User login
Focus on long-COVID: Perimenopause and post-COVID chronic fatigue
Long COVID (postacute sequelae of SARS-CoV-2 infection, or PASC) is an emerging syndrome that affects 50% to 70% of people who survive COVID-19 for up to 3 months or longer after acute disease.1 It is a multisystem condition that causes dysfunction of respiratory, cardiac, and nervous tissue, at least in part likely due to alterations in cellular energy metabolism and reduced oxygen supply to tissue.3 Patients who have had SARS-CoV-2 infection report persistent symptoms and signs that affect their quality of life. These may include neurocognitive, cardiorespiratory, gastrointestinal, and musculoskeletal symptoms; loss of taste and smell; and constitutional symptoms.2 There is no one test to determine if symptoms are due to COVID-19.3
Acute COVID-19 mortality risk factors include increasing age, chronic comorbidities, and male sex. However, long COVID risk factors are quite different. A meta-analysis and review of 20 articles that met inclusion criteria (n = 13,340 study participants), limited by pooling of crude estimates, found that risk factors were female sex and severity of acute disease.4 A second meta-analysis of 37 studies with 1 preprint found that female sex and comorbidities such as pulmonary disease, diabetes, and obesity were risk factors for long COVID.5 Qualitative analysis of single studies (n = 18 study participants) suggested that older adults can develop more long COVID symptoms than younger adults, but this association between advancing age and long COVID was not supported when data were pooled into a meta-analysis.3 However, both single studies (n = 16 study participants) and the meta-analysis (n = 7 study participants) did support female sex as a risk factor for long COVID, along with single studies suggesting increased risk with medical comorbidities for pulmonary disease, diabetes, and organ transplantation.
Perimenopause
Perimenopause: A temporary disruption to physiologic ovarian steroid hormone production following COVID could acutely exacerbate symptoms of perimenopause and menopause.
JoAnn V. Pinkerton, MD, MSCP
The higher prevalence of long COVID in women younger than 50 years6 supports the overlap that studies have shown between symptoms of long COVID and perimenopause,7 as the median age of natural menopause is 51 years. Thus, health care providers need to differentiate between long COVID and other conditions, such as perimenopause, which share similar symptoms (FIGURE). Perimenopause might be diagnosed as long COVID, or the 2 might affect each other.
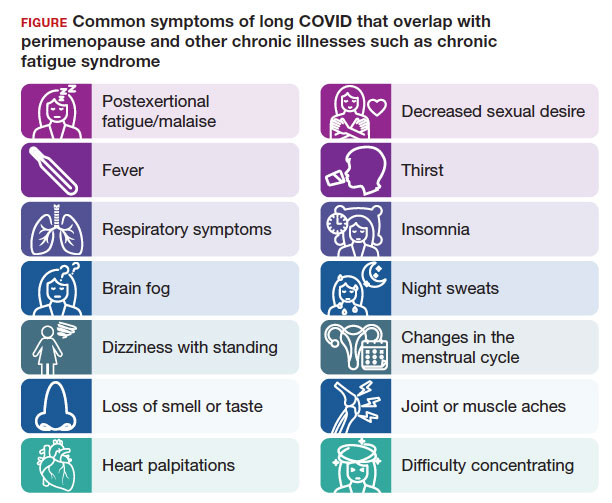
Symptoms of long COVID include fatigue, brain fog, and increased heart rate after recovering from COVID-19 and may continue or increase after an initial infection.8 Common symptoms of perimenopause and menopause, which also could be seen with long COVID, include typical menopausal symptoms such as hot flashes, night sweats, or disrupted sleep; changes in mood including dysthymia, depression, anxiety, or emotional lability; cognitive concerns such as brain fog or decreased concentration; and decreased stamina, fatigue, joint and muscle pains, or more frequent headaches. Therefore, women in their 40s or 50s with persistent symptoms after having COVID-19 without an alternative diagnosis, and who present with menstrual irregularity,9 hot flashes, or night sweats, could be having an exacerbation of perimenopausal symptoms, or they could be experiencing a combination of long COVID and perimenopausal symptoms.
- Consider long COVID, versus perimenopause, or both, in women aged younger than 50 years
- Estradiol, which has been shown to alleviate perimenopausal and menopausal symptoms, also has been shown to have beneficial effects during acute COVID-19 infection
- Hormone therapy could improve symptoms of perimenopause and long COVID if some of the symptoms are due to changes in ovary function
Continue to: Potential pathophysiology...
Potential pathophysiology
Inflammation is likely to be critical in the pathogenesis of postacute sequelae of SARS-CoV-2 infection, or PASC. Individuals with long COVID have elevated inflammatory markers for several months.10 The chronic inflammation associated with long COVID could cause disturbances in the ovary and ovarian hormone production.2,10,11
During perimenopause, the ovary is more sensitive to illnesses such as COVID-19and to stress. The current theory is that COVID-19 affects the ovary with declines in ovarian reserve and ovarian function7 and with potential disruptions to the menstrual cycle, gonadal function, and ovarian sufficiency that lead to issues with menopause or fertility, as well as symptom exacerbation around menstruation.12 Another theory is that SARS-CoV-2 infection affects ovary hormone production, as there is an abundance of angiotensin-converting enzyme-2 receptors on ovarian and endometrial tissue.11 Thus, it makes sense that long COVID could bring on symptoms of perimenopause or menopause more acutely or more severely or lengthen the duration of perimenopausal symptoms.
Perimenopause is the transitional period prior to menopause, when the ovaries gradually produce fewer hormones and is associated with erratic hormonal fluctuations. The length of this transitional period varies from 4 to 10 years. Ethnic variations in the duration of hot flashes have been found, noting that Black and Hispanic women have them for an average of 8 to 10 years (longer), White women for an average of 7 years, and Asian, Japanese, and Chinese women for an average of 5 to 6 years (shorter).17
What should health care providers ask?
Distinguishing perimenopause from long COVID. It is important to try to differentiate between perimenopause and long COVID, and it is possible to have both, with long COVID exacerbating the menopausal symptoms.7,8 Health care providers should be alert to consider perimenopause if women present with shorter or longer cycles (21-40 days), missed periods (particularly 60 days or 2 months), or worsening perimenopausal mood, migraines, insomnia, or hot flashes. Clinicians should actively enquire about all of these symptoms.
Moreover, if a perimenopausal woman reports acutely worsening symptoms after COVID-19, health care providers should address the perimenopausal symptoms and determine whether hormone therapy is appropriate and could improve their symptoms. Women do not need to wait until they go 1 year without a period to be treated with hormone therapy to improve perimenopausal and menopausal symptoms. If women with long COVID have perimenopause or menopause symptoms, they should have access to evidence-based information and discuss menopausal hormone therapy if appropriate. Hormone therapy could improve both perimenopausal symptoms and the long COVID symptoms if some of the symptoms are due to changes in ovary function. Health care providers could consider progesterone or antidepressants during the second half of the cycle (luteal phase) or estrogen combined with progesterone for the entire cycle.18
For health care providers working in long COVID clinics, in addition to asking when symptoms started, what makes symptoms worse, the frequency of symptoms, and which activities are affected, ask about perimenopausal and menopausal symptoms. If a woman has irregular periods, sleep disturbances, fatigue, or mood changes, consider that these could be related to long COVID, perimenopause, or both.8,18 Be able to offer treatment or refer patients to a women’s health specialist who can assess and offer treatment.
A role for vitamin D? A recent retrospective case-matched study found that 6 months after hospital discharge, patients with long COVID had lower levels of 25(OH) vitamin D with the most notable symptom being brain fog.19 Thus, there may be a role for vitamin D supplementation as a preventive strategy in those being discharged after hospitalization. Vitamin D levels and supplementation have not been otherwise evaluated to date.
Lifestyle strategies for women with perimenopause and long COVID
Lifestyle strategies should be encouraged for women during perimenopause and long COVID. This includes good nutrition (avoiding carbs and sweets, particularly before menses), getting at least 7 hours of sleep and using sleep hygiene (regular bedtimes, sleep regimen, no late screens), getting regular exercise 5 days per week, reducing stress, avoiding excess alcohol, and not smoking. All of these factors can help women and their ovarian function during this period of ovarian fluctuations.
The timing of menopause and COVID may coincide with midlife stressors, including relationship issues (separations or divorce), health issues for the individual or their partner, widowhood, parenting challenges (care of young children, struggles with adolescents, grown children returning home), being childless, concerns about aging parents and caregiving responsibilities, as well as midlife career, community, or education issues—all of which make both long COVID and perimenopause more challenging to navigate.
Need for research
There is a need for future research to understand the epidemiologic basis and underlying biological mechanisms of sex differences seen in women with long COVID. Studying the effects of COVID-19 on ovarian function could lead to a better understanding of perimenopause, what causes ovarian failure to speed up, and possibly ways to slow it down8 since there are health risks of early menopause.16
References
- Fernández-de-Las-Peñas C, Palacios-Ceña D, GómezMayordomo V, et al. Defining post-COVID symptoms (postacute COVID, long COVID, persistent post-COVID): an integrative classification. Int J Environ Res Public Health. 2021;18:2621. doi: 10.3390/ijerph18052621
- Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601-615. doi: 10.1038/s41591 -021-01283-z
- Davis HE, McCorkell L, Vogel JM, et al. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133-146. doi: 10.1038/s41579-022-00846-2
- Maglietta G, Diodati F, Puntoni M, et al. Prognostic factors for post-COVID-19 syndrome: a systematic review and meta-analysis. J Clin Med. 2022;11:1541. doi: 10.3390 /jcm11061541
- Notarte KI, de Oliveira MHS, Peligro PJ, et al. Age, sex and previous comorbidities as risk factors not associated with SARS-CoV-2 infection for long COVID-19: a systematic review and meta-analysis. J Clin Med. 2022;11:7314. doi: 10.3390 /jcm11247314
- Sigfrid L, Drake TM, Pauley E, et al. Long COVID in adults discharged from UK hospitals after COVID-19: a prospective, multicentre cohort study using the ISARIC WHO Clinical Characterisation Protocol. Lancet Reg Health Eur. 2021;8:100186. doi: 10.1016/j.lanepe.2021.100186
- Pollack B, von Saltza E, McCorkell L, et al. Female reproductive health impacts of long COVID and associated illnesses including ME/CFS, POTS, and connective tissue disorders: a literature review. Front Rehabil Sci. 2023;4:1122673. doi: 10.3389/fresc.2023.1122673
- Stewart S, Newson L, Briggs TA, et al. Long COVID risk - a signal to address sex hormones and women’s health. Lancet Reg Health Eur. 2021;11:100242. doi: 10.1016 /j.lanepe.2021.100242
- Li K, Chen G, Hou H, et al. Analysis of sex hormones and menstruation in COVID-19 women of child-bearing age. Reprod Biomed Online. 2021;42:260-267. doi: 10.1016 /j.rbmo.2020.09.020
- Phetsouphanh C, Darley DR, Wilson DB, et al. Immunological dysfunction persists for 8 months following initial mild-tomoderate SARS-CoV-2 infection. Nat Immunol. 2022;23:210216. doi: 10.1038/s41590-021-01113-x
- Sharp GC, Fraser A, Sawyer G, et al. The COVID-19 pandemic and the menstrual cycle: research gaps and opportunities. Int J Epidemiol. 2022;51:691-700. doi: 10.1093/ije/dyab239
- Ding T, Wang T, Zhang J, et al. Analysis of ovarian injury associated with COVID-19 disease in reproductive-aged women in Wuhan, China: an observational study. Front Med (Lausanne). 2021;8:635255. doi: 10.3389/fmed.2021.635255
- Huang B, Cai Y, Li N, et al. Sex-based clinical and immunological differences in COVID-19. BMC Infect Dis. 2021;21:647. doi: 10.1186/s12879-021-06313-2
- Connor J, Madhavan S, Mokashi M, et al. Health risks and outcomes that disproportionately affect women during the Covid-19 pandemic: a review. Soc Sci Med. 2020;266:113364. doi: 10.1016/j.socscimed.2020.113364
- Mauvais-Jarvis F, Klein SL, Levin ER. Estradiol, progesterone, immunomodulation, and COVID-19 outcomes. Endocrinology. 2020;161:bqaa127. doi:10.1210/endocr/bqaa127
- The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767-794. doi: 10.1097/GME.0000000000002028
- Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175:531-539. doi:10.1001 /jamainternmed.2014.8063
- Newson L, Lewis R, O’Hara M. Long COVID and menopause - the important role of hormones in long COVID must be considered. Maturitas. 2021;152:74. doi: 10.1016 /j.maturitas.2021.08.026
- di Filippo L, Frara S, Nannipieri F, et al. Low Vitamin D levels are associated with long COVID syndrome in COVID-19 survivors. J Clin Endocrinol Metab. 2023;108:e1106-e1116. doi: 10.1210/clinem/dgad207
Continue to: Chronic fatigue syndrome...
Chronic fatigue syndrome
Chronic fatigue syndrome: A large number of patients have “post-COVID conditions” affecting everyday function, including depression/anxiety, insomnia, and chronic fatigue (with a 3:1 female predominance)
Alexandra Kadl, MD
After 3 years battling acute COVID-19 infections, we encounter now a large number of patients with PASC— also known as “long COVID,” “COVID long-hauler syndrome,” and “post-COVID conditions”—a persistent multisystem syndrome that impacts everyday function.1 As of October 2023, there are more than 100 million COVID-19 survivors reported in the United States; 10% to 85% of COVID survivors2-4 may show lingering, life-altering symptoms after recovery. Common reported symptoms include fatigue, depression/ anxiety, insomnia, and brain fog/difficulty concentrating, which are particularly high in women who often had experienced only mild acute COVID-19 disease and were not even hospitalized. More recently, chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) has been recognized as major component of PASC5 with a 3:1 female predominance.6 Up to 75% of patients with this diagnosis are not able to maintain their jobs and normal life, and up to 25% are so disabled that they are bedbound.6
Diagnosis
Although illnesses resembling CFS have been reported for more than 200 years,7 the diagnosis of CFS/ME remains difficult to make. There is a likely underreporting due to fear of being labeled as malingering when reaching out to health care providers, and there is a reporting bias toward higher socioeconomic groups due to better access to health care. The current criteria for the diagnosis of CFS/ME include the following 3 components8:
- substantial impairment in the ability to function for more than 6 months, accompanied by profound fatigue, not alleviated by rest
- post-exertional malaise (PEM; prolonged, disabling exacerbation of the patient’s baseline symptoms after exercise)
- non-refreshing sleep, PLUS either cognitive impairment or orthostatic intolerance.
Pathophysiology
Originally found to evolve in a small patient population with Epstein-Barr virus infection and Lyme disease, CFS/ME has moved to centerstage after the COVID-19 pandemic. While the diagnosis of COVID-19–related CFS/ME has advanced in the field, a clear mechanistic explanation of why it occurs is still missing. Certain risk factors have been identified for the development of CFS/ME, including female sex, reactivation of herpesviruses, and presence of connective tissue disorders; however, about one-third of patients with CFS/ME do not have identifiable risk factors.9,10 Persistence of viral particles11 and prolonged inflammatory states are speculated to affect the nervous system and mitochondrial function and metabolism. Interestingly, there is no correlation between severity of initial COVID-19 illness and the development of CFS/ME, similar to observations in non–COVID-19–related CFS/ME.
Proposed therapy
There is currently no proven therapy for CFS/ME. At this time, several immunomodulatory, antiviral, and neuromodulator drugs are being tested in clinical trial networks around the world.12 Usual physical therapy with near maximum intensity has been shown to exacerbate symptoms and often results in PEM, which is described as a “crash” or “full collapse” by patients. The time for recovery after such episodes can be several days.13
Instead, the focus should be on addressing “treatable” concomitant symptoms, such as sleep disorders, anxiety and depression, and chronic pain. Lifestyle changes, avoidance of triggers, and exercise without over exertion are currently recommended to avoid incapacitating PEM.
Gaps in knowledge
There is a large knowledge gap regarding the pathophysiology, prevention, and therapy for CFS/ME. Many health care practitioners are not familiar with the disease and have focused on measurable parameters of exercise limitations and fatigue, such as anemias and lung and cardiac impairments, thus treating CFS/ME as a form of deconditioning. Given the large number of patients who recovered from acute COVID-19 that are now disabled due to CFS/ME, a patient-centered research opportunity has arisen. Biomedical/mechanistic research is ongoing, and well-designed clinical trials evaluating pharmacologic intervention as well as tailored exercise programs are needed.
Conclusion
General practitioners and women’s health specialists need to be aware of CFS/ME, especially when managing patients with long COVID. They also need to know that typical physical therapy may worsen symptoms. Furthermore, clinicians should shy away from trial drugs with a theoretical benefit outside of a clinical trial. ●
- Chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) has been recognized as a major component of PASC
- Typical physical therapy has been shown to exacerbate symptoms of CFS/ME
- Treatment should focus on addressing “treatable” concomitant symptoms, lifestyle changes, avoidance of triggers, and exercise without over exertion
References
- Soriano JB, Murthy S, Marshall JC, et al. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22:e102-e107. doi: 10.1016 /S1473-3099(21)00703-9
- Chen C, Haupert SR, Zimmermann L, et al. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: a meta-analysis and systematic review. J Infect Dis. 2022;226:1593-1607. doi: 10.1093/infdis/jiac136
- Davis HE, McCorkell L, Vogel JM, et al. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133-146. doi: 10.1038/s41579-022 -00846-2
- Pavli A, Theodoridou M, Maltezou HC. Post-COVID syndrome: incidence, clinical spectrum, and challenges for primary healthcare professionals. Arch Med Res. 2021;52:575-581. doi: 10.1016/j.arcmed.2021.03.010
- Kedor C, Freitag H, Meyer-Arndt L, et al. A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat Commun. 2022;13:5104. doi: 10.1038/s41467-022-32507-6
- Bateman L, Bested AC, Bonilla HF, et al. Myalgic encephalomyelitis/chronic fatigue syndrome: essentials of diagnosis and management. Mayo Clin Proc. 2021;96:28612878. doi: 10.1016/j.mayocp.2021.07.004
- Wessely S. History of postviral fatigue syndrome. Br Med Bull. 1991;47:919-941. doi: 10.1093/oxfordjournals.bmb.a072521
- Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; Board on the Health of Select Populations; Institute of Medicine. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness. National Academies Press; 2015. doi: 10.17226/19012
- Ceban F, Ling S, Lui LMW, et al. Fatigue and cognitive impairment in post-COVID-19 syndrome: a systematic review and meta-analysis. Brain Behav Immun. 2022;101:93135. doi: 10.1016/j.bbi.2021.12.020
- Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019
- Hanson MR. The viral origin of myalgic encephalomyelitis/ chronic fatigue syndrome. PLoS Pathog. 2023;19:e1011523. doi: 10.1371/journal.ppat.1011523
- Scheibenbogen C, Bellmann-Strobl JT, Heindrich C, et al. Fighting post-COVID and ME/CFS—development of curative therapies. Front Med (Lausanne). 2023;10:1194754. doi: 10.3389/fmed.2023.1194754
- Stussman B, Williams A, Snow J, et al. Characterization of post-exertional malaise in patients with myalgic encephalomyelitis/chronic fatigue syndrome. Front Neurol. 2020;11:1025. doi: 10.3389/fneur.2020.01025
Long COVID (postacute sequelae of SARS-CoV-2 infection, or PASC) is an emerging syndrome that affects 50% to 70% of people who survive COVID-19 for up to 3 months or longer after acute disease.1 It is a multisystem condition that causes dysfunction of respiratory, cardiac, and nervous tissue, at least in part likely due to alterations in cellular energy metabolism and reduced oxygen supply to tissue.3 Patients who have had SARS-CoV-2 infection report persistent symptoms and signs that affect their quality of life. These may include neurocognitive, cardiorespiratory, gastrointestinal, and musculoskeletal symptoms; loss of taste and smell; and constitutional symptoms.2 There is no one test to determine if symptoms are due to COVID-19.3
Acute COVID-19 mortality risk factors include increasing age, chronic comorbidities, and male sex. However, long COVID risk factors are quite different. A meta-analysis and review of 20 articles that met inclusion criteria (n = 13,340 study participants), limited by pooling of crude estimates, found that risk factors were female sex and severity of acute disease.4 A second meta-analysis of 37 studies with 1 preprint found that female sex and comorbidities such as pulmonary disease, diabetes, and obesity were risk factors for long COVID.5 Qualitative analysis of single studies (n = 18 study participants) suggested that older adults can develop more long COVID symptoms than younger adults, but this association between advancing age and long COVID was not supported when data were pooled into a meta-analysis.3 However, both single studies (n = 16 study participants) and the meta-analysis (n = 7 study participants) did support female sex as a risk factor for long COVID, along with single studies suggesting increased risk with medical comorbidities for pulmonary disease, diabetes, and organ transplantation.
Perimenopause
Perimenopause: A temporary disruption to physiologic ovarian steroid hormone production following COVID could acutely exacerbate symptoms of perimenopause and menopause.
JoAnn V. Pinkerton, MD, MSCP
The higher prevalence of long COVID in women younger than 50 years6 supports the overlap that studies have shown between symptoms of long COVID and perimenopause,7 as the median age of natural menopause is 51 years. Thus, health care providers need to differentiate between long COVID and other conditions, such as perimenopause, which share similar symptoms (FIGURE). Perimenopause might be diagnosed as long COVID, or the 2 might affect each other.

Symptoms of long COVID include fatigue, brain fog, and increased heart rate after recovering from COVID-19 and may continue or increase after an initial infection.8 Common symptoms of perimenopause and menopause, which also could be seen with long COVID, include typical menopausal symptoms such as hot flashes, night sweats, or disrupted sleep; changes in mood including dysthymia, depression, anxiety, or emotional lability; cognitive concerns such as brain fog or decreased concentration; and decreased stamina, fatigue, joint and muscle pains, or more frequent headaches. Therefore, women in their 40s or 50s with persistent symptoms after having COVID-19 without an alternative diagnosis, and who present with menstrual irregularity,9 hot flashes, or night sweats, could be having an exacerbation of perimenopausal symptoms, or they could be experiencing a combination of long COVID and perimenopausal symptoms.
- Consider long COVID, versus perimenopause, or both, in women aged younger than 50 years
- Estradiol, which has been shown to alleviate perimenopausal and menopausal symptoms, also has been shown to have beneficial effects during acute COVID-19 infection
- Hormone therapy could improve symptoms of perimenopause and long COVID if some of the symptoms are due to changes in ovary function
Continue to: Potential pathophysiology...
Potential pathophysiology
Inflammation is likely to be critical in the pathogenesis of postacute sequelae of SARS-CoV-2 infection, or PASC. Individuals with long COVID have elevated inflammatory markers for several months.10 The chronic inflammation associated with long COVID could cause disturbances in the ovary and ovarian hormone production.2,10,11
During perimenopause, the ovary is more sensitive to illnesses such as COVID-19and to stress. The current theory is that COVID-19 affects the ovary with declines in ovarian reserve and ovarian function7 and with potential disruptions to the menstrual cycle, gonadal function, and ovarian sufficiency that lead to issues with menopause or fertility, as well as symptom exacerbation around menstruation.12 Another theory is that SARS-CoV-2 infection affects ovary hormone production, as there is an abundance of angiotensin-converting enzyme-2 receptors on ovarian and endometrial tissue.11 Thus, it makes sense that long COVID could bring on symptoms of perimenopause or menopause more acutely or more severely or lengthen the duration of perimenopausal symptoms.
Perimenopause is the transitional period prior to menopause, when the ovaries gradually produce fewer hormones and is associated with erratic hormonal fluctuations. The length of this transitional period varies from 4 to 10 years. Ethnic variations in the duration of hot flashes have been found, noting that Black and Hispanic women have them for an average of 8 to 10 years (longer), White women for an average of 7 years, and Asian, Japanese, and Chinese women for an average of 5 to 6 years (shorter).17
What should health care providers ask?
Distinguishing perimenopause from long COVID. It is important to try to differentiate between perimenopause and long COVID, and it is possible to have both, with long COVID exacerbating the menopausal symptoms.7,8 Health care providers should be alert to consider perimenopause if women present with shorter or longer cycles (21-40 days), missed periods (particularly 60 days or 2 months), or worsening perimenopausal mood, migraines, insomnia, or hot flashes. Clinicians should actively enquire about all of these symptoms.
Moreover, if a perimenopausal woman reports acutely worsening symptoms after COVID-19, health care providers should address the perimenopausal symptoms and determine whether hormone therapy is appropriate and could improve their symptoms. Women do not need to wait until they go 1 year without a period to be treated with hormone therapy to improve perimenopausal and menopausal symptoms. If women with long COVID have perimenopause or menopause symptoms, they should have access to evidence-based information and discuss menopausal hormone therapy if appropriate. Hormone therapy could improve both perimenopausal symptoms and the long COVID symptoms if some of the symptoms are due to changes in ovary function. Health care providers could consider progesterone or antidepressants during the second half of the cycle (luteal phase) or estrogen combined with progesterone for the entire cycle.18
For health care providers working in long COVID clinics, in addition to asking when symptoms started, what makes symptoms worse, the frequency of symptoms, and which activities are affected, ask about perimenopausal and menopausal symptoms. If a woman has irregular periods, sleep disturbances, fatigue, or mood changes, consider that these could be related to long COVID, perimenopause, or both.8,18 Be able to offer treatment or refer patients to a women’s health specialist who can assess and offer treatment.
A role for vitamin D? A recent retrospective case-matched study found that 6 months after hospital discharge, patients with long COVID had lower levels of 25(OH) vitamin D with the most notable symptom being brain fog.19 Thus, there may be a role for vitamin D supplementation as a preventive strategy in those being discharged after hospitalization. Vitamin D levels and supplementation have not been otherwise evaluated to date.
Lifestyle strategies for women with perimenopause and long COVID
Lifestyle strategies should be encouraged for women during perimenopause and long COVID. This includes good nutrition (avoiding carbs and sweets, particularly before menses), getting at least 7 hours of sleep and using sleep hygiene (regular bedtimes, sleep regimen, no late screens), getting regular exercise 5 days per week, reducing stress, avoiding excess alcohol, and not smoking. All of these factors can help women and their ovarian function during this period of ovarian fluctuations.
The timing of menopause and COVID may coincide with midlife stressors, including relationship issues (separations or divorce), health issues for the individual or their partner, widowhood, parenting challenges (care of young children, struggles with adolescents, grown children returning home), being childless, concerns about aging parents and caregiving responsibilities, as well as midlife career, community, or education issues—all of which make both long COVID and perimenopause more challenging to navigate.
Need for research
There is a need for future research to understand the epidemiologic basis and underlying biological mechanisms of sex differences seen in women with long COVID. Studying the effects of COVID-19 on ovarian function could lead to a better understanding of perimenopause, what causes ovarian failure to speed up, and possibly ways to slow it down8 since there are health risks of early menopause.16
References
- Fernández-de-Las-Peñas C, Palacios-Ceña D, GómezMayordomo V, et al. Defining post-COVID symptoms (postacute COVID, long COVID, persistent post-COVID): an integrative classification. Int J Environ Res Public Health. 2021;18:2621. doi: 10.3390/ijerph18052621
- Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601-615. doi: 10.1038/s41591 -021-01283-z
- Davis HE, McCorkell L, Vogel JM, et al. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133-146. doi: 10.1038/s41579-022-00846-2
- Maglietta G, Diodati F, Puntoni M, et al. Prognostic factors for post-COVID-19 syndrome: a systematic review and meta-analysis. J Clin Med. 2022;11:1541. doi: 10.3390 /jcm11061541
- Notarte KI, de Oliveira MHS, Peligro PJ, et al. Age, sex and previous comorbidities as risk factors not associated with SARS-CoV-2 infection for long COVID-19: a systematic review and meta-analysis. J Clin Med. 2022;11:7314. doi: 10.3390 /jcm11247314
- Sigfrid L, Drake TM, Pauley E, et al. Long COVID in adults discharged from UK hospitals after COVID-19: a prospective, multicentre cohort study using the ISARIC WHO Clinical Characterisation Protocol. Lancet Reg Health Eur. 2021;8:100186. doi: 10.1016/j.lanepe.2021.100186
- Pollack B, von Saltza E, McCorkell L, et al. Female reproductive health impacts of long COVID and associated illnesses including ME/CFS, POTS, and connective tissue disorders: a literature review. Front Rehabil Sci. 2023;4:1122673. doi: 10.3389/fresc.2023.1122673
- Stewart S, Newson L, Briggs TA, et al. Long COVID risk - a signal to address sex hormones and women’s health. Lancet Reg Health Eur. 2021;11:100242. doi: 10.1016 /j.lanepe.2021.100242
- Li K, Chen G, Hou H, et al. Analysis of sex hormones and menstruation in COVID-19 women of child-bearing age. Reprod Biomed Online. 2021;42:260-267. doi: 10.1016 /j.rbmo.2020.09.020
- Phetsouphanh C, Darley DR, Wilson DB, et al. Immunological dysfunction persists for 8 months following initial mild-tomoderate SARS-CoV-2 infection. Nat Immunol. 2022;23:210216. doi: 10.1038/s41590-021-01113-x
- Sharp GC, Fraser A, Sawyer G, et al. The COVID-19 pandemic and the menstrual cycle: research gaps and opportunities. Int J Epidemiol. 2022;51:691-700. doi: 10.1093/ije/dyab239
- Ding T, Wang T, Zhang J, et al. Analysis of ovarian injury associated with COVID-19 disease in reproductive-aged women in Wuhan, China: an observational study. Front Med (Lausanne). 2021;8:635255. doi: 10.3389/fmed.2021.635255
- Huang B, Cai Y, Li N, et al. Sex-based clinical and immunological differences in COVID-19. BMC Infect Dis. 2021;21:647. doi: 10.1186/s12879-021-06313-2
- Connor J, Madhavan S, Mokashi M, et al. Health risks and outcomes that disproportionately affect women during the Covid-19 pandemic: a review. Soc Sci Med. 2020;266:113364. doi: 10.1016/j.socscimed.2020.113364
- Mauvais-Jarvis F, Klein SL, Levin ER. Estradiol, progesterone, immunomodulation, and COVID-19 outcomes. Endocrinology. 2020;161:bqaa127. doi:10.1210/endocr/bqaa127
- The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767-794. doi: 10.1097/GME.0000000000002028
- Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175:531-539. doi:10.1001 /jamainternmed.2014.8063
- Newson L, Lewis R, O’Hara M. Long COVID and menopause - the important role of hormones in long COVID must be considered. Maturitas. 2021;152:74. doi: 10.1016 /j.maturitas.2021.08.026
- di Filippo L, Frara S, Nannipieri F, et al. Low Vitamin D levels are associated with long COVID syndrome in COVID-19 survivors. J Clin Endocrinol Metab. 2023;108:e1106-e1116. doi: 10.1210/clinem/dgad207
Continue to: Chronic fatigue syndrome...
Chronic fatigue syndrome
Chronic fatigue syndrome: A large number of patients have “post-COVID conditions” affecting everyday function, including depression/anxiety, insomnia, and chronic fatigue (with a 3:1 female predominance)
Alexandra Kadl, MD
After 3 years battling acute COVID-19 infections, we encounter now a large number of patients with PASC— also known as “long COVID,” “COVID long-hauler syndrome,” and “post-COVID conditions”—a persistent multisystem syndrome that impacts everyday function.1 As of October 2023, there are more than 100 million COVID-19 survivors reported in the United States; 10% to 85% of COVID survivors2-4 may show lingering, life-altering symptoms after recovery. Common reported symptoms include fatigue, depression/ anxiety, insomnia, and brain fog/difficulty concentrating, which are particularly high in women who often had experienced only mild acute COVID-19 disease and were not even hospitalized. More recently, chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) has been recognized as major component of PASC5 with a 3:1 female predominance.6 Up to 75% of patients with this diagnosis are not able to maintain their jobs and normal life, and up to 25% are so disabled that they are bedbound.6
Diagnosis
Although illnesses resembling CFS have been reported for more than 200 years,7 the diagnosis of CFS/ME remains difficult to make. There is a likely underreporting due to fear of being labeled as malingering when reaching out to health care providers, and there is a reporting bias toward higher socioeconomic groups due to better access to health care. The current criteria for the diagnosis of CFS/ME include the following 3 components8:
- substantial impairment in the ability to function for more than 6 months, accompanied by profound fatigue, not alleviated by rest
- post-exertional malaise (PEM; prolonged, disabling exacerbation of the patient’s baseline symptoms after exercise)
- non-refreshing sleep, PLUS either cognitive impairment or orthostatic intolerance.
Pathophysiology
Originally found to evolve in a small patient population with Epstein-Barr virus infection and Lyme disease, CFS/ME has moved to centerstage after the COVID-19 pandemic. While the diagnosis of COVID-19–related CFS/ME has advanced in the field, a clear mechanistic explanation of why it occurs is still missing. Certain risk factors have been identified for the development of CFS/ME, including female sex, reactivation of herpesviruses, and presence of connective tissue disorders; however, about one-third of patients with CFS/ME do not have identifiable risk factors.9,10 Persistence of viral particles11 and prolonged inflammatory states are speculated to affect the nervous system and mitochondrial function and metabolism. Interestingly, there is no correlation between severity of initial COVID-19 illness and the development of CFS/ME, similar to observations in non–COVID-19–related CFS/ME.
Proposed therapy
There is currently no proven therapy for CFS/ME. At this time, several immunomodulatory, antiviral, and neuromodulator drugs are being tested in clinical trial networks around the world.12 Usual physical therapy with near maximum intensity has been shown to exacerbate symptoms and often results in PEM, which is described as a “crash” or “full collapse” by patients. The time for recovery after such episodes can be several days.13
Instead, the focus should be on addressing “treatable” concomitant symptoms, such as sleep disorders, anxiety and depression, and chronic pain. Lifestyle changes, avoidance of triggers, and exercise without over exertion are currently recommended to avoid incapacitating PEM.
Gaps in knowledge
There is a large knowledge gap regarding the pathophysiology, prevention, and therapy for CFS/ME. Many health care practitioners are not familiar with the disease and have focused on measurable parameters of exercise limitations and fatigue, such as anemias and lung and cardiac impairments, thus treating CFS/ME as a form of deconditioning. Given the large number of patients who recovered from acute COVID-19 that are now disabled due to CFS/ME, a patient-centered research opportunity has arisen. Biomedical/mechanistic research is ongoing, and well-designed clinical trials evaluating pharmacologic intervention as well as tailored exercise programs are needed.
Conclusion
General practitioners and women’s health specialists need to be aware of CFS/ME, especially when managing patients with long COVID. They also need to know that typical physical therapy may worsen symptoms. Furthermore, clinicians should shy away from trial drugs with a theoretical benefit outside of a clinical trial. ●
- Chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) has been recognized as a major component of PASC
- Typical physical therapy has been shown to exacerbate symptoms of CFS/ME
- Treatment should focus on addressing “treatable” concomitant symptoms, lifestyle changes, avoidance of triggers, and exercise without over exertion
References
- Soriano JB, Murthy S, Marshall JC, et al. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22:e102-e107. doi: 10.1016 /S1473-3099(21)00703-9
- Chen C, Haupert SR, Zimmermann L, et al. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: a meta-analysis and systematic review. J Infect Dis. 2022;226:1593-1607. doi: 10.1093/infdis/jiac136
- Davis HE, McCorkell L, Vogel JM, et al. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133-146. doi: 10.1038/s41579-022 -00846-2
- Pavli A, Theodoridou M, Maltezou HC. Post-COVID syndrome: incidence, clinical spectrum, and challenges for primary healthcare professionals. Arch Med Res. 2021;52:575-581. doi: 10.1016/j.arcmed.2021.03.010
- Kedor C, Freitag H, Meyer-Arndt L, et al. A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat Commun. 2022;13:5104. doi: 10.1038/s41467-022-32507-6
- Bateman L, Bested AC, Bonilla HF, et al. Myalgic encephalomyelitis/chronic fatigue syndrome: essentials of diagnosis and management. Mayo Clin Proc. 2021;96:28612878. doi: 10.1016/j.mayocp.2021.07.004
- Wessely S. History of postviral fatigue syndrome. Br Med Bull. 1991;47:919-941. doi: 10.1093/oxfordjournals.bmb.a072521
- Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; Board on the Health of Select Populations; Institute of Medicine. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness. National Academies Press; 2015. doi: 10.17226/19012
- Ceban F, Ling S, Lui LMW, et al. Fatigue and cognitive impairment in post-COVID-19 syndrome: a systematic review and meta-analysis. Brain Behav Immun. 2022;101:93135. doi: 10.1016/j.bbi.2021.12.020
- Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019
- Hanson MR. The viral origin of myalgic encephalomyelitis/ chronic fatigue syndrome. PLoS Pathog. 2023;19:e1011523. doi: 10.1371/journal.ppat.1011523
- Scheibenbogen C, Bellmann-Strobl JT, Heindrich C, et al. Fighting post-COVID and ME/CFS—development of curative therapies. Front Med (Lausanne). 2023;10:1194754. doi: 10.3389/fmed.2023.1194754
- Stussman B, Williams A, Snow J, et al. Characterization of post-exertional malaise in patients with myalgic encephalomyelitis/chronic fatigue syndrome. Front Neurol. 2020;11:1025. doi: 10.3389/fneur.2020.01025
Long COVID (postacute sequelae of SARS-CoV-2 infection, or PASC) is an emerging syndrome that affects 50% to 70% of people who survive COVID-19 for up to 3 months or longer after acute disease.1 It is a multisystem condition that causes dysfunction of respiratory, cardiac, and nervous tissue, at least in part likely due to alterations in cellular energy metabolism and reduced oxygen supply to tissue.3 Patients who have had SARS-CoV-2 infection report persistent symptoms and signs that affect their quality of life. These may include neurocognitive, cardiorespiratory, gastrointestinal, and musculoskeletal symptoms; loss of taste and smell; and constitutional symptoms.2 There is no one test to determine if symptoms are due to COVID-19.3
Acute COVID-19 mortality risk factors include increasing age, chronic comorbidities, and male sex. However, long COVID risk factors are quite different. A meta-analysis and review of 20 articles that met inclusion criteria (n = 13,340 study participants), limited by pooling of crude estimates, found that risk factors were female sex and severity of acute disease.4 A second meta-analysis of 37 studies with 1 preprint found that female sex and comorbidities such as pulmonary disease, diabetes, and obesity were risk factors for long COVID.5 Qualitative analysis of single studies (n = 18 study participants) suggested that older adults can develop more long COVID symptoms than younger adults, but this association between advancing age and long COVID was not supported when data were pooled into a meta-analysis.3 However, both single studies (n = 16 study participants) and the meta-analysis (n = 7 study participants) did support female sex as a risk factor for long COVID, along with single studies suggesting increased risk with medical comorbidities for pulmonary disease, diabetes, and organ transplantation.
Perimenopause
Perimenopause: A temporary disruption to physiologic ovarian steroid hormone production following COVID could acutely exacerbate symptoms of perimenopause and menopause.
JoAnn V. Pinkerton, MD, MSCP
The higher prevalence of long COVID in women younger than 50 years6 supports the overlap that studies have shown between symptoms of long COVID and perimenopause,7 as the median age of natural menopause is 51 years. Thus, health care providers need to differentiate between long COVID and other conditions, such as perimenopause, which share similar symptoms (FIGURE). Perimenopause might be diagnosed as long COVID, or the 2 might affect each other.

Symptoms of long COVID include fatigue, brain fog, and increased heart rate after recovering from COVID-19 and may continue or increase after an initial infection.8 Common symptoms of perimenopause and menopause, which also could be seen with long COVID, include typical menopausal symptoms such as hot flashes, night sweats, or disrupted sleep; changes in mood including dysthymia, depression, anxiety, or emotional lability; cognitive concerns such as brain fog or decreased concentration; and decreased stamina, fatigue, joint and muscle pains, or more frequent headaches. Therefore, women in their 40s or 50s with persistent symptoms after having COVID-19 without an alternative diagnosis, and who present with menstrual irregularity,9 hot flashes, or night sweats, could be having an exacerbation of perimenopausal symptoms, or they could be experiencing a combination of long COVID and perimenopausal symptoms.
- Consider long COVID, versus perimenopause, or both, in women aged younger than 50 years
- Estradiol, which has been shown to alleviate perimenopausal and menopausal symptoms, also has been shown to have beneficial effects during acute COVID-19 infection
- Hormone therapy could improve symptoms of perimenopause and long COVID if some of the symptoms are due to changes in ovary function
Continue to: Potential pathophysiology...
Potential pathophysiology
Inflammation is likely to be critical in the pathogenesis of postacute sequelae of SARS-CoV-2 infection, or PASC. Individuals with long COVID have elevated inflammatory markers for several months.10 The chronic inflammation associated with long COVID could cause disturbances in the ovary and ovarian hormone production.2,10,11
During perimenopause, the ovary is more sensitive to illnesses such as COVID-19and to stress. The current theory is that COVID-19 affects the ovary with declines in ovarian reserve and ovarian function7 and with potential disruptions to the menstrual cycle, gonadal function, and ovarian sufficiency that lead to issues with menopause or fertility, as well as symptom exacerbation around menstruation.12 Another theory is that SARS-CoV-2 infection affects ovary hormone production, as there is an abundance of angiotensin-converting enzyme-2 receptors on ovarian and endometrial tissue.11 Thus, it makes sense that long COVID could bring on symptoms of perimenopause or menopause more acutely or more severely or lengthen the duration of perimenopausal symptoms.
Perimenopause is the transitional period prior to menopause, when the ovaries gradually produce fewer hormones and is associated with erratic hormonal fluctuations. The length of this transitional period varies from 4 to 10 years. Ethnic variations in the duration of hot flashes have been found, noting that Black and Hispanic women have them for an average of 8 to 10 years (longer), White women for an average of 7 years, and Asian, Japanese, and Chinese women for an average of 5 to 6 years (shorter).17
What should health care providers ask?
Distinguishing perimenopause from long COVID. It is important to try to differentiate between perimenopause and long COVID, and it is possible to have both, with long COVID exacerbating the menopausal symptoms.7,8 Health care providers should be alert to consider perimenopause if women present with shorter or longer cycles (21-40 days), missed periods (particularly 60 days or 2 months), or worsening perimenopausal mood, migraines, insomnia, or hot flashes. Clinicians should actively enquire about all of these symptoms.
Moreover, if a perimenopausal woman reports acutely worsening symptoms after COVID-19, health care providers should address the perimenopausal symptoms and determine whether hormone therapy is appropriate and could improve their symptoms. Women do not need to wait until they go 1 year without a period to be treated with hormone therapy to improve perimenopausal and menopausal symptoms. If women with long COVID have perimenopause or menopause symptoms, they should have access to evidence-based information and discuss menopausal hormone therapy if appropriate. Hormone therapy could improve both perimenopausal symptoms and the long COVID symptoms if some of the symptoms are due to changes in ovary function. Health care providers could consider progesterone or antidepressants during the second half of the cycle (luteal phase) or estrogen combined with progesterone for the entire cycle.18
For health care providers working in long COVID clinics, in addition to asking when symptoms started, what makes symptoms worse, the frequency of symptoms, and which activities are affected, ask about perimenopausal and menopausal symptoms. If a woman has irregular periods, sleep disturbances, fatigue, or mood changes, consider that these could be related to long COVID, perimenopause, or both.8,18 Be able to offer treatment or refer patients to a women’s health specialist who can assess and offer treatment.
A role for vitamin D? A recent retrospective case-matched study found that 6 months after hospital discharge, patients with long COVID had lower levels of 25(OH) vitamin D with the most notable symptom being brain fog.19 Thus, there may be a role for vitamin D supplementation as a preventive strategy in those being discharged after hospitalization. Vitamin D levels and supplementation have not been otherwise evaluated to date.
Lifestyle strategies for women with perimenopause and long COVID
Lifestyle strategies should be encouraged for women during perimenopause and long COVID. This includes good nutrition (avoiding carbs and sweets, particularly before menses), getting at least 7 hours of sleep and using sleep hygiene (regular bedtimes, sleep regimen, no late screens), getting regular exercise 5 days per week, reducing stress, avoiding excess alcohol, and not smoking. All of these factors can help women and their ovarian function during this period of ovarian fluctuations.
The timing of menopause and COVID may coincide with midlife stressors, including relationship issues (separations or divorce), health issues for the individual or their partner, widowhood, parenting challenges (care of young children, struggles with adolescents, grown children returning home), being childless, concerns about aging parents and caregiving responsibilities, as well as midlife career, community, or education issues—all of which make both long COVID and perimenopause more challenging to navigate.
Need for research
There is a need for future research to understand the epidemiologic basis and underlying biological mechanisms of sex differences seen in women with long COVID. Studying the effects of COVID-19 on ovarian function could lead to a better understanding of perimenopause, what causes ovarian failure to speed up, and possibly ways to slow it down8 since there are health risks of early menopause.16
References
- Fernández-de-Las-Peñas C, Palacios-Ceña D, GómezMayordomo V, et al. Defining post-COVID symptoms (postacute COVID, long COVID, persistent post-COVID): an integrative classification. Int J Environ Res Public Health. 2021;18:2621. doi: 10.3390/ijerph18052621
- Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601-615. doi: 10.1038/s41591 -021-01283-z
- Davis HE, McCorkell L, Vogel JM, et al. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133-146. doi: 10.1038/s41579-022-00846-2
- Maglietta G, Diodati F, Puntoni M, et al. Prognostic factors for post-COVID-19 syndrome: a systematic review and meta-analysis. J Clin Med. 2022;11:1541. doi: 10.3390 /jcm11061541
- Notarte KI, de Oliveira MHS, Peligro PJ, et al. Age, sex and previous comorbidities as risk factors not associated with SARS-CoV-2 infection for long COVID-19: a systematic review and meta-analysis. J Clin Med. 2022;11:7314. doi: 10.3390 /jcm11247314
- Sigfrid L, Drake TM, Pauley E, et al. Long COVID in adults discharged from UK hospitals after COVID-19: a prospective, multicentre cohort study using the ISARIC WHO Clinical Characterisation Protocol. Lancet Reg Health Eur. 2021;8:100186. doi: 10.1016/j.lanepe.2021.100186
- Pollack B, von Saltza E, McCorkell L, et al. Female reproductive health impacts of long COVID and associated illnesses including ME/CFS, POTS, and connective tissue disorders: a literature review. Front Rehabil Sci. 2023;4:1122673. doi: 10.3389/fresc.2023.1122673
- Stewart S, Newson L, Briggs TA, et al. Long COVID risk - a signal to address sex hormones and women’s health. Lancet Reg Health Eur. 2021;11:100242. doi: 10.1016 /j.lanepe.2021.100242
- Li K, Chen G, Hou H, et al. Analysis of sex hormones and menstruation in COVID-19 women of child-bearing age. Reprod Biomed Online. 2021;42:260-267. doi: 10.1016 /j.rbmo.2020.09.020
- Phetsouphanh C, Darley DR, Wilson DB, et al. Immunological dysfunction persists for 8 months following initial mild-tomoderate SARS-CoV-2 infection. Nat Immunol. 2022;23:210216. doi: 10.1038/s41590-021-01113-x
- Sharp GC, Fraser A, Sawyer G, et al. The COVID-19 pandemic and the menstrual cycle: research gaps and opportunities. Int J Epidemiol. 2022;51:691-700. doi: 10.1093/ije/dyab239
- Ding T, Wang T, Zhang J, et al. Analysis of ovarian injury associated with COVID-19 disease in reproductive-aged women in Wuhan, China: an observational study. Front Med (Lausanne). 2021;8:635255. doi: 10.3389/fmed.2021.635255
- Huang B, Cai Y, Li N, et al. Sex-based clinical and immunological differences in COVID-19. BMC Infect Dis. 2021;21:647. doi: 10.1186/s12879-021-06313-2
- Connor J, Madhavan S, Mokashi M, et al. Health risks and outcomes that disproportionately affect women during the Covid-19 pandemic: a review. Soc Sci Med. 2020;266:113364. doi: 10.1016/j.socscimed.2020.113364
- Mauvais-Jarvis F, Klein SL, Levin ER. Estradiol, progesterone, immunomodulation, and COVID-19 outcomes. Endocrinology. 2020;161:bqaa127. doi:10.1210/endocr/bqaa127
- The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767-794. doi: 10.1097/GME.0000000000002028
- Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175:531-539. doi:10.1001 /jamainternmed.2014.8063
- Newson L, Lewis R, O’Hara M. Long COVID and menopause - the important role of hormones in long COVID must be considered. Maturitas. 2021;152:74. doi: 10.1016 /j.maturitas.2021.08.026
- di Filippo L, Frara S, Nannipieri F, et al. Low Vitamin D levels are associated with long COVID syndrome in COVID-19 survivors. J Clin Endocrinol Metab. 2023;108:e1106-e1116. doi: 10.1210/clinem/dgad207
Continue to: Chronic fatigue syndrome...
Chronic fatigue syndrome
Chronic fatigue syndrome: A large number of patients have “post-COVID conditions” affecting everyday function, including depression/anxiety, insomnia, and chronic fatigue (with a 3:1 female predominance)
Alexandra Kadl, MD
After 3 years battling acute COVID-19 infections, we encounter now a large number of patients with PASC— also known as “long COVID,” “COVID long-hauler syndrome,” and “post-COVID conditions”—a persistent multisystem syndrome that impacts everyday function.1 As of October 2023, there are more than 100 million COVID-19 survivors reported in the United States; 10% to 85% of COVID survivors2-4 may show lingering, life-altering symptoms after recovery. Common reported symptoms include fatigue, depression/ anxiety, insomnia, and brain fog/difficulty concentrating, which are particularly high in women who often had experienced only mild acute COVID-19 disease and were not even hospitalized. More recently, chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) has been recognized as major component of PASC5 with a 3:1 female predominance.6 Up to 75% of patients with this diagnosis are not able to maintain their jobs and normal life, and up to 25% are so disabled that they are bedbound.6
Diagnosis
Although illnesses resembling CFS have been reported for more than 200 years,7 the diagnosis of CFS/ME remains difficult to make. There is a likely underreporting due to fear of being labeled as malingering when reaching out to health care providers, and there is a reporting bias toward higher socioeconomic groups due to better access to health care. The current criteria for the diagnosis of CFS/ME include the following 3 components8:
- substantial impairment in the ability to function for more than 6 months, accompanied by profound fatigue, not alleviated by rest
- post-exertional malaise (PEM; prolonged, disabling exacerbation of the patient’s baseline symptoms after exercise)
- non-refreshing sleep, PLUS either cognitive impairment or orthostatic intolerance.
Pathophysiology
Originally found to evolve in a small patient population with Epstein-Barr virus infection and Lyme disease, CFS/ME has moved to centerstage after the COVID-19 pandemic. While the diagnosis of COVID-19–related CFS/ME has advanced in the field, a clear mechanistic explanation of why it occurs is still missing. Certain risk factors have been identified for the development of CFS/ME, including female sex, reactivation of herpesviruses, and presence of connective tissue disorders; however, about one-third of patients with CFS/ME do not have identifiable risk factors.9,10 Persistence of viral particles11 and prolonged inflammatory states are speculated to affect the nervous system and mitochondrial function and metabolism. Interestingly, there is no correlation between severity of initial COVID-19 illness and the development of CFS/ME, similar to observations in non–COVID-19–related CFS/ME.
Proposed therapy
There is currently no proven therapy for CFS/ME. At this time, several immunomodulatory, antiviral, and neuromodulator drugs are being tested in clinical trial networks around the world.12 Usual physical therapy with near maximum intensity has been shown to exacerbate symptoms and often results in PEM, which is described as a “crash” or “full collapse” by patients. The time for recovery after such episodes can be several days.13
Instead, the focus should be on addressing “treatable” concomitant symptoms, such as sleep disorders, anxiety and depression, and chronic pain. Lifestyle changes, avoidance of triggers, and exercise without over exertion are currently recommended to avoid incapacitating PEM.
Gaps in knowledge
There is a large knowledge gap regarding the pathophysiology, prevention, and therapy for CFS/ME. Many health care practitioners are not familiar with the disease and have focused on measurable parameters of exercise limitations and fatigue, such as anemias and lung and cardiac impairments, thus treating CFS/ME as a form of deconditioning. Given the large number of patients who recovered from acute COVID-19 that are now disabled due to CFS/ME, a patient-centered research opportunity has arisen. Biomedical/mechanistic research is ongoing, and well-designed clinical trials evaluating pharmacologic intervention as well as tailored exercise programs are needed.
Conclusion
General practitioners and women’s health specialists need to be aware of CFS/ME, especially when managing patients with long COVID. They also need to know that typical physical therapy may worsen symptoms. Furthermore, clinicians should shy away from trial drugs with a theoretical benefit outside of a clinical trial. ●
- Chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) has been recognized as a major component of PASC
- Typical physical therapy has been shown to exacerbate symptoms of CFS/ME
- Treatment should focus on addressing “treatable” concomitant symptoms, lifestyle changes, avoidance of triggers, and exercise without over exertion
References
- Soriano JB, Murthy S, Marshall JC, et al. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22:e102-e107. doi: 10.1016 /S1473-3099(21)00703-9
- Chen C, Haupert SR, Zimmermann L, et al. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: a meta-analysis and systematic review. J Infect Dis. 2022;226:1593-1607. doi: 10.1093/infdis/jiac136
- Davis HE, McCorkell L, Vogel JM, et al. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133-146. doi: 10.1038/s41579-022 -00846-2
- Pavli A, Theodoridou M, Maltezou HC. Post-COVID syndrome: incidence, clinical spectrum, and challenges for primary healthcare professionals. Arch Med Res. 2021;52:575-581. doi: 10.1016/j.arcmed.2021.03.010
- Kedor C, Freitag H, Meyer-Arndt L, et al. A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat Commun. 2022;13:5104. doi: 10.1038/s41467-022-32507-6
- Bateman L, Bested AC, Bonilla HF, et al. Myalgic encephalomyelitis/chronic fatigue syndrome: essentials of diagnosis and management. Mayo Clin Proc. 2021;96:28612878. doi: 10.1016/j.mayocp.2021.07.004
- Wessely S. History of postviral fatigue syndrome. Br Med Bull. 1991;47:919-941. doi: 10.1093/oxfordjournals.bmb.a072521
- Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; Board on the Health of Select Populations; Institute of Medicine. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness. National Academies Press; 2015. doi: 10.17226/19012
- Ceban F, Ling S, Lui LMW, et al. Fatigue and cognitive impairment in post-COVID-19 syndrome: a systematic review and meta-analysis. Brain Behav Immun. 2022;101:93135. doi: 10.1016/j.bbi.2021.12.020
- Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019
- Hanson MR. The viral origin of myalgic encephalomyelitis/ chronic fatigue syndrome. PLoS Pathog. 2023;19:e1011523. doi: 10.1371/journal.ppat.1011523
- Scheibenbogen C, Bellmann-Strobl JT, Heindrich C, et al. Fighting post-COVID and ME/CFS—development of curative therapies. Front Med (Lausanne). 2023;10:1194754. doi: 10.3389/fmed.2023.1194754
- Stussman B, Williams A, Snow J, et al. Characterization of post-exertional malaise in patients with myalgic encephalomyelitis/chronic fatigue syndrome. Front Neurol. 2020;11:1025. doi: 10.3389/fneur.2020.01025