User login
Would you be able to recognize the signs and symptoms of this particular drug overdose?
CASE 1
Two days after reviving her boyfriend with naloxone, a woman and her 30-year-old boyfriend presented to our family medicine clinic. They explained that he had injected heroin and shortly thereafter he stopped breathing and his lips turned blue. The patient’s girlfriend did not call emergency medical services (EMS) at the time because she was afraid of getting arrested due to past incarceration for possession of illegal drugs. Instead, she revived him with naloxone that she found in his bag.
Both the patient and his girlfriend were scared and surprised by his “terrible reaction,” as he had previously purchased heroin from the same dealer and used the same dose without similar effects. However, the patient did note that the drug he purchased this time had a bright white tinge, when normally the drug was light yellow.
On physical examination, the patient’s heart rate and blood pressure were normal. There were needle track marks on both forearms, elbows, and upper arms. A laboratory workup obtained during this visit revealed anemia and a normal basic metabolic panel. A hepatitis C virus antibody test was positive, and a hepatic function panel revealed elevated transaminase levels. Urine toxicology was positive for opioids and negative for other substances.
CASE 2
A 58-year-old man with a history of chronic hepatitis C, polysubstance abuse, and schizophrenia was transported to the emergency department by EMS after his family found him unresponsive in his bedroom. The patient had agonal breathing when EMS arrived, so they administered naloxone (4 mg intranasal and 4 mg intravenous). His breathing improved, but his mental status did not. He was still obtunded upon arrival in the emergency department and vomited 4 tan-colored patches. The patient was tachycardic (heart rate, 108 beats/min), hypertensive (blood pressure, 189/95 mm Hg), and had rapid shallow breathing (respiratory rate, 38 breaths/min). He was intubated for airway protection, at which time 2 more tan-colored patches were removed from his pharynx.
Laboratory evaluation revealed an acute kidney injury with a high anion metabolic acidosis. A hepatic function panel showed elevated transaminase levels. Plasma acetaminophen and salicylate levels were normal. A computed tomography head scan was normal. Urine toxicology was negative for opioids but was positive for cocaine and benzodiazepines.
THE DIAGNOSIS
Opioid overdose caused the acute respiratory depression in both cases. In Case 1, the patient unknowingly overdosed on heroin laced with fentanyl, known as China White, which likely caused the drug’s bright white tinge. In Case 2, the patient’s overdose was the result of oral ingestion of fentanyl patches. (Limited urine toxicology was negative for opiates because fentanyl is a fully synthetic opioid that shows up only with a specific or extended assay. More on this in a bit.)
DISCUSSION
The fatal drug overdose epidemic in the United States is growing. From 2000 to 2014, the mortality rate from drug overdose increased by 137%, including a 200% increase in the rate of overdose deaths related to opioids (ie, pain medications, heroin).1 Between 2013 and 2014, the age-adjusted mortality rate related to methadone, a synthetic opioid, remained unchanged; however, age-adjusted mortality rates related to natural and semisynthetic opioid pain medications, heroin, and synthetic opioids other than methadone (eg, fentanyl) increased by 9%, 26%, and 80%, respectively. In 2014, a sharp increase in overdose deaths related to synthetic opioids other than methadone coincided with law enforcement reports of increased availability of illegal fentanyl; however, the toxicology panel used by coroners and medical examiners at that time could not distinguish between illegal and prescription fentanyl.1
Continue to: Among 70,237 drug overdose deaths...
Among 70,237 drug overdose deaths in the United States in 2017, 47,600 (67.8%) involved an opioid. From 2013 to 2017, drug overdose death rates increased in 35 of 50 states and the District of Columbia, and significant increases in death rates involving synthetic opioids occurred in 15 out of 20 states, likely driven by illicitly manufactured fentanyl.2
Fentanyl-laced heroin: More common, but not new
In October 1991, 3-methylfentanyl was identified in 16 fatal drug overdoses in Allegheny County, Pennsylvania, contributing to a 4-fold increase in overdose deaths compared to the previous year. Fentanyl mixed with heroin and other drugs is commonly found in the Midwest, Northeast, and Southern regions of the United States; in 2014, more than 80% of fentanyl confiscations occurred in 10 states within these regions, with the highest incidence occurring in Ohio.3
When combined with fentanyl, heroin becomes 50 to 100 times more potent, resulting in a subjective high with exaggerated central nervous system depression manifesting as lethargy, miosis, and respiratory depression.4 Most drug users are unaware and unable to identify when heroin is laced with fentanyl, which may contribute to the rise in deaths from unintentional drug overdose.1,5,6
Oral abuse of fentanyl patches can be fatal
Outcomes from oral abuse of fentanyl patches have ranged from transient overdose symptoms, such as lethargy and respiratory depression, to death.7-9 When administered in a medical setting, transbuccal fentanyl has a bioavailability of 50% to 65% across the buccal membrane. Nearly 20% of the drug escapes hepatic first pass metabolism when fentanyl patches are ingested orally and enters the systemic circulation, resulting in severe overdose and potentially death. Prolonged chewing and sucking on fentanyl patches increases the contact time with the buccal membrane, resulting in increased systemic absorption compared to oral ingestion without chewing/sucking.7-9
Urine toxicology screening detects compounds based on a chemical assay for drugs—generally codeine, morphine, and their metabolites. Because fentanyl is a fully synthetic opioid, its structure is not
Continue to: Survival of fentanyl overdose depends on naloxone availability
Survival of fentanyl overdose depends on naloxone availability
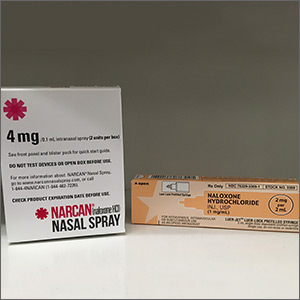
Naloxone is a safe and effective antidote to an opioid overdose. It comes in 3 preparations, including intramuscular and subcutaneous injections and an intranasal spray.12 Concerns that naloxone will harm patients with opioid dependence are unfounded. Naloxone can induce symptoms of opioid withdrawal, such as yawning, lacrimation, piloerection, diaphoresis, myalgia, vomiting, and diarrhea. While these withdrawal symptoms are unpleasant, they are not life threatening.12 Due to its high potency, large doses of naloxone (ie, 4–16 mg) are required to reverse the effects of a fentanyl overdose.13 Intranasal naloxone hydrochloride 4 mg delivered in a single spray is preferred due to the ease of administration. Repeat doses may be necessary if respiratory depression continues or recurs prior to the arrival of emergency medical services. Increasing the availability of naloxone to first responders has the potential to save many lives.6
THE TAKEAWAY
Fentanyl is a major contributor to the growing drug overdose crisis in the United States. When laced with heroin or consumed orally in the form of transdermal patches, fentanyl becomes more potent and is increasingly fatal. It’s crucial that primary care physicians be able to identify and educate at-risk patients about the fatal consequences of fentanyl overdose and coordinate care to help get them into an appropriate rehabilitation program.
In order to quickly recognize the signs of fentanyl-related overdose, it’s important to be alert for this possibility. At the bedside, the most easily recognized abnormality associated with fentanyl or other opioid overdose is a decline in respiratory rate culminating in apnea.10 A respiratory rate of 12 breaths/min or less in a patient who is not in physiologic sleep strongly suggests acute opioid intoxication, particularly when accompanied by miosis or stupor. Other signs include bradycardia, hypotension, and seizures from anoxia.10
Apart from the severity of symptoms, it is hard to clinically distinguish fentanyl overdose from other opiate overdose incidents. Given the degree to which illegal opiates are contaminated with fentanyl in the United States,3 it is appropriate to screen for fentanyl with extended panel urine toxicology testing in patients with suspected opioid overdose.
CORRESPONDENCE
Jaividhya Dasarathy, MD, 2500 MetroHealth Medical Center, Cleveland, OH 44109; Jdasarathy@metrohealth.org
1. Rudd RA, Aleshire N, Zibbell JE, et al. Increases in drug and opioid overdose deaths—United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2016;64:1378-1382.
2. Scholl L, Seth P, Kariisa M, et al. Drug and opioid-involved overdose deaths—United States, 2013–2017. MMWR Morb Mortal Wkly Rep. 2019;67:1419-1427.
3. Hibbs J, Perper J, Winek CL. An outbreak of designer drug-related deaths in Pennsylvania. JAMA. 1991;265:1011-1013.
4. Increases in fentanyl drug confiscations and fentanyl-related overdose fatalities. Centers for Disease Control and Prevention Web site. https://emergency.cdc.gov/han/han00384.asp. Published October 26, 2015. Accessed May 3, 2019.
5. Fentanyl. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/drugoverdose/opioids/fentanyl.html. Updated December 19, 2018. Accessed May 3, 2019.
6. Peterson AB, Gladden RM, Delcher C, et al. Increases in fentanyl-related overdose deaths—Florida and Ohio, 2013–2015. MMWR Morb Mortal Wkly Rep. 2016;65:844-849.
7. Streisand JB, Varvel JR, Stanski DR, et al. Absorption and bioavailability of oral transmucosal fentanyl citrate. Anesthesiology. 1991;75:223-229.
8. Kharasch ED, Whittington D, Hoffer C. Influence of hepatic and intestinal cytochrome P4503A activity on the acute disposition and effects of oral transmucosal fentanyl citrate. Anesthesiology. 2004;101:729-737.
9. Woodall KL, Martin TL, McLellan BA. Oral abuse of fentanyl patches (Duragesic): seven case reports. J Forensic Sci. 2008;53:222-225.
10. Moeller KE, Lee KC, Kissack JC. Urine drug screening: practical guide for clinicians. Mayo Clin Proc. 2008;83:66-76.
11. Appropriate Use of Drug Testing in Clinical Addiction Medicine. American Society of Addiction Medicine Web site. https://www.asam.org/docs/default-source/quality-science/appropriate_use_of_drug_testing_in_clinical-1-(7).pdf?sfvrsn=2. Published April 5, 2017. Accessed May 30, 2019.
12. Boyer EW. Management of opioid analgesic overdose. N Engl J Med. 2012;367:146-155.
13. Drugs@FDA: FDA approved drug products. US Food and Drug Administration Web site. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=208411. Accessed May 22, 2019.
CASE 1
Two days after reviving her boyfriend with naloxone, a woman and her 30-year-old boyfriend presented to our family medicine clinic. They explained that he had injected heroin and shortly thereafter he stopped breathing and his lips turned blue. The patient’s girlfriend did not call emergency medical services (EMS) at the time because she was afraid of getting arrested due to past incarceration for possession of illegal drugs. Instead, she revived him with naloxone that she found in his bag.
Both the patient and his girlfriend were scared and surprised by his “terrible reaction,” as he had previously purchased heroin from the same dealer and used the same dose without similar effects. However, the patient did note that the drug he purchased this time had a bright white tinge, when normally the drug was light yellow.
On physical examination, the patient’s heart rate and blood pressure were normal. There were needle track marks on both forearms, elbows, and upper arms. A laboratory workup obtained during this visit revealed anemia and a normal basic metabolic panel. A hepatitis C virus antibody test was positive, and a hepatic function panel revealed elevated transaminase levels. Urine toxicology was positive for opioids and negative for other substances.
CASE 2
A 58-year-old man with a history of chronic hepatitis C, polysubstance abuse, and schizophrenia was transported to the emergency department by EMS after his family found him unresponsive in his bedroom. The patient had agonal breathing when EMS arrived, so they administered naloxone (4 mg intranasal and 4 mg intravenous). His breathing improved, but his mental status did not. He was still obtunded upon arrival in the emergency department and vomited 4 tan-colored patches. The patient was tachycardic (heart rate, 108 beats/min), hypertensive (blood pressure, 189/95 mm Hg), and had rapid shallow breathing (respiratory rate, 38 breaths/min). He was intubated for airway protection, at which time 2 more tan-colored patches were removed from his pharynx.
Laboratory evaluation revealed an acute kidney injury with a high anion metabolic acidosis. A hepatic function panel showed elevated transaminase levels. Plasma acetaminophen and salicylate levels were normal. A computed tomography head scan was normal. Urine toxicology was negative for opioids but was positive for cocaine and benzodiazepines.
THE DIAGNOSIS
Opioid overdose caused the acute respiratory depression in both cases. In Case 1, the patient unknowingly overdosed on heroin laced with fentanyl, known as China White, which likely caused the drug’s bright white tinge. In Case 2, the patient’s overdose was the result of oral ingestion of fentanyl patches. (Limited urine toxicology was negative for opiates because fentanyl is a fully synthetic opioid that shows up only with a specific or extended assay. More on this in a bit.)
DISCUSSION
The fatal drug overdose epidemic in the United States is growing. From 2000 to 2014, the mortality rate from drug overdose increased by 137%, including a 200% increase in the rate of overdose deaths related to opioids (ie, pain medications, heroin).1 Between 2013 and 2014, the age-adjusted mortality rate related to methadone, a synthetic opioid, remained unchanged; however, age-adjusted mortality rates related to natural and semisynthetic opioid pain medications, heroin, and synthetic opioids other than methadone (eg, fentanyl) increased by 9%, 26%, and 80%, respectively. In 2014, a sharp increase in overdose deaths related to synthetic opioids other than methadone coincided with law enforcement reports of increased availability of illegal fentanyl; however, the toxicology panel used by coroners and medical examiners at that time could not distinguish between illegal and prescription fentanyl.1
Continue to: Among 70,237 drug overdose deaths...
Among 70,237 drug overdose deaths in the United States in 2017, 47,600 (67.8%) involved an opioid. From 2013 to 2017, drug overdose death rates increased in 35 of 50 states and the District of Columbia, and significant increases in death rates involving synthetic opioids occurred in 15 out of 20 states, likely driven by illicitly manufactured fentanyl.2
Fentanyl-laced heroin: More common, but not new
In October 1991, 3-methylfentanyl was identified in 16 fatal drug overdoses in Allegheny County, Pennsylvania, contributing to a 4-fold increase in overdose deaths compared to the previous year. Fentanyl mixed with heroin and other drugs is commonly found in the Midwest, Northeast, and Southern regions of the United States; in 2014, more than 80% of fentanyl confiscations occurred in 10 states within these regions, with the highest incidence occurring in Ohio.3
When combined with fentanyl, heroin becomes 50 to 100 times more potent, resulting in a subjective high with exaggerated central nervous system depression manifesting as lethargy, miosis, and respiratory depression.4 Most drug users are unaware and unable to identify when heroin is laced with fentanyl, which may contribute to the rise in deaths from unintentional drug overdose.1,5,6
Oral abuse of fentanyl patches can be fatal
Outcomes from oral abuse of fentanyl patches have ranged from transient overdose symptoms, such as lethargy and respiratory depression, to death.7-9 When administered in a medical setting, transbuccal fentanyl has a bioavailability of 50% to 65% across the buccal membrane. Nearly 20% of the drug escapes hepatic first pass metabolism when fentanyl patches are ingested orally and enters the systemic circulation, resulting in severe overdose and potentially death. Prolonged chewing and sucking on fentanyl patches increases the contact time with the buccal membrane, resulting in increased systemic absorption compared to oral ingestion without chewing/sucking.7-9
Urine toxicology screening detects compounds based on a chemical assay for drugs—generally codeine, morphine, and their metabolites. Because fentanyl is a fully synthetic opioid, its structure is not
Continue to: Survival of fentanyl overdose depends on naloxone availability
Survival of fentanyl overdose depends on naloxone availability
Naloxone is a safe and effective antidote to an opioid overdose. It comes in 3 preparations, including intramuscular and subcutaneous injections and an intranasal spray.12 Concerns that naloxone will harm patients with opioid dependence are unfounded. Naloxone can induce symptoms of opioid withdrawal, such as yawning, lacrimation, piloerection, diaphoresis, myalgia, vomiting, and diarrhea. While these withdrawal symptoms are unpleasant, they are not life threatening.12 Due to its high potency, large doses of naloxone (ie, 4–16 mg) are required to reverse the effects of a fentanyl overdose.13 Intranasal naloxone hydrochloride 4 mg delivered in a single spray is preferred due to the ease of administration. Repeat doses may be necessary if respiratory depression continues or recurs prior to the arrival of emergency medical services. Increasing the availability of naloxone to first responders has the potential to save many lives.6
THE TAKEAWAY
Fentanyl is a major contributor to the growing drug overdose crisis in the United States. When laced with heroin or consumed orally in the form of transdermal patches, fentanyl becomes more potent and is increasingly fatal. It’s crucial that primary care physicians be able to identify and educate at-risk patients about the fatal consequences of fentanyl overdose and coordinate care to help get them into an appropriate rehabilitation program.
In order to quickly recognize the signs of fentanyl-related overdose, it’s important to be alert for this possibility. At the bedside, the most easily recognized abnormality associated with fentanyl or other opioid overdose is a decline in respiratory rate culminating in apnea.10 A respiratory rate of 12 breaths/min or less in a patient who is not in physiologic sleep strongly suggests acute opioid intoxication, particularly when accompanied by miosis or stupor. Other signs include bradycardia, hypotension, and seizures from anoxia.10
Apart from the severity of symptoms, it is hard to clinically distinguish fentanyl overdose from other opiate overdose incidents. Given the degree to which illegal opiates are contaminated with fentanyl in the United States,3 it is appropriate to screen for fentanyl with extended panel urine toxicology testing in patients with suspected opioid overdose.
CORRESPONDENCE
Jaividhya Dasarathy, MD, 2500 MetroHealth Medical Center, Cleveland, OH 44109; Jdasarathy@metrohealth.org
CASE 1
Two days after reviving her boyfriend with naloxone, a woman and her 30-year-old boyfriend presented to our family medicine clinic. They explained that he had injected heroin and shortly thereafter he stopped breathing and his lips turned blue. The patient’s girlfriend did not call emergency medical services (EMS) at the time because she was afraid of getting arrested due to past incarceration for possession of illegal drugs. Instead, she revived him with naloxone that she found in his bag.
Both the patient and his girlfriend were scared and surprised by his “terrible reaction,” as he had previously purchased heroin from the same dealer and used the same dose without similar effects. However, the patient did note that the drug he purchased this time had a bright white tinge, when normally the drug was light yellow.
On physical examination, the patient’s heart rate and blood pressure were normal. There were needle track marks on both forearms, elbows, and upper arms. A laboratory workup obtained during this visit revealed anemia and a normal basic metabolic panel. A hepatitis C virus antibody test was positive, and a hepatic function panel revealed elevated transaminase levels. Urine toxicology was positive for opioids and negative for other substances.
CASE 2
A 58-year-old man with a history of chronic hepatitis C, polysubstance abuse, and schizophrenia was transported to the emergency department by EMS after his family found him unresponsive in his bedroom. The patient had agonal breathing when EMS arrived, so they administered naloxone (4 mg intranasal and 4 mg intravenous). His breathing improved, but his mental status did not. He was still obtunded upon arrival in the emergency department and vomited 4 tan-colored patches. The patient was tachycardic (heart rate, 108 beats/min), hypertensive (blood pressure, 189/95 mm Hg), and had rapid shallow breathing (respiratory rate, 38 breaths/min). He was intubated for airway protection, at which time 2 more tan-colored patches were removed from his pharynx.
Laboratory evaluation revealed an acute kidney injury with a high anion metabolic acidosis. A hepatic function panel showed elevated transaminase levels. Plasma acetaminophen and salicylate levels were normal. A computed tomography head scan was normal. Urine toxicology was negative for opioids but was positive for cocaine and benzodiazepines.
THE DIAGNOSIS
Opioid overdose caused the acute respiratory depression in both cases. In Case 1, the patient unknowingly overdosed on heroin laced with fentanyl, known as China White, which likely caused the drug’s bright white tinge. In Case 2, the patient’s overdose was the result of oral ingestion of fentanyl patches. (Limited urine toxicology was negative for opiates because fentanyl is a fully synthetic opioid that shows up only with a specific or extended assay. More on this in a bit.)
DISCUSSION
The fatal drug overdose epidemic in the United States is growing. From 2000 to 2014, the mortality rate from drug overdose increased by 137%, including a 200% increase in the rate of overdose deaths related to opioids (ie, pain medications, heroin).1 Between 2013 and 2014, the age-adjusted mortality rate related to methadone, a synthetic opioid, remained unchanged; however, age-adjusted mortality rates related to natural and semisynthetic opioid pain medications, heroin, and synthetic opioids other than methadone (eg, fentanyl) increased by 9%, 26%, and 80%, respectively. In 2014, a sharp increase in overdose deaths related to synthetic opioids other than methadone coincided with law enforcement reports of increased availability of illegal fentanyl; however, the toxicology panel used by coroners and medical examiners at that time could not distinguish between illegal and prescription fentanyl.1
Continue to: Among 70,237 drug overdose deaths...
Among 70,237 drug overdose deaths in the United States in 2017, 47,600 (67.8%) involved an opioid. From 2013 to 2017, drug overdose death rates increased in 35 of 50 states and the District of Columbia, and significant increases in death rates involving synthetic opioids occurred in 15 out of 20 states, likely driven by illicitly manufactured fentanyl.2
Fentanyl-laced heroin: More common, but not new
In October 1991, 3-methylfentanyl was identified in 16 fatal drug overdoses in Allegheny County, Pennsylvania, contributing to a 4-fold increase in overdose deaths compared to the previous year. Fentanyl mixed with heroin and other drugs is commonly found in the Midwest, Northeast, and Southern regions of the United States; in 2014, more than 80% of fentanyl confiscations occurred in 10 states within these regions, with the highest incidence occurring in Ohio.3
When combined with fentanyl, heroin becomes 50 to 100 times more potent, resulting in a subjective high with exaggerated central nervous system depression manifesting as lethargy, miosis, and respiratory depression.4 Most drug users are unaware and unable to identify when heroin is laced with fentanyl, which may contribute to the rise in deaths from unintentional drug overdose.1,5,6
Oral abuse of fentanyl patches can be fatal
Outcomes from oral abuse of fentanyl patches have ranged from transient overdose symptoms, such as lethargy and respiratory depression, to death.7-9 When administered in a medical setting, transbuccal fentanyl has a bioavailability of 50% to 65% across the buccal membrane. Nearly 20% of the drug escapes hepatic first pass metabolism when fentanyl patches are ingested orally and enters the systemic circulation, resulting in severe overdose and potentially death. Prolonged chewing and sucking on fentanyl patches increases the contact time with the buccal membrane, resulting in increased systemic absorption compared to oral ingestion without chewing/sucking.7-9
Urine toxicology screening detects compounds based on a chemical assay for drugs—generally codeine, morphine, and their metabolites. Because fentanyl is a fully synthetic opioid, its structure is not
Continue to: Survival of fentanyl overdose depends on naloxone availability
Survival of fentanyl overdose depends on naloxone availability
Naloxone is a safe and effective antidote to an opioid overdose. It comes in 3 preparations, including intramuscular and subcutaneous injections and an intranasal spray.12 Concerns that naloxone will harm patients with opioid dependence are unfounded. Naloxone can induce symptoms of opioid withdrawal, such as yawning, lacrimation, piloerection, diaphoresis, myalgia, vomiting, and diarrhea. While these withdrawal symptoms are unpleasant, they are not life threatening.12 Due to its high potency, large doses of naloxone (ie, 4–16 mg) are required to reverse the effects of a fentanyl overdose.13 Intranasal naloxone hydrochloride 4 mg delivered in a single spray is preferred due to the ease of administration. Repeat doses may be necessary if respiratory depression continues or recurs prior to the arrival of emergency medical services. Increasing the availability of naloxone to first responders has the potential to save many lives.6
THE TAKEAWAY
Fentanyl is a major contributor to the growing drug overdose crisis in the United States. When laced with heroin or consumed orally in the form of transdermal patches, fentanyl becomes more potent and is increasingly fatal. It’s crucial that primary care physicians be able to identify and educate at-risk patients about the fatal consequences of fentanyl overdose and coordinate care to help get them into an appropriate rehabilitation program.
In order to quickly recognize the signs of fentanyl-related overdose, it’s important to be alert for this possibility. At the bedside, the most easily recognized abnormality associated with fentanyl or other opioid overdose is a decline in respiratory rate culminating in apnea.10 A respiratory rate of 12 breaths/min or less in a patient who is not in physiologic sleep strongly suggests acute opioid intoxication, particularly when accompanied by miosis or stupor. Other signs include bradycardia, hypotension, and seizures from anoxia.10
Apart from the severity of symptoms, it is hard to clinically distinguish fentanyl overdose from other opiate overdose incidents. Given the degree to which illegal opiates are contaminated with fentanyl in the United States,3 it is appropriate to screen for fentanyl with extended panel urine toxicology testing in patients with suspected opioid overdose.
CORRESPONDENCE
Jaividhya Dasarathy, MD, 2500 MetroHealth Medical Center, Cleveland, OH 44109; Jdasarathy@metrohealth.org
1. Rudd RA, Aleshire N, Zibbell JE, et al. Increases in drug and opioid overdose deaths—United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2016;64:1378-1382.
2. Scholl L, Seth P, Kariisa M, et al. Drug and opioid-involved overdose deaths—United States, 2013–2017. MMWR Morb Mortal Wkly Rep. 2019;67:1419-1427.
3. Hibbs J, Perper J, Winek CL. An outbreak of designer drug-related deaths in Pennsylvania. JAMA. 1991;265:1011-1013.
4. Increases in fentanyl drug confiscations and fentanyl-related overdose fatalities. Centers for Disease Control and Prevention Web site. https://emergency.cdc.gov/han/han00384.asp. Published October 26, 2015. Accessed May 3, 2019.
5. Fentanyl. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/drugoverdose/opioids/fentanyl.html. Updated December 19, 2018. Accessed May 3, 2019.
6. Peterson AB, Gladden RM, Delcher C, et al. Increases in fentanyl-related overdose deaths—Florida and Ohio, 2013–2015. MMWR Morb Mortal Wkly Rep. 2016;65:844-849.
7. Streisand JB, Varvel JR, Stanski DR, et al. Absorption and bioavailability of oral transmucosal fentanyl citrate. Anesthesiology. 1991;75:223-229.
8. Kharasch ED, Whittington D, Hoffer C. Influence of hepatic and intestinal cytochrome P4503A activity on the acute disposition and effects of oral transmucosal fentanyl citrate. Anesthesiology. 2004;101:729-737.
9. Woodall KL, Martin TL, McLellan BA. Oral abuse of fentanyl patches (Duragesic): seven case reports. J Forensic Sci. 2008;53:222-225.
10. Moeller KE, Lee KC, Kissack JC. Urine drug screening: practical guide for clinicians. Mayo Clin Proc. 2008;83:66-76.
11. Appropriate Use of Drug Testing in Clinical Addiction Medicine. American Society of Addiction Medicine Web site. https://www.asam.org/docs/default-source/quality-science/appropriate_use_of_drug_testing_in_clinical-1-(7).pdf?sfvrsn=2. Published April 5, 2017. Accessed May 30, 2019.
12. Boyer EW. Management of opioid analgesic overdose. N Engl J Med. 2012;367:146-155.
13. Drugs@FDA: FDA approved drug products. US Food and Drug Administration Web site. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=208411. Accessed May 22, 2019.
1. Rudd RA, Aleshire N, Zibbell JE, et al. Increases in drug and opioid overdose deaths—United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2016;64:1378-1382.
2. Scholl L, Seth P, Kariisa M, et al. Drug and opioid-involved overdose deaths—United States, 2013–2017. MMWR Morb Mortal Wkly Rep. 2019;67:1419-1427.
3. Hibbs J, Perper J, Winek CL. An outbreak of designer drug-related deaths in Pennsylvania. JAMA. 1991;265:1011-1013.
4. Increases in fentanyl drug confiscations and fentanyl-related overdose fatalities. Centers for Disease Control and Prevention Web site. https://emergency.cdc.gov/han/han00384.asp. Published October 26, 2015. Accessed May 3, 2019.
5. Fentanyl. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/drugoverdose/opioids/fentanyl.html. Updated December 19, 2018. Accessed May 3, 2019.
6. Peterson AB, Gladden RM, Delcher C, et al. Increases in fentanyl-related overdose deaths—Florida and Ohio, 2013–2015. MMWR Morb Mortal Wkly Rep. 2016;65:844-849.
7. Streisand JB, Varvel JR, Stanski DR, et al. Absorption and bioavailability of oral transmucosal fentanyl citrate. Anesthesiology. 1991;75:223-229.
8. Kharasch ED, Whittington D, Hoffer C. Influence of hepatic and intestinal cytochrome P4503A activity on the acute disposition and effects of oral transmucosal fentanyl citrate. Anesthesiology. 2004;101:729-737.
9. Woodall KL, Martin TL, McLellan BA. Oral abuse of fentanyl patches (Duragesic): seven case reports. J Forensic Sci. 2008;53:222-225.
10. Moeller KE, Lee KC, Kissack JC. Urine drug screening: practical guide for clinicians. Mayo Clin Proc. 2008;83:66-76.
11. Appropriate Use of Drug Testing in Clinical Addiction Medicine. American Society of Addiction Medicine Web site. https://www.asam.org/docs/default-source/quality-science/appropriate_use_of_drug_testing_in_clinical-1-(7).pdf?sfvrsn=2. Published April 5, 2017. Accessed May 30, 2019.
12. Boyer EW. Management of opioid analgesic overdose. N Engl J Med. 2012;367:146-155.
13. Drugs@FDA: FDA approved drug products. US Food and Drug Administration Web site. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=208411. Accessed May 22, 2019.
Depigmented patches, mild scaling on newborn • Dx?
THE CASE
A 21-year-old G3P2 mother gave birth to an African American girl via vaginal delivery. Labor had been induced due to gestational hypertension at term. She’d also had a stillborn at term at the age of 16 followed by a second live term birth 3 years ago. During this most recent pregnancy, she’d received adequate prenatal care and had been treated for chlamydia with a single dose of oral azithromycin 1 g.
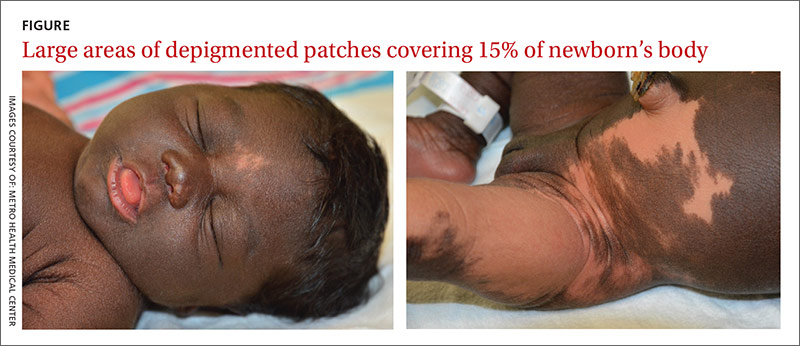
The newborn had an Apgar score of 9 out of 9 and weighed 6.7 pounds at birth. During a physical examination in the nursery, the infant was found to have large areas of smooth depigmentation on her forehead, right forearm, lower abdomen, and left thigh, with surrounding areas of thickened skin that had mild scaling and hyperpigmentation (FIGURE). The depigmented areas involved approximately 15% of the newborn’s body. The father and paternal grandmother, who were present at the time of delivery, also had depigmented areas of their skin.
The newborn’s tongue was pink and her mucus membranes were moist. No macules or patches were noted on either the oral or vaginal mucosa. Cardiac, pulmonary, and ocular examinations (including evaluation of the retina by ophthalmoscopy) were normal. There was no nystagmus or strabismus. The newborn’s extremities were normal, symmetric, and moveable, and she was easily consoled.
THE DIAGNOSIS
We diagnosed the newborn with piebaldism based on her appearance. Piebaldism consists of hypopigmented/depigmented areas and is a clinical diagnosis; no testing is required.
Concerned about the areas of hyperpigmentation, we decided to get a dermatologist’s opinion. The dermatology team briefly considered the diagnosis of a large melanocytic nevus with sparing of some areas, but a skin biopsy of a hyperpigmented area on the left leg came back with a normal number of melanocytes.
DISCUSSION
Piebaldism is a rare autosomal dominant disorder characterized by the congenital absence of melanocytes in affected areas of the skin and hair due to mutations of the c-kit gene. The c-kit gene affects the differentiation and migration of melanoblasts from the neural crest during embryonic life.1 The incidence of piebaldism is estimated to be less than one in 20,000.2 Both males and females are equally affected, and no race is spared.2,3
Affected individuals present with a white forelock and relatively stable, persistent depigmentation of skin with a characteristic distribution from birth.3 A white forelock arising from a triangular, elongated, or diamond-shaped midline or depigmented macule on the forehead may be the only manifestation in 80% to 90% of cases.3 The characteristic distribution of depigmented macules includes a central macule on the forehead, the anterior abdomen extending to the chest, the lateral trunk sparing the dorsal spine, and the mid-arms and legs sparing the hands and feet.2
Depigmented macules are rectangular, rhomboid, or irregular in shape and usually have a symmetrical distribution. Typically, islands of hyperpigmentation are present within and at the border of depigmented areas.4 Piebaldism is associated in rare instances with neurofibromatosis type 1, Hirschsprung’s disease, hearing loss, and Waardenburg syndrome.4
Histologically, melanocytes are absent or considerably reduced in the depigmented areas and are normal in number in the hyperpigmented areas.5
The differential diagnosis of piebaldism includes mosaicism, albinism, and vitiligo.
Cutaneous mosaicism stems from a gene mutation that occurs during embryogenesis and the lesions are distributed along certain patterns and forms. A chromosomal analysis of our patient showed a normal female karyotype that excluded mosaicism.
Albinism is a genetically inherited disorder characterized by partial or complete absence of melanin production in the skin, hair, and eyes.6 Eye and fundus examinations were normal in our patient, which excluded albinism.
Vitiligo is rarely present at birth but is usually acquired later in life. It results from an immune-mediated destruction of melanocytes and is not genetically inherited, although familial incidence has been reported.7
There are no effective therapies
A combination of dermabrasion and grafting of pigmented skin into depigmented areas, with or without phototherapy, has been used in select patients, although no solid data are available on its effectiveness.3 The lack of effective and safe therapies can make treatment challenging. Piebaldism is usually not medically harmful, but the emotional and psychological effects on the family and the patient as they grow up can be devastating. Therefore, supportive counseling is recommended.
Our patient. Supportive counseling and a follow-up appointment with a dermatologist was planned for our patient and her family.
THE TAKEAWAY
The clinical diagnosis of piebaldism is straightforward based on the presence of a white forelock in the frontal region, the appearance of depigmented macules since birth that stay relatively stable, and the presence of a similar pattern of depigmented macules in other family members. Histologic or genetic testing is not necessary to establish the diagnosis. Rarely, cases of piebaldism are associated with hearing loss, necessitating a hearing assessment and an audiology exam. Unfortunately, there are no effective treatments for piebaldism.
1. Ward KA, Moss C, Sanders DS. Human piebaldism: relationship between phenotype and site of kit gene mutation. Br J Dermatol. 1995;132:929-935.
2. Agarwal S, Ojha A. Piebaldism: A brief report and review of the literature. Indian Dermatol Online J. 2012;3:144-147.
3. Oiso N, Fukai K, Kawada A, et al. Piebaldism. J Dermatol. 2013;40:330-335.
4. Spritz RA, Itin PH, Gutmann DH. Piebaldism and neurofibromatosis type 1: horses of very different colors. J Invest Dermatol. 2004;122:xxxiv-xxxv.
5. Makino T, Yanagihara M, Oiso N, et al. Repigmentation of the epidermis around the acrosyringium in piebald skin: an ultrastructural examination. Br J Dermatol. 2013;168:910-912.
6. Karaman A. Oculocutaneous albinism type 1A: a case report. Dermatol Online J. 2008;14:13.
7. Plensdorf S, Martinez J. Common pigmentation disorders. Am Fam Physician. 2009;79:109-116.
THE CASE
A 21-year-old G3P2 mother gave birth to an African American girl via vaginal delivery. Labor had been induced due to gestational hypertension at term. She’d also had a stillborn at term at the age of 16 followed by a second live term birth 3 years ago. During this most recent pregnancy, she’d received adequate prenatal care and had been treated for chlamydia with a single dose of oral azithromycin 1 g.
The newborn had an Apgar score of 9 out of 9 and weighed 6.7 pounds at birth. During a physical examination in the nursery, the infant was found to have large areas of smooth depigmentation on her forehead, right forearm, lower abdomen, and left thigh, with surrounding areas of thickened skin that had mild scaling and hyperpigmentation (FIGURE). The depigmented areas involved approximately 15% of the newborn’s body. The father and paternal grandmother, who were present at the time of delivery, also had depigmented areas of their skin.
The newborn’s tongue was pink and her mucus membranes were moist. No macules or patches were noted on either the oral or vaginal mucosa. Cardiac, pulmonary, and ocular examinations (including evaluation of the retina by ophthalmoscopy) were normal. There was no nystagmus or strabismus. The newborn’s extremities were normal, symmetric, and moveable, and she was easily consoled.
THE DIAGNOSIS
We diagnosed the newborn with piebaldism based on her appearance. Piebaldism consists of hypopigmented/depigmented areas and is a clinical diagnosis; no testing is required.
Concerned about the areas of hyperpigmentation, we decided to get a dermatologist’s opinion. The dermatology team briefly considered the diagnosis of a large melanocytic nevus with sparing of some areas, but a skin biopsy of a hyperpigmented area on the left leg came back with a normal number of melanocytes.
DISCUSSION
Piebaldism is a rare autosomal dominant disorder characterized by the congenital absence of melanocytes in affected areas of the skin and hair due to mutations of the c-kit gene. The c-kit gene affects the differentiation and migration of melanoblasts from the neural crest during embryonic life.1 The incidence of piebaldism is estimated to be less than one in 20,000.2 Both males and females are equally affected, and no race is spared.2,3
Affected individuals present with a white forelock and relatively stable, persistent depigmentation of skin with a characteristic distribution from birth.3 A white forelock arising from a triangular, elongated, or diamond-shaped midline or depigmented macule on the forehead may be the only manifestation in 80% to 90% of cases.3 The characteristic distribution of depigmented macules includes a central macule on the forehead, the anterior abdomen extending to the chest, the lateral trunk sparing the dorsal spine, and the mid-arms and legs sparing the hands and feet.2
Depigmented macules are rectangular, rhomboid, or irregular in shape and usually have a symmetrical distribution. Typically, islands of hyperpigmentation are present within and at the border of depigmented areas.4 Piebaldism is associated in rare instances with neurofibromatosis type 1, Hirschsprung’s disease, hearing loss, and Waardenburg syndrome.4
Histologically, melanocytes are absent or considerably reduced in the depigmented areas and are normal in number in the hyperpigmented areas.5
The differential diagnosis of piebaldism includes mosaicism, albinism, and vitiligo.
Cutaneous mosaicism stems from a gene mutation that occurs during embryogenesis and the lesions are distributed along certain patterns and forms. A chromosomal analysis of our patient showed a normal female karyotype that excluded mosaicism.
Albinism is a genetically inherited disorder characterized by partial or complete absence of melanin production in the skin, hair, and eyes.6 Eye and fundus examinations were normal in our patient, which excluded albinism.
Vitiligo is rarely present at birth but is usually acquired later in life. It results from an immune-mediated destruction of melanocytes and is not genetically inherited, although familial incidence has been reported.7
There are no effective therapies
A combination of dermabrasion and grafting of pigmented skin into depigmented areas, with or without phototherapy, has been used in select patients, although no solid data are available on its effectiveness.3 The lack of effective and safe therapies can make treatment challenging. Piebaldism is usually not medically harmful, but the emotional and psychological effects on the family and the patient as they grow up can be devastating. Therefore, supportive counseling is recommended.
Our patient. Supportive counseling and a follow-up appointment with a dermatologist was planned for our patient and her family.
THE TAKEAWAY
The clinical diagnosis of piebaldism is straightforward based on the presence of a white forelock in the frontal region, the appearance of depigmented macules since birth that stay relatively stable, and the presence of a similar pattern of depigmented macules in other family members. Histologic or genetic testing is not necessary to establish the diagnosis. Rarely, cases of piebaldism are associated with hearing loss, necessitating a hearing assessment and an audiology exam. Unfortunately, there are no effective treatments for piebaldism.
THE CASE
A 21-year-old G3P2 mother gave birth to an African American girl via vaginal delivery. Labor had been induced due to gestational hypertension at term. She’d also had a stillborn at term at the age of 16 followed by a second live term birth 3 years ago. During this most recent pregnancy, she’d received adequate prenatal care and had been treated for chlamydia with a single dose of oral azithromycin 1 g.
The newborn had an Apgar score of 9 out of 9 and weighed 6.7 pounds at birth. During a physical examination in the nursery, the infant was found to have large areas of smooth depigmentation on her forehead, right forearm, lower abdomen, and left thigh, with surrounding areas of thickened skin that had mild scaling and hyperpigmentation (FIGURE). The depigmented areas involved approximately 15% of the newborn’s body. The father and paternal grandmother, who were present at the time of delivery, also had depigmented areas of their skin.
The newborn’s tongue was pink and her mucus membranes were moist. No macules or patches were noted on either the oral or vaginal mucosa. Cardiac, pulmonary, and ocular examinations (including evaluation of the retina by ophthalmoscopy) were normal. There was no nystagmus or strabismus. The newborn’s extremities were normal, symmetric, and moveable, and she was easily consoled.
THE DIAGNOSIS
We diagnosed the newborn with piebaldism based on her appearance. Piebaldism consists of hypopigmented/depigmented areas and is a clinical diagnosis; no testing is required.
Concerned about the areas of hyperpigmentation, we decided to get a dermatologist’s opinion. The dermatology team briefly considered the diagnosis of a large melanocytic nevus with sparing of some areas, but a skin biopsy of a hyperpigmented area on the left leg came back with a normal number of melanocytes.
DISCUSSION
Piebaldism is a rare autosomal dominant disorder characterized by the congenital absence of melanocytes in affected areas of the skin and hair due to mutations of the c-kit gene. The c-kit gene affects the differentiation and migration of melanoblasts from the neural crest during embryonic life.1 The incidence of piebaldism is estimated to be less than one in 20,000.2 Both males and females are equally affected, and no race is spared.2,3
Affected individuals present with a white forelock and relatively stable, persistent depigmentation of skin with a characteristic distribution from birth.3 A white forelock arising from a triangular, elongated, or diamond-shaped midline or depigmented macule on the forehead may be the only manifestation in 80% to 90% of cases.3 The characteristic distribution of depigmented macules includes a central macule on the forehead, the anterior abdomen extending to the chest, the lateral trunk sparing the dorsal spine, and the mid-arms and legs sparing the hands and feet.2
Depigmented macules are rectangular, rhomboid, or irregular in shape and usually have a symmetrical distribution. Typically, islands of hyperpigmentation are present within and at the border of depigmented areas.4 Piebaldism is associated in rare instances with neurofibromatosis type 1, Hirschsprung’s disease, hearing loss, and Waardenburg syndrome.4
Histologically, melanocytes are absent or considerably reduced in the depigmented areas and are normal in number in the hyperpigmented areas.5
The differential diagnosis of piebaldism includes mosaicism, albinism, and vitiligo.
Cutaneous mosaicism stems from a gene mutation that occurs during embryogenesis and the lesions are distributed along certain patterns and forms. A chromosomal analysis of our patient showed a normal female karyotype that excluded mosaicism.
Albinism is a genetically inherited disorder characterized by partial or complete absence of melanin production in the skin, hair, and eyes.6 Eye and fundus examinations were normal in our patient, which excluded albinism.
Vitiligo is rarely present at birth but is usually acquired later in life. It results from an immune-mediated destruction of melanocytes and is not genetically inherited, although familial incidence has been reported.7
There are no effective therapies
A combination of dermabrasion and grafting of pigmented skin into depigmented areas, with or without phototherapy, has been used in select patients, although no solid data are available on its effectiveness.3 The lack of effective and safe therapies can make treatment challenging. Piebaldism is usually not medically harmful, but the emotional and psychological effects on the family and the patient as they grow up can be devastating. Therefore, supportive counseling is recommended.
Our patient. Supportive counseling and a follow-up appointment with a dermatologist was planned for our patient and her family.
THE TAKEAWAY
The clinical diagnosis of piebaldism is straightforward based on the presence of a white forelock in the frontal region, the appearance of depigmented macules since birth that stay relatively stable, and the presence of a similar pattern of depigmented macules in other family members. Histologic or genetic testing is not necessary to establish the diagnosis. Rarely, cases of piebaldism are associated with hearing loss, necessitating a hearing assessment and an audiology exam. Unfortunately, there are no effective treatments for piebaldism.
1. Ward KA, Moss C, Sanders DS. Human piebaldism: relationship between phenotype and site of kit gene mutation. Br J Dermatol. 1995;132:929-935.
2. Agarwal S, Ojha A. Piebaldism: A brief report and review of the literature. Indian Dermatol Online J. 2012;3:144-147.
3. Oiso N, Fukai K, Kawada A, et al. Piebaldism. J Dermatol. 2013;40:330-335.
4. Spritz RA, Itin PH, Gutmann DH. Piebaldism and neurofibromatosis type 1: horses of very different colors. J Invest Dermatol. 2004;122:xxxiv-xxxv.
5. Makino T, Yanagihara M, Oiso N, et al. Repigmentation of the epidermis around the acrosyringium in piebald skin: an ultrastructural examination. Br J Dermatol. 2013;168:910-912.
6. Karaman A. Oculocutaneous albinism type 1A: a case report. Dermatol Online J. 2008;14:13.
7. Plensdorf S, Martinez J. Common pigmentation disorders. Am Fam Physician. 2009;79:109-116.
1. Ward KA, Moss C, Sanders DS. Human piebaldism: relationship between phenotype and site of kit gene mutation. Br J Dermatol. 1995;132:929-935.
2. Agarwal S, Ojha A. Piebaldism: A brief report and review of the literature. Indian Dermatol Online J. 2012;3:144-147.
3. Oiso N, Fukai K, Kawada A, et al. Piebaldism. J Dermatol. 2013;40:330-335.
4. Spritz RA, Itin PH, Gutmann DH. Piebaldism and neurofibromatosis type 1: horses of very different colors. J Invest Dermatol. 2004;122:xxxiv-xxxv.
5. Makino T, Yanagihara M, Oiso N, et al. Repigmentation of the epidermis around the acrosyringium in piebald skin: an ultrastructural examination. Br J Dermatol. 2013;168:910-912.
6. Karaman A. Oculocutaneous albinism type 1A: a case report. Dermatol Online J. 2008;14:13.
7. Plensdorf S, Martinez J. Common pigmentation disorders. Am Fam Physician. 2009;79:109-116.