User login
Does surgery for carpal tunnel syndrome improve outcomes?
Good evidence supports the use of surgery for carpal tunnel syndrome over nonsurgical therapies such as wrist splints, nonsteroidal anti-inflammatory drugs (NSAIDs), physical therapy, occupational therapy, local steroid injections, work modification, and oral vitamin B6 (Grade of Recommendation: A, based on extrapolation from a systematic review of 1 randomized controlled trial [RCT], 1 additional recent RCT, and 2 cohort studies). Surgery is likely worth the extra costs when conservative therapy (up to 3 months) fails to improve symptoms and return of function, because delayed surgery is as successful as surgery performed shortly after diagnosis. Closed endoscopic release and open release surgery are equally effective therapies for controlling symptoms (Grade of Recommendation: C, based on extrapolation from a systematic review of RCTs). However, whether endoscopic release results in more rapid regain of function and return to work is unclear.
See the Patient Information at the end of this article.
Evidence summary
A recent Cochrane review based on only 1 RCT of 22 patients published in 1964 concluded that surgical treatment of carpal tunnel syndrome appears to be more effective than wrist splinting.1 A well-designed RCT of 176 patients published since that Cochrane review stated that with regard to overall improvement of symptoms and function status, surgical treatment of carpal tunnel syndrome was more effective than wrist splinting 18 months posttreatment.2 The investigators found that surgery resulted in worse short-term outcomes at 1 month follow-up (29% vs 42% success), but by 3 months the improvement in all outcomes was greater in the surgery group (80% vs 54% success). The number needed to treat (NNT) over 18 months was only 2 patients in the treatment-received (per protocol) analysis (92% vs 37% success) and 7 in the intention-to-treat analysis (90% vs 75% success). Patients in the conservative treatment group who underwent surgery after splinting had failed had a higher success rate after 18 months follow-up than patients who did not have surgery (94% vs 62% success rate; NNT = 3).
One cohort study of 90 patients concluded that with respect to symptom control and return to function, open release surgery was as effective as local steroid injection at 1 month follow-up.3 However, at 4 to 6 months after the operation, surgery patients were found to have significantly improved symptom and function scores, with continued improvement compared with patients who received the steroid injection. One other cohort study of 429 patients found that surgery (open or closed endoscopic) was more effective with respect to symptom relief and functional status than various nonsurgical therapies (NSAIDs, splints, physical or occupational therapy, local steroid injections, work modification, or vitamin B6) at 30 months follow-up.4 In both cohort studies, the patients’ pretreatment symptom and functioning scores were worse in the surgery group than in the nonsurgical group. The investigators in the first study3 did not report controlling for these scores. In the second study,4 the authors controlled for functional status scores, but not for symptom severity.
One recent systematic review of 14 RCTs comparing types of surgical therapies for carpal tunnel syndrome concluded that none of the alternative surgical procedures, including closed endoscopic release, appeared to give better symptom relief than open release; and that the evidence is conflicting as to whether endoscopic release results in earlier return to work or improved level of function.5
Recommendations from others
The American Society of Plastic and Reconstructive Surgeons recommends surgical release in the following situations6: (1) failed or incomplete conservative therapy; (2) motor weakness or thenar atrophy; (3) lumbrical pattern symptoms (occur when the metacarpophalangeal joints are held at 90 degrees, eg, driving, letter writing, holding a magazine, pinching, using a small tool); (4) severe pattern on electrical studies (not defined); (5) space-occupying lesions requiring excision; (6) acute carpal tunnel syndrome with symptoms lasting longer than 6 to 8 hours; and (7) progressive or severe symptoms lasting longer than 12 months. The Society did not recommend one surgical procedure over another.
Maureen O’Reilly Brown, MD, MPH
Swedish Family Medicine Residency Program Seattle, Washington
In my practice, many patients have carpal tunnel syndrome and we regularly struggle with the question of whether and when to suggest surgical consultation. This review will make that struggle easier. With at least 33% of cases responding to splinting alone, an initial trial of conservative treatment seems appropriate for most patients. However, early surgical referral when a conservative approach has failed can now be easily justified, given the 90% or better success rate with surgery. The authors also include guidelines from the American Society of Plastic and Reconstructive Surgeons, which may be helpful in selecting which patients should go directly to surgical release.
Patient Information
What is carpal tunnel syndrome?
Carpal tunnel syndrome is felt as pain, tingling, a burning sensation, or loss of sensation that occurs throughout all or part of the hand. These symptoms may be worse at night and can wake you from sleep. You may feel the pain in just the hand, or it may travel up the arm.
How it’s diagnosed
Carpal tunnel syndrome can be challenging to diagnose.
Your doctor will ask you to describe your symptoms and may ask you to perform specific motions with your hand or wrist to see how they affect your symptoms.
Your doctor may arrange for a nerve conduction study—a test to determine how well the nerves in your hand are working. The test can detect if the pressure on the nerve is enough to affect how well it works.
How it’s treated
Your doctor may ask you to wear wrist splints at night or during work, and may advise you to reduce those activities that make the problem worse. Steroid injections into the carpal tunnel may also help. If such conservative treatment does not help, your doctor may talk to you about a simple surgical procedure to relieve pressure on the nerve. The surgeon cuts the ligament over the carpal tunnel, which releases the pressure on the nerve. This surgery works well to relieve the symptoms of carpal tunnel syndrome.
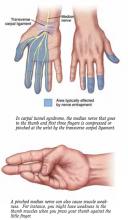
The carpal “tunnel” is the space in which nerves, tendons, and blood vessels pass through the bones of the wrist. Anything that narrows the tunnel, such as swelling of tendons, can compress the nerve and cause carpal tunnel syndrome.
1. Verdugo RJ, Salinas RS, Castillo J, Cea JG. Surgical versus non-surgical treatment for carpal tunnel syndrome. Cochrane Database Syst Rev 2002;(2):CD001552.-
2. Gerritsen AA, de Vet HC, Scholten RJ, Bertelsmann FW, de Krom MC, Bouter LM. Splinting vs surgery in the treatment of carpal tunnel syndrome: a randomized controlled trial. JAMA 2002;288:1245-51.
3. Demirci S, Kutluhan S, Koyuncuoglu HR, et al. Comparison of open carpal tunnel release and local steroid treatment outcomes in idiopathic carpal tunnel syndrome. Rheumatol Int 2002;22:33-7.
4. Katz JN, Keller RB, Simmons BP, et al. Maine carpal tunnel study: outcomes of operative and nonoperative therapy for carpal tunnel syndrome in a community-based cohort. J Hand Surg [Am] 1998;23:697-710.
5. Gerritsen AA, Uitdehaag BM, van Geldere D, Scholten RJ, de Vet HC, Bouter LM. Systematic review of randomized clinical trials of surgical treatment for carpal tunnel syndrome. Br J Surg 2001;88:1285-95.
6. American Society of Plastic and Reconstructive Surgeons. Carpal Tunnel Syndrome (Guidelines). Arlington Heights, IL: American Society of Plastic and Reconstructive Surgeons; 1998.
Good evidence supports the use of surgery for carpal tunnel syndrome over nonsurgical therapies such as wrist splints, nonsteroidal anti-inflammatory drugs (NSAIDs), physical therapy, occupational therapy, local steroid injections, work modification, and oral vitamin B6 (Grade of Recommendation: A, based on extrapolation from a systematic review of 1 randomized controlled trial [RCT], 1 additional recent RCT, and 2 cohort studies). Surgery is likely worth the extra costs when conservative therapy (up to 3 months) fails to improve symptoms and return of function, because delayed surgery is as successful as surgery performed shortly after diagnosis. Closed endoscopic release and open release surgery are equally effective therapies for controlling symptoms (Grade of Recommendation: C, based on extrapolation from a systematic review of RCTs). However, whether endoscopic release results in more rapid regain of function and return to work is unclear.
See the Patient Information at the end of this article.
Evidence summary
A recent Cochrane review based on only 1 RCT of 22 patients published in 1964 concluded that surgical treatment of carpal tunnel syndrome appears to be more effective than wrist splinting.1 A well-designed RCT of 176 patients published since that Cochrane review stated that with regard to overall improvement of symptoms and function status, surgical treatment of carpal tunnel syndrome was more effective than wrist splinting 18 months posttreatment.2 The investigators found that surgery resulted in worse short-term outcomes at 1 month follow-up (29% vs 42% success), but by 3 months the improvement in all outcomes was greater in the surgery group (80% vs 54% success). The number needed to treat (NNT) over 18 months was only 2 patients in the treatment-received (per protocol) analysis (92% vs 37% success) and 7 in the intention-to-treat analysis (90% vs 75% success). Patients in the conservative treatment group who underwent surgery after splinting had failed had a higher success rate after 18 months follow-up than patients who did not have surgery (94% vs 62% success rate; NNT = 3).
One cohort study of 90 patients concluded that with respect to symptom control and return to function, open release surgery was as effective as local steroid injection at 1 month follow-up.3 However, at 4 to 6 months after the operation, surgery patients were found to have significantly improved symptom and function scores, with continued improvement compared with patients who received the steroid injection. One other cohort study of 429 patients found that surgery (open or closed endoscopic) was more effective with respect to symptom relief and functional status than various nonsurgical therapies (NSAIDs, splints, physical or occupational therapy, local steroid injections, work modification, or vitamin B6) at 30 months follow-up.4 In both cohort studies, the patients’ pretreatment symptom and functioning scores were worse in the surgery group than in the nonsurgical group. The investigators in the first study3 did not report controlling for these scores. In the second study,4 the authors controlled for functional status scores, but not for symptom severity.
One recent systematic review of 14 RCTs comparing types of surgical therapies for carpal tunnel syndrome concluded that none of the alternative surgical procedures, including closed endoscopic release, appeared to give better symptom relief than open release; and that the evidence is conflicting as to whether endoscopic release results in earlier return to work or improved level of function.5
Recommendations from others
The American Society of Plastic and Reconstructive Surgeons recommends surgical release in the following situations6: (1) failed or incomplete conservative therapy; (2) motor weakness or thenar atrophy; (3) lumbrical pattern symptoms (occur when the metacarpophalangeal joints are held at 90 degrees, eg, driving, letter writing, holding a magazine, pinching, using a small tool); (4) severe pattern on electrical studies (not defined); (5) space-occupying lesions requiring excision; (6) acute carpal tunnel syndrome with symptoms lasting longer than 6 to 8 hours; and (7) progressive or severe symptoms lasting longer than 12 months. The Society did not recommend one surgical procedure over another.
Maureen O’Reilly Brown, MD, MPH
Swedish Family Medicine Residency Program Seattle, Washington
In my practice, many patients have carpal tunnel syndrome and we regularly struggle with the question of whether and when to suggest surgical consultation. This review will make that struggle easier. With at least 33% of cases responding to splinting alone, an initial trial of conservative treatment seems appropriate for most patients. However, early surgical referral when a conservative approach has failed can now be easily justified, given the 90% or better success rate with surgery. The authors also include guidelines from the American Society of Plastic and Reconstructive Surgeons, which may be helpful in selecting which patients should go directly to surgical release.
Patient Information
What is carpal tunnel syndrome?
Carpal tunnel syndrome is felt as pain, tingling, a burning sensation, or loss of sensation that occurs throughout all or part of the hand. These symptoms may be worse at night and can wake you from sleep. You may feel the pain in just the hand, or it may travel up the arm.
How it’s diagnosed
Carpal tunnel syndrome can be challenging to diagnose.
Your doctor will ask you to describe your symptoms and may ask you to perform specific motions with your hand or wrist to see how they affect your symptoms.
Your doctor may arrange for a nerve conduction study—a test to determine how well the nerves in your hand are working. The test can detect if the pressure on the nerve is enough to affect how well it works.
How it’s treated
Your doctor may ask you to wear wrist splints at night or during work, and may advise you to reduce those activities that make the problem worse. Steroid injections into the carpal tunnel may also help. If such conservative treatment does not help, your doctor may talk to you about a simple surgical procedure to relieve pressure on the nerve. The surgeon cuts the ligament over the carpal tunnel, which releases the pressure on the nerve. This surgery works well to relieve the symptoms of carpal tunnel syndrome.
The carpal “tunnel” is the space in which nerves, tendons, and blood vessels pass through the bones of the wrist. Anything that narrows the tunnel, such as swelling of tendons, can compress the nerve and cause carpal tunnel syndrome.
Good evidence supports the use of surgery for carpal tunnel syndrome over nonsurgical therapies such as wrist splints, nonsteroidal anti-inflammatory drugs (NSAIDs), physical therapy, occupational therapy, local steroid injections, work modification, and oral vitamin B6 (Grade of Recommendation: A, based on extrapolation from a systematic review of 1 randomized controlled trial [RCT], 1 additional recent RCT, and 2 cohort studies). Surgery is likely worth the extra costs when conservative therapy (up to 3 months) fails to improve symptoms and return of function, because delayed surgery is as successful as surgery performed shortly after diagnosis. Closed endoscopic release and open release surgery are equally effective therapies for controlling symptoms (Grade of Recommendation: C, based on extrapolation from a systematic review of RCTs). However, whether endoscopic release results in more rapid regain of function and return to work is unclear.
See the Patient Information at the end of this article.
Evidence summary
A recent Cochrane review based on only 1 RCT of 22 patients published in 1964 concluded that surgical treatment of carpal tunnel syndrome appears to be more effective than wrist splinting.1 A well-designed RCT of 176 patients published since that Cochrane review stated that with regard to overall improvement of symptoms and function status, surgical treatment of carpal tunnel syndrome was more effective than wrist splinting 18 months posttreatment.2 The investigators found that surgery resulted in worse short-term outcomes at 1 month follow-up (29% vs 42% success), but by 3 months the improvement in all outcomes was greater in the surgery group (80% vs 54% success). The number needed to treat (NNT) over 18 months was only 2 patients in the treatment-received (per protocol) analysis (92% vs 37% success) and 7 in the intention-to-treat analysis (90% vs 75% success). Patients in the conservative treatment group who underwent surgery after splinting had failed had a higher success rate after 18 months follow-up than patients who did not have surgery (94% vs 62% success rate; NNT = 3).
One cohort study of 90 patients concluded that with respect to symptom control and return to function, open release surgery was as effective as local steroid injection at 1 month follow-up.3 However, at 4 to 6 months after the operation, surgery patients were found to have significantly improved symptom and function scores, with continued improvement compared with patients who received the steroid injection. One other cohort study of 429 patients found that surgery (open or closed endoscopic) was more effective with respect to symptom relief and functional status than various nonsurgical therapies (NSAIDs, splints, physical or occupational therapy, local steroid injections, work modification, or vitamin B6) at 30 months follow-up.4 In both cohort studies, the patients’ pretreatment symptom and functioning scores were worse in the surgery group than in the nonsurgical group. The investigators in the first study3 did not report controlling for these scores. In the second study,4 the authors controlled for functional status scores, but not for symptom severity.
One recent systematic review of 14 RCTs comparing types of surgical therapies for carpal tunnel syndrome concluded that none of the alternative surgical procedures, including closed endoscopic release, appeared to give better symptom relief than open release; and that the evidence is conflicting as to whether endoscopic release results in earlier return to work or improved level of function.5
Recommendations from others
The American Society of Plastic and Reconstructive Surgeons recommends surgical release in the following situations6: (1) failed or incomplete conservative therapy; (2) motor weakness or thenar atrophy; (3) lumbrical pattern symptoms (occur when the metacarpophalangeal joints are held at 90 degrees, eg, driving, letter writing, holding a magazine, pinching, using a small tool); (4) severe pattern on electrical studies (not defined); (5) space-occupying lesions requiring excision; (6) acute carpal tunnel syndrome with symptoms lasting longer than 6 to 8 hours; and (7) progressive or severe symptoms lasting longer than 12 months. The Society did not recommend one surgical procedure over another.
Maureen O’Reilly Brown, MD, MPH
Swedish Family Medicine Residency Program Seattle, Washington
In my practice, many patients have carpal tunnel syndrome and we regularly struggle with the question of whether and when to suggest surgical consultation. This review will make that struggle easier. With at least 33% of cases responding to splinting alone, an initial trial of conservative treatment seems appropriate for most patients. However, early surgical referral when a conservative approach has failed can now be easily justified, given the 90% or better success rate with surgery. The authors also include guidelines from the American Society of Plastic and Reconstructive Surgeons, which may be helpful in selecting which patients should go directly to surgical release.
Patient Information
What is carpal tunnel syndrome?
Carpal tunnel syndrome is felt as pain, tingling, a burning sensation, or loss of sensation that occurs throughout all or part of the hand. These symptoms may be worse at night and can wake you from sleep. You may feel the pain in just the hand, or it may travel up the arm.
How it’s diagnosed
Carpal tunnel syndrome can be challenging to diagnose.
Your doctor will ask you to describe your symptoms and may ask you to perform specific motions with your hand or wrist to see how they affect your symptoms.
Your doctor may arrange for a nerve conduction study—a test to determine how well the nerves in your hand are working. The test can detect if the pressure on the nerve is enough to affect how well it works.
How it’s treated
Your doctor may ask you to wear wrist splints at night or during work, and may advise you to reduce those activities that make the problem worse. Steroid injections into the carpal tunnel may also help. If such conservative treatment does not help, your doctor may talk to you about a simple surgical procedure to relieve pressure on the nerve. The surgeon cuts the ligament over the carpal tunnel, which releases the pressure on the nerve. This surgery works well to relieve the symptoms of carpal tunnel syndrome.
The carpal “tunnel” is the space in which nerves, tendons, and blood vessels pass through the bones of the wrist. Anything that narrows the tunnel, such as swelling of tendons, can compress the nerve and cause carpal tunnel syndrome.
1. Verdugo RJ, Salinas RS, Castillo J, Cea JG. Surgical versus non-surgical treatment for carpal tunnel syndrome. Cochrane Database Syst Rev 2002;(2):CD001552.-
2. Gerritsen AA, de Vet HC, Scholten RJ, Bertelsmann FW, de Krom MC, Bouter LM. Splinting vs surgery in the treatment of carpal tunnel syndrome: a randomized controlled trial. JAMA 2002;288:1245-51.
3. Demirci S, Kutluhan S, Koyuncuoglu HR, et al. Comparison of open carpal tunnel release and local steroid treatment outcomes in idiopathic carpal tunnel syndrome. Rheumatol Int 2002;22:33-7.
4. Katz JN, Keller RB, Simmons BP, et al. Maine carpal tunnel study: outcomes of operative and nonoperative therapy for carpal tunnel syndrome in a community-based cohort. J Hand Surg [Am] 1998;23:697-710.
5. Gerritsen AA, Uitdehaag BM, van Geldere D, Scholten RJ, de Vet HC, Bouter LM. Systematic review of randomized clinical trials of surgical treatment for carpal tunnel syndrome. Br J Surg 2001;88:1285-95.
6. American Society of Plastic and Reconstructive Surgeons. Carpal Tunnel Syndrome (Guidelines). Arlington Heights, IL: American Society of Plastic and Reconstructive Surgeons; 1998.
1. Verdugo RJ, Salinas RS, Castillo J, Cea JG. Surgical versus non-surgical treatment for carpal tunnel syndrome. Cochrane Database Syst Rev 2002;(2):CD001552.-
2. Gerritsen AA, de Vet HC, Scholten RJ, Bertelsmann FW, de Krom MC, Bouter LM. Splinting vs surgery in the treatment of carpal tunnel syndrome: a randomized controlled trial. JAMA 2002;288:1245-51.
3. Demirci S, Kutluhan S, Koyuncuoglu HR, et al. Comparison of open carpal tunnel release and local steroid treatment outcomes in idiopathic carpal tunnel syndrome. Rheumatol Int 2002;22:33-7.
4. Katz JN, Keller RB, Simmons BP, et al. Maine carpal tunnel study: outcomes of operative and nonoperative therapy for carpal tunnel syndrome in a community-based cohort. J Hand Surg [Am] 1998;23:697-710.
5. Gerritsen AA, Uitdehaag BM, van Geldere D, Scholten RJ, de Vet HC, Bouter LM. Systematic review of randomized clinical trials of surgical treatment for carpal tunnel syndrome. Br J Surg 2001;88:1285-95.
6. American Society of Plastic and Reconstructive Surgeons. Carpal Tunnel Syndrome (Guidelines). Arlington Heights, IL: American Society of Plastic and Reconstructive Surgeons; 1998.
Evidence-based answers from the Family Physicians Inquiries Network
What influences family physicians’ cancer screening decisions when practice guidelines are unclear or conflicting?
OBJECTIVES: To determine: a) the respondents’ perceptions of 4 unclear or conflicting cancer screening guidelines: prostate specific antigen (PSA) for men over age 50, mammography for women ages 40-49, colorectal screening by fecal occult blood testing (FOBT), and colonoscopy for patients over age 40; and b) the influence of various patient and physician factors on the decision to order these tests.
STUDY DESIGN: National Canadian mail survey of randomly selected family physicians.
POPULATION: Family physicians in active practice (n=565) selected from rural and urban family medicine sites in 5 provinces representing the main regions in Canada: British Columbia, Alberta, Ontario, Quebec, Nova Scotia.
OUTCOMES MEASURED: Agreement with guideline statements, and decision to order screening test in 6 clinical vignettes.
RESULTS: Of 565 surveys mailed, 351 (62.1%) were returned. Most respondents agreed with the Canadian Task Force recommendations, and the majority believed that various guidelines for 3 of the 4 screens were conflicting (PSA 86.6%; mammography 67.5%; FOBT 62.4%). Patient anxiety about cancer, patient expectations of being tested, and a positive family history of cancer significantly increased the odds that the 4 tests would be ordered. A good quality patient-MD relationship significantly decreased the odds of ordering a mammogram. Screening decisions were also significantly influenced by the respondents’ beliefs about whether screening was recommended and whether screening could cause more harm than good. A physician’s sensitivity to his or her colleagues’ practice influenced screening decisions regarding PSA and mammography.
CONCLUSIONS: These results suggest a conceptual framework for understanding the determinants of screening behavior when guidelines are unclear or conflicting.
Four factors are significant determinants, independently, of a physician’s decision to order a screening test when recommendations are unclear or conflicting: a patient’s anxiety about having cancer; a patient’s expectation to undergo screening; a family history of cancer; and (in most cases) the quality of the patient-physician relationship. Particularly in the context of breast cancer screening, when a patient and physician have a good relationship, they are more likely than when the relationship is poor to discuss the pros and cons of a conflicting screening guideline and reach a mutually agreeable decision.
In instances of conflicting recommendations, the importance physicians attribute to the practice of colleagues influences their screening decisions.
Although most studies of the determinants of physicians’ cancer screening behavior have dealt with facilitators or barriers to the adoption of guidelines with clear recommendations, virtually no studies have examined factors affecting physician practice when guidelines are unclear or conflicting. When guidelines are unclear, many physicians are left with little direction. By performing cancer screening procedures that are not clearly effective,1 physicians are diverting limited resources to areas where there is uncertain or no benefit to patients.
We studied physician decision-making in cancer screening when guidelines are “unclear” or conflicting. We defined an unclear guideline as a C recommendation (insufficient evidence to recommend the maneuver or not) from the Canadian Task Force on the Periodic Health Examination.2,3 We defined a “conflicting” guideline as one for which there were different recommendations from at least 2 different organizations for the same cancer screening maneuver.
The authors conducted a qualitative study of 10 focus groups across Canada, and identified factors that influence family physicians’ cancer-screening decisions when guidelines are unclear or conflicting.4 The findings supported a conceptual model with 8 factors: 1) patient factors (patient anxiety, expectations, and family history); 2) physician factors (perception of guidelines, clinical practice experience, influence of family physician and specialist colleagues, and time/financial costs; 3) the patient-physician relationship (quality of rapport). Four of these 8 factors were considered the most influential: patient anxiety about having cancer, patient expectations to have a screening test, family history of cancer, and the quality of the patient-physician relationship.
Although we know of many factors that determine cancer-screening decisions, it is not known how much each of these factors contributes to physicians’ decisions to perform tests in specific situations. The aim of this study was to verify these determining factors and to quantify the strength of the influence of each one on cancer screening decisions.
Methods
We conducted a national survey of family physicians in Canada because they are the main preventive care providers in Canada and because a physician’s recommendation is the strongest predictor of an individual’s decision to have a screening test.5
The self-administered questionnaire was mailed to a random sample of 600 family physicians, 120 from each of 5 provincial licensing bodies from 5 regions in Canada: British Columbia, Alberta, Ontario, Quebec, and Nova Scotia. We stratified by postal codes to ensure equal representation of urban and rural physicians (oversampled) to permit subgroup analysis. Ethical approval was obtained from all participating institutions. We followed a modified 4-step Dillman6 method, using initial full mailing, follow up reminder postcards, second full mailing, and phone call reminders.
The questionnaire was composed of 2 parts. Part 1 contained 40 single-item questions on physicians’ perceptions of guideline recommendations for cancers of interest, and the perceived influence of various factors on their decision to order screening tests (all factors identified in the literature and in our previous study). The questionnaire also contained items on practice characteristics, demographics, and respondents’ personal experience with cancer or cancer screening tests.
Part 2 contained 6 clinical case vignettes; 2 for prostate specific antigen (PSA), 2 for mammography, and 2 for fecal occult blood testing (FOBT) and colonoscopy, for which recommendations can be unclear according to Canadian guidelines, or conflicting.2 As for screening for prostate cancer with PSA for men over age 50, there is fair evidence for when not to screen, but conflicting recommendations from at least 2 major organizations. Mammography for breast cancer screening in women age 40 to 49 has conflicting recommendations (different recommendations from at least 2 different organizations). FOBT and colonoscopy for colorectal cancer detection over age 40 are unclear C recommendations (insufficient evidence to either recommend or not).
The Clinical Case Vignettes
Clinical case vignettes have been shown to be a useful, inexpensive, and effective method for eliciting physicians’ decision-making behavior in a simulated situation.7 Case vignettes have been used to examine physicians’ practice behavior with cancer patients.8-11For research purposes, the usefulness of the clinical case vignette rests on the ability to vary specific factors (relevant independent variables under study) from one vignette to another, while keeping constant the surrounding factors of the case presented (the frame).
For each clinical case vignette, the dependent variable was the physician’s decision to order the screening test presented or not. The independent variables were the 4 most influential factors identified in the prior qualitative study,4 embedded within the description of each clinical case vignette. Each independent variable had 2 levels: presence or absence of patient anxiety, patient expectations for testing, and family history of cancer, and easy or difficult relationship. This enabled 16 different versions of each clinical case vignette frame, and 2 frames were developed for each cancer screening. The clinical case vignettes were developed and tested in 4 steps. First, 6 investigators (R.G., F.T., C.H., A.K., M.O., J.B.B.) generated case vignettes from their own clinical experience that reflected specified levels of the factors. Second, 12 family physician colleagues empirically validated the descriptions in the case vignettes. A minimum of 9 of these physicians had to correctly identify the intended level of each of the factors in question. Third, factors not attracting 75% agreement were corrected or replaced. Fourth, the modified clinical case vignettes were submitted to another group of 12 family physicians for their perceptions of the intended levels of the relevant factors. The final versions of the vignettes reflected concordance between the perceived and the intended levels in the factors for each case vignette. Figure 1.
Our design was “fractional” in the sense that we sampled from only a fraction of all possible combinations of independent variables. We had estimated that each family physician could respond to no more than 6 case vignettes (2 per cancer screening). The reduced set represented the vignettes that were clinically realistic. As a result, each physician received set of 6 vignettes offering a clinically meaningful spread of possible levels of the independent variables to maximize the opportunity to detect practice behavior variation. The clinical case vignettes were presented in random order to avoid sequence bias. This design ensured that each physician had 1 vignette with all independent factors absent, 1 with all factors present, and the remaining 4 with a diversity of the possible combinations of levels of patient factors.
Analyses
The analysis of the binary response for each test (order versus not order screening test) included the factors listed in the theoretical framework, with an additional random effect to take into account possible correlations among responses to 2 vignettes from the same physician. The estimation of each model’s parameters was performed using the Generalized Estimating Equation approach of SAS; this variant of logistic regression accounts for the non-independence of observations. We examined first the effect of each of the 4 principal factors-individually and together-on the decision to order a screening test. Then we looked for additional significant effects of physician demographics and perceptions of guidelines. We looked for interactions between the quality of the relationship with other factors. For each screening test, we developed a final parsimonious model which included all factors that were statistically significant at P = 0.05.
Results
Of the original 600 physicians, there were 351 respondents, 214 non-respondents, and 35 ineligibles (16 were not in full-time practice, defined as < 15 hours a week; 8 were not practicing; 6 were in another specialty; 4 had moved out of the jurisdiction; and 1 had died). The final response rate was 62.1% (351/565). The respondents’ demographic characteristics Table 1 reflected the Canadian family physician population, except that there were more certificants of the College of Family Physicians of Canada (akin to Board certification in the US) among the respondents.
By [the fractional factorial] design, the versions of the vignettes with all patient factors present or all absent were the most frequent versions of the vignettes, and the frequency of the remaining versions were uniformly distributed for each vignette. There was no evidence of a systematic under-representation of any versions as a result of non-response.
Perceptions of Guidelines
The respondents’ perceptions of the guideline recommendations for the 4 cancer screening tests are shown in Table 2. Although the respondents’ perceptions of guidelines agreed with the Canadian Task Force guidelines for PSA and mammography, they diverged for colorectal cancer screening. For example, 83.5% of respondents thought colonoscopy was not recommended for patients over 40. A majority of respondents believed that the guidelines for PSA, mammography, and FOBT were conflicting.
The Influence of the Four Principal Factors
Individually, the 4 principal factors were significant determinants of the physician’s decision to order the screening test when the evidence was unclear or conflicting Table 3. The patient’s anxiety about having cancer, their expectations of having a screening test, the quality of the patient-physician-relationship (in most cases), and a positive family history of the relevant cancer all increased the odds of screening. When all 4 factors were analyzed as a combined group adjusting for the presence of other factors Table 3, the principal factors that remained significant determinants of the physician’s decision to order the screening were as follows: anxiety for PSA and mammography; patient expectations for PSA, mammography, and FOBT; a high quality patient-physician relationship for mammography (reduced the likelihood of ordering); and positive family history for all but mammography.
The Combined Influence of the Principal Factors and Physician Factors Physician variables were added to the initial logistic regression models to derive a final parsimonious model for each screening test. Table 4 shows that for each of the screening maneuvers, there were differences not only in the factors that significantly influenced the decision to screen, but also in the magnitude of influence as manifested by the odds ratios. The direction of the influence was similar across examples: all the factors increased the odds of screening except perception that the test is not recommended or does more harm than good, and a good patient-doctor relationship (in the mammography example). PSA and mammography had a similar pattern: patient anxiety, expectations, family history, the physician’s perception of the level of recommendation of the test, whether it creates more harm than good, and the influence of colleagues all significantly influenced the decision to screen. For FOBT, patient expectations, the level of perceived recommendations and the perception of harm were significant. For colonoscopy, patient anxiety, family history, and the perception of the level of recommendation were significant determinants.
Discussion
The results of this study add to the findings from the focus groups and suggest a conceptual framework or model for understanding the determinants of screening behaviour in unclear and conflicting recommendation situations. Although this model offers a more complex picture of the determinants of cancer screening in these instances, there is a great deal of consistency. Patient anxiety, patient expectations, family history of cancer, physicians’ perceptions of the relevant guideline, and physicians’ perceptions of the benefit or harm in screening were all important determinants of screening decisions. One of the important differences in the 2 studies is the relative strength of the influence of family history in this survey study, in particular for mammography and colonoscopy.
Family physicians are trained to heed patient anxiety, but it has only been described as an indirect determinant of cancer screening.12 Patient expectations has been described in the literature in a number of studies as an important determinant of screening.1,13 In addition, other patient-specific factors have been shown to be associated with physician adoption of guidelines, such as patient concerns about finances, quality of life, and location of care.14 Recent research has found an increase in physicians’ wish for more patient involvement in the development of clinical guidelines, and they have suggested that practice guidelines should reflect patient preferences.15
In the final model, the quality of the patient-physician relationship was related to one cancer screening maneuver: mammography for women aged 40-49. It is interesting that a good relationship halved the odds of screening tests being ordered when accounting for other patient factors. The importance of the influence of the patient-physician relationship on screening has been described in previous studies.12,16 In a good patient-physician relationship, patient and physician are more likely to discuss the pros and cons of a conflicting screening guideline and to find common ground than when the relationship is poor.16
The patient-physician relationship did not appear to be an important determinant in the prostate and colorectal screening examples. For PSA screening it may be due to the unique character of the relationship male patients have with their physician. A recent study found that male patients experience many barriers to seeking help, and they find it difficult to discuss their health concerns and preventive care issues with their physicians.17 For colorectal screening by colonoscopy the relationship may not have been an important determinant because 2 other determinants appeared to be so important and may overshadow any others: the great majority of respondents believed that it was not recommended (83.5%); and family history played a very important role in influencing screening.
In the final statistical modeling, several additional physician factors appeared to influence screening decisions. In particular, both the perception of whether the screening test was recommended and the belief that the screening test could cause more harm than good contributed independently to the screening decision. The same factors were noted in our qualitative study, a finding supported by many examples in the literature.1,18,19 In addition, the importance that physicians attribute to the practice of colleagues appeared to influence screening decisions in the 2 conflicting examples (from a Canadian perspective)-PSA screening and mammography. This suggests an important role of colleagues in conflicting examples. Previous research has suggested that social influences play an important role-in particular, when uncertainty is high, or when the evidence is still evolving and recommendations based on the evidence are not in common practice.20
Our emerging model Figure 2 shows that there are more than just cognitive processes at work in this sort of decision-making. The findings suggest that aspects of the patient-physician relationship and the influence of colleagues affect decision-making as well. Further, our findings indicate that these determinants are important when the guidelines are unclear or conflicting.
Many of the factors identified in this study have been described previously.1,13,21, 34 There are also recent theories to help explain how and why physicians decide to screen their patients for cancer, including whether they agree with and adhere to recommended guidelines.24,35However, these theories were developed within the context of clinical decision making when the guidelines are clear. The unique contribution of our study and emerging model is that it concerns screening decisions with unclear or conflicting guidelines. The impact of uncertainty on this aspect of physician decision-making is important. Physicians need to make decisions in the face of uncertainty. They appear to do this by believing one side of the argument or another, by balancing the perceived good or harm from screening, and by looking for support from colleagues to bolster their decision. In addition, their patients play a key role in influencing these decisions, with the doctor and patient finding common ground, often resulting in a shared decision.
Limitations
We represented the clinical factors with dichotomous situations, when, in real encounters, there would be a much greater range in the level of intensity of factors such as patient anxiety, expression of expectation, and quality of the relationship. Also, even though the case vignettes provided some background, for the physician respondent it was a “one of” situation which does not reflect a typical primary care situation that includes continuing care of patients who have a variety of coexisting clinical issues. The magnitude of the influence of these factors may be considerably underestimated or overestimated with the use of clinical case vignettes.
The generalizability of the respondents may be a limitation, as they were younger (1.7 years, not significant) and more likely to be certificants than the non-respondents. The latter difference may have contributed to a trend that stressed the influence of patient anxiety and wishes, which reflects residency training issues in family medicine. Last, although the study was done in Canada, we believe the findings likely apply to US family physicians, as graduate training is quite similar in the two countries.
Conclusions
This study underlies the importance of the cognitive component in decision making-in particular, of perceptions of guidelines, and of the influence of patients and their needs and the patient-physician relationship.
Our results verify our model in general terms, but also build on and advance the conceptual model that evolved from our qualitative findings. It provides a useful framework for understanding clinical decision-making in the face of uncertainty or controversy, and may be applicable to other clinical domains.
In future research we plan to test the effect of race and cultural aspects of the patient and of the physician on physicians’ screening decisions. Ultimately, the model could be used to design interventions to assist with the implementation of preventive services guidelines, and to be included in future CME programs for practicing physicians.
Acknowledgements
Funding/support: This study was funded by the Medical Research Council of Canada and the Prince Edward Island Cancer Research Council.
1. Zyzanski SJ, Stange KC, Kelly R, Flocke S, Shank JC, Chao J, Jaen CR, Smith CK. Family physicians’ disagreements with the US Preventive Services Task Force recommendations. J Fam Pract 1994;39:140-147.
2. Canadian Task Force on the Periodic Health Examination. The Canadian Guide to Clinical Preventive Health Care. Health Canada, Ottawa, 1994.
3. U.S. Preventive Services Task Force. Guide to Clinical Preventive Services. Report of the U.S. Preventive Services Task Force. Williams & Wilkins, Baltimore, 1996.
4. Tudiver F, Brown BB, Medved W, Herbert C, Ritvo P, Guibert R, Haggerty J, Goel V, Smith P, O’Beirne M, Katz A, Moliner P, Ciampi A, Williams JI. Making decisions about cancer screening when the guidelines are unclear or conflicting. J Fam Pract 2001;50:682-687.
5. White E, Urban N, Taylor V. Mammography utilization, public health impact, and cost effectiveness in the United States. Ann Rev Pub Health 1993;14:605-633.
6. Dillman DA. Mail and Telephone Surveys: the Total Design Method. New York, John Wiley and Sons, 1978.
7. Peabody JW, Luck J, Glassman P, Dresselhaus TR, Lee M. Comparison of vignettes, standardized patients, and chart abstraction: a prospective validation study of 3 methods for measuring quality. J Am Med Assoc 2000;283:1715-1722.
8. Deber RB, Thompson GG. Who still prefers aggressive surgery for breast cancer? Implications for the clinical applications of clinical trials. Arch Int Med 1987;147:1543-7.
9. Sawka CA, O’Connor AM, Llewellyn Thomas HA, To T, Pinfold SP, Harrison Woermke D. Appropriateness of adjuvant systemic therapy for axillary node negative breast cancer: a physician opinion survey. J Clin Oncol 1995;13:1459-69.
10. Tannock IF, Belanger D. Use of a physician directed questionnaire to define a consensus about management of breast cancer: implications for assessing costs and benefits of treatment. J National Cancer Inst Monographs 1992;11:137-42.
11. Sutherland HJ, Lockwood GA, Minkin S, Tritchler DL, Till JE, Llewellyn Thomas HA. Measuring satisfaction with health care: a comparison of single with paired rating strategies. Soc Sci Med 1989;28:53-58.
12. Jones I, Morrell D. General practitioners’ background knowledge of their patients. Fam Pract 1995;12:49-53.
13. Langley GR, Tritchler DL, Llewellyn-Thomas HA, Till JE. Use of written cases to study factors associated with regional variations in referral rates. J Clin Epidem 1991;44:391-402.
14. Shekelle PG, Kravitz RL, Beart J, Marger M, Wang M, Lee M. Are nonspecific practice guidelines potentially harmful A randomized comparison of the effect of nonspecific versus specific guidelines on physician decision making. Health Serv Res 2000;34:1429-1448.
15. Grol R, Dalhuijsen J, Thomas S, Veld C, Rutten G, Mokkink H. Attributes of clinical guidelines that influence use of guidelines in general practice: observational study. BMJ 1998;317:858-861.
16. McWilliam CL, Brown JB, Stewart M. Breast cancer patients’ experiences of patient-doctor communication: a working relationship. Patient Educ Couns 2000;39:191-204.
17. Tudiver F, Talbot Y. Why don’t men go to physicians? Family physicians’ perspectives on help seeking behavior of men. J Fam Pract 1999;48:47-52.
18. Brownson RC, Davis JR, Simms SG, Kern TG, Harmon RG. Cancer control knowledge and priorities among primary care physicians. J Cancer Educ 1993;8:35-41.
19. Battista RN, Williams JI, MacFarlane LA. Determinants of preventive practices in fee for-service primary care. Am J Prev Med 1990;6:6-11.
20. Mittman BS, Tonesk X, Jacobson PD. Implementing clinical practice CPGs: social influence strategies and practitioner behavior change. QRB 1992;Dec:413-422.
21. Battista RN, Williams JI, MacFarlane LA. Determinants of primary medical practice in adult cancer prevention. Med Care 1986;24:216-226.
22. Burack RC. Barriers to clinical preventive medicine. Prim Care 1989;16:245-250.
23. Frame PS. Breast Cancer Screening in Older Women: the family practice perspective. J Gerontol 1992;47, Spec.:No. 131-3.
24. Pathman DE, Konrad TR, Freed GL, Freeman VA, Koch GG. The awareness-to-adherence model of the steps to clinical guideline compliance. Med Care 1996;34:873-889.
25. Stange KC, Kelly R, Chao J, Zyzanski SJ, Shank JC, Jaen CR, Melnikow J, Flocke S. Physician agreement with US Preventive Services Task Force recommendations. J Fam Pract 1992;34:409-416.
26. Brownson RC, Davis JR, Simms SG, Kern TG, Harmon RG. Cancer control knowledge and priorities among primary care physicians. J Cancer Educ 1993;8:35-41.
27. Costanza M.E, Stoddard A.M, Zapks J.G, Gaw V.P, Barth R. Physician compliance with mammography guidelines: Barriers and enhancers. J Am Board Fam Pract 1992;5:143-52.
28. Weingarten S, Stone E, Hayward R, Tunis S, Pelter M, Huang H, Kristopaitis R. The adoption of preventive care practice guidelines by primary care physicians. J Gen Int Med 1990;10:138-144.
29. Young MJ, Fried LS, Eisenberg J, Hershey J, Williams S. Do cardiologists have higher thresholds for recommending coronary arteriography than family physicians? Health Serv Res 1987;22:623-635.
30. Smith HE, Herbert CP. Preventive practice among primary care physicians in British Columbia: relation to recommendations of the Canadian Task Force on the Periodic Health Examination. Canad Med Assoc J 1993;149:1795-800.
31. Triezenberg D.J, Smith M.A, Holmes T.M. Cancer screening and detection in family practice: A MIRNET study. J Fam Pract 1995;40:27-33.
32. Summerton N. Positive and negative factors in defensive medicine: A questionnaire study of general practitioners. BMJ 1995;310:27-9.
33. Jones I, Morrell D. General practitioners’ background knowledge of their patients. Fam Pract 1995;12:49-53.
34. Cabana MD, Rand CS, Powe NR, WU AW, Wilson MH, Abboud PA, Rubin HR. Why don’t physicians follow clinical practice guidelines? A framework for improvement. J Am Med Assoc 1999;282:1458-1465.
35. Mandelblatt JS, Yabroff KR, Kerner JF. Equitable access to cancer care services. Cancer 1999;86:2378-2390.
Address reprint requests to Fred Tudiver, MD, Department of Family Medicine, East Tennessee State University , Box 70621, Johnson City, TN 37614. Email: tudiverf@etsu.edu.
To submit a letter to the editor on this topic, click here: jfp@fammed.uc.edu.
OBJECTIVES: To determine: a) the respondents’ perceptions of 4 unclear or conflicting cancer screening guidelines: prostate specific antigen (PSA) for men over age 50, mammography for women ages 40-49, colorectal screening by fecal occult blood testing (FOBT), and colonoscopy for patients over age 40; and b) the influence of various patient and physician factors on the decision to order these tests.
STUDY DESIGN: National Canadian mail survey of randomly selected family physicians.
POPULATION: Family physicians in active practice (n=565) selected from rural and urban family medicine sites in 5 provinces representing the main regions in Canada: British Columbia, Alberta, Ontario, Quebec, Nova Scotia.
OUTCOMES MEASURED: Agreement with guideline statements, and decision to order screening test in 6 clinical vignettes.
RESULTS: Of 565 surveys mailed, 351 (62.1%) were returned. Most respondents agreed with the Canadian Task Force recommendations, and the majority believed that various guidelines for 3 of the 4 screens were conflicting (PSA 86.6%; mammography 67.5%; FOBT 62.4%). Patient anxiety about cancer, patient expectations of being tested, and a positive family history of cancer significantly increased the odds that the 4 tests would be ordered. A good quality patient-MD relationship significantly decreased the odds of ordering a mammogram. Screening decisions were also significantly influenced by the respondents’ beliefs about whether screening was recommended and whether screening could cause more harm than good. A physician’s sensitivity to his or her colleagues’ practice influenced screening decisions regarding PSA and mammography.
CONCLUSIONS: These results suggest a conceptual framework for understanding the determinants of screening behavior when guidelines are unclear or conflicting.
Four factors are significant determinants, independently, of a physician’s decision to order a screening test when recommendations are unclear or conflicting: a patient’s anxiety about having cancer; a patient’s expectation to undergo screening; a family history of cancer; and (in most cases) the quality of the patient-physician relationship. Particularly in the context of breast cancer screening, when a patient and physician have a good relationship, they are more likely than when the relationship is poor to discuss the pros and cons of a conflicting screening guideline and reach a mutually agreeable decision.
In instances of conflicting recommendations, the importance physicians attribute to the practice of colleagues influences their screening decisions.
Although most studies of the determinants of physicians’ cancer screening behavior have dealt with facilitators or barriers to the adoption of guidelines with clear recommendations, virtually no studies have examined factors affecting physician practice when guidelines are unclear or conflicting. When guidelines are unclear, many physicians are left with little direction. By performing cancer screening procedures that are not clearly effective,1 physicians are diverting limited resources to areas where there is uncertain or no benefit to patients.
We studied physician decision-making in cancer screening when guidelines are “unclear” or conflicting. We defined an unclear guideline as a C recommendation (insufficient evidence to recommend the maneuver or not) from the Canadian Task Force on the Periodic Health Examination.2,3 We defined a “conflicting” guideline as one for which there were different recommendations from at least 2 different organizations for the same cancer screening maneuver.
The authors conducted a qualitative study of 10 focus groups across Canada, and identified factors that influence family physicians’ cancer-screening decisions when guidelines are unclear or conflicting.4 The findings supported a conceptual model with 8 factors: 1) patient factors (patient anxiety, expectations, and family history); 2) physician factors (perception of guidelines, clinical practice experience, influence of family physician and specialist colleagues, and time/financial costs; 3) the patient-physician relationship (quality of rapport). Four of these 8 factors were considered the most influential: patient anxiety about having cancer, patient expectations to have a screening test, family history of cancer, and the quality of the patient-physician relationship.
Although we know of many factors that determine cancer-screening decisions, it is not known how much each of these factors contributes to physicians’ decisions to perform tests in specific situations. The aim of this study was to verify these determining factors and to quantify the strength of the influence of each one on cancer screening decisions.
Methods
We conducted a national survey of family physicians in Canada because they are the main preventive care providers in Canada and because a physician’s recommendation is the strongest predictor of an individual’s decision to have a screening test.5
The self-administered questionnaire was mailed to a random sample of 600 family physicians, 120 from each of 5 provincial licensing bodies from 5 regions in Canada: British Columbia, Alberta, Ontario, Quebec, and Nova Scotia. We stratified by postal codes to ensure equal representation of urban and rural physicians (oversampled) to permit subgroup analysis. Ethical approval was obtained from all participating institutions. We followed a modified 4-step Dillman6 method, using initial full mailing, follow up reminder postcards, second full mailing, and phone call reminders.
The questionnaire was composed of 2 parts. Part 1 contained 40 single-item questions on physicians’ perceptions of guideline recommendations for cancers of interest, and the perceived influence of various factors on their decision to order screening tests (all factors identified in the literature and in our previous study). The questionnaire also contained items on practice characteristics, demographics, and respondents’ personal experience with cancer or cancer screening tests.
Part 2 contained 6 clinical case vignettes; 2 for prostate specific antigen (PSA), 2 for mammography, and 2 for fecal occult blood testing (FOBT) and colonoscopy, for which recommendations can be unclear according to Canadian guidelines, or conflicting.2 As for screening for prostate cancer with PSA for men over age 50, there is fair evidence for when not to screen, but conflicting recommendations from at least 2 major organizations. Mammography for breast cancer screening in women age 40 to 49 has conflicting recommendations (different recommendations from at least 2 different organizations). FOBT and colonoscopy for colorectal cancer detection over age 40 are unclear C recommendations (insufficient evidence to either recommend or not).
The Clinical Case Vignettes
Clinical case vignettes have been shown to be a useful, inexpensive, and effective method for eliciting physicians’ decision-making behavior in a simulated situation.7 Case vignettes have been used to examine physicians’ practice behavior with cancer patients.8-11For research purposes, the usefulness of the clinical case vignette rests on the ability to vary specific factors (relevant independent variables under study) from one vignette to another, while keeping constant the surrounding factors of the case presented (the frame).
For each clinical case vignette, the dependent variable was the physician’s decision to order the screening test presented or not. The independent variables were the 4 most influential factors identified in the prior qualitative study,4 embedded within the description of each clinical case vignette. Each independent variable had 2 levels: presence or absence of patient anxiety, patient expectations for testing, and family history of cancer, and easy or difficult relationship. This enabled 16 different versions of each clinical case vignette frame, and 2 frames were developed for each cancer screening. The clinical case vignettes were developed and tested in 4 steps. First, 6 investigators (R.G., F.T., C.H., A.K., M.O., J.B.B.) generated case vignettes from their own clinical experience that reflected specified levels of the factors. Second, 12 family physician colleagues empirically validated the descriptions in the case vignettes. A minimum of 9 of these physicians had to correctly identify the intended level of each of the factors in question. Third, factors not attracting 75% agreement were corrected or replaced. Fourth, the modified clinical case vignettes were submitted to another group of 12 family physicians for their perceptions of the intended levels of the relevant factors. The final versions of the vignettes reflected concordance between the perceived and the intended levels in the factors for each case vignette. Figure 1.
Our design was “fractional” in the sense that we sampled from only a fraction of all possible combinations of independent variables. We had estimated that each family physician could respond to no more than 6 case vignettes (2 per cancer screening). The reduced set represented the vignettes that were clinically realistic. As a result, each physician received set of 6 vignettes offering a clinically meaningful spread of possible levels of the independent variables to maximize the opportunity to detect practice behavior variation. The clinical case vignettes were presented in random order to avoid sequence bias. This design ensured that each physician had 1 vignette with all independent factors absent, 1 with all factors present, and the remaining 4 with a diversity of the possible combinations of levels of patient factors.
Analyses
The analysis of the binary response for each test (order versus not order screening test) included the factors listed in the theoretical framework, with an additional random effect to take into account possible correlations among responses to 2 vignettes from the same physician. The estimation of each model’s parameters was performed using the Generalized Estimating Equation approach of SAS; this variant of logistic regression accounts for the non-independence of observations. We examined first the effect of each of the 4 principal factors-individually and together-on the decision to order a screening test. Then we looked for additional significant effects of physician demographics and perceptions of guidelines. We looked for interactions between the quality of the relationship with other factors. For each screening test, we developed a final parsimonious model which included all factors that were statistically significant at P = 0.05.
Results
Of the original 600 physicians, there were 351 respondents, 214 non-respondents, and 35 ineligibles (16 were not in full-time practice, defined as < 15 hours a week; 8 were not practicing; 6 were in another specialty; 4 had moved out of the jurisdiction; and 1 had died). The final response rate was 62.1% (351/565). The respondents’ demographic characteristics Table 1 reflected the Canadian family physician population, except that there were more certificants of the College of Family Physicians of Canada (akin to Board certification in the US) among the respondents.
By [the fractional factorial] design, the versions of the vignettes with all patient factors present or all absent were the most frequent versions of the vignettes, and the frequency of the remaining versions were uniformly distributed for each vignette. There was no evidence of a systematic under-representation of any versions as a result of non-response.
Perceptions of Guidelines
The respondents’ perceptions of the guideline recommendations for the 4 cancer screening tests are shown in Table 2. Although the respondents’ perceptions of guidelines agreed with the Canadian Task Force guidelines for PSA and mammography, they diverged for colorectal cancer screening. For example, 83.5% of respondents thought colonoscopy was not recommended for patients over 40. A majority of respondents believed that the guidelines for PSA, mammography, and FOBT were conflicting.
The Influence of the Four Principal Factors
Individually, the 4 principal factors were significant determinants of the physician’s decision to order the screening test when the evidence was unclear or conflicting Table 3. The patient’s anxiety about having cancer, their expectations of having a screening test, the quality of the patient-physician-relationship (in most cases), and a positive family history of the relevant cancer all increased the odds of screening. When all 4 factors were analyzed as a combined group adjusting for the presence of other factors Table 3, the principal factors that remained significant determinants of the physician’s decision to order the screening were as follows: anxiety for PSA and mammography; patient expectations for PSA, mammography, and FOBT; a high quality patient-physician relationship for mammography (reduced the likelihood of ordering); and positive family history for all but mammography.
The Combined Influence of the Principal Factors and Physician Factors Physician variables were added to the initial logistic regression models to derive a final parsimonious model for each screening test. Table 4 shows that for each of the screening maneuvers, there were differences not only in the factors that significantly influenced the decision to screen, but also in the magnitude of influence as manifested by the odds ratios. The direction of the influence was similar across examples: all the factors increased the odds of screening except perception that the test is not recommended or does more harm than good, and a good patient-doctor relationship (in the mammography example). PSA and mammography had a similar pattern: patient anxiety, expectations, family history, the physician’s perception of the level of recommendation of the test, whether it creates more harm than good, and the influence of colleagues all significantly influenced the decision to screen. For FOBT, patient expectations, the level of perceived recommendations and the perception of harm were significant. For colonoscopy, patient anxiety, family history, and the perception of the level of recommendation were significant determinants.
Discussion
The results of this study add to the findings from the focus groups and suggest a conceptual framework or model for understanding the determinants of screening behaviour in unclear and conflicting recommendation situations. Although this model offers a more complex picture of the determinants of cancer screening in these instances, there is a great deal of consistency. Patient anxiety, patient expectations, family history of cancer, physicians’ perceptions of the relevant guideline, and physicians’ perceptions of the benefit or harm in screening were all important determinants of screening decisions. One of the important differences in the 2 studies is the relative strength of the influence of family history in this survey study, in particular for mammography and colonoscopy.
Family physicians are trained to heed patient anxiety, but it has only been described as an indirect determinant of cancer screening.12 Patient expectations has been described in the literature in a number of studies as an important determinant of screening.1,13 In addition, other patient-specific factors have been shown to be associated with physician adoption of guidelines, such as patient concerns about finances, quality of life, and location of care.14 Recent research has found an increase in physicians’ wish for more patient involvement in the development of clinical guidelines, and they have suggested that practice guidelines should reflect patient preferences.15
In the final model, the quality of the patient-physician relationship was related to one cancer screening maneuver: mammography for women aged 40-49. It is interesting that a good relationship halved the odds of screening tests being ordered when accounting for other patient factors. The importance of the influence of the patient-physician relationship on screening has been described in previous studies.12,16 In a good patient-physician relationship, patient and physician are more likely to discuss the pros and cons of a conflicting screening guideline and to find common ground than when the relationship is poor.16
The patient-physician relationship did not appear to be an important determinant in the prostate and colorectal screening examples. For PSA screening it may be due to the unique character of the relationship male patients have with their physician. A recent study found that male patients experience many barriers to seeking help, and they find it difficult to discuss their health concerns and preventive care issues with their physicians.17 For colorectal screening by colonoscopy the relationship may not have been an important determinant because 2 other determinants appeared to be so important and may overshadow any others: the great majority of respondents believed that it was not recommended (83.5%); and family history played a very important role in influencing screening.
In the final statistical modeling, several additional physician factors appeared to influence screening decisions. In particular, both the perception of whether the screening test was recommended and the belief that the screening test could cause more harm than good contributed independently to the screening decision. The same factors were noted in our qualitative study, a finding supported by many examples in the literature.1,18,19 In addition, the importance that physicians attribute to the practice of colleagues appeared to influence screening decisions in the 2 conflicting examples (from a Canadian perspective)-PSA screening and mammography. This suggests an important role of colleagues in conflicting examples. Previous research has suggested that social influences play an important role-in particular, when uncertainty is high, or when the evidence is still evolving and recommendations based on the evidence are not in common practice.20
Our emerging model Figure 2 shows that there are more than just cognitive processes at work in this sort of decision-making. The findings suggest that aspects of the patient-physician relationship and the influence of colleagues affect decision-making as well. Further, our findings indicate that these determinants are important when the guidelines are unclear or conflicting.
Many of the factors identified in this study have been described previously.1,13,21, 34 There are also recent theories to help explain how and why physicians decide to screen their patients for cancer, including whether they agree with and adhere to recommended guidelines.24,35However, these theories were developed within the context of clinical decision making when the guidelines are clear. The unique contribution of our study and emerging model is that it concerns screening decisions with unclear or conflicting guidelines. The impact of uncertainty on this aspect of physician decision-making is important. Physicians need to make decisions in the face of uncertainty. They appear to do this by believing one side of the argument or another, by balancing the perceived good or harm from screening, and by looking for support from colleagues to bolster their decision. In addition, their patients play a key role in influencing these decisions, with the doctor and patient finding common ground, often resulting in a shared decision.
Limitations
We represented the clinical factors with dichotomous situations, when, in real encounters, there would be a much greater range in the level of intensity of factors such as patient anxiety, expression of expectation, and quality of the relationship. Also, even though the case vignettes provided some background, for the physician respondent it was a “one of” situation which does not reflect a typical primary care situation that includes continuing care of patients who have a variety of coexisting clinical issues. The magnitude of the influence of these factors may be considerably underestimated or overestimated with the use of clinical case vignettes.
The generalizability of the respondents may be a limitation, as they were younger (1.7 years, not significant) and more likely to be certificants than the non-respondents. The latter difference may have contributed to a trend that stressed the influence of patient anxiety and wishes, which reflects residency training issues in family medicine. Last, although the study was done in Canada, we believe the findings likely apply to US family physicians, as graduate training is quite similar in the two countries.
Conclusions
This study underlies the importance of the cognitive component in decision making-in particular, of perceptions of guidelines, and of the influence of patients and their needs and the patient-physician relationship.
Our results verify our model in general terms, but also build on and advance the conceptual model that evolved from our qualitative findings. It provides a useful framework for understanding clinical decision-making in the face of uncertainty or controversy, and may be applicable to other clinical domains.
In future research we plan to test the effect of race and cultural aspects of the patient and of the physician on physicians’ screening decisions. Ultimately, the model could be used to design interventions to assist with the implementation of preventive services guidelines, and to be included in future CME programs for practicing physicians.
Acknowledgements
Funding/support: This study was funded by the Medical Research Council of Canada and the Prince Edward Island Cancer Research Council.
OBJECTIVES: To determine: a) the respondents’ perceptions of 4 unclear or conflicting cancer screening guidelines: prostate specific antigen (PSA) for men over age 50, mammography for women ages 40-49, colorectal screening by fecal occult blood testing (FOBT), and colonoscopy for patients over age 40; and b) the influence of various patient and physician factors on the decision to order these tests.
STUDY DESIGN: National Canadian mail survey of randomly selected family physicians.
POPULATION: Family physicians in active practice (n=565) selected from rural and urban family medicine sites in 5 provinces representing the main regions in Canada: British Columbia, Alberta, Ontario, Quebec, Nova Scotia.
OUTCOMES MEASURED: Agreement with guideline statements, and decision to order screening test in 6 clinical vignettes.
RESULTS: Of 565 surveys mailed, 351 (62.1%) were returned. Most respondents agreed with the Canadian Task Force recommendations, and the majority believed that various guidelines for 3 of the 4 screens were conflicting (PSA 86.6%; mammography 67.5%; FOBT 62.4%). Patient anxiety about cancer, patient expectations of being tested, and a positive family history of cancer significantly increased the odds that the 4 tests would be ordered. A good quality patient-MD relationship significantly decreased the odds of ordering a mammogram. Screening decisions were also significantly influenced by the respondents’ beliefs about whether screening was recommended and whether screening could cause more harm than good. A physician’s sensitivity to his or her colleagues’ practice influenced screening decisions regarding PSA and mammography.
CONCLUSIONS: These results suggest a conceptual framework for understanding the determinants of screening behavior when guidelines are unclear or conflicting.
Four factors are significant determinants, independently, of a physician’s decision to order a screening test when recommendations are unclear or conflicting: a patient’s anxiety about having cancer; a patient’s expectation to undergo screening; a family history of cancer; and (in most cases) the quality of the patient-physician relationship. Particularly in the context of breast cancer screening, when a patient and physician have a good relationship, they are more likely than when the relationship is poor to discuss the pros and cons of a conflicting screening guideline and reach a mutually agreeable decision.
In instances of conflicting recommendations, the importance physicians attribute to the practice of colleagues influences their screening decisions.
Although most studies of the determinants of physicians’ cancer screening behavior have dealt with facilitators or barriers to the adoption of guidelines with clear recommendations, virtually no studies have examined factors affecting physician practice when guidelines are unclear or conflicting. When guidelines are unclear, many physicians are left with little direction. By performing cancer screening procedures that are not clearly effective,1 physicians are diverting limited resources to areas where there is uncertain or no benefit to patients.
We studied physician decision-making in cancer screening when guidelines are “unclear” or conflicting. We defined an unclear guideline as a C recommendation (insufficient evidence to recommend the maneuver or not) from the Canadian Task Force on the Periodic Health Examination.2,3 We defined a “conflicting” guideline as one for which there were different recommendations from at least 2 different organizations for the same cancer screening maneuver.
The authors conducted a qualitative study of 10 focus groups across Canada, and identified factors that influence family physicians’ cancer-screening decisions when guidelines are unclear or conflicting.4 The findings supported a conceptual model with 8 factors: 1) patient factors (patient anxiety, expectations, and family history); 2) physician factors (perception of guidelines, clinical practice experience, influence of family physician and specialist colleagues, and time/financial costs; 3) the patient-physician relationship (quality of rapport). Four of these 8 factors were considered the most influential: patient anxiety about having cancer, patient expectations to have a screening test, family history of cancer, and the quality of the patient-physician relationship.
Although we know of many factors that determine cancer-screening decisions, it is not known how much each of these factors contributes to physicians’ decisions to perform tests in specific situations. The aim of this study was to verify these determining factors and to quantify the strength of the influence of each one on cancer screening decisions.
Methods
We conducted a national survey of family physicians in Canada because they are the main preventive care providers in Canada and because a physician’s recommendation is the strongest predictor of an individual’s decision to have a screening test.5
The self-administered questionnaire was mailed to a random sample of 600 family physicians, 120 from each of 5 provincial licensing bodies from 5 regions in Canada: British Columbia, Alberta, Ontario, Quebec, and Nova Scotia. We stratified by postal codes to ensure equal representation of urban and rural physicians (oversampled) to permit subgroup analysis. Ethical approval was obtained from all participating institutions. We followed a modified 4-step Dillman6 method, using initial full mailing, follow up reminder postcards, second full mailing, and phone call reminders.
The questionnaire was composed of 2 parts. Part 1 contained 40 single-item questions on physicians’ perceptions of guideline recommendations for cancers of interest, and the perceived influence of various factors on their decision to order screening tests (all factors identified in the literature and in our previous study). The questionnaire also contained items on practice characteristics, demographics, and respondents’ personal experience with cancer or cancer screening tests.
Part 2 contained 6 clinical case vignettes; 2 for prostate specific antigen (PSA), 2 for mammography, and 2 for fecal occult blood testing (FOBT) and colonoscopy, for which recommendations can be unclear according to Canadian guidelines, or conflicting.2 As for screening for prostate cancer with PSA for men over age 50, there is fair evidence for when not to screen, but conflicting recommendations from at least 2 major organizations. Mammography for breast cancer screening in women age 40 to 49 has conflicting recommendations (different recommendations from at least 2 different organizations). FOBT and colonoscopy for colorectal cancer detection over age 40 are unclear C recommendations (insufficient evidence to either recommend or not).
The Clinical Case Vignettes
Clinical case vignettes have been shown to be a useful, inexpensive, and effective method for eliciting physicians’ decision-making behavior in a simulated situation.7 Case vignettes have been used to examine physicians’ practice behavior with cancer patients.8-11For research purposes, the usefulness of the clinical case vignette rests on the ability to vary specific factors (relevant independent variables under study) from one vignette to another, while keeping constant the surrounding factors of the case presented (the frame).
For each clinical case vignette, the dependent variable was the physician’s decision to order the screening test presented or not. The independent variables were the 4 most influential factors identified in the prior qualitative study,4 embedded within the description of each clinical case vignette. Each independent variable had 2 levels: presence or absence of patient anxiety, patient expectations for testing, and family history of cancer, and easy or difficult relationship. This enabled 16 different versions of each clinical case vignette frame, and 2 frames were developed for each cancer screening. The clinical case vignettes were developed and tested in 4 steps. First, 6 investigators (R.G., F.T., C.H., A.K., M.O., J.B.B.) generated case vignettes from their own clinical experience that reflected specified levels of the factors. Second, 12 family physician colleagues empirically validated the descriptions in the case vignettes. A minimum of 9 of these physicians had to correctly identify the intended level of each of the factors in question. Third, factors not attracting 75% agreement were corrected or replaced. Fourth, the modified clinical case vignettes were submitted to another group of 12 family physicians for their perceptions of the intended levels of the relevant factors. The final versions of the vignettes reflected concordance between the perceived and the intended levels in the factors for each case vignette. Figure 1.
Our design was “fractional” in the sense that we sampled from only a fraction of all possible combinations of independent variables. We had estimated that each family physician could respond to no more than 6 case vignettes (2 per cancer screening). The reduced set represented the vignettes that were clinically realistic. As a result, each physician received set of 6 vignettes offering a clinically meaningful spread of possible levels of the independent variables to maximize the opportunity to detect practice behavior variation. The clinical case vignettes were presented in random order to avoid sequence bias. This design ensured that each physician had 1 vignette with all independent factors absent, 1 with all factors present, and the remaining 4 with a diversity of the possible combinations of levels of patient factors.
Analyses
The analysis of the binary response for each test (order versus not order screening test) included the factors listed in the theoretical framework, with an additional random effect to take into account possible correlations among responses to 2 vignettes from the same physician. The estimation of each model’s parameters was performed using the Generalized Estimating Equation approach of SAS; this variant of logistic regression accounts for the non-independence of observations. We examined first the effect of each of the 4 principal factors-individually and together-on the decision to order a screening test. Then we looked for additional significant effects of physician demographics and perceptions of guidelines. We looked for interactions between the quality of the relationship with other factors. For each screening test, we developed a final parsimonious model which included all factors that were statistically significant at P = 0.05.
Results
Of the original 600 physicians, there were 351 respondents, 214 non-respondents, and 35 ineligibles (16 were not in full-time practice, defined as < 15 hours a week; 8 were not practicing; 6 were in another specialty; 4 had moved out of the jurisdiction; and 1 had died). The final response rate was 62.1% (351/565). The respondents’ demographic characteristics Table 1 reflected the Canadian family physician population, except that there were more certificants of the College of Family Physicians of Canada (akin to Board certification in the US) among the respondents.
By [the fractional factorial] design, the versions of the vignettes with all patient factors present or all absent were the most frequent versions of the vignettes, and the frequency of the remaining versions were uniformly distributed for each vignette. There was no evidence of a systematic under-representation of any versions as a result of non-response.
Perceptions of Guidelines
The respondents’ perceptions of the guideline recommendations for the 4 cancer screening tests are shown in Table 2. Although the respondents’ perceptions of guidelines agreed with the Canadian Task Force guidelines for PSA and mammography, they diverged for colorectal cancer screening. For example, 83.5% of respondents thought colonoscopy was not recommended for patients over 40. A majority of respondents believed that the guidelines for PSA, mammography, and FOBT were conflicting.
The Influence of the Four Principal Factors
Individually, the 4 principal factors were significant determinants of the physician’s decision to order the screening test when the evidence was unclear or conflicting Table 3. The patient’s anxiety about having cancer, their expectations of having a screening test, the quality of the patient-physician-relationship (in most cases), and a positive family history of the relevant cancer all increased the odds of screening. When all 4 factors were analyzed as a combined group adjusting for the presence of other factors Table 3, the principal factors that remained significant determinants of the physician’s decision to order the screening were as follows: anxiety for PSA and mammography; patient expectations for PSA, mammography, and FOBT; a high quality patient-physician relationship for mammography (reduced the likelihood of ordering); and positive family history for all but mammography.
The Combined Influence of the Principal Factors and Physician Factors Physician variables were added to the initial logistic regression models to derive a final parsimonious model for each screening test. Table 4 shows that for each of the screening maneuvers, there were differences not only in the factors that significantly influenced the decision to screen, but also in the magnitude of influence as manifested by the odds ratios. The direction of the influence was similar across examples: all the factors increased the odds of screening except perception that the test is not recommended or does more harm than good, and a good patient-doctor relationship (in the mammography example). PSA and mammography had a similar pattern: patient anxiety, expectations, family history, the physician’s perception of the level of recommendation of the test, whether it creates more harm than good, and the influence of colleagues all significantly influenced the decision to screen. For FOBT, patient expectations, the level of perceived recommendations and the perception of harm were significant. For colonoscopy, patient anxiety, family history, and the perception of the level of recommendation were significant determinants.
Discussion
The results of this study add to the findings from the focus groups and suggest a conceptual framework or model for understanding the determinants of screening behaviour in unclear and conflicting recommendation situations. Although this model offers a more complex picture of the determinants of cancer screening in these instances, there is a great deal of consistency. Patient anxiety, patient expectations, family history of cancer, physicians’ perceptions of the relevant guideline, and physicians’ perceptions of the benefit or harm in screening were all important determinants of screening decisions. One of the important differences in the 2 studies is the relative strength of the influence of family history in this survey study, in particular for mammography and colonoscopy.
Family physicians are trained to heed patient anxiety, but it has only been described as an indirect determinant of cancer screening.12 Patient expectations has been described in the literature in a number of studies as an important determinant of screening.1,13 In addition, other patient-specific factors have been shown to be associated with physician adoption of guidelines, such as patient concerns about finances, quality of life, and location of care.14 Recent research has found an increase in physicians’ wish for more patient involvement in the development of clinical guidelines, and they have suggested that practice guidelines should reflect patient preferences.15
In the final model, the quality of the patient-physician relationship was related to one cancer screening maneuver: mammography for women aged 40-49. It is interesting that a good relationship halved the odds of screening tests being ordered when accounting for other patient factors. The importance of the influence of the patient-physician relationship on screening has been described in previous studies.12,16 In a good patient-physician relationship, patient and physician are more likely to discuss the pros and cons of a conflicting screening guideline and to find common ground than when the relationship is poor.16
The patient-physician relationship did not appear to be an important determinant in the prostate and colorectal screening examples. For PSA screening it may be due to the unique character of the relationship male patients have with their physician. A recent study found that male patients experience many barriers to seeking help, and they find it difficult to discuss their health concerns and preventive care issues with their physicians.17 For colorectal screening by colonoscopy the relationship may not have been an important determinant because 2 other determinants appeared to be so important and may overshadow any others: the great majority of respondents believed that it was not recommended (83.5%); and family history played a very important role in influencing screening.
In the final statistical modeling, several additional physician factors appeared to influence screening decisions. In particular, both the perception of whether the screening test was recommended and the belief that the screening test could cause more harm than good contributed independently to the screening decision. The same factors were noted in our qualitative study, a finding supported by many examples in the literature.1,18,19 In addition, the importance that physicians attribute to the practice of colleagues appeared to influence screening decisions in the 2 conflicting examples (from a Canadian perspective)-PSA screening and mammography. This suggests an important role of colleagues in conflicting examples. Previous research has suggested that social influences play an important role-in particular, when uncertainty is high, or when the evidence is still evolving and recommendations based on the evidence are not in common practice.20
Our emerging model Figure 2 shows that there are more than just cognitive processes at work in this sort of decision-making. The findings suggest that aspects of the patient-physician relationship and the influence of colleagues affect decision-making as well. Further, our findings indicate that these determinants are important when the guidelines are unclear or conflicting.
Many of the factors identified in this study have been described previously.1,13,21, 34 There are also recent theories to help explain how and why physicians decide to screen their patients for cancer, including whether they agree with and adhere to recommended guidelines.24,35However, these theories were developed within the context of clinical decision making when the guidelines are clear. The unique contribution of our study and emerging model is that it concerns screening decisions with unclear or conflicting guidelines. The impact of uncertainty on this aspect of physician decision-making is important. Physicians need to make decisions in the face of uncertainty. They appear to do this by believing one side of the argument or another, by balancing the perceived good or harm from screening, and by looking for support from colleagues to bolster their decision. In addition, their patients play a key role in influencing these decisions, with the doctor and patient finding common ground, often resulting in a shared decision.
Limitations
We represented the clinical factors with dichotomous situations, when, in real encounters, there would be a much greater range in the level of intensity of factors such as patient anxiety, expression of expectation, and quality of the relationship. Also, even though the case vignettes provided some background, for the physician respondent it was a “one of” situation which does not reflect a typical primary care situation that includes continuing care of patients who have a variety of coexisting clinical issues. The magnitude of the influence of these factors may be considerably underestimated or overestimated with the use of clinical case vignettes.
The generalizability of the respondents may be a limitation, as they were younger (1.7 years, not significant) and more likely to be certificants than the non-respondents. The latter difference may have contributed to a trend that stressed the influence of patient anxiety and wishes, which reflects residency training issues in family medicine. Last, although the study was done in Canada, we believe the findings likely apply to US family physicians, as graduate training is quite similar in the two countries.
Conclusions
This study underlies the importance of the cognitive component in decision making-in particular, of perceptions of guidelines, and of the influence of patients and their needs and the patient-physician relationship.
Our results verify our model in general terms, but also build on and advance the conceptual model that evolved from our qualitative findings. It provides a useful framework for understanding clinical decision-making in the face of uncertainty or controversy, and may be applicable to other clinical domains.
In future research we plan to test the effect of race and cultural aspects of the patient and of the physician on physicians’ screening decisions. Ultimately, the model could be used to design interventions to assist with the implementation of preventive services guidelines, and to be included in future CME programs for practicing physicians.
Acknowledgements
Funding/support: This study was funded by the Medical Research Council of Canada and the Prince Edward Island Cancer Research Council.
1. Zyzanski SJ, Stange KC, Kelly R, Flocke S, Shank JC, Chao J, Jaen CR, Smith CK. Family physicians’ disagreements with the US Preventive Services Task Force recommendations. J Fam Pract 1994;39:140-147.
2. Canadian Task Force on the Periodic Health Examination. The Canadian Guide to Clinical Preventive Health Care. Health Canada, Ottawa, 1994.
3. U.S. Preventive Services Task Force. Guide to Clinical Preventive Services. Report of the U.S. Preventive Services Task Force. Williams & Wilkins, Baltimore, 1996.
4. Tudiver F, Brown BB, Medved W, Herbert C, Ritvo P, Guibert R, Haggerty J, Goel V, Smith P, O’Beirne M, Katz A, Moliner P, Ciampi A, Williams JI. Making decisions about cancer screening when the guidelines are unclear or conflicting. J Fam Pract 2001;50:682-687.
5. White E, Urban N, Taylor V. Mammography utilization, public health impact, and cost effectiveness in the United States. Ann Rev Pub Health 1993;14:605-633.
6. Dillman DA. Mail and Telephone Surveys: the Total Design Method. New York, John Wiley and Sons, 1978.
7. Peabody JW, Luck J, Glassman P, Dresselhaus TR, Lee M. Comparison of vignettes, standardized patients, and chart abstraction: a prospective validation study of 3 methods for measuring quality. J Am Med Assoc 2000;283:1715-1722.
8. Deber RB, Thompson GG. Who still prefers aggressive surgery for breast cancer? Implications for the clinical applications of clinical trials. Arch Int Med 1987;147:1543-7.
9. Sawka CA, O’Connor AM, Llewellyn Thomas HA, To T, Pinfold SP, Harrison Woermke D. Appropriateness of adjuvant systemic therapy for axillary node negative breast cancer: a physician opinion survey. J Clin Oncol 1995;13:1459-69.
10. Tannock IF, Belanger D. Use of a physician directed questionnaire to define a consensus about management of breast cancer: implications for assessing costs and benefits of treatment. J National Cancer Inst Monographs 1992;11:137-42.
11. Sutherland HJ, Lockwood GA, Minkin S, Tritchler DL, Till JE, Llewellyn Thomas HA. Measuring satisfaction with health care: a comparison of single with paired rating strategies. Soc Sci Med 1989;28:53-58.
12. Jones I, Morrell D. General practitioners’ background knowledge of their patients. Fam Pract 1995;12:49-53.
13. Langley GR, Tritchler DL, Llewellyn-Thomas HA, Till JE. Use of written cases to study factors associated with regional variations in referral rates. J Clin Epidem 1991;44:391-402.
14. Shekelle PG, Kravitz RL, Beart J, Marger M, Wang M, Lee M. Are nonspecific practice guidelines potentially harmful A randomized comparison of the effect of nonspecific versus specific guidelines on physician decision making. Health Serv Res 2000;34:1429-1448.
15. Grol R, Dalhuijsen J, Thomas S, Veld C, Rutten G, Mokkink H. Attributes of clinical guidelines that influence use of guidelines in general practice: observational study. BMJ 1998;317:858-861.
16. McWilliam CL, Brown JB, Stewart M. Breast cancer patients’ experiences of patient-doctor communication: a working relationship. Patient Educ Couns 2000;39:191-204.
17. Tudiver F, Talbot Y. Why don’t men go to physicians? Family physicians’ perspectives on help seeking behavior of men. J Fam Pract 1999;48:47-52.
18. Brownson RC, Davis JR, Simms SG, Kern TG, Harmon RG. Cancer control knowledge and priorities among primary care physicians. J Cancer Educ 1993;8:35-41.
19. Battista RN, Williams JI, MacFarlane LA. Determinants of preventive practices in fee for-service primary care. Am J Prev Med 1990;6:6-11.
20. Mittman BS, Tonesk X, Jacobson PD. Implementing clinical practice CPGs: social influence strategies and practitioner behavior change. QRB 1992;Dec:413-422.
21. Battista RN, Williams JI, MacFarlane LA. Determinants of primary medical practice in adult cancer prevention. Med Care 1986;24:216-226.
22. Burack RC. Barriers to clinical preventive medicine. Prim Care 1989;16:245-250.
23. Frame PS. Breast Cancer Screening in Older Women: the family practice perspective. J Gerontol 1992;47, Spec.:No. 131-3.
24. Pathman DE, Konrad TR, Freed GL, Freeman VA, Koch GG. The awareness-to-adherence model of the steps to clinical guideline compliance. Med Care 1996;34:873-889.
25. Stange KC, Kelly R, Chao J, Zyzanski SJ, Shank JC, Jaen CR, Melnikow J, Flocke S. Physician agreement with US Preventive Services Task Force recommendations. J Fam Pract 1992;34:409-416.
26. Brownson RC, Davis JR, Simms SG, Kern TG, Harmon RG. Cancer control knowledge and priorities among primary care physicians. J Cancer Educ 1993;8:35-41.
27. Costanza M.E, Stoddard A.M, Zapks J.G, Gaw V.P, Barth R. Physician compliance with mammography guidelines: Barriers and enhancers. J Am Board Fam Pract 1992;5:143-52.
28. Weingarten S, Stone E, Hayward R, Tunis S, Pelter M, Huang H, Kristopaitis R. The adoption of preventive care practice guidelines by primary care physicians. J Gen Int Med 1990;10:138-144.
29. Young MJ, Fried LS, Eisenberg J, Hershey J, Williams S. Do cardiologists have higher thresholds for recommending coronary arteriography than family physicians? Health Serv Res 1987;22:623-635.
30. Smith HE, Herbert CP. Preventive practice among primary care physicians in British Columbia: relation to recommendations of the Canadian Task Force on the Periodic Health Examination. Canad Med Assoc J 1993;149:1795-800.
31. Triezenberg D.J, Smith M.A, Holmes T.M. Cancer screening and detection in family practice: A MIRNET study. J Fam Pract 1995;40:27-33.
32. Summerton N. Positive and negative factors in defensive medicine: A questionnaire study of general practitioners. BMJ 1995;310:27-9.
33. Jones I, Morrell D. General practitioners’ background knowledge of their patients. Fam Pract 1995;12:49-53.
34. Cabana MD, Rand CS, Powe NR, WU AW, Wilson MH, Abboud PA, Rubin HR. Why don’t physicians follow clinical practice guidelines? A framework for improvement. J Am Med Assoc 1999;282:1458-1465.
35. Mandelblatt JS, Yabroff KR, Kerner JF. Equitable access to cancer care services. Cancer 1999;86:2378-2390.
Address reprint requests to Fred Tudiver, MD, Department of Family Medicine, East Tennessee State University , Box 70621, Johnson City, TN 37614. Email: tudiverf@etsu.edu.
To submit a letter to the editor on this topic, click here: jfp@fammed.uc.edu.
1. Zyzanski SJ, Stange KC, Kelly R, Flocke S, Shank JC, Chao J, Jaen CR, Smith CK. Family physicians’ disagreements with the US Preventive Services Task Force recommendations. J Fam Pract 1994;39:140-147.
2. Canadian Task Force on the Periodic Health Examination. The Canadian Guide to Clinical Preventive Health Care. Health Canada, Ottawa, 1994.
3. U.S. Preventive Services Task Force. Guide to Clinical Preventive Services. Report of the U.S. Preventive Services Task Force. Williams & Wilkins, Baltimore, 1996.
4. Tudiver F, Brown BB, Medved W, Herbert C, Ritvo P, Guibert R, Haggerty J, Goel V, Smith P, O’Beirne M, Katz A, Moliner P, Ciampi A, Williams JI. Making decisions about cancer screening when the guidelines are unclear or conflicting. J Fam Pract 2001;50:682-687.
5. White E, Urban N, Taylor V. Mammography utilization, public health impact, and cost effectiveness in the United States. Ann Rev Pub Health 1993;14:605-633.
6. Dillman DA. Mail and Telephone Surveys: the Total Design Method. New York, John Wiley and Sons, 1978.
7. Peabody JW, Luck J, Glassman P, Dresselhaus TR, Lee M. Comparison of vignettes, standardized patients, and chart abstraction: a prospective validation study of 3 methods for measuring quality. J Am Med Assoc 2000;283:1715-1722.
8. Deber RB, Thompson GG. Who still prefers aggressive surgery for breast cancer? Implications for the clinical applications of clinical trials. Arch Int Med 1987;147:1543-7.
9. Sawka CA, O’Connor AM, Llewellyn Thomas HA, To T, Pinfold SP, Harrison Woermke D. Appropriateness of adjuvant systemic therapy for axillary node negative breast cancer: a physician opinion survey. J Clin Oncol 1995;13:1459-69.
10. Tannock IF, Belanger D. Use of a physician directed questionnaire to define a consensus about management of breast cancer: implications for assessing costs and benefits of treatment. J National Cancer Inst Monographs 1992;11:137-42.
11. Sutherland HJ, Lockwood GA, Minkin S, Tritchler DL, Till JE, Llewellyn Thomas HA. Measuring satisfaction with health care: a comparison of single with paired rating strategies. Soc Sci Med 1989;28:53-58.
12. Jones I, Morrell D. General practitioners’ background knowledge of their patients. Fam Pract 1995;12:49-53.
13. Langley GR, Tritchler DL, Llewellyn-Thomas HA, Till JE. Use of written cases to study factors associated with regional variations in referral rates. J Clin Epidem 1991;44:391-402.
14. Shekelle PG, Kravitz RL, Beart J, Marger M, Wang M, Lee M. Are nonspecific practice guidelines potentially harmful A randomized comparison of the effect of nonspecific versus specific guidelines on physician decision making. Health Serv Res 2000;34:1429-1448.
15. Grol R, Dalhuijsen J, Thomas S, Veld C, Rutten G, Mokkink H. Attributes of clinical guidelines that influence use of guidelines in general practice: observational study. BMJ 1998;317:858-861.
16. McWilliam CL, Brown JB, Stewart M. Breast cancer patients’ experiences of patient-doctor communication: a working relationship. Patient Educ Couns 2000;39:191-204.
17. Tudiver F, Talbot Y. Why don’t men go to physicians? Family physicians’ perspectives on help seeking behavior of men. J Fam Pract 1999;48:47-52.
18. Brownson RC, Davis JR, Simms SG, Kern TG, Harmon RG. Cancer control knowledge and priorities among primary care physicians. J Cancer Educ 1993;8:35-41.
19. Battista RN, Williams JI, MacFarlane LA. Determinants of preventive practices in fee for-service primary care. Am J Prev Med 1990;6:6-11.
20. Mittman BS, Tonesk X, Jacobson PD. Implementing clinical practice CPGs: social influence strategies and practitioner behavior change. QRB 1992;Dec:413-422.
21. Battista RN, Williams JI, MacFarlane LA. Determinants of primary medical practice in adult cancer prevention. Med Care 1986;24:216-226.
22. Burack RC. Barriers to clinical preventive medicine. Prim Care 1989;16:245-250.
23. Frame PS. Breast Cancer Screening in Older Women: the family practice perspective. J Gerontol 1992;47, Spec.:No. 131-3.
24. Pathman DE, Konrad TR, Freed GL, Freeman VA, Koch GG. The awareness-to-adherence model of the steps to clinical guideline compliance. Med Care 1996;34:873-889.
25. Stange KC, Kelly R, Chao J, Zyzanski SJ, Shank JC, Jaen CR, Melnikow J, Flocke S. Physician agreement with US Preventive Services Task Force recommendations. J Fam Pract 1992;34:409-416.
26. Brownson RC, Davis JR, Simms SG, Kern TG, Harmon RG. Cancer control knowledge and priorities among primary care physicians. J Cancer Educ 1993;8:35-41.
27. Costanza M.E, Stoddard A.M, Zapks J.G, Gaw V.P, Barth R. Physician compliance with mammography guidelines: Barriers and enhancers. J Am Board Fam Pract 1992;5:143-52.
28. Weingarten S, Stone E, Hayward R, Tunis S, Pelter M, Huang H, Kristopaitis R. The adoption of preventive care practice guidelines by primary care physicians. J Gen Int Med 1990;10:138-144.
29. Young MJ, Fried LS, Eisenberg J, Hershey J, Williams S. Do cardiologists have higher thresholds for recommending coronary arteriography than family physicians? Health Serv Res 1987;22:623-635.
30. Smith HE, Herbert CP. Preventive practice among primary care physicians in British Columbia: relation to recommendations of the Canadian Task Force on the Periodic Health Examination. Canad Med Assoc J 1993;149:1795-800.
31. Triezenberg D.J, Smith M.A, Holmes T.M. Cancer screening and detection in family practice: A MIRNET study. J Fam Pract 1995;40:27-33.
32. Summerton N. Positive and negative factors in defensive medicine: A questionnaire study of general practitioners. BMJ 1995;310:27-9.
33. Jones I, Morrell D. General practitioners’ background knowledge of their patients. Fam Pract 1995;12:49-53.
34. Cabana MD, Rand CS, Powe NR, WU AW, Wilson MH, Abboud PA, Rubin HR. Why don’t physicians follow clinical practice guidelines? A framework for improvement. J Am Med Assoc 1999;282:1458-1465.
35. Mandelblatt JS, Yabroff KR, Kerner JF. Equitable access to cancer care services. Cancer 1999;86:2378-2390.
Address reprint requests to Fred Tudiver, MD, Department of Family Medicine, East Tennessee State University , Box 70621, Johnson City, TN 37614. Email: tudiverf@etsu.edu.
To submit a letter to the editor on this topic, click here: jfp@fammed.uc.edu.
Making Decisions About Cancer Screening When the Guidelines Are Unclear or Conflicting
STUDY DESIGN: We analyzed discussions with focus groups using a constant comparative approach.
POPULATION: A total of 73 family physicians in active practice participated in 10 focus groups (1 urban group and 1 rural group in each of 5 Canadian provinces).
OUTCOME MEASURES: Our main outcome measures were participants’ perceptions regarding cancer screening when the guidelines were unclear or conflicting.
RESULTS: We propose a model of the determinants of cancer screening decision making with regard to unclear and conflicting guidelines. This model is rooted in the physician-patient relationship, and is an interactive process influenced by patient factors (anxiety, expectations, and family history) and physician factors (perception of guidelines, clinical practice experience, influence of colleagues, distinction between the screening styles of specialists and family physicians, and the amount of time and financial costs involved in performing the maneuver).
CONCLUSIONS: Our model is unique, because it is embedded in the physician-patient relationship. Ultimately, a modified model could be used to design interventions to assist with the implementation of preventive services guidelines.
Every year physicians and patients receive hundreds of messages about guidelines for cancer screening. Ideally, physicians will adopt and adhere to the evidence-based clinical practice guidelines. By doing so, there is maximum application of a proven technology to those who can most benefit, and valuable resources are not wasted in examinations that are not based on good or fair evidence. However, many physicians are not adhering to cancer screening guidelines backed by good evidence.1,2 Also, many are performing cancer screening procedures that are not recommended (either because of a lack of evidence or because they have been shown to be ineffective).3
Most of the literature on physician cancer screening has dealt with facilitators or barriers to the adoption of commonly recommended guidelines. These studies did not address the factors that affect physician practice when the guidelines are unclear or conflicting, or when they clearly recommend against the procedure. We defined an “unclear” guideline as a “C” recommendation (insufficient evidence to recommend the maneuver) from the Canadian Task Force on the Periodic Health Examination (CTFPHE).4 Guidelines were “conflicting” when at least 2 organizations gave different recommendations for the same cancer screening examination.
Despite the CTFPHE guidelines,4 inconsistencies in practice remain. Although the CTFPHE recommends that breast cancer screening begin at age 50 years, 59% of women aged 40 to 49 years reported having mammograms in 1994,5 a rate nearly equivalent to those aged 50 to 59 in Ontario.6 It is clear that family physicians—the major cancer screeners in many countries—are frequently not following guidelines. The use of ineffective procedures or those for which the evidence is unclear can waste scarce health resources and lead to harm for those whose test results are false positive. The objective of our study7 was to determine the factors involved in the cancer screening decisions of family physicians in situations where the clinical practice guidelines were unclear or conflicting (prostate-specific antigen testing, mammography for ages 40 to 49 years, colorectal tests) as opposed to when they were clear and uncontroversial.
Methods
Ten focus groups7 were conducted with 1 urban group and 1 rural group in each of 5 Canadian provinces: British Columbia (BC), Alberta (AB), Ontario (ON), Quebec (QC), and Prince Edward Island (PEI). Ethical approval was obtained from all participating institutions. We focused on family physicians because they are the main preventive health care providers in Canada, and physician recommendation is the most important predictor of whether an individual obtains a particular screening test.8 Eight focus groups were conducted face to face, and 2 were done by teleconference because of the geographic remoteness of 2 rural areas. Each focus group was co-facilitated by a local research assistant and 1 of the investigators. The focus group moderators participated in a 2-hour training session to ensure standardization across sites. The group sessions lasted approximately 60 to 90 minutes; all were audiotaped and transcribed verbatim.*Table w1
Recruitment and Sampling
We used maximum variation sampling to ensure heterogeneity within the groups and to recruit physicians who would serve as information-rich participants with a wide range for age, practice type, location, and education.9,10 Recruitment involved a 2-step process:11 First, urban and rural family physicians were randomly selected from lists provided by each local area’s licensing body; and second, physician recruiters (“leader figures”) from each local area identified physicians who they believed would provide an adequate variance of opinions.
Data Collection and Analysis
Data collection and analysis occurred iteratively.12-14 After every focus group 3 investigators reviewed transcripts independently to identify the central issues that emerged. Over several meetings they compared and combined their independent analyses. Emerging themes were explored and expanded in subsequent focus groups. Although saturation15 had been achieved by the 8th focus group, we completed the final 2 groups to ensure regional representation. The second step in the analysis involved determining the similarities, differences, and potential connections among key words, phrases, and concepts within and among each focus group transcript. Finally, the themes and subcategories of all focus groups were compared and contrasted, and the quotes that most accurately illustrated the themes were identified.
Trustworthiness and Validation
All groups were audiotaped and transcribed verbatim, and extensive field notes were made during the focus groups and throughout the analysis. Validation of the data was achieved by conducting member-checking interviews16 with 15 information-rich participants from the focus groups after completion of the initial analysis. We then refined the themes.
Results
The physicians’ demographics Table 1 reflect the Canadian family physician population.17 Three major themes emerged from the analysis as determinants of cancer screening with unclear or controversial guidelines: patient factors, physician factors, and physician-patient relationship factors Table 2.
Patient Factors
Patient factors included expectations, anxieties, family history, peers, and media influences. Many of the physician participants commented that patient expectations and demands for screening were major determinants of their decision to screen when guidelines were unclear. Although they expressed discomfort with this behavior, physicians acknowledged being frequently swayed by patient demands. One said, “I think that if the patient comes into my office and he wants something, that influences me a hell of a lot.” (QC rural)
The physicians also suggested that patients’ anxieties about cancer were important. The higher the perceived anxiety, the more likely they were to order the relevant cancer screening test, even if the recommendations were unclear. A participant said, “If a patient came in with a particular anxiety and would be allayed by [screening]…I would go ahead and recommend it.” (BC rural)
The presence of any positive family history appeared to influence the physicians’ screening decisions, even if it was not a recognized risk factor in the cancer screening guideline. Physicians also felt that the media is an important influence on patients’ requests for screening. One of the physicians said, “I think the media really influences a lot of patients, and unfortunately it doesn’t always give them the correct information.” (ON urban)
Physician Factors
Physician factors included the perception of guidelines, clinical practice experience, the influence of colleagues, the distinction between the screening styles of specialists and family physicians, and the time and financial costs involved in performing the screening maneuver. The 2 most important determinants appeared to be the physicians’ perceptions of guidelines and their clinical experience.
The physicians’ perception of guidelines had 5 components Table 2. First, many physicians saw guidelines as just guidelines and not as directives. This was most evident when the guideline was viewed as unclear or conflicting. Second, many indicated that unclear guidelines are not guidelines at all and that their task was to individualize the screening decisions to patients and their situations. A participant said, “If they’re unclear, then you have to use your judgment in terms of the patient, your patient population, their follow-up ability, what their risk factors, age, etcetera, are.” (AB rural)
The third perception of guidelines was confusion due to the multiplicity and changing nature of guidelines. One physician said, “As far as breast cancer goes, it appears…things are still…in flux…changing all the time.” (ON urban)
The physicians’ degree of trust in the source of the guideline was the fourth component. A participant said, “If you get a guideline from a consensus group where…a group of specialists get together…including some family docs…certainly I would take that with more…clout.” (AB rural)
The fifth component was the perceived effectiveness of a particular screening maneuver. One physician said, “In the…years that we’ve been [screening] we have found cancers at the stage A and B…that have been easily looked after…. We have not had 1 patient pass away.” (AB rural)
Physicians viewed their clinical experience as influencing their cancer screening decisions, and many felt that they were much more likely to order screening tests early in their careers. A participant said, “In terms of screening there’s a tendency, especially when you’re young and keen and scared, that you’re gonna miss something.” (AB urban)
Physicians were concerned about missing a diagnosis of cancer. If they actually had such an experience, it subsequently lowered their threshold for cancer screening for some time afterward. One physician said, “Suppose you missed a case of colorectal cancer, and someone else finds it; then you tend to run gun shy for a long time and perhaps overinvestigate and over-refer for a time.” (BC rural)
Colleagues could positively or negatively influence screening decisions. A participant said, “Some guidelines come out, and somebody will say, ‘Oh that’s trash. I’m not going to do that.’ And then it’s a little hard for the rest of us to easily incorporate that.” (BC rural)
Family physicians also felt that they had a unique screening style compared with specialists, stemming from their continuing long-term relationships with their patients. One physician said, “The specialists will tend to jump on the blood test wagon a lot faster than I think we will, because again they don’t know the patients.” (AB urban)
Time and financial costs were also identified as important practice factors in the decision-making process. A participant said, “Economics also plays a part…because it can take…half an hour to explain to a patient why you don’t want to do something. It can take 2 minutes to do it.” (ON rural)
The Physician-Patient Relationship
Decisions about cancer screening took place within an interactive relationship between the patient and physician. Physicians characterized the relationship as one of varying intensity and depth, and there appeared to be 3 key points about the relationship in terms of cancer screening. First, the stronger and more positive the relationship, the more likely that the physician would feel free to engage the patient in a discussion about not performing a test that is based on an unclear or negative guideline. One physician said, “If you’ve known somebody for a long time and they come to you with something that you don’t think is right, it’s a little bit easier to talk to them.” (PEI rural)
Conversely, if the relationship was new or tenuous, physicians felt “The lack of a good relationship has an impact…they tend not to go along with your recommendations.” (AB rural)
The second point regarding the physician-patient relationship was that when a guideline was unclear, it often called for a different interaction than when the guideline was clear. It involved more information giving, presented in a manner that assisted the patient. One physician said, “I try to give the patient as much information as I have, in words that they will understand, so that they can come to an informed decision. That’s what I do when the guidelines are unclear.” (ON urban)
The process of information-giving promoted finding common ground, particularly when patients were requesting a screening maneuver not backed by clear evidence. One participant said:[For] patients who want tests that we don’t necessarily think are indicated, I follow the evidence, and that’s a negotiation. …an explanation of the evidence and then almost throw it back at the patient...it’s not medical-legal. It’s not economic. It’s between me and my patient. (QC urban)
Finally, many physician participants observed that even when the guidelines are clear, many cancer screening decisions are not. As a result, they noted that this often necessitated a process of finding common ground by engaging patients in mutual decision making.
Discussion
Many of the factors we identified have been described previously.18-36 However, to the best of our knowledge they have not been combined into a comprehensive typology for cancer screening decision making that includes the physician-patient relationship and that deals with unclear and conflicting guidelines. One conceptual framework for the determinants of screening behavior22 is based on pediatric vaccinations and does not include unclear or controversial guidelines. Another more recent model is based on cancer care, not screening, but it does include some elements of communication between provider and patient.23 Our proposed model of decision making regarding cancer screening Figure 1 is a modification of these frameworks based on our findings and is specific to decisions about cancer screening.
One unique feature of our model is that it is embedded in the physician-patient relationship. In particular, the quality of this relationship and the clarity of the recommendation appears to be most important. It involves an interactive process and mutual discussion with the patient. This ultimately includes finding agreement and culminates in a mutual agreement between the patient and the physician about the cancer screening maneuver.37,38 Our findings are also in concordance with other literature on physician test-ordering. The concern about missing a diagnosis of cancer is similar to “chagrin bias” —when physicians are more likely to order inappropriate chest radiographs if they anticipated feeling regret if they missed a diagnosis of pneumonia.39
Limitations
Although attempts were made to have regional representation from the entire country (Canada), the findings may not be transferable to other family medicine settings. Two of the 10 focus groups were conducted by teleconference, which may bias results, because it is a different data collection method. However, previous experience with telephone focus groups had been successful. (C.H., personal communication) The 2 teleconference groups did not provide markedly different data from those conducted in-person. Also, because of budget restraints, 5 different moderators were used. The investigators organized training sessions to standardize focus group moderation across sites; however, it is difficult to estimate the potential bias, given that moderators have their own styles. Finally, the data were based on the perspective of physicians and not patients.
Future Research
In the next phase of our study we will test the model’s factors quantitatively on a random sample of physicians and go through the same steps with a patient/consumer sample. Ultimately, we will use a modified model to design interventions to assist with the implementation of preventive services guidelines.
Conclusions
Our findings are of importance for those implementing preventive care guidelines. The focus group participants were clearly less happy with guidelines that were equivocal, and were less likely to follow them. Patient factors and the physician-patient relationship appear to be important in such cases. Although patient-oriented decision aids could help physicians in these situations, it is clearly more difficult to develop aids to guide patients in settings when the evidence is unclear, because the information required is more complex. The family physicians’ perceptions of the effectiveness of a particular screening test was very important, perhaps more important to the participants than the scientific evidence behind a guideline. Although personal experience is a weak and unscientific level of evidence subject to many biases, it is likely an important influence on cancer screening decision making in primary care, particularly when the evidence is uncertain. Future education efforts directed at primary care providers should address the influence of personal experience as well as the failure to attend to the level of evidence behind recommendations.
Acknowledgments
Our project was funded by a peer-reviewed grant from the Medical Research Council of Canada (grant number 14673) and by the Prince Edward Island Cancer Research Council. We wish to thank the staff of the Department of Family and Community Medicine, University of Toronto, for their tireless support of this project.
1. Main DS, Cohen SJ, DiClemente CC. Measuring physician readiness to change cancer screening: preliminary results. Am J Prev Med 1995;11:54-58.
2. Lomas J, Anderson GM, Domnick-Pierre K, Vayda E, Enkin MW, Hannah WJ. Do practice guidelines guide practice? The effects of a consensus statement on the practice of physicians. N Engl J Med 1989;321:1306-11.
3. Zyzanski SJ, Stange KC, Kelly R, et al. Family physicians’ disagreements with the US Preventive Services Task Force recommendations. J Fam Pract 1994;39:140-47.
4. Canadian Task Force on the Periodic Health Examination The Canadian guide to clinical preventive health care. Ottawa, Canada: Health Canada; 1994.
5. Statistics Canada 1994 national population health survey. Public use data file; 1995.
6. Goel V. Whose guidelines are they anyways? Mammography screening in Ontario. Can J Publ Health 1996;87:181-82.
7. Brown JB. The use of focus groups in clinical research. In: Crabtree BF, Miller WL, eds. Doing qualitative research. Newbury Park, Calif: Sage Publications; 1999.
8. White E, Urban N, Taylor V. Mammography utilization, public health impact, and cost-effectiveness in the United States. Ann Rev Pub Health 1993;14:605-33.
9. Patton M. Qualitative evaluation and research methods. 2nd ed. Newbury Park, Calif: Sage Publications; 1990.
10. Kuzel AJ, Like RC. Standards of trustworthiness for qualitative studies in primary care. In: Norton PG, Stewart M, Tudiver F, Bass MF, Dunn EV, eds. Primary care research: traditional and innovative approaches. Newbury Park, Calif: Sage Publications; 1991.
11. Borgiel AE, Dunn EV, Lamont CT, et al. Recruiting family physicians as participants in research. Fam Pract 1989;6:168-72.
12. Morgan DL. Focus groups as qualitative research. Newbury Park, Calif: Sage Publications; 1988.
13. Morgan DL. Successful focus groups: advancing the state of the art. Newbury Park, Calif: Sage Publications; 1993.
14. Strauss AL, Corbin J. Basics of qualitative research: grounded theory, procedure and techniques. Beverly Hills, Calif: Sage Publication; 1990.
15. Kuzel A. Sampling in qualitative theory. In: Crabtree BF, Miller WL. Doing qualitative research. Newbury Park, Calif: Sage Publications; 1999;41-4217.-
16. Gilchrist VJ, Williams RL. Key informant interviews. In: Crabtree BF, Miller WL. Doing qualitative research. Newbury Park, Calif: Sage Publications; 1999:81.
17. Southam Directories Group. National MD select profiler version. Toronto, Ontario, Canada: Don Mills Southam Directories Group; 1999.
18. Battista RN, Williams JI, MacFarlane LA. Determinants of primary medical practice in adult cancer prevention. Med Care 1986;24:216-26.
19. Battista RN, Williams JI, MacFarlane LA. Determinants of preventive practices in fee-for-service primary care. Am J Prev Med 1990;6:6-11.
20. Burack RC. Barriers to clinical preventive medicine. Prim Care 1989;16:245-50.
21. Frame PS. Breast cancer screening in older women: the family practice perspective. J Geronol 1992;47:131-33.
22. Pathman DE, Konrad TR, Freed GL, Freeman VA, Koch GG. The awareness-to-adherence model of the steps to clinical guideline compliance. Med Care 1996;34:873-89.
23. Mandelblatt JS, Yabroff KR, Kerner JF. Equitable access to cancer services: a review of barriers to quality care. Cancer 1999;86:2378-90.
24. Langley GR, Tritchler DL, Llewellyn-Thomas HA, Till JE. Use of written cases to study factors associated with regional variations in referral rates. J Clin Epidemiol 1991;44:391-402.
25. Zyzanski SJ, Stange KC, Kelly R, et al. Family physicians’ disagreements with the US Preventive Services Task Force recommendations. J Fam Pract 1994;39:140-47.
26. Stange KC, Kelly R, Chao J, et al. Physician agreement with US Preventive Services Task Force recommendations. J Fam Pract 1992;34:409-16.
27. Mittman BS, Tonesk X, Jacobson PD. Implementing clinical practice CPGs: social influence strategies and practitioner behavior change. QRB 1992;18:413-22.
28. Brownson RC, Davis JR, Simms SG, Kern TG, Harmon RG. Cancer control knowledge and priorities among primary care physicians. J Cancer Educ 1993;8:35-41.
29. Costanza ME, Stoddard AM, Zapks JG, Gaw VP, Barth R. Physician compliance with mammography guidelines: barriers and enhancers. J Am Board Fam Pract 1992;5:143-52.
30. Weingarten S, Stone E, Hayward R, et al. The adoption of preventive care practice guidelines by primary care physicians. J Gen Intern Med 1990;10:138-44.
31. Young MJ, Fried LS, Eisenberg J, Hershey J, Williams S. Do cardiologists have higher thresholds for recommending coronary arteriography than family physicians? Health Serv Res 1987;22:623-35.
32. Smith HE, Herbert CP. Preventive practice among primary care physicians in British Columbia: relation to recommendations of the Canadian Task Force on the Periodic Health Examination. Can Med Assoc J 1993;149:1795-800.
33. Triezenberg DJ, Smith MA, Holmes TM. Cancer screening and detection in family practice: a MIRNET study. J Fam Pract 1995;40:27-33.
34. Summerton N. Positive and negative factors in defensive medicine: a questionnaire study of general practitioners. BMJ 1995;310:27-29.
35. Jones I, Morrell D. General practitioners’ background knowledge of their patients. Fam Pract 1995;12:49-53.
36. Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. J Am Med Assoc 1999;282:1458-65.
37. Stewart M, Brown JB, Boon H, Galajda J, Meredith L, Sangster M. Evidence on patient-doctor communication. Cancer Prev Control 1999;3:25-30.
38. Stewart M, Brown JB, Weston WW, McWhinney IR, McWilliam CL, Freeman TR. Patient-centered medicine: transforming the clinical method. Thousand Oaks, Calif: Sage Publications; 1995.
39. Heckerling PS, Tape TG, Wigton RS. Relation of physicians’ predicted probabilities of pneumonia to their utilities for ordering chest x-rays to detect pneumonia. Med Decis Making 1992;12:32-38.
STUDY DESIGN: We analyzed discussions with focus groups using a constant comparative approach.
POPULATION: A total of 73 family physicians in active practice participated in 10 focus groups (1 urban group and 1 rural group in each of 5 Canadian provinces).
OUTCOME MEASURES: Our main outcome measures were participants’ perceptions regarding cancer screening when the guidelines were unclear or conflicting.
RESULTS: We propose a model of the determinants of cancer screening decision making with regard to unclear and conflicting guidelines. This model is rooted in the physician-patient relationship, and is an interactive process influenced by patient factors (anxiety, expectations, and family history) and physician factors (perception of guidelines, clinical practice experience, influence of colleagues, distinction between the screening styles of specialists and family physicians, and the amount of time and financial costs involved in performing the maneuver).
CONCLUSIONS: Our model is unique, because it is embedded in the physician-patient relationship. Ultimately, a modified model could be used to design interventions to assist with the implementation of preventive services guidelines.
Every year physicians and patients receive hundreds of messages about guidelines for cancer screening. Ideally, physicians will adopt and adhere to the evidence-based clinical practice guidelines. By doing so, there is maximum application of a proven technology to those who can most benefit, and valuable resources are not wasted in examinations that are not based on good or fair evidence. However, many physicians are not adhering to cancer screening guidelines backed by good evidence.1,2 Also, many are performing cancer screening procedures that are not recommended (either because of a lack of evidence or because they have been shown to be ineffective).3
Most of the literature on physician cancer screening has dealt with facilitators or barriers to the adoption of commonly recommended guidelines. These studies did not address the factors that affect physician practice when the guidelines are unclear or conflicting, or when they clearly recommend against the procedure. We defined an “unclear” guideline as a “C” recommendation (insufficient evidence to recommend the maneuver) from the Canadian Task Force on the Periodic Health Examination (CTFPHE).4 Guidelines were “conflicting” when at least 2 organizations gave different recommendations for the same cancer screening examination.
Despite the CTFPHE guidelines,4 inconsistencies in practice remain. Although the CTFPHE recommends that breast cancer screening begin at age 50 years, 59% of women aged 40 to 49 years reported having mammograms in 1994,5 a rate nearly equivalent to those aged 50 to 59 in Ontario.6 It is clear that family physicians—the major cancer screeners in many countries—are frequently not following guidelines. The use of ineffective procedures or those for which the evidence is unclear can waste scarce health resources and lead to harm for those whose test results are false positive. The objective of our study7 was to determine the factors involved in the cancer screening decisions of family physicians in situations where the clinical practice guidelines were unclear or conflicting (prostate-specific antigen testing, mammography for ages 40 to 49 years, colorectal tests) as opposed to when they were clear and uncontroversial.
Methods
Ten focus groups7 were conducted with 1 urban group and 1 rural group in each of 5 Canadian provinces: British Columbia (BC), Alberta (AB), Ontario (ON), Quebec (QC), and Prince Edward Island (PEI). Ethical approval was obtained from all participating institutions. We focused on family physicians because they are the main preventive health care providers in Canada, and physician recommendation is the most important predictor of whether an individual obtains a particular screening test.8 Eight focus groups were conducted face to face, and 2 were done by teleconference because of the geographic remoteness of 2 rural areas. Each focus group was co-facilitated by a local research assistant and 1 of the investigators. The focus group moderators participated in a 2-hour training session to ensure standardization across sites. The group sessions lasted approximately 60 to 90 minutes; all were audiotaped and transcribed verbatim.*Table w1
Recruitment and Sampling
We used maximum variation sampling to ensure heterogeneity within the groups and to recruit physicians who would serve as information-rich participants with a wide range for age, practice type, location, and education.9,10 Recruitment involved a 2-step process:11 First, urban and rural family physicians were randomly selected from lists provided by each local area’s licensing body; and second, physician recruiters (“leader figures”) from each local area identified physicians who they believed would provide an adequate variance of opinions.
Data Collection and Analysis
Data collection and analysis occurred iteratively.12-14 After every focus group 3 investigators reviewed transcripts independently to identify the central issues that emerged. Over several meetings they compared and combined their independent analyses. Emerging themes were explored and expanded in subsequent focus groups. Although saturation15 had been achieved by the 8th focus group, we completed the final 2 groups to ensure regional representation. The second step in the analysis involved determining the similarities, differences, and potential connections among key words, phrases, and concepts within and among each focus group transcript. Finally, the themes and subcategories of all focus groups were compared and contrasted, and the quotes that most accurately illustrated the themes were identified.
Trustworthiness and Validation
All groups were audiotaped and transcribed verbatim, and extensive field notes were made during the focus groups and throughout the analysis. Validation of the data was achieved by conducting member-checking interviews16 with 15 information-rich participants from the focus groups after completion of the initial analysis. We then refined the themes.
Results
The physicians’ demographics Table 1 reflect the Canadian family physician population.17 Three major themes emerged from the analysis as determinants of cancer screening with unclear or controversial guidelines: patient factors, physician factors, and physician-patient relationship factors Table 2.
Patient Factors
Patient factors included expectations, anxieties, family history, peers, and media influences. Many of the physician participants commented that patient expectations and demands for screening were major determinants of their decision to screen when guidelines were unclear. Although they expressed discomfort with this behavior, physicians acknowledged being frequently swayed by patient demands. One said, “I think that if the patient comes into my office and he wants something, that influences me a hell of a lot.” (QC rural)
The physicians also suggested that patients’ anxieties about cancer were important. The higher the perceived anxiety, the more likely they were to order the relevant cancer screening test, even if the recommendations were unclear. A participant said, “If a patient came in with a particular anxiety and would be allayed by [screening]…I would go ahead and recommend it.” (BC rural)
The presence of any positive family history appeared to influence the physicians’ screening decisions, even if it was not a recognized risk factor in the cancer screening guideline. Physicians also felt that the media is an important influence on patients’ requests for screening. One of the physicians said, “I think the media really influences a lot of patients, and unfortunately it doesn’t always give them the correct information.” (ON urban)
Physician Factors
Physician factors included the perception of guidelines, clinical practice experience, the influence of colleagues, the distinction between the screening styles of specialists and family physicians, and the time and financial costs involved in performing the screening maneuver. The 2 most important determinants appeared to be the physicians’ perceptions of guidelines and their clinical experience.
The physicians’ perception of guidelines had 5 components Table 2. First, many physicians saw guidelines as just guidelines and not as directives. This was most evident when the guideline was viewed as unclear or conflicting. Second, many indicated that unclear guidelines are not guidelines at all and that their task was to individualize the screening decisions to patients and their situations. A participant said, “If they’re unclear, then you have to use your judgment in terms of the patient, your patient population, their follow-up ability, what their risk factors, age, etcetera, are.” (AB rural)
The third perception of guidelines was confusion due to the multiplicity and changing nature of guidelines. One physician said, “As far as breast cancer goes, it appears…things are still…in flux…changing all the time.” (ON urban)
The physicians’ degree of trust in the source of the guideline was the fourth component. A participant said, “If you get a guideline from a consensus group where…a group of specialists get together…including some family docs…certainly I would take that with more…clout.” (AB rural)
The fifth component was the perceived effectiveness of a particular screening maneuver. One physician said, “In the…years that we’ve been [screening] we have found cancers at the stage A and B…that have been easily looked after…. We have not had 1 patient pass away.” (AB rural)
Physicians viewed their clinical experience as influencing their cancer screening decisions, and many felt that they were much more likely to order screening tests early in their careers. A participant said, “In terms of screening there’s a tendency, especially when you’re young and keen and scared, that you’re gonna miss something.” (AB urban)
Physicians were concerned about missing a diagnosis of cancer. If they actually had such an experience, it subsequently lowered their threshold for cancer screening for some time afterward. One physician said, “Suppose you missed a case of colorectal cancer, and someone else finds it; then you tend to run gun shy for a long time and perhaps overinvestigate and over-refer for a time.” (BC rural)
Colleagues could positively or negatively influence screening decisions. A participant said, “Some guidelines come out, and somebody will say, ‘Oh that’s trash. I’m not going to do that.’ And then it’s a little hard for the rest of us to easily incorporate that.” (BC rural)
Family physicians also felt that they had a unique screening style compared with specialists, stemming from their continuing long-term relationships with their patients. One physician said, “The specialists will tend to jump on the blood test wagon a lot faster than I think we will, because again they don’t know the patients.” (AB urban)
Time and financial costs were also identified as important practice factors in the decision-making process. A participant said, “Economics also plays a part…because it can take…half an hour to explain to a patient why you don’t want to do something. It can take 2 minutes to do it.” (ON rural)
The Physician-Patient Relationship
Decisions about cancer screening took place within an interactive relationship between the patient and physician. Physicians characterized the relationship as one of varying intensity and depth, and there appeared to be 3 key points about the relationship in terms of cancer screening. First, the stronger and more positive the relationship, the more likely that the physician would feel free to engage the patient in a discussion about not performing a test that is based on an unclear or negative guideline. One physician said, “If you’ve known somebody for a long time and they come to you with something that you don’t think is right, it’s a little bit easier to talk to them.” (PEI rural)
Conversely, if the relationship was new or tenuous, physicians felt “The lack of a good relationship has an impact…they tend not to go along with your recommendations.” (AB rural)
The second point regarding the physician-patient relationship was that when a guideline was unclear, it often called for a different interaction than when the guideline was clear. It involved more information giving, presented in a manner that assisted the patient. One physician said, “I try to give the patient as much information as I have, in words that they will understand, so that they can come to an informed decision. That’s what I do when the guidelines are unclear.” (ON urban)
The process of information-giving promoted finding common ground, particularly when patients were requesting a screening maneuver not backed by clear evidence. One participant said:[For] patients who want tests that we don’t necessarily think are indicated, I follow the evidence, and that’s a negotiation. …an explanation of the evidence and then almost throw it back at the patient...it’s not medical-legal. It’s not economic. It’s between me and my patient. (QC urban)
Finally, many physician participants observed that even when the guidelines are clear, many cancer screening decisions are not. As a result, they noted that this often necessitated a process of finding common ground by engaging patients in mutual decision making.
Discussion
Many of the factors we identified have been described previously.18-36 However, to the best of our knowledge they have not been combined into a comprehensive typology for cancer screening decision making that includes the physician-patient relationship and that deals with unclear and conflicting guidelines. One conceptual framework for the determinants of screening behavior22 is based on pediatric vaccinations and does not include unclear or controversial guidelines. Another more recent model is based on cancer care, not screening, but it does include some elements of communication between provider and patient.23 Our proposed model of decision making regarding cancer screening Figure 1 is a modification of these frameworks based on our findings and is specific to decisions about cancer screening.
One unique feature of our model is that it is embedded in the physician-patient relationship. In particular, the quality of this relationship and the clarity of the recommendation appears to be most important. It involves an interactive process and mutual discussion with the patient. This ultimately includes finding agreement and culminates in a mutual agreement between the patient and the physician about the cancer screening maneuver.37,38 Our findings are also in concordance with other literature on physician test-ordering. The concern about missing a diagnosis of cancer is similar to “chagrin bias” —when physicians are more likely to order inappropriate chest radiographs if they anticipated feeling regret if they missed a diagnosis of pneumonia.39
Limitations
Although attempts were made to have regional representation from the entire country (Canada), the findings may not be transferable to other family medicine settings. Two of the 10 focus groups were conducted by teleconference, which may bias results, because it is a different data collection method. However, previous experience with telephone focus groups had been successful. (C.H., personal communication) The 2 teleconference groups did not provide markedly different data from those conducted in-person. Also, because of budget restraints, 5 different moderators were used. The investigators organized training sessions to standardize focus group moderation across sites; however, it is difficult to estimate the potential bias, given that moderators have their own styles. Finally, the data were based on the perspective of physicians and not patients.
Future Research
In the next phase of our study we will test the model’s factors quantitatively on a random sample of physicians and go through the same steps with a patient/consumer sample. Ultimately, we will use a modified model to design interventions to assist with the implementation of preventive services guidelines.
Conclusions
Our findings are of importance for those implementing preventive care guidelines. The focus group participants were clearly less happy with guidelines that were equivocal, and were less likely to follow them. Patient factors and the physician-patient relationship appear to be important in such cases. Although patient-oriented decision aids could help physicians in these situations, it is clearly more difficult to develop aids to guide patients in settings when the evidence is unclear, because the information required is more complex. The family physicians’ perceptions of the effectiveness of a particular screening test was very important, perhaps more important to the participants than the scientific evidence behind a guideline. Although personal experience is a weak and unscientific level of evidence subject to many biases, it is likely an important influence on cancer screening decision making in primary care, particularly when the evidence is uncertain. Future education efforts directed at primary care providers should address the influence of personal experience as well as the failure to attend to the level of evidence behind recommendations.
Acknowledgments
Our project was funded by a peer-reviewed grant from the Medical Research Council of Canada (grant number 14673) and by the Prince Edward Island Cancer Research Council. We wish to thank the staff of the Department of Family and Community Medicine, University of Toronto, for their tireless support of this project.
STUDY DESIGN: We analyzed discussions with focus groups using a constant comparative approach.
POPULATION: A total of 73 family physicians in active practice participated in 10 focus groups (1 urban group and 1 rural group in each of 5 Canadian provinces).
OUTCOME MEASURES: Our main outcome measures were participants’ perceptions regarding cancer screening when the guidelines were unclear or conflicting.
RESULTS: We propose a model of the determinants of cancer screening decision making with regard to unclear and conflicting guidelines. This model is rooted in the physician-patient relationship, and is an interactive process influenced by patient factors (anxiety, expectations, and family history) and physician factors (perception of guidelines, clinical practice experience, influence of colleagues, distinction between the screening styles of specialists and family physicians, and the amount of time and financial costs involved in performing the maneuver).
CONCLUSIONS: Our model is unique, because it is embedded in the physician-patient relationship. Ultimately, a modified model could be used to design interventions to assist with the implementation of preventive services guidelines.
Every year physicians and patients receive hundreds of messages about guidelines for cancer screening. Ideally, physicians will adopt and adhere to the evidence-based clinical practice guidelines. By doing so, there is maximum application of a proven technology to those who can most benefit, and valuable resources are not wasted in examinations that are not based on good or fair evidence. However, many physicians are not adhering to cancer screening guidelines backed by good evidence.1,2 Also, many are performing cancer screening procedures that are not recommended (either because of a lack of evidence or because they have been shown to be ineffective).3
Most of the literature on physician cancer screening has dealt with facilitators or barriers to the adoption of commonly recommended guidelines. These studies did not address the factors that affect physician practice when the guidelines are unclear or conflicting, or when they clearly recommend against the procedure. We defined an “unclear” guideline as a “C” recommendation (insufficient evidence to recommend the maneuver) from the Canadian Task Force on the Periodic Health Examination (CTFPHE).4 Guidelines were “conflicting” when at least 2 organizations gave different recommendations for the same cancer screening examination.
Despite the CTFPHE guidelines,4 inconsistencies in practice remain. Although the CTFPHE recommends that breast cancer screening begin at age 50 years, 59% of women aged 40 to 49 years reported having mammograms in 1994,5 a rate nearly equivalent to those aged 50 to 59 in Ontario.6 It is clear that family physicians—the major cancer screeners in many countries—are frequently not following guidelines. The use of ineffective procedures or those for which the evidence is unclear can waste scarce health resources and lead to harm for those whose test results are false positive. The objective of our study7 was to determine the factors involved in the cancer screening decisions of family physicians in situations where the clinical practice guidelines were unclear or conflicting (prostate-specific antigen testing, mammography for ages 40 to 49 years, colorectal tests) as opposed to when they were clear and uncontroversial.
Methods
Ten focus groups7 were conducted with 1 urban group and 1 rural group in each of 5 Canadian provinces: British Columbia (BC), Alberta (AB), Ontario (ON), Quebec (QC), and Prince Edward Island (PEI). Ethical approval was obtained from all participating institutions. We focused on family physicians because they are the main preventive health care providers in Canada, and physician recommendation is the most important predictor of whether an individual obtains a particular screening test.8 Eight focus groups were conducted face to face, and 2 were done by teleconference because of the geographic remoteness of 2 rural areas. Each focus group was co-facilitated by a local research assistant and 1 of the investigators. The focus group moderators participated in a 2-hour training session to ensure standardization across sites. The group sessions lasted approximately 60 to 90 minutes; all were audiotaped and transcribed verbatim.*Table w1
Recruitment and Sampling
We used maximum variation sampling to ensure heterogeneity within the groups and to recruit physicians who would serve as information-rich participants with a wide range for age, practice type, location, and education.9,10 Recruitment involved a 2-step process:11 First, urban and rural family physicians were randomly selected from lists provided by each local area’s licensing body; and second, physician recruiters (“leader figures”) from each local area identified physicians who they believed would provide an adequate variance of opinions.
Data Collection and Analysis
Data collection and analysis occurred iteratively.12-14 After every focus group 3 investigators reviewed transcripts independently to identify the central issues that emerged. Over several meetings they compared and combined their independent analyses. Emerging themes were explored and expanded in subsequent focus groups. Although saturation15 had been achieved by the 8th focus group, we completed the final 2 groups to ensure regional representation. The second step in the analysis involved determining the similarities, differences, and potential connections among key words, phrases, and concepts within and among each focus group transcript. Finally, the themes and subcategories of all focus groups were compared and contrasted, and the quotes that most accurately illustrated the themes were identified.
Trustworthiness and Validation
All groups were audiotaped and transcribed verbatim, and extensive field notes were made during the focus groups and throughout the analysis. Validation of the data was achieved by conducting member-checking interviews16 with 15 information-rich participants from the focus groups after completion of the initial analysis. We then refined the themes.
Results
The physicians’ demographics Table 1 reflect the Canadian family physician population.17 Three major themes emerged from the analysis as determinants of cancer screening with unclear or controversial guidelines: patient factors, physician factors, and physician-patient relationship factors Table 2.
Patient Factors
Patient factors included expectations, anxieties, family history, peers, and media influences. Many of the physician participants commented that patient expectations and demands for screening were major determinants of their decision to screen when guidelines were unclear. Although they expressed discomfort with this behavior, physicians acknowledged being frequently swayed by patient demands. One said, “I think that if the patient comes into my office and he wants something, that influences me a hell of a lot.” (QC rural)
The physicians also suggested that patients’ anxieties about cancer were important. The higher the perceived anxiety, the more likely they were to order the relevant cancer screening test, even if the recommendations were unclear. A participant said, “If a patient came in with a particular anxiety and would be allayed by [screening]…I would go ahead and recommend it.” (BC rural)
The presence of any positive family history appeared to influence the physicians’ screening decisions, even if it was not a recognized risk factor in the cancer screening guideline. Physicians also felt that the media is an important influence on patients’ requests for screening. One of the physicians said, “I think the media really influences a lot of patients, and unfortunately it doesn’t always give them the correct information.” (ON urban)
Physician Factors
Physician factors included the perception of guidelines, clinical practice experience, the influence of colleagues, the distinction between the screening styles of specialists and family physicians, and the time and financial costs involved in performing the screening maneuver. The 2 most important determinants appeared to be the physicians’ perceptions of guidelines and their clinical experience.
The physicians’ perception of guidelines had 5 components Table 2. First, many physicians saw guidelines as just guidelines and not as directives. This was most evident when the guideline was viewed as unclear or conflicting. Second, many indicated that unclear guidelines are not guidelines at all and that their task was to individualize the screening decisions to patients and their situations. A participant said, “If they’re unclear, then you have to use your judgment in terms of the patient, your patient population, their follow-up ability, what their risk factors, age, etcetera, are.” (AB rural)
The third perception of guidelines was confusion due to the multiplicity and changing nature of guidelines. One physician said, “As far as breast cancer goes, it appears…things are still…in flux…changing all the time.” (ON urban)
The physicians’ degree of trust in the source of the guideline was the fourth component. A participant said, “If you get a guideline from a consensus group where…a group of specialists get together…including some family docs…certainly I would take that with more…clout.” (AB rural)
The fifth component was the perceived effectiveness of a particular screening maneuver. One physician said, “In the…years that we’ve been [screening] we have found cancers at the stage A and B…that have been easily looked after…. We have not had 1 patient pass away.” (AB rural)
Physicians viewed their clinical experience as influencing their cancer screening decisions, and many felt that they were much more likely to order screening tests early in their careers. A participant said, “In terms of screening there’s a tendency, especially when you’re young and keen and scared, that you’re gonna miss something.” (AB urban)
Physicians were concerned about missing a diagnosis of cancer. If they actually had such an experience, it subsequently lowered their threshold for cancer screening for some time afterward. One physician said, “Suppose you missed a case of colorectal cancer, and someone else finds it; then you tend to run gun shy for a long time and perhaps overinvestigate and over-refer for a time.” (BC rural)
Colleagues could positively or negatively influence screening decisions. A participant said, “Some guidelines come out, and somebody will say, ‘Oh that’s trash. I’m not going to do that.’ And then it’s a little hard for the rest of us to easily incorporate that.” (BC rural)
Family physicians also felt that they had a unique screening style compared with specialists, stemming from their continuing long-term relationships with their patients. One physician said, “The specialists will tend to jump on the blood test wagon a lot faster than I think we will, because again they don’t know the patients.” (AB urban)
Time and financial costs were also identified as important practice factors in the decision-making process. A participant said, “Economics also plays a part…because it can take…half an hour to explain to a patient why you don’t want to do something. It can take 2 minutes to do it.” (ON rural)
The Physician-Patient Relationship
Decisions about cancer screening took place within an interactive relationship between the patient and physician. Physicians characterized the relationship as one of varying intensity and depth, and there appeared to be 3 key points about the relationship in terms of cancer screening. First, the stronger and more positive the relationship, the more likely that the physician would feel free to engage the patient in a discussion about not performing a test that is based on an unclear or negative guideline. One physician said, “If you’ve known somebody for a long time and they come to you with something that you don’t think is right, it’s a little bit easier to talk to them.” (PEI rural)
Conversely, if the relationship was new or tenuous, physicians felt “The lack of a good relationship has an impact…they tend not to go along with your recommendations.” (AB rural)
The second point regarding the physician-patient relationship was that when a guideline was unclear, it often called for a different interaction than when the guideline was clear. It involved more information giving, presented in a manner that assisted the patient. One physician said, “I try to give the patient as much information as I have, in words that they will understand, so that they can come to an informed decision. That’s what I do when the guidelines are unclear.” (ON urban)
The process of information-giving promoted finding common ground, particularly when patients were requesting a screening maneuver not backed by clear evidence. One participant said:[For] patients who want tests that we don’t necessarily think are indicated, I follow the evidence, and that’s a negotiation. …an explanation of the evidence and then almost throw it back at the patient...it’s not medical-legal. It’s not economic. It’s between me and my patient. (QC urban)
Finally, many physician participants observed that even when the guidelines are clear, many cancer screening decisions are not. As a result, they noted that this often necessitated a process of finding common ground by engaging patients in mutual decision making.
Discussion
Many of the factors we identified have been described previously.18-36 However, to the best of our knowledge they have not been combined into a comprehensive typology for cancer screening decision making that includes the physician-patient relationship and that deals with unclear and conflicting guidelines. One conceptual framework for the determinants of screening behavior22 is based on pediatric vaccinations and does not include unclear or controversial guidelines. Another more recent model is based on cancer care, not screening, but it does include some elements of communication between provider and patient.23 Our proposed model of decision making regarding cancer screening Figure 1 is a modification of these frameworks based on our findings and is specific to decisions about cancer screening.
One unique feature of our model is that it is embedded in the physician-patient relationship. In particular, the quality of this relationship and the clarity of the recommendation appears to be most important. It involves an interactive process and mutual discussion with the patient. This ultimately includes finding agreement and culminates in a mutual agreement between the patient and the physician about the cancer screening maneuver.37,38 Our findings are also in concordance with other literature on physician test-ordering. The concern about missing a diagnosis of cancer is similar to “chagrin bias” —when physicians are more likely to order inappropriate chest radiographs if they anticipated feeling regret if they missed a diagnosis of pneumonia.39
Limitations
Although attempts were made to have regional representation from the entire country (Canada), the findings may not be transferable to other family medicine settings. Two of the 10 focus groups were conducted by teleconference, which may bias results, because it is a different data collection method. However, previous experience with telephone focus groups had been successful. (C.H., personal communication) The 2 teleconference groups did not provide markedly different data from those conducted in-person. Also, because of budget restraints, 5 different moderators were used. The investigators organized training sessions to standardize focus group moderation across sites; however, it is difficult to estimate the potential bias, given that moderators have their own styles. Finally, the data were based on the perspective of physicians and not patients.
Future Research
In the next phase of our study we will test the model’s factors quantitatively on a random sample of physicians and go through the same steps with a patient/consumer sample. Ultimately, we will use a modified model to design interventions to assist with the implementation of preventive services guidelines.
Conclusions
Our findings are of importance for those implementing preventive care guidelines. The focus group participants were clearly less happy with guidelines that were equivocal, and were less likely to follow them. Patient factors and the physician-patient relationship appear to be important in such cases. Although patient-oriented decision aids could help physicians in these situations, it is clearly more difficult to develop aids to guide patients in settings when the evidence is unclear, because the information required is more complex. The family physicians’ perceptions of the effectiveness of a particular screening test was very important, perhaps more important to the participants than the scientific evidence behind a guideline. Although personal experience is a weak and unscientific level of evidence subject to many biases, it is likely an important influence on cancer screening decision making in primary care, particularly when the evidence is uncertain. Future education efforts directed at primary care providers should address the influence of personal experience as well as the failure to attend to the level of evidence behind recommendations.
Acknowledgments
Our project was funded by a peer-reviewed grant from the Medical Research Council of Canada (grant number 14673) and by the Prince Edward Island Cancer Research Council. We wish to thank the staff of the Department of Family and Community Medicine, University of Toronto, for their tireless support of this project.
1. Main DS, Cohen SJ, DiClemente CC. Measuring physician readiness to change cancer screening: preliminary results. Am J Prev Med 1995;11:54-58.
2. Lomas J, Anderson GM, Domnick-Pierre K, Vayda E, Enkin MW, Hannah WJ. Do practice guidelines guide practice? The effects of a consensus statement on the practice of physicians. N Engl J Med 1989;321:1306-11.
3. Zyzanski SJ, Stange KC, Kelly R, et al. Family physicians’ disagreements with the US Preventive Services Task Force recommendations. J Fam Pract 1994;39:140-47.
4. Canadian Task Force on the Periodic Health Examination The Canadian guide to clinical preventive health care. Ottawa, Canada: Health Canada; 1994.
5. Statistics Canada 1994 national population health survey. Public use data file; 1995.
6. Goel V. Whose guidelines are they anyways? Mammography screening in Ontario. Can J Publ Health 1996;87:181-82.
7. Brown JB. The use of focus groups in clinical research. In: Crabtree BF, Miller WL, eds. Doing qualitative research. Newbury Park, Calif: Sage Publications; 1999.
8. White E, Urban N, Taylor V. Mammography utilization, public health impact, and cost-effectiveness in the United States. Ann Rev Pub Health 1993;14:605-33.
9. Patton M. Qualitative evaluation and research methods. 2nd ed. Newbury Park, Calif: Sage Publications; 1990.
10. Kuzel AJ, Like RC. Standards of trustworthiness for qualitative studies in primary care. In: Norton PG, Stewart M, Tudiver F, Bass MF, Dunn EV, eds. Primary care research: traditional and innovative approaches. Newbury Park, Calif: Sage Publications; 1991.
11. Borgiel AE, Dunn EV, Lamont CT, et al. Recruiting family physicians as participants in research. Fam Pract 1989;6:168-72.
12. Morgan DL. Focus groups as qualitative research. Newbury Park, Calif: Sage Publications; 1988.
13. Morgan DL. Successful focus groups: advancing the state of the art. Newbury Park, Calif: Sage Publications; 1993.
14. Strauss AL, Corbin J. Basics of qualitative research: grounded theory, procedure and techniques. Beverly Hills, Calif: Sage Publication; 1990.
15. Kuzel A. Sampling in qualitative theory. In: Crabtree BF, Miller WL. Doing qualitative research. Newbury Park, Calif: Sage Publications; 1999;41-4217.-
16. Gilchrist VJ, Williams RL. Key informant interviews. In: Crabtree BF, Miller WL. Doing qualitative research. Newbury Park, Calif: Sage Publications; 1999:81.
17. Southam Directories Group. National MD select profiler version. Toronto, Ontario, Canada: Don Mills Southam Directories Group; 1999.
18. Battista RN, Williams JI, MacFarlane LA. Determinants of primary medical practice in adult cancer prevention. Med Care 1986;24:216-26.
19. Battista RN, Williams JI, MacFarlane LA. Determinants of preventive practices in fee-for-service primary care. Am J Prev Med 1990;6:6-11.
20. Burack RC. Barriers to clinical preventive medicine. Prim Care 1989;16:245-50.
21. Frame PS. Breast cancer screening in older women: the family practice perspective. J Geronol 1992;47:131-33.
22. Pathman DE, Konrad TR, Freed GL, Freeman VA, Koch GG. The awareness-to-adherence model of the steps to clinical guideline compliance. Med Care 1996;34:873-89.
23. Mandelblatt JS, Yabroff KR, Kerner JF. Equitable access to cancer services: a review of barriers to quality care. Cancer 1999;86:2378-90.
24. Langley GR, Tritchler DL, Llewellyn-Thomas HA, Till JE. Use of written cases to study factors associated with regional variations in referral rates. J Clin Epidemiol 1991;44:391-402.
25. Zyzanski SJ, Stange KC, Kelly R, et al. Family physicians’ disagreements with the US Preventive Services Task Force recommendations. J Fam Pract 1994;39:140-47.
26. Stange KC, Kelly R, Chao J, et al. Physician agreement with US Preventive Services Task Force recommendations. J Fam Pract 1992;34:409-16.
27. Mittman BS, Tonesk X, Jacobson PD. Implementing clinical practice CPGs: social influence strategies and practitioner behavior change. QRB 1992;18:413-22.
28. Brownson RC, Davis JR, Simms SG, Kern TG, Harmon RG. Cancer control knowledge and priorities among primary care physicians. J Cancer Educ 1993;8:35-41.
29. Costanza ME, Stoddard AM, Zapks JG, Gaw VP, Barth R. Physician compliance with mammography guidelines: barriers and enhancers. J Am Board Fam Pract 1992;5:143-52.
30. Weingarten S, Stone E, Hayward R, et al. The adoption of preventive care practice guidelines by primary care physicians. J Gen Intern Med 1990;10:138-44.
31. Young MJ, Fried LS, Eisenberg J, Hershey J, Williams S. Do cardiologists have higher thresholds for recommending coronary arteriography than family physicians? Health Serv Res 1987;22:623-35.
32. Smith HE, Herbert CP. Preventive practice among primary care physicians in British Columbia: relation to recommendations of the Canadian Task Force on the Periodic Health Examination. Can Med Assoc J 1993;149:1795-800.
33. Triezenberg DJ, Smith MA, Holmes TM. Cancer screening and detection in family practice: a MIRNET study. J Fam Pract 1995;40:27-33.
34. Summerton N. Positive and negative factors in defensive medicine: a questionnaire study of general practitioners. BMJ 1995;310:27-29.
35. Jones I, Morrell D. General practitioners’ background knowledge of their patients. Fam Pract 1995;12:49-53.
36. Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. J Am Med Assoc 1999;282:1458-65.
37. Stewart M, Brown JB, Boon H, Galajda J, Meredith L, Sangster M. Evidence on patient-doctor communication. Cancer Prev Control 1999;3:25-30.
38. Stewart M, Brown JB, Weston WW, McWhinney IR, McWilliam CL, Freeman TR. Patient-centered medicine: transforming the clinical method. Thousand Oaks, Calif: Sage Publications; 1995.
39. Heckerling PS, Tape TG, Wigton RS. Relation of physicians’ predicted probabilities of pneumonia to their utilities for ordering chest x-rays to detect pneumonia. Med Decis Making 1992;12:32-38.
1. Main DS, Cohen SJ, DiClemente CC. Measuring physician readiness to change cancer screening: preliminary results. Am J Prev Med 1995;11:54-58.
2. Lomas J, Anderson GM, Domnick-Pierre K, Vayda E, Enkin MW, Hannah WJ. Do practice guidelines guide practice? The effects of a consensus statement on the practice of physicians. N Engl J Med 1989;321:1306-11.
3. Zyzanski SJ, Stange KC, Kelly R, et al. Family physicians’ disagreements with the US Preventive Services Task Force recommendations. J Fam Pract 1994;39:140-47.
4. Canadian Task Force on the Periodic Health Examination The Canadian guide to clinical preventive health care. Ottawa, Canada: Health Canada; 1994.
5. Statistics Canada 1994 national population health survey. Public use data file; 1995.
6. Goel V. Whose guidelines are they anyways? Mammography screening in Ontario. Can J Publ Health 1996;87:181-82.
7. Brown JB. The use of focus groups in clinical research. In: Crabtree BF, Miller WL, eds. Doing qualitative research. Newbury Park, Calif: Sage Publications; 1999.
8. White E, Urban N, Taylor V. Mammography utilization, public health impact, and cost-effectiveness in the United States. Ann Rev Pub Health 1993;14:605-33.
9. Patton M. Qualitative evaluation and research methods. 2nd ed. Newbury Park, Calif: Sage Publications; 1990.
10. Kuzel AJ, Like RC. Standards of trustworthiness for qualitative studies in primary care. In: Norton PG, Stewart M, Tudiver F, Bass MF, Dunn EV, eds. Primary care research: traditional and innovative approaches. Newbury Park, Calif: Sage Publications; 1991.
11. Borgiel AE, Dunn EV, Lamont CT, et al. Recruiting family physicians as participants in research. Fam Pract 1989;6:168-72.
12. Morgan DL. Focus groups as qualitative research. Newbury Park, Calif: Sage Publications; 1988.
13. Morgan DL. Successful focus groups: advancing the state of the art. Newbury Park, Calif: Sage Publications; 1993.
14. Strauss AL, Corbin J. Basics of qualitative research: grounded theory, procedure and techniques. Beverly Hills, Calif: Sage Publication; 1990.
15. Kuzel A. Sampling in qualitative theory. In: Crabtree BF, Miller WL. Doing qualitative research. Newbury Park, Calif: Sage Publications; 1999;41-4217.-
16. Gilchrist VJ, Williams RL. Key informant interviews. In: Crabtree BF, Miller WL. Doing qualitative research. Newbury Park, Calif: Sage Publications; 1999:81.
17. Southam Directories Group. National MD select profiler version. Toronto, Ontario, Canada: Don Mills Southam Directories Group; 1999.
18. Battista RN, Williams JI, MacFarlane LA. Determinants of primary medical practice in adult cancer prevention. Med Care 1986;24:216-26.
19. Battista RN, Williams JI, MacFarlane LA. Determinants of preventive practices in fee-for-service primary care. Am J Prev Med 1990;6:6-11.
20. Burack RC. Barriers to clinical preventive medicine. Prim Care 1989;16:245-50.
21. Frame PS. Breast cancer screening in older women: the family practice perspective. J Geronol 1992;47:131-33.
22. Pathman DE, Konrad TR, Freed GL, Freeman VA, Koch GG. The awareness-to-adherence model of the steps to clinical guideline compliance. Med Care 1996;34:873-89.
23. Mandelblatt JS, Yabroff KR, Kerner JF. Equitable access to cancer services: a review of barriers to quality care. Cancer 1999;86:2378-90.
24. Langley GR, Tritchler DL, Llewellyn-Thomas HA, Till JE. Use of written cases to study factors associated with regional variations in referral rates. J Clin Epidemiol 1991;44:391-402.
25. Zyzanski SJ, Stange KC, Kelly R, et al. Family physicians’ disagreements with the US Preventive Services Task Force recommendations. J Fam Pract 1994;39:140-47.
26. Stange KC, Kelly R, Chao J, et al. Physician agreement with US Preventive Services Task Force recommendations. J Fam Pract 1992;34:409-16.
27. Mittman BS, Tonesk X, Jacobson PD. Implementing clinical practice CPGs: social influence strategies and practitioner behavior change. QRB 1992;18:413-22.
28. Brownson RC, Davis JR, Simms SG, Kern TG, Harmon RG. Cancer control knowledge and priorities among primary care physicians. J Cancer Educ 1993;8:35-41.
29. Costanza ME, Stoddard AM, Zapks JG, Gaw VP, Barth R. Physician compliance with mammography guidelines: barriers and enhancers. J Am Board Fam Pract 1992;5:143-52.
30. Weingarten S, Stone E, Hayward R, et al. The adoption of preventive care practice guidelines by primary care physicians. J Gen Intern Med 1990;10:138-44.
31. Young MJ, Fried LS, Eisenberg J, Hershey J, Williams S. Do cardiologists have higher thresholds for recommending coronary arteriography than family physicians? Health Serv Res 1987;22:623-35.
32. Smith HE, Herbert CP. Preventive practice among primary care physicians in British Columbia: relation to recommendations of the Canadian Task Force on the Periodic Health Examination. Can Med Assoc J 1993;149:1795-800.
33. Triezenberg DJ, Smith MA, Holmes TM. Cancer screening and detection in family practice: a MIRNET study. J Fam Pract 1995;40:27-33.
34. Summerton N. Positive and negative factors in defensive medicine: a questionnaire study of general practitioners. BMJ 1995;310:27-29.
35. Jones I, Morrell D. General practitioners’ background knowledge of their patients. Fam Pract 1995;12:49-53.
36. Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. J Am Med Assoc 1999;282:1458-65.
37. Stewart M, Brown JB, Boon H, Galajda J, Meredith L, Sangster M. Evidence on patient-doctor communication. Cancer Prev Control 1999;3:25-30.
38. Stewart M, Brown JB, Weston WW, McWhinney IR, McWilliam CL, Freeman TR. Patient-centered medicine: transforming the clinical method. Thousand Oaks, Calif: Sage Publications; 1995.
39. Heckerling PS, Tape TG, Wigton RS. Relation of physicians’ predicted probabilities of pneumonia to their utilities for ordering chest x-rays to detect pneumonia. Med Decis Making 1992;12:32-38.