizer, Regeneron Pharmaceuticals, Sanofi Genzyme, SUN Pharmaceuticals, and UCB.
.
The success of telemedicine in managing chronic inflammatory skin conditions including psoriasis during the COVID-19 pandemic “highlighted that teledermatology can be used beyond the context of a global health crisis to provide continuity of care and improve access to health care more broadly,” the task force wrote in a paper published online in JAAD International.
Co–senior author George Han, MD, PhD, said in an interview that the impetus for the guidelines came from NPF patient advocates, who realized that the organization needed something to take to payers and governmental agencies to advocate for better access to dermatologic care. He is associate professor of dermatology and director of teledermatology at the Hofstra/Northwell department of dermatology, Hyde Park, New York.
“We realized that, in many places around the country, people don’t have access to dermatology.” In upstate New York, said Dr. Han, his anecdotal research has revealed wait times of 6 months or more.
As a guiding principle, the authors pronounce teledermatology “a reasonable alternative for providing long-term management of patients with psoriasis.” Research shows that nearly all dermatologists used teledermatology during the pandemic, the authors noted, and that well-run programs improve Psoriasis Area and Severity Index (PASI) scores and other measures on par with in-person care. Telemedicine may be especially useful for initial visits, they added, particularly when distance, patient incapacity, and circumstances prevent face-to-face evaluation.
Additional position statements emphasize that teledermatology should support rather than supplant in-person visits, and that this balance may be particularly important in cases involving psoriatic arthritis (PsA). “Even though we can’t do a physical exam and palpate some of those joints in person,” said Dr. Han, “tools have been developed that, through a series of questions the patient can answer, can guide you towards whether there is a high index of suspicion for psoriatic arthritis.” Such patients require in-person evaluation with urgency, he said, because delays in PsA diagnosis and treatment can lead to irreversible joint damage and significant functional impairment.
Another motivation for producing the guidelines, said Dr. Han, was that, even when underserved patients get a dermatology appointment, some providers may not have all the latest tools or medicines available for treating psoriasis. In such cases, telemedicine may allow dermatologists specializing in psoriasis care to extend their reach in comanaging patients with primary care physicians and community dermatologists.
Before the appointment, guidelines suggest determining what form of teledermatology will best suit each patient. Authors recommended gauging patients’ savviness with computers and cameras, and counseling patients regarding available virtual evaluation tools – such as live video visits, store-and-forward photo strategies, and assessment-tool training videos.
A subsequent guideline underscores the importance of continuously improving technology to support expeditious image capture and workflows that emulate in-person practice. Dr. Han explained, “we wanted to make sure that on the back end there’s adequate support such that – if through teledermatology, we determine that the patient should get, say, a systemic treatment – the patient is able to get the appropriate lab tests, get the medicine, and know how to inject it.”
Regarding reimbursement, Dr. Han said that policies varied prepandemic, but many commercial insurers covered telemedicine at a rate 20% lower than the in-person rate. During the pandemic, he said, insurers shifted to provide the higher rate for telemedicine, consistent with policies adopted by the Centers for Medicare & Medicaid Services.
“There are differences in coverage and reimbursement from plan to plan,” Dr. Han added. “And even within the same plan, there are carve-outs so that some plans don’t allow certain services. The big picture is that for the most part these services are covered at a level comparable to an in-person visit at present.”
With the Department of Health & Human Services’ public health emergency declaration expiring in May, he said, physicians have worried that some of the allowances made by CMS – such as lifting requirements that Medicare patients in rural areas be seen at care sites – will expire. “It seems that some of those limitations have been addressed, and those allowances are going to be extended until Congress is able to pass something that gives us durable access to telemedicine care. We think that based on the current environment telemedicine is here to stay.”
The study was funded by the NPF. Dr. Han has been an investigator, adviser, speaker, or researcher for AbbVie, Amgen, Apogee Therapeutics, Arcutis, Athenex, Bausch Health, Beiersdorf, Boehringer Ingelheim, Bond Avillion, Bristol Myers Squibb, Celgene, CeraVe, Dermavant, DermTech, Eli Lilly, EPI Health, Janssen Pharmaceuticals, LEO Pharma, L’Oreal, MC2 Therapeutics, Novartis, Ortho Dermatologics, PellePharm, Pfizer, Regeneron Pharmaceuticals, Sanofi Genzyme, SUN Pharmaceuticals, and UCB.
.
The success of telemedicine in managing chronic inflammatory skin conditions including psoriasis during the COVID-19 pandemic “highlighted that teledermatology can be used beyond the context of a global health crisis to provide continuity of care and improve access to health care more broadly,” the task force wrote in a paper published online in JAAD International.
Co–senior author George Han, MD, PhD, said in an interview that the impetus for the guidelines came from NPF patient advocates, who realized that the organization needed something to take to payers and governmental agencies to advocate for better access to dermatologic care. He is associate professor of dermatology and director of teledermatology at the Hofstra/Northwell department of dermatology, Hyde Park, New York.
“We realized that, in many places around the country, people don’t have access to dermatology.” In upstate New York, said Dr. Han, his anecdotal research has revealed wait times of 6 months or more.
As a guiding principle, the authors pronounce teledermatology “a reasonable alternative for providing long-term management of patients with psoriasis.” Research shows that nearly all dermatologists used teledermatology during the pandemic, the authors noted, and that well-run programs improve Psoriasis Area and Severity Index (PASI) scores and other measures on par with in-person care. Telemedicine may be especially useful for initial visits, they added, particularly when distance, patient incapacity, and circumstances prevent face-to-face evaluation.
Additional position statements emphasize that teledermatology should support rather than supplant in-person visits, and that this balance may be particularly important in cases involving psoriatic arthritis (PsA). “Even though we can’t do a physical exam and palpate some of those joints in person,” said Dr. Han, “tools have been developed that, through a series of questions the patient can answer, can guide you towards whether there is a high index of suspicion for psoriatic arthritis.” Such patients require in-person evaluation with urgency, he said, because delays in PsA diagnosis and treatment can lead to irreversible joint damage and significant functional impairment.
Another motivation for producing the guidelines, said Dr. Han, was that, even when underserved patients get a dermatology appointment, some providers may not have all the latest tools or medicines available for treating psoriasis. In such cases, telemedicine may allow dermatologists specializing in psoriasis care to extend their reach in comanaging patients with primary care physicians and community dermatologists.
Before the appointment, guidelines suggest determining what form of teledermatology will best suit each patient. Authors recommended gauging patients’ savviness with computers and cameras, and counseling patients regarding available virtual evaluation tools – such as live video visits, store-and-forward photo strategies, and assessment-tool training videos.
A subsequent guideline underscores the importance of continuously improving technology to support expeditious image capture and workflows that emulate in-person practice. Dr. Han explained, “we wanted to make sure that on the back end there’s adequate support such that – if through teledermatology, we determine that the patient should get, say, a systemic treatment – the patient is able to get the appropriate lab tests, get the medicine, and know how to inject it.”
Regarding reimbursement, Dr. Han said that policies varied prepandemic, but many commercial insurers covered telemedicine at a rate 20% lower than the in-person rate. During the pandemic, he said, insurers shifted to provide the higher rate for telemedicine, consistent with policies adopted by the Centers for Medicare & Medicaid Services.
“There are differences in coverage and reimbursement from plan to plan,” Dr. Han added. “And even within the same plan, there are carve-outs so that some plans don’t allow certain services. The big picture is that for the most part these services are covered at a level comparable to an in-person visit at present.”
With the Department of Health & Human Services’ public health emergency declaration expiring in May, he said, physicians have worried that some of the allowances made by CMS – such as lifting requirements that Medicare patients in rural areas be seen at care sites – will expire. “It seems that some of those limitations have been addressed, and those allowances are going to be extended until Congress is able to pass something that gives us durable access to telemedicine care. We think that based on the current environment telemedicine is here to stay.”
The study was funded by the NPF. Dr. Han has been an investigator, adviser, speaker, or researcher for AbbVie, Amgen, Apogee Therapeutics, Arcutis, Athenex, Bausch Health, Beiersdorf, Boehringer Ingelheim, Bond Avillion, Bristol Myers Squibb, Celgene, CeraVe, Dermavant, DermTech, Eli Lilly, EPI Health, Janssen Pharmaceuticals, LEO Pharma, L’Oreal, MC2 Therapeutics, Novartis, Ortho Dermatologics, PellePharm, Pfizer, Regeneron Pharmaceuticals, Sanofi Genzyme, SUN Pharmaceuticals, and UCB.
FROM JAAD INTERNATIONAL
Pooled safety data analysis of tralokinumab reported
The most , according to a review published in the British Journal of Dermatology.
These findings underscore the mechanistic elegance of interleukin (IL)-13 inhibition and highlight potential advantages of flexible dosing, according to the study’s lead author, Eric Simpson, MD, MCR. Overall, the pooled analysis of safety data from five phase 2 and 3 trials shows that “blockade of a single cytokine provides excellent short- and long-term safety, which is useful for a severe chronic disease,” said Dr. Simpson, professor of dermatology at Oregon Health & Science University in Portland.
Most patients with AD require years of treatment. “So for clinicians to confidently report to patients the low rates of serious adverse events (AEs) and lack of immune suppression side-effect profile is very encouraging for both the provider and patient,” Dr. Simpson said, noting there were no new signals or concerning short-term AEs.
Tralokinumab (Adbry), an IL-13 antagonist administered subcutaneously, was approved by the Food and Drug Administration for treatment of moderate to severe AD in adults in December 2021.
Minor differences vs. placebo
In the pooled analysis involving 1,605 patients treated for 16 weeks with tralokinumab and 680 who received placebo, frequency of any AE was 65.7% and 67.2%, respectively. Severe AEs occurred in 4.6% and 6.3% of patients, respectively.
The most common AE overall was AD, which occurred less often in tralokinumab-treated patients (15.4%) than those on placebo (26.2%). Other common AEs that occurred more frequently with tralokinumab included viral upper respiratory tract infections (15.7% vs. 12.2%), upper respiratory tract infections (URTI, 5.6% vs. 4.8%), conjunctivitis (5.4% vs. 1.9%), and injection-site reactions (3.5% vs. 0.3%).
AEs that occurred less often with tralokinumab than placebo included skin infections (3.7% vs. 9.2%, respectively) and infected dermatitis (1.6% vs. 6.4%).
Regarding safety areas of special interest, eye disorders classified as conjunctivitis, keratoconjunctivitis, or keratitis occurred more commonly with tralokinumab (7.9%) than placebo (3.4%). Most eye disorders were mild or moderate and resolved during the study. During maintenance treatment up to 52 weeks, AE rates mirrored those in the initial treatment period and did not increase with treatment duration.
In fact, Dr. Simpson said, the low rate of AEs that are known to accompany type 2 blockade, such as conjunctivitis, do not increase but rather appear to drop with longer-term use. The fact that skin infections were reduced vs. placebo and decreased over time suggests that long-term IL-13 blockade with tralokinumab positively impacts skin infections, a well-known comorbidity in uncontrolled AD, he added.
Raj Chovatiya, MD, PhD, who was asked to comment on the study, said, “These findings provide additional data supporting the safety and tolerability of tralokinumab and support my personal real-world experience with tralokinumab as a safe and effective biologic therapy for patients with moderate to severe AD.”
Dr. Chovatiya is assistant professor, director of the Center for Eczema and Itch, and medical director of clinical trials at Northwestern University in Chicago.
Four-week dosing
Consistent with ECZTRA 3, the rates of URTIs and conjunctivitis were lower with maintenance dosing 300 mg every 4 weeks, consideration of which is approved for responders weighing less than 220 pounds, vs. 300 mg every 2 weeks. Specifically, 6.7% of patients on every 4-week dosing schedule experienced URTIs, vs. 9.4% on the every 2-week dosing schedule and 7% of those on the every 2-week dosing schedule plus optional topical corticosteroids. Corresponding figures for conjunctivitis were 3%, 5%, and 5.6%, respectively.
“Four-week dosing is a possibility in your patients with a good clinical response at 16 weeks,” Dr. Simpson said. Advantages include improved convenience for patients, he added, and this analysis shows that dosing every 4 weeks may improve tolerability, with a lower rate of conjunctivitis.
Although it is difficult to directly compare review data to other studies, said Dr. Chovatiya, findings also suggest that tralokinumab may be associated with reduced infections and conjunctivitis compared with other advanced AD therapies. Head-to-head trials and real-world studies are needed to better understand comparative safety, he added.
Some patients will lose a degree of response with the 4-week dosing schedule, Dr. Simpson said. In ECZTRA 1 and 2, 55.9% of patients who achieved investigator global assessment (IGA) scores of 0 or 1 after 16 weeks of dosing every 2 weeks maintained this response level through week 52, vs. 42.4% of responders who switched from dosing every 2 weeks to every 4 weeks after week 16. But according to data that Dr. Simpson recently presented, 95% of patients switched to monthly dosing who relapsed and returned to dosing every 2 weeks regained their original response level within approximately 4 weeks.
In his personal practice, Dr. Simpson has prescribed tralokinumab for patients with AD for up to a year. However, he and fellow investigators have been following much larger populations for more than 2 years and are planning additional publications. “Safety data will continue to accrue” said Dr. Simpson, “but I don’t expect any surprises.”
The clinical trials were sponsored by MedImmune (phase 2b) and LEO Pharma ( ECZTRA phase 3 trials), which also sponsored the review. Dr. Simpson reports grants and personal fees from numerous pharmaceutical companies. Dr. Chovatiya has been an advisory board member, consultant, investigator, and speaker for numerous pharmaceutical companies including LEO Pharma.
A version of this article first appeared on Medscape.com.
The most , according to a review published in the British Journal of Dermatology.
These findings underscore the mechanistic elegance of interleukin (IL)-13 inhibition and highlight potential advantages of flexible dosing, according to the study’s lead author, Eric Simpson, MD, MCR. Overall, the pooled analysis of safety data from five phase 2 and 3 trials shows that “blockade of a single cytokine provides excellent short- and long-term safety, which is useful for a severe chronic disease,” said Dr. Simpson, professor of dermatology at Oregon Health & Science University in Portland.
Most patients with AD require years of treatment. “So for clinicians to confidently report to patients the low rates of serious adverse events (AEs) and lack of immune suppression side-effect profile is very encouraging for both the provider and patient,” Dr. Simpson said, noting there were no new signals or concerning short-term AEs.
Tralokinumab (Adbry), an IL-13 antagonist administered subcutaneously, was approved by the Food and Drug Administration for treatment of moderate to severe AD in adults in December 2021.
Minor differences vs. placebo
In the pooled analysis involving 1,605 patients treated for 16 weeks with tralokinumab and 680 who received placebo, frequency of any AE was 65.7% and 67.2%, respectively. Severe AEs occurred in 4.6% and 6.3% of patients, respectively.
The most common AE overall was AD, which occurred less often in tralokinumab-treated patients (15.4%) than those on placebo (26.2%). Other common AEs that occurred more frequently with tralokinumab included viral upper respiratory tract infections (15.7% vs. 12.2%), upper respiratory tract infections (URTI, 5.6% vs. 4.8%), conjunctivitis (5.4% vs. 1.9%), and injection-site reactions (3.5% vs. 0.3%).
AEs that occurred less often with tralokinumab than placebo included skin infections (3.7% vs. 9.2%, respectively) and infected dermatitis (1.6% vs. 6.4%).
Regarding safety areas of special interest, eye disorders classified as conjunctivitis, keratoconjunctivitis, or keratitis occurred more commonly with tralokinumab (7.9%) than placebo (3.4%). Most eye disorders were mild or moderate and resolved during the study. During maintenance treatment up to 52 weeks, AE rates mirrored those in the initial treatment period and did not increase with treatment duration.
In fact, Dr. Simpson said, the low rate of AEs that are known to accompany type 2 blockade, such as conjunctivitis, do not increase but rather appear to drop with longer-term use. The fact that skin infections were reduced vs. placebo and decreased over time suggests that long-term IL-13 blockade with tralokinumab positively impacts skin infections, a well-known comorbidity in uncontrolled AD, he added.
Raj Chovatiya, MD, PhD, who was asked to comment on the study, said, “These findings provide additional data supporting the safety and tolerability of tralokinumab and support my personal real-world experience with tralokinumab as a safe and effective biologic therapy for patients with moderate to severe AD.”
Dr. Chovatiya is assistant professor, director of the Center for Eczema and Itch, and medical director of clinical trials at Northwestern University in Chicago.
Four-week dosing
Consistent with ECZTRA 3, the rates of URTIs and conjunctivitis were lower with maintenance dosing 300 mg every 4 weeks, consideration of which is approved for responders weighing less than 220 pounds, vs. 300 mg every 2 weeks. Specifically, 6.7% of patients on every 4-week dosing schedule experienced URTIs, vs. 9.4% on the every 2-week dosing schedule and 7% of those on the every 2-week dosing schedule plus optional topical corticosteroids. Corresponding figures for conjunctivitis were 3%, 5%, and 5.6%, respectively.
“Four-week dosing is a possibility in your patients with a good clinical response at 16 weeks,” Dr. Simpson said. Advantages include improved convenience for patients, he added, and this analysis shows that dosing every 4 weeks may improve tolerability, with a lower rate of conjunctivitis.
Although it is difficult to directly compare review data to other studies, said Dr. Chovatiya, findings also suggest that tralokinumab may be associated with reduced infections and conjunctivitis compared with other advanced AD therapies. Head-to-head trials and real-world studies are needed to better understand comparative safety, he added.
Some patients will lose a degree of response with the 4-week dosing schedule, Dr. Simpson said. In ECZTRA 1 and 2, 55.9% of patients who achieved investigator global assessment (IGA) scores of 0 or 1 after 16 weeks of dosing every 2 weeks maintained this response level through week 52, vs. 42.4% of responders who switched from dosing every 2 weeks to every 4 weeks after week 16. But according to data that Dr. Simpson recently presented, 95% of patients switched to monthly dosing who relapsed and returned to dosing every 2 weeks regained their original response level within approximately 4 weeks.
In his personal practice, Dr. Simpson has prescribed tralokinumab for patients with AD for up to a year. However, he and fellow investigators have been following much larger populations for more than 2 years and are planning additional publications. “Safety data will continue to accrue” said Dr. Simpson, “but I don’t expect any surprises.”
The clinical trials were sponsored by MedImmune (phase 2b) and LEO Pharma ( ECZTRA phase 3 trials), which also sponsored the review. Dr. Simpson reports grants and personal fees from numerous pharmaceutical companies. Dr. Chovatiya has been an advisory board member, consultant, investigator, and speaker for numerous pharmaceutical companies including LEO Pharma.
A version of this article first appeared on Medscape.com.
The most , according to a review published in the British Journal of Dermatology.
These findings underscore the mechanistic elegance of interleukin (IL)-13 inhibition and highlight potential advantages of flexible dosing, according to the study’s lead author, Eric Simpson, MD, MCR. Overall, the pooled analysis of safety data from five phase 2 and 3 trials shows that “blockade of a single cytokine provides excellent short- and long-term safety, which is useful for a severe chronic disease,” said Dr. Simpson, professor of dermatology at Oregon Health & Science University in Portland.
Most patients with AD require years of treatment. “So for clinicians to confidently report to patients the low rates of serious adverse events (AEs) and lack of immune suppression side-effect profile is very encouraging for both the provider and patient,” Dr. Simpson said, noting there were no new signals or concerning short-term AEs.
Tralokinumab (Adbry), an IL-13 antagonist administered subcutaneously, was approved by the Food and Drug Administration for treatment of moderate to severe AD in adults in December 2021.
Minor differences vs. placebo
In the pooled analysis involving 1,605 patients treated for 16 weeks with tralokinumab and 680 who received placebo, frequency of any AE was 65.7% and 67.2%, respectively. Severe AEs occurred in 4.6% and 6.3% of patients, respectively.
The most common AE overall was AD, which occurred less often in tralokinumab-treated patients (15.4%) than those on placebo (26.2%). Other common AEs that occurred more frequently with tralokinumab included viral upper respiratory tract infections (15.7% vs. 12.2%), upper respiratory tract infections (URTI, 5.6% vs. 4.8%), conjunctivitis (5.4% vs. 1.9%), and injection-site reactions (3.5% vs. 0.3%).
AEs that occurred less often with tralokinumab than placebo included skin infections (3.7% vs. 9.2%, respectively) and infected dermatitis (1.6% vs. 6.4%).
Regarding safety areas of special interest, eye disorders classified as conjunctivitis, keratoconjunctivitis, or keratitis occurred more commonly with tralokinumab (7.9%) than placebo (3.4%). Most eye disorders were mild or moderate and resolved during the study. During maintenance treatment up to 52 weeks, AE rates mirrored those in the initial treatment period and did not increase with treatment duration.
In fact, Dr. Simpson said, the low rate of AEs that are known to accompany type 2 blockade, such as conjunctivitis, do not increase but rather appear to drop with longer-term use. The fact that skin infections were reduced vs. placebo and decreased over time suggests that long-term IL-13 blockade with tralokinumab positively impacts skin infections, a well-known comorbidity in uncontrolled AD, he added.
Raj Chovatiya, MD, PhD, who was asked to comment on the study, said, “These findings provide additional data supporting the safety and tolerability of tralokinumab and support my personal real-world experience with tralokinumab as a safe and effective biologic therapy for patients with moderate to severe AD.”
Dr. Chovatiya is assistant professor, director of the Center for Eczema and Itch, and medical director of clinical trials at Northwestern University in Chicago.
Four-week dosing
Consistent with ECZTRA 3, the rates of URTIs and conjunctivitis were lower with maintenance dosing 300 mg every 4 weeks, consideration of which is approved for responders weighing less than 220 pounds, vs. 300 mg every 2 weeks. Specifically, 6.7% of patients on every 4-week dosing schedule experienced URTIs, vs. 9.4% on the every 2-week dosing schedule and 7% of those on the every 2-week dosing schedule plus optional topical corticosteroids. Corresponding figures for conjunctivitis were 3%, 5%, and 5.6%, respectively.
“Four-week dosing is a possibility in your patients with a good clinical response at 16 weeks,” Dr. Simpson said. Advantages include improved convenience for patients, he added, and this analysis shows that dosing every 4 weeks may improve tolerability, with a lower rate of conjunctivitis.
Although it is difficult to directly compare review data to other studies, said Dr. Chovatiya, findings also suggest that tralokinumab may be associated with reduced infections and conjunctivitis compared with other advanced AD therapies. Head-to-head trials and real-world studies are needed to better understand comparative safety, he added.
Some patients will lose a degree of response with the 4-week dosing schedule, Dr. Simpson said. In ECZTRA 1 and 2, 55.9% of patients who achieved investigator global assessment (IGA) scores of 0 or 1 after 16 weeks of dosing every 2 weeks maintained this response level through week 52, vs. 42.4% of responders who switched from dosing every 2 weeks to every 4 weeks after week 16. But according to data that Dr. Simpson recently presented, 95% of patients switched to monthly dosing who relapsed and returned to dosing every 2 weeks regained their original response level within approximately 4 weeks.
In his personal practice, Dr. Simpson has prescribed tralokinumab for patients with AD for up to a year. However, he and fellow investigators have been following much larger populations for more than 2 years and are planning additional publications. “Safety data will continue to accrue” said Dr. Simpson, “but I don’t expect any surprises.”
The clinical trials were sponsored by MedImmune (phase 2b) and LEO Pharma ( ECZTRA phase 3 trials), which also sponsored the review. Dr. Simpson reports grants and personal fees from numerous pharmaceutical companies. Dr. Chovatiya has been an advisory board member, consultant, investigator, and speaker for numerous pharmaceutical companies including LEO Pharma.
A version of this article first appeared on Medscape.com.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
NRS grants target rosacea’s underlying mechanisms
Two new , according to an announcement by the NRS.
As part of the NRS research grants program, the organization recently awarded $10,000 to Emanual Maverakis, MD, professor of dermatology, University of California, Davis, and research fellow Samantha Herbert, MSPH. Their project will characterize rosacea pathophysiology using single-cell RNA sequencing. This novel analytical technique provides specific information on the signals expressed by different cell types and will help researchers better understand the role each subtype may play in rosacea, along with how these cells interact with each other, according to the NRS press release. New knowledge in the foregoing areas may fuel development of better therapies, the release added.
The NRS awarded its second new-research grant to Arisa Ortiz, MD, director of laser and cosmetic dermatology and associate professor of dermatology, University of California, San Diego. She was awarded $5,000 to examine whether laser therapy affects the skin microbiome, the complex ecosystem of bacteria and other microorganisms that reside on the skin. Studies have detected significant differences – such as higher levels of Demodex folliculorum and Staphylococcus epidermidis and lower levels of Cutibacterium acnes – in the microbiome of skin with rosacea compared with healthy skin. Dr. Ortiz’s research also will probe how blood vessels, which laser therapy often target, contribute to the rosacea disease process.
The NRS also renewed its support of an ongoing study led by Sezen Karakus, MD, assistant professor of ophthalmology at the Johns Hopkins Wilmer Eye Institute, Baltimore. She is studying the role of the ocular-surface microbiome in rosacea pathogenesis. Because ocular rosacea can lead to vision-threatening corneal complications, Dr. Karakus said in the press release, identifying microorganisms present on the ocular surface may spur development of targeted treatment strategies.
A second ongoing study for which the NRS renewed funding is investigating whether certain intracellular signals recently found to be elevated in rosacea lesions may drive skin inflammation, which may be a root cause of rosacea. Emmanuel Contassot, PhD, project leader in the dermatology department at the University Hospital of Basel, Switzerland, is leading the study.
To date, the NRS research grants program has awarded more than $1.6 million to research designed to further elucidate potential causes and other key aspects of rosacea with the goal of advancing treatment, prevention, or potential cure of rosacea.
For interested researchers, the deadline to submit proposals for next year’s grants is June 16, 2023. Forms and instructions are available through the research grants section of the NRS website or by contacting the NRS at 4619 N. Ravenswood Ave., Suite 103, Chicago, IL 60640; 888-662-5874; or info@rosacea.org.
Two new , according to an announcement by the NRS.
As part of the NRS research grants program, the organization recently awarded $10,000 to Emanual Maverakis, MD, professor of dermatology, University of California, Davis, and research fellow Samantha Herbert, MSPH. Their project will characterize rosacea pathophysiology using single-cell RNA sequencing. This novel analytical technique provides specific information on the signals expressed by different cell types and will help researchers better understand the role each subtype may play in rosacea, along with how these cells interact with each other, according to the NRS press release. New knowledge in the foregoing areas may fuel development of better therapies, the release added.
The NRS awarded its second new-research grant to Arisa Ortiz, MD, director of laser and cosmetic dermatology and associate professor of dermatology, University of California, San Diego. She was awarded $5,000 to examine whether laser therapy affects the skin microbiome, the complex ecosystem of bacteria and other microorganisms that reside on the skin. Studies have detected significant differences – such as higher levels of Demodex folliculorum and Staphylococcus epidermidis and lower levels of Cutibacterium acnes – in the microbiome of skin with rosacea compared with healthy skin. Dr. Ortiz’s research also will probe how blood vessels, which laser therapy often target, contribute to the rosacea disease process.
The NRS also renewed its support of an ongoing study led by Sezen Karakus, MD, assistant professor of ophthalmology at the Johns Hopkins Wilmer Eye Institute, Baltimore. She is studying the role of the ocular-surface microbiome in rosacea pathogenesis. Because ocular rosacea can lead to vision-threatening corneal complications, Dr. Karakus said in the press release, identifying microorganisms present on the ocular surface may spur development of targeted treatment strategies.
A second ongoing study for which the NRS renewed funding is investigating whether certain intracellular signals recently found to be elevated in rosacea lesions may drive skin inflammation, which may be a root cause of rosacea. Emmanuel Contassot, PhD, project leader in the dermatology department at the University Hospital of Basel, Switzerland, is leading the study.
To date, the NRS research grants program has awarded more than $1.6 million to research designed to further elucidate potential causes and other key aspects of rosacea with the goal of advancing treatment, prevention, or potential cure of rosacea.
For interested researchers, the deadline to submit proposals for next year’s grants is June 16, 2023. Forms and instructions are available through the research grants section of the NRS website or by contacting the NRS at 4619 N. Ravenswood Ave., Suite 103, Chicago, IL 60640; 888-662-5874; or info@rosacea.org.
Two new , according to an announcement by the NRS.
As part of the NRS research grants program, the organization recently awarded $10,000 to Emanual Maverakis, MD, professor of dermatology, University of California, Davis, and research fellow Samantha Herbert, MSPH. Their project will characterize rosacea pathophysiology using single-cell RNA sequencing. This novel analytical technique provides specific information on the signals expressed by different cell types and will help researchers better understand the role each subtype may play in rosacea, along with how these cells interact with each other, according to the NRS press release. New knowledge in the foregoing areas may fuel development of better therapies, the release added.
The NRS awarded its second new-research grant to Arisa Ortiz, MD, director of laser and cosmetic dermatology and associate professor of dermatology, University of California, San Diego. She was awarded $5,000 to examine whether laser therapy affects the skin microbiome, the complex ecosystem of bacteria and other microorganisms that reside on the skin. Studies have detected significant differences – such as higher levels of Demodex folliculorum and Staphylococcus epidermidis and lower levels of Cutibacterium acnes – in the microbiome of skin with rosacea compared with healthy skin. Dr. Ortiz’s research also will probe how blood vessels, which laser therapy often target, contribute to the rosacea disease process.
The NRS also renewed its support of an ongoing study led by Sezen Karakus, MD, assistant professor of ophthalmology at the Johns Hopkins Wilmer Eye Institute, Baltimore. She is studying the role of the ocular-surface microbiome in rosacea pathogenesis. Because ocular rosacea can lead to vision-threatening corneal complications, Dr. Karakus said in the press release, identifying microorganisms present on the ocular surface may spur development of targeted treatment strategies.
A second ongoing study for which the NRS renewed funding is investigating whether certain intracellular signals recently found to be elevated in rosacea lesions may drive skin inflammation, which may be a root cause of rosacea. Emmanuel Contassot, PhD, project leader in the dermatology department at the University Hospital of Basel, Switzerland, is leading the study.
To date, the NRS research grants program has awarded more than $1.6 million to research designed to further elucidate potential causes and other key aspects of rosacea with the goal of advancing treatment, prevention, or potential cure of rosacea.
For interested researchers, the deadline to submit proposals for next year’s grants is June 16, 2023. Forms and instructions are available through the research grants section of the NRS website or by contacting the NRS at 4619 N. Ravenswood Ave., Suite 103, Chicago, IL 60640; 888-662-5874; or info@rosacea.org.
Serum dupilumab levels do not predict clinical response
The finding that serum levels, according to a study published in JAMA Dermatology.
The study results mean that researchers should continue exploring potential AD drugs with novel mechanisms to help patients who fail type 2 inflammatory inhibition, experts told this news organization. The search for accurate augurs of clinical performance also must continue.
Addressing inadequate response
Quantifying nonresponse and incomplete response levels with dupilumab is difficult, said Jonathan I. Silverberg, MD, PhD, MPH, offering perspective on the study. “True nonresponse is probably less than 20%, but many other patients are inadequate responders even if they are having partial response.” Dr. Silverberg, professor of dermatology and director of clinical research, at George Washington University, Washington, was not an investigator.
Robert Sidbury, MD, MPH, added, “When a patient doesn’t respond to a medication that you expect they should, we always ask ourselves why.” Dermatologists have long assumed that, as with biologics for psoriasis, low blood levels were to blame for dupilumab nonresponse, said Dr. Sidbury, who is division chief of dermatology at Seattle Children’s Hospital and was not involved with the study. “This study showed that there was no correlation between response and blood levels.”
In the study, Lotte S. Spekhorst, MD, of National Expertise Center for Atopic Dermatitis, department of dermatology and allergology, University Medical Center Utrecht (the Netherlands) and coinvestigators prospectively followed 295 consecutive adult patients with moderate AD who were treated with dupilumab for 1 year. All patients received the same loading (600 mg) and biweekly (300 mg) doses.
The median dupilumab level at 16 weeks was 86.6 mcg/mL, which is higher than serum levels observed with other monoclonal antibodies used for other indications, such as psoriasis and inflammatory bowel disease, the authors noted. More importantly, researchers found no significant relationship between median week 16 dupilumab levels and 1-year clinical responses measured either discretely (Eczema Area and Severity Index [EASI] < 50, 50, 75, or 90; P = .18) or as quartiles (P = .06).
“It may be that response is dependent on target availability of the IL-4R-alpha, with an interpatient variability producing heterogeneity in response,” the authors wrote. But because serum dupilumab levels were relatively high, they said, all patients’ IL-4R-alpha “was likely fully saturated” at 16 weeks.
“This would explain why serum dupilumab levels were not related to effectiveness,” they noted, “although we cannot rule out differential effects in the tissue associated with heterogeneity in serum dupilumab levels.”
The study helps explain why some patients do not fully respond to dupilumab, said Eric L. Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland, who was not involved with the study.
“One hypothesis would be that drug serum levels differ due to metabolism or absorption reasons,” Dr. Simpson said in an interview. Results also suggest that heterogeneity in disease biology, such as other uninhibited cytokine pathways, might explain differences in clinical results. “Thus, more therapeutics that target different inflammatory pathways are needed to capture responses in patients not adequately responding to type 2 inflammatory blockade,” he said.
Assessing AEs
As with response levels, serum dupilumab levels at week 16 did not predict AEs including dupilumab-associated ocular surface disease (DAOSD), which impacted 46.4% of 216 patients who reported AEs. These findings also contradict what happens with biologics in other diseases such as psoriasis and inflammatory bowel disease, said Dr. Sidbury, wherein serum drug levels may predict both clinical response and side-effect risks.
A previous study showed that lowering dupilumab levels led to improvement in DAOSD. Authors of the current study therefore surmised that DAOSD development might be more associated with interpatient variability in IL-4R-alpha expression than with serum drug levels. “More research is necessary to confirm the hypothesis of interpatient variability of the IL-4Ra and the pharmacokinetics of dupilumab,” they concluded.
For now, said Dr. Sidbury, the study helps clinicians look beyond serum drug levels when patients respond inadequately to dupilumab. Moreover, added Dr. Silverberg, study results mean that physicians must find other ways to predict dupilumab response levels. “We need better predictors of clinical response – theranostic markers that we could test the patient to and understand how well they’re going to do,” he said.
Be it dupilumab or any other medication, he said, physicians lack even confirmatory biomarkers to reflect when a drug is working well. “Right now, we go with clinical assessments. But if it’s not drug levels, we have to figure out why some patients do markedly better than others.”
It was not unreasonable, Dr. Silverberg said, for the investigators to seek a biomarker in blood rather than tissue. “But in this disease, we believe that the more important place to look for biomarkers and drug levels would be in the skin itself. So we are still left with the issue” that drug levels in tissue might reflect response when serum levels do not.
The study was supported by grants from AbbVie, Eli Lilly, Leo Pharma, Pfizer, and Sanofi. Study patients participated in the BioDay Registry, which is sponsored by Sanofi, Regeneron, AbbVie, Eli Lilly, LEO Pharma, and Pfizer; the sponsors had no role in the study design and conduct. Dr. Spekhorst discloses receiving speaking fees from Abbvie outside the work; disclosures of other authors included receiving advisory, speaking consulting, and/or investigator fees from Sanofi Genzyme during the study. Several authors had no disclosures.
Dr. Simpson has been an investigator and consultant for Regeneron and Sanofi, makers of dupilumab. Dr. Silverberg has been an investigator, consultant, and speaker for Regeneron and Sanofi. Dr. Sidbury has been a clinical investigator for all dupilumab pediatric trials. (His institution has a contract with Regeneron and Sanofi, but he receives no money from the arrangement.)
The finding that serum levels, according to a study published in JAMA Dermatology.
The study results mean that researchers should continue exploring potential AD drugs with novel mechanisms to help patients who fail type 2 inflammatory inhibition, experts told this news organization. The search for accurate augurs of clinical performance also must continue.
Addressing inadequate response
Quantifying nonresponse and incomplete response levels with dupilumab is difficult, said Jonathan I. Silverberg, MD, PhD, MPH, offering perspective on the study. “True nonresponse is probably less than 20%, but many other patients are inadequate responders even if they are having partial response.” Dr. Silverberg, professor of dermatology and director of clinical research, at George Washington University, Washington, was not an investigator.
Robert Sidbury, MD, MPH, added, “When a patient doesn’t respond to a medication that you expect they should, we always ask ourselves why.” Dermatologists have long assumed that, as with biologics for psoriasis, low blood levels were to blame for dupilumab nonresponse, said Dr. Sidbury, who is division chief of dermatology at Seattle Children’s Hospital and was not involved with the study. “This study showed that there was no correlation between response and blood levels.”
In the study, Lotte S. Spekhorst, MD, of National Expertise Center for Atopic Dermatitis, department of dermatology and allergology, University Medical Center Utrecht (the Netherlands) and coinvestigators prospectively followed 295 consecutive adult patients with moderate AD who were treated with dupilumab for 1 year. All patients received the same loading (600 mg) and biweekly (300 mg) doses.
The median dupilumab level at 16 weeks was 86.6 mcg/mL, which is higher than serum levels observed with other monoclonal antibodies used for other indications, such as psoriasis and inflammatory bowel disease, the authors noted. More importantly, researchers found no significant relationship between median week 16 dupilumab levels and 1-year clinical responses measured either discretely (Eczema Area and Severity Index [EASI] < 50, 50, 75, or 90; P = .18) or as quartiles (P = .06).
“It may be that response is dependent on target availability of the IL-4R-alpha, with an interpatient variability producing heterogeneity in response,” the authors wrote. But because serum dupilumab levels were relatively high, they said, all patients’ IL-4R-alpha “was likely fully saturated” at 16 weeks.
“This would explain why serum dupilumab levels were not related to effectiveness,” they noted, “although we cannot rule out differential effects in the tissue associated with heterogeneity in serum dupilumab levels.”
The study helps explain why some patients do not fully respond to dupilumab, said Eric L. Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland, who was not involved with the study.
“One hypothesis would be that drug serum levels differ due to metabolism or absorption reasons,” Dr. Simpson said in an interview. Results also suggest that heterogeneity in disease biology, such as other uninhibited cytokine pathways, might explain differences in clinical results. “Thus, more therapeutics that target different inflammatory pathways are needed to capture responses in patients not adequately responding to type 2 inflammatory blockade,” he said.
Assessing AEs
As with response levels, serum dupilumab levels at week 16 did not predict AEs including dupilumab-associated ocular surface disease (DAOSD), which impacted 46.4% of 216 patients who reported AEs. These findings also contradict what happens with biologics in other diseases such as psoriasis and inflammatory bowel disease, said Dr. Sidbury, wherein serum drug levels may predict both clinical response and side-effect risks.
A previous study showed that lowering dupilumab levels led to improvement in DAOSD. Authors of the current study therefore surmised that DAOSD development might be more associated with interpatient variability in IL-4R-alpha expression than with serum drug levels. “More research is necessary to confirm the hypothesis of interpatient variability of the IL-4Ra and the pharmacokinetics of dupilumab,” they concluded.
For now, said Dr. Sidbury, the study helps clinicians look beyond serum drug levels when patients respond inadequately to dupilumab. Moreover, added Dr. Silverberg, study results mean that physicians must find other ways to predict dupilumab response levels. “We need better predictors of clinical response – theranostic markers that we could test the patient to and understand how well they’re going to do,” he said.
Be it dupilumab or any other medication, he said, physicians lack even confirmatory biomarkers to reflect when a drug is working well. “Right now, we go with clinical assessments. But if it’s not drug levels, we have to figure out why some patients do markedly better than others.”
It was not unreasonable, Dr. Silverberg said, for the investigators to seek a biomarker in blood rather than tissue. “But in this disease, we believe that the more important place to look for biomarkers and drug levels would be in the skin itself. So we are still left with the issue” that drug levels in tissue might reflect response when serum levels do not.
The study was supported by grants from AbbVie, Eli Lilly, Leo Pharma, Pfizer, and Sanofi. Study patients participated in the BioDay Registry, which is sponsored by Sanofi, Regeneron, AbbVie, Eli Lilly, LEO Pharma, and Pfizer; the sponsors had no role in the study design and conduct. Dr. Spekhorst discloses receiving speaking fees from Abbvie outside the work; disclosures of other authors included receiving advisory, speaking consulting, and/or investigator fees from Sanofi Genzyme during the study. Several authors had no disclosures.
Dr. Simpson has been an investigator and consultant for Regeneron and Sanofi, makers of dupilumab. Dr. Silverberg has been an investigator, consultant, and speaker for Regeneron and Sanofi. Dr. Sidbury has been a clinical investigator for all dupilumab pediatric trials. (His institution has a contract with Regeneron and Sanofi, but he receives no money from the arrangement.)
The finding that serum levels, according to a study published in JAMA Dermatology.
The study results mean that researchers should continue exploring potential AD drugs with novel mechanisms to help patients who fail type 2 inflammatory inhibition, experts told this news organization. The search for accurate augurs of clinical performance also must continue.
Addressing inadequate response
Quantifying nonresponse and incomplete response levels with dupilumab is difficult, said Jonathan I. Silverberg, MD, PhD, MPH, offering perspective on the study. “True nonresponse is probably less than 20%, but many other patients are inadequate responders even if they are having partial response.” Dr. Silverberg, professor of dermatology and director of clinical research, at George Washington University, Washington, was not an investigator.
Robert Sidbury, MD, MPH, added, “When a patient doesn’t respond to a medication that you expect they should, we always ask ourselves why.” Dermatologists have long assumed that, as with biologics for psoriasis, low blood levels were to blame for dupilumab nonresponse, said Dr. Sidbury, who is division chief of dermatology at Seattle Children’s Hospital and was not involved with the study. “This study showed that there was no correlation between response and blood levels.”
In the study, Lotte S. Spekhorst, MD, of National Expertise Center for Atopic Dermatitis, department of dermatology and allergology, University Medical Center Utrecht (the Netherlands) and coinvestigators prospectively followed 295 consecutive adult patients with moderate AD who were treated with dupilumab for 1 year. All patients received the same loading (600 mg) and biweekly (300 mg) doses.
The median dupilumab level at 16 weeks was 86.6 mcg/mL, which is higher than serum levels observed with other monoclonal antibodies used for other indications, such as psoriasis and inflammatory bowel disease, the authors noted. More importantly, researchers found no significant relationship between median week 16 dupilumab levels and 1-year clinical responses measured either discretely (Eczema Area and Severity Index [EASI] < 50, 50, 75, or 90; P = .18) or as quartiles (P = .06).
“It may be that response is dependent on target availability of the IL-4R-alpha, with an interpatient variability producing heterogeneity in response,” the authors wrote. But because serum dupilumab levels were relatively high, they said, all patients’ IL-4R-alpha “was likely fully saturated” at 16 weeks.
“This would explain why serum dupilumab levels were not related to effectiveness,” they noted, “although we cannot rule out differential effects in the tissue associated with heterogeneity in serum dupilumab levels.”
The study helps explain why some patients do not fully respond to dupilumab, said Eric L. Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland, who was not involved with the study.
“One hypothesis would be that drug serum levels differ due to metabolism or absorption reasons,” Dr. Simpson said in an interview. Results also suggest that heterogeneity in disease biology, such as other uninhibited cytokine pathways, might explain differences in clinical results. “Thus, more therapeutics that target different inflammatory pathways are needed to capture responses in patients not adequately responding to type 2 inflammatory blockade,” he said.
Assessing AEs
As with response levels, serum dupilumab levels at week 16 did not predict AEs including dupilumab-associated ocular surface disease (DAOSD), which impacted 46.4% of 216 patients who reported AEs. These findings also contradict what happens with biologics in other diseases such as psoriasis and inflammatory bowel disease, said Dr. Sidbury, wherein serum drug levels may predict both clinical response and side-effect risks.
A previous study showed that lowering dupilumab levels led to improvement in DAOSD. Authors of the current study therefore surmised that DAOSD development might be more associated with interpatient variability in IL-4R-alpha expression than with serum drug levels. “More research is necessary to confirm the hypothesis of interpatient variability of the IL-4Ra and the pharmacokinetics of dupilumab,” they concluded.
For now, said Dr. Sidbury, the study helps clinicians look beyond serum drug levels when patients respond inadequately to dupilumab. Moreover, added Dr. Silverberg, study results mean that physicians must find other ways to predict dupilumab response levels. “We need better predictors of clinical response – theranostic markers that we could test the patient to and understand how well they’re going to do,” he said.
Be it dupilumab or any other medication, he said, physicians lack even confirmatory biomarkers to reflect when a drug is working well. “Right now, we go with clinical assessments. But if it’s not drug levels, we have to figure out why some patients do markedly better than others.”
It was not unreasonable, Dr. Silverberg said, for the investigators to seek a biomarker in blood rather than tissue. “But in this disease, we believe that the more important place to look for biomarkers and drug levels would be in the skin itself. So we are still left with the issue” that drug levels in tissue might reflect response when serum levels do not.
The study was supported by grants from AbbVie, Eli Lilly, Leo Pharma, Pfizer, and Sanofi. Study patients participated in the BioDay Registry, which is sponsored by Sanofi, Regeneron, AbbVie, Eli Lilly, LEO Pharma, and Pfizer; the sponsors had no role in the study design and conduct. Dr. Spekhorst discloses receiving speaking fees from Abbvie outside the work; disclosures of other authors included receiving advisory, speaking consulting, and/or investigator fees from Sanofi Genzyme during the study. Several authors had no disclosures.
Dr. Simpson has been an investigator and consultant for Regeneron and Sanofi, makers of dupilumab. Dr. Silverberg has been an investigator, consultant, and speaker for Regeneron and Sanofi. Dr. Sidbury has been a clinical investigator for all dupilumab pediatric trials. (His institution has a contract with Regeneron and Sanofi, but he receives no money from the arrangement.)
FROM JAMA DERMATOLOGY