User login
Can history and exam alone reliably predict pneumonia?
- Models based on clinical information do not reliably predict the presence of pneumonia. Testing for elevated C-reactive protein added limited value.
Background Prediction rules based on clinical information have been developed to support the diagnosis of pneumonia and help limit the use of expensive diagnostic tests. However, these prediction rules need to be validated in the primary care setting.
Methods Adults who met our definition of lower respiratory tract infection (LRTI) were recruited for a prospective study on the causes of LRTI, between November 15, 1998 and June 1, 2001 in the Leiden region of the Netherlands. Clinical information was collected and chest radiography was performed. A literature search was also done to find prediction rules for pneumonia.
Results 129 patients—26 with pneumonia and 103 without—were included, and 6 prediction rules were applied. Only the model with the addition of a test for C-reactive protein had a significant area under the curve of 0.69 (95% confidence interval [CI], 0.58–0.80), with a positive predictive value of 47% (95% CI, 23–71) and a negative predictive value of 84% (95% CI, 77–91). The pretest probabilities for the presence and absence of pneumonia were 20% and 80%, respectively.
Conclusions Models based only on clinical information do not reliably predict the presence of pneumonia. The addition of an elevated C-reactive protein level seems of little value.
Few patients with lower respiratory tract infections (LRTIs) are actually diagnosed with pneumonia after a chest X-ray. Studies in general practice show radiographically confirmed pneumonia in 6% to 39% of these patients, depending on inclusion criteria.1-5
Despite the vital role that X-rays play in separating those who have this lung ailment from those who do not, this imaging tool is not a standard of care throughout the world in the diagnosis of pneumonia. For instance, primary care physicians in the Netherlands usually diagnose pneumonia based on medical history and physical examination, despite Dutch guidelines6 that call for X-rays in cases of suspected pneumonia. The reason: patients have to be sent to a hospital for an X-ray. This contrasts sharply to the US, where most family practice settings have radiographic equipment “down the hall.”
Regardless, though, of whether a physician is in the Netherlands or the US, it would certainly be welcome news if physicians could turn to a reliable prediction model that would reduce our reliance on medical imaging that can be costly—and in the case of the Netherlands, involve a trip to the hospital.
The value of prediction rules
Several investigators created prediction rules for pneumonia using information from the clinical history, physical examination, and simple laboratory tests.5,7-12 Although the variables in these prediction rules vary considerably, most include fever, dyspnea, and any abnormality on auscultation (the signs and symptoms of these rules are given in TABLE 1). However, these rules are not used much in primary care, even in Europe. They have proven their value, however, in emergency departments in Europe and the US, where they are used to guide treatment and to predict the prognosis of the disease.13,14
Validation of the prediction rules is necessary to create reliable tools for clinicians to use in the general practice setting. Only one rule10 had already been validated in other populations. In this study, the value of published prediction rules was tested in our group of patients with LRTI in a general practice setting.15
TABLE 1
Signs, symptoms, and values for 6 prediction models
| MODEL | REGRESSION EQUATION AND VARIABLES |
|---|---|
| Singal8 | Y=–3.539 |
| + 0.884 for cough | |
| + 0.681 for fever | |
| + 0.464 for crackles | |
| + 0.030 for 20.16 (pretest probability of pneumonia)* | |
| Heckerling10 | Y=–1.705 |
| + 0.494 for temperature >37.7°C | |
| + 0.428 for pulse >100 beats/min | |
| + 0.658 for rales | |
| + 0.638 for decreased breath sounds | |
| + 0.691 for absence of asthma | |
| Melbye11 | Y=+ 4.7 for fever (reported by patient) with duration of illness of 1 week or more |
| – 4.5 for coryza | |
| – 2.1 for sore throat | |
| + 5.0 for dyspnea | |
| + 8.2 for chest pain, lateral | |
| + 0.9 for crackles | |
| González Ortiz12 | Y=–1.87 |
| 1.3 for pathologic auscultation | |
| + 1.64 for neutrophilia | |
| + 1.70 for pleural pain | |
| + 1.21 for dyspnea | |
| Hopstaken I5 | Y=–2.74 |
| + 1.02 for dry cough | |
| + 1.78 for diarrhea | |
| + 1.13 for temperature ≥38°C | |
| Hopstaken II5 | Y=–4.15 |
| + 0.91 for dry cough | |
| + 1.01 for diarrhea | |
| + 0.64 for temperature ≥38°C | |
| + 2.78 for C-reactive protein ≥20 mg/L | |
| “Fever” means self-reported fever; “temperature” means taken by physician. | |
| *For the pretest probability of pneumonia, the frequency (20.16%) of patients with pneumonia found in our dataset was used. | |
Methods
Recruiting the patients
The study was conducted in the Leiden region of the Netherlands between November 15, 1998 and June 1, 2001 (with a summer break in June, July, and August 2000), with the assistance of 23 primary care practitioners serving a total population of 27,000 people. Patients were recruited as part of a study of the causes of LRTI.15 We included patients who were 18 years of age and older who consulted their primary care physician for signs and symptoms of LRTI and met the following criteria for it:14
- any abnormality on pulmonary auscultation, and
- at least 2 of 3 signs and symptoms: (1) a self-report of fever >38°C, or fever in the past 48 hours, (2) dyspnea or cough (productive or nonproductive), (3) tachypnea, malaise, or confusion.
Patients coming to the Leiden University Medical Center as well as those seen on home visits were included. We excluded patients who were pregnant and patients who had diseases that could have made follow-up difficult—for instance, those with an advanced malignancy.
History and exam. An investigator (primarily AWG) visited the patients at home within 24 hours of diagnosis by their primary care physician. The investigator took a standard history and did a physical examination. Sputum samples, throat swabs, and blood samples were collected for microbiological analysis; blood was also taken for erythrocyte sedimentaion rate (ESR) and C-reactive protein (CRP). An investigator visited the patients again 10 to 14 days later, at which time she took a second blood sample. (The management of the illness remained the primary care physician’s responsibility. Information on patients, microbiological assays, and criteria for microbiological diagnosis are given in detail in an earlier study.15)
Chest radiographs. In accordance with the study protocol, chest radiographs (posteroanterior and lateral) were taken 5 to 7 days after the history and exam were taken, in 1 of 4 nearby hospitals. Local radiologists made the first assessment during routine daily practice. The radiologists were asked to assess the existence of a consolidation on the radiographs. This study’s radiologist (FEJAW), who was aware of the clinical details but not informed about the results of the first assessment, reviewed the radiographs systematically. In case of a discrepancy between the 2 assessments, a third radiologist (HMZ) was asked to judge. The aim was to reach consensus. If previous radiographs were available, they were used for comparison.
The finding of a consolidation was regarded as evidence of pneumonia and served as the reference standard.
Literature search
Prediction models for pneumonia from the literature were identified by searching Medline from 1966 to June 2003, supplemented by checking article references.
The search was limited to studies with adult patients. Prediction models needed to be:
- From original prospective studies into the accuracy or precision of history and exam in a general practice or ambulatory setting, with inclusion criteria comparable with our definition of LRTI
- Developed with the use of multivariate techniques
- Not focused on hospital admission, hospital-acquired pneumonia, pediatric pneumonia, specific pneumonia (eg, tuberculosis), or AIDS-related pneumonia.
The search revealed 5 papers that met our criteria, from which we obtained 6 prediction rules: Singal,8 Heckerling,10 Melbye,11 González Ortiz,12 Hopstaken I,5 and Hopstaken II.5 The signs and symptoms used for diagnosis as well as the regression equations of these rules are given in TABLE 1.
Two further prediction models, though considered in 2 other reviews (Metlay et al16 and Zaat et al17), were not applied. These were models by Diehr,7 whose inclusion criteria (patients with cough) did not fit our definition for LRTI, and Gennis et al,9 which had only a univariate analysis of variables in the prediction of pneumonia.
Statistical analysis
As the patients’ data were collected for a prospective study on causes of LRTI,15 it offered us the opportunity to apply these prediction rules to our data set. We analyzed the data with SPSS version 11.0 for Windows (SPSS, Inc, Chicago, Ill). The 6 models were applied on our data set.
For this purpose, the regression scores corresponding to the different models for each patient were computed. The regression scores were used to calculate receiver operating characteristic (ROC) curves with areas under the curve. Positive and negative predictive values of the models were calculated, with a predicted probability for pneumonia >50% (ie, score on the regression equation of 0) was used as a cutoff point.
Results
Pneumonia rates in our patients
A total of 145 patients with LRTI were included in the study.15 For 137, a chest radiograph was taken. From these radiographs, 129 could be reviewed and 8 were lost after the first assessment. The mean age of the patients was 50 years (standard deviation=14); 86 (53%) patients were female and 63 (49%) had comorbidity, predominantly cardiovascular or pulmonary diseases (6 had both).
Of these 129 patients, 26 were diagnosed (by chest radiograph) to have pneumonia. The mean time between onset of symptoms and chest x-ray was 14 days; the mean time between chest x-ray and inclusion in the study was 9 days. All patients but 1 were treated with antibiotics.
How did the prediction models do?
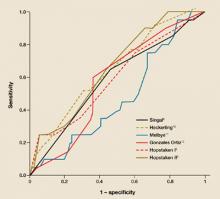
We applied the selected models to our data set; the results are shown in TABLE 2. Hopstaken II,5 which included an elevated CRP (≥20 mg/L), was the only model with a significant area under the curve of ROC (FIGURE).
Looking at the distribution of the scores on the regression equation of Hopstaken II,5 we observed that patients without pneumonia more often had a low score. For example, 29% of the patients without pneumonia had a score below –3, compared with 8% of the patients with pneumonia. Nine percent of the patients without pneumonia and 32% of the patients with pneumonia had a score above 0 (data not shown).
The model Hopstaken II showed a positive predictive value of 47% and negative predictive value of 84% (TABLE 2). The pretest probabilities for the presence and absence of pneumonia were 20% and 80%, respectively.
FIGURE
ROC curves: How did the predictive models do?
Tho ROC curves of the models as applied to the patients in this study. This graph plots the fraction of true positives vs the fraction of false positives (1 – specificity).
TABLE 2
Predictive values of the 6 models
| MODEL | ROC AREA (95% CI ) | ROC AREA (95% CI) AS GIVEN IN ARTICLES | POSITIVE PREDICTIVE VALUE (95% CI ) | NEGATIVE PREDICTIVE VALUE (95% CI ) | |
|---|---|---|---|---|---|
| Singal8 | 0.58 (0.45–0.70) | 0.75 (0.71–0.79) | —* | 80% (73%–87%) | |
| Heckerling10 | 0.63 (0.50–0.75) | 0.82 (0.78–0.86) | 24% (11%–38%) | 85% (77%–93%) | |
| Melbye11 | 0.49 (0.37–0.62)† | Not given | 17% (6%–36%) | 79% (70%–86%) | |
| González Ortiz12 | 0.57 (0.45–0.68) | 0.84 (CI not given) | 23% (15%–31%) | 88% (74%–100%) | |
| Hopstaken I5 | 0.62 (0.50–0.75) | 0.76 (CI not given) | 43% (17%–69%) | 83% (76%–90%) | |
| Hopstaken II5 | 0.69 (0.58–0.80)‡ | 0.80 (CI not given) | 47% (23%–71%) | 84% (77%–91%) | |
| The pretest probability for the presence of pneumonia was 20%; the pretest probability for the absence pneumonia was 80%. ROC, receiver operating characteristic; CI, confidence interval. | |||||
| * No patients had a value above the cutoff point for the regression equation of 0. | |||||
| † The cutoff point was set at 9.7. At this point 20.2% of the patients had pneumonia. | |||||
| ‡ P value <.05. | |||||
Discussion
History and physical exam cannot reliably predict pneumonia
The results of this study show that models only using medical history and physical examination do not reliably predict the presence of pneumonia compared with the gold standard: presence of a consolidation on chest radiograph. The model from the Hopstaken paper5 that used an elevated CRP in addition to the other information did better. However, this model’s predictive value for pneumonia was still limited. Given a population with a pretest probability of 20%, the post-test probability for a positive test result is 47%. The negative predictive value of this model is 84%, given a pretest probability of no pneumonia of 80%.
Addition of CRP improved the positive predictive value from 43% (Hopstaken I) to 47% (Hopstaken II).5 Several investigators18-21 have confirmed the value of CRP measurement in the diagnosis of infectious diseases. In our analyses to find predictive variables for the presence of pneumonia, we found a significant association for elevated CRP levels, as was shown by the results for the model Hopstaken II.5 However, this association is of limited value.
Limitations of this study
Possible bias in setting, inclusion criteria. Our study was conducted in a general practice setting in the Netherlands, as was Hopstaken.5 The studies by Singal,8 Heckering,10 and González Ortiz12 recruited patients from emergency departments in the US and Spain. In these countries, the organization of medical care is different from the Netherlands, and it is possible that the setting of the studies influenced the results. Note that only the predicting rule by Hopstaken, in general practice setting, shows significant results.
As a prerequisite for the inclusion of patients, we applied “abnormality on auscultation,” which had not been the case in other studies. González Ortiz12 used fever >38°C, and Hopstaken5 used cough as a prerequisite for inclusion; Singal8 and Heckering10 only included patients in whom a chest X-ray had been done. This could have introduced some selection bias. Although there were different inclusion criteria, all the patients were suspected of having LRTI. It is unclear how these differences in inclusion criteria may have influenced the results.
Radiography may have missed cases. Chest radiography was used as the standard reference to confirm the diagnosis of pneumonia because of its low cost and general accessibility. However, the reliability of this test is debated. In this study, chest radiographs were reviewed by several radiologists to increase the reliability of the diagnosis. The chest radiographs were taken about 5 to 7 days after inclusion in the study, which was 2 weeks after onset of symptoms on average.
A study by Macfarlane et al22 showed that abnormalities on X-ray generally persist for quite a long time—4 weeks after the diagnosis of pneumonia only 50% of the abnormalities had resolved. However, a study by Mittl et al23 showed complete resolution in 50% of the patients after 2 weeks, with even more rapid clearance in the younger age groups (up to 60% at the age of 20). Interpolation of these findings showed resolution of symptoms after 1 week of 5% to 10% and 5% to 30%, respectively. In our study, a possible 3-week delay between onset of symptoms and chest radiographs could have resulted in resolution of pneumonia in a few patients.
However, our patient population, with a mean age of 50 years, is somewhat older than that in Mittl’s population,23 with its mean age of 40 years. The study by Mittl showed that resolution of pneumonia occurred less rapidly in older patients. Because our population was older Mittl’s population, the resolution of pneumonia could have been less in our population.
Treatment may have influenced results. How quickly antibiotic use affected radiologic findings remains unclear. Nearly all patients in our study were treated with antibiotics, as in the study by Mittl.23 In our study the mean time between onset of the symptoms and inclusion was 9 days; Macfarlane22 and Mittl23 did not show these data, so we could not compare findings.
Acknowledgments
We wish to thank the patients and GPs who participated in the study. We would also like to thank Professor W J J Assendelft (Leiden university Medical Center) for his constructive criticism and H Bolk-Lucieer for reviewing the manuscript. This work was made possible by a grant from the european Commission Framework V and from Pfizer. Ethical approval has been granted by the Medical Ethics Committee of the Leiden University Medical Center.
Correspondence
A W Graffelman, MD, PhD, Department of General Practice and Nursing Home Medicine, LUMC, PO Box 2088, 2301 CB Leiden, The Netherlands a.w.graffelman@lumc.nl
1. Macfarlane J, Holmes W, Gard P, et al. Prospective study of the incidence, etiology and outcome of adult lower respiratory tract illness in the community. Thorax 2001;56:109-114.
2. Macfarlane JT, Colville A, Guion A, Macfarlane RM, Rose DH. Prospective study of etiology and outcome of adult lower-respiratory-tract infections in the community. Lancet 1993;341:511-514.
3. Lieberman D, Lieberman D, Korsonsky I, et al. A comparative study of the etiology of adult upper and lower respiratory tract infections in the community. Diagn Microbiol Infect Dis 2002;42:21-28.
4. Woodhead MA, Macfarlane JT, McCracken JS, Rose DH, Finch RG. Prospective study of etiology and outcome of pneumonia in the community. Lancet 1987;i:671-674.
5. Hopstaken RM, Muris JWM, Knottnerus JA, Kester ADM, Rinkens PELM, Dinant GJ. Contributions of symptoms, signs, erythrocyte sedimentation rate, and C-reactive protein to a diagnosis of pneumonia in acute lower respiratory tract infection. Br J Gen Pract 2003;53:358-364.
6. Verheij TJM, Salomé PL, Bindels PJ, et al. NHG-Standaard acuut hoesten. Huisarts Wet 2003;46:496-506.
7. Diehr P, Wood RW, Bushyhead J, Krueger L, Wolcott B, Tomkins RK. Prediction of pneumonia in outpatients with acute cough: a statistical approach. J Chron Dis 1984;37:215-225.
8. Singal BM, Hedges JR, Radack KL. Decision rules and clinical prediction of pneumonia: evaluation of low-yield criteria. Ann Emerg Med 1989;18:37-44.
9. Gennis P, Gallagher J, Falvo C, Baker S, Than W. Clinical criteria for the detection of pneumonia in adults: guidelines for ordering chest roentgenograms in the emergency department. J Emerg Med 1989;7:263-268.
10. Heckerling PS, Tape TG, Wigton RS, et al. Clinical prediction rule for pulmonary infiltrates. Ann Intern Med 1990;113:664-670.
11. Melbye H, Straume B, Aasebo U, Dale K. Diagnosis of pneumonia in adults in general practice: Relative importance of typical symptoms and abnormal chest signs evaluated against a radiographic reference standard. Scand J Prim Health Care 1992;10:226-233.
12. González Ortiz MA, Carnicero Bujarrabal M, Verela Entrecanales M. Prediction of existence of pneumonia in adults with fever. Med Clin (Barc) 1995;105:521-524.
13. Aujesky D, Auble Te, Yealy DM, et al. Prospective comparison of three validated prediction rules for prognosis in community-acquired pneumonia. Am J Med 2005;118:384-392.
14. Woodhead M, Blasi F, Ewig S, et al. Guidelines for the management of adult lower respiratory tract infections. Eur Respir J 2005;26:1138-1180.
15. Graffelman AW, Knuistingh Neven A, Le Cessie S, Kroes ACM, Springer MP, Van den Broek PJ. Pathogens involved in lower respiratory tract infections in general practice. B J Gen Pract 2004;54:15-19.
16. Metlay JP, Kapoor WN, Fine MJ. Does this patient have community-acquired pneumonia? Diagnosing pneumonia by history and physical examination. JAMA 1997;278:1440-1445.
17. Zaat JOM, Stalman WAB, Assendelft WJJ. Hoort, wie klopt daar? Een systematische literatuurstudie naar de waarde van anamnese en lichamelijk onderzoek bij verdenking op een pneumonie. Huisarts Wet 1998;41:461-469.
18. Melbye H, Straume B, Aasebo U, Brox J. The diagnosis of adult pneumonia in general practice. The diagnostic value of history, physical examination and some blood tests. Scand J Prim Health Care. 1988;6:111-117.
19. Lindback S, Hellgren U, Julander I, Hansson LO. The value of C-reactive protein as a marker of bacterial infections in patients with septicaemia, endocarditis and influenza. Scand J Infect Dis 1989;21:543-549.
20. Hjortdahl P, Landaas S, Urdal P, Steinbakk M, Fuglerud P, Nygaard B. C-reactive protein: a new rapid assay for managing infectious disease in primary health care. Scand J Prim Health Care 1991;9:3-10.
21. Dahler Eriksen BS, Lauritzen T, Lassen JF, Lund ED, Brandslund I. Near patient test for C-reactive protein in general practice: assessment of clinical, organizational and economic outcomes. Clin Chem 1999;45:478-485.
22. Macfarlane JT, Miller AC, Roderick Smith WH, Morris AH, Rose DH. Comparative radiographic features of community acquired Legionnaires’ disease, pneumococcal pneumonia, mycoplasma pneumonia, and psittacosis. Thorax 1984;39:28-33.
23. Mittl RL, Schwab RJ, Duchin JS, Goin JE, Albeida SM, Miller WT. Radiographic resolution of community-acquired pneumonia. Am J Respir Crit Care Med 1994;194:630-635.
- Models based on clinical information do not reliably predict the presence of pneumonia. Testing for elevated C-reactive protein added limited value.
Background Prediction rules based on clinical information have been developed to support the diagnosis of pneumonia and help limit the use of expensive diagnostic tests. However, these prediction rules need to be validated in the primary care setting.
Methods Adults who met our definition of lower respiratory tract infection (LRTI) were recruited for a prospective study on the causes of LRTI, between November 15, 1998 and June 1, 2001 in the Leiden region of the Netherlands. Clinical information was collected and chest radiography was performed. A literature search was also done to find prediction rules for pneumonia.
Results 129 patients—26 with pneumonia and 103 without—were included, and 6 prediction rules were applied. Only the model with the addition of a test for C-reactive protein had a significant area under the curve of 0.69 (95% confidence interval [CI], 0.58–0.80), with a positive predictive value of 47% (95% CI, 23–71) and a negative predictive value of 84% (95% CI, 77–91). The pretest probabilities for the presence and absence of pneumonia were 20% and 80%, respectively.
Conclusions Models based only on clinical information do not reliably predict the presence of pneumonia. The addition of an elevated C-reactive protein level seems of little value.
Few patients with lower respiratory tract infections (LRTIs) are actually diagnosed with pneumonia after a chest X-ray. Studies in general practice show radiographically confirmed pneumonia in 6% to 39% of these patients, depending on inclusion criteria.1-5
Despite the vital role that X-rays play in separating those who have this lung ailment from those who do not, this imaging tool is not a standard of care throughout the world in the diagnosis of pneumonia. For instance, primary care physicians in the Netherlands usually diagnose pneumonia based on medical history and physical examination, despite Dutch guidelines6 that call for X-rays in cases of suspected pneumonia. The reason: patients have to be sent to a hospital for an X-ray. This contrasts sharply to the US, where most family practice settings have radiographic equipment “down the hall.”
Regardless, though, of whether a physician is in the Netherlands or the US, it would certainly be welcome news if physicians could turn to a reliable prediction model that would reduce our reliance on medical imaging that can be costly—and in the case of the Netherlands, involve a trip to the hospital.
The value of prediction rules
Several investigators created prediction rules for pneumonia using information from the clinical history, physical examination, and simple laboratory tests.5,7-12 Although the variables in these prediction rules vary considerably, most include fever, dyspnea, and any abnormality on auscultation (the signs and symptoms of these rules are given in TABLE 1). However, these rules are not used much in primary care, even in Europe. They have proven their value, however, in emergency departments in Europe and the US, where they are used to guide treatment and to predict the prognosis of the disease.13,14
Validation of the prediction rules is necessary to create reliable tools for clinicians to use in the general practice setting. Only one rule10 had already been validated in other populations. In this study, the value of published prediction rules was tested in our group of patients with LRTI in a general practice setting.15
TABLE 1
Signs, symptoms, and values for 6 prediction models
| MODEL | REGRESSION EQUATION AND VARIABLES |
|---|---|
| Singal8 | Y=–3.539 |
| + 0.884 for cough | |
| + 0.681 for fever | |
| + 0.464 for crackles | |
| + 0.030 for 20.16 (pretest probability of pneumonia)* | |
| Heckerling10 | Y=–1.705 |
| + 0.494 for temperature >37.7°C | |
| + 0.428 for pulse >100 beats/min | |
| + 0.658 for rales | |
| + 0.638 for decreased breath sounds | |
| + 0.691 for absence of asthma | |
| Melbye11 | Y=+ 4.7 for fever (reported by patient) with duration of illness of 1 week or more |
| – 4.5 for coryza | |
| – 2.1 for sore throat | |
| + 5.0 for dyspnea | |
| + 8.2 for chest pain, lateral | |
| + 0.9 for crackles | |
| González Ortiz12 | Y=–1.87 |
| 1.3 for pathologic auscultation | |
| + 1.64 for neutrophilia | |
| + 1.70 for pleural pain | |
| + 1.21 for dyspnea | |
| Hopstaken I5 | Y=–2.74 |
| + 1.02 for dry cough | |
| + 1.78 for diarrhea | |
| + 1.13 for temperature ≥38°C | |
| Hopstaken II5 | Y=–4.15 |
| + 0.91 for dry cough | |
| + 1.01 for diarrhea | |
| + 0.64 for temperature ≥38°C | |
| + 2.78 for C-reactive protein ≥20 mg/L | |
| “Fever” means self-reported fever; “temperature” means taken by physician. | |
| *For the pretest probability of pneumonia, the frequency (20.16%) of patients with pneumonia found in our dataset was used. | |
Methods
Recruiting the patients
The study was conducted in the Leiden region of the Netherlands between November 15, 1998 and June 1, 2001 (with a summer break in June, July, and August 2000), with the assistance of 23 primary care practitioners serving a total population of 27,000 people. Patients were recruited as part of a study of the causes of LRTI.15 We included patients who were 18 years of age and older who consulted their primary care physician for signs and symptoms of LRTI and met the following criteria for it:14
- any abnormality on pulmonary auscultation, and
- at least 2 of 3 signs and symptoms: (1) a self-report of fever >38°C, or fever in the past 48 hours, (2) dyspnea or cough (productive or nonproductive), (3) tachypnea, malaise, or confusion.
Patients coming to the Leiden University Medical Center as well as those seen on home visits were included. We excluded patients who were pregnant and patients who had diseases that could have made follow-up difficult—for instance, those with an advanced malignancy.
History and exam. An investigator (primarily AWG) visited the patients at home within 24 hours of diagnosis by their primary care physician. The investigator took a standard history and did a physical examination. Sputum samples, throat swabs, and blood samples were collected for microbiological analysis; blood was also taken for erythrocyte sedimentaion rate (ESR) and C-reactive protein (CRP). An investigator visited the patients again 10 to 14 days later, at which time she took a second blood sample. (The management of the illness remained the primary care physician’s responsibility. Information on patients, microbiological assays, and criteria for microbiological diagnosis are given in detail in an earlier study.15)
Chest radiographs. In accordance with the study protocol, chest radiographs (posteroanterior and lateral) were taken 5 to 7 days after the history and exam were taken, in 1 of 4 nearby hospitals. Local radiologists made the first assessment during routine daily practice. The radiologists were asked to assess the existence of a consolidation on the radiographs. This study’s radiologist (FEJAW), who was aware of the clinical details but not informed about the results of the first assessment, reviewed the radiographs systematically. In case of a discrepancy between the 2 assessments, a third radiologist (HMZ) was asked to judge. The aim was to reach consensus. If previous radiographs were available, they were used for comparison.
The finding of a consolidation was regarded as evidence of pneumonia and served as the reference standard.
Literature search
Prediction models for pneumonia from the literature were identified by searching Medline from 1966 to June 2003, supplemented by checking article references.
The search was limited to studies with adult patients. Prediction models needed to be:
- From original prospective studies into the accuracy or precision of history and exam in a general practice or ambulatory setting, with inclusion criteria comparable with our definition of LRTI
- Developed with the use of multivariate techniques
- Not focused on hospital admission, hospital-acquired pneumonia, pediatric pneumonia, specific pneumonia (eg, tuberculosis), or AIDS-related pneumonia.
The search revealed 5 papers that met our criteria, from which we obtained 6 prediction rules: Singal,8 Heckerling,10 Melbye,11 González Ortiz,12 Hopstaken I,5 and Hopstaken II.5 The signs and symptoms used for diagnosis as well as the regression equations of these rules are given in TABLE 1.
Two further prediction models, though considered in 2 other reviews (Metlay et al16 and Zaat et al17), were not applied. These were models by Diehr,7 whose inclusion criteria (patients with cough) did not fit our definition for LRTI, and Gennis et al,9 which had only a univariate analysis of variables in the prediction of pneumonia.
Statistical analysis
As the patients’ data were collected for a prospective study on causes of LRTI,15 it offered us the opportunity to apply these prediction rules to our data set. We analyzed the data with SPSS version 11.0 for Windows (SPSS, Inc, Chicago, Ill). The 6 models were applied on our data set.
For this purpose, the regression scores corresponding to the different models for each patient were computed. The regression scores were used to calculate receiver operating characteristic (ROC) curves with areas under the curve. Positive and negative predictive values of the models were calculated, with a predicted probability for pneumonia >50% (ie, score on the regression equation of 0) was used as a cutoff point.
Results
Pneumonia rates in our patients
A total of 145 patients with LRTI were included in the study.15 For 137, a chest radiograph was taken. From these radiographs, 129 could be reviewed and 8 were lost after the first assessment. The mean age of the patients was 50 years (standard deviation=14); 86 (53%) patients were female and 63 (49%) had comorbidity, predominantly cardiovascular or pulmonary diseases (6 had both).
Of these 129 patients, 26 were diagnosed (by chest radiograph) to have pneumonia. The mean time between onset of symptoms and chest x-ray was 14 days; the mean time between chest x-ray and inclusion in the study was 9 days. All patients but 1 were treated with antibiotics.
How did the prediction models do?
We applied the selected models to our data set; the results are shown in TABLE 2. Hopstaken II,5 which included an elevated CRP (≥20 mg/L), was the only model with a significant area under the curve of ROC (FIGURE).
Looking at the distribution of the scores on the regression equation of Hopstaken II,5 we observed that patients without pneumonia more often had a low score. For example, 29% of the patients without pneumonia had a score below –3, compared with 8% of the patients with pneumonia. Nine percent of the patients without pneumonia and 32% of the patients with pneumonia had a score above 0 (data not shown).
The model Hopstaken II showed a positive predictive value of 47% and negative predictive value of 84% (TABLE 2). The pretest probabilities for the presence and absence of pneumonia were 20% and 80%, respectively.
FIGURE
ROC curves: How did the predictive models do?
Tho ROC curves of the models as applied to the patients in this study. This graph plots the fraction of true positives vs the fraction of false positives (1 – specificity).
TABLE 2
Predictive values of the 6 models
| MODEL | ROC AREA (95% CI ) | ROC AREA (95% CI) AS GIVEN IN ARTICLES | POSITIVE PREDICTIVE VALUE (95% CI ) | NEGATIVE PREDICTIVE VALUE (95% CI ) | |
|---|---|---|---|---|---|
| Singal8 | 0.58 (0.45–0.70) | 0.75 (0.71–0.79) | —* | 80% (73%–87%) | |
| Heckerling10 | 0.63 (0.50–0.75) | 0.82 (0.78–0.86) | 24% (11%–38%) | 85% (77%–93%) | |
| Melbye11 | 0.49 (0.37–0.62)† | Not given | 17% (6%–36%) | 79% (70%–86%) | |
| González Ortiz12 | 0.57 (0.45–0.68) | 0.84 (CI not given) | 23% (15%–31%) | 88% (74%–100%) | |
| Hopstaken I5 | 0.62 (0.50–0.75) | 0.76 (CI not given) | 43% (17%–69%) | 83% (76%–90%) | |
| Hopstaken II5 | 0.69 (0.58–0.80)‡ | 0.80 (CI not given) | 47% (23%–71%) | 84% (77%–91%) | |
| The pretest probability for the presence of pneumonia was 20%; the pretest probability for the absence pneumonia was 80%. ROC, receiver operating characteristic; CI, confidence interval. | |||||
| * No patients had a value above the cutoff point for the regression equation of 0. | |||||
| † The cutoff point was set at 9.7. At this point 20.2% of the patients had pneumonia. | |||||
| ‡ P value <.05. | |||||
Discussion
History and physical exam cannot reliably predict pneumonia
The results of this study show that models only using medical history and physical examination do not reliably predict the presence of pneumonia compared with the gold standard: presence of a consolidation on chest radiograph. The model from the Hopstaken paper5 that used an elevated CRP in addition to the other information did better. However, this model’s predictive value for pneumonia was still limited. Given a population with a pretest probability of 20%, the post-test probability for a positive test result is 47%. The negative predictive value of this model is 84%, given a pretest probability of no pneumonia of 80%.
Addition of CRP improved the positive predictive value from 43% (Hopstaken I) to 47% (Hopstaken II).5 Several investigators18-21 have confirmed the value of CRP measurement in the diagnosis of infectious diseases. In our analyses to find predictive variables for the presence of pneumonia, we found a significant association for elevated CRP levels, as was shown by the results for the model Hopstaken II.5 However, this association is of limited value.
Limitations of this study
Possible bias in setting, inclusion criteria. Our study was conducted in a general practice setting in the Netherlands, as was Hopstaken.5 The studies by Singal,8 Heckering,10 and González Ortiz12 recruited patients from emergency departments in the US and Spain. In these countries, the organization of medical care is different from the Netherlands, and it is possible that the setting of the studies influenced the results. Note that only the predicting rule by Hopstaken, in general practice setting, shows significant results.
As a prerequisite for the inclusion of patients, we applied “abnormality on auscultation,” which had not been the case in other studies. González Ortiz12 used fever >38°C, and Hopstaken5 used cough as a prerequisite for inclusion; Singal8 and Heckering10 only included patients in whom a chest X-ray had been done. This could have introduced some selection bias. Although there were different inclusion criteria, all the patients were suspected of having LRTI. It is unclear how these differences in inclusion criteria may have influenced the results.
Radiography may have missed cases. Chest radiography was used as the standard reference to confirm the diagnosis of pneumonia because of its low cost and general accessibility. However, the reliability of this test is debated. In this study, chest radiographs were reviewed by several radiologists to increase the reliability of the diagnosis. The chest radiographs were taken about 5 to 7 days after inclusion in the study, which was 2 weeks after onset of symptoms on average.
A study by Macfarlane et al22 showed that abnormalities on X-ray generally persist for quite a long time—4 weeks after the diagnosis of pneumonia only 50% of the abnormalities had resolved. However, a study by Mittl et al23 showed complete resolution in 50% of the patients after 2 weeks, with even more rapid clearance in the younger age groups (up to 60% at the age of 20). Interpolation of these findings showed resolution of symptoms after 1 week of 5% to 10% and 5% to 30%, respectively. In our study, a possible 3-week delay between onset of symptoms and chest radiographs could have resulted in resolution of pneumonia in a few patients.
However, our patient population, with a mean age of 50 years, is somewhat older than that in Mittl’s population,23 with its mean age of 40 years. The study by Mittl showed that resolution of pneumonia occurred less rapidly in older patients. Because our population was older Mittl’s population, the resolution of pneumonia could have been less in our population.
Treatment may have influenced results. How quickly antibiotic use affected radiologic findings remains unclear. Nearly all patients in our study were treated with antibiotics, as in the study by Mittl.23 In our study the mean time between onset of the symptoms and inclusion was 9 days; Macfarlane22 and Mittl23 did not show these data, so we could not compare findings.
Acknowledgments
We wish to thank the patients and GPs who participated in the study. We would also like to thank Professor W J J Assendelft (Leiden university Medical Center) for his constructive criticism and H Bolk-Lucieer for reviewing the manuscript. This work was made possible by a grant from the european Commission Framework V and from Pfizer. Ethical approval has been granted by the Medical Ethics Committee of the Leiden University Medical Center.
Correspondence
A W Graffelman, MD, PhD, Department of General Practice and Nursing Home Medicine, LUMC, PO Box 2088, 2301 CB Leiden, The Netherlands a.w.graffelman@lumc.nl
- Models based on clinical information do not reliably predict the presence of pneumonia. Testing for elevated C-reactive protein added limited value.
Background Prediction rules based on clinical information have been developed to support the diagnosis of pneumonia and help limit the use of expensive diagnostic tests. However, these prediction rules need to be validated in the primary care setting.
Methods Adults who met our definition of lower respiratory tract infection (LRTI) were recruited for a prospective study on the causes of LRTI, between November 15, 1998 and June 1, 2001 in the Leiden region of the Netherlands. Clinical information was collected and chest radiography was performed. A literature search was also done to find prediction rules for pneumonia.
Results 129 patients—26 with pneumonia and 103 without—were included, and 6 prediction rules were applied. Only the model with the addition of a test for C-reactive protein had a significant area under the curve of 0.69 (95% confidence interval [CI], 0.58–0.80), with a positive predictive value of 47% (95% CI, 23–71) and a negative predictive value of 84% (95% CI, 77–91). The pretest probabilities for the presence and absence of pneumonia were 20% and 80%, respectively.
Conclusions Models based only on clinical information do not reliably predict the presence of pneumonia. The addition of an elevated C-reactive protein level seems of little value.
Few patients with lower respiratory tract infections (LRTIs) are actually diagnosed with pneumonia after a chest X-ray. Studies in general practice show radiographically confirmed pneumonia in 6% to 39% of these patients, depending on inclusion criteria.1-5
Despite the vital role that X-rays play in separating those who have this lung ailment from those who do not, this imaging tool is not a standard of care throughout the world in the diagnosis of pneumonia. For instance, primary care physicians in the Netherlands usually diagnose pneumonia based on medical history and physical examination, despite Dutch guidelines6 that call for X-rays in cases of suspected pneumonia. The reason: patients have to be sent to a hospital for an X-ray. This contrasts sharply to the US, where most family practice settings have radiographic equipment “down the hall.”
Regardless, though, of whether a physician is in the Netherlands or the US, it would certainly be welcome news if physicians could turn to a reliable prediction model that would reduce our reliance on medical imaging that can be costly—and in the case of the Netherlands, involve a trip to the hospital.
The value of prediction rules
Several investigators created prediction rules for pneumonia using information from the clinical history, physical examination, and simple laboratory tests.5,7-12 Although the variables in these prediction rules vary considerably, most include fever, dyspnea, and any abnormality on auscultation (the signs and symptoms of these rules are given in TABLE 1). However, these rules are not used much in primary care, even in Europe. They have proven their value, however, in emergency departments in Europe and the US, where they are used to guide treatment and to predict the prognosis of the disease.13,14
Validation of the prediction rules is necessary to create reliable tools for clinicians to use in the general practice setting. Only one rule10 had already been validated in other populations. In this study, the value of published prediction rules was tested in our group of patients with LRTI in a general practice setting.15
TABLE 1
Signs, symptoms, and values for 6 prediction models
| MODEL | REGRESSION EQUATION AND VARIABLES |
|---|---|
| Singal8 | Y=–3.539 |
| + 0.884 for cough | |
| + 0.681 for fever | |
| + 0.464 for crackles | |
| + 0.030 for 20.16 (pretest probability of pneumonia)* | |
| Heckerling10 | Y=–1.705 |
| + 0.494 for temperature >37.7°C | |
| + 0.428 for pulse >100 beats/min | |
| + 0.658 for rales | |
| + 0.638 for decreased breath sounds | |
| + 0.691 for absence of asthma | |
| Melbye11 | Y=+ 4.7 for fever (reported by patient) with duration of illness of 1 week or more |
| – 4.5 for coryza | |
| – 2.1 for sore throat | |
| + 5.0 for dyspnea | |
| + 8.2 for chest pain, lateral | |
| + 0.9 for crackles | |
| González Ortiz12 | Y=–1.87 |
| 1.3 for pathologic auscultation | |
| + 1.64 for neutrophilia | |
| + 1.70 for pleural pain | |
| + 1.21 for dyspnea | |
| Hopstaken I5 | Y=–2.74 |
| + 1.02 for dry cough | |
| + 1.78 for diarrhea | |
| + 1.13 for temperature ≥38°C | |
| Hopstaken II5 | Y=–4.15 |
| + 0.91 for dry cough | |
| + 1.01 for diarrhea | |
| + 0.64 for temperature ≥38°C | |
| + 2.78 for C-reactive protein ≥20 mg/L | |
| “Fever” means self-reported fever; “temperature” means taken by physician. | |
| *For the pretest probability of pneumonia, the frequency (20.16%) of patients with pneumonia found in our dataset was used. | |
Methods
Recruiting the patients
The study was conducted in the Leiden region of the Netherlands between November 15, 1998 and June 1, 2001 (with a summer break in June, July, and August 2000), with the assistance of 23 primary care practitioners serving a total population of 27,000 people. Patients were recruited as part of a study of the causes of LRTI.15 We included patients who were 18 years of age and older who consulted their primary care physician for signs and symptoms of LRTI and met the following criteria for it:14
- any abnormality on pulmonary auscultation, and
- at least 2 of 3 signs and symptoms: (1) a self-report of fever >38°C, or fever in the past 48 hours, (2) dyspnea or cough (productive or nonproductive), (3) tachypnea, malaise, or confusion.
Patients coming to the Leiden University Medical Center as well as those seen on home visits were included. We excluded patients who were pregnant and patients who had diseases that could have made follow-up difficult—for instance, those with an advanced malignancy.
History and exam. An investigator (primarily AWG) visited the patients at home within 24 hours of diagnosis by their primary care physician. The investigator took a standard history and did a physical examination. Sputum samples, throat swabs, and blood samples were collected for microbiological analysis; blood was also taken for erythrocyte sedimentaion rate (ESR) and C-reactive protein (CRP). An investigator visited the patients again 10 to 14 days later, at which time she took a second blood sample. (The management of the illness remained the primary care physician’s responsibility. Information on patients, microbiological assays, and criteria for microbiological diagnosis are given in detail in an earlier study.15)
Chest radiographs. In accordance with the study protocol, chest radiographs (posteroanterior and lateral) were taken 5 to 7 days after the history and exam were taken, in 1 of 4 nearby hospitals. Local radiologists made the first assessment during routine daily practice. The radiologists were asked to assess the existence of a consolidation on the radiographs. This study’s radiologist (FEJAW), who was aware of the clinical details but not informed about the results of the first assessment, reviewed the radiographs systematically. In case of a discrepancy between the 2 assessments, a third radiologist (HMZ) was asked to judge. The aim was to reach consensus. If previous radiographs were available, they were used for comparison.
The finding of a consolidation was regarded as evidence of pneumonia and served as the reference standard.
Literature search
Prediction models for pneumonia from the literature were identified by searching Medline from 1966 to June 2003, supplemented by checking article references.
The search was limited to studies with adult patients. Prediction models needed to be:
- From original prospective studies into the accuracy or precision of history and exam in a general practice or ambulatory setting, with inclusion criteria comparable with our definition of LRTI
- Developed with the use of multivariate techniques
- Not focused on hospital admission, hospital-acquired pneumonia, pediatric pneumonia, specific pneumonia (eg, tuberculosis), or AIDS-related pneumonia.
The search revealed 5 papers that met our criteria, from which we obtained 6 prediction rules: Singal,8 Heckerling,10 Melbye,11 González Ortiz,12 Hopstaken I,5 and Hopstaken II.5 The signs and symptoms used for diagnosis as well as the regression equations of these rules are given in TABLE 1.
Two further prediction models, though considered in 2 other reviews (Metlay et al16 and Zaat et al17), were not applied. These were models by Diehr,7 whose inclusion criteria (patients with cough) did not fit our definition for LRTI, and Gennis et al,9 which had only a univariate analysis of variables in the prediction of pneumonia.
Statistical analysis
As the patients’ data were collected for a prospective study on causes of LRTI,15 it offered us the opportunity to apply these prediction rules to our data set. We analyzed the data with SPSS version 11.0 for Windows (SPSS, Inc, Chicago, Ill). The 6 models were applied on our data set.
For this purpose, the regression scores corresponding to the different models for each patient were computed. The regression scores were used to calculate receiver operating characteristic (ROC) curves with areas under the curve. Positive and negative predictive values of the models were calculated, with a predicted probability for pneumonia >50% (ie, score on the regression equation of 0) was used as a cutoff point.
Results
Pneumonia rates in our patients
A total of 145 patients with LRTI were included in the study.15 For 137, a chest radiograph was taken. From these radiographs, 129 could be reviewed and 8 were lost after the first assessment. The mean age of the patients was 50 years (standard deviation=14); 86 (53%) patients were female and 63 (49%) had comorbidity, predominantly cardiovascular or pulmonary diseases (6 had both).
Of these 129 patients, 26 were diagnosed (by chest radiograph) to have pneumonia. The mean time between onset of symptoms and chest x-ray was 14 days; the mean time between chest x-ray and inclusion in the study was 9 days. All patients but 1 were treated with antibiotics.
How did the prediction models do?
We applied the selected models to our data set; the results are shown in TABLE 2. Hopstaken II,5 which included an elevated CRP (≥20 mg/L), was the only model with a significant area under the curve of ROC (FIGURE).
Looking at the distribution of the scores on the regression equation of Hopstaken II,5 we observed that patients without pneumonia more often had a low score. For example, 29% of the patients without pneumonia had a score below –3, compared with 8% of the patients with pneumonia. Nine percent of the patients without pneumonia and 32% of the patients with pneumonia had a score above 0 (data not shown).
The model Hopstaken II showed a positive predictive value of 47% and negative predictive value of 84% (TABLE 2). The pretest probabilities for the presence and absence of pneumonia were 20% and 80%, respectively.
FIGURE
ROC curves: How did the predictive models do?
Tho ROC curves of the models as applied to the patients in this study. This graph plots the fraction of true positives vs the fraction of false positives (1 – specificity).
TABLE 2
Predictive values of the 6 models
| MODEL | ROC AREA (95% CI ) | ROC AREA (95% CI) AS GIVEN IN ARTICLES | POSITIVE PREDICTIVE VALUE (95% CI ) | NEGATIVE PREDICTIVE VALUE (95% CI ) | |
|---|---|---|---|---|---|
| Singal8 | 0.58 (0.45–0.70) | 0.75 (0.71–0.79) | —* | 80% (73%–87%) | |
| Heckerling10 | 0.63 (0.50–0.75) | 0.82 (0.78–0.86) | 24% (11%–38%) | 85% (77%–93%) | |
| Melbye11 | 0.49 (0.37–0.62)† | Not given | 17% (6%–36%) | 79% (70%–86%) | |
| González Ortiz12 | 0.57 (0.45–0.68) | 0.84 (CI not given) | 23% (15%–31%) | 88% (74%–100%) | |
| Hopstaken I5 | 0.62 (0.50–0.75) | 0.76 (CI not given) | 43% (17%–69%) | 83% (76%–90%) | |
| Hopstaken II5 | 0.69 (0.58–0.80)‡ | 0.80 (CI not given) | 47% (23%–71%) | 84% (77%–91%) | |
| The pretest probability for the presence of pneumonia was 20%; the pretest probability for the absence pneumonia was 80%. ROC, receiver operating characteristic; CI, confidence interval. | |||||
| * No patients had a value above the cutoff point for the regression equation of 0. | |||||
| † The cutoff point was set at 9.7. At this point 20.2% of the patients had pneumonia. | |||||
| ‡ P value <.05. | |||||
Discussion
History and physical exam cannot reliably predict pneumonia
The results of this study show that models only using medical history and physical examination do not reliably predict the presence of pneumonia compared with the gold standard: presence of a consolidation on chest radiograph. The model from the Hopstaken paper5 that used an elevated CRP in addition to the other information did better. However, this model’s predictive value for pneumonia was still limited. Given a population with a pretest probability of 20%, the post-test probability for a positive test result is 47%. The negative predictive value of this model is 84%, given a pretest probability of no pneumonia of 80%.
Addition of CRP improved the positive predictive value from 43% (Hopstaken I) to 47% (Hopstaken II).5 Several investigators18-21 have confirmed the value of CRP measurement in the diagnosis of infectious diseases. In our analyses to find predictive variables for the presence of pneumonia, we found a significant association for elevated CRP levels, as was shown by the results for the model Hopstaken II.5 However, this association is of limited value.
Limitations of this study
Possible bias in setting, inclusion criteria. Our study was conducted in a general practice setting in the Netherlands, as was Hopstaken.5 The studies by Singal,8 Heckering,10 and González Ortiz12 recruited patients from emergency departments in the US and Spain. In these countries, the organization of medical care is different from the Netherlands, and it is possible that the setting of the studies influenced the results. Note that only the predicting rule by Hopstaken, in general practice setting, shows significant results.
As a prerequisite for the inclusion of patients, we applied “abnormality on auscultation,” which had not been the case in other studies. González Ortiz12 used fever >38°C, and Hopstaken5 used cough as a prerequisite for inclusion; Singal8 and Heckering10 only included patients in whom a chest X-ray had been done. This could have introduced some selection bias. Although there were different inclusion criteria, all the patients were suspected of having LRTI. It is unclear how these differences in inclusion criteria may have influenced the results.
Radiography may have missed cases. Chest radiography was used as the standard reference to confirm the diagnosis of pneumonia because of its low cost and general accessibility. However, the reliability of this test is debated. In this study, chest radiographs were reviewed by several radiologists to increase the reliability of the diagnosis. The chest radiographs were taken about 5 to 7 days after inclusion in the study, which was 2 weeks after onset of symptoms on average.
A study by Macfarlane et al22 showed that abnormalities on X-ray generally persist for quite a long time—4 weeks after the diagnosis of pneumonia only 50% of the abnormalities had resolved. However, a study by Mittl et al23 showed complete resolution in 50% of the patients after 2 weeks, with even more rapid clearance in the younger age groups (up to 60% at the age of 20). Interpolation of these findings showed resolution of symptoms after 1 week of 5% to 10% and 5% to 30%, respectively. In our study, a possible 3-week delay between onset of symptoms and chest radiographs could have resulted in resolution of pneumonia in a few patients.
However, our patient population, with a mean age of 50 years, is somewhat older than that in Mittl’s population,23 with its mean age of 40 years. The study by Mittl showed that resolution of pneumonia occurred less rapidly in older patients. Because our population was older Mittl’s population, the resolution of pneumonia could have been less in our population.
Treatment may have influenced results. How quickly antibiotic use affected radiologic findings remains unclear. Nearly all patients in our study were treated with antibiotics, as in the study by Mittl.23 In our study the mean time between onset of the symptoms and inclusion was 9 days; Macfarlane22 and Mittl23 did not show these data, so we could not compare findings.
Acknowledgments
We wish to thank the patients and GPs who participated in the study. We would also like to thank Professor W J J Assendelft (Leiden university Medical Center) for his constructive criticism and H Bolk-Lucieer for reviewing the manuscript. This work was made possible by a grant from the european Commission Framework V and from Pfizer. Ethical approval has been granted by the Medical Ethics Committee of the Leiden University Medical Center.
Correspondence
A W Graffelman, MD, PhD, Department of General Practice and Nursing Home Medicine, LUMC, PO Box 2088, 2301 CB Leiden, The Netherlands a.w.graffelman@lumc.nl
1. Macfarlane J, Holmes W, Gard P, et al. Prospective study of the incidence, etiology and outcome of adult lower respiratory tract illness in the community. Thorax 2001;56:109-114.
2. Macfarlane JT, Colville A, Guion A, Macfarlane RM, Rose DH. Prospective study of etiology and outcome of adult lower-respiratory-tract infections in the community. Lancet 1993;341:511-514.
3. Lieberman D, Lieberman D, Korsonsky I, et al. A comparative study of the etiology of adult upper and lower respiratory tract infections in the community. Diagn Microbiol Infect Dis 2002;42:21-28.
4. Woodhead MA, Macfarlane JT, McCracken JS, Rose DH, Finch RG. Prospective study of etiology and outcome of pneumonia in the community. Lancet 1987;i:671-674.
5. Hopstaken RM, Muris JWM, Knottnerus JA, Kester ADM, Rinkens PELM, Dinant GJ. Contributions of symptoms, signs, erythrocyte sedimentation rate, and C-reactive protein to a diagnosis of pneumonia in acute lower respiratory tract infection. Br J Gen Pract 2003;53:358-364.
6. Verheij TJM, Salomé PL, Bindels PJ, et al. NHG-Standaard acuut hoesten. Huisarts Wet 2003;46:496-506.
7. Diehr P, Wood RW, Bushyhead J, Krueger L, Wolcott B, Tomkins RK. Prediction of pneumonia in outpatients with acute cough: a statistical approach. J Chron Dis 1984;37:215-225.
8. Singal BM, Hedges JR, Radack KL. Decision rules and clinical prediction of pneumonia: evaluation of low-yield criteria. Ann Emerg Med 1989;18:37-44.
9. Gennis P, Gallagher J, Falvo C, Baker S, Than W. Clinical criteria for the detection of pneumonia in adults: guidelines for ordering chest roentgenograms in the emergency department. J Emerg Med 1989;7:263-268.
10. Heckerling PS, Tape TG, Wigton RS, et al. Clinical prediction rule for pulmonary infiltrates. Ann Intern Med 1990;113:664-670.
11. Melbye H, Straume B, Aasebo U, Dale K. Diagnosis of pneumonia in adults in general practice: Relative importance of typical symptoms and abnormal chest signs evaluated against a radiographic reference standard. Scand J Prim Health Care 1992;10:226-233.
12. González Ortiz MA, Carnicero Bujarrabal M, Verela Entrecanales M. Prediction of existence of pneumonia in adults with fever. Med Clin (Barc) 1995;105:521-524.
13. Aujesky D, Auble Te, Yealy DM, et al. Prospective comparison of three validated prediction rules for prognosis in community-acquired pneumonia. Am J Med 2005;118:384-392.
14. Woodhead M, Blasi F, Ewig S, et al. Guidelines for the management of adult lower respiratory tract infections. Eur Respir J 2005;26:1138-1180.
15. Graffelman AW, Knuistingh Neven A, Le Cessie S, Kroes ACM, Springer MP, Van den Broek PJ. Pathogens involved in lower respiratory tract infections in general practice. B J Gen Pract 2004;54:15-19.
16. Metlay JP, Kapoor WN, Fine MJ. Does this patient have community-acquired pneumonia? Diagnosing pneumonia by history and physical examination. JAMA 1997;278:1440-1445.
17. Zaat JOM, Stalman WAB, Assendelft WJJ. Hoort, wie klopt daar? Een systematische literatuurstudie naar de waarde van anamnese en lichamelijk onderzoek bij verdenking op een pneumonie. Huisarts Wet 1998;41:461-469.
18. Melbye H, Straume B, Aasebo U, Brox J. The diagnosis of adult pneumonia in general practice. The diagnostic value of history, physical examination and some blood tests. Scand J Prim Health Care. 1988;6:111-117.
19. Lindback S, Hellgren U, Julander I, Hansson LO. The value of C-reactive protein as a marker of bacterial infections in patients with septicaemia, endocarditis and influenza. Scand J Infect Dis 1989;21:543-549.
20. Hjortdahl P, Landaas S, Urdal P, Steinbakk M, Fuglerud P, Nygaard B. C-reactive protein: a new rapid assay for managing infectious disease in primary health care. Scand J Prim Health Care 1991;9:3-10.
21. Dahler Eriksen BS, Lauritzen T, Lassen JF, Lund ED, Brandslund I. Near patient test for C-reactive protein in general practice: assessment of clinical, organizational and economic outcomes. Clin Chem 1999;45:478-485.
22. Macfarlane JT, Miller AC, Roderick Smith WH, Morris AH, Rose DH. Comparative radiographic features of community acquired Legionnaires’ disease, pneumococcal pneumonia, mycoplasma pneumonia, and psittacosis. Thorax 1984;39:28-33.
23. Mittl RL, Schwab RJ, Duchin JS, Goin JE, Albeida SM, Miller WT. Radiographic resolution of community-acquired pneumonia. Am J Respir Crit Care Med 1994;194:630-635.
1. Macfarlane J, Holmes W, Gard P, et al. Prospective study of the incidence, etiology and outcome of adult lower respiratory tract illness in the community. Thorax 2001;56:109-114.
2. Macfarlane JT, Colville A, Guion A, Macfarlane RM, Rose DH. Prospective study of etiology and outcome of adult lower-respiratory-tract infections in the community. Lancet 1993;341:511-514.
3. Lieberman D, Lieberman D, Korsonsky I, et al. A comparative study of the etiology of adult upper and lower respiratory tract infections in the community. Diagn Microbiol Infect Dis 2002;42:21-28.
4. Woodhead MA, Macfarlane JT, McCracken JS, Rose DH, Finch RG. Prospective study of etiology and outcome of pneumonia in the community. Lancet 1987;i:671-674.
5. Hopstaken RM, Muris JWM, Knottnerus JA, Kester ADM, Rinkens PELM, Dinant GJ. Contributions of symptoms, signs, erythrocyte sedimentation rate, and C-reactive protein to a diagnosis of pneumonia in acute lower respiratory tract infection. Br J Gen Pract 2003;53:358-364.
6. Verheij TJM, Salomé PL, Bindels PJ, et al. NHG-Standaard acuut hoesten. Huisarts Wet 2003;46:496-506.
7. Diehr P, Wood RW, Bushyhead J, Krueger L, Wolcott B, Tomkins RK. Prediction of pneumonia in outpatients with acute cough: a statistical approach. J Chron Dis 1984;37:215-225.
8. Singal BM, Hedges JR, Radack KL. Decision rules and clinical prediction of pneumonia: evaluation of low-yield criteria. Ann Emerg Med 1989;18:37-44.
9. Gennis P, Gallagher J, Falvo C, Baker S, Than W. Clinical criteria for the detection of pneumonia in adults: guidelines for ordering chest roentgenograms in the emergency department. J Emerg Med 1989;7:263-268.
10. Heckerling PS, Tape TG, Wigton RS, et al. Clinical prediction rule for pulmonary infiltrates. Ann Intern Med 1990;113:664-670.
11. Melbye H, Straume B, Aasebo U, Dale K. Diagnosis of pneumonia in adults in general practice: Relative importance of typical symptoms and abnormal chest signs evaluated against a radiographic reference standard. Scand J Prim Health Care 1992;10:226-233.
12. González Ortiz MA, Carnicero Bujarrabal M, Verela Entrecanales M. Prediction of existence of pneumonia in adults with fever. Med Clin (Barc) 1995;105:521-524.
13. Aujesky D, Auble Te, Yealy DM, et al. Prospective comparison of three validated prediction rules for prognosis in community-acquired pneumonia. Am J Med 2005;118:384-392.
14. Woodhead M, Blasi F, Ewig S, et al. Guidelines for the management of adult lower respiratory tract infections. Eur Respir J 2005;26:1138-1180.
15. Graffelman AW, Knuistingh Neven A, Le Cessie S, Kroes ACM, Springer MP, Van den Broek PJ. Pathogens involved in lower respiratory tract infections in general practice. B J Gen Pract 2004;54:15-19.
16. Metlay JP, Kapoor WN, Fine MJ. Does this patient have community-acquired pneumonia? Diagnosing pneumonia by history and physical examination. JAMA 1997;278:1440-1445.
17. Zaat JOM, Stalman WAB, Assendelft WJJ. Hoort, wie klopt daar? Een systematische literatuurstudie naar de waarde van anamnese en lichamelijk onderzoek bij verdenking op een pneumonie. Huisarts Wet 1998;41:461-469.
18. Melbye H, Straume B, Aasebo U, Brox J. The diagnosis of adult pneumonia in general practice. The diagnostic value of history, physical examination and some blood tests. Scand J Prim Health Care. 1988;6:111-117.
19. Lindback S, Hellgren U, Julander I, Hansson LO. The value of C-reactive protein as a marker of bacterial infections in patients with septicaemia, endocarditis and influenza. Scand J Infect Dis 1989;21:543-549.
20. Hjortdahl P, Landaas S, Urdal P, Steinbakk M, Fuglerud P, Nygaard B. C-reactive protein: a new rapid assay for managing infectious disease in primary health care. Scand J Prim Health Care 1991;9:3-10.
21. Dahler Eriksen BS, Lauritzen T, Lassen JF, Lund ED, Brandslund I. Near patient test for C-reactive protein in general practice: assessment of clinical, organizational and economic outcomes. Clin Chem 1999;45:478-485.
22. Macfarlane JT, Miller AC, Roderick Smith WH, Morris AH, Rose DH. Comparative radiographic features of community acquired Legionnaires’ disease, pneumococcal pneumonia, mycoplasma pneumonia, and psittacosis. Thorax 1984;39:28-33.
23. Mittl RL, Schwab RJ, Duchin JS, Goin JE, Albeida SM, Miller WT. Radiographic resolution of community-acquired pneumonia. Am J Respir Crit Care Med 1994;194:630-635.