User login
Type 2 diabetes: The role of basal insulin therapy
- Patients should be screened for diabetes at age 45 years—earlier if they are overweight and have at least 1 other risk factor.
- Management of type 2 diabetes requires a multifactorial approach that includes not only glycemic control but also addresses such risk factors as hypertension, dyslipidemia, renal impairment, and obesity.
- Tight glucose control (A1C <7%) may require intensive therapy with more than one antiglycemic agent. Early addition of basal insulin may be an efficient way to achieve A1C targets in some patients.
According to the latest estimates by the American Diabetes Association (ADA), more than 12.1 million persons in the United States have been diagnosed with diabetes and about 6 million remain undiagnosed.1 Type 2 diabetes, which comprises the majority (up to 95%) of all diabetes cases,2 has a profound impact on patient health and quality of life and places significant burdens on the health care system. For example, it confers a risk for myocardial infarction (MI) and cardiovascular mortality that is comparable to that for patients who have previously had an MI.3
Economically, the costs of diabetes in the United States are overwhelming: direct and indirect medical expenditures related to diabetes totaled $132 billion in 2002 and are expected to increase to $192 billion annually by 2020.1 Demographically adjusted, per-capita expenses for persons diagnosed with diabetes are more than double those of persons without diabetes.1
Clinical challenge
Type 2 diabetes poses a major challenge to primary care physicians, who are the main providers of care for about 80% of patients with this disease.4 Patients may present with a variety of complications, including neurologic, peripheral vascular, cardiovascular (eg, MI, stroke), renal, and ophthalmic disorders.1 Often, both microvascular and macrovascular complications precede the initial diagnosis of diabetes.5
Results of randomized clinical trials and population-based studies confirm that secondary preventive measures (ie, ameliorating risk factors in patients with known diabetes) and improved glycemic control can help reduce diabetes complications.3,6 A multifactorial approach that both achieves glycemic control and addresses other risk factors, such as dyslipidemia, hypertension, and microalbuminuria, has been demonstrated to be important, particularly for reducing macrovascular complications.7-9 Treatment goals for patients with type 2 diabetes include: an A1C value of <7.0%, a low-density lipoprotein cholesterol level of <100 mg/dL, and a blood pressure level of <130/80 mm Hg.10-12
The United Kingdom Prospective Diabetes Study (UKPDS 35)—in a posthoc observational substudy—suggested that there is “no threshold” for A1C lowering for any type of diabetes complication, indicating that treatment probably should aim to bring the A1C level as close to normal as possible.6 Thus, the American College of Endocrinology (ACE), for example, has set an even more aggressive A1C goal of ≤6.5%.13 Organizations such as the ADA and ACE believe that lower A1C targets not only are beneficial but that they also are achievable with currently available therapeutic agents. Ultimately, an individual patient’s comorbid conditions, life expectancy, and preferences must be considered when determining goals.14
Despite compelling outcomes data, the prevalence of diabetes remains high and clinical control remains suboptimal. The Health Plan Employer Data and Information Set, a widely used tool for measuring quality of health care, currently considers an A1C of >9.5% as “poorly controlled” disease.15 According to data from the Third National Health and Nutrition Examination Survey, 18% of patients with diabetes have an A1C of >9.5%.16 In areas where obesity is highly prevalent, such as New Orleans, the percentages are even higher, with up to 43% of obese patients treated at urban teaching hospitals in that area having A1C values of >9.5%.17 The Diabetes Quality Improvement Project, which governs 20 public and private health care organizations, found that 35% of its patients had a mean A1C of >9.5%.18 Considering that 9.5% is well above the ADA goal of <7.0%, data such as these underscore the need for more widespread diabetes screening and earlier intervention.
Identifying diabetes
The ADA recommends that screening with a fasting plasma glucose test be considered in all patients aged 45 years and older, particularly those with a body mass index of 25 kg/m2 or more.19 If the results are normal, screening should be repeated every 3 years. However, patients who are thought to be at increased risk should be considered for more frequent screening. The ADA recommendations have been endorsed by the American Academy of Pediatrics. The American Academy of Family Physicians follows the recommendations established by the US Preventive Services Task Force: screening is suggested in adults with hypertension or dyslipidemia.20
Routine screening for type 1 diabetes among healthy children is not expected to yield many cases and is not recommended. However, because the incidence of type 2 diabetes is increasing among children, it has been suggested that those who are overweight (defined as weight for height >85th percentile or weight >120% of ideal for height) with 2 or more additional risk factors should be screened every 2 years, beginning at age 10 years or at the onset of puberty.19
The ADA criteria for diagnosis of diabetes are listed in Table 1 . Whichever criterion is used, diagnostic test results must be confirmed on a subsequent day.19
Patients who do not meet the diagnostic criteria but have either impaired fasting glucose (IFG) or impaired glucose tolerance (IGT) are considered to have prediabetes (see Table 1 ).19,21 Based on recent estimates, more than 12 million adults in the United States have prediabetes.22 Of great importance is regular monitoring of patients with IGT or IFG because in addition to diabetes, they are at a heightened risk of MI or stroke compared with normoglycemic persons.
TABLE 1
Diagnostic criteria for prediabetes and diabetes
| Prediabetes | Diabetes |
|---|---|
| One or both of the following: | One or more of the following: |
|
|
| *Oral glucose tolerance test should use glucose load containing equivalent of 75 g anhydrous glucose dissolved in water. | |
| Adapted from American Diabetes Association.19 | |
Intensive management
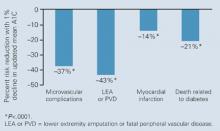
The UKPDS showed that intensive glycemic control in type 2 diabetes significantly reduced the risk of microvascular complications.23,24 In UKPDS 33, patients with newly diagnosed type 2 diabetes whose disease remained uncontrolled after completing 3 months of dietary management were initially randomized to intensive treatment (goal: FPG <108 mg/dL) with sulfonylurea or insulin monotherapy or to conventional treatment (goal: FPG <270 mg/dL) that began with diet. After 10 years, the median A1C was 7.0% in the intensive group and 7.9% in the conventional group.23 The reduction in A1C was associated with a 25% reduction of microvascular endpoints, mainly a reduced need for retinal photocoagulation (relative risk [RR] for intensive therapy, 0.75; 95% CI, 0.60–0.93; P<.01). While there was a 16% reduction in the risk of MI, this difference was not statistically significant (RR, 0.84; 95% CI, 0.71–1.00; P=.052). The UKPDS 35, which was an epidemiologic analysis of data (ie, not a direct comparison of the 2 treatment arms), demonstrated that each 1% reduction in mean A1C was associated with a 21% decrease in risk for any diabetes-related endpoint (95% CI, 17%–24%; P<.0001), a 21% decrease in diabetes-related deaths (95% CI, 15%–27%; P<.0001), a 37% decrease in the risk for microvascular complications (95% CI, 33%–41%; P<.0001), and a 14% decrease in the risk of MI (95% CI, 8%–21%; P<.0001) ( Figure 1 ).6
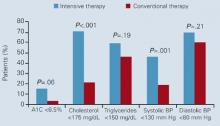
Another study that supports intensive management of type 2 diabetes is the Steno-2 study, a randomized controlled trial of 160 patients with type 2 diabetes and microalbuminuria.8 After 7.8 years of follow-up, 54% of conventionally treated and 57% of intensively treated patients were receiving insulin. Decreases in risk factors (eg, A1C, blood pressure, lipids, and albumin excretion) were significantly greater in patients receiving intensive therapy ( Figure 2 ). Significant reductions also were seen in the risk of cardiovascular disease (RR, 0.47; 95% CI, 0.24–0.73), nephropathy (RR, 0.39; 95% CI, 0.17–0.87), retinopathy (RR, 0.42; 95% CI, 0.21–0.86), and autonomic neuropathy (RR, 0.37; 95% CI, 0.18–0.79). Overall, intensive therapy in the Steno-2 study reduced risk of cardiovascular and microvascular events by about 50%.
FIGURE 1
UKPDS 35: Risk reductions associated with each 1% decrease in updated mean A1C
FIGURE 2 Percentage of patients in Steno-2 who reached treatment goals at mean follow-up of 7.8 years
Role of insulin in achieving targets
Another finding of the UKPDS was that type 2 diabetes is routinely progressive and that treatment with a single agent is unlikely to be successful for more than 5 years. Such observations mirror the physiologic progression from insulin resistance to absolute insulin deficiency. Thus, lifestyle modification often needs to be supplemented with oral antihyperglycemic therapy. In some cases, early treatment with insulin is preferable to relieve symptoms of hyperglycemia (eg, blurred vision, frequent thirst), which are suggestive of glucotoxicity and deteriorating β-cell function. As absolute insulin deficiency occurs, multiple oral agents, with or without insulin, may be required for optimal control of the disease.4,25
In UKPDS 57, 826 patients with type 2 diabetes were randomized to diet (n=242), ultralente insulin, a long-acting basal insulin (n=245), or sulfonylurea with the addition of ultralente insulin if FPG remained >108 mg/dL at maximal sulfonylurea dosage (n=339). Over 6 years, 53% of those started on sulfonylurea required addition of insulin.26 Patients treated with insulin monotherapy achieved a median A1C of 7.1%, while those on insulin plus sulfonylurea had a median A1C of 6.6% (P<.01). Episodes of major hypoglycemia occurred at a rate of 1.6% per year in the combined therapy group, compared with 3.2% a year in the insulin monotherapy group (P=0.17). In view of the observed progression of β-cell dysfunction and the failure of oral agents to maintain glycemic control, early addition of insulin to existing oral therapy may be a successful strategy in patients with type 2 diabetes.
Surmounting barriers
Insulin therapy may be delayed for a variety of reasons, including concerns about weight gain and hypo-glycemia as well as misconceptions that it increases the risk of cardiovascular disease.27 UKPDS 33 noted that relatively low proportions of patients treated with insulin experienced major (needing third-party help or medical intervention) hypoglycemic events (1.8% per year; intent-to-treat analysis).23 While both hypoglycemia and weight gain may occur with any form of insulin therapy or use of oral secretagogues, the benefits of effective glycemic control should be considered; moreover, tactics that may reduce these problems are available.28 For example, combined therapy with metformin and insulin can reduce or abolish the weight gain that otherwise may occur when insulin monotherapy is started or intensified.29 Treatment-associated hypoglycemia is generally mild to moderate in type 2 diabetes, and can be addressed with self–blood-glucose monitoring and patient education regarding recognition of symptoms and self-treatment. Also, the new long-acting insulin analogue, insulin glargine, causes fewer episodes of hypoglycemia than does neutral protamine Hagedorn (NPH) insulin.30-32
It is sometimes thought that insulin therapy may be associated with increased insulin resistance; however, studies examining peripheral insulin sensitivity have demonstrated that restoration of glycemic control with insulin improved insulin sensitivity.27,33 Also, the Diabetes Mellitus Insulin-Glucose Infusion in Acute Myocardial Infarction trial showed that intensive glycemic control with insulin therapy after an MI reduced the risk of adverse cardiovascular outcomes.6,34
Patient barriers to insulin therapy also include fear of needles and injections or a belief that insulin therapy represents failure, punishment, or a worsened prognosis.35 The notion that starting insulin therapy is a penalty for failure may be reinforced by physicians’ misconceptions about the role of insulin in type 2 diabetes.35 Setting the appropriate expectation that type 2 diabetes is a progressive disease that often eventually requires insulin can help to prevent such negative attitudes.
Finally, both patients and physicians may perceive insulin therapy as a complex and time-consuming treatment.27 Lack of office personnel and time to provide appropriate patient education for insulin therapy may contribute to such a perception. Concerns over complexity may be ameliorated by use of devices such as insulin pens and jet injectors that provide a precise dose and simplify self-administration. Premixed insulins are given twice daily and provide a measure of convenience, although flexibility of meal timing is limited in order to prevent hypoglycemia. Newer treatment regimens may also reduce patient apprehension concerning complexity and increase compliance.4 Notably, a simple and effective approach is to add once-daily basal insulin to established oral therapy.
Basal insulin studies
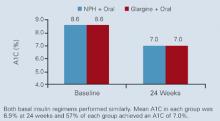
The efficiency and efficacy of adding basal insulin to existing oral antidiabetic agents was demonstrated in the randomized, open-label Treat-to-Target Trial.32 Overweight patients (N=756) who had been inadequately controlled (A1C >7.5%) on 1 or 2 oral agents (sulfonylurea, metformin, or thiazolidinedione) received insulin glargine or NPH insulin. The insulin dosage, taken at bedtime, was systematically titrated, based on before-breakfast glucose tests performed daily by the patients. Mean A1C levels fell from 8.6% to 6.9% after 24 weeks of therapy ( Figure 3 ). Both insulins performed similarly in terms of glycemic control, with 57% of patients in each group achieving an A1C of ≥7.0%. However, there was a significant difference in the incidence of hypoglycemia. Complete treatment success, defined as reaching target A1C without a single episode of nocturnal hypoglycemia (≤72 mg/dL), was achieved in 33% of the glargine patients and in 27% of the NPH group (P<.05). Overall rates of hypoglycemia, expressed as events per patient per year, were as follows: 14 symptomatic events in glargine patients vs 18 in NPH patients (P<.02), 9 confirmed events of ≤72 mg/dL in glargine patients vs 13 in NPH patients (P<.005), and 3 confirmed events of ≤56 mg/dL in glargine patients vs 5 in NPH patients (P<.003).
In another recent multicenter trial, 695 patients were randomized to 24 weeks of treatment with glimepiride plus 1 of 3 insulin regimens: morning insulin glargine, bedtime insulin glargine, or bedtime NPH insulin.36 Mean A1C levels decreased by 1.24% with morning glargine, 0.96% with bedtime glargine, and 0.84% with bedtime NPH. The percentage of patients achieving an A1C of ≤7.5% was 43% with morning glargine, 33% with bedtime glargine (P=.021 vs morning glargine), and 32% with bedtime NPH (P=.017 vs morning glargine). The percentage of patients who experienced nocturnal hypoglycemia was 17% with morning glargine, 23% with bedtime glargine, and 38% with bedtime NPH (P=.001 vs both glargine regimens). Finally, the percentage of patients who experienced symptomatic hypoglycemia was 56% with morning glargine (P=.004 vs bedtime glargine), 43% with bedtime glargine, and 58% with bedtime NPH (P=.001 vs bedtime glargine).
FIGURE 3 Treat-to-Target Trial: Rapid achievement of A1C goal
Practical aspects
Most commonly, insulin (Table 2) is introduced when single- or multipleagent oral therapy has failed to maintain glycemic control. Insulin may be added to existing oral therapy or, less typically, used as monotherapy. Of the 3 available insulins that have been used for basal therapy (NPH insulin, insulin glargine, and ultralente [insulin zinc extended]), ultralente may not be ideal since considerable variation has been noted in its pharmacokinetic and pharmacodynamic effects.37 Either NPH or glargine can be started at a dose of 10 U, at bedtime, while oral agents are continued at previous dosages. To provide 24-hour coverage, NPH may be needed twice daily whereas a single dose of glargine usually provides 24-hour coverage when administered at the same time each day.38 Patients usually need a total daily basal insulin dose of 0.5 to 0.6 U/kg (typically about 45 U daily for a 100-kg person).39 The dose may be adjusted weekly according to the patient’s FPG level.
Cost is a consideration in insulin therapy. Although more expensive than NPH, basal glargine is associated with 25% fewer episodes of nocturnal hypoglycemia, improved postdinner control, and slightly less weight gain.31,40 A retrospective review of medical and pharmacy claims demonstrated that considerable direct costs result from treatment of hypoglycemia associated with antidiabetic therapy, suggesting that therapies with less potential for inducing hypoglycemia would likely reduce these costs.41
The intensive insulin titration schedule used in the Treat-to-Target Trial is shown in Table 3. In clinical practice, a somewhat less intensive approach may work well. For example, the dose may be increased weekly by 4 U if the FPG level is >140 mg/dL on 3 consecutive occasions, or by 2 U if the FPG level is 120 to 140 mg/dL on 3 consecutive occasions.
Insulin glargine should not be mixed in the same syringe with other insulins as the pharmacokinetic profile may be altered. In the case of NPH, careful rolling or turning of the vial prior to injection to fully resuspend the crystals is advisable to minimize the variability of effect that may otherwise occur. Glargine, as with the regular and rapid-acting insulin analogues lispro and aspart, is a clear solution that does not need to be resuspended before administration. The addition of rapid-acting insulin analogues prandially to a regimen of basal insulin or basal-bolus therapy can be used to more closely mimic physiologic insulin secretion and may be needed when basal insulin replacement alone is insufficient to reach target levels of glycemic control.4
TABLE 2
Insulin formulations
| Insulin* | Onset | Peak | Effective duration (h) |
|---|---|---|---|
| Rapid-acting | |||
| Lispro | 5–15 min | 30–90 min | 5 |
| Aspart | 5–15 min | 30–90 min | 5 |
| Short-acting | |||
| Regular U100 | 30–60 min | 2–3 h | 5–8 |
| Regular U500 | 30–60 min | 2–3 h | 5–8 |
| Intermediate-acting | |||
| Isophane insulin (NPH) | 2–4 h | 4–10 h | 10–16 |
| Insulin zinc | 2–4 h | 4–12 h | 12–18 |
| Long-acting | |||
| Insulin zinc extended | 6–10 h | 10–16 h | 18–24 |
| Insulin glargine | 2–4 h† | No pronounced peak | 20–24 |
| Premixed | |||
| 70% NPH/30% regular | 30–60 min | Dual | 10–16 |
| 50% NPH/50% regular | 30–60 min | Dual | 10–16 |
| 75% NPL/25% lispro | 5–15 min | Dual | 10–16 |
| 70% NP/30% aspart | 5–15 min | Dual | 10–16 |
| *Assuming 0.1–0.2 U/kg per injection; onset and duration may vary by injection site (except for insulin glargine). | |||
| †Time to steady state. | |||
| NPH = neutral protamine Hagedorn; NPL = neutral protamine lispro; NP = neutral protamine. | |||
| Adapted from DeWitt and Hirsch.4 | |||
TABLE 3
Basal insulin: Initiation and dosage adjustment
| Forced titration schedule | |||
|---|---|---|---|
| |||
| Self-monitored FPG (mg/dL) | Dosage increase (IU/day) in Treat-to-Target Trial | ||
| ≥180 | 8 | ||
| ≥140 – <180 | 6 | ||
| ≥120 – <140 | 4 | ||
| >100 – <120 | 2 | ||
| *No increase if plasma glucose is <72 mg/dL in the preceding week; decrease dosage (2–4 IU/day) if plasma glucose is <56 mg/dL or severe hypoglycemia (requiring assistance) occurred within the preceding week. | |||
| FPG = fasting plasma glucose. | |||
| Adapted from Riddle et al.32 | |||
Summary
New evidence and methods continue to alter management of patients with type 2 diabetes. Appropriate screening and earlier intervention may help reduce the incidence and progression of microvascular and macrovascular complications. A multifactorial approach that addresses such risk factors as blood pressure, lipids, and glycemia has demonstrated reduced morbidity and mortality. While glycemic targets are getting lower, they can be efficiently attained with combined basal insulin and oral antidiabetic therapy. Insulin, too often considered a therapy of last resort, is an important intervention that can be used safely and effectively earlier in the course of type 2 diabetes.
1. American Diabetes Association. Economic costs of diabetes in the U.S. in 2002. Diabetes Care. 2003;26:917-932.
2. National Institute of Diabetes and Digestive and Kidney Diseases. National diabetes statistics fact sheet: general information and national estimates on diabetes in the United States, 2000. Bethesda, Md: U.S. Department of Health and Human Services, National Institutes of Health, 2002. National Institute of Diabetes and Digestive and Kidney Diseases, 2002.
3. Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229-234.
4. DeWitt DE, Hirsch IB. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus: scientific review. JAMA. 2003;289:2254-2264.
5. Dagogo-Jack S, Santiago JV. Pathophysiology of type 2 diabetes and modes of action of therapeutic interventions. Arch Intern Med. 1997;157:1802-1817.
6. Stratton IM, Adler AI, Neil HAW, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. Br Med J. 2000;321:405-412.
7. Gaede P, Vedel P, Parving H-H, Pedersen O. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno-2 randomised study. Lancet. 1999;353:617-622.
8. Gaede P, Vedel P, Larsen N, Jensen GVH, Parving H-H, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383-393.
9. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. Br Med J. 1998;317:703-713.
10. American Diabetes Association. Clinical practice recommendations 2003. Diabetes Care. 2003;26(suppl 1):S1-S156.
11. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
12. National Institutes of Health, National Heart Lung and Blood Institute. JNC 7 Express: The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Bethesda, Md: US Dept of Health and Human Services; May 2003. NIH publication 03-5233. Available at: http://www.nhlbi.nih.gov/guidelines/hypertension/express. Accessed November 14, 2003.
13. American Association of Clinical Endocrinologists medical guidelines for the management of diabetes mellitus: the AACE system of intensive diabetes self-management: 2000 update. Endocr Pract. 2000;8(suppl 1):40-82.
14. Woolf SH, Davidson MB, Greenfield S, et al. Controlling blood glucose levels in patients with type 2 diabetes mellitus: an evidence-based policy statement by the American Academy of Family Physicians and American Diabetes Association. J Fam Pract. 2000;49:453-460.
15. National Committee for Quality Assurance. The State of Health Care Quality: 2003. Washington, DC: NCQA; 2003;1-34.
16. Saaddine JB, Engelgau MM, Beckles GL, Gregg EW, Thompson TJ, Venkat Narayan KMV. A diabetes report card for the United States: quality of care in the 1990s. Ann Intern Med. 2002;136:565-574.
17. Suwattee P, Lynch JC, Pendergrass ML. Quality of care for diabetic patients in a large urban public hospital. Diabetes Care. 2003;26:563-568.
18. Fleming BB, Greenfield S, Engelgau MM, Pogach LM, Clauser SB, Parrott MA. The Diabetes Quality Improvement Project: moving science into health policy to gain an edge on the diabetes epidemic. Diabetes Care. 2001;24:1815-1820.
19. American Diabetes Association. Screening for type 2 diabetes. Diabetes Care. 2004;27:S11-S14.
20. U.S. Preventive Services Task Force. Screening for type 2 diabetes mellitus in adults: recommendations and rationale. Ann Intern Med. 2003;138:212-214.
21. Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160-3167.
22. Centers for Disease Control and Prevention. Prevalence of diabetes and impaired fasting glucose in adults: United States, 1999-2000. Morb Mortal Wkly Rep. 2003;52:833-837.
23. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837-853.
24. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854-865.
25. Feld S. AACE diabetes guidelines. Endocr Pract. 2002;8(suppl 1):41-65.
26. Wright A, Burden AC, Paisey RB, Cull CA, Holman RR. Sulfonylurea inadequacy: efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the UK Prospective Diabetes Study (UKPDS 57). Diabetes Care. 2002;25:330-336.
27. Riddle MC. The underuse of insulin therapy in North America. Diabetes Metab Res Rev. 2002;18:S42-S49.
28. Rosenstock J. Insulin therapy: optimizing control in type 1 and type 2 diabetes. Clin Cornerstone. 2001;4:50-64.
29. Avilés-Santa L, Sinding J, Raskin P. Effects of metformin in patients with poorly controlled, insulin-treated type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1999;131:182-188.
30. Ratner RE, Hirsch IB, Neifing JL, Garg SK, Mecca TE, Wilson CA. Less hypoglycemia with insulin glargine in intensive insulin therapy for type 1 diabetes. U.S. Study Group of Insulin Glargine in Type 1 Diabetes. Diabetes Care. 2000;23:639-643.
31. Yki-Jarvinen H, Dressler A, Ziemen M. Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. HOE 901/3002 Study Group. Diabetes Care. 2000;23:1130-1136.
32. Riddle MC, Rosenstock J, Gerich J. The Treat-to-Target Trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26:3080-3086.
33. Scarlett JA, Gray RS, Griffin J, Olefsky JM, Kolterman OG. Insulin treatment reverses the insulin resistance of type II diabetes mellitus. Diabetes Care. 1982;5:353-363.
34. Malmberg K. and the DIGAMI (Diabetes Mellitus Insulin-Glucose Infusion in Acute Myocardial Infarction) Study Group Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus. Br Med J. 1997;314:1512-1515.
35. Hunt LM, Valenzuela MA, Pugh JA. NIDDM patients’ fears and hopes about insulin therapy: the basis of patient reluctance. Diabetes Care. 1997;20:292-298.
36. Fritsche A, Schweitzer MA, Haring HU. Glimepiride combined with morning insulin glargine, bedtime neutral protamine Hagedorn insulin, or bedtime insulin glargine in patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med. 2003;138:952-959.
37. Lepore M, Pampanelli S, Fanelli C, et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes. 2000;49:2142-2148.
38. Full U.S. Prescribing Information for Lantus. Available at: http://www.lantus.com/professional/home.jsp .Accessed March 5, 2003.
39. DeWitt DE, Dugdale DC. Using new insulin strategies in the outpatient treatment of diabetes: clinical applications. JAMA. 2003;289:2265-2269.
40. Rosenstock J, Schwartz SL, Clark CM, Jr.,, Park GD, Donley DW, Edwards MB. Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care. 2001;24:631-636.
41. Raut M, Sung JCY, Law AW. Cost of treating hypoglycemia among patients with diabetes [abstract]. Diabetes Care. 2003;26(suppl 2):A1152.-
Disclosures: Dr LeRoith serves as a consultant to Aventis Pharmaceuticals, Pfizer Inc, and Novo Nordisk Pharmaceuticals, Inc. and on the speakers’ bureaus of Aventis Pharmaceuticals, Pfizer Inc, and Eli Lilly and Co. Dr Levetan serves as a consultant to Eli Lilly and Co. and Novo Nordisk Pharmaceuticals, Inc. and on the speakers’ bureaus of Aventis Pharmaceuticals and Novo Nordisk Pharmaceuticals, Inc. Dr Hirsch serves as a consultant to Eli Lilly and Co., Novo Nordisk Pharmaceuticals, Inc., Aventis Pharmaceuticals, and Medtronic MiniMed. Dr Riddle has received grant/research support from Amylin Pharmaceuticals, Aventis Pharmaceuticals, and Pfizer Inc. He serves as a consultant to and is on the speakers’ bureaus of Amylin Pharmaceuticals, Aventis Pharmaceuticals, GlaxoSmithKline, and Novo Nordisk Pharmaceuticals, Inc. Corresponding author: Derek Le Roith, MD, PhD, Room 8D12, Bldg 10, National Institutes of Health, MSC 1758, Bethesda MD 20892-1758. E-mail: leroith@comcast.net.
- Patients should be screened for diabetes at age 45 years—earlier if they are overweight and have at least 1 other risk factor.
- Management of type 2 diabetes requires a multifactorial approach that includes not only glycemic control but also addresses such risk factors as hypertension, dyslipidemia, renal impairment, and obesity.
- Tight glucose control (A1C <7%) may require intensive therapy with more than one antiglycemic agent. Early addition of basal insulin may be an efficient way to achieve A1C targets in some patients.
According to the latest estimates by the American Diabetes Association (ADA), more than 12.1 million persons in the United States have been diagnosed with diabetes and about 6 million remain undiagnosed.1 Type 2 diabetes, which comprises the majority (up to 95%) of all diabetes cases,2 has a profound impact on patient health and quality of life and places significant burdens on the health care system. For example, it confers a risk for myocardial infarction (MI) and cardiovascular mortality that is comparable to that for patients who have previously had an MI.3
Economically, the costs of diabetes in the United States are overwhelming: direct and indirect medical expenditures related to diabetes totaled $132 billion in 2002 and are expected to increase to $192 billion annually by 2020.1 Demographically adjusted, per-capita expenses for persons diagnosed with diabetes are more than double those of persons without diabetes.1
Clinical challenge
Type 2 diabetes poses a major challenge to primary care physicians, who are the main providers of care for about 80% of patients with this disease.4 Patients may present with a variety of complications, including neurologic, peripheral vascular, cardiovascular (eg, MI, stroke), renal, and ophthalmic disorders.1 Often, both microvascular and macrovascular complications precede the initial diagnosis of diabetes.5
Results of randomized clinical trials and population-based studies confirm that secondary preventive measures (ie, ameliorating risk factors in patients with known diabetes) and improved glycemic control can help reduce diabetes complications.3,6 A multifactorial approach that both achieves glycemic control and addresses other risk factors, such as dyslipidemia, hypertension, and microalbuminuria, has been demonstrated to be important, particularly for reducing macrovascular complications.7-9 Treatment goals for patients with type 2 diabetes include: an A1C value of <7.0%, a low-density lipoprotein cholesterol level of <100 mg/dL, and a blood pressure level of <130/80 mm Hg.10-12
The United Kingdom Prospective Diabetes Study (UKPDS 35)—in a posthoc observational substudy—suggested that there is “no threshold” for A1C lowering for any type of diabetes complication, indicating that treatment probably should aim to bring the A1C level as close to normal as possible.6 Thus, the American College of Endocrinology (ACE), for example, has set an even more aggressive A1C goal of ≤6.5%.13 Organizations such as the ADA and ACE believe that lower A1C targets not only are beneficial but that they also are achievable with currently available therapeutic agents. Ultimately, an individual patient’s comorbid conditions, life expectancy, and preferences must be considered when determining goals.14
Despite compelling outcomes data, the prevalence of diabetes remains high and clinical control remains suboptimal. The Health Plan Employer Data and Information Set, a widely used tool for measuring quality of health care, currently considers an A1C of >9.5% as “poorly controlled” disease.15 According to data from the Third National Health and Nutrition Examination Survey, 18% of patients with diabetes have an A1C of >9.5%.16 In areas where obesity is highly prevalent, such as New Orleans, the percentages are even higher, with up to 43% of obese patients treated at urban teaching hospitals in that area having A1C values of >9.5%.17 The Diabetes Quality Improvement Project, which governs 20 public and private health care organizations, found that 35% of its patients had a mean A1C of >9.5%.18 Considering that 9.5% is well above the ADA goal of <7.0%, data such as these underscore the need for more widespread diabetes screening and earlier intervention.
Identifying diabetes
The ADA recommends that screening with a fasting plasma glucose test be considered in all patients aged 45 years and older, particularly those with a body mass index of 25 kg/m2 or more.19 If the results are normal, screening should be repeated every 3 years. However, patients who are thought to be at increased risk should be considered for more frequent screening. The ADA recommendations have been endorsed by the American Academy of Pediatrics. The American Academy of Family Physicians follows the recommendations established by the US Preventive Services Task Force: screening is suggested in adults with hypertension or dyslipidemia.20
Routine screening for type 1 diabetes among healthy children is not expected to yield many cases and is not recommended. However, because the incidence of type 2 diabetes is increasing among children, it has been suggested that those who are overweight (defined as weight for height >85th percentile or weight >120% of ideal for height) with 2 or more additional risk factors should be screened every 2 years, beginning at age 10 years or at the onset of puberty.19
The ADA criteria for diagnosis of diabetes are listed in Table 1 . Whichever criterion is used, diagnostic test results must be confirmed on a subsequent day.19
Patients who do not meet the diagnostic criteria but have either impaired fasting glucose (IFG) or impaired glucose tolerance (IGT) are considered to have prediabetes (see Table 1 ).19,21 Based on recent estimates, more than 12 million adults in the United States have prediabetes.22 Of great importance is regular monitoring of patients with IGT or IFG because in addition to diabetes, they are at a heightened risk of MI or stroke compared with normoglycemic persons.
TABLE 1
Diagnostic criteria for prediabetes and diabetes
| Prediabetes | Diabetes |
|---|---|
| One or both of the following: | One or more of the following: |
|
|
| *Oral glucose tolerance test should use glucose load containing equivalent of 75 g anhydrous glucose dissolved in water. | |
| Adapted from American Diabetes Association.19 | |
Intensive management
The UKPDS showed that intensive glycemic control in type 2 diabetes significantly reduced the risk of microvascular complications.23,24 In UKPDS 33, patients with newly diagnosed type 2 diabetes whose disease remained uncontrolled after completing 3 months of dietary management were initially randomized to intensive treatment (goal: FPG <108 mg/dL) with sulfonylurea or insulin monotherapy or to conventional treatment (goal: FPG <270 mg/dL) that began with diet. After 10 years, the median A1C was 7.0% in the intensive group and 7.9% in the conventional group.23 The reduction in A1C was associated with a 25% reduction of microvascular endpoints, mainly a reduced need for retinal photocoagulation (relative risk [RR] for intensive therapy, 0.75; 95% CI, 0.60–0.93; P<.01). While there was a 16% reduction in the risk of MI, this difference was not statistically significant (RR, 0.84; 95% CI, 0.71–1.00; P=.052). The UKPDS 35, which was an epidemiologic analysis of data (ie, not a direct comparison of the 2 treatment arms), demonstrated that each 1% reduction in mean A1C was associated with a 21% decrease in risk for any diabetes-related endpoint (95% CI, 17%–24%; P<.0001), a 21% decrease in diabetes-related deaths (95% CI, 15%–27%; P<.0001), a 37% decrease in the risk for microvascular complications (95% CI, 33%–41%; P<.0001), and a 14% decrease in the risk of MI (95% CI, 8%–21%; P<.0001) ( Figure 1 ).6
Another study that supports intensive management of type 2 diabetes is the Steno-2 study, a randomized controlled trial of 160 patients with type 2 diabetes and microalbuminuria.8 After 7.8 years of follow-up, 54% of conventionally treated and 57% of intensively treated patients were receiving insulin. Decreases in risk factors (eg, A1C, blood pressure, lipids, and albumin excretion) were significantly greater in patients receiving intensive therapy ( Figure 2 ). Significant reductions also were seen in the risk of cardiovascular disease (RR, 0.47; 95% CI, 0.24–0.73), nephropathy (RR, 0.39; 95% CI, 0.17–0.87), retinopathy (RR, 0.42; 95% CI, 0.21–0.86), and autonomic neuropathy (RR, 0.37; 95% CI, 0.18–0.79). Overall, intensive therapy in the Steno-2 study reduced risk of cardiovascular and microvascular events by about 50%.
FIGURE 1
UKPDS 35: Risk reductions associated with each 1% decrease in updated mean A1C
FIGURE 2 Percentage of patients in Steno-2 who reached treatment goals at mean follow-up of 7.8 years
Role of insulin in achieving targets
Another finding of the UKPDS was that type 2 diabetes is routinely progressive and that treatment with a single agent is unlikely to be successful for more than 5 years. Such observations mirror the physiologic progression from insulin resistance to absolute insulin deficiency. Thus, lifestyle modification often needs to be supplemented with oral antihyperglycemic therapy. In some cases, early treatment with insulin is preferable to relieve symptoms of hyperglycemia (eg, blurred vision, frequent thirst), which are suggestive of glucotoxicity and deteriorating β-cell function. As absolute insulin deficiency occurs, multiple oral agents, with or without insulin, may be required for optimal control of the disease.4,25
In UKPDS 57, 826 patients with type 2 diabetes were randomized to diet (n=242), ultralente insulin, a long-acting basal insulin (n=245), or sulfonylurea with the addition of ultralente insulin if FPG remained >108 mg/dL at maximal sulfonylurea dosage (n=339). Over 6 years, 53% of those started on sulfonylurea required addition of insulin.26 Patients treated with insulin monotherapy achieved a median A1C of 7.1%, while those on insulin plus sulfonylurea had a median A1C of 6.6% (P<.01). Episodes of major hypoglycemia occurred at a rate of 1.6% per year in the combined therapy group, compared with 3.2% a year in the insulin monotherapy group (P=0.17). In view of the observed progression of β-cell dysfunction and the failure of oral agents to maintain glycemic control, early addition of insulin to existing oral therapy may be a successful strategy in patients with type 2 diabetes.
Surmounting barriers
Insulin therapy may be delayed for a variety of reasons, including concerns about weight gain and hypo-glycemia as well as misconceptions that it increases the risk of cardiovascular disease.27 UKPDS 33 noted that relatively low proportions of patients treated with insulin experienced major (needing third-party help or medical intervention) hypoglycemic events (1.8% per year; intent-to-treat analysis).23 While both hypoglycemia and weight gain may occur with any form of insulin therapy or use of oral secretagogues, the benefits of effective glycemic control should be considered; moreover, tactics that may reduce these problems are available.28 For example, combined therapy with metformin and insulin can reduce or abolish the weight gain that otherwise may occur when insulin monotherapy is started or intensified.29 Treatment-associated hypoglycemia is generally mild to moderate in type 2 diabetes, and can be addressed with self–blood-glucose monitoring and patient education regarding recognition of symptoms and self-treatment. Also, the new long-acting insulin analogue, insulin glargine, causes fewer episodes of hypoglycemia than does neutral protamine Hagedorn (NPH) insulin.30-32
It is sometimes thought that insulin therapy may be associated with increased insulin resistance; however, studies examining peripheral insulin sensitivity have demonstrated that restoration of glycemic control with insulin improved insulin sensitivity.27,33 Also, the Diabetes Mellitus Insulin-Glucose Infusion in Acute Myocardial Infarction trial showed that intensive glycemic control with insulin therapy after an MI reduced the risk of adverse cardiovascular outcomes.6,34
Patient barriers to insulin therapy also include fear of needles and injections or a belief that insulin therapy represents failure, punishment, or a worsened prognosis.35 The notion that starting insulin therapy is a penalty for failure may be reinforced by physicians’ misconceptions about the role of insulin in type 2 diabetes.35 Setting the appropriate expectation that type 2 diabetes is a progressive disease that often eventually requires insulin can help to prevent such negative attitudes.
Finally, both patients and physicians may perceive insulin therapy as a complex and time-consuming treatment.27 Lack of office personnel and time to provide appropriate patient education for insulin therapy may contribute to such a perception. Concerns over complexity may be ameliorated by use of devices such as insulin pens and jet injectors that provide a precise dose and simplify self-administration. Premixed insulins are given twice daily and provide a measure of convenience, although flexibility of meal timing is limited in order to prevent hypoglycemia. Newer treatment regimens may also reduce patient apprehension concerning complexity and increase compliance.4 Notably, a simple and effective approach is to add once-daily basal insulin to established oral therapy.
Basal insulin studies
The efficiency and efficacy of adding basal insulin to existing oral antidiabetic agents was demonstrated in the randomized, open-label Treat-to-Target Trial.32 Overweight patients (N=756) who had been inadequately controlled (A1C >7.5%) on 1 or 2 oral agents (sulfonylurea, metformin, or thiazolidinedione) received insulin glargine or NPH insulin. The insulin dosage, taken at bedtime, was systematically titrated, based on before-breakfast glucose tests performed daily by the patients. Mean A1C levels fell from 8.6% to 6.9% after 24 weeks of therapy ( Figure 3 ). Both insulins performed similarly in terms of glycemic control, with 57% of patients in each group achieving an A1C of ≥7.0%. However, there was a significant difference in the incidence of hypoglycemia. Complete treatment success, defined as reaching target A1C without a single episode of nocturnal hypoglycemia (≤72 mg/dL), was achieved in 33% of the glargine patients and in 27% of the NPH group (P<.05). Overall rates of hypoglycemia, expressed as events per patient per year, were as follows: 14 symptomatic events in glargine patients vs 18 in NPH patients (P<.02), 9 confirmed events of ≤72 mg/dL in glargine patients vs 13 in NPH patients (P<.005), and 3 confirmed events of ≤56 mg/dL in glargine patients vs 5 in NPH patients (P<.003).
In another recent multicenter trial, 695 patients were randomized to 24 weeks of treatment with glimepiride plus 1 of 3 insulin regimens: morning insulin glargine, bedtime insulin glargine, or bedtime NPH insulin.36 Mean A1C levels decreased by 1.24% with morning glargine, 0.96% with bedtime glargine, and 0.84% with bedtime NPH. The percentage of patients achieving an A1C of ≤7.5% was 43% with morning glargine, 33% with bedtime glargine (P=.021 vs morning glargine), and 32% with bedtime NPH (P=.017 vs morning glargine). The percentage of patients who experienced nocturnal hypoglycemia was 17% with morning glargine, 23% with bedtime glargine, and 38% with bedtime NPH (P=.001 vs both glargine regimens). Finally, the percentage of patients who experienced symptomatic hypoglycemia was 56% with morning glargine (P=.004 vs bedtime glargine), 43% with bedtime glargine, and 58% with bedtime NPH (P=.001 vs bedtime glargine).
FIGURE 3 Treat-to-Target Trial: Rapid achievement of A1C goal
Practical aspects
Most commonly, insulin (Table 2) is introduced when single- or multipleagent oral therapy has failed to maintain glycemic control. Insulin may be added to existing oral therapy or, less typically, used as monotherapy. Of the 3 available insulins that have been used for basal therapy (NPH insulin, insulin glargine, and ultralente [insulin zinc extended]), ultralente may not be ideal since considerable variation has been noted in its pharmacokinetic and pharmacodynamic effects.37 Either NPH or glargine can be started at a dose of 10 U, at bedtime, while oral agents are continued at previous dosages. To provide 24-hour coverage, NPH may be needed twice daily whereas a single dose of glargine usually provides 24-hour coverage when administered at the same time each day.38 Patients usually need a total daily basal insulin dose of 0.5 to 0.6 U/kg (typically about 45 U daily for a 100-kg person).39 The dose may be adjusted weekly according to the patient’s FPG level.
Cost is a consideration in insulin therapy. Although more expensive than NPH, basal glargine is associated with 25% fewer episodes of nocturnal hypoglycemia, improved postdinner control, and slightly less weight gain.31,40 A retrospective review of medical and pharmacy claims demonstrated that considerable direct costs result from treatment of hypoglycemia associated with antidiabetic therapy, suggesting that therapies with less potential for inducing hypoglycemia would likely reduce these costs.41
The intensive insulin titration schedule used in the Treat-to-Target Trial is shown in Table 3. In clinical practice, a somewhat less intensive approach may work well. For example, the dose may be increased weekly by 4 U if the FPG level is >140 mg/dL on 3 consecutive occasions, or by 2 U if the FPG level is 120 to 140 mg/dL on 3 consecutive occasions.
Insulin glargine should not be mixed in the same syringe with other insulins as the pharmacokinetic profile may be altered. In the case of NPH, careful rolling or turning of the vial prior to injection to fully resuspend the crystals is advisable to minimize the variability of effect that may otherwise occur. Glargine, as with the regular and rapid-acting insulin analogues lispro and aspart, is a clear solution that does not need to be resuspended before administration. The addition of rapid-acting insulin analogues prandially to a regimen of basal insulin or basal-bolus therapy can be used to more closely mimic physiologic insulin secretion and may be needed when basal insulin replacement alone is insufficient to reach target levels of glycemic control.4
TABLE 2
Insulin formulations
| Insulin* | Onset | Peak | Effective duration (h) |
|---|---|---|---|
| Rapid-acting | |||
| Lispro | 5–15 min | 30–90 min | 5 |
| Aspart | 5–15 min | 30–90 min | 5 |
| Short-acting | |||
| Regular U100 | 30–60 min | 2–3 h | 5–8 |
| Regular U500 | 30–60 min | 2–3 h | 5–8 |
| Intermediate-acting | |||
| Isophane insulin (NPH) | 2–4 h | 4–10 h | 10–16 |
| Insulin zinc | 2–4 h | 4–12 h | 12–18 |
| Long-acting | |||
| Insulin zinc extended | 6–10 h | 10–16 h | 18–24 |
| Insulin glargine | 2–4 h† | No pronounced peak | 20–24 |
| Premixed | |||
| 70% NPH/30% regular | 30–60 min | Dual | 10–16 |
| 50% NPH/50% regular | 30–60 min | Dual | 10–16 |
| 75% NPL/25% lispro | 5–15 min | Dual | 10–16 |
| 70% NP/30% aspart | 5–15 min | Dual | 10–16 |
| *Assuming 0.1–0.2 U/kg per injection; onset and duration may vary by injection site (except for insulin glargine). | |||
| †Time to steady state. | |||
| NPH = neutral protamine Hagedorn; NPL = neutral protamine lispro; NP = neutral protamine. | |||
| Adapted from DeWitt and Hirsch.4 | |||
TABLE 3
Basal insulin: Initiation and dosage adjustment
| Forced titration schedule | |||
|---|---|---|---|
| |||
| Self-monitored FPG (mg/dL) | Dosage increase (IU/day) in Treat-to-Target Trial | ||
| ≥180 | 8 | ||
| ≥140 – <180 | 6 | ||
| ≥120 – <140 | 4 | ||
| >100 – <120 | 2 | ||
| *No increase if plasma glucose is <72 mg/dL in the preceding week; decrease dosage (2–4 IU/day) if plasma glucose is <56 mg/dL or severe hypoglycemia (requiring assistance) occurred within the preceding week. | |||
| FPG = fasting plasma glucose. | |||
| Adapted from Riddle et al.32 | |||
Summary
New evidence and methods continue to alter management of patients with type 2 diabetes. Appropriate screening and earlier intervention may help reduce the incidence and progression of microvascular and macrovascular complications. A multifactorial approach that addresses such risk factors as blood pressure, lipids, and glycemia has demonstrated reduced morbidity and mortality. While glycemic targets are getting lower, they can be efficiently attained with combined basal insulin and oral antidiabetic therapy. Insulin, too often considered a therapy of last resort, is an important intervention that can be used safely and effectively earlier in the course of type 2 diabetes.
- Patients should be screened for diabetes at age 45 years—earlier if they are overweight and have at least 1 other risk factor.
- Management of type 2 diabetes requires a multifactorial approach that includes not only glycemic control but also addresses such risk factors as hypertension, dyslipidemia, renal impairment, and obesity.
- Tight glucose control (A1C <7%) may require intensive therapy with more than one antiglycemic agent. Early addition of basal insulin may be an efficient way to achieve A1C targets in some patients.
According to the latest estimates by the American Diabetes Association (ADA), more than 12.1 million persons in the United States have been diagnosed with diabetes and about 6 million remain undiagnosed.1 Type 2 diabetes, which comprises the majority (up to 95%) of all diabetes cases,2 has a profound impact on patient health and quality of life and places significant burdens on the health care system. For example, it confers a risk for myocardial infarction (MI) and cardiovascular mortality that is comparable to that for patients who have previously had an MI.3
Economically, the costs of diabetes in the United States are overwhelming: direct and indirect medical expenditures related to diabetes totaled $132 billion in 2002 and are expected to increase to $192 billion annually by 2020.1 Demographically adjusted, per-capita expenses for persons diagnosed with diabetes are more than double those of persons without diabetes.1
Clinical challenge
Type 2 diabetes poses a major challenge to primary care physicians, who are the main providers of care for about 80% of patients with this disease.4 Patients may present with a variety of complications, including neurologic, peripheral vascular, cardiovascular (eg, MI, stroke), renal, and ophthalmic disorders.1 Often, both microvascular and macrovascular complications precede the initial diagnosis of diabetes.5
Results of randomized clinical trials and population-based studies confirm that secondary preventive measures (ie, ameliorating risk factors in patients with known diabetes) and improved glycemic control can help reduce diabetes complications.3,6 A multifactorial approach that both achieves glycemic control and addresses other risk factors, such as dyslipidemia, hypertension, and microalbuminuria, has been demonstrated to be important, particularly for reducing macrovascular complications.7-9 Treatment goals for patients with type 2 diabetes include: an A1C value of <7.0%, a low-density lipoprotein cholesterol level of <100 mg/dL, and a blood pressure level of <130/80 mm Hg.10-12
The United Kingdom Prospective Diabetes Study (UKPDS 35)—in a posthoc observational substudy—suggested that there is “no threshold” for A1C lowering for any type of diabetes complication, indicating that treatment probably should aim to bring the A1C level as close to normal as possible.6 Thus, the American College of Endocrinology (ACE), for example, has set an even more aggressive A1C goal of ≤6.5%.13 Organizations such as the ADA and ACE believe that lower A1C targets not only are beneficial but that they also are achievable with currently available therapeutic agents. Ultimately, an individual patient’s comorbid conditions, life expectancy, and preferences must be considered when determining goals.14
Despite compelling outcomes data, the prevalence of diabetes remains high and clinical control remains suboptimal. The Health Plan Employer Data and Information Set, a widely used tool for measuring quality of health care, currently considers an A1C of >9.5% as “poorly controlled” disease.15 According to data from the Third National Health and Nutrition Examination Survey, 18% of patients with diabetes have an A1C of >9.5%.16 In areas where obesity is highly prevalent, such as New Orleans, the percentages are even higher, with up to 43% of obese patients treated at urban teaching hospitals in that area having A1C values of >9.5%.17 The Diabetes Quality Improvement Project, which governs 20 public and private health care organizations, found that 35% of its patients had a mean A1C of >9.5%.18 Considering that 9.5% is well above the ADA goal of <7.0%, data such as these underscore the need for more widespread diabetes screening and earlier intervention.
Identifying diabetes
The ADA recommends that screening with a fasting plasma glucose test be considered in all patients aged 45 years and older, particularly those with a body mass index of 25 kg/m2 or more.19 If the results are normal, screening should be repeated every 3 years. However, patients who are thought to be at increased risk should be considered for more frequent screening. The ADA recommendations have been endorsed by the American Academy of Pediatrics. The American Academy of Family Physicians follows the recommendations established by the US Preventive Services Task Force: screening is suggested in adults with hypertension or dyslipidemia.20
Routine screening for type 1 diabetes among healthy children is not expected to yield many cases and is not recommended. However, because the incidence of type 2 diabetes is increasing among children, it has been suggested that those who are overweight (defined as weight for height >85th percentile or weight >120% of ideal for height) with 2 or more additional risk factors should be screened every 2 years, beginning at age 10 years or at the onset of puberty.19
The ADA criteria for diagnosis of diabetes are listed in Table 1 . Whichever criterion is used, diagnostic test results must be confirmed on a subsequent day.19
Patients who do not meet the diagnostic criteria but have either impaired fasting glucose (IFG) or impaired glucose tolerance (IGT) are considered to have prediabetes (see Table 1 ).19,21 Based on recent estimates, more than 12 million adults in the United States have prediabetes.22 Of great importance is regular monitoring of patients with IGT or IFG because in addition to diabetes, they are at a heightened risk of MI or stroke compared with normoglycemic persons.
TABLE 1
Diagnostic criteria for prediabetes and diabetes
| Prediabetes | Diabetes |
|---|---|
| One or both of the following: | One or more of the following: |
|
|
| *Oral glucose tolerance test should use glucose load containing equivalent of 75 g anhydrous glucose dissolved in water. | |
| Adapted from American Diabetes Association.19 | |
Intensive management
The UKPDS showed that intensive glycemic control in type 2 diabetes significantly reduced the risk of microvascular complications.23,24 In UKPDS 33, patients with newly diagnosed type 2 diabetes whose disease remained uncontrolled after completing 3 months of dietary management were initially randomized to intensive treatment (goal: FPG <108 mg/dL) with sulfonylurea or insulin monotherapy or to conventional treatment (goal: FPG <270 mg/dL) that began with diet. After 10 years, the median A1C was 7.0% in the intensive group and 7.9% in the conventional group.23 The reduction in A1C was associated with a 25% reduction of microvascular endpoints, mainly a reduced need for retinal photocoagulation (relative risk [RR] for intensive therapy, 0.75; 95% CI, 0.60–0.93; P<.01). While there was a 16% reduction in the risk of MI, this difference was not statistically significant (RR, 0.84; 95% CI, 0.71–1.00; P=.052). The UKPDS 35, which was an epidemiologic analysis of data (ie, not a direct comparison of the 2 treatment arms), demonstrated that each 1% reduction in mean A1C was associated with a 21% decrease in risk for any diabetes-related endpoint (95% CI, 17%–24%; P<.0001), a 21% decrease in diabetes-related deaths (95% CI, 15%–27%; P<.0001), a 37% decrease in the risk for microvascular complications (95% CI, 33%–41%; P<.0001), and a 14% decrease in the risk of MI (95% CI, 8%–21%; P<.0001) ( Figure 1 ).6
Another study that supports intensive management of type 2 diabetes is the Steno-2 study, a randomized controlled trial of 160 patients with type 2 diabetes and microalbuminuria.8 After 7.8 years of follow-up, 54% of conventionally treated and 57% of intensively treated patients were receiving insulin. Decreases in risk factors (eg, A1C, blood pressure, lipids, and albumin excretion) were significantly greater in patients receiving intensive therapy ( Figure 2 ). Significant reductions also were seen in the risk of cardiovascular disease (RR, 0.47; 95% CI, 0.24–0.73), nephropathy (RR, 0.39; 95% CI, 0.17–0.87), retinopathy (RR, 0.42; 95% CI, 0.21–0.86), and autonomic neuropathy (RR, 0.37; 95% CI, 0.18–0.79). Overall, intensive therapy in the Steno-2 study reduced risk of cardiovascular and microvascular events by about 50%.
FIGURE 1
UKPDS 35: Risk reductions associated with each 1% decrease in updated mean A1C
FIGURE 2 Percentage of patients in Steno-2 who reached treatment goals at mean follow-up of 7.8 years
Role of insulin in achieving targets
Another finding of the UKPDS was that type 2 diabetes is routinely progressive and that treatment with a single agent is unlikely to be successful for more than 5 years. Such observations mirror the physiologic progression from insulin resistance to absolute insulin deficiency. Thus, lifestyle modification often needs to be supplemented with oral antihyperglycemic therapy. In some cases, early treatment with insulin is preferable to relieve symptoms of hyperglycemia (eg, blurred vision, frequent thirst), which are suggestive of glucotoxicity and deteriorating β-cell function. As absolute insulin deficiency occurs, multiple oral agents, with or without insulin, may be required for optimal control of the disease.4,25
In UKPDS 57, 826 patients with type 2 diabetes were randomized to diet (n=242), ultralente insulin, a long-acting basal insulin (n=245), or sulfonylurea with the addition of ultralente insulin if FPG remained >108 mg/dL at maximal sulfonylurea dosage (n=339). Over 6 years, 53% of those started on sulfonylurea required addition of insulin.26 Patients treated with insulin monotherapy achieved a median A1C of 7.1%, while those on insulin plus sulfonylurea had a median A1C of 6.6% (P<.01). Episodes of major hypoglycemia occurred at a rate of 1.6% per year in the combined therapy group, compared with 3.2% a year in the insulin monotherapy group (P=0.17). In view of the observed progression of β-cell dysfunction and the failure of oral agents to maintain glycemic control, early addition of insulin to existing oral therapy may be a successful strategy in patients with type 2 diabetes.
Surmounting barriers
Insulin therapy may be delayed for a variety of reasons, including concerns about weight gain and hypo-glycemia as well as misconceptions that it increases the risk of cardiovascular disease.27 UKPDS 33 noted that relatively low proportions of patients treated with insulin experienced major (needing third-party help or medical intervention) hypoglycemic events (1.8% per year; intent-to-treat analysis).23 While both hypoglycemia and weight gain may occur with any form of insulin therapy or use of oral secretagogues, the benefits of effective glycemic control should be considered; moreover, tactics that may reduce these problems are available.28 For example, combined therapy with metformin and insulin can reduce or abolish the weight gain that otherwise may occur when insulin monotherapy is started or intensified.29 Treatment-associated hypoglycemia is generally mild to moderate in type 2 diabetes, and can be addressed with self–blood-glucose monitoring and patient education regarding recognition of symptoms and self-treatment. Also, the new long-acting insulin analogue, insulin glargine, causes fewer episodes of hypoglycemia than does neutral protamine Hagedorn (NPH) insulin.30-32
It is sometimes thought that insulin therapy may be associated with increased insulin resistance; however, studies examining peripheral insulin sensitivity have demonstrated that restoration of glycemic control with insulin improved insulin sensitivity.27,33 Also, the Diabetes Mellitus Insulin-Glucose Infusion in Acute Myocardial Infarction trial showed that intensive glycemic control with insulin therapy after an MI reduced the risk of adverse cardiovascular outcomes.6,34
Patient barriers to insulin therapy also include fear of needles and injections or a belief that insulin therapy represents failure, punishment, or a worsened prognosis.35 The notion that starting insulin therapy is a penalty for failure may be reinforced by physicians’ misconceptions about the role of insulin in type 2 diabetes.35 Setting the appropriate expectation that type 2 diabetes is a progressive disease that often eventually requires insulin can help to prevent such negative attitudes.
Finally, both patients and physicians may perceive insulin therapy as a complex and time-consuming treatment.27 Lack of office personnel and time to provide appropriate patient education for insulin therapy may contribute to such a perception. Concerns over complexity may be ameliorated by use of devices such as insulin pens and jet injectors that provide a precise dose and simplify self-administration. Premixed insulins are given twice daily and provide a measure of convenience, although flexibility of meal timing is limited in order to prevent hypoglycemia. Newer treatment regimens may also reduce patient apprehension concerning complexity and increase compliance.4 Notably, a simple and effective approach is to add once-daily basal insulin to established oral therapy.
Basal insulin studies
The efficiency and efficacy of adding basal insulin to existing oral antidiabetic agents was demonstrated in the randomized, open-label Treat-to-Target Trial.32 Overweight patients (N=756) who had been inadequately controlled (A1C >7.5%) on 1 or 2 oral agents (sulfonylurea, metformin, or thiazolidinedione) received insulin glargine or NPH insulin. The insulin dosage, taken at bedtime, was systematically titrated, based on before-breakfast glucose tests performed daily by the patients. Mean A1C levels fell from 8.6% to 6.9% after 24 weeks of therapy ( Figure 3 ). Both insulins performed similarly in terms of glycemic control, with 57% of patients in each group achieving an A1C of ≥7.0%. However, there was a significant difference in the incidence of hypoglycemia. Complete treatment success, defined as reaching target A1C without a single episode of nocturnal hypoglycemia (≤72 mg/dL), was achieved in 33% of the glargine patients and in 27% of the NPH group (P<.05). Overall rates of hypoglycemia, expressed as events per patient per year, were as follows: 14 symptomatic events in glargine patients vs 18 in NPH patients (P<.02), 9 confirmed events of ≤72 mg/dL in glargine patients vs 13 in NPH patients (P<.005), and 3 confirmed events of ≤56 mg/dL in glargine patients vs 5 in NPH patients (P<.003).
In another recent multicenter trial, 695 patients were randomized to 24 weeks of treatment with glimepiride plus 1 of 3 insulin regimens: morning insulin glargine, bedtime insulin glargine, or bedtime NPH insulin.36 Mean A1C levels decreased by 1.24% with morning glargine, 0.96% with bedtime glargine, and 0.84% with bedtime NPH. The percentage of patients achieving an A1C of ≤7.5% was 43% with morning glargine, 33% with bedtime glargine (P=.021 vs morning glargine), and 32% with bedtime NPH (P=.017 vs morning glargine). The percentage of patients who experienced nocturnal hypoglycemia was 17% with morning glargine, 23% with bedtime glargine, and 38% with bedtime NPH (P=.001 vs both glargine regimens). Finally, the percentage of patients who experienced symptomatic hypoglycemia was 56% with morning glargine (P=.004 vs bedtime glargine), 43% with bedtime glargine, and 58% with bedtime NPH (P=.001 vs bedtime glargine).
FIGURE 3 Treat-to-Target Trial: Rapid achievement of A1C goal
Practical aspects
Most commonly, insulin (Table 2) is introduced when single- or multipleagent oral therapy has failed to maintain glycemic control. Insulin may be added to existing oral therapy or, less typically, used as monotherapy. Of the 3 available insulins that have been used for basal therapy (NPH insulin, insulin glargine, and ultralente [insulin zinc extended]), ultralente may not be ideal since considerable variation has been noted in its pharmacokinetic and pharmacodynamic effects.37 Either NPH or glargine can be started at a dose of 10 U, at bedtime, while oral agents are continued at previous dosages. To provide 24-hour coverage, NPH may be needed twice daily whereas a single dose of glargine usually provides 24-hour coverage when administered at the same time each day.38 Patients usually need a total daily basal insulin dose of 0.5 to 0.6 U/kg (typically about 45 U daily for a 100-kg person).39 The dose may be adjusted weekly according to the patient’s FPG level.
Cost is a consideration in insulin therapy. Although more expensive than NPH, basal glargine is associated with 25% fewer episodes of nocturnal hypoglycemia, improved postdinner control, and slightly less weight gain.31,40 A retrospective review of medical and pharmacy claims demonstrated that considerable direct costs result from treatment of hypoglycemia associated with antidiabetic therapy, suggesting that therapies with less potential for inducing hypoglycemia would likely reduce these costs.41
The intensive insulin titration schedule used in the Treat-to-Target Trial is shown in Table 3. In clinical practice, a somewhat less intensive approach may work well. For example, the dose may be increased weekly by 4 U if the FPG level is >140 mg/dL on 3 consecutive occasions, or by 2 U if the FPG level is 120 to 140 mg/dL on 3 consecutive occasions.
Insulin glargine should not be mixed in the same syringe with other insulins as the pharmacokinetic profile may be altered. In the case of NPH, careful rolling or turning of the vial prior to injection to fully resuspend the crystals is advisable to minimize the variability of effect that may otherwise occur. Glargine, as with the regular and rapid-acting insulin analogues lispro and aspart, is a clear solution that does not need to be resuspended before administration. The addition of rapid-acting insulin analogues prandially to a regimen of basal insulin or basal-bolus therapy can be used to more closely mimic physiologic insulin secretion and may be needed when basal insulin replacement alone is insufficient to reach target levels of glycemic control.4
TABLE 2
Insulin formulations
| Insulin* | Onset | Peak | Effective duration (h) |
|---|---|---|---|
| Rapid-acting | |||
| Lispro | 5–15 min | 30–90 min | 5 |
| Aspart | 5–15 min | 30–90 min | 5 |
| Short-acting | |||
| Regular U100 | 30–60 min | 2–3 h | 5–8 |
| Regular U500 | 30–60 min | 2–3 h | 5–8 |
| Intermediate-acting | |||
| Isophane insulin (NPH) | 2–4 h | 4–10 h | 10–16 |
| Insulin zinc | 2–4 h | 4–12 h | 12–18 |
| Long-acting | |||
| Insulin zinc extended | 6–10 h | 10–16 h | 18–24 |
| Insulin glargine | 2–4 h† | No pronounced peak | 20–24 |
| Premixed | |||
| 70% NPH/30% regular | 30–60 min | Dual | 10–16 |
| 50% NPH/50% regular | 30–60 min | Dual | 10–16 |
| 75% NPL/25% lispro | 5–15 min | Dual | 10–16 |
| 70% NP/30% aspart | 5–15 min | Dual | 10–16 |
| *Assuming 0.1–0.2 U/kg per injection; onset and duration may vary by injection site (except for insulin glargine). | |||
| †Time to steady state. | |||
| NPH = neutral protamine Hagedorn; NPL = neutral protamine lispro; NP = neutral protamine. | |||
| Adapted from DeWitt and Hirsch.4 | |||
TABLE 3
Basal insulin: Initiation and dosage adjustment
| Forced titration schedule | |||
|---|---|---|---|
| |||
| Self-monitored FPG (mg/dL) | Dosage increase (IU/day) in Treat-to-Target Trial | ||
| ≥180 | 8 | ||
| ≥140 – <180 | 6 | ||
| ≥120 – <140 | 4 | ||
| >100 – <120 | 2 | ||
| *No increase if plasma glucose is <72 mg/dL in the preceding week; decrease dosage (2–4 IU/day) if plasma glucose is <56 mg/dL or severe hypoglycemia (requiring assistance) occurred within the preceding week. | |||
| FPG = fasting plasma glucose. | |||
| Adapted from Riddle et al.32 | |||
Summary
New evidence and methods continue to alter management of patients with type 2 diabetes. Appropriate screening and earlier intervention may help reduce the incidence and progression of microvascular and macrovascular complications. A multifactorial approach that addresses such risk factors as blood pressure, lipids, and glycemia has demonstrated reduced morbidity and mortality. While glycemic targets are getting lower, they can be efficiently attained with combined basal insulin and oral antidiabetic therapy. Insulin, too often considered a therapy of last resort, is an important intervention that can be used safely and effectively earlier in the course of type 2 diabetes.
1. American Diabetes Association. Economic costs of diabetes in the U.S. in 2002. Diabetes Care. 2003;26:917-932.
2. National Institute of Diabetes and Digestive and Kidney Diseases. National diabetes statistics fact sheet: general information and national estimates on diabetes in the United States, 2000. Bethesda, Md: U.S. Department of Health and Human Services, National Institutes of Health, 2002. National Institute of Diabetes and Digestive and Kidney Diseases, 2002.
3. Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229-234.
4. DeWitt DE, Hirsch IB. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus: scientific review. JAMA. 2003;289:2254-2264.
5. Dagogo-Jack S, Santiago JV. Pathophysiology of type 2 diabetes and modes of action of therapeutic interventions. Arch Intern Med. 1997;157:1802-1817.
6. Stratton IM, Adler AI, Neil HAW, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. Br Med J. 2000;321:405-412.
7. Gaede P, Vedel P, Parving H-H, Pedersen O. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno-2 randomised study. Lancet. 1999;353:617-622.
8. Gaede P, Vedel P, Larsen N, Jensen GVH, Parving H-H, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383-393.
9. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. Br Med J. 1998;317:703-713.
10. American Diabetes Association. Clinical practice recommendations 2003. Diabetes Care. 2003;26(suppl 1):S1-S156.
11. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
12. National Institutes of Health, National Heart Lung and Blood Institute. JNC 7 Express: The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Bethesda, Md: US Dept of Health and Human Services; May 2003. NIH publication 03-5233. Available at: http://www.nhlbi.nih.gov/guidelines/hypertension/express. Accessed November 14, 2003.
13. American Association of Clinical Endocrinologists medical guidelines for the management of diabetes mellitus: the AACE system of intensive diabetes self-management: 2000 update. Endocr Pract. 2000;8(suppl 1):40-82.
14. Woolf SH, Davidson MB, Greenfield S, et al. Controlling blood glucose levels in patients with type 2 diabetes mellitus: an evidence-based policy statement by the American Academy of Family Physicians and American Diabetes Association. J Fam Pract. 2000;49:453-460.
15. National Committee for Quality Assurance. The State of Health Care Quality: 2003. Washington, DC: NCQA; 2003;1-34.
16. Saaddine JB, Engelgau MM, Beckles GL, Gregg EW, Thompson TJ, Venkat Narayan KMV. A diabetes report card for the United States: quality of care in the 1990s. Ann Intern Med. 2002;136:565-574.
17. Suwattee P, Lynch JC, Pendergrass ML. Quality of care for diabetic patients in a large urban public hospital. Diabetes Care. 2003;26:563-568.
18. Fleming BB, Greenfield S, Engelgau MM, Pogach LM, Clauser SB, Parrott MA. The Diabetes Quality Improvement Project: moving science into health policy to gain an edge on the diabetes epidemic. Diabetes Care. 2001;24:1815-1820.
19. American Diabetes Association. Screening for type 2 diabetes. Diabetes Care. 2004;27:S11-S14.
20. U.S. Preventive Services Task Force. Screening for type 2 diabetes mellitus in adults: recommendations and rationale. Ann Intern Med. 2003;138:212-214.
21. Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160-3167.
22. Centers for Disease Control and Prevention. Prevalence of diabetes and impaired fasting glucose in adults: United States, 1999-2000. Morb Mortal Wkly Rep. 2003;52:833-837.
23. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837-853.
24. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854-865.
25. Feld S. AACE diabetes guidelines. Endocr Pract. 2002;8(suppl 1):41-65.
26. Wright A, Burden AC, Paisey RB, Cull CA, Holman RR. Sulfonylurea inadequacy: efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the UK Prospective Diabetes Study (UKPDS 57). Diabetes Care. 2002;25:330-336.
27. Riddle MC. The underuse of insulin therapy in North America. Diabetes Metab Res Rev. 2002;18:S42-S49.
28. Rosenstock J. Insulin therapy: optimizing control in type 1 and type 2 diabetes. Clin Cornerstone. 2001;4:50-64.
29. Avilés-Santa L, Sinding J, Raskin P. Effects of metformin in patients with poorly controlled, insulin-treated type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1999;131:182-188.
30. Ratner RE, Hirsch IB, Neifing JL, Garg SK, Mecca TE, Wilson CA. Less hypoglycemia with insulin glargine in intensive insulin therapy for type 1 diabetes. U.S. Study Group of Insulin Glargine in Type 1 Diabetes. Diabetes Care. 2000;23:639-643.
31. Yki-Jarvinen H, Dressler A, Ziemen M. Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. HOE 901/3002 Study Group. Diabetes Care. 2000;23:1130-1136.
32. Riddle MC, Rosenstock J, Gerich J. The Treat-to-Target Trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26:3080-3086.
33. Scarlett JA, Gray RS, Griffin J, Olefsky JM, Kolterman OG. Insulin treatment reverses the insulin resistance of type II diabetes mellitus. Diabetes Care. 1982;5:353-363.
34. Malmberg K. and the DIGAMI (Diabetes Mellitus Insulin-Glucose Infusion in Acute Myocardial Infarction) Study Group Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus. Br Med J. 1997;314:1512-1515.
35. Hunt LM, Valenzuela MA, Pugh JA. NIDDM patients’ fears and hopes about insulin therapy: the basis of patient reluctance. Diabetes Care. 1997;20:292-298.
36. Fritsche A, Schweitzer MA, Haring HU. Glimepiride combined with morning insulin glargine, bedtime neutral protamine Hagedorn insulin, or bedtime insulin glargine in patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med. 2003;138:952-959.
37. Lepore M, Pampanelli S, Fanelli C, et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes. 2000;49:2142-2148.
38. Full U.S. Prescribing Information for Lantus. Available at: http://www.lantus.com/professional/home.jsp .Accessed March 5, 2003.
39. DeWitt DE, Dugdale DC. Using new insulin strategies in the outpatient treatment of diabetes: clinical applications. JAMA. 2003;289:2265-2269.
40. Rosenstock J, Schwartz SL, Clark CM, Jr.,, Park GD, Donley DW, Edwards MB. Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care. 2001;24:631-636.
41. Raut M, Sung JCY, Law AW. Cost of treating hypoglycemia among patients with diabetes [abstract]. Diabetes Care. 2003;26(suppl 2):A1152.-
Disclosures: Dr LeRoith serves as a consultant to Aventis Pharmaceuticals, Pfizer Inc, and Novo Nordisk Pharmaceuticals, Inc. and on the speakers’ bureaus of Aventis Pharmaceuticals, Pfizer Inc, and Eli Lilly and Co. Dr Levetan serves as a consultant to Eli Lilly and Co. and Novo Nordisk Pharmaceuticals, Inc. and on the speakers’ bureaus of Aventis Pharmaceuticals and Novo Nordisk Pharmaceuticals, Inc. Dr Hirsch serves as a consultant to Eli Lilly and Co., Novo Nordisk Pharmaceuticals, Inc., Aventis Pharmaceuticals, and Medtronic MiniMed. Dr Riddle has received grant/research support from Amylin Pharmaceuticals, Aventis Pharmaceuticals, and Pfizer Inc. He serves as a consultant to and is on the speakers’ bureaus of Amylin Pharmaceuticals, Aventis Pharmaceuticals, GlaxoSmithKline, and Novo Nordisk Pharmaceuticals, Inc. Corresponding author: Derek Le Roith, MD, PhD, Room 8D12, Bldg 10, National Institutes of Health, MSC 1758, Bethesda MD 20892-1758. E-mail: leroith@comcast.net.
1. American Diabetes Association. Economic costs of diabetes in the U.S. in 2002. Diabetes Care. 2003;26:917-932.
2. National Institute of Diabetes and Digestive and Kidney Diseases. National diabetes statistics fact sheet: general information and national estimates on diabetes in the United States, 2000. Bethesda, Md: U.S. Department of Health and Human Services, National Institutes of Health, 2002. National Institute of Diabetes and Digestive and Kidney Diseases, 2002.
3. Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229-234.
4. DeWitt DE, Hirsch IB. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus: scientific review. JAMA. 2003;289:2254-2264.
5. Dagogo-Jack S, Santiago JV. Pathophysiology of type 2 diabetes and modes of action of therapeutic interventions. Arch Intern Med. 1997;157:1802-1817.
6. Stratton IM, Adler AI, Neil HAW, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. Br Med J. 2000;321:405-412.
7. Gaede P, Vedel P, Parving H-H, Pedersen O. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno-2 randomised study. Lancet. 1999;353:617-622.
8. Gaede P, Vedel P, Larsen N, Jensen GVH, Parving H-H, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383-393.
9. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. Br Med J. 1998;317:703-713.
10. American Diabetes Association. Clinical practice recommendations 2003. Diabetes Care. 2003;26(suppl 1):S1-S156.
11. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
12. National Institutes of Health, National Heart Lung and Blood Institute. JNC 7 Express: The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Bethesda, Md: US Dept of Health and Human Services; May 2003. NIH publication 03-5233. Available at: http://www.nhlbi.nih.gov/guidelines/hypertension/express. Accessed November 14, 2003.
13. American Association of Clinical Endocrinologists medical guidelines for the management of diabetes mellitus: the AACE system of intensive diabetes self-management: 2000 update. Endocr Pract. 2000;8(suppl 1):40-82.
14. Woolf SH, Davidson MB, Greenfield S, et al. Controlling blood glucose levels in patients with type 2 diabetes mellitus: an evidence-based policy statement by the American Academy of Family Physicians and American Diabetes Association. J Fam Pract. 2000;49:453-460.
15. National Committee for Quality Assurance. The State of Health Care Quality: 2003. Washington, DC: NCQA; 2003;1-34.
16. Saaddine JB, Engelgau MM, Beckles GL, Gregg EW, Thompson TJ, Venkat Narayan KMV. A diabetes report card for the United States: quality of care in the 1990s. Ann Intern Med. 2002;136:565-574.
17. Suwattee P, Lynch JC, Pendergrass ML. Quality of care for diabetic patients in a large urban public hospital. Diabetes Care. 2003;26:563-568.
18. Fleming BB, Greenfield S, Engelgau MM, Pogach LM, Clauser SB, Parrott MA. The Diabetes Quality Improvement Project: moving science into health policy to gain an edge on the diabetes epidemic. Diabetes Care. 2001;24:1815-1820.
19. American Diabetes Association. Screening for type 2 diabetes. Diabetes Care. 2004;27:S11-S14.
20. U.S. Preventive Services Task Force. Screening for type 2 diabetes mellitus in adults: recommendations and rationale. Ann Intern Med. 2003;138:212-214.
21. Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160-3167.
22. Centers for Disease Control and Prevention. Prevalence of diabetes and impaired fasting glucose in adults: United States, 1999-2000. Morb Mortal Wkly Rep. 2003;52:833-837.
23. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837-853.
24. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854-865.
25. Feld S. AACE diabetes guidelines. Endocr Pract. 2002;8(suppl 1):41-65.
26. Wright A, Burden AC, Paisey RB, Cull CA, Holman RR. Sulfonylurea inadequacy: efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the UK Prospective Diabetes Study (UKPDS 57). Diabetes Care. 2002;25:330-336.
27. Riddle MC. The underuse of insulin therapy in North America. Diabetes Metab Res Rev. 2002;18:S42-S49.
28. Rosenstock J. Insulin therapy: optimizing control in type 1 and type 2 diabetes. Clin Cornerstone. 2001;4:50-64.
29. Avilés-Santa L, Sinding J, Raskin P. Effects of metformin in patients with poorly controlled, insulin-treated type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1999;131:182-188.
30. Ratner RE, Hirsch IB, Neifing JL, Garg SK, Mecca TE, Wilson CA. Less hypoglycemia with insulin glargine in intensive insulin therapy for type 1 diabetes. U.S. Study Group of Insulin Glargine in Type 1 Diabetes. Diabetes Care. 2000;23:639-643.
31. Yki-Jarvinen H, Dressler A, Ziemen M. Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. HOE 901/3002 Study Group. Diabetes Care. 2000;23:1130-1136.
32. Riddle MC, Rosenstock J, Gerich J. The Treat-to-Target Trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26:3080-3086.
33. Scarlett JA, Gray RS, Griffin J, Olefsky JM, Kolterman OG. Insulin treatment reverses the insulin resistance of type II diabetes mellitus. Diabetes Care. 1982;5:353-363.
34. Malmberg K. and the DIGAMI (Diabetes Mellitus Insulin-Glucose Infusion in Acute Myocardial Infarction) Study Group Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus. Br Med J. 1997;314:1512-1515.
35. Hunt LM, Valenzuela MA, Pugh JA. NIDDM patients’ fears and hopes about insulin therapy: the basis of patient reluctance. Diabetes Care. 1997;20:292-298.
36. Fritsche A, Schweitzer MA, Haring HU. Glimepiride combined with morning insulin glargine, bedtime neutral protamine Hagedorn insulin, or bedtime insulin glargine in patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med. 2003;138:952-959.
37. Lepore M, Pampanelli S, Fanelli C, et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes. 2000;49:2142-2148.
38. Full U.S. Prescribing Information for Lantus. Available at: http://www.lantus.com/professional/home.jsp .Accessed March 5, 2003.
39. DeWitt DE, Dugdale DC. Using new insulin strategies in the outpatient treatment of diabetes: clinical applications. JAMA. 2003;289:2265-2269.
40. Rosenstock J, Schwartz SL, Clark CM, Jr.,, Park GD, Donley DW, Edwards MB. Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care. 2001;24:631-636.
41. Raut M, Sung JCY, Law AW. Cost of treating hypoglycemia among patients with diabetes [abstract]. Diabetes Care. 2003;26(suppl 2):A1152.-
Disclosures: Dr LeRoith serves as a consultant to Aventis Pharmaceuticals, Pfizer Inc, and Novo Nordisk Pharmaceuticals, Inc. and on the speakers’ bureaus of Aventis Pharmaceuticals, Pfizer Inc, and Eli Lilly and Co. Dr Levetan serves as a consultant to Eli Lilly and Co. and Novo Nordisk Pharmaceuticals, Inc. and on the speakers’ bureaus of Aventis Pharmaceuticals and Novo Nordisk Pharmaceuticals, Inc. Dr Hirsch serves as a consultant to Eli Lilly and Co., Novo Nordisk Pharmaceuticals, Inc., Aventis Pharmaceuticals, and Medtronic MiniMed. Dr Riddle has received grant/research support from Amylin Pharmaceuticals, Aventis Pharmaceuticals, and Pfizer Inc. He serves as a consultant to and is on the speakers’ bureaus of Amylin Pharmaceuticals, Aventis Pharmaceuticals, GlaxoSmithKline, and Novo Nordisk Pharmaceuticals, Inc. Corresponding author: Derek Le Roith, MD, PhD, Room 8D12, Bldg 10, National Institutes of Health, MSC 1758, Bethesda MD 20892-1758. E-mail: leroith@comcast.net.