User login
Electrical vagus nerve stimulation for the treatment of chronic heart failure
Autonomic imbalance characterized by sustained sympathetic overdrive and by parasympathetic withdrawal is a key maladaptation of the heart failure (HF) state. This autonomic dysregulation has long been recognized as a mediator of increased mortality and morbidity in myocardial infarction and HF.1,2 Sympathovagal imbalance in HF can lead to increased heart rate, excess release of proinflammatory cytokines, dysregulation of nitric oxide (NO) pathways, and arrythmogenesis. Diminished vagal activity reflected in increased heart rate is a predictor of high mortality in HF.3,4 Sustained increase of sympathetic activity contributes to progressive left ventricular (LV) dysfunction in HF and promotes progressive LV remodeling.5,6 Pharmacologic agents that reduce heart rate, such as beta-blockers and, more recently, specific and selective inhibitors of the cardiac pacemaker current If, have been shown to improve survival and prevent or attenuate progressive LV remodeling in animals with HF.4,5,7,8
During the past two to three decades, the emphasis on modulation of neurohumoral activition for treatment of chronic HF gave rise to angiotensin-converting enzyme inhibitors, beta-adrenergic receptor blockers, and aldosterone antagonists. In recent years, renewed interest has emerged in modulating parasympathetic or vagal activity as a therapeutic target for treating chronic HF. An alteration in cardiac vagal efferent activity through peripheral cardiac nerve stimulation can produce bradycardia and can modify atrial as well as ventricular contractile function.9,10
Electrical vagus nerve stimulation (VNS) was shown to prevent sudden cardiac death in dogs with myocardial infarction and to improve long-term survival in rats with chronic HF.11,12 VNS has also been shown to suppress arrhythmias in conscious rats with chronic HF secondary to myocardial infarction.13
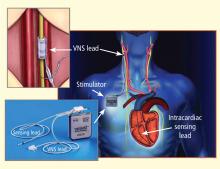
This article focuses primarily on the effects of chronic VNS on LV dysfunction and remodeling in dogs with HF produced by multiple sequential intracoronary microembolizations14 or by high-rate ventricular pacing15 and on the safety, feasibility, and efficacy trends of VNS in patients with advanced HF.16
VNS IN DOGS WITH MICROEMBOLIZATION-INDUCED HEART FAILURE
Monotherapy with VNS
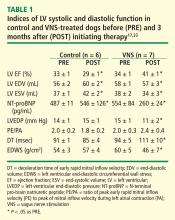
Long-term VNS therapy also elicited improvements in indices of LV diastolic function. VNS significantly decreased LV end-diastolic pressure (Table 1), increased deceleration time of rapid mitral inflow velocity, tended to increase the ratio of peak mitral inflow velocity during early LV filling to peak mitral inflow velocity during left atrial contraction (PE/PA), and significantly reduced LV end-diastolic circumferential wall stress, a determinant of myocardial oxygen consumption (Table 1). These measures suggest that VNS can reduce preload, improve LV relaxation and improve LV function without increasing myocardial oxygen consumption.
VNS in combination with beta-blockade
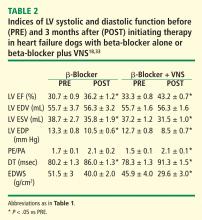
The effects of VNS in combination with beta-blockade were examined in dogs with HF. Dogs with LV ejection fraction of approximately 35% were randomized to 3 months of therapy with a beta-blocker alone (metoprolol succinate, 100 mg once daily, n = 6) or to metoprolol (100 mg once daily) combined with active VNS with CardioFit (n = 6). As with the monotherapy study, the CardioFit VNS system was operated in the feedback on-demand heart rate responsive mode. Dogs were started on oral metoprolol therapy 2 weeks prior to randomization to VNS therapy. After randomization, all dogs continued to receive metoprolol succinate once daily for the duration of the study.18
The improvements in LV systolic and diastolic function with combination therapy were associated with important changes in heart rate. Twenty-four–hour ambulatory ECG Holter monitoring studies showed no differences in minimum and average heart rate between dogs treated with metoprolol and those treated with combination therapy with VNS. Maximum heart rate, however, was significantly lower in dogs treated with the combination therapy (114 ± 12 vs 149 ± 8 beats/min, P < .05). These observations suggest that preventing heart rate escape at the high end may further improve LV systolic function compared with beta-blockade alone. This added benefit of the combination of VNS and beta-blockade was likely the result of reducing the adverse impact of increased cardiac workload and increased myocardial oxygen consumption elicited by the heart rate increase.
VNS and left ventricular remodeling
VNS and proinflammatory cytokines, nitric oxide, and gap junction proteins
Nitric oxide (NO) is formed by a family of NO synthases (NOS). The three isoforms of NOS identified to date are endothelin NOS (eNOS), inducible NOS (iNOS), and neuronal NOS (nNOS). The three isoforms have differing characteristics and roles:
eNOS. NO produced by eNOS plays an important role in the regulation of cell growth and apoptosis22 and can enhance myocardial relaxation and regulate contractility.22,23
iNOS. Overexpression of iNOS in cardiomyocytes in mice results in peroxynitrite generation associated with fibrosis, LV hypertrophy, chamber dilation, cardiomyopathic phenotype, heart block, and sudden cardiac death.24
nNOS. nNOS has been shown to be upregulated in the human failing heart and in rats following myocardial infarction.25 In rats with HF, inhibition of nNOS leads to increased sensitivity of the myocardium to beta-adrenergic stimulation,26 suggesting a role for nNOS in the autocrine regulation of myocardial contractility.26
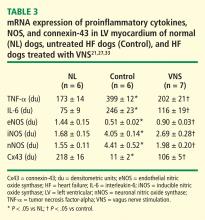
In dogs with coronary microembolization-induced HF, mRNA and protein expression of eNOS in LV myocardium is significantly downregulated compared with normal dogs; therapy with VNS significantly improves the expression of eNOS (Table 3).27 Both iNOS and nNOS are significantly upregulated, and their expression tends to be normalized by long-term VNS therapy.27
Gap junction proteins or connexins are reduced or redistributed from intercalated disks to lateral cell borders in a variety of cardiac diseases, including HF.28 This so-called “gap junction remodeling” is considered highly arrhythmogenic. In mammals, gap junctions exclusively contain connexin-43 (Cx43). Reduced expression of Cx43 occurs in the failing human heart and has been shown to result in slowed transmural conduction and dispersion of action potential duration with increased susceptibility to arrhythmia and sudden cardiac death.29,30 In dogs with coronary microembolization-induced HF, mRNA and protein expression of Cx43 in LV myocardium was shown to be markedly downregulated compared with normal dogs, and long-term therapy with VNS was associated with a significant increase in the expression of Cx43 in LV myocardium (Table 3).31
VNS IN DOGS WITH RAPID PACING–INDUCED HEART FAILURE
Electrical VNS as a potential therapy for HF was examined in dogs with HF secondary to high-rate ventricular pacing using the Cyberonics VNS system (Cyberonics Inc., Houston, TX), which does not operate on a negative feedback mechanism.15 In this study, VNS therapy was delivered continuously for the duration of the study with a duty cycle of 14 seconds on and 12 seconds off. VNS signals were delivered to the right cervical vagus nerve at a frequency of 20 Hz and a pulse width of 0.5 msec.15 Dogs were randomized to control (n = 7) or to monotherapy with VNS (n = 8) and followed for 8 weeks. All measurements were made approximately 15 minutes after temporarily turning off the ventricular pacemaker and the vagus nerve stimulator.15 VNS therapy resulted in a significant decrease in LV end-diastolic and end-systolic volumes and a significant increase in LV ejection fraction compared with controls.15 This improvement was associated with significant reduction in plasma levels of norepinephrine, angiotensin II, and C-reactive protein. The study also demonstrated the effectiveness of VNS in restoring baroreflex sensitivity, thus improving cardiac autonomic control.15 Because rapid pacing was maintained throughout the study except for short periods when measurements were made, one can argue that the benefits of VNS therapy in this model of HF are independent of heart rate.15
SAFETY AND TOLERABILITY OF VNS IN PATIENTS WITH ADVANCED HEART FAILURE
In patients with HF, reduced vagal activity is associated with increased mortality.1 Vagal withdrawal has also been shown to precede episodes of acute decompensation.32 In a recently published study, De Ferrari et al, on behalf of the CardioFit Multicenter Trial Investigators, examined the safety and tolerability of chronic VNS in 32 patients with symptomatic HF and severe LV dysfunction using the CardioFit system.16 The CardioFit system used in this study differed from that used in dogs with microembolization-induced HF in that it did not operate on a negative feedback principle. A bradycardia limit causing interruption of VNS was set at 55 beats/min. A 3-week uptitration period was used to maximize current amplitude and duty cycle based on patient sensation. The intensity of the stimulation reached 4.1 ± 1.2 mA at the end of the titration period.16
This multicenter, open-label, phase 2 trial involved 3 to 6 months of followup with an optional 1 year followup. The results suggested that VNS may be safe and tolerable in HF patients with severe LV dysfunction. Trends for efficacy were also favorable, bearing in mind the nonrandomized and unblinded nature of the study design. The study showed significant improvements in New York Heart Association HF classification, 6-minute walk test, LV ejection fraction, and LV systolic volumes.16
CONCLUSIONS
A wealth of preclinical and clinical studies supports the concept that electrical VNS can favorably modify the underlying pathophysiology and course of evolving HF. In animals with HF, VNS improves LV function, attenuates LV remodeling and may prevent arrhythmias that provoke sudden cardiac death. VNS derives these potential clinical benefits from multiple mechanisms of action that include reduced heart rate and normalization of sympathetic overdrive. VNS also appears to have a favorable impact on other signaling pathways that are likely to elicit beneficial effects in patients with HF. These include restoration of baroreflex sensitivity, suppression of proinflammatory cytokines, normalization of NO signaling pathways, and suppression of gap junction remodeling. At present, there is no evidence to implicate a single mechanism of action for the benefits derived from VNS. Instead, it is likely that all of the mechanisms listed above act in concert to elicit the global benefit seen with VNS. In humans with HF, VNS may be safe, feasible, and apparently well tolerated. Full appreciation of its efficacy in treating chronic HF must await completion of pivotal randomized clinical trials.
- Schwartz PJ, Vanoli E, Stramba-Badiale M, De Ferrari GM, Billman GE, Foreman RD. Autonomic mechanisms and sudden death. New insights from analysis of baroreceptor reflexes in conscious dogs with and without myocardial infarction. Circulation 1988; 78:969–979.
- Mortara A, La Rovere MT, Pinna GD, et al. Arterial baroreflex modulation of heart rate in chronic heart failure: clinical and hemodynamic correlates and prognostic implications. Circulation 1997; 96:3450–3458.
- La Rovere MT, Bigger JT Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) investigators. Lancet. 1998; 351:478–484.
- Lechat P, Hulot JS, Escolano S, et al. Heart rate and cardiac rhythm relationships with bisoprolol benefit in chronic heart failure in CIBIS II trial. Circulation 2001; 103:1428–1433.
- Sabbah HN, Shimoyama H, Kono T, et al. Effects of long-term monotherapy with enalapril, metoprolol and digoxin on the progression of left ventricular dysfunction and dilation in dogs with reduced ejection fraction. Circulation 1994; 89:2852–2859.
- Sabbah HN, Stanley WC, Sharov VG, et al. Effects of dopamine beta-hydroxylase inhibition with nepicastat on the progression of left ventricular dysfunction and remodeling in dogs with chronic heart failure. Circulation 2000; 102:1990–1995.
- Cheng Y, George I, Yi GH, et al. Bradycardic therapy improves left ventricular function and remodeling in dogs with coronary embolization-induced chronic heart failure. J Pharmacol Exp Ther 2007; 321:469–476.
- Swedberg K, Komajda M, Bohm M, et al, on behalf of the SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 2010; 376:875–885.
- Kunze DL. Reflex discharge patterns of cardiac vagal efferent fibres. J Physiol 1972; 222:1–15.
- Harman MA, Reeves TJ. Effects of vagus nerve stimulation on atrial and ventricular function. Am J Physiol 1968; 215:1210–1217.
- Vanoli E, De Ferrari GM, Stramba-Badiale M, Hull SS Jr, Foreman RD, Schwartz PJ. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ Res 1991; 68:1471–1481.
- Li M, Zheng C, Sato T, Kawada T, Sugimachi N, Sunagawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation 2004; 109:120–124.
- Zheng C, Li M, Inagaki M, Kawada T, Sunagawa K, Sugimachi M. Vagal stimulation markedly suppresses arrhythmias in conscious rats with chronic heart failure after myocardial infarction. Conf Proc IEEE Eng Med Biol Soc 2005; 7:7072–7075.
- Sabbah HN, Stein PD, Kono T, et al. A canine model of chronic heart failure produced by multiple sequential coronary microembolizations. Am J Physiol 1991; 260:H1379–H1384.
- Zhang Y, Popovic ZB, Bibevski S, et al. Chronic vagus nerve stimulation improves autonomic control and attenuates systemic inflammation and heart failure progression in a canine high-rate pacing model. Circ Heart Fail 2009; 2:692–699.
- De Ferrari GM, Crijns HJ, Borggrefe M, et al, on behalf of CardioFit Multicenter Trial Investigators. Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur Heart J 2011; 32:847–855.
- Sabbah HN, Rastogi S, Mishra S, et al. Long-term therapy with neuroselective electric vagus nerve stimulation improves LV function and attenuates global LV remodelling in dogs with chronic heart failure. Eur J Heart Fail Supplements 2005; 4(suppl):166–167. Abstract 744.
- Sabbah HN, Imai M, Zaretsky A, et al. Therapy with vagus nerve electrical stimulation combined with beta-blockade improves left ventricular systolic function in dogs with heart failure beyond that seen with beta-blockade alone. Eur J Heart Fail Supplements 2007; 6(suppl):114. Abstract 509.
- Liu YH, Yang XP, Sharov VG, et al. Effects of angiotensin-converting enzyme inhibitors and angiotensin ii type 1 receptor antagonists in rats with heart failure. Role of kinins and angiotensin ii type 2 receptors. J Clin Invest 1997; 99:1926–1935.
- Wang H, Yu M, Ochani M, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 2003; 421:384–388.
- Sabbah H, Rastogi S, Mishra S, Imai M, Gupta RC. Chronic therapy with neuroselective electric vagus nerve stimulation attenuates mRNA expression of pro-inflammatory cytokines in dogs with heart failure. Eur Heart J Suppl 2005; 26(suppl 1):65.
- Feng Q, Song W, Lu X, et al. Development of heart failure and congenital septal defects in mice lacking endothelial nitric oxide synthase. Circulation 2002; 106:873–879.
- Kelly RA, Balligand JL, Smith TW. Nitric oxide and cardiac function. Circ Res 1996; 79:363–380.
- Mungrue IN, Gros R, You X, et al. Cardiomyocyte overexpression of iNOS in mice results in peroxynitrite generation, heart block, and sudden death. J Clin Invest 2002; 109:735–743.
- Damy T, Ratajczak P, Shah AM, et al. Increased neuronal nitric oxide synthase-derived NO production in the failing human heart. Lancet 2004; 363:1365–1367.
- Bendall JK, Damy T, Ratajczak P, et al. Role of myocardial neuronal nitric oxide synthase-derived nitric oxide in beta-adrenergic hyporesponsiveness after myocardial infarction-induced heart failure in rat. Circulation 2004; 110:2368–2375.
- Gupta RC, Mishra S, Rastogi S, Imai M, Zaca V, Sabbah HN. Chronic therapy with electric vagus nerve stimulation normalizes mRNA and protein expression of nitric oxide synthase in myocardium of dogs with heart failure. Eur Heart J 2006; 27:477. Abstract.
- Severs NJ, Bruce AF, Dupont E, Rothery S. Remodelling of gap junctions and connexin expression in diseased myocardium. Cardiovasc Res 2008; 80:9–19.
- Wang XJ, Gerdes AM. Chronic pressure overload cardiac hypertrophy and failure in guinea pigs: III. Intercalated disc remodeling. J Mol Cell Cardiol 1999; 31:333–343.
- Ai X, Pogwizd M. Connexin 43 downregulation and dephosphorylation in nonischemic heart failure is associated with enhanced colocalized protein phosphatase type 2A. Circ Res 2005; 96:54–63.
- Rastogi S, Mishra S, Ilsar I, Zaretsky A, Sabbah HN. Chronic therapy with electric vagus nerve stimulation normalizes mRNA and protein expression of connexin-40, -43 and -45 in left ventricular myocardium of dogs with heart failure. Circulation 2007; 116:II_218. Abstract 1089.
- Adamson PB, Smith AL, Abraham WT, et al. Continuous autonomic assessment in patients with symptomatic heart failure: prognostic value of heart rate variability measured by an implanted cardiac resynchronization device. Circulation 2004; 110:2389–2394.
- Sabbah HN, Ilsar I, Zaretsky A, Rastogi S, Wang M, Gupta RC. Vagus nerve stimulation in experimental heart failure. Heart Fail Rev 2011; 16:171–178.
- Schwartz PJ, Vanoli E, Stramba-Badiale M, De Ferrari GM, Billman GE, Foreman RD. Autonomic mechanisms and sudden death. New insights from analysis of baroreceptor reflexes in conscious dogs with and without myocardial infarction. Circulation 1988; 78:969–979.
- Mortara A, La Rovere MT, Pinna GD, et al Arterial baroreflex modulation of heart rate in chronic heart failure: clinical and hemodynamic correlates and prognostic implications. Circulation 1997; 96:3450–3458.
- La Rovere MT, Bigger JT, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) investigators. Lancet. 1998; 351:478–484.
- Lechat P, Hulot JS, Escolano S, et al Heart rate and cardiac rhythm relationships with bisoprolol benefit in chronic heart failure in CIBIS II trial. Circulation 2001; 103:1428–1433.
- Sabbah HN, Shimoyama H, Kono T, et al Effects of long-term monotherapy with enalapril, metoprolol and digoxin on the progression of left ventricular dysfunction and dilation in dogs with reduced ejection fraction. Circulation 1994; 89:2852–2859.
- Sabbah HN, Stanley WC, Sharov VG, et al Effects of dopamine β-hydroxylase inhibition with nepicastat on the progression of left ventricular dysfunction and remodeling in dogs with chronic heart failure. Circulation 2000; 102:1990–1995.
- Cheng Y, George I, Yi GH, et al Bradycardic therapy improves left ventricular function and remodeling in dogs with coronary embolization-induced chronic heart failure. J Pharmacol Exp Ther 2007; 321:469–476.
- Swedberg K, Komajda M, Bohm M, et al., on behalf of the SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 2010; 376:875–885.
- Kunze DL. Reflex discharge patterns of cardiac vagal efferent fibres. J Physiol 1972; 222:1–15.
- Harman MA, Reeves TJ. Effects of vagus nerve stimulation on atrial and ventricular function. Am J Physiol 1968; 215:1210–1217.
- Vanoli E, De Ferrari GM, Stramba-Badiale M, Hull SS, Foreman RD, Schwartz PJ. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ Res 1991; 68:1471–1481.
- Li M, Zheng C, Sato T, Kawada T, Sugimachi N, Sunagawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation 2004; 109:120–124.
- Zheng C, Li M, Inagaki M, Kawada T, Sunagawa K, Sugimachi M. Vagal stimulation markedly suppresses arrhythmias in conscious rats with chronic heart failure after myocardial infarction. Conf Proc IEEE Eng Med Biol Soc 2005; 7:7072–7075.
- Sabbah HN, Stein PD, Kono T, et al A canine model of chronic heart failure produced by multiple sequential coronary microembolizations. Am J Physiol 1991; 260:H1379–H1384.
- Zhang Y, Popovic ZB, Bibevski S, et al Chronic vagus nerve stimulation improves autonomic control and attenuates systemic inflammation and heart failure progression in a canine high-rate pacing model. Circ Heart Fail 2009; 2:692–699.
- De Ferrari GM, Crijns HJ, Borggrefe M, et al., on behalf of CardioFit Multicenter Trial Investigators. Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur Heart J 2011; 32:847–855.
- Sabbah HN, Rastogi S, Mishra S, et al Long-term therapy with neuroselective electric vagus nerve stimulation improves LV function and attenuates global LV remodelling in dogs with chronic heart failure. Eur J Heart Fail Supplements 2005; 4( suppl):166–167. Abstract 744.
- Sabbah HN, Imai M, Zaretsky A, et al Therapy with vagus nerve electrical stimulation combined with beta-blockade improves left ventricular systolic function in dogs with heart failure beyond that seen with beta-blockade alone. Eur J Heart Fail Supplements 2007; 6( suppl):114. Abstract 509.
- Liu YH, Yang XP, Sharov VG, et al Effects of angiotensin-converting enzyme inhibitors and angiotensin II type 1 receptor antagonists in rats with heart failure. Role of kinins and angiotensin II type 2 receptors. J Clin Invest 1997; 99:1926–1935.
- Wang H, Yu M, Ochani M, et al Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 2003; 421:384–388.
- Sabbah H, Rastogi S, Mishra S, Imai M, Gupta RC. Chronic therapy with neuroselective electric vagus nerve stimulation attenuates mRNA expression of pro-inflammatory cytokines in dogs with heart failure. Eur Heart J Suppl 2005; 26( suppl 1):65.
- Feng Q, Song W, Lu X, et al Development of heart failure and congenital septal defects in mice lacking endothelial nitric oxide synthase. Circulation 2002; 106:873–879.
- Kelly RA, Balligand JL, Smith TW. Nitric oxide and cardiac function. Circ Res 1996; 79:363–380.
- Mungrue IN, Gros R, You X, et al Cardiomyocyte overexpression of iNOS in mice results in peroxynitrite generation, heart block, and sudden death. J Clin Invest 2002; 109:735–743.
- Damy T, Ratajczak P, Shah AM, et al Increased neuronal nitric oxide synthase-derived NO production in the failing human heart. Lancet 2004; 363:1365–1367.
- Bendall JK, Damy T, Ratajczak P, et al Role of myocardial neuronal nitric oxide synthase-derived nitric oxide in β-adrenergic hyporesponsiveness after myocardial infarction-induced heart failure in rat. Circulation 2004; 110:2368–2375.
- Gupta RC, Mishra S, Rastogi S, Imai M, Zaca V, Sabbah HN. Chronic therapy with electric vagus nerve stimulation normalizes mRNA and protein expression of nitric oxide synthase in myocardium of dogs with heart failure. Eur Heart J 2006; 27:477. Abstract.
- Severs NJ, Bruce AF, Dupont E, Rothery S. Remodelling of gap junctions and connexin expression in diseased myocardium. Cardio vasc Res 2008; 80:9–19.
- Wang XJ, Gerdes AM. Chronic pressure overload cardiac hypertrophy and failure in guinea pigs: III. Intercalated disc remodeling. J Mol Cell Cardiol 1999; 31:333–343.
- Ai X, Pogwizd M. Connexin 43 downregulation and dephosphorylation in nonischemic heart failure is associated with enhanced colocalized protein phosphatase type 2A. Circ Res 2005; 96:54–63.
- Rastogi S, Mishra S, Ilsar I, Zaretsky A, Sabbah HN. Chronic therapy with electric vagus nerve stimulation normalizes mRNA and protein expression of connexin-40, -43 and -45 in left ventricular myocardium of dogs with heart failure. Circulation 2007; 116:II_218. Abstract 1089.
- Adamson PB, Smith AL, Abraham WT, et al Continuous autonomic assessment in patients with symptomatic heart failure: prognostic value of heart rate variability measured by an implanted cardiac resynchronization device. Circulation 2004; 110:2389–2394.
- Sabbah HN, Ilsar I, Zaretsky A, Rastogi S, Wang M, Gupta RC. Vagus nerve stimulation in experimental heart failure. Heart Fail Rev 2011; 16:171–178.
Autonomic imbalance characterized by sustained sympathetic overdrive and by parasympathetic withdrawal is a key maladaptation of the heart failure (HF) state. This autonomic dysregulation has long been recognized as a mediator of increased mortality and morbidity in myocardial infarction and HF.1,2 Sympathovagal imbalance in HF can lead to increased heart rate, excess release of proinflammatory cytokines, dysregulation of nitric oxide (NO) pathways, and arrythmogenesis. Diminished vagal activity reflected in increased heart rate is a predictor of high mortality in HF.3,4 Sustained increase of sympathetic activity contributes to progressive left ventricular (LV) dysfunction in HF and promotes progressive LV remodeling.5,6 Pharmacologic agents that reduce heart rate, such as beta-blockers and, more recently, specific and selective inhibitors of the cardiac pacemaker current If, have been shown to improve survival and prevent or attenuate progressive LV remodeling in animals with HF.4,5,7,8
During the past two to three decades, the emphasis on modulation of neurohumoral activition for treatment of chronic HF gave rise to angiotensin-converting enzyme inhibitors, beta-adrenergic receptor blockers, and aldosterone antagonists. In recent years, renewed interest has emerged in modulating parasympathetic or vagal activity as a therapeutic target for treating chronic HF. An alteration in cardiac vagal efferent activity through peripheral cardiac nerve stimulation can produce bradycardia and can modify atrial as well as ventricular contractile function.9,10
Electrical vagus nerve stimulation (VNS) was shown to prevent sudden cardiac death in dogs with myocardial infarction and to improve long-term survival in rats with chronic HF.11,12 VNS has also been shown to suppress arrhythmias in conscious rats with chronic HF secondary to myocardial infarction.13
This article focuses primarily on the effects of chronic VNS on LV dysfunction and remodeling in dogs with HF produced by multiple sequential intracoronary microembolizations14 or by high-rate ventricular pacing15 and on the safety, feasibility, and efficacy trends of VNS in patients with advanced HF.16
VNS IN DOGS WITH MICROEMBOLIZATION-INDUCED HEART FAILURE
Monotherapy with VNS
Long-term VNS therapy also elicited improvements in indices of LV diastolic function. VNS significantly decreased LV end-diastolic pressure (Table 1), increased deceleration time of rapid mitral inflow velocity, tended to increase the ratio of peak mitral inflow velocity during early LV filling to peak mitral inflow velocity during left atrial contraction (PE/PA), and significantly reduced LV end-diastolic circumferential wall stress, a determinant of myocardial oxygen consumption (Table 1). These measures suggest that VNS can reduce preload, improve LV relaxation and improve LV function without increasing myocardial oxygen consumption.
VNS in combination with beta-blockade
The effects of VNS in combination with beta-blockade were examined in dogs with HF. Dogs with LV ejection fraction of approximately 35% were randomized to 3 months of therapy with a beta-blocker alone (metoprolol succinate, 100 mg once daily, n = 6) or to metoprolol (100 mg once daily) combined with active VNS with CardioFit (n = 6). As with the monotherapy study, the CardioFit VNS system was operated in the feedback on-demand heart rate responsive mode. Dogs were started on oral metoprolol therapy 2 weeks prior to randomization to VNS therapy. After randomization, all dogs continued to receive metoprolol succinate once daily for the duration of the study.18
The improvements in LV systolic and diastolic function with combination therapy were associated with important changes in heart rate. Twenty-four–hour ambulatory ECG Holter monitoring studies showed no differences in minimum and average heart rate between dogs treated with metoprolol and those treated with combination therapy with VNS. Maximum heart rate, however, was significantly lower in dogs treated with the combination therapy (114 ± 12 vs 149 ± 8 beats/min, P < .05). These observations suggest that preventing heart rate escape at the high end may further improve LV systolic function compared with beta-blockade alone. This added benefit of the combination of VNS and beta-blockade was likely the result of reducing the adverse impact of increased cardiac workload and increased myocardial oxygen consumption elicited by the heart rate increase.
VNS and left ventricular remodeling
VNS and proinflammatory cytokines, nitric oxide, and gap junction proteins
Nitric oxide (NO) is formed by a family of NO synthases (NOS). The three isoforms of NOS identified to date are endothelin NOS (eNOS), inducible NOS (iNOS), and neuronal NOS (nNOS). The three isoforms have differing characteristics and roles:
eNOS. NO produced by eNOS plays an important role in the regulation of cell growth and apoptosis22 and can enhance myocardial relaxation and regulate contractility.22,23
iNOS. Overexpression of iNOS in cardiomyocytes in mice results in peroxynitrite generation associated with fibrosis, LV hypertrophy, chamber dilation, cardiomyopathic phenotype, heart block, and sudden cardiac death.24
nNOS. nNOS has been shown to be upregulated in the human failing heart and in rats following myocardial infarction.25 In rats with HF, inhibition of nNOS leads to increased sensitivity of the myocardium to beta-adrenergic stimulation,26 suggesting a role for nNOS in the autocrine regulation of myocardial contractility.26
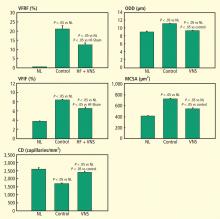
In dogs with coronary microembolization-induced HF, mRNA and protein expression of eNOS in LV myocardium is significantly downregulated compared with normal dogs; therapy with VNS significantly improves the expression of eNOS (Table 3).27 Both iNOS and nNOS are significantly upregulated, and their expression tends to be normalized by long-term VNS therapy.27
Gap junction proteins or connexins are reduced or redistributed from intercalated disks to lateral cell borders in a variety of cardiac diseases, including HF.28 This so-called “gap junction remodeling” is considered highly arrhythmogenic. In mammals, gap junctions exclusively contain connexin-43 (Cx43). Reduced expression of Cx43 occurs in the failing human heart and has been shown to result in slowed transmural conduction and dispersion of action potential duration with increased susceptibility to arrhythmia and sudden cardiac death.29,30 In dogs with coronary microembolization-induced HF, mRNA and protein expression of Cx43 in LV myocardium was shown to be markedly downregulated compared with normal dogs, and long-term therapy with VNS was associated with a significant increase in the expression of Cx43 in LV myocardium (Table 3).31
VNS IN DOGS WITH RAPID PACING–INDUCED HEART FAILURE
Electrical VNS as a potential therapy for HF was examined in dogs with HF secondary to high-rate ventricular pacing using the Cyberonics VNS system (Cyberonics Inc., Houston, TX), which does not operate on a negative feedback mechanism.15 In this study, VNS therapy was delivered continuously for the duration of the study with a duty cycle of 14 seconds on and 12 seconds off. VNS signals were delivered to the right cervical vagus nerve at a frequency of 20 Hz and a pulse width of 0.5 msec.15 Dogs were randomized to control (n = 7) or to monotherapy with VNS (n = 8) and followed for 8 weeks. All measurements were made approximately 15 minutes after temporarily turning off the ventricular pacemaker and the vagus nerve stimulator.15 VNS therapy resulted in a significant decrease in LV end-diastolic and end-systolic volumes and a significant increase in LV ejection fraction compared with controls.15 This improvement was associated with significant reduction in plasma levels of norepinephrine, angiotensin II, and C-reactive protein. The study also demonstrated the effectiveness of VNS in restoring baroreflex sensitivity, thus improving cardiac autonomic control.15 Because rapid pacing was maintained throughout the study except for short periods when measurements were made, one can argue that the benefits of VNS therapy in this model of HF are independent of heart rate.15
SAFETY AND TOLERABILITY OF VNS IN PATIENTS WITH ADVANCED HEART FAILURE
In patients with HF, reduced vagal activity is associated with increased mortality.1 Vagal withdrawal has also been shown to precede episodes of acute decompensation.32 In a recently published study, De Ferrari et al, on behalf of the CardioFit Multicenter Trial Investigators, examined the safety and tolerability of chronic VNS in 32 patients with symptomatic HF and severe LV dysfunction using the CardioFit system.16 The CardioFit system used in this study differed from that used in dogs with microembolization-induced HF in that it did not operate on a negative feedback principle. A bradycardia limit causing interruption of VNS was set at 55 beats/min. A 3-week uptitration period was used to maximize current amplitude and duty cycle based on patient sensation. The intensity of the stimulation reached 4.1 ± 1.2 mA at the end of the titration period.16
This multicenter, open-label, phase 2 trial involved 3 to 6 months of followup with an optional 1 year followup. The results suggested that VNS may be safe and tolerable in HF patients with severe LV dysfunction. Trends for efficacy were also favorable, bearing in mind the nonrandomized and unblinded nature of the study design. The study showed significant improvements in New York Heart Association HF classification, 6-minute walk test, LV ejection fraction, and LV systolic volumes.16
CONCLUSIONS
A wealth of preclinical and clinical studies supports the concept that electrical VNS can favorably modify the underlying pathophysiology and course of evolving HF. In animals with HF, VNS improves LV function, attenuates LV remodeling and may prevent arrhythmias that provoke sudden cardiac death. VNS derives these potential clinical benefits from multiple mechanisms of action that include reduced heart rate and normalization of sympathetic overdrive. VNS also appears to have a favorable impact on other signaling pathways that are likely to elicit beneficial effects in patients with HF. These include restoration of baroreflex sensitivity, suppression of proinflammatory cytokines, normalization of NO signaling pathways, and suppression of gap junction remodeling. At present, there is no evidence to implicate a single mechanism of action for the benefits derived from VNS. Instead, it is likely that all of the mechanisms listed above act in concert to elicit the global benefit seen with VNS. In humans with HF, VNS may be safe, feasible, and apparently well tolerated. Full appreciation of its efficacy in treating chronic HF must await completion of pivotal randomized clinical trials.
Autonomic imbalance characterized by sustained sympathetic overdrive and by parasympathetic withdrawal is a key maladaptation of the heart failure (HF) state. This autonomic dysregulation has long been recognized as a mediator of increased mortality and morbidity in myocardial infarction and HF.1,2 Sympathovagal imbalance in HF can lead to increased heart rate, excess release of proinflammatory cytokines, dysregulation of nitric oxide (NO) pathways, and arrythmogenesis. Diminished vagal activity reflected in increased heart rate is a predictor of high mortality in HF.3,4 Sustained increase of sympathetic activity contributes to progressive left ventricular (LV) dysfunction in HF and promotes progressive LV remodeling.5,6 Pharmacologic agents that reduce heart rate, such as beta-blockers and, more recently, specific and selective inhibitors of the cardiac pacemaker current If, have been shown to improve survival and prevent or attenuate progressive LV remodeling in animals with HF.4,5,7,8
During the past two to three decades, the emphasis on modulation of neurohumoral activition for treatment of chronic HF gave rise to angiotensin-converting enzyme inhibitors, beta-adrenergic receptor blockers, and aldosterone antagonists. In recent years, renewed interest has emerged in modulating parasympathetic or vagal activity as a therapeutic target for treating chronic HF. An alteration in cardiac vagal efferent activity through peripheral cardiac nerve stimulation can produce bradycardia and can modify atrial as well as ventricular contractile function.9,10
Electrical vagus nerve stimulation (VNS) was shown to prevent sudden cardiac death in dogs with myocardial infarction and to improve long-term survival in rats with chronic HF.11,12 VNS has also been shown to suppress arrhythmias in conscious rats with chronic HF secondary to myocardial infarction.13
This article focuses primarily on the effects of chronic VNS on LV dysfunction and remodeling in dogs with HF produced by multiple sequential intracoronary microembolizations14 or by high-rate ventricular pacing15 and on the safety, feasibility, and efficacy trends of VNS in patients with advanced HF.16
VNS IN DOGS WITH MICROEMBOLIZATION-INDUCED HEART FAILURE
Monotherapy with VNS
Long-term VNS therapy also elicited improvements in indices of LV diastolic function. VNS significantly decreased LV end-diastolic pressure (Table 1), increased deceleration time of rapid mitral inflow velocity, tended to increase the ratio of peak mitral inflow velocity during early LV filling to peak mitral inflow velocity during left atrial contraction (PE/PA), and significantly reduced LV end-diastolic circumferential wall stress, a determinant of myocardial oxygen consumption (Table 1). These measures suggest that VNS can reduce preload, improve LV relaxation and improve LV function without increasing myocardial oxygen consumption.
VNS in combination with beta-blockade
The effects of VNS in combination with beta-blockade were examined in dogs with HF. Dogs with LV ejection fraction of approximately 35% were randomized to 3 months of therapy with a beta-blocker alone (metoprolol succinate, 100 mg once daily, n = 6) or to metoprolol (100 mg once daily) combined with active VNS with CardioFit (n = 6). As with the monotherapy study, the CardioFit VNS system was operated in the feedback on-demand heart rate responsive mode. Dogs were started on oral metoprolol therapy 2 weeks prior to randomization to VNS therapy. After randomization, all dogs continued to receive metoprolol succinate once daily for the duration of the study.18
The improvements in LV systolic and diastolic function with combination therapy were associated with important changes in heart rate. Twenty-four–hour ambulatory ECG Holter monitoring studies showed no differences in minimum and average heart rate between dogs treated with metoprolol and those treated with combination therapy with VNS. Maximum heart rate, however, was significantly lower in dogs treated with the combination therapy (114 ± 12 vs 149 ± 8 beats/min, P < .05). These observations suggest that preventing heart rate escape at the high end may further improve LV systolic function compared with beta-blockade alone. This added benefit of the combination of VNS and beta-blockade was likely the result of reducing the adverse impact of increased cardiac workload and increased myocardial oxygen consumption elicited by the heart rate increase.
VNS and left ventricular remodeling
VNS and proinflammatory cytokines, nitric oxide, and gap junction proteins
Nitric oxide (NO) is formed by a family of NO synthases (NOS). The three isoforms of NOS identified to date are endothelin NOS (eNOS), inducible NOS (iNOS), and neuronal NOS (nNOS). The three isoforms have differing characteristics and roles:
eNOS. NO produced by eNOS plays an important role in the regulation of cell growth and apoptosis22 and can enhance myocardial relaxation and regulate contractility.22,23
iNOS. Overexpression of iNOS in cardiomyocytes in mice results in peroxynitrite generation associated with fibrosis, LV hypertrophy, chamber dilation, cardiomyopathic phenotype, heart block, and sudden cardiac death.24
nNOS. nNOS has been shown to be upregulated in the human failing heart and in rats following myocardial infarction.25 In rats with HF, inhibition of nNOS leads to increased sensitivity of the myocardium to beta-adrenergic stimulation,26 suggesting a role for nNOS in the autocrine regulation of myocardial contractility.26
In dogs with coronary microembolization-induced HF, mRNA and protein expression of eNOS in LV myocardium is significantly downregulated compared with normal dogs; therapy with VNS significantly improves the expression of eNOS (Table 3).27 Both iNOS and nNOS are significantly upregulated, and their expression tends to be normalized by long-term VNS therapy.27
Gap junction proteins or connexins are reduced or redistributed from intercalated disks to lateral cell borders in a variety of cardiac diseases, including HF.28 This so-called “gap junction remodeling” is considered highly arrhythmogenic. In mammals, gap junctions exclusively contain connexin-43 (Cx43). Reduced expression of Cx43 occurs in the failing human heart and has been shown to result in slowed transmural conduction and dispersion of action potential duration with increased susceptibility to arrhythmia and sudden cardiac death.29,30 In dogs with coronary microembolization-induced HF, mRNA and protein expression of Cx43 in LV myocardium was shown to be markedly downregulated compared with normal dogs, and long-term therapy with VNS was associated with a significant increase in the expression of Cx43 in LV myocardium (Table 3).31
VNS IN DOGS WITH RAPID PACING–INDUCED HEART FAILURE
Electrical VNS as a potential therapy for HF was examined in dogs with HF secondary to high-rate ventricular pacing using the Cyberonics VNS system (Cyberonics Inc., Houston, TX), which does not operate on a negative feedback mechanism.15 In this study, VNS therapy was delivered continuously for the duration of the study with a duty cycle of 14 seconds on and 12 seconds off. VNS signals were delivered to the right cervical vagus nerve at a frequency of 20 Hz and a pulse width of 0.5 msec.15 Dogs were randomized to control (n = 7) or to monotherapy with VNS (n = 8) and followed for 8 weeks. All measurements were made approximately 15 minutes after temporarily turning off the ventricular pacemaker and the vagus nerve stimulator.15 VNS therapy resulted in a significant decrease in LV end-diastolic and end-systolic volumes and a significant increase in LV ejection fraction compared with controls.15 This improvement was associated with significant reduction in plasma levels of norepinephrine, angiotensin II, and C-reactive protein. The study also demonstrated the effectiveness of VNS in restoring baroreflex sensitivity, thus improving cardiac autonomic control.15 Because rapid pacing was maintained throughout the study except for short periods when measurements were made, one can argue that the benefits of VNS therapy in this model of HF are independent of heart rate.15
SAFETY AND TOLERABILITY OF VNS IN PATIENTS WITH ADVANCED HEART FAILURE
In patients with HF, reduced vagal activity is associated with increased mortality.1 Vagal withdrawal has also been shown to precede episodes of acute decompensation.32 In a recently published study, De Ferrari et al, on behalf of the CardioFit Multicenter Trial Investigators, examined the safety and tolerability of chronic VNS in 32 patients with symptomatic HF and severe LV dysfunction using the CardioFit system.16 The CardioFit system used in this study differed from that used in dogs with microembolization-induced HF in that it did not operate on a negative feedback principle. A bradycardia limit causing interruption of VNS was set at 55 beats/min. A 3-week uptitration period was used to maximize current amplitude and duty cycle based on patient sensation. The intensity of the stimulation reached 4.1 ± 1.2 mA at the end of the titration period.16
This multicenter, open-label, phase 2 trial involved 3 to 6 months of followup with an optional 1 year followup. The results suggested that VNS may be safe and tolerable in HF patients with severe LV dysfunction. Trends for efficacy were also favorable, bearing in mind the nonrandomized and unblinded nature of the study design. The study showed significant improvements in New York Heart Association HF classification, 6-minute walk test, LV ejection fraction, and LV systolic volumes.16
CONCLUSIONS
A wealth of preclinical and clinical studies supports the concept that electrical VNS can favorably modify the underlying pathophysiology and course of evolving HF. In animals with HF, VNS improves LV function, attenuates LV remodeling and may prevent arrhythmias that provoke sudden cardiac death. VNS derives these potential clinical benefits from multiple mechanisms of action that include reduced heart rate and normalization of sympathetic overdrive. VNS also appears to have a favorable impact on other signaling pathways that are likely to elicit beneficial effects in patients with HF. These include restoration of baroreflex sensitivity, suppression of proinflammatory cytokines, normalization of NO signaling pathways, and suppression of gap junction remodeling. At present, there is no evidence to implicate a single mechanism of action for the benefits derived from VNS. Instead, it is likely that all of the mechanisms listed above act in concert to elicit the global benefit seen with VNS. In humans with HF, VNS may be safe, feasible, and apparently well tolerated. Full appreciation of its efficacy in treating chronic HF must await completion of pivotal randomized clinical trials.
- Schwartz PJ, Vanoli E, Stramba-Badiale M, De Ferrari GM, Billman GE, Foreman RD. Autonomic mechanisms and sudden death. New insights from analysis of baroreceptor reflexes in conscious dogs with and without myocardial infarction. Circulation 1988; 78:969–979.
- Mortara A, La Rovere MT, Pinna GD, et al. Arterial baroreflex modulation of heart rate in chronic heart failure: clinical and hemodynamic correlates and prognostic implications. Circulation 1997; 96:3450–3458.
- La Rovere MT, Bigger JT Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) investigators. Lancet. 1998; 351:478–484.
- Lechat P, Hulot JS, Escolano S, et al. Heart rate and cardiac rhythm relationships with bisoprolol benefit in chronic heart failure in CIBIS II trial. Circulation 2001; 103:1428–1433.
- Sabbah HN, Shimoyama H, Kono T, et al. Effects of long-term monotherapy with enalapril, metoprolol and digoxin on the progression of left ventricular dysfunction and dilation in dogs with reduced ejection fraction. Circulation 1994; 89:2852–2859.
- Sabbah HN, Stanley WC, Sharov VG, et al. Effects of dopamine beta-hydroxylase inhibition with nepicastat on the progression of left ventricular dysfunction and remodeling in dogs with chronic heart failure. Circulation 2000; 102:1990–1995.
- Cheng Y, George I, Yi GH, et al. Bradycardic therapy improves left ventricular function and remodeling in dogs with coronary embolization-induced chronic heart failure. J Pharmacol Exp Ther 2007; 321:469–476.
- Swedberg K, Komajda M, Bohm M, et al, on behalf of the SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 2010; 376:875–885.
- Kunze DL. Reflex discharge patterns of cardiac vagal efferent fibres. J Physiol 1972; 222:1–15.
- Harman MA, Reeves TJ. Effects of vagus nerve stimulation on atrial and ventricular function. Am J Physiol 1968; 215:1210–1217.
- Vanoli E, De Ferrari GM, Stramba-Badiale M, Hull SS Jr, Foreman RD, Schwartz PJ. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ Res 1991; 68:1471–1481.
- Li M, Zheng C, Sato T, Kawada T, Sugimachi N, Sunagawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation 2004; 109:120–124.
- Zheng C, Li M, Inagaki M, Kawada T, Sunagawa K, Sugimachi M. Vagal stimulation markedly suppresses arrhythmias in conscious rats with chronic heart failure after myocardial infarction. Conf Proc IEEE Eng Med Biol Soc 2005; 7:7072–7075.
- Sabbah HN, Stein PD, Kono T, et al. A canine model of chronic heart failure produced by multiple sequential coronary microembolizations. Am J Physiol 1991; 260:H1379–H1384.
- Zhang Y, Popovic ZB, Bibevski S, et al. Chronic vagus nerve stimulation improves autonomic control and attenuates systemic inflammation and heart failure progression in a canine high-rate pacing model. Circ Heart Fail 2009; 2:692–699.
- De Ferrari GM, Crijns HJ, Borggrefe M, et al, on behalf of CardioFit Multicenter Trial Investigators. Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur Heart J 2011; 32:847–855.
- Sabbah HN, Rastogi S, Mishra S, et al. Long-term therapy with neuroselective electric vagus nerve stimulation improves LV function and attenuates global LV remodelling in dogs with chronic heart failure. Eur J Heart Fail Supplements 2005; 4(suppl):166–167. Abstract 744.
- Sabbah HN, Imai M, Zaretsky A, et al. Therapy with vagus nerve electrical stimulation combined with beta-blockade improves left ventricular systolic function in dogs with heart failure beyond that seen with beta-blockade alone. Eur J Heart Fail Supplements 2007; 6(suppl):114. Abstract 509.
- Liu YH, Yang XP, Sharov VG, et al. Effects of angiotensin-converting enzyme inhibitors and angiotensin ii type 1 receptor antagonists in rats with heart failure. Role of kinins and angiotensin ii type 2 receptors. J Clin Invest 1997; 99:1926–1935.
- Wang H, Yu M, Ochani M, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 2003; 421:384–388.
- Sabbah H, Rastogi S, Mishra S, Imai M, Gupta RC. Chronic therapy with neuroselective electric vagus nerve stimulation attenuates mRNA expression of pro-inflammatory cytokines in dogs with heart failure. Eur Heart J Suppl 2005; 26(suppl 1):65.
- Feng Q, Song W, Lu X, et al. Development of heart failure and congenital septal defects in mice lacking endothelial nitric oxide synthase. Circulation 2002; 106:873–879.
- Kelly RA, Balligand JL, Smith TW. Nitric oxide and cardiac function. Circ Res 1996; 79:363–380.
- Mungrue IN, Gros R, You X, et al. Cardiomyocyte overexpression of iNOS in mice results in peroxynitrite generation, heart block, and sudden death. J Clin Invest 2002; 109:735–743.
- Damy T, Ratajczak P, Shah AM, et al. Increased neuronal nitric oxide synthase-derived NO production in the failing human heart. Lancet 2004; 363:1365–1367.
- Bendall JK, Damy T, Ratajczak P, et al. Role of myocardial neuronal nitric oxide synthase-derived nitric oxide in beta-adrenergic hyporesponsiveness after myocardial infarction-induced heart failure in rat. Circulation 2004; 110:2368–2375.
- Gupta RC, Mishra S, Rastogi S, Imai M, Zaca V, Sabbah HN. Chronic therapy with electric vagus nerve stimulation normalizes mRNA and protein expression of nitric oxide synthase in myocardium of dogs with heart failure. Eur Heart J 2006; 27:477. Abstract.
- Severs NJ, Bruce AF, Dupont E, Rothery S. Remodelling of gap junctions and connexin expression in diseased myocardium. Cardiovasc Res 2008; 80:9–19.
- Wang XJ, Gerdes AM. Chronic pressure overload cardiac hypertrophy and failure in guinea pigs: III. Intercalated disc remodeling. J Mol Cell Cardiol 1999; 31:333–343.
- Ai X, Pogwizd M. Connexin 43 downregulation and dephosphorylation in nonischemic heart failure is associated with enhanced colocalized protein phosphatase type 2A. Circ Res 2005; 96:54–63.
- Rastogi S, Mishra S, Ilsar I, Zaretsky A, Sabbah HN. Chronic therapy with electric vagus nerve stimulation normalizes mRNA and protein expression of connexin-40, -43 and -45 in left ventricular myocardium of dogs with heart failure. Circulation 2007; 116:II_218. Abstract 1089.
- Adamson PB, Smith AL, Abraham WT, et al. Continuous autonomic assessment in patients with symptomatic heart failure: prognostic value of heart rate variability measured by an implanted cardiac resynchronization device. Circulation 2004; 110:2389–2394.
- Sabbah HN, Ilsar I, Zaretsky A, Rastogi S, Wang M, Gupta RC. Vagus nerve stimulation in experimental heart failure. Heart Fail Rev 2011; 16:171–178.
- Schwartz PJ, Vanoli E, Stramba-Badiale M, De Ferrari GM, Billman GE, Foreman RD. Autonomic mechanisms and sudden death. New insights from analysis of baroreceptor reflexes in conscious dogs with and without myocardial infarction. Circulation 1988; 78:969–979.
- Mortara A, La Rovere MT, Pinna GD, et al Arterial baroreflex modulation of heart rate in chronic heart failure: clinical and hemodynamic correlates and prognostic implications. Circulation 1997; 96:3450–3458.
- La Rovere MT, Bigger JT, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) investigators. Lancet. 1998; 351:478–484.
- Lechat P, Hulot JS, Escolano S, et al Heart rate and cardiac rhythm relationships with bisoprolol benefit in chronic heart failure in CIBIS II trial. Circulation 2001; 103:1428–1433.
- Sabbah HN, Shimoyama H, Kono T, et al Effects of long-term monotherapy with enalapril, metoprolol and digoxin on the progression of left ventricular dysfunction and dilation in dogs with reduced ejection fraction. Circulation 1994; 89:2852–2859.
- Sabbah HN, Stanley WC, Sharov VG, et al Effects of dopamine β-hydroxylase inhibition with nepicastat on the progression of left ventricular dysfunction and remodeling in dogs with chronic heart failure. Circulation 2000; 102:1990–1995.
- Cheng Y, George I, Yi GH, et al Bradycardic therapy improves left ventricular function and remodeling in dogs with coronary embolization-induced chronic heart failure. J Pharmacol Exp Ther 2007; 321:469–476.
- Swedberg K, Komajda M, Bohm M, et al., on behalf of the SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 2010; 376:875–885.
- Kunze DL. Reflex discharge patterns of cardiac vagal efferent fibres. J Physiol 1972; 222:1–15.
- Harman MA, Reeves TJ. Effects of vagus nerve stimulation on atrial and ventricular function. Am J Physiol 1968; 215:1210–1217.
- Vanoli E, De Ferrari GM, Stramba-Badiale M, Hull SS, Foreman RD, Schwartz PJ. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ Res 1991; 68:1471–1481.
- Li M, Zheng C, Sato T, Kawada T, Sugimachi N, Sunagawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation 2004; 109:120–124.
- Zheng C, Li M, Inagaki M, Kawada T, Sunagawa K, Sugimachi M. Vagal stimulation markedly suppresses arrhythmias in conscious rats with chronic heart failure after myocardial infarction. Conf Proc IEEE Eng Med Biol Soc 2005; 7:7072–7075.
- Sabbah HN, Stein PD, Kono T, et al A canine model of chronic heart failure produced by multiple sequential coronary microembolizations. Am J Physiol 1991; 260:H1379–H1384.
- Zhang Y, Popovic ZB, Bibevski S, et al Chronic vagus nerve stimulation improves autonomic control and attenuates systemic inflammation and heart failure progression in a canine high-rate pacing model. Circ Heart Fail 2009; 2:692–699.
- De Ferrari GM, Crijns HJ, Borggrefe M, et al., on behalf of CardioFit Multicenter Trial Investigators. Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur Heart J 2011; 32:847–855.
- Sabbah HN, Rastogi S, Mishra S, et al Long-term therapy with neuroselective electric vagus nerve stimulation improves LV function and attenuates global LV remodelling in dogs with chronic heart failure. Eur J Heart Fail Supplements 2005; 4( suppl):166–167. Abstract 744.
- Sabbah HN, Imai M, Zaretsky A, et al Therapy with vagus nerve electrical stimulation combined with beta-blockade improves left ventricular systolic function in dogs with heart failure beyond that seen with beta-blockade alone. Eur J Heart Fail Supplements 2007; 6( suppl):114. Abstract 509.
- Liu YH, Yang XP, Sharov VG, et al Effects of angiotensin-converting enzyme inhibitors and angiotensin II type 1 receptor antagonists in rats with heart failure. Role of kinins and angiotensin II type 2 receptors. J Clin Invest 1997; 99:1926–1935.
- Wang H, Yu M, Ochani M, et al Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 2003; 421:384–388.
- Sabbah H, Rastogi S, Mishra S, Imai M, Gupta RC. Chronic therapy with neuroselective electric vagus nerve stimulation attenuates mRNA expression of pro-inflammatory cytokines in dogs with heart failure. Eur Heart J Suppl 2005; 26( suppl 1):65.
- Feng Q, Song W, Lu X, et al Development of heart failure and congenital septal defects in mice lacking endothelial nitric oxide synthase. Circulation 2002; 106:873–879.
- Kelly RA, Balligand JL, Smith TW. Nitric oxide and cardiac function. Circ Res 1996; 79:363–380.
- Mungrue IN, Gros R, You X, et al Cardiomyocyte overexpression of iNOS in mice results in peroxynitrite generation, heart block, and sudden death. J Clin Invest 2002; 109:735–743.
- Damy T, Ratajczak P, Shah AM, et al Increased neuronal nitric oxide synthase-derived NO production in the failing human heart. Lancet 2004; 363:1365–1367.
- Bendall JK, Damy T, Ratajczak P, et al Role of myocardial neuronal nitric oxide synthase-derived nitric oxide in β-adrenergic hyporesponsiveness after myocardial infarction-induced heart failure in rat. Circulation 2004; 110:2368–2375.
- Gupta RC, Mishra S, Rastogi S, Imai M, Zaca V, Sabbah HN. Chronic therapy with electric vagus nerve stimulation normalizes mRNA and protein expression of nitric oxide synthase in myocardium of dogs with heart failure. Eur Heart J 2006; 27:477. Abstract.
- Severs NJ, Bruce AF, Dupont E, Rothery S. Remodelling of gap junctions and connexin expression in diseased myocardium. Cardio vasc Res 2008; 80:9–19.
- Wang XJ, Gerdes AM. Chronic pressure overload cardiac hypertrophy and failure in guinea pigs: III. Intercalated disc remodeling. J Mol Cell Cardiol 1999; 31:333–343.
- Ai X, Pogwizd M. Connexin 43 downregulation and dephosphorylation in nonischemic heart failure is associated with enhanced colocalized protein phosphatase type 2A. Circ Res 2005; 96:54–63.
- Rastogi S, Mishra S, Ilsar I, Zaretsky A, Sabbah HN. Chronic therapy with electric vagus nerve stimulation normalizes mRNA and protein expression of connexin-40, -43 and -45 in left ventricular myocardium of dogs with heart failure. Circulation 2007; 116:II_218. Abstract 1089.
- Adamson PB, Smith AL, Abraham WT, et al Continuous autonomic assessment in patients with symptomatic heart failure: prognostic value of heart rate variability measured by an implanted cardiac resynchronization device. Circulation 2004; 110:2389–2394.
- Sabbah HN, Ilsar I, Zaretsky A, Rastogi S, Wang M, Gupta RC. Vagus nerve stimulation in experimental heart failure. Heart Fail Rev 2011; 16:171–178.
- Schwartz PJ, Vanoli E, Stramba-Badiale M, De Ferrari GM, Billman GE, Foreman RD. Autonomic mechanisms and sudden death. New insights from analysis of baroreceptor reflexes in conscious dogs with and without myocardial infarction. Circulation 1988; 78:969–979.
- Mortara A, La Rovere MT, Pinna GD, et al. Arterial baroreflex modulation of heart rate in chronic heart failure: clinical and hemodynamic correlates and prognostic implications. Circulation 1997; 96:3450–3458.
- La Rovere MT, Bigger JT Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) investigators. Lancet. 1998; 351:478–484.
- Lechat P, Hulot JS, Escolano S, et al. Heart rate and cardiac rhythm relationships with bisoprolol benefit in chronic heart failure in CIBIS II trial. Circulation 2001; 103:1428–1433.
- Sabbah HN, Shimoyama H, Kono T, et al. Effects of long-term monotherapy with enalapril, metoprolol and digoxin on the progression of left ventricular dysfunction and dilation in dogs with reduced ejection fraction. Circulation 1994; 89:2852–2859.
- Sabbah HN, Stanley WC, Sharov VG, et al. Effects of dopamine beta-hydroxylase inhibition with nepicastat on the progression of left ventricular dysfunction and remodeling in dogs with chronic heart failure. Circulation 2000; 102:1990–1995.
- Cheng Y, George I, Yi GH, et al. Bradycardic therapy improves left ventricular function and remodeling in dogs with coronary embolization-induced chronic heart failure. J Pharmacol Exp Ther 2007; 321:469–476.
- Swedberg K, Komajda M, Bohm M, et al, on behalf of the SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 2010; 376:875–885.
- Kunze DL. Reflex discharge patterns of cardiac vagal efferent fibres. J Physiol 1972; 222:1–15.
- Harman MA, Reeves TJ. Effects of vagus nerve stimulation on atrial and ventricular function. Am J Physiol 1968; 215:1210–1217.
- Vanoli E, De Ferrari GM, Stramba-Badiale M, Hull SS Jr, Foreman RD, Schwartz PJ. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ Res 1991; 68:1471–1481.
- Li M, Zheng C, Sato T, Kawada T, Sugimachi N, Sunagawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation 2004; 109:120–124.
- Zheng C, Li M, Inagaki M, Kawada T, Sunagawa K, Sugimachi M. Vagal stimulation markedly suppresses arrhythmias in conscious rats with chronic heart failure after myocardial infarction. Conf Proc IEEE Eng Med Biol Soc 2005; 7:7072–7075.
- Sabbah HN, Stein PD, Kono T, et al. A canine model of chronic heart failure produced by multiple sequential coronary microembolizations. Am J Physiol 1991; 260:H1379–H1384.
- Zhang Y, Popovic ZB, Bibevski S, et al. Chronic vagus nerve stimulation improves autonomic control and attenuates systemic inflammation and heart failure progression in a canine high-rate pacing model. Circ Heart Fail 2009; 2:692–699.
- De Ferrari GM, Crijns HJ, Borggrefe M, et al, on behalf of CardioFit Multicenter Trial Investigators. Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur Heart J 2011; 32:847–855.
- Sabbah HN, Rastogi S, Mishra S, et al. Long-term therapy with neuroselective electric vagus nerve stimulation improves LV function and attenuates global LV remodelling in dogs with chronic heart failure. Eur J Heart Fail Supplements 2005; 4(suppl):166–167. Abstract 744.
- Sabbah HN, Imai M, Zaretsky A, et al. Therapy with vagus nerve electrical stimulation combined with beta-blockade improves left ventricular systolic function in dogs with heart failure beyond that seen with beta-blockade alone. Eur J Heart Fail Supplements 2007; 6(suppl):114. Abstract 509.
- Liu YH, Yang XP, Sharov VG, et al. Effects of angiotensin-converting enzyme inhibitors and angiotensin ii type 1 receptor antagonists in rats with heart failure. Role of kinins and angiotensin ii type 2 receptors. J Clin Invest 1997; 99:1926–1935.
- Wang H, Yu M, Ochani M, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 2003; 421:384–388.
- Sabbah H, Rastogi S, Mishra S, Imai M, Gupta RC. Chronic therapy with neuroselective electric vagus nerve stimulation attenuates mRNA expression of pro-inflammatory cytokines in dogs with heart failure. Eur Heart J Suppl 2005; 26(suppl 1):65.
- Feng Q, Song W, Lu X, et al. Development of heart failure and congenital septal defects in mice lacking endothelial nitric oxide synthase. Circulation 2002; 106:873–879.
- Kelly RA, Balligand JL, Smith TW. Nitric oxide and cardiac function. Circ Res 1996; 79:363–380.
- Mungrue IN, Gros R, You X, et al. Cardiomyocyte overexpression of iNOS in mice results in peroxynitrite generation, heart block, and sudden death. J Clin Invest 2002; 109:735–743.
- Damy T, Ratajczak P, Shah AM, et al. Increased neuronal nitric oxide synthase-derived NO production in the failing human heart. Lancet 2004; 363:1365–1367.
- Bendall JK, Damy T, Ratajczak P, et al. Role of myocardial neuronal nitric oxide synthase-derived nitric oxide in beta-adrenergic hyporesponsiveness after myocardial infarction-induced heart failure in rat. Circulation 2004; 110:2368–2375.
- Gupta RC, Mishra S, Rastogi S, Imai M, Zaca V, Sabbah HN. Chronic therapy with electric vagus nerve stimulation normalizes mRNA and protein expression of nitric oxide synthase in myocardium of dogs with heart failure. Eur Heart J 2006; 27:477. Abstract.
- Severs NJ, Bruce AF, Dupont E, Rothery S. Remodelling of gap junctions and connexin expression in diseased myocardium. Cardiovasc Res 2008; 80:9–19.
- Wang XJ, Gerdes AM. Chronic pressure overload cardiac hypertrophy and failure in guinea pigs: III. Intercalated disc remodeling. J Mol Cell Cardiol 1999; 31:333–343.
- Ai X, Pogwizd M. Connexin 43 downregulation and dephosphorylation in nonischemic heart failure is associated with enhanced colocalized protein phosphatase type 2A. Circ Res 2005; 96:54–63.
- Rastogi S, Mishra S, Ilsar I, Zaretsky A, Sabbah HN. Chronic therapy with electric vagus nerve stimulation normalizes mRNA and protein expression of connexin-40, -43 and -45 in left ventricular myocardium of dogs with heart failure. Circulation 2007; 116:II_218. Abstract 1089.
- Adamson PB, Smith AL, Abraham WT, et al. Continuous autonomic assessment in patients with symptomatic heart failure: prognostic value of heart rate variability measured by an implanted cardiac resynchronization device. Circulation 2004; 110:2389–2394.
- Sabbah HN, Ilsar I, Zaretsky A, Rastogi S, Wang M, Gupta RC. Vagus nerve stimulation in experimental heart failure. Heart Fail Rev 2011; 16:171–178.
- Schwartz PJ, Vanoli E, Stramba-Badiale M, De Ferrari GM, Billman GE, Foreman RD. Autonomic mechanisms and sudden death. New insights from analysis of baroreceptor reflexes in conscious dogs with and without myocardial infarction. Circulation 1988; 78:969–979.
- Mortara A, La Rovere MT, Pinna GD, et al Arterial baroreflex modulation of heart rate in chronic heart failure: clinical and hemodynamic correlates and prognostic implications. Circulation 1997; 96:3450–3458.
- La Rovere MT, Bigger JT, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) investigators. Lancet. 1998; 351:478–484.
- Lechat P, Hulot JS, Escolano S, et al Heart rate and cardiac rhythm relationships with bisoprolol benefit in chronic heart failure in CIBIS II trial. Circulation 2001; 103:1428–1433.
- Sabbah HN, Shimoyama H, Kono T, et al Effects of long-term monotherapy with enalapril, metoprolol and digoxin on the progression of left ventricular dysfunction and dilation in dogs with reduced ejection fraction. Circulation 1994; 89:2852–2859.
- Sabbah HN, Stanley WC, Sharov VG, et al Effects of dopamine β-hydroxylase inhibition with nepicastat on the progression of left ventricular dysfunction and remodeling in dogs with chronic heart failure. Circulation 2000; 102:1990–1995.
- Cheng Y, George I, Yi GH, et al Bradycardic therapy improves left ventricular function and remodeling in dogs with coronary embolization-induced chronic heart failure. J Pharmacol Exp Ther 2007; 321:469–476.
- Swedberg K, Komajda M, Bohm M, et al., on behalf of the SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 2010; 376:875–885.
- Kunze DL. Reflex discharge patterns of cardiac vagal efferent fibres. J Physiol 1972; 222:1–15.
- Harman MA, Reeves TJ. Effects of vagus nerve stimulation on atrial and ventricular function. Am J Physiol 1968; 215:1210–1217.
- Vanoli E, De Ferrari GM, Stramba-Badiale M, Hull SS, Foreman RD, Schwartz PJ. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ Res 1991; 68:1471–1481.
- Li M, Zheng C, Sato T, Kawada T, Sugimachi N, Sunagawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation 2004; 109:120–124.
- Zheng C, Li M, Inagaki M, Kawada T, Sunagawa K, Sugimachi M. Vagal stimulation markedly suppresses arrhythmias in conscious rats with chronic heart failure after myocardial infarction. Conf Proc IEEE Eng Med Biol Soc 2005; 7:7072–7075.
- Sabbah HN, Stein PD, Kono T, et al A canine model of chronic heart failure produced by multiple sequential coronary microembolizations. Am J Physiol 1991; 260:H1379–H1384.
- Zhang Y, Popovic ZB, Bibevski S, et al Chronic vagus nerve stimulation improves autonomic control and attenuates systemic inflammation and heart failure progression in a canine high-rate pacing model. Circ Heart Fail 2009; 2:692–699.
- De Ferrari GM, Crijns HJ, Borggrefe M, et al., on behalf of CardioFit Multicenter Trial Investigators. Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur Heart J 2011; 32:847–855.
- Sabbah HN, Rastogi S, Mishra S, et al Long-term therapy with neuroselective electric vagus nerve stimulation improves LV function and attenuates global LV remodelling in dogs with chronic heart failure. Eur J Heart Fail Supplements 2005; 4( suppl):166–167. Abstract 744.
- Sabbah HN, Imai M, Zaretsky A, et al Therapy with vagus nerve electrical stimulation combined with beta-blockade improves left ventricular systolic function in dogs with heart failure beyond that seen with beta-blockade alone. Eur J Heart Fail Supplements 2007; 6( suppl):114. Abstract 509.
- Liu YH, Yang XP, Sharov VG, et al Effects of angiotensin-converting enzyme inhibitors and angiotensin II type 1 receptor antagonists in rats with heart failure. Role of kinins and angiotensin II type 2 receptors. J Clin Invest 1997; 99:1926–1935.
- Wang H, Yu M, Ochani M, et al Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 2003; 421:384–388.
- Sabbah H, Rastogi S, Mishra S, Imai M, Gupta RC. Chronic therapy with neuroselective electric vagus nerve stimulation attenuates mRNA expression of pro-inflammatory cytokines in dogs with heart failure. Eur Heart J Suppl 2005; 26( suppl 1):65.
- Feng Q, Song W, Lu X, et al Development of heart failure and congenital septal defects in mice lacking endothelial nitric oxide synthase. Circulation 2002; 106:873–879.
- Kelly RA, Balligand JL, Smith TW. Nitric oxide and cardiac function. Circ Res 1996; 79:363–380.
- Mungrue IN, Gros R, You X, et al Cardiomyocyte overexpression of iNOS in mice results in peroxynitrite generation, heart block, and sudden death. J Clin Invest 2002; 109:735–743.
- Damy T, Ratajczak P, Shah AM, et al Increased neuronal nitric oxide synthase-derived NO production in the failing human heart. Lancet 2004; 363:1365–1367.
- Bendall JK, Damy T, Ratajczak P, et al Role of myocardial neuronal nitric oxide synthase-derived nitric oxide in β-adrenergic hyporesponsiveness after myocardial infarction-induced heart failure in rat. Circulation 2004; 110:2368–2375.
- Gupta RC, Mishra S, Rastogi S, Imai M, Zaca V, Sabbah HN. Chronic therapy with electric vagus nerve stimulation normalizes mRNA and protein expression of nitric oxide synthase in myocardium of dogs with heart failure. Eur Heart J 2006; 27:477. Abstract.
- Severs NJ, Bruce AF, Dupont E, Rothery S. Remodelling of gap junctions and connexin expression in diseased myocardium. Cardio vasc Res 2008; 80:9–19.
- Wang XJ, Gerdes AM. Chronic pressure overload cardiac hypertrophy and failure in guinea pigs: III. Intercalated disc remodeling. J Mol Cell Cardiol 1999; 31:333–343.
- Ai X, Pogwizd M. Connexin 43 downregulation and dephosphorylation in nonischemic heart failure is associated with enhanced colocalized protein phosphatase type 2A. Circ Res 2005; 96:54–63.
- Rastogi S, Mishra S, Ilsar I, Zaretsky A, Sabbah HN. Chronic therapy with electric vagus nerve stimulation normalizes mRNA and protein expression of connexin-40, -43 and -45 in left ventricular myocardium of dogs with heart failure. Circulation 2007; 116:II_218. Abstract 1089.
- Adamson PB, Smith AL, Abraham WT, et al Continuous autonomic assessment in patients with symptomatic heart failure: prognostic value of heart rate variability measured by an implanted cardiac resynchronization device. Circulation 2004; 110:2389–2394.
- Sabbah HN, Ilsar I, Zaretsky A, Rastogi S, Wang M, Gupta RC. Vagus nerve stimulation in experimental heart failure. Heart Fail Rev 2011; 16:171–178.