User login
Overview of breast cancer staging and surgical treatment options
In the late 19th century, breast cancer was considered a fatal disease. That began to change in the 1880s when W.S. Halsted described the radical mastectomy as the way to treat patients with breast cancer.1 This aggressive surgical treatment—in which the breast, axillary lymph nodes, and chest muscles are all removed—remained the standard of care throughout much of the 20th century; as late as the early 1970s, nearly half (48%) of breast cancer patients were treated with radical mastectomy. During the 1970s, however, the Halsted radical mastectomy was largely abandoned for a less-disfiguring muscle-sparing technique called the modified radical mastectomy; by 1981, only 3% of patients underwent the Halsted mastectomy.2
The 1980s heralded even more minimally invasive techniques with the advent of breast conservation therapy, in which an incision is made over the tumor and the tumor is completely removed with negative margins, leaving behind normal breast tissue. (This procedure has been referred to by many different names, including definitive excision, lumpectomy, quadrantectomy, and partial mastectomy; since they all mean the same thing, for clarity and consistency this article will use “breast conservation therapy” throughout.) During the 1990s, surgical invasiveness was further minimized with the emergence of sentinel lymph node excision.
An important contributor to this evolution in the standard of breast cancer therapy since the 1970s has been the National Surgical Adjuvant Breast and Bowel Project (NSABP), a National Cancer Institute–funded clinical trials cooperative group. NSABP studies have been the driving force to show that the extent of surgery could be reduced without compromising outcome.3 These studies, along with several other trials, have resulted in a marked reduction in surgical aggressiveness and a multitude of adjuvant therapies for women with breast cancer. This article will briefly explore where this evolution has brought us in terms of the surgical options available for treatment of breast cancer today. We also discuss other key components in the management of women with newly diagnosed breast cancer, including cancer staging, patient counseling, and assessment of axillary lymph nodes.
BREAST CANCER CLASSIFICATION AND STAGING
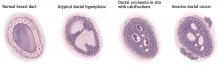
Pathologic classification
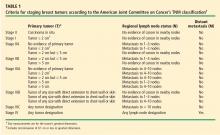
Cancer staging
A simpler method relies on the National Cancer Institute’s SEER (Surveillance, Epidemiology, and End Results) summary staging system.5 This system classifies tumors as “localized” (contained in the breast, either in situ or invasive), “regional” (identified in regional lymph nodes), or “metastatic” (spread to distant organ systems).
Of course, patients cannot be told their stage until after surgery, when a final pathologic report detailing tumor size and nodal status is available. Some patients will never be definitively staged—for instance, those who undergo neoadjuvant chemotherapy for locally advanced disease prior to lymph node dissection, or those who do not have a metastatic work-up. The metastatic work-up involves ordering of additional tests to assess for metastasis, but only when prompted by specific patient symptoms. Thus, if the patient has shortness of breath, a chest radiograph or a chest computed tomograph (CT) needs to be ordered; for elevated liver enzymes, CTs of the abdomen and pelvis are ordered; for central nervous system symptoms, brain magnetic resonance imaging (MRI) is ordered; and for back pain or bone pain, a bone scan is ordered to rule out metastatic disease to bone.
INITIAL PATIENT ASSESSMENT AND COUNSELING
Relationship-building is fundamental
Following an initial diagnosis of breast cancer, the patient must undergo an assessment for local and systemic disease. The surgeon, as a member of a multi-disciplinary breast cancer treatment team, often spearheads this initial assessment. This first visit must go beyond mere clinical evaluation, however, and include thorough discussion and relationship-building with the patient, as this early meeting establishes a relationship with the patient that will carry through her entire process of cancer care. For a true understanding between patient and surgeon to occur, it is critical for patients to be comfortable in sharing their fears, expectations, and lifestyle needs. Following a diagnosis of breast cancer, the initial reactions women go through include both fear and realization of one’s own mortality. Although these responses may no longer be justified by the reality of patient outcomes in most cases, they are normal and fully understandable reactions. For this reason, clinicians must be sensitive to these reactions while being supportive about the efficacy of the treatment options available.
History, breast exam, and review of imaging studies
In addition to the establishment of communication and understanding, the vital components of this first meeting include a detailed medical history, a clinical breast examination, a review of imaging studies, and a discussion of treatment options.
The history should include all aspects of the patient’s reproductive history, her family history of breast cancer, and any comorbidities and medications being taken.
The clinical breast examination should give special attention to the shape (asymmetry), appearance (eg, dimpling, erythema, nipple inversion), and overall feel of the breasts. A palpable mass must be recorded in terms of its location in relation to the skin, the nipple-areola complex, and the chest wall, as well as the quadrant of the breast in which it lies. The regional lymph node basins need to be examined closely, including the axilla and supraclavicular nodes.
Imaging studies also need to be reviewed closely. Patients today frequently present with multiple types of imaging studies, including mammography, ultrasonography, and MRI. Occasionally patients also may present with nuclear medicine exam results, CTs, thermographic images, positron emission tomography studies, and bone scans. All radiology studies need to be reviewed closely and examined in the context of what they were ordered for and what utility they potentially provide.
Treatment options: Surgery is first step in most cases
Once the above components are addressed, the patient should be engaged in a discussion of treatment options. Most women with breast cancer will undergo some type of surgery in conjunction with radiation therapy, chemotherapy, or both. Generally, surgery takes place as the first part of a multiple-component therapy plan. The main goal of surgery is to remove the cancer and accurately define the stage of the disease.
Consider plastic surgery consultation
When indicated and available, consultation with a plastic surgery team may be appropriate at this stage to provide support and comfort to the patient so that she better understands her options for breast reconstruction along with those for breast cancer surgery. Recent data show that most general surgeons do not discuss reconstruction with their breast cancer patients before surgical breast cancer therapy, but that when such discussions do occur, they significantly influence patients’ treatment choices.6 Giving patients the chance to learn about reconstructive options through discussion with a plastic surgeon represents a good opportunity to provide complete patient care in a multidisciplinary way.
OVERVIEW OF SURGICAL OPTIONS
Two general approaches, no difference in survival
The two mainstays of surgical treatment today are (1) breast conservation therapy, generally followed by total or partial breast irradiation, and (2) mastectomy.
The prospective randomized trial data obtained from the NSABP trials have demonstrated no survival differences between patients with early-stage breast cancer based on whether they were treated with breast conservation therapy or mastectomy.2 Beyond this fundamental issue of survival, there are a number of nuances, many of them logistical, related to the success of either operation that the clinician must keep in mind when presenting these surgical choices to patients. These considerations are reviewed below.
Breast conservation therapy
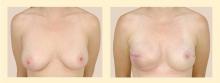
For breast conservation therapy, the ratio of tumor size to breast size must be small enough to ensure complete tumor removal with an acceptable cosmetic outcome. In general, it is estimated that up to 25% of the breast can be removed while still ensuring a “good” cosmetic outcome. Advances in closure techniques allowing for more tissue to be removed with even better cosmetic outcomes are known as oncoplastic closure. These techniques are mostly performed by breast oncologic surgeons, often in consultation or conjunction with plastic surgeons. (Reconstructive options following breast conservation therapy are reviewed in a subsequent article in this supplement.) Additionally, the patient must agree and be deemed a candidate for postoperative radiation therapy. The patient must be able to be followed clinically to enable early detection of a potential local recurrence.
Mastectomy
A second surgical option for patients is mastectomy. Today “mastectomy” can refer to any of several subtypes of surgical procedures, which are outlined below and should be considered on a patient-by-patient basis. Mastectomy is appropriate when breast conservation therapy is not possible (due to a large or multicentric tumor) or would result in poor cosmetic outcome, or when the patient specifically chooses a mastectomy.
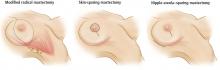
Simple mastectomy involves removal of the breast only, without removal of lymph nodes. Either of the incisions depicted in the left and center panels of Figure 3 can be used. Both modified radical mastectomy and simple mastectomy involve removal of the nipple and areola (nipple-areola complex).
Skin-sparing mastectomy (Figure 3, center) is performed when a patient is undergoing immediate breast reconstruction (using either a silicone or saline implant or autologous tissue). The goal is to remove all breast tissue, along with the nipple-areola complex, while preserving as much viable skin as possible to optimize the cosmetic outcome.7,8
Nipple-areola–sparing mastectomy. There is increasing experience with attempts to preserve the nipple-areola complex. These procedures attempt to preserve either the whole complex, termed nipple-areola–sparing mastectomy (sometimes called simply nipple-sparing mastectomy) (Figure 3, right), or just the areola, with removal of the nipple (areola-sparing mastectomy). These procedures are also performed in a skin-sparing fashion.
There is some controversy surrounding these techniques to spare the nipple and/or areola, including debate over which technique.nipple-areola–sparing mastectomy or areola-sparing mastectomy.may be more oncologically safe. Currently the literature shows that both are probably safe oncologic alternatives for remote tumors that do not have an extensive intraductal component. Generally, frozen sections are performed intraoperatively on the retroareola tissue to document that there is no evidence of tumor.9
SURGICAL COMPLICATIONS
Breast procedures are fairly safe operations, but every operation has a risk of complications. Reported complications of breast surgery include the following:
- Bleeding
- Infection (including both cellulitis and abscess)
- Seroma
- Arm morbidity (including lymphedema)
- Phantom breast syndrome
- Injury to the motor nerves.
Seromas often occur in patients after mastectomy or lymph node surgery. Prolonged lymphatic drainage is usually exacerbated by extensive axillary node involvement and obesity.
Arm morbidity can present in different ways. Lymphedema is the most common manifestation, with reported incidences of approximately 15% to 20% when axillary lymph node dissection is performed versus 7% when sentinel lymph node biopsy is done.10 The risk of lymphedema can be reduced by avoiding blood pressure measurements, venipunctures, and intravenous insertions in the arm on the side of the operation, as well as by wearing a compression sleeve on the affected arm during airplane flights.
Phantom breast syndrome is rare but may manifest as pain that may also involve itching, nipple sensation, erotic sensations, or premenstrual-type soreness.
Many surgeons have historically removed the intercostobrachial nerves but are now trying to preserve these nerves, which when removed cause loss of sensation in the upper inner arm. Although rare, nerve injury during an axillary procedure has been reported. It may involve the long thoracic nerve (denervating the serratus anterior muscle and causing a winged scapula) or the thoracodorsal bundle (denervating the latissimus dorsi muscle and causing difficulty with arm/shoulder adduction).
LOCAL CANCER RECURRENCE
Among women undergoing mastectomy for breast cancer, 10% to 15% will have a recurrence of cancer in the chest wall or axillary lymph nodes within 10 years.11 Similarly, among women undergoing breast conservation therapy plus radiation therapy, 10% to 15% will have in-breast cancer recurrence or recurrence in axillary lymph nodes within 10 years, although women who undergo breast conservation therapy without radiation have a much higher recurrence rate.11 Considerations for screening the surgically altered breast are discussed in the previous article in this supplement.
ASSESSMENT OF AXILLARY LYMPH NODES FOR METASTASIS
Even when patients have a known histologic diagnosis of breast cancer and have made a firm decision regarding the surgical option for removal of their cancer, the status of their axillary lymph nodes remains a great unanswered question until after the surgical procedure is completed. Lymph node status—ie, determining whether the cancer has spread to the axillary lymph nodes—still serves as the critical determinant for guiding adjuvant treatment, predicting survival, and assessing the risk of recurrence.
Axillary lymph node dissection
The standard approach for evaluating lymph node status has been a complete dissection of the axillary space, or axillary lymph node dissection. As briefly noted above, the axillary lymph nodes are anatomically classified into three levels as defined by their location relative to the pectoralis minor muscle. The extent of a nodal dissection can be defined by the number of nodes removed.
Sentinel node biopsy: A less-invasive alternative
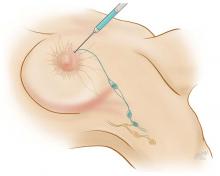
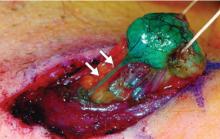
Axillary lymph node dissection has been called into question over the last 15 years due to its invasiveness and the potential morbidity associated with it (including lymphedema and paresthesias). As a result, sentinel lymph node biopsy, a minimally invasive technique for identifying axillary metastasis, was developed to avoid the need for (and risk of complications from) axillary lymph node dissection in patients who have a low probability of axillary metastasis.
Overall, however, it is now accepted that intraoperative lymph node mapping with sentinel lymphadenectomy is an effective and minimally invasive alternative to axillary lymph node dissection for identifying nodes containing metastases.
CONCLUSIONS
Decisions surrounding the choice of breast surgery procedure must be individualized to the patient and her desires and based on comprehensive patient evaluation and thorough patient counseling. Optimal results for the patient—oncologically, psychologically, and in terms of cosmetic outcomes—require consultation and collaboration among general surgeons, medical oncologists, genetic counselors, radiation oncologists, radiologists, and plastic surgeons to clarify the risks and benefits of various intervention options. Striving for this multidisciplinary collaboration will promote optimal patient management and the most favorable clinical outcomes.
- Bland CS. The Halsted mastectomy: present illness and past history. West J Med 1981; 134:549–555.
- Frykberg ER, Bland KI. Evolution of surgical principles and techniques for the management of breast cancer. In: Bland KI, Copeland EM III, eds. The Breast: Comprehensive Management of Benign and Malignant Disorders. 3rd ed. St. Louis, MO: Saunders; 2004:759–785.
- Newman LA, Mamounas EP. Review of breast cancer clinical trials conducted by the National Surgical Adjuvant Breast Project. Surg Clin N Am 2007; 87:279–305.
- Greene FL, Page DL, Fleming ID, et al, eds. Breast. In: AJCC Cancer Staging Manual. 6th ed. New York, NY: Springer; 2002:223–240.
- Young JJ, Roffers S, Gloeckler Ries L, et al. SEER Summary Staging Manual 2000: Codes and Coding Instructions. NIH Publication No. 01-4969. Bethesda, MD: National Institutes of Health; 2000.
- Alderman AK, Hawley ST, Waljee J, Mujahid M, Morrow M, Katz SJ. Understanding the impact of breast reconstruction on the surgical decision-making process for breast cancer. Cancer 2007; 112:489–494.
- Toth BA, Lappert P. Modified skin incisions for mastectomy: the need for plastic surgical input in preoperative planning. Plast Reconstr Surg 1991; 87:1048–1053.
- Cunnick GH, Mokbel K. Skin-sparing mastectomy. Am J Surg 2004; 188:78–84.
- Crowe JP Jr, Kim JA, Yetman R, et al. Nipple-sparing mastectomy: technique and results of 54 procedures. Arch Surg 2004; 139:148–150.
- Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst 2006; 98:599–609.
- Jacobson JA, Danforth DN, Cowan KH, et al. Ten-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancer. N Engl J Med 1995; 332:907–911.
- Tanis PJ, Nieweg OE, Valdés Olmos RA, et al. History of sentinel node and validation of the technique. Breast Cancer Res 2001; 3:109–112.
- Chagpar AB, Martin RC, Scoggins CR, et al. Factors predicting failure to identify a sentinel lymph node in breast cancer. Surgery 2005; 138:56–63.
- McMasters KM, Wong SL, Chao C, et al. Defining the optimal surgeon experience for breast cancer sentinel lymph node biopsy: a model for implementation of new surgical techniques. Ann Surg 2001; 234:292–300.
In the late 19th century, breast cancer was considered a fatal disease. That began to change in the 1880s when W.S. Halsted described the radical mastectomy as the way to treat patients with breast cancer.1 This aggressive surgical treatment—in which the breast, axillary lymph nodes, and chest muscles are all removed—remained the standard of care throughout much of the 20th century; as late as the early 1970s, nearly half (48%) of breast cancer patients were treated with radical mastectomy. During the 1970s, however, the Halsted radical mastectomy was largely abandoned for a less-disfiguring muscle-sparing technique called the modified radical mastectomy; by 1981, only 3% of patients underwent the Halsted mastectomy.2
The 1980s heralded even more minimally invasive techniques with the advent of breast conservation therapy, in which an incision is made over the tumor and the tumor is completely removed with negative margins, leaving behind normal breast tissue. (This procedure has been referred to by many different names, including definitive excision, lumpectomy, quadrantectomy, and partial mastectomy; since they all mean the same thing, for clarity and consistency this article will use “breast conservation therapy” throughout.) During the 1990s, surgical invasiveness was further minimized with the emergence of sentinel lymph node excision.
An important contributor to this evolution in the standard of breast cancer therapy since the 1970s has been the National Surgical Adjuvant Breast and Bowel Project (NSABP), a National Cancer Institute–funded clinical trials cooperative group. NSABP studies have been the driving force to show that the extent of surgery could be reduced without compromising outcome.3 These studies, along with several other trials, have resulted in a marked reduction in surgical aggressiveness and a multitude of adjuvant therapies for women with breast cancer. This article will briefly explore where this evolution has brought us in terms of the surgical options available for treatment of breast cancer today. We also discuss other key components in the management of women with newly diagnosed breast cancer, including cancer staging, patient counseling, and assessment of axillary lymph nodes.
BREAST CANCER CLASSIFICATION AND STAGING
Pathologic classification
Cancer staging
A simpler method relies on the National Cancer Institute’s SEER (Surveillance, Epidemiology, and End Results) summary staging system.5 This system classifies tumors as “localized” (contained in the breast, either in situ or invasive), “regional” (identified in regional lymph nodes), or “metastatic” (spread to distant organ systems).
Of course, patients cannot be told their stage until after surgery, when a final pathologic report detailing tumor size and nodal status is available. Some patients will never be definitively staged—for instance, those who undergo neoadjuvant chemotherapy for locally advanced disease prior to lymph node dissection, or those who do not have a metastatic work-up. The metastatic work-up involves ordering of additional tests to assess for metastasis, but only when prompted by specific patient symptoms. Thus, if the patient has shortness of breath, a chest radiograph or a chest computed tomograph (CT) needs to be ordered; for elevated liver enzymes, CTs of the abdomen and pelvis are ordered; for central nervous system symptoms, brain magnetic resonance imaging (MRI) is ordered; and for back pain or bone pain, a bone scan is ordered to rule out metastatic disease to bone.
INITIAL PATIENT ASSESSMENT AND COUNSELING
Relationship-building is fundamental
Following an initial diagnosis of breast cancer, the patient must undergo an assessment for local and systemic disease. The surgeon, as a member of a multi-disciplinary breast cancer treatment team, often spearheads this initial assessment. This first visit must go beyond mere clinical evaluation, however, and include thorough discussion and relationship-building with the patient, as this early meeting establishes a relationship with the patient that will carry through her entire process of cancer care. For a true understanding between patient and surgeon to occur, it is critical for patients to be comfortable in sharing their fears, expectations, and lifestyle needs. Following a diagnosis of breast cancer, the initial reactions women go through include both fear and realization of one’s own mortality. Although these responses may no longer be justified by the reality of patient outcomes in most cases, they are normal and fully understandable reactions. For this reason, clinicians must be sensitive to these reactions while being supportive about the efficacy of the treatment options available.
History, breast exam, and review of imaging studies
In addition to the establishment of communication and understanding, the vital components of this first meeting include a detailed medical history, a clinical breast examination, a review of imaging studies, and a discussion of treatment options.
The history should include all aspects of the patient’s reproductive history, her family history of breast cancer, and any comorbidities and medications being taken.
The clinical breast examination should give special attention to the shape (asymmetry), appearance (eg, dimpling, erythema, nipple inversion), and overall feel of the breasts. A palpable mass must be recorded in terms of its location in relation to the skin, the nipple-areola complex, and the chest wall, as well as the quadrant of the breast in which it lies. The regional lymph node basins need to be examined closely, including the axilla and supraclavicular nodes.
Imaging studies also need to be reviewed closely. Patients today frequently present with multiple types of imaging studies, including mammography, ultrasonography, and MRI. Occasionally patients also may present with nuclear medicine exam results, CTs, thermographic images, positron emission tomography studies, and bone scans. All radiology studies need to be reviewed closely and examined in the context of what they were ordered for and what utility they potentially provide.
Treatment options: Surgery is first step in most cases
Once the above components are addressed, the patient should be engaged in a discussion of treatment options. Most women with breast cancer will undergo some type of surgery in conjunction with radiation therapy, chemotherapy, or both. Generally, surgery takes place as the first part of a multiple-component therapy plan. The main goal of surgery is to remove the cancer and accurately define the stage of the disease.
Consider plastic surgery consultation
When indicated and available, consultation with a plastic surgery team may be appropriate at this stage to provide support and comfort to the patient so that she better understands her options for breast reconstruction along with those for breast cancer surgery. Recent data show that most general surgeons do not discuss reconstruction with their breast cancer patients before surgical breast cancer therapy, but that when such discussions do occur, they significantly influence patients’ treatment choices.6 Giving patients the chance to learn about reconstructive options through discussion with a plastic surgeon represents a good opportunity to provide complete patient care in a multidisciplinary way.
OVERVIEW OF SURGICAL OPTIONS
Two general approaches, no difference in survival
The two mainstays of surgical treatment today are (1) breast conservation therapy, generally followed by total or partial breast irradiation, and (2) mastectomy.
The prospective randomized trial data obtained from the NSABP trials have demonstrated no survival differences between patients with early-stage breast cancer based on whether they were treated with breast conservation therapy or mastectomy.2 Beyond this fundamental issue of survival, there are a number of nuances, many of them logistical, related to the success of either operation that the clinician must keep in mind when presenting these surgical choices to patients. These considerations are reviewed below.
Breast conservation therapy
For breast conservation therapy, the ratio of tumor size to breast size must be small enough to ensure complete tumor removal with an acceptable cosmetic outcome. In general, it is estimated that up to 25% of the breast can be removed while still ensuring a “good” cosmetic outcome. Advances in closure techniques allowing for more tissue to be removed with even better cosmetic outcomes are known as oncoplastic closure. These techniques are mostly performed by breast oncologic surgeons, often in consultation or conjunction with plastic surgeons. (Reconstructive options following breast conservation therapy are reviewed in a subsequent article in this supplement.) Additionally, the patient must agree and be deemed a candidate for postoperative radiation therapy. The patient must be able to be followed clinically to enable early detection of a potential local recurrence.
Mastectomy
A second surgical option for patients is mastectomy. Today “mastectomy” can refer to any of several subtypes of surgical procedures, which are outlined below and should be considered on a patient-by-patient basis. Mastectomy is appropriate when breast conservation therapy is not possible (due to a large or multicentric tumor) or would result in poor cosmetic outcome, or when the patient specifically chooses a mastectomy.
Simple mastectomy involves removal of the breast only, without removal of lymph nodes. Either of the incisions depicted in the left and center panels of Figure 3 can be used. Both modified radical mastectomy and simple mastectomy involve removal of the nipple and areola (nipple-areola complex).
Skin-sparing mastectomy (Figure 3, center) is performed when a patient is undergoing immediate breast reconstruction (using either a silicone or saline implant or autologous tissue). The goal is to remove all breast tissue, along with the nipple-areola complex, while preserving as much viable skin as possible to optimize the cosmetic outcome.7,8
Nipple-areola–sparing mastectomy. There is increasing experience with attempts to preserve the nipple-areola complex. These procedures attempt to preserve either the whole complex, termed nipple-areola–sparing mastectomy (sometimes called simply nipple-sparing mastectomy) (Figure 3, right), or just the areola, with removal of the nipple (areola-sparing mastectomy). These procedures are also performed in a skin-sparing fashion.
There is some controversy surrounding these techniques to spare the nipple and/or areola, including debate over which technique.nipple-areola–sparing mastectomy or areola-sparing mastectomy.may be more oncologically safe. Currently the literature shows that both are probably safe oncologic alternatives for remote tumors that do not have an extensive intraductal component. Generally, frozen sections are performed intraoperatively on the retroareola tissue to document that there is no evidence of tumor.9
SURGICAL COMPLICATIONS
Breast procedures are fairly safe operations, but every operation has a risk of complications. Reported complications of breast surgery include the following:
- Bleeding
- Infection (including both cellulitis and abscess)
- Seroma
- Arm morbidity (including lymphedema)
- Phantom breast syndrome
- Injury to the motor nerves.
Seromas often occur in patients after mastectomy or lymph node surgery. Prolonged lymphatic drainage is usually exacerbated by extensive axillary node involvement and obesity.
Arm morbidity can present in different ways. Lymphedema is the most common manifestation, with reported incidences of approximately 15% to 20% when axillary lymph node dissection is performed versus 7% when sentinel lymph node biopsy is done.10 The risk of lymphedema can be reduced by avoiding blood pressure measurements, venipunctures, and intravenous insertions in the arm on the side of the operation, as well as by wearing a compression sleeve on the affected arm during airplane flights.
Phantom breast syndrome is rare but may manifest as pain that may also involve itching, nipple sensation, erotic sensations, or premenstrual-type soreness.
Many surgeons have historically removed the intercostobrachial nerves but are now trying to preserve these nerves, which when removed cause loss of sensation in the upper inner arm. Although rare, nerve injury during an axillary procedure has been reported. It may involve the long thoracic nerve (denervating the serratus anterior muscle and causing a winged scapula) or the thoracodorsal bundle (denervating the latissimus dorsi muscle and causing difficulty with arm/shoulder adduction).
LOCAL CANCER RECURRENCE
Among women undergoing mastectomy for breast cancer, 10% to 15% will have a recurrence of cancer in the chest wall or axillary lymph nodes within 10 years.11 Similarly, among women undergoing breast conservation therapy plus radiation therapy, 10% to 15% will have in-breast cancer recurrence or recurrence in axillary lymph nodes within 10 years, although women who undergo breast conservation therapy without radiation have a much higher recurrence rate.11 Considerations for screening the surgically altered breast are discussed in the previous article in this supplement.
ASSESSMENT OF AXILLARY LYMPH NODES FOR METASTASIS
Even when patients have a known histologic diagnosis of breast cancer and have made a firm decision regarding the surgical option for removal of their cancer, the status of their axillary lymph nodes remains a great unanswered question until after the surgical procedure is completed. Lymph node status—ie, determining whether the cancer has spread to the axillary lymph nodes—still serves as the critical determinant for guiding adjuvant treatment, predicting survival, and assessing the risk of recurrence.
Axillary lymph node dissection
The standard approach for evaluating lymph node status has been a complete dissection of the axillary space, or axillary lymph node dissection. As briefly noted above, the axillary lymph nodes are anatomically classified into three levels as defined by their location relative to the pectoralis minor muscle. The extent of a nodal dissection can be defined by the number of nodes removed.
Sentinel node biopsy: A less-invasive alternative
Axillary lymph node dissection has been called into question over the last 15 years due to its invasiveness and the potential morbidity associated with it (including lymphedema and paresthesias). As a result, sentinel lymph node biopsy, a minimally invasive technique for identifying axillary metastasis, was developed to avoid the need for (and risk of complications from) axillary lymph node dissection in patients who have a low probability of axillary metastasis.
Overall, however, it is now accepted that intraoperative lymph node mapping with sentinel lymphadenectomy is an effective and minimally invasive alternative to axillary lymph node dissection for identifying nodes containing metastases.
CONCLUSIONS
Decisions surrounding the choice of breast surgery procedure must be individualized to the patient and her desires and based on comprehensive patient evaluation and thorough patient counseling. Optimal results for the patient—oncologically, psychologically, and in terms of cosmetic outcomes—require consultation and collaboration among general surgeons, medical oncologists, genetic counselors, radiation oncologists, radiologists, and plastic surgeons to clarify the risks and benefits of various intervention options. Striving for this multidisciplinary collaboration will promote optimal patient management and the most favorable clinical outcomes.
In the late 19th century, breast cancer was considered a fatal disease. That began to change in the 1880s when W.S. Halsted described the radical mastectomy as the way to treat patients with breast cancer.1 This aggressive surgical treatment—in which the breast, axillary lymph nodes, and chest muscles are all removed—remained the standard of care throughout much of the 20th century; as late as the early 1970s, nearly half (48%) of breast cancer patients were treated with radical mastectomy. During the 1970s, however, the Halsted radical mastectomy was largely abandoned for a less-disfiguring muscle-sparing technique called the modified radical mastectomy; by 1981, only 3% of patients underwent the Halsted mastectomy.2
The 1980s heralded even more minimally invasive techniques with the advent of breast conservation therapy, in which an incision is made over the tumor and the tumor is completely removed with negative margins, leaving behind normal breast tissue. (This procedure has been referred to by many different names, including definitive excision, lumpectomy, quadrantectomy, and partial mastectomy; since they all mean the same thing, for clarity and consistency this article will use “breast conservation therapy” throughout.) During the 1990s, surgical invasiveness was further minimized with the emergence of sentinel lymph node excision.
An important contributor to this evolution in the standard of breast cancer therapy since the 1970s has been the National Surgical Adjuvant Breast and Bowel Project (NSABP), a National Cancer Institute–funded clinical trials cooperative group. NSABP studies have been the driving force to show that the extent of surgery could be reduced without compromising outcome.3 These studies, along with several other trials, have resulted in a marked reduction in surgical aggressiveness and a multitude of adjuvant therapies for women with breast cancer. This article will briefly explore where this evolution has brought us in terms of the surgical options available for treatment of breast cancer today. We also discuss other key components in the management of women with newly diagnosed breast cancer, including cancer staging, patient counseling, and assessment of axillary lymph nodes.
BREAST CANCER CLASSIFICATION AND STAGING
Pathologic classification
Cancer staging
A simpler method relies on the National Cancer Institute’s SEER (Surveillance, Epidemiology, and End Results) summary staging system.5 This system classifies tumors as “localized” (contained in the breast, either in situ or invasive), “regional” (identified in regional lymph nodes), or “metastatic” (spread to distant organ systems).
Of course, patients cannot be told their stage until after surgery, when a final pathologic report detailing tumor size and nodal status is available. Some patients will never be definitively staged—for instance, those who undergo neoadjuvant chemotherapy for locally advanced disease prior to lymph node dissection, or those who do not have a metastatic work-up. The metastatic work-up involves ordering of additional tests to assess for metastasis, but only when prompted by specific patient symptoms. Thus, if the patient has shortness of breath, a chest radiograph or a chest computed tomograph (CT) needs to be ordered; for elevated liver enzymes, CTs of the abdomen and pelvis are ordered; for central nervous system symptoms, brain magnetic resonance imaging (MRI) is ordered; and for back pain or bone pain, a bone scan is ordered to rule out metastatic disease to bone.
INITIAL PATIENT ASSESSMENT AND COUNSELING
Relationship-building is fundamental
Following an initial diagnosis of breast cancer, the patient must undergo an assessment for local and systemic disease. The surgeon, as a member of a multi-disciplinary breast cancer treatment team, often spearheads this initial assessment. This first visit must go beyond mere clinical evaluation, however, and include thorough discussion and relationship-building with the patient, as this early meeting establishes a relationship with the patient that will carry through her entire process of cancer care. For a true understanding between patient and surgeon to occur, it is critical for patients to be comfortable in sharing their fears, expectations, and lifestyle needs. Following a diagnosis of breast cancer, the initial reactions women go through include both fear and realization of one’s own mortality. Although these responses may no longer be justified by the reality of patient outcomes in most cases, they are normal and fully understandable reactions. For this reason, clinicians must be sensitive to these reactions while being supportive about the efficacy of the treatment options available.
History, breast exam, and review of imaging studies
In addition to the establishment of communication and understanding, the vital components of this first meeting include a detailed medical history, a clinical breast examination, a review of imaging studies, and a discussion of treatment options.
The history should include all aspects of the patient’s reproductive history, her family history of breast cancer, and any comorbidities and medications being taken.
The clinical breast examination should give special attention to the shape (asymmetry), appearance (eg, dimpling, erythema, nipple inversion), and overall feel of the breasts. A palpable mass must be recorded in terms of its location in relation to the skin, the nipple-areola complex, and the chest wall, as well as the quadrant of the breast in which it lies. The regional lymph node basins need to be examined closely, including the axilla and supraclavicular nodes.
Imaging studies also need to be reviewed closely. Patients today frequently present with multiple types of imaging studies, including mammography, ultrasonography, and MRI. Occasionally patients also may present with nuclear medicine exam results, CTs, thermographic images, positron emission tomography studies, and bone scans. All radiology studies need to be reviewed closely and examined in the context of what they were ordered for and what utility they potentially provide.
Treatment options: Surgery is first step in most cases
Once the above components are addressed, the patient should be engaged in a discussion of treatment options. Most women with breast cancer will undergo some type of surgery in conjunction with radiation therapy, chemotherapy, or both. Generally, surgery takes place as the first part of a multiple-component therapy plan. The main goal of surgery is to remove the cancer and accurately define the stage of the disease.
Consider plastic surgery consultation
When indicated and available, consultation with a plastic surgery team may be appropriate at this stage to provide support and comfort to the patient so that she better understands her options for breast reconstruction along with those for breast cancer surgery. Recent data show that most general surgeons do not discuss reconstruction with their breast cancer patients before surgical breast cancer therapy, but that when such discussions do occur, they significantly influence patients’ treatment choices.6 Giving patients the chance to learn about reconstructive options through discussion with a plastic surgeon represents a good opportunity to provide complete patient care in a multidisciplinary way.
OVERVIEW OF SURGICAL OPTIONS
Two general approaches, no difference in survival
The two mainstays of surgical treatment today are (1) breast conservation therapy, generally followed by total or partial breast irradiation, and (2) mastectomy.
The prospective randomized trial data obtained from the NSABP trials have demonstrated no survival differences between patients with early-stage breast cancer based on whether they were treated with breast conservation therapy or mastectomy.2 Beyond this fundamental issue of survival, there are a number of nuances, many of them logistical, related to the success of either operation that the clinician must keep in mind when presenting these surgical choices to patients. These considerations are reviewed below.
Breast conservation therapy
For breast conservation therapy, the ratio of tumor size to breast size must be small enough to ensure complete tumor removal with an acceptable cosmetic outcome. In general, it is estimated that up to 25% of the breast can be removed while still ensuring a “good” cosmetic outcome. Advances in closure techniques allowing for more tissue to be removed with even better cosmetic outcomes are known as oncoplastic closure. These techniques are mostly performed by breast oncologic surgeons, often in consultation or conjunction with plastic surgeons. (Reconstructive options following breast conservation therapy are reviewed in a subsequent article in this supplement.) Additionally, the patient must agree and be deemed a candidate for postoperative radiation therapy. The patient must be able to be followed clinically to enable early detection of a potential local recurrence.
Mastectomy
A second surgical option for patients is mastectomy. Today “mastectomy” can refer to any of several subtypes of surgical procedures, which are outlined below and should be considered on a patient-by-patient basis. Mastectomy is appropriate when breast conservation therapy is not possible (due to a large or multicentric tumor) or would result in poor cosmetic outcome, or when the patient specifically chooses a mastectomy.
Simple mastectomy involves removal of the breast only, without removal of lymph nodes. Either of the incisions depicted in the left and center panels of Figure 3 can be used. Both modified radical mastectomy and simple mastectomy involve removal of the nipple and areola (nipple-areola complex).
Skin-sparing mastectomy (Figure 3, center) is performed when a patient is undergoing immediate breast reconstruction (using either a silicone or saline implant or autologous tissue). The goal is to remove all breast tissue, along with the nipple-areola complex, while preserving as much viable skin as possible to optimize the cosmetic outcome.7,8
Nipple-areola–sparing mastectomy. There is increasing experience with attempts to preserve the nipple-areola complex. These procedures attempt to preserve either the whole complex, termed nipple-areola–sparing mastectomy (sometimes called simply nipple-sparing mastectomy) (Figure 3, right), or just the areola, with removal of the nipple (areola-sparing mastectomy). These procedures are also performed in a skin-sparing fashion.
There is some controversy surrounding these techniques to spare the nipple and/or areola, including debate over which technique.nipple-areola–sparing mastectomy or areola-sparing mastectomy.may be more oncologically safe. Currently the literature shows that both are probably safe oncologic alternatives for remote tumors that do not have an extensive intraductal component. Generally, frozen sections are performed intraoperatively on the retroareola tissue to document that there is no evidence of tumor.9
SURGICAL COMPLICATIONS
Breast procedures are fairly safe operations, but every operation has a risk of complications. Reported complications of breast surgery include the following:
- Bleeding
- Infection (including both cellulitis and abscess)
- Seroma
- Arm morbidity (including lymphedema)
- Phantom breast syndrome
- Injury to the motor nerves.
Seromas often occur in patients after mastectomy or lymph node surgery. Prolonged lymphatic drainage is usually exacerbated by extensive axillary node involvement and obesity.
Arm morbidity can present in different ways. Lymphedema is the most common manifestation, with reported incidences of approximately 15% to 20% when axillary lymph node dissection is performed versus 7% when sentinel lymph node biopsy is done.10 The risk of lymphedema can be reduced by avoiding blood pressure measurements, venipunctures, and intravenous insertions in the arm on the side of the operation, as well as by wearing a compression sleeve on the affected arm during airplane flights.
Phantom breast syndrome is rare but may manifest as pain that may also involve itching, nipple sensation, erotic sensations, or premenstrual-type soreness.
Many surgeons have historically removed the intercostobrachial nerves but are now trying to preserve these nerves, which when removed cause loss of sensation in the upper inner arm. Although rare, nerve injury during an axillary procedure has been reported. It may involve the long thoracic nerve (denervating the serratus anterior muscle and causing a winged scapula) or the thoracodorsal bundle (denervating the latissimus dorsi muscle and causing difficulty with arm/shoulder adduction).
LOCAL CANCER RECURRENCE
Among women undergoing mastectomy for breast cancer, 10% to 15% will have a recurrence of cancer in the chest wall or axillary lymph nodes within 10 years.11 Similarly, among women undergoing breast conservation therapy plus radiation therapy, 10% to 15% will have in-breast cancer recurrence or recurrence in axillary lymph nodes within 10 years, although women who undergo breast conservation therapy without radiation have a much higher recurrence rate.11 Considerations for screening the surgically altered breast are discussed in the previous article in this supplement.
ASSESSMENT OF AXILLARY LYMPH NODES FOR METASTASIS
Even when patients have a known histologic diagnosis of breast cancer and have made a firm decision regarding the surgical option for removal of their cancer, the status of their axillary lymph nodes remains a great unanswered question until after the surgical procedure is completed. Lymph node status—ie, determining whether the cancer has spread to the axillary lymph nodes—still serves as the critical determinant for guiding adjuvant treatment, predicting survival, and assessing the risk of recurrence.
Axillary lymph node dissection
The standard approach for evaluating lymph node status has been a complete dissection of the axillary space, or axillary lymph node dissection. As briefly noted above, the axillary lymph nodes are anatomically classified into three levels as defined by their location relative to the pectoralis minor muscle. The extent of a nodal dissection can be defined by the number of nodes removed.
Sentinel node biopsy: A less-invasive alternative
Axillary lymph node dissection has been called into question over the last 15 years due to its invasiveness and the potential morbidity associated with it (including lymphedema and paresthesias). As a result, sentinel lymph node biopsy, a minimally invasive technique for identifying axillary metastasis, was developed to avoid the need for (and risk of complications from) axillary lymph node dissection in patients who have a low probability of axillary metastasis.
Overall, however, it is now accepted that intraoperative lymph node mapping with sentinel lymphadenectomy is an effective and minimally invasive alternative to axillary lymph node dissection for identifying nodes containing metastases.
CONCLUSIONS
Decisions surrounding the choice of breast surgery procedure must be individualized to the patient and her desires and based on comprehensive patient evaluation and thorough patient counseling. Optimal results for the patient—oncologically, psychologically, and in terms of cosmetic outcomes—require consultation and collaboration among general surgeons, medical oncologists, genetic counselors, radiation oncologists, radiologists, and plastic surgeons to clarify the risks and benefits of various intervention options. Striving for this multidisciplinary collaboration will promote optimal patient management and the most favorable clinical outcomes.
- Bland CS. The Halsted mastectomy: present illness and past history. West J Med 1981; 134:549–555.
- Frykberg ER, Bland KI. Evolution of surgical principles and techniques for the management of breast cancer. In: Bland KI, Copeland EM III, eds. The Breast: Comprehensive Management of Benign and Malignant Disorders. 3rd ed. St. Louis, MO: Saunders; 2004:759–785.
- Newman LA, Mamounas EP. Review of breast cancer clinical trials conducted by the National Surgical Adjuvant Breast Project. Surg Clin N Am 2007; 87:279–305.
- Greene FL, Page DL, Fleming ID, et al, eds. Breast. In: AJCC Cancer Staging Manual. 6th ed. New York, NY: Springer; 2002:223–240.
- Young JJ, Roffers S, Gloeckler Ries L, et al. SEER Summary Staging Manual 2000: Codes and Coding Instructions. NIH Publication No. 01-4969. Bethesda, MD: National Institutes of Health; 2000.
- Alderman AK, Hawley ST, Waljee J, Mujahid M, Morrow M, Katz SJ. Understanding the impact of breast reconstruction on the surgical decision-making process for breast cancer. Cancer 2007; 112:489–494.
- Toth BA, Lappert P. Modified skin incisions for mastectomy: the need for plastic surgical input in preoperative planning. Plast Reconstr Surg 1991; 87:1048–1053.
- Cunnick GH, Mokbel K. Skin-sparing mastectomy. Am J Surg 2004; 188:78–84.
- Crowe JP Jr, Kim JA, Yetman R, et al. Nipple-sparing mastectomy: technique and results of 54 procedures. Arch Surg 2004; 139:148–150.
- Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst 2006; 98:599–609.
- Jacobson JA, Danforth DN, Cowan KH, et al. Ten-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancer. N Engl J Med 1995; 332:907–911.
- Tanis PJ, Nieweg OE, Valdés Olmos RA, et al. History of sentinel node and validation of the technique. Breast Cancer Res 2001; 3:109–112.
- Chagpar AB, Martin RC, Scoggins CR, et al. Factors predicting failure to identify a sentinel lymph node in breast cancer. Surgery 2005; 138:56–63.
- McMasters KM, Wong SL, Chao C, et al. Defining the optimal surgeon experience for breast cancer sentinel lymph node biopsy: a model for implementation of new surgical techniques. Ann Surg 2001; 234:292–300.
- Bland CS. The Halsted mastectomy: present illness and past history. West J Med 1981; 134:549–555.
- Frykberg ER, Bland KI. Evolution of surgical principles and techniques for the management of breast cancer. In: Bland KI, Copeland EM III, eds. The Breast: Comprehensive Management of Benign and Malignant Disorders. 3rd ed. St. Louis, MO: Saunders; 2004:759–785.
- Newman LA, Mamounas EP. Review of breast cancer clinical trials conducted by the National Surgical Adjuvant Breast Project. Surg Clin N Am 2007; 87:279–305.
- Greene FL, Page DL, Fleming ID, et al, eds. Breast. In: AJCC Cancer Staging Manual. 6th ed. New York, NY: Springer; 2002:223–240.
- Young JJ, Roffers S, Gloeckler Ries L, et al. SEER Summary Staging Manual 2000: Codes and Coding Instructions. NIH Publication No. 01-4969. Bethesda, MD: National Institutes of Health; 2000.
- Alderman AK, Hawley ST, Waljee J, Mujahid M, Morrow M, Katz SJ. Understanding the impact of breast reconstruction on the surgical decision-making process for breast cancer. Cancer 2007; 112:489–494.
- Toth BA, Lappert P. Modified skin incisions for mastectomy: the need for plastic surgical input in preoperative planning. Plast Reconstr Surg 1991; 87:1048–1053.
- Cunnick GH, Mokbel K. Skin-sparing mastectomy. Am J Surg 2004; 188:78–84.
- Crowe JP Jr, Kim JA, Yetman R, et al. Nipple-sparing mastectomy: technique and results of 54 procedures. Arch Surg 2004; 139:148–150.
- Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst 2006; 98:599–609.
- Jacobson JA, Danforth DN, Cowan KH, et al. Ten-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancer. N Engl J Med 1995; 332:907–911.
- Tanis PJ, Nieweg OE, Valdés Olmos RA, et al. History of sentinel node and validation of the technique. Breast Cancer Res 2001; 3:109–112.
- Chagpar AB, Martin RC, Scoggins CR, et al. Factors predicting failure to identify a sentinel lymph node in breast cancer. Surgery 2005; 138:56–63.
- McMasters KM, Wong SL, Chao C, et al. Defining the optimal surgeon experience for breast cancer sentinel lymph node biopsy: a model for implementation of new surgical techniques. Ann Surg 2001; 234:292–300.