User login
Kidney Patients With Diabetes: Managing Their Medication
CE/CME No: CR-1402
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Discuss the incidence and staging of chronic kidney disease (CKD).
• Enumerate the treatment goals for the CKD patient with diabetes.
• Describe the hypoglycemic medications that can be used at each stage of CKD.
FACULTY
Cheryl Gilmartin is a Clinical Pharmacist in Nephrology and a Clinical Assistant Professor in the College of Pharmacy at the University of Illinois at Chicago. Jane S. Davis, a member of the Clinician Reviews editorial board, is a nurse practitioner in the Division of Nephrology at the University of Alabama at Birmingham and is the communications chairperson for the National Kidney Foundation’s Council of Advanced Practitioners (NKF-CAP). Kim Zuber, past chair of the NKF-CAP, is a physician assistant with Metropolitan Nephrology in Alexandria, Virginia, and Clinton, Maryland.
ACCREDITATION STATEMENT
Article begins on next page >>
Because the vast majority of patients with chronic kidney disease (CKD) are diabetic and thus take hypoglycemic medications, knowledge of renal dosing for these medications, their mechanisms of action, and their safety profiles, as well as consideration of A1C goals, is vital for the practicing clinician. Management of the diabetic CKD patient, identified by stage of kidney disease, is outlined, with dosing regimens as determined by the glomerular filtration rate. Special attention is given to insulin management.
Diabetes is the most common cause of chronic kidney disease (CKD) throughout the world and leaves in its wake huge financial and social burdens.1 Diabetes has been the main cause of kidney failure in the United States for a number of years; as of 2012, diabetes became the most common cause of kidney failure in the world.2
The cost of caring for the rapidly increasing diabetes population in the US has been enormous. In 2012, the price tag for treating diabetes was $245 billion for medical care alone.3 The societal cost—loss in life years, loss of productivity caused by an increase in early retirement and disability, and burden of caregiving on families—is immeasurable.
In addition to CKD, diabetes is a major risk factor for heart disease and can lead to blindness and amputations. The rising incidence of diabetes has resulted in a new sense of urgency and has prompted the health care community to find new ways to reduce the burden on the patient, as well as encourage more aggressive research on halting progression of the disease, preventing end-stage kidney failure, and avoiding the need for dialysis.
Nearly two out of three (59%) adults in the US will be diagnosed with CKD stages 3 to 5 during their lifetimes.4 Some of this can be attributed to normal loss of kidney function associated with aging. However, much is due to the double burden of diabetes and hypertension.5 Patients with CKD require ongoing medical care, and much of it will be provided in primary care practices.6 This article discusses the Kidney Disease: Improving Global Outcomes (KDIGO) 2012 staging guidelines and explains which oral and injectable diabetes medications are acceptable at each stage of CKD (as defined by the glomerular filtration rate [GFR]) and how to simplify dosing.
On the next page: Staging CKD >>
STAGING CHRONIC KIDNEY DISEASE
The increasing incidence of CKD was first recognized in the 1990s. The need to care for the patient with CKD was understood, but there was a dearth of standardized management practices. Research was hindered by the many names used to identify kidney disease in the literature: from mildly elevated serum creatinine to low clearance to renal insufficiency.7 To rectify this, the National Kidney Foundation (NKF) convened an expert panel to develop a standard language and units of measurement to define kidney disease. The panel was tasked with developing classifications and an evaluation system for renal disease; identifying ways to attain more timely referrals to nephrology; clarifying research objectives; and delineating how to improve medication dosing. Ultimately, the main objective was to improve management of the patient with CKD.
In 2002, the NKF’s Dialysis Outcomes Quality Initiative (K/DOQI) defined the stages of kidney disease based on assessment of GFR and other kidney disease markers.8 GFR is a mathematical calculation for determining kidney function that corrects for loss of kidney function and incorporates the patient’s gender, race, age, and body size—the nonmodifiable risk factors known in 2001. GFR is expressed in mL/min/1.73 m2. Classifying patients with CKD according to the stage of kidney disease by GFR provides a mechanism for studying the efficacy and adverse reactions of medications while correlating them to each stage. This is of particular importance when treating patients with diabetes and kidney disease, as it can help to avoid adverse effects associated with inappropriate renal dosing. Many medications are renally eliminated; as kidney function diminishes, the pharmacokinetics of drugs are altered, potentially causing toxicity and an increased risk for drug interactions. As the GFR falls, even drugs not eliminated by the kidney may have altered pharmacokinetics and pharmacodynamics that may cause renal or systemic toxicity.1
Determining the precise method for renally dosing medication has been one of the most vigorously debated topics in CKD. Many medications were prescribed using the patient’s serum creatinine (SCr) concentration, a marker but not an exact measure of kidney disease.8 The Cockcroft-Gault equation, which provides an estimate of creatinine clearance (eCrCl; an alternate GFR measurement), was primarily used to determine renal drug dosing. 9
In 1999, a multisite study group worked to develop an equation to measure loss of kidney function in the patient with CKD.10,11 The new formula, known as the MDRD (Modification of Diet in Renal Disease) equation, standardized the measurement of loss of kidney function and provided an estimated GFR (eGFR) that was more accurate then eCrCl. At that time, the National Kidney Disease Education Program (NKDEP) decided to use the MDRD equation to assess renal function and use the eCrCl for dosing medications. The latter was chosen because most drugs were analyzed using eCrCl.9 More recently, the NKDEP stated that either the eCrCl or the eGFR is acceptable for renal drug dosing. Hudson and Nyman9 advise that when discrepancies occur between eGFR and eCrCl, clinical judgment should be applied in the elderly and in those with extremes in body weight. In those cases, therapeutic outcomes and risk versus benefit need to be considered when determining renal dosing.
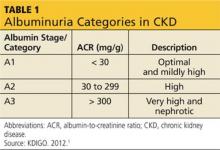
The publication of CKD stages by K/DOQI in 2002 sparked research that has uncovered additional criteria that might be useful in slowing the progression and improving the management of CKD. Thus, in 2013, new guidelines by KDIGO incorporating the latest international findings were published.1 KDIGO expands the definition of CKD to include albuminuria (ALB) as an added risk factor (see Figure) and has modified K/DOQI stages 3 and 5. ALB was included because it has predictive value for progression of CKD to kidney failure. In the new classification, there are three ranges defined for ALB measured in mg/g (see Table 1). The KDIGO guidelines, like K/DOQI, classify kidney disease using a 1-to-5 staging system; however, the stage numbers in the KDIGO classification are prefaced by a G (ie, G1, G2). Also, as mentioned above, stages G3 and G5 in the KDIGO classification have been further categorized.
CKD stage 3 was divided for practical reasons. It was found that the original CKD stage 3 was too large and diverse a classification to use for research studies, tracking of hospitalizations, and precise dosing of medications.12 K/DOQI stage 3 encompassed a GFR from 30 to 59 mL/min/1.73 m2. The KDIGO guideline divides K/DOQI stage 3 into G3a (GFR of 45 to 59 mL/min/1.73 m2) and G3b (GFR of 30 to 44 mL/min/1.73 m2). The staging is then further classified by the presence or absence of ALB. The patient with a GFR of 56 mL/min/1.73 m2 with ALB is in CKD stage 3aA3 (very high and nephrotic ALB) and has a greater risk for disease progression than the patient with a GFR of 56 mL/min/1.73 m2 without ALB. The latter patient is in CKD stage 3aA1(optimal and mildly high ALB). Finally, many medication dosages that were acceptable at a GFR of 56 mL/min/1.73 m2 had to be further adjusted for patients with a GFR of 30 mL/min/1.73 m2 to avoid toxicity. This further justified the KDIGO reclassification of stage 3.
Stage 5 from K/DOQI only reflected a GFR < 15 mL/min/1.73 m2. KDIGO utilizes the same GFR but splits the stage into patients not receiving dialysis and those receiving dialysis, G5 and G5D, respectively. The dosing and timing of medications due to drug dialyzability—including renal-specific medications—may be quite different for patients on dialysis as compared to those with a GFR < 15 mL/min/1.73 m2 not on dialysis, making the distinction beneficial.
On the next page: Diabetes management >>
DIABETES MANAGEMENT
The treatment of patients early in diabetic CKD is often the responsibility of primary care providers, who are faced with the daunting task of addressing the renal dosing of medications. The eGFR or eCrCl needs to be utilized to adjust diabetic medications, largely to avoid hypoglycemia. To alleviate some of the difficulty, the KDIGO CKD stage and the eCrCl and eGFR for diabetic medications are delineated in Table 2.1
Note that the guidelines are not meant to be strictly adhered to but rather are intended as a tool for managing the patient with CKD. Clinically significant patient factors need to be considered to adequately adjust drugs in CKD. Also, as new data become available from postmarketing reports, dosing adjustments, added precautions, or updated risk factors in these fragile patients may need to be incorporated into the guidelines. As new diabetic medications are approved by the FDA, kidney-specific dosing will continue to be a moving target.
While making lifestyle changes is very important early in diabetes, by the time diabetic nephropathy manifests, more aggressive action is warranted.12 A part of the diabetic microvascular triad (along with retinopathy and neuropathy), nephropathy signals existing and irreversible organ damage. Before instituting treatment to delay diabetic nephropathy progression, an A1C goal needs to be established. The long-standing goal has been an A1C < 7%, according to guidelines established by the American Diabetes Association, or 6.5%, as recognized by the American Association of Clinical Endocrinologists.13 The Diabetes Control and Complications Trial (DCCT) demonstrated microvascular benefit of tight glycemic control in type 1 diabetic patients when the A1C was maintained at an average of 7.2% (versus 9.1% in conventional therapy).14 The United Kingdom Prospective Diabetes Study similarly demonstrated microvascular benefit with intensive blood glucose control in type 2 diabetic patients who were maintained at a median A1C of 7% compared to the conventional treatment group maintained at an A1C of 7.9%.15
In January 2013, Perkovic and colleagues, in a post hoc analysis of the ADVANCE Trial, showed that maintaining an A1C between 6.5% and 7% in a patient with diabetes and macroalbuminuria (UACR > 300 μg/mg) slows progression to kidney failure.16 The time needed to treat with intensive glucose control was five years, and the number needed to treat was 41. Thus, for every 41 patients with diabetes, CKD, and ALB whose A1C is below 7%, one will not progress to kidney failure in five years of treatment.
The five-year lead time is troublesome, however. It discourages many patients from taking steps to achieve strict glycemic control; a significant number fail to follow their diabetic diets or take their medications until the damage is done. For the elderly kidney patient with diabetes and multiple other comorbidities, aggressively managing diabetes may result in hypoglycemia.12 On balance, the slight loss of kidney function five years hence is less problematic than the present risk for a fall and resulting hip fracture due to hypoglycemia. For this reason, the KDOQI glycemic guidelines suggest an A1C between 7% to 7.5% as the goal for those with significant comorbidities.12
On the next page: Diabetic medication dosing for CKD >>
DIABETIC MEDICATION DOSING FOR CKD
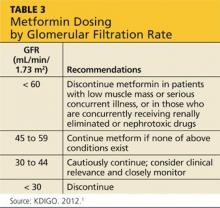
Glycemic control is important in delaying the progression of kidney failure in the patient with CKD. Hypoglycemic medications by definition may cause low blood glucose levels. This is of particular concern in patients with diminishing renal function, particularly the elderly and CKD patients. Antidiabetes drugs for patients in CKD stages 1 and 2 have few renal precautions, although care must be taken with metformin (see Table 3). Metformin is the drug of choice for the patient newly diagnosed with diabetes. It is inexpensive and effective, causes hypoglycemia only with intensive exercise, is taken orally, and is generally well tolerated. However, the package insert (PI) indicates stopping it in patients whose kidney disease has progressed to a GFR < 60 mL/min/1.73 m2 because it causes an increased risk for lactic acidosis.17,18
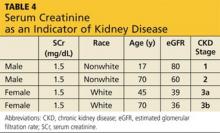
Metformin was developed prior to 1998, when the approval of the dosage regimen was tied to SCr levels rather than the presently accepted eGFR.19 As a result, the PI states that metformin should not be used in women with an SCr > 1.4 mg/dL or in men with an SCr > 1.5 mg/dL.17 However, as noted, SCr concentration alone is a very poor indicator of kidney disease (see Table 4).
Currently, the KDIGO guidelines recommend assessing the GFR for metformin dosing. When the GFR is < 60 mL/min/1.73 m2, KDIGO recommends metformin be discontinued in patients with low muscle mass or serious concurrent illness, or those who are concurrently receiving renally eliminated or nephrotoxic drugs (this includes, but is not limited to, renin-angiotensin-aldosterone system [RAAS] inhibitors, NSAIDs, and diuretics).1 In those patients who do not have the exclusions delineated above, KDIGO recommends that metformin may be continued when the GFR is > 45 mL/min/1.73 m2 (stages G1 to G3a), closely monitored and reconsidered when the GFR is 30 to 44 mL/min/1.73 m2 (stage G3b), and discontinued when the GFR is < 30 mL/min/1.73 m2 (stages G4 to G5). The KDIGO differs from other sources that recommend metformin be stopped when the GFR is < 50 to 70 ml/min/1.73 m2.20-22 The metformin PI also recommends stopping metformin the day of or day before and two days after procedures in which radiographic iodinated contrast dye is administered, to avoid acute kidney failure. Finally, it is important to note that any combination medication formulated with metformin should be stopped when the GFR is 50 mL/min/1.73 m2 because lactic acidosis has also been seen in patients taking these medications.21
At stage 3a (GFR, 45 to 60 mL/min/1.73 m2), the sulfonylureas require dosing changes (see Table 2). Glipizide is often the oral medication of choice during CKD, and it may be used in all stages, including dialysis, but does require renal dosing and monitoring.23 Although the insulins are more effective than glipizide in glycemic control, many practitioners prescribe it for patients who will not accept an injectable medication. Glyburide is not used at this stage, as it can lead to complications in patients with a GFR < 60 mL/min/1.73 m2, particularly hypoglycemia.
Like the sulfonylureas, the meglitinides enhance insulin secretion.13,18 Repaglinide and nateglinide are fast-acting and need to be taken with meals. Because it is recommended that the dose of the meglitinides be omitted when a meal is skipped, they are good for patients who eat meals irregularly. The meglitinides require initiation at a low dose in severe renal insufficiency.
The thiazolidinediones (TDZs) are used with caution in patients with CKD, not only because of controversial adverse cardiac effects, but also for peripheral edema that interferes with CKD management.18 The TDZs (rosiglitazone and pioglitazone) require no dose adjustment and appear to have a beneficial effect in obese patients and in patients who experienced weight gain with other hypoglycemic agents.
The newest of the diabetic drugs, canagliflozin, requires a working kidney to be effective.24,25 It is used with precaution when GFR is 45 to 59 mL/min/1.73 m2 at stage G3a and is contraindicated when GFR is < 45 mL/min/1.73 m2 in stage G3b. Canagliflozin's effects are felt in early segment of proximal convoluted tubule where it blocks sodium-glucose co-transporter 2, thereby lowering reabsorption of glucose and increasing excretion of glucose in the urine. In practical terms, it can affect kidney function and, due to the inhibition of the glucose-sodium pathway, induce hyperkalemia.25 Monitoring of both the GFR and potassium is vital when administering this drug. As data from postmarketing reports come in and practitioners gain experience, use of canaglifozin in the patient with CKD will be further delineated.
At a GFR between 30 and 45 mL/min/1.73 m2, CKD stage 3b, alpha-glucosidase inhibitors acarbose and miglitol are contraindicated. The GLP-1 mimetic liraglutide needs to be avoided when the GFR is < 60 mL/min/1.73 m2 (stage G3a). While exenatide requires close monitoring and caution when the GFR is < 50 mL/min/1.73 m2, it needs to be discontinued at a GFR
< 30 mL/min/1.73 m2 (stage 4). DPP-4 inhibitors become problematic at a GFR < 60 mL/min/
1.73 m2 beginning at stage G3a. All except the DPP-4 inhibitor linagliptin require dosing changes as the loss of GFR continues. Exact dosing adjustments for the drug categories are found in Table 2.
In stage 4 CKD (GFR, 15 to 30 mL/min/1.73 m2), more medications are eliminated from consideration. The amylinomimetic pramlintide cannot be used for a patient with a GFR < 15 mL/min/1.73 m2 (see Table 2). Both GLP-1 mimetics, exenatide and liraglutide, must be stopped when the GFR drops to 30 mL/min/1.73 m2. DDP-4 inhibitors need to be dosed according to the GFR but are still acceptable at CKD stage 4. Ideally, patients with diabetes should be referred to nephrology at stage 3, but referral is vital by stage 4.
Although oral medications are not as commonly prescribed as insulins at CKD stages 4 and 5, in stage 5 CKD (GFR, < 15 mL/min/1.73 m2), a limited number of oral diabetic agents are available (see Table 2). Glipizide is inexpensive and generic; therefore, it is the most commonly used oral medication in this stage. A recent study found linagliptin was safe to use throughout all stages of kidney disease without adjustment for GFR.26 While nateglinide is still acceptable in stage 5, it is used less frequently than glipizide.
As the kidney fails, the need for diabetic medications also decreases and dosing needs to take into account any residual renal function. Because glucose is produced in the proximal tubules of the kidney (known as gluconeogenesis), the level of glucose falls as the kidney fails. Excretion of insulin and other diabetes medications decreases, and thus the amount of insulin remaining in circulation is increased. This, in combination with the reduced glucose, increases the patient’s risk for hypoglycemia.13 Although protocols for medication adjustment are tied to GFR levels, close follow-up by the practitioner is required because GFR is not a reliable measure of kidney function when creatinine levels change rapidly.27
On the next page: Insulins >>
INSULINS
Many patients start insulin prior to reaching CKD stage 4 or 5 because it is the best choice for managing diabetes through all stages of CKD.28 Insulin can slow the progression to kidney failure by providing better A1C control, and all patients should be encouraged to start insulin as soon as it is appropriate.29 Many patients are very reluctant to use injectables, but by stage 5 they are willing to use insulin if it will slow progression of disease and help prevent the need for dialysis.2
The long-acting basal insulins (detemir and glargine) or a combination of basal and oral medication can work quite well at stage 5. Many patients will require a low dose of the long-acting basal insulins and short-acting insulin with meals. As the kidney fails, the short-acting doses can often be discontinued.29 A protocol that mixes long- and short-acting insulin with the largest meal is very effective at this stage. The long-acting insulin dose will need to be decreased as the kidney continues to fail. The American College of Physicians protocol for insulin suggests a 25% decrease at stage 4 and a 50% decrease at stage 5. Each patient is different, however, and dosing must be determined individually.23
On the next page: Patient education >>
PATIENT EDUCATION
There is a correlation between patients who participate in kidney disease education programs and a decrease in the progression of kidney disease (see “Case Study: The Recalcitrant Patient With Diabetes"). These classes can be taught by a physician, physician assistant, nurse practitioner, or clinical nurse specialist. To alleviate the burden of end-stage kidney disease in the US, Medicare Part B pays for six outpatient kidney disease patient education classes per lifetime of the beneficiary.30
Since 2010, when kidney disease education programs were rolled out, over 10,000 classes have been taught.31 Many practitioners report that patients are more compliant and understand their disease better when they attend classes.32 Data are being gathered to determine the effectiveness of the classes on patient outcomes.
On the next page: Conclusion >>
CONCLUSION
Knowing how to manage the patient with both diabetes and kidney disease is increasingly vital as this patient population grows. Much of the management of these patients will fall to the primary care practitioner.6 The most effective way to start treatment is to identify which CKD stage the patient fits into.
Once the patient is properly categorized, safe and effective diabetic medications can be selected and dosed according to stage. Although the new classification system may be difficult to incorporate into some electronic medical record systems and practitioner behavior, ultimately, it will allow safer management of the patient with CKD and a better predictive power of outcome.
1. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney International Suppl. 2013;3:1-150.
2. Brazie M. Finding the sweet spot: trouble-shooting diabetic dilemmas. Oral presentation at NKF Spring Clinical Meetings; May 2012, Washington, DC.
3. Estimated cost of diabetes care $245 billion in U.S. in 2012. Renal & Urology News. March 9, 2013. www.renalandurologynews.com/estimated-cost-of-diabetes-245-billion-in-us-in-2012/article/283616/. Accessed January 17, 2013.
4. Grams ME, Chow EK, Segev DL, Coresh J. Lifetime incidence of CKD stages 3-5 in the United States. Am J Kidney Dis. 2013;62(2):245-252.
5. Greenberg A, ed. National Kidney Foundation Primer of Kidney Diseases. 5th ed. Philadelphia, PA: Saunders Elsevier; 2009.
6. Spann SJ, Nutting PA, Galliher JM, et al. Management of type 2 diabetes in the primary care setting: a practice-based research study. Ann Fam Med. 2006;4(1):23-31.
7. Hsu CY, Chertow GM. Chronic renal confusion: insufficiency, failure, dysfunction, or disease. Am J Kidney Dis. 2000;49(3):482-496.
8. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J Kidney Dis. 2002;39(suppl 1):S1-S266.
9. Hudson JQ, Nyman HA. Use of the estimated glomerular filtration rate for drug dosing in the chronic kidney disease patient. Curr Opin Nephrol Hypertens. 2011;20:482-491.
10. Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461-470.
11. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612.
12. NKF-KDOQI clinical practice guideline for diabetes and CKD, guideline 2: management of hyperglycemia and general diabetes care in CKD. Am J Kidney Dis. 2012;60(5):850-886. www.kidney.org/professionals/kdoqi/guideline_diabetes/guide2.htm. Accessed January 2, 2014.
13. Abe M, Okada K, Soma M. Antidiabetic agents in patients with chronic kidney disease and end-stage renal disease on dialysis: metabolism and clinical practice. Curr Drug Metab. 2011;12(1):57-69.
14. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med. 2000;342:381-389.
15. Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405-411.
16. Perkovic V, Heerspink HL, Chalmers J, et al. Intensive glucose control improves kidney outcomes in patients with type 2 diabetes. Kidney Int. 2013;83(3):517-23.
17. Metformin [package insert]. US Department of Health and Human Services, US Food and Drug Administration. www.fda.gov/ohrms/dockets/dailys/02/May02/053102/800471e6.pdf. Accessed January 17, 2013.
18. Triplitt CL, Reasner CA. Chapter 83. Diabetes Mellitus. In: Wells BG, ed. Pharmacotherapy: A Pathophysiologic Approach. 8th ed. New York, NY: McGraw-Hill; 2011.
19. FDA. Guidance for industry: pharmacokinetics in patients with impaired renal function—study design, data analysis, and impact on dosing and labeling. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulato ryInformation/Guidances/ucm072127.pdf. Accessed January 17, 2013.
20. Lacy CF, Armstrong LL, Goldman MP, Lance LL. Drug Information Handbook. 20th ed. Hudson, OH: Lexi-Comp, Inc.; 2011.
21. Rocha A, Almeida M, Santos J, Carvalho A. Metformin in patients with chronic kidney disease: strengths and weaknesses. J Nephrol. 2013; 26(10):55-60.
22. Gilbert SJ, Weiner DE, eds. National Kidney Foundation Primer on Kidney Diseases. 6th ed. Philadelphia, PA: Elsevier Saunders; 2014.
23. Aronoff GR, Bennett WM, Berns JS, et al. Drug Prescribing in Renal Failure: Dosing Guidelines for Adults and Children. 5th ed. Philadelphia, PA: American College of Physicians; 2007.
24. Valentine V. The role of the kidney and the sodium-glucose co-transporter inhibition in diabetes management. Clin Diabetes. 2012;30(4):151-155.
25. Invokana [package insert]. Titusville, NJ: Janssen Pharmaceuticals; 2013.
26. McGill JB, Sloan L, Newman J, et al. Long-term efficacy and safety of linagliptin in patients with type 2 diabetes and severe renal impairment. Diabetes Care. 2013;36(2):237-244.
27. National Kidney Disease and Education Program (NKDEP). Estimated glomerular filtration rate (eGFR) info sheet. NIH publication No.10-6286, March 2010.
28. Thummel K, Shen D, Isoherranen N, et al. Design and optimization of dosage regimens: pharmacokinetic data. In: Hardman J, Limbird L, Goodman G (eds). Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 11th ed. New York, NY: McGraw-Hill; 2006.
29. Snyder RW, Berns JS. Use of insulin and oral hypoglycemic medications in patients with diabetes mellitus and advanced kidney disease. Semin Dial. 2004;17(5):365-70.
30. Centers for Medicare and Medicaid Services. Medicare program: revisions to payment policies under the Physician Fee Schedule, Clinical Laboratory Fee Schedule & other revisions to Part B for CY 2014; Final Rule. Federal Register. 2009;74(226):61738-62188. www.gpo.gov/fdsys/pkg/FR-2009-11-25/html/E9-26502.htm. Accessed January 17, 2013.
31. Zuber K, Davis J. Kidney disease education: a niche for PAs and NPs. JAAPA. 2013;26(7):42-47.
32. Zuber K, Davis J. Stories from the trenches: the first year experience with kidney disease education. Nephrol News Issues. 2012;26(2):20-21.
CE/CME No: CR-1402
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Discuss the incidence and staging of chronic kidney disease (CKD).
• Enumerate the treatment goals for the CKD patient with diabetes.
• Describe the hypoglycemic medications that can be used at each stage of CKD.
FACULTY
Cheryl Gilmartin is a Clinical Pharmacist in Nephrology and a Clinical Assistant Professor in the College of Pharmacy at the University of Illinois at Chicago. Jane S. Davis, a member of the Clinician Reviews editorial board, is a nurse practitioner in the Division of Nephrology at the University of Alabama at Birmingham and is the communications chairperson for the National Kidney Foundation’s Council of Advanced Practitioners (NKF-CAP). Kim Zuber, past chair of the NKF-CAP, is a physician assistant with Metropolitan Nephrology in Alexandria, Virginia, and Clinton, Maryland.
ACCREDITATION STATEMENT
Article begins on next page >>
Because the vast majority of patients with chronic kidney disease (CKD) are diabetic and thus take hypoglycemic medications, knowledge of renal dosing for these medications, their mechanisms of action, and their safety profiles, as well as consideration of A1C goals, is vital for the practicing clinician. Management of the diabetic CKD patient, identified by stage of kidney disease, is outlined, with dosing regimens as determined by the glomerular filtration rate. Special attention is given to insulin management.
Diabetes is the most common cause of chronic kidney disease (CKD) throughout the world and leaves in its wake huge financial and social burdens.1 Diabetes has been the main cause of kidney failure in the United States for a number of years; as of 2012, diabetes became the most common cause of kidney failure in the world.2
The cost of caring for the rapidly increasing diabetes population in the US has been enormous. In 2012, the price tag for treating diabetes was $245 billion for medical care alone.3 The societal cost—loss in life years, loss of productivity caused by an increase in early retirement and disability, and burden of caregiving on families—is immeasurable.
In addition to CKD, diabetes is a major risk factor for heart disease and can lead to blindness and amputations. The rising incidence of diabetes has resulted in a new sense of urgency and has prompted the health care community to find new ways to reduce the burden on the patient, as well as encourage more aggressive research on halting progression of the disease, preventing end-stage kidney failure, and avoiding the need for dialysis.
Nearly two out of three (59%) adults in the US will be diagnosed with CKD stages 3 to 5 during their lifetimes.4 Some of this can be attributed to normal loss of kidney function associated with aging. However, much is due to the double burden of diabetes and hypertension.5 Patients with CKD require ongoing medical care, and much of it will be provided in primary care practices.6 This article discusses the Kidney Disease: Improving Global Outcomes (KDIGO) 2012 staging guidelines and explains which oral and injectable diabetes medications are acceptable at each stage of CKD (as defined by the glomerular filtration rate [GFR]) and how to simplify dosing.
On the next page: Staging CKD >>
STAGING CHRONIC KIDNEY DISEASE
The increasing incidence of CKD was first recognized in the 1990s. The need to care for the patient with CKD was understood, but there was a dearth of standardized management practices. Research was hindered by the many names used to identify kidney disease in the literature: from mildly elevated serum creatinine to low clearance to renal insufficiency.7 To rectify this, the National Kidney Foundation (NKF) convened an expert panel to develop a standard language and units of measurement to define kidney disease. The panel was tasked with developing classifications and an evaluation system for renal disease; identifying ways to attain more timely referrals to nephrology; clarifying research objectives; and delineating how to improve medication dosing. Ultimately, the main objective was to improve management of the patient with CKD.
In 2002, the NKF’s Dialysis Outcomes Quality Initiative (K/DOQI) defined the stages of kidney disease based on assessment of GFR and other kidney disease markers.8 GFR is a mathematical calculation for determining kidney function that corrects for loss of kidney function and incorporates the patient’s gender, race, age, and body size—the nonmodifiable risk factors known in 2001. GFR is expressed in mL/min/1.73 m2. Classifying patients with CKD according to the stage of kidney disease by GFR provides a mechanism for studying the efficacy and adverse reactions of medications while correlating them to each stage. This is of particular importance when treating patients with diabetes and kidney disease, as it can help to avoid adverse effects associated with inappropriate renal dosing. Many medications are renally eliminated; as kidney function diminishes, the pharmacokinetics of drugs are altered, potentially causing toxicity and an increased risk for drug interactions. As the GFR falls, even drugs not eliminated by the kidney may have altered pharmacokinetics and pharmacodynamics that may cause renal or systemic toxicity.1
Determining the precise method for renally dosing medication has been one of the most vigorously debated topics in CKD. Many medications were prescribed using the patient’s serum creatinine (SCr) concentration, a marker but not an exact measure of kidney disease.8 The Cockcroft-Gault equation, which provides an estimate of creatinine clearance (eCrCl; an alternate GFR measurement), was primarily used to determine renal drug dosing. 9
In 1999, a multisite study group worked to develop an equation to measure loss of kidney function in the patient with CKD.10,11 The new formula, known as the MDRD (Modification of Diet in Renal Disease) equation, standardized the measurement of loss of kidney function and provided an estimated GFR (eGFR) that was more accurate then eCrCl. At that time, the National Kidney Disease Education Program (NKDEP) decided to use the MDRD equation to assess renal function and use the eCrCl for dosing medications. The latter was chosen because most drugs were analyzed using eCrCl.9 More recently, the NKDEP stated that either the eCrCl or the eGFR is acceptable for renal drug dosing. Hudson and Nyman9 advise that when discrepancies occur between eGFR and eCrCl, clinical judgment should be applied in the elderly and in those with extremes in body weight. In those cases, therapeutic outcomes and risk versus benefit need to be considered when determining renal dosing.
The publication of CKD stages by K/DOQI in 2002 sparked research that has uncovered additional criteria that might be useful in slowing the progression and improving the management of CKD. Thus, in 2013, new guidelines by KDIGO incorporating the latest international findings were published.1 KDIGO expands the definition of CKD to include albuminuria (ALB) as an added risk factor (see Figure) and has modified K/DOQI stages 3 and 5. ALB was included because it has predictive value for progression of CKD to kidney failure. In the new classification, there are three ranges defined for ALB measured in mg/g (see Table 1). The KDIGO guidelines, like K/DOQI, classify kidney disease using a 1-to-5 staging system; however, the stage numbers in the KDIGO classification are prefaced by a G (ie, G1, G2). Also, as mentioned above, stages G3 and G5 in the KDIGO classification have been further categorized.
CKD stage 3 was divided for practical reasons. It was found that the original CKD stage 3 was too large and diverse a classification to use for research studies, tracking of hospitalizations, and precise dosing of medications.12 K/DOQI stage 3 encompassed a GFR from 30 to 59 mL/min/1.73 m2. The KDIGO guideline divides K/DOQI stage 3 into G3a (GFR of 45 to 59 mL/min/1.73 m2) and G3b (GFR of 30 to 44 mL/min/1.73 m2). The staging is then further classified by the presence or absence of ALB. The patient with a GFR of 56 mL/min/1.73 m2 with ALB is in CKD stage 3aA3 (very high and nephrotic ALB) and has a greater risk for disease progression than the patient with a GFR of 56 mL/min/1.73 m2 without ALB. The latter patient is in CKD stage 3aA1(optimal and mildly high ALB). Finally, many medication dosages that were acceptable at a GFR of 56 mL/min/1.73 m2 had to be further adjusted for patients with a GFR of 30 mL/min/1.73 m2 to avoid toxicity. This further justified the KDIGO reclassification of stage 3.
Stage 5 from K/DOQI only reflected a GFR < 15 mL/min/1.73 m2. KDIGO utilizes the same GFR but splits the stage into patients not receiving dialysis and those receiving dialysis, G5 and G5D, respectively. The dosing and timing of medications due to drug dialyzability—including renal-specific medications—may be quite different for patients on dialysis as compared to those with a GFR < 15 mL/min/1.73 m2 not on dialysis, making the distinction beneficial.
On the next page: Diabetes management >>
DIABETES MANAGEMENT
The treatment of patients early in diabetic CKD is often the responsibility of primary care providers, who are faced with the daunting task of addressing the renal dosing of medications. The eGFR or eCrCl needs to be utilized to adjust diabetic medications, largely to avoid hypoglycemia. To alleviate some of the difficulty, the KDIGO CKD stage and the eCrCl and eGFR for diabetic medications are delineated in Table 2.1
Note that the guidelines are not meant to be strictly adhered to but rather are intended as a tool for managing the patient with CKD. Clinically significant patient factors need to be considered to adequately adjust drugs in CKD. Also, as new data become available from postmarketing reports, dosing adjustments, added precautions, or updated risk factors in these fragile patients may need to be incorporated into the guidelines. As new diabetic medications are approved by the FDA, kidney-specific dosing will continue to be a moving target.
While making lifestyle changes is very important early in diabetes, by the time diabetic nephropathy manifests, more aggressive action is warranted.12 A part of the diabetic microvascular triad (along with retinopathy and neuropathy), nephropathy signals existing and irreversible organ damage. Before instituting treatment to delay diabetic nephropathy progression, an A1C goal needs to be established. The long-standing goal has been an A1C < 7%, according to guidelines established by the American Diabetes Association, or 6.5%, as recognized by the American Association of Clinical Endocrinologists.13 The Diabetes Control and Complications Trial (DCCT) demonstrated microvascular benefit of tight glycemic control in type 1 diabetic patients when the A1C was maintained at an average of 7.2% (versus 9.1% in conventional therapy).14 The United Kingdom Prospective Diabetes Study similarly demonstrated microvascular benefit with intensive blood glucose control in type 2 diabetic patients who were maintained at a median A1C of 7% compared to the conventional treatment group maintained at an A1C of 7.9%.15
In January 2013, Perkovic and colleagues, in a post hoc analysis of the ADVANCE Trial, showed that maintaining an A1C between 6.5% and 7% in a patient with diabetes and macroalbuminuria (UACR > 300 μg/mg) slows progression to kidney failure.16 The time needed to treat with intensive glucose control was five years, and the number needed to treat was 41. Thus, for every 41 patients with diabetes, CKD, and ALB whose A1C is below 7%, one will not progress to kidney failure in five years of treatment.
The five-year lead time is troublesome, however. It discourages many patients from taking steps to achieve strict glycemic control; a significant number fail to follow their diabetic diets or take their medications until the damage is done. For the elderly kidney patient with diabetes and multiple other comorbidities, aggressively managing diabetes may result in hypoglycemia.12 On balance, the slight loss of kidney function five years hence is less problematic than the present risk for a fall and resulting hip fracture due to hypoglycemia. For this reason, the KDOQI glycemic guidelines suggest an A1C between 7% to 7.5% as the goal for those with significant comorbidities.12
On the next page: Diabetic medication dosing for CKD >>
DIABETIC MEDICATION DOSING FOR CKD
Glycemic control is important in delaying the progression of kidney failure in the patient with CKD. Hypoglycemic medications by definition may cause low blood glucose levels. This is of particular concern in patients with diminishing renal function, particularly the elderly and CKD patients. Antidiabetes drugs for patients in CKD stages 1 and 2 have few renal precautions, although care must be taken with metformin (see Table 3). Metformin is the drug of choice for the patient newly diagnosed with diabetes. It is inexpensive and effective, causes hypoglycemia only with intensive exercise, is taken orally, and is generally well tolerated. However, the package insert (PI) indicates stopping it in patients whose kidney disease has progressed to a GFR < 60 mL/min/1.73 m2 because it causes an increased risk for lactic acidosis.17,18
Metformin was developed prior to 1998, when the approval of the dosage regimen was tied to SCr levels rather than the presently accepted eGFR.19 As a result, the PI states that metformin should not be used in women with an SCr > 1.4 mg/dL or in men with an SCr > 1.5 mg/dL.17 However, as noted, SCr concentration alone is a very poor indicator of kidney disease (see Table 4).
Currently, the KDIGO guidelines recommend assessing the GFR for metformin dosing. When the GFR is < 60 mL/min/1.73 m2, KDIGO recommends metformin be discontinued in patients with low muscle mass or serious concurrent illness, or those who are concurrently receiving renally eliminated or nephrotoxic drugs (this includes, but is not limited to, renin-angiotensin-aldosterone system [RAAS] inhibitors, NSAIDs, and diuretics).1 In those patients who do not have the exclusions delineated above, KDIGO recommends that metformin may be continued when the GFR is > 45 mL/min/1.73 m2 (stages G1 to G3a), closely monitored and reconsidered when the GFR is 30 to 44 mL/min/1.73 m2 (stage G3b), and discontinued when the GFR is < 30 mL/min/1.73 m2 (stages G4 to G5). The KDIGO differs from other sources that recommend metformin be stopped when the GFR is < 50 to 70 ml/min/1.73 m2.20-22 The metformin PI also recommends stopping metformin the day of or day before and two days after procedures in which radiographic iodinated contrast dye is administered, to avoid acute kidney failure. Finally, it is important to note that any combination medication formulated with metformin should be stopped when the GFR is 50 mL/min/1.73 m2 because lactic acidosis has also been seen in patients taking these medications.21
At stage 3a (GFR, 45 to 60 mL/min/1.73 m2), the sulfonylureas require dosing changes (see Table 2). Glipizide is often the oral medication of choice during CKD, and it may be used in all stages, including dialysis, but does require renal dosing and monitoring.23 Although the insulins are more effective than glipizide in glycemic control, many practitioners prescribe it for patients who will not accept an injectable medication. Glyburide is not used at this stage, as it can lead to complications in patients with a GFR < 60 mL/min/1.73 m2, particularly hypoglycemia.
Like the sulfonylureas, the meglitinides enhance insulin secretion.13,18 Repaglinide and nateglinide are fast-acting and need to be taken with meals. Because it is recommended that the dose of the meglitinides be omitted when a meal is skipped, they are good for patients who eat meals irregularly. The meglitinides require initiation at a low dose in severe renal insufficiency.
The thiazolidinediones (TDZs) are used with caution in patients with CKD, not only because of controversial adverse cardiac effects, but also for peripheral edema that interferes with CKD management.18 The TDZs (rosiglitazone and pioglitazone) require no dose adjustment and appear to have a beneficial effect in obese patients and in patients who experienced weight gain with other hypoglycemic agents.
The newest of the diabetic drugs, canagliflozin, requires a working kidney to be effective.24,25 It is used with precaution when GFR is 45 to 59 mL/min/1.73 m2 at stage G3a and is contraindicated when GFR is < 45 mL/min/1.73 m2 in stage G3b. Canagliflozin's effects are felt in early segment of proximal convoluted tubule where it blocks sodium-glucose co-transporter 2, thereby lowering reabsorption of glucose and increasing excretion of glucose in the urine. In practical terms, it can affect kidney function and, due to the inhibition of the glucose-sodium pathway, induce hyperkalemia.25 Monitoring of both the GFR and potassium is vital when administering this drug. As data from postmarketing reports come in and practitioners gain experience, use of canaglifozin in the patient with CKD will be further delineated.
At a GFR between 30 and 45 mL/min/1.73 m2, CKD stage 3b, alpha-glucosidase inhibitors acarbose and miglitol are contraindicated. The GLP-1 mimetic liraglutide needs to be avoided when the GFR is < 60 mL/min/1.73 m2 (stage G3a). While exenatide requires close monitoring and caution when the GFR is < 50 mL/min/1.73 m2, it needs to be discontinued at a GFR
< 30 mL/min/1.73 m2 (stage 4). DPP-4 inhibitors become problematic at a GFR < 60 mL/min/
1.73 m2 beginning at stage G3a. All except the DPP-4 inhibitor linagliptin require dosing changes as the loss of GFR continues. Exact dosing adjustments for the drug categories are found in Table 2.
In stage 4 CKD (GFR, 15 to 30 mL/min/1.73 m2), more medications are eliminated from consideration. The amylinomimetic pramlintide cannot be used for a patient with a GFR < 15 mL/min/1.73 m2 (see Table 2). Both GLP-1 mimetics, exenatide and liraglutide, must be stopped when the GFR drops to 30 mL/min/1.73 m2. DDP-4 inhibitors need to be dosed according to the GFR but are still acceptable at CKD stage 4. Ideally, patients with diabetes should be referred to nephrology at stage 3, but referral is vital by stage 4.
Although oral medications are not as commonly prescribed as insulins at CKD stages 4 and 5, in stage 5 CKD (GFR, < 15 mL/min/1.73 m2), a limited number of oral diabetic agents are available (see Table 2). Glipizide is inexpensive and generic; therefore, it is the most commonly used oral medication in this stage. A recent study found linagliptin was safe to use throughout all stages of kidney disease without adjustment for GFR.26 While nateglinide is still acceptable in stage 5, it is used less frequently than glipizide.
As the kidney fails, the need for diabetic medications also decreases and dosing needs to take into account any residual renal function. Because glucose is produced in the proximal tubules of the kidney (known as gluconeogenesis), the level of glucose falls as the kidney fails. Excretion of insulin and other diabetes medications decreases, and thus the amount of insulin remaining in circulation is increased. This, in combination with the reduced glucose, increases the patient’s risk for hypoglycemia.13 Although protocols for medication adjustment are tied to GFR levels, close follow-up by the practitioner is required because GFR is not a reliable measure of kidney function when creatinine levels change rapidly.27
On the next page: Insulins >>
INSULINS
Many patients start insulin prior to reaching CKD stage 4 or 5 because it is the best choice for managing diabetes through all stages of CKD.28 Insulin can slow the progression to kidney failure by providing better A1C control, and all patients should be encouraged to start insulin as soon as it is appropriate.29 Many patients are very reluctant to use injectables, but by stage 5 they are willing to use insulin if it will slow progression of disease and help prevent the need for dialysis.2
The long-acting basal insulins (detemir and glargine) or a combination of basal and oral medication can work quite well at stage 5. Many patients will require a low dose of the long-acting basal insulins and short-acting insulin with meals. As the kidney fails, the short-acting doses can often be discontinued.29 A protocol that mixes long- and short-acting insulin with the largest meal is very effective at this stage. The long-acting insulin dose will need to be decreased as the kidney continues to fail. The American College of Physicians protocol for insulin suggests a 25% decrease at stage 4 and a 50% decrease at stage 5. Each patient is different, however, and dosing must be determined individually.23
On the next page: Patient education >>
PATIENT EDUCATION
There is a correlation between patients who participate in kidney disease education programs and a decrease in the progression of kidney disease (see “Case Study: The Recalcitrant Patient With Diabetes"). These classes can be taught by a physician, physician assistant, nurse practitioner, or clinical nurse specialist. To alleviate the burden of end-stage kidney disease in the US, Medicare Part B pays for six outpatient kidney disease patient education classes per lifetime of the beneficiary.30
Since 2010, when kidney disease education programs were rolled out, over 10,000 classes have been taught.31 Many practitioners report that patients are more compliant and understand their disease better when they attend classes.32 Data are being gathered to determine the effectiveness of the classes on patient outcomes.
On the next page: Conclusion >>
CONCLUSION
Knowing how to manage the patient with both diabetes and kidney disease is increasingly vital as this patient population grows. Much of the management of these patients will fall to the primary care practitioner.6 The most effective way to start treatment is to identify which CKD stage the patient fits into.
Once the patient is properly categorized, safe and effective diabetic medications can be selected and dosed according to stage. Although the new classification system may be difficult to incorporate into some electronic medical record systems and practitioner behavior, ultimately, it will allow safer management of the patient with CKD and a better predictive power of outcome.
CE/CME No: CR-1402
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Discuss the incidence and staging of chronic kidney disease (CKD).
• Enumerate the treatment goals for the CKD patient with diabetes.
• Describe the hypoglycemic medications that can be used at each stage of CKD.
FACULTY
Cheryl Gilmartin is a Clinical Pharmacist in Nephrology and a Clinical Assistant Professor in the College of Pharmacy at the University of Illinois at Chicago. Jane S. Davis, a member of the Clinician Reviews editorial board, is a nurse practitioner in the Division of Nephrology at the University of Alabama at Birmingham and is the communications chairperson for the National Kidney Foundation’s Council of Advanced Practitioners (NKF-CAP). Kim Zuber, past chair of the NKF-CAP, is a physician assistant with Metropolitan Nephrology in Alexandria, Virginia, and Clinton, Maryland.
ACCREDITATION STATEMENT
Article begins on next page >>
Because the vast majority of patients with chronic kidney disease (CKD) are diabetic and thus take hypoglycemic medications, knowledge of renal dosing for these medications, their mechanisms of action, and their safety profiles, as well as consideration of A1C goals, is vital for the practicing clinician. Management of the diabetic CKD patient, identified by stage of kidney disease, is outlined, with dosing regimens as determined by the glomerular filtration rate. Special attention is given to insulin management.
Diabetes is the most common cause of chronic kidney disease (CKD) throughout the world and leaves in its wake huge financial and social burdens.1 Diabetes has been the main cause of kidney failure in the United States for a number of years; as of 2012, diabetes became the most common cause of kidney failure in the world.2
The cost of caring for the rapidly increasing diabetes population in the US has been enormous. In 2012, the price tag for treating diabetes was $245 billion for medical care alone.3 The societal cost—loss in life years, loss of productivity caused by an increase in early retirement and disability, and burden of caregiving on families—is immeasurable.
In addition to CKD, diabetes is a major risk factor for heart disease and can lead to blindness and amputations. The rising incidence of diabetes has resulted in a new sense of urgency and has prompted the health care community to find new ways to reduce the burden on the patient, as well as encourage more aggressive research on halting progression of the disease, preventing end-stage kidney failure, and avoiding the need for dialysis.
Nearly two out of three (59%) adults in the US will be diagnosed with CKD stages 3 to 5 during their lifetimes.4 Some of this can be attributed to normal loss of kidney function associated with aging. However, much is due to the double burden of diabetes and hypertension.5 Patients with CKD require ongoing medical care, and much of it will be provided in primary care practices.6 This article discusses the Kidney Disease: Improving Global Outcomes (KDIGO) 2012 staging guidelines and explains which oral and injectable diabetes medications are acceptable at each stage of CKD (as defined by the glomerular filtration rate [GFR]) and how to simplify dosing.
On the next page: Staging CKD >>
STAGING CHRONIC KIDNEY DISEASE
The increasing incidence of CKD was first recognized in the 1990s. The need to care for the patient with CKD was understood, but there was a dearth of standardized management practices. Research was hindered by the many names used to identify kidney disease in the literature: from mildly elevated serum creatinine to low clearance to renal insufficiency.7 To rectify this, the National Kidney Foundation (NKF) convened an expert panel to develop a standard language and units of measurement to define kidney disease. The panel was tasked with developing classifications and an evaluation system for renal disease; identifying ways to attain more timely referrals to nephrology; clarifying research objectives; and delineating how to improve medication dosing. Ultimately, the main objective was to improve management of the patient with CKD.
In 2002, the NKF’s Dialysis Outcomes Quality Initiative (K/DOQI) defined the stages of kidney disease based on assessment of GFR and other kidney disease markers.8 GFR is a mathematical calculation for determining kidney function that corrects for loss of kidney function and incorporates the patient’s gender, race, age, and body size—the nonmodifiable risk factors known in 2001. GFR is expressed in mL/min/1.73 m2. Classifying patients with CKD according to the stage of kidney disease by GFR provides a mechanism for studying the efficacy and adverse reactions of medications while correlating them to each stage. This is of particular importance when treating patients with diabetes and kidney disease, as it can help to avoid adverse effects associated with inappropriate renal dosing. Many medications are renally eliminated; as kidney function diminishes, the pharmacokinetics of drugs are altered, potentially causing toxicity and an increased risk for drug interactions. As the GFR falls, even drugs not eliminated by the kidney may have altered pharmacokinetics and pharmacodynamics that may cause renal or systemic toxicity.1
Determining the precise method for renally dosing medication has been one of the most vigorously debated topics in CKD. Many medications were prescribed using the patient’s serum creatinine (SCr) concentration, a marker but not an exact measure of kidney disease.8 The Cockcroft-Gault equation, which provides an estimate of creatinine clearance (eCrCl; an alternate GFR measurement), was primarily used to determine renal drug dosing. 9
In 1999, a multisite study group worked to develop an equation to measure loss of kidney function in the patient with CKD.10,11 The new formula, known as the MDRD (Modification of Diet in Renal Disease) equation, standardized the measurement of loss of kidney function and provided an estimated GFR (eGFR) that was more accurate then eCrCl. At that time, the National Kidney Disease Education Program (NKDEP) decided to use the MDRD equation to assess renal function and use the eCrCl for dosing medications. The latter was chosen because most drugs were analyzed using eCrCl.9 More recently, the NKDEP stated that either the eCrCl or the eGFR is acceptable for renal drug dosing. Hudson and Nyman9 advise that when discrepancies occur between eGFR and eCrCl, clinical judgment should be applied in the elderly and in those with extremes in body weight. In those cases, therapeutic outcomes and risk versus benefit need to be considered when determining renal dosing.
The publication of CKD stages by K/DOQI in 2002 sparked research that has uncovered additional criteria that might be useful in slowing the progression and improving the management of CKD. Thus, in 2013, new guidelines by KDIGO incorporating the latest international findings were published.1 KDIGO expands the definition of CKD to include albuminuria (ALB) as an added risk factor (see Figure) and has modified K/DOQI stages 3 and 5. ALB was included because it has predictive value for progression of CKD to kidney failure. In the new classification, there are three ranges defined for ALB measured in mg/g (see Table 1). The KDIGO guidelines, like K/DOQI, classify kidney disease using a 1-to-5 staging system; however, the stage numbers in the KDIGO classification are prefaced by a G (ie, G1, G2). Also, as mentioned above, stages G3 and G5 in the KDIGO classification have been further categorized.
CKD stage 3 was divided for practical reasons. It was found that the original CKD stage 3 was too large and diverse a classification to use for research studies, tracking of hospitalizations, and precise dosing of medications.12 K/DOQI stage 3 encompassed a GFR from 30 to 59 mL/min/1.73 m2. The KDIGO guideline divides K/DOQI stage 3 into G3a (GFR of 45 to 59 mL/min/1.73 m2) and G3b (GFR of 30 to 44 mL/min/1.73 m2). The staging is then further classified by the presence or absence of ALB. The patient with a GFR of 56 mL/min/1.73 m2 with ALB is in CKD stage 3aA3 (very high and nephrotic ALB) and has a greater risk for disease progression than the patient with a GFR of 56 mL/min/1.73 m2 without ALB. The latter patient is in CKD stage 3aA1(optimal and mildly high ALB). Finally, many medication dosages that were acceptable at a GFR of 56 mL/min/1.73 m2 had to be further adjusted for patients with a GFR of 30 mL/min/1.73 m2 to avoid toxicity. This further justified the KDIGO reclassification of stage 3.
Stage 5 from K/DOQI only reflected a GFR < 15 mL/min/1.73 m2. KDIGO utilizes the same GFR but splits the stage into patients not receiving dialysis and those receiving dialysis, G5 and G5D, respectively. The dosing and timing of medications due to drug dialyzability—including renal-specific medications—may be quite different for patients on dialysis as compared to those with a GFR < 15 mL/min/1.73 m2 not on dialysis, making the distinction beneficial.
On the next page: Diabetes management >>
DIABETES MANAGEMENT
The treatment of patients early in diabetic CKD is often the responsibility of primary care providers, who are faced with the daunting task of addressing the renal dosing of medications. The eGFR or eCrCl needs to be utilized to adjust diabetic medications, largely to avoid hypoglycemia. To alleviate some of the difficulty, the KDIGO CKD stage and the eCrCl and eGFR for diabetic medications are delineated in Table 2.1
Note that the guidelines are not meant to be strictly adhered to but rather are intended as a tool for managing the patient with CKD. Clinically significant patient factors need to be considered to adequately adjust drugs in CKD. Also, as new data become available from postmarketing reports, dosing adjustments, added precautions, or updated risk factors in these fragile patients may need to be incorporated into the guidelines. As new diabetic medications are approved by the FDA, kidney-specific dosing will continue to be a moving target.
While making lifestyle changes is very important early in diabetes, by the time diabetic nephropathy manifests, more aggressive action is warranted.12 A part of the diabetic microvascular triad (along with retinopathy and neuropathy), nephropathy signals existing and irreversible organ damage. Before instituting treatment to delay diabetic nephropathy progression, an A1C goal needs to be established. The long-standing goal has been an A1C < 7%, according to guidelines established by the American Diabetes Association, or 6.5%, as recognized by the American Association of Clinical Endocrinologists.13 The Diabetes Control and Complications Trial (DCCT) demonstrated microvascular benefit of tight glycemic control in type 1 diabetic patients when the A1C was maintained at an average of 7.2% (versus 9.1% in conventional therapy).14 The United Kingdom Prospective Diabetes Study similarly demonstrated microvascular benefit with intensive blood glucose control in type 2 diabetic patients who were maintained at a median A1C of 7% compared to the conventional treatment group maintained at an A1C of 7.9%.15
In January 2013, Perkovic and colleagues, in a post hoc analysis of the ADVANCE Trial, showed that maintaining an A1C between 6.5% and 7% in a patient with diabetes and macroalbuminuria (UACR > 300 μg/mg) slows progression to kidney failure.16 The time needed to treat with intensive glucose control was five years, and the number needed to treat was 41. Thus, for every 41 patients with diabetes, CKD, and ALB whose A1C is below 7%, one will not progress to kidney failure in five years of treatment.
The five-year lead time is troublesome, however. It discourages many patients from taking steps to achieve strict glycemic control; a significant number fail to follow their diabetic diets or take their medications until the damage is done. For the elderly kidney patient with diabetes and multiple other comorbidities, aggressively managing diabetes may result in hypoglycemia.12 On balance, the slight loss of kidney function five years hence is less problematic than the present risk for a fall and resulting hip fracture due to hypoglycemia. For this reason, the KDOQI glycemic guidelines suggest an A1C between 7% to 7.5% as the goal for those with significant comorbidities.12
On the next page: Diabetic medication dosing for CKD >>
DIABETIC MEDICATION DOSING FOR CKD
Glycemic control is important in delaying the progression of kidney failure in the patient with CKD. Hypoglycemic medications by definition may cause low blood glucose levels. This is of particular concern in patients with diminishing renal function, particularly the elderly and CKD patients. Antidiabetes drugs for patients in CKD stages 1 and 2 have few renal precautions, although care must be taken with metformin (see Table 3). Metformin is the drug of choice for the patient newly diagnosed with diabetes. It is inexpensive and effective, causes hypoglycemia only with intensive exercise, is taken orally, and is generally well tolerated. However, the package insert (PI) indicates stopping it in patients whose kidney disease has progressed to a GFR < 60 mL/min/1.73 m2 because it causes an increased risk for lactic acidosis.17,18
Metformin was developed prior to 1998, when the approval of the dosage regimen was tied to SCr levels rather than the presently accepted eGFR.19 As a result, the PI states that metformin should not be used in women with an SCr > 1.4 mg/dL or in men with an SCr > 1.5 mg/dL.17 However, as noted, SCr concentration alone is a very poor indicator of kidney disease (see Table 4).
Currently, the KDIGO guidelines recommend assessing the GFR for metformin dosing. When the GFR is < 60 mL/min/1.73 m2, KDIGO recommends metformin be discontinued in patients with low muscle mass or serious concurrent illness, or those who are concurrently receiving renally eliminated or nephrotoxic drugs (this includes, but is not limited to, renin-angiotensin-aldosterone system [RAAS] inhibitors, NSAIDs, and diuretics).1 In those patients who do not have the exclusions delineated above, KDIGO recommends that metformin may be continued when the GFR is > 45 mL/min/1.73 m2 (stages G1 to G3a), closely monitored and reconsidered when the GFR is 30 to 44 mL/min/1.73 m2 (stage G3b), and discontinued when the GFR is < 30 mL/min/1.73 m2 (stages G4 to G5). The KDIGO differs from other sources that recommend metformin be stopped when the GFR is < 50 to 70 ml/min/1.73 m2.20-22 The metformin PI also recommends stopping metformin the day of or day before and two days after procedures in which radiographic iodinated contrast dye is administered, to avoid acute kidney failure. Finally, it is important to note that any combination medication formulated with metformin should be stopped when the GFR is 50 mL/min/1.73 m2 because lactic acidosis has also been seen in patients taking these medications.21
At stage 3a (GFR, 45 to 60 mL/min/1.73 m2), the sulfonylureas require dosing changes (see Table 2). Glipizide is often the oral medication of choice during CKD, and it may be used in all stages, including dialysis, but does require renal dosing and monitoring.23 Although the insulins are more effective than glipizide in glycemic control, many practitioners prescribe it for patients who will not accept an injectable medication. Glyburide is not used at this stage, as it can lead to complications in patients with a GFR < 60 mL/min/1.73 m2, particularly hypoglycemia.
Like the sulfonylureas, the meglitinides enhance insulin secretion.13,18 Repaglinide and nateglinide are fast-acting and need to be taken with meals. Because it is recommended that the dose of the meglitinides be omitted when a meal is skipped, they are good for patients who eat meals irregularly. The meglitinides require initiation at a low dose in severe renal insufficiency.
The thiazolidinediones (TDZs) are used with caution in patients with CKD, not only because of controversial adverse cardiac effects, but also for peripheral edema that interferes with CKD management.18 The TDZs (rosiglitazone and pioglitazone) require no dose adjustment and appear to have a beneficial effect in obese patients and in patients who experienced weight gain with other hypoglycemic agents.
The newest of the diabetic drugs, canagliflozin, requires a working kidney to be effective.24,25 It is used with precaution when GFR is 45 to 59 mL/min/1.73 m2 at stage G3a and is contraindicated when GFR is < 45 mL/min/1.73 m2 in stage G3b. Canagliflozin's effects are felt in early segment of proximal convoluted tubule where it blocks sodium-glucose co-transporter 2, thereby lowering reabsorption of glucose and increasing excretion of glucose in the urine. In practical terms, it can affect kidney function and, due to the inhibition of the glucose-sodium pathway, induce hyperkalemia.25 Monitoring of both the GFR and potassium is vital when administering this drug. As data from postmarketing reports come in and practitioners gain experience, use of canaglifozin in the patient with CKD will be further delineated.
At a GFR between 30 and 45 mL/min/1.73 m2, CKD stage 3b, alpha-glucosidase inhibitors acarbose and miglitol are contraindicated. The GLP-1 mimetic liraglutide needs to be avoided when the GFR is < 60 mL/min/1.73 m2 (stage G3a). While exenatide requires close monitoring and caution when the GFR is < 50 mL/min/1.73 m2, it needs to be discontinued at a GFR
< 30 mL/min/1.73 m2 (stage 4). DPP-4 inhibitors become problematic at a GFR < 60 mL/min/
1.73 m2 beginning at stage G3a. All except the DPP-4 inhibitor linagliptin require dosing changes as the loss of GFR continues. Exact dosing adjustments for the drug categories are found in Table 2.
In stage 4 CKD (GFR, 15 to 30 mL/min/1.73 m2), more medications are eliminated from consideration. The amylinomimetic pramlintide cannot be used for a patient with a GFR < 15 mL/min/1.73 m2 (see Table 2). Both GLP-1 mimetics, exenatide and liraglutide, must be stopped when the GFR drops to 30 mL/min/1.73 m2. DDP-4 inhibitors need to be dosed according to the GFR but are still acceptable at CKD stage 4. Ideally, patients with diabetes should be referred to nephrology at stage 3, but referral is vital by stage 4.
Although oral medications are not as commonly prescribed as insulins at CKD stages 4 and 5, in stage 5 CKD (GFR, < 15 mL/min/1.73 m2), a limited number of oral diabetic agents are available (see Table 2). Glipizide is inexpensive and generic; therefore, it is the most commonly used oral medication in this stage. A recent study found linagliptin was safe to use throughout all stages of kidney disease without adjustment for GFR.26 While nateglinide is still acceptable in stage 5, it is used less frequently than glipizide.
As the kidney fails, the need for diabetic medications also decreases and dosing needs to take into account any residual renal function. Because glucose is produced in the proximal tubules of the kidney (known as gluconeogenesis), the level of glucose falls as the kidney fails. Excretion of insulin and other diabetes medications decreases, and thus the amount of insulin remaining in circulation is increased. This, in combination with the reduced glucose, increases the patient’s risk for hypoglycemia.13 Although protocols for medication adjustment are tied to GFR levels, close follow-up by the practitioner is required because GFR is not a reliable measure of kidney function when creatinine levels change rapidly.27
On the next page: Insulins >>
INSULINS
Many patients start insulin prior to reaching CKD stage 4 or 5 because it is the best choice for managing diabetes through all stages of CKD.28 Insulin can slow the progression to kidney failure by providing better A1C control, and all patients should be encouraged to start insulin as soon as it is appropriate.29 Many patients are very reluctant to use injectables, but by stage 5 they are willing to use insulin if it will slow progression of disease and help prevent the need for dialysis.2
The long-acting basal insulins (detemir and glargine) or a combination of basal and oral medication can work quite well at stage 5. Many patients will require a low dose of the long-acting basal insulins and short-acting insulin with meals. As the kidney fails, the short-acting doses can often be discontinued.29 A protocol that mixes long- and short-acting insulin with the largest meal is very effective at this stage. The long-acting insulin dose will need to be decreased as the kidney continues to fail. The American College of Physicians protocol for insulin suggests a 25% decrease at stage 4 and a 50% decrease at stage 5. Each patient is different, however, and dosing must be determined individually.23
On the next page: Patient education >>
PATIENT EDUCATION
There is a correlation between patients who participate in kidney disease education programs and a decrease in the progression of kidney disease (see “Case Study: The Recalcitrant Patient With Diabetes"). These classes can be taught by a physician, physician assistant, nurse practitioner, or clinical nurse specialist. To alleviate the burden of end-stage kidney disease in the US, Medicare Part B pays for six outpatient kidney disease patient education classes per lifetime of the beneficiary.30
Since 2010, when kidney disease education programs were rolled out, over 10,000 classes have been taught.31 Many practitioners report that patients are more compliant and understand their disease better when they attend classes.32 Data are being gathered to determine the effectiveness of the classes on patient outcomes.
On the next page: Conclusion >>
CONCLUSION
Knowing how to manage the patient with both diabetes and kidney disease is increasingly vital as this patient population grows. Much of the management of these patients will fall to the primary care practitioner.6 The most effective way to start treatment is to identify which CKD stage the patient fits into.
Once the patient is properly categorized, safe and effective diabetic medications can be selected and dosed according to stage. Although the new classification system may be difficult to incorporate into some electronic medical record systems and practitioner behavior, ultimately, it will allow safer management of the patient with CKD and a better predictive power of outcome.
1. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney International Suppl. 2013;3:1-150.
2. Brazie M. Finding the sweet spot: trouble-shooting diabetic dilemmas. Oral presentation at NKF Spring Clinical Meetings; May 2012, Washington, DC.
3. Estimated cost of diabetes care $245 billion in U.S. in 2012. Renal & Urology News. March 9, 2013. www.renalandurologynews.com/estimated-cost-of-diabetes-245-billion-in-us-in-2012/article/283616/. Accessed January 17, 2013.
4. Grams ME, Chow EK, Segev DL, Coresh J. Lifetime incidence of CKD stages 3-5 in the United States. Am J Kidney Dis. 2013;62(2):245-252.
5. Greenberg A, ed. National Kidney Foundation Primer of Kidney Diseases. 5th ed. Philadelphia, PA: Saunders Elsevier; 2009.
6. Spann SJ, Nutting PA, Galliher JM, et al. Management of type 2 diabetes in the primary care setting: a practice-based research study. Ann Fam Med. 2006;4(1):23-31.
7. Hsu CY, Chertow GM. Chronic renal confusion: insufficiency, failure, dysfunction, or disease. Am J Kidney Dis. 2000;49(3):482-496.
8. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J Kidney Dis. 2002;39(suppl 1):S1-S266.
9. Hudson JQ, Nyman HA. Use of the estimated glomerular filtration rate for drug dosing in the chronic kidney disease patient. Curr Opin Nephrol Hypertens. 2011;20:482-491.
10. Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461-470.
11. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612.
12. NKF-KDOQI clinical practice guideline for diabetes and CKD, guideline 2: management of hyperglycemia and general diabetes care in CKD. Am J Kidney Dis. 2012;60(5):850-886. www.kidney.org/professionals/kdoqi/guideline_diabetes/guide2.htm. Accessed January 2, 2014.
13. Abe M, Okada K, Soma M. Antidiabetic agents in patients with chronic kidney disease and end-stage renal disease on dialysis: metabolism and clinical practice. Curr Drug Metab. 2011;12(1):57-69.
14. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med. 2000;342:381-389.
15. Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405-411.
16. Perkovic V, Heerspink HL, Chalmers J, et al. Intensive glucose control improves kidney outcomes in patients with type 2 diabetes. Kidney Int. 2013;83(3):517-23.
17. Metformin [package insert]. US Department of Health and Human Services, US Food and Drug Administration. www.fda.gov/ohrms/dockets/dailys/02/May02/053102/800471e6.pdf. Accessed January 17, 2013.
18. Triplitt CL, Reasner CA. Chapter 83. Diabetes Mellitus. In: Wells BG, ed. Pharmacotherapy: A Pathophysiologic Approach. 8th ed. New York, NY: McGraw-Hill; 2011.
19. FDA. Guidance for industry: pharmacokinetics in patients with impaired renal function—study design, data analysis, and impact on dosing and labeling. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulato ryInformation/Guidances/ucm072127.pdf. Accessed January 17, 2013.
20. Lacy CF, Armstrong LL, Goldman MP, Lance LL. Drug Information Handbook. 20th ed. Hudson, OH: Lexi-Comp, Inc.; 2011.
21. Rocha A, Almeida M, Santos J, Carvalho A. Metformin in patients with chronic kidney disease: strengths and weaknesses. J Nephrol. 2013; 26(10):55-60.
22. Gilbert SJ, Weiner DE, eds. National Kidney Foundation Primer on Kidney Diseases. 6th ed. Philadelphia, PA: Elsevier Saunders; 2014.
23. Aronoff GR, Bennett WM, Berns JS, et al. Drug Prescribing in Renal Failure: Dosing Guidelines for Adults and Children. 5th ed. Philadelphia, PA: American College of Physicians; 2007.
24. Valentine V. The role of the kidney and the sodium-glucose co-transporter inhibition in diabetes management. Clin Diabetes. 2012;30(4):151-155.
25. Invokana [package insert]. Titusville, NJ: Janssen Pharmaceuticals; 2013.
26. McGill JB, Sloan L, Newman J, et al. Long-term efficacy and safety of linagliptin in patients with type 2 diabetes and severe renal impairment. Diabetes Care. 2013;36(2):237-244.
27. National Kidney Disease and Education Program (NKDEP). Estimated glomerular filtration rate (eGFR) info sheet. NIH publication No.10-6286, March 2010.
28. Thummel K, Shen D, Isoherranen N, et al. Design and optimization of dosage regimens: pharmacokinetic data. In: Hardman J, Limbird L, Goodman G (eds). Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 11th ed. New York, NY: McGraw-Hill; 2006.
29. Snyder RW, Berns JS. Use of insulin and oral hypoglycemic medications in patients with diabetes mellitus and advanced kidney disease. Semin Dial. 2004;17(5):365-70.
30. Centers for Medicare and Medicaid Services. Medicare program: revisions to payment policies under the Physician Fee Schedule, Clinical Laboratory Fee Schedule & other revisions to Part B for CY 2014; Final Rule. Federal Register. 2009;74(226):61738-62188. www.gpo.gov/fdsys/pkg/FR-2009-11-25/html/E9-26502.htm. Accessed January 17, 2013.
31. Zuber K, Davis J. Kidney disease education: a niche for PAs and NPs. JAAPA. 2013;26(7):42-47.
32. Zuber K, Davis J. Stories from the trenches: the first year experience with kidney disease education. Nephrol News Issues. 2012;26(2):20-21.
1. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney International Suppl. 2013;3:1-150.
2. Brazie M. Finding the sweet spot: trouble-shooting diabetic dilemmas. Oral presentation at NKF Spring Clinical Meetings; May 2012, Washington, DC.
3. Estimated cost of diabetes care $245 billion in U.S. in 2012. Renal & Urology News. March 9, 2013. www.renalandurologynews.com/estimated-cost-of-diabetes-245-billion-in-us-in-2012/article/283616/. Accessed January 17, 2013.
4. Grams ME, Chow EK, Segev DL, Coresh J. Lifetime incidence of CKD stages 3-5 in the United States. Am J Kidney Dis. 2013;62(2):245-252.
5. Greenberg A, ed. National Kidney Foundation Primer of Kidney Diseases. 5th ed. Philadelphia, PA: Saunders Elsevier; 2009.
6. Spann SJ, Nutting PA, Galliher JM, et al. Management of type 2 diabetes in the primary care setting: a practice-based research study. Ann Fam Med. 2006;4(1):23-31.
7. Hsu CY, Chertow GM. Chronic renal confusion: insufficiency, failure, dysfunction, or disease. Am J Kidney Dis. 2000;49(3):482-496.
8. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J Kidney Dis. 2002;39(suppl 1):S1-S266.
9. Hudson JQ, Nyman HA. Use of the estimated glomerular filtration rate for drug dosing in the chronic kidney disease patient. Curr Opin Nephrol Hypertens. 2011;20:482-491.
10. Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461-470.
11. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612.
12. NKF-KDOQI clinical practice guideline for diabetes and CKD, guideline 2: management of hyperglycemia and general diabetes care in CKD. Am J Kidney Dis. 2012;60(5):850-886. www.kidney.org/professionals/kdoqi/guideline_diabetes/guide2.htm. Accessed January 2, 2014.
13. Abe M, Okada K, Soma M. Antidiabetic agents in patients with chronic kidney disease and end-stage renal disease on dialysis: metabolism and clinical practice. Curr Drug Metab. 2011;12(1):57-69.
14. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med. 2000;342:381-389.
15. Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405-411.
16. Perkovic V, Heerspink HL, Chalmers J, et al. Intensive glucose control improves kidney outcomes in patients with type 2 diabetes. Kidney Int. 2013;83(3):517-23.
17. Metformin [package insert]. US Department of Health and Human Services, US Food and Drug Administration. www.fda.gov/ohrms/dockets/dailys/02/May02/053102/800471e6.pdf. Accessed January 17, 2013.
18. Triplitt CL, Reasner CA. Chapter 83. Diabetes Mellitus. In: Wells BG, ed. Pharmacotherapy: A Pathophysiologic Approach. 8th ed. New York, NY: McGraw-Hill; 2011.
19. FDA. Guidance for industry: pharmacokinetics in patients with impaired renal function—study design, data analysis, and impact on dosing and labeling. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulato ryInformation/Guidances/ucm072127.pdf. Accessed January 17, 2013.
20. Lacy CF, Armstrong LL, Goldman MP, Lance LL. Drug Information Handbook. 20th ed. Hudson, OH: Lexi-Comp, Inc.; 2011.
21. Rocha A, Almeida M, Santos J, Carvalho A. Metformin in patients with chronic kidney disease: strengths and weaknesses. J Nephrol. 2013; 26(10):55-60.
22. Gilbert SJ, Weiner DE, eds. National Kidney Foundation Primer on Kidney Diseases. 6th ed. Philadelphia, PA: Elsevier Saunders; 2014.
23. Aronoff GR, Bennett WM, Berns JS, et al. Drug Prescribing in Renal Failure: Dosing Guidelines for Adults and Children. 5th ed. Philadelphia, PA: American College of Physicians; 2007.
24. Valentine V. The role of the kidney and the sodium-glucose co-transporter inhibition in diabetes management. Clin Diabetes. 2012;30(4):151-155.
25. Invokana [package insert]. Titusville, NJ: Janssen Pharmaceuticals; 2013.
26. McGill JB, Sloan L, Newman J, et al. Long-term efficacy and safety of linagliptin in patients with type 2 diabetes and severe renal impairment. Diabetes Care. 2013;36(2):237-244.
27. National Kidney Disease and Education Program (NKDEP). Estimated glomerular filtration rate (eGFR) info sheet. NIH publication No.10-6286, March 2010.
28. Thummel K, Shen D, Isoherranen N, et al. Design and optimization of dosage regimens: pharmacokinetic data. In: Hardman J, Limbird L, Goodman G (eds). Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 11th ed. New York, NY: McGraw-Hill; 2006.
29. Snyder RW, Berns JS. Use of insulin and oral hypoglycemic medications in patients with diabetes mellitus and advanced kidney disease. Semin Dial. 2004;17(5):365-70.
30. Centers for Medicare and Medicaid Services. Medicare program: revisions to payment policies under the Physician Fee Schedule, Clinical Laboratory Fee Schedule & other revisions to Part B for CY 2014; Final Rule. Federal Register. 2009;74(226):61738-62188. www.gpo.gov/fdsys/pkg/FR-2009-11-25/html/E9-26502.htm. Accessed January 17, 2013.
31. Zuber K, Davis J. Kidney disease education: a niche for PAs and NPs. JAAPA. 2013;26(7):42-47.
32. Zuber K, Davis J. Stories from the trenches: the first year experience with kidney disease education. Nephrol News Issues. 2012;26(2):20-21.