User login
Impact of the MTHFR C677T genetic variant on depression
Ms. T, age 55, presents to her psychiatrist’s clinic with a chief complaint of ongoing symptoms of anhedonia and lethargy related to her diagnosis of major depressive disorder (MDD). She also has a history of peripheral arterial disease, hypothyroidism, and generalized anxiety disorder. Her current antidepressant regimen is duloxetine, 60 mg/d, and mirtazapine, 15 mg at night. She recently elected to undergo pharmacogenetic testing, which showed that she is heterozygous for the methylenetetrahydrofolate reductase (MTHFR) C677T mutation (MTHFR C677T CT carrier). Her test report states that she may have impaired folate metabolism. Her psychiatrist adds L-methylfolate, 15 mg/d, to her current antidepressant regimen.
What is the relationship between folic acid and MTHFR?
Methylenetetrahydrofolate reductase is an intracellular enzyme responsible for one of several steps involved in converting dietary folic acid to its physiologically active form, L-methylfolate.1 Once active, L-methylfolate can be transported into the CNS, where it participates in one-carbon transfer reactions.2,3 Mutations in the MTHFR gene have been associated with decreased activity of the enzyme, which has been shown to result in accumulation of homocysteine and may lead to decreased synthesis of neurotransmitters.2,4Commercial pharmacogenetic testing panels may offer MTHFR genetic testing to assist with prescribing decisions for patients with mental illness. The most well-characterized mutation currently is C677T (rsID1801133), which is a single amino acid base pair change (cytosine [C] to thymine [T]) that leads to increased thermolability and instability of the enzyme.5 Carrying 1 or 2 T alleles can lead to a 35% or 70% reduction in enzyme activity, respectively. The T variant allele is most frequent in Hispanics (20% to 25%), Asians (up to 63%), and Caucasians (8% to 20%); however, it is relatively uncommon in African Americans (<2%).5,6 Another variant, A1289C (rs1801131), has also been associated with decreased enzyme function, particularly when analyzed in combination with C677T. However, carrying the 1289C variant allele does not appear to result in as large of a reduction of enzyme function as the 677T variant.7
What is the relationship between MTHFR C677T and depression?
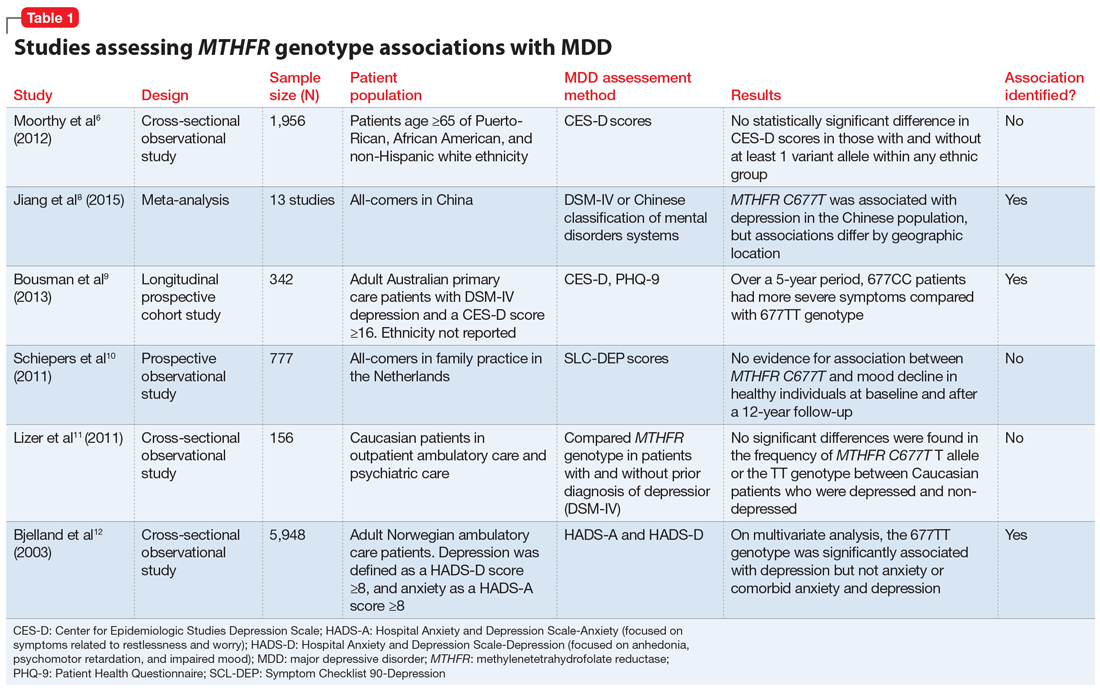
Some researchers have proposed that the C677T mutation in MTHFR may be associated with depression as a result of decreased neurotransmitter synthesis, but studies have not consistently supported this hypothesis. Several studies suggest an association between MTHFR mutations and MDD8-10:
Jiang et al8 performed a meta-analysis of 13 studies including 1,295 Chinese patients and found that having at least 1 C677T variant allele was significantly associated with an increased risk of depression (for T vs C odds ratio 1.52, 95% confidence interval 1.24 to 1.85). The authors noted a stronger association identified in the Northern Chinese population compared with the Southern Chinese population.8
Bousman et al9 found that American patients with MDD and the 677CC genotype had greater Patient Health Questionnaire-9 (PHQ-9) scores at assessments at 24, 36, and 48 months post-baseline compared with those with the 677TT genotype (P = .024), which was unexpected based on previously reported associations.9
Schiepers et al10 also assessed the association between the MTHFR genotype in a Dutch ambulatory care population over 12 years. There was no association identified between scores on the depression subscale of the Symptom Checklist 90 and C677T diplotype.10
Table 16,8-12 provides summaries of these and other selected studies on MTHFR and MDD. Overall, although a pathophysiological basis for depression and decreased MTHFR function has been proposed, the current body of literature does not indicate a consistent link between MTHFR C677T genetic variants alone and depression.
Continue to: Medication changes based on MTHFR: What is the evidence?
Medication changes based on MTHFR: What is the evidence?
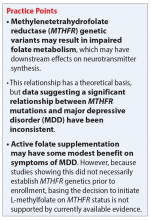
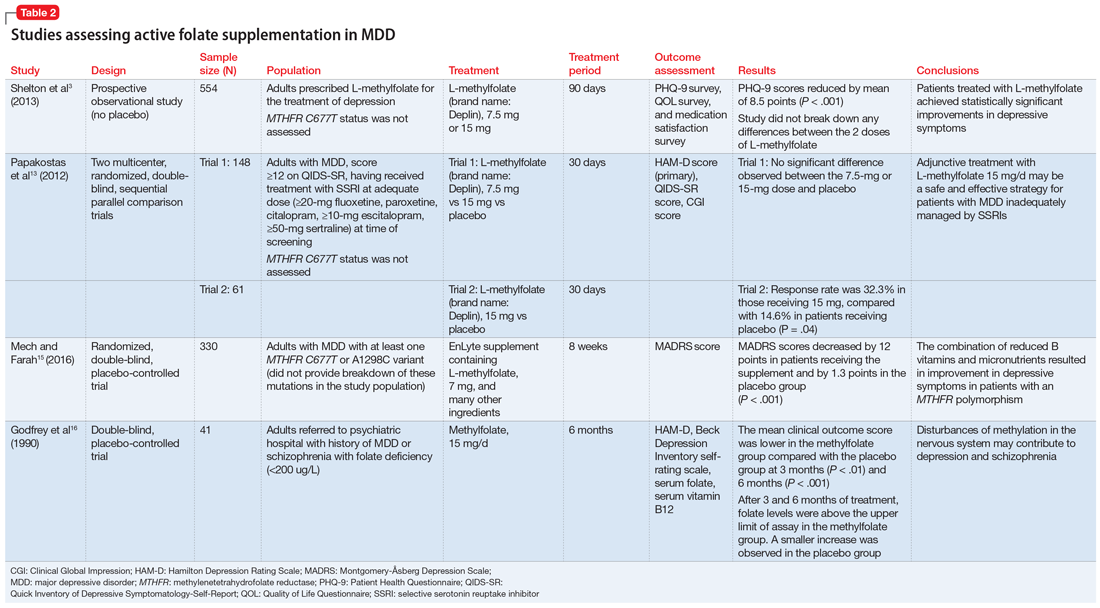
Some evidence supports the use of active folate supplementation to improve symptoms of MDD.
Shelton et al3 conducted an observational study that assessed the effects of adding L-methylfolate (brand name: Deplin), 7.5 or 15 mg, to existing antidepressant therapy in 502 patients with MDD who had baseline PHQ-9 scores of at least 5. After an average 95 days of therapy, PHQ-9 scores were reduced by a mean of 8.5 points, with 67.9% of patients achieving at least a 50% reduction in PHQ-9 scores. The study did not take into account patients’ MTHFR genotype or differentiate results between the 2 doses of L-methylfolate.3
Papakostas et al13 performed 2 randomized, double-blind, parallel-sequential, placebo-controlled trials of L-methylfolate for patients with MDD. The first compared L-methylfolate, 7.5 and 15 mg, to placebo, without regard to MTHFR genotype.13 There was no significant difference between the 7.5-mg dose and placebo, or the 15-mg dose and placebo. However, among the group receiving the 15-mg dose, the response rate was 24%, vs 9% in the placebo group, which approached significance (P = .1). Papakostas et al13 followed up with a smaller trial comparing the 15-mg dose alone to placebo, and found the response rate was 32.3% in patients treated with L-methylfolate compared with 14.6% in the placebo group (P = .04).13
Although the Shelton et al3 and Papakostas et al13 studies showed some improvement in depressive symptom scores among patients who received L-methylfolate supplementation, an important consideration is if MTHFR genotype may predict patient response to this therapy.
Papakostas et al14 performed a post hoc analysis of their earlier study to assess potential associations amongst multiple other biomarkers of inflammation and metabolic disturbances hypothesized by the authors to be associated with MDD, as well as body mass index (BMI), with treatment outcome.14 When change in the Hamilton Depression Rating Scale-28 (HDRS-28) was analyzed by C677T and A1298C variant groups (677 CT vs TT and 1298 AC vs CC), no statistically significant improvements were identified (C677T mean change from baseline −3.8 points, P = .087; A1298C mean change from baseline −0.5 points, P = .807).14 However, statistically significant improvements in HDRS-28 scores were observed compared with baseline when the C677T genotype was pooled with other biomarkers, including methionine synthase (MTR 2756 AG/GG, −23.3 points vs baseline, P < .001) and a voltage-dependent calcium channel (CACNAIC AG/AA, −9 points vs baseline, P < .001), as well as with BMI ≥ 30 kg/m2 (−9.9 points vs baseline, P = .001).14
Continue to: Mech and Farah...
Mech and Farah15 performed a randomized, double-blind, placebo-controlled study of the use of EnLyte, a supplement containing 7-mg L-methylfolate, in patients with at least 1 variant of MTHFR (either C677T or A1298C) over an 8-week period. In addition to L-methylfolate, this supplement contains other active ingredients, including leucovorin (or folinic acid), magnesium ascorbate, and ferrous glycine cysteinate. Montgomery-Åsberg Depression Scale (MADRS) scores improved by 12 points in patients who received the supplement and by 1.3 points in patients who received placebo. However, because the supplement contained many ingredients, the response observed in this study cannot be attributed to L-methylfolate alone.15
Table 23,13,15,16 contains summaries of these and other selected studies assessing active folate supplementation in MDD.
CASE CONTINUED
Over the next several weeks, Ms. T experiences some modest improvement in mood while taking L-methylfolate and her antidepressant regimen, and she experiences no notable adverse effects. Unfortunately, after 3 months, Ms. T discontinues the supplement due to the cost.
The value of MTHFR testing
Ms. T’s case is an example of how clinicians may respond to MTHFR pharmacogenetic testing. Although L-methylfolate has shown some benefit in several randomized clinical trials, available data do not confirm the relevance of MTHFR functional status to symptom response. Additionally, there is likely interplay among multiple factors affecting patients’ response to L-methylfolate. Larger randomized trials prospectively assessing other pharmacogenetic and lifestyle factors may shed more light on which patients would benefit.
Based on available data, the decision to prescribe L-methylfolate should not necessarily hinge on MTHFR genetics alone. Both patients and clinicians must be aware of the potentially prohibitive cost if L-methylfolate is recommended, as prescription insurance may not provide coverage (eg, a recent search on GoodRx.com showed that generic L-methylfolate was approximately $40 for 30 tablets; prices may vary). Additionally, clinicians should be aware that L-methylfolate is regulated as a medical food product and is not subject to strict quality standards required for prescription medications. Future prospective studies assessing the use of L-methylfolate specifically in patients with a MTHFR variants while investigating other relevant covariates may help identify which specific patient populations would benefit from supplementation.
Continue to: Related Resources
Related Resources
- Gilbody S, Lewis S, Lightfoot T. Methylenetetrahydrofolate reductase (MTHFR) genetic polymorphisms and psychiatric disorders: a HuGE review. Am J Epidemiol. 2007;165(1):1-13.
- Trimmer E. Methylenetetrahydrofolate reductase: biochemical characterization and medical significance. Current Pharmaceutical Design. 2013;19(4):2574-3595.
Drug Brand Names
Citalopram • Celexa
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
L-methylfolate • Deplin
Mirtazapine • Remeron
Paroxetine • Paxil
Sertraline • Zoloft
1. Scaglione F, Panzavolta G. Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica. 2014;44(5):480-488.
2. Jadavji N, Wieske F, Dirnagl U, et al. Methylenetetrahydrofolate reductase deficiency alters levels of glutamate and gamma-aminobutyric acid in brain tissue. Molecular Genetics and Metabolism Reports. 2015;3(Issue C):1-4.
3. Shelton R, Manning J, Barrentine L, et al. Assessing effects of L-methylfolate in depression management: results of a real-world patient experience trial. Prim Care Companion CNS Disord. 2013;15(4):pii:PCC.13m01520. doi: 10.4088/PCC.13m01520.
4. Brustolin S, Giugliani R, Felix T. Genetics of homocysteine metabolism and associated disorders. Braz J Med Biol Res. 2010;43(1):1-7.
5. Blom H, Smulders Y. Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects. J Inherit Metab Dis. 2011;34:75-81.
6. Moorthy D, Peter I, Scott T, et al. Status of vitamins B-12 and B-6 but not of folate, homocysteine, and the methylenetetrahydrofolate reductase C677T polymorphism are associated with impaired cognition and depression in adults. J Nutr. 2012;142:1554-1560.
7. Lievers K, Boers G, Verhoef P, et al. A second common variant in the methylenetetrahydrofolate reductase (MTHFR) gene and its relationship to MTHFR enzyme activity, homocysteine, and cardiovascular disease risk. J Mol Med (Berl). 2001;79(9):522-528.
8. Jiang W, Xu J, Lu X, et al. Association between MTHFR C677T polymorphism and depression: a meta-analysis in the Chinese population. Psychol Health Med. 2015;21(6):675-685.
9. Bousman C, Potiriadis M, Everall I, et al. Methylenetetrahydrofolate reductase (MTHFR) genetic variation and major depressive disorder prognosis: a five-year prospective cohort study of primary care attendees. Am J Med Genet B Neuropsychiatr Genet. 2014;165B(1):68-76.
10. Schiepers O, Van Boxtel M, de Groot R, et al. Genetic variation in folate metabolism is not associated with cognitive functioning or mood in healthy adults. Prog Neuro-Psychopharmacol Biol Psychiatry. 2011;35(7):1682-1688.
11. Lizer M, Bogdan R, Kidd R. Comparison of the frequency of the methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism in depressed versus nondepressed patients. J Psychiatr Pract. 2011;17(6):404-409.
12. Bjelland I, Tell G, Vollset S, et al. Folate, vitamin B12, homocysteine, and the MTHFR 677C->T polymorphism in anxiety and depression: the Hordaland Homocysteine Study. Arch Gen Psychiatry. 2003;60(6):618-626.
13. Papakostas G, Shelton R, Zajecka J, et al. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel sequential trials. Am J Psychiatry. 2012;169(12):1267-1274.
14. Papakostas G, Shelton R, Zajecka J, et al. Effect of adjunctive L-methylfolate 15 mg among inadequate responders to SSRIs in depressed patients who were stratified by biomarker levels and genotype: results from a randomized clinical trial. J Clin Psychiatry. 2014;75(8):855-863.
15. Mech A, Farah A. Correlation of clinical response with homocysteine reduction during therapy with reduced B vitamins in patients with MDD who are positive for MTHFR C677T or A1298C polymorphism: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2016;77(5):668-671.
16. Godfrey P, Toone B, Carney M, et al. Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 1990;336(8712):392-395.
Ms. T, age 55, presents to her psychiatrist’s clinic with a chief complaint of ongoing symptoms of anhedonia and lethargy related to her diagnosis of major depressive disorder (MDD). She also has a history of peripheral arterial disease, hypothyroidism, and generalized anxiety disorder. Her current antidepressant regimen is duloxetine, 60 mg/d, and mirtazapine, 15 mg at night. She recently elected to undergo pharmacogenetic testing, which showed that she is heterozygous for the methylenetetrahydrofolate reductase (MTHFR) C677T mutation (MTHFR C677T CT carrier). Her test report states that she may have impaired folate metabolism. Her psychiatrist adds L-methylfolate, 15 mg/d, to her current antidepressant regimen.
What is the relationship between folic acid and MTHFR?
Methylenetetrahydrofolate reductase is an intracellular enzyme responsible for one of several steps involved in converting dietary folic acid to its physiologically active form, L-methylfolate.1 Once active, L-methylfolate can be transported into the CNS, where it participates in one-carbon transfer reactions.2,3 Mutations in the MTHFR gene have been associated with decreased activity of the enzyme, which has been shown to result in accumulation of homocysteine and may lead to decreased synthesis of neurotransmitters.2,4Commercial pharmacogenetic testing panels may offer MTHFR genetic testing to assist with prescribing decisions for patients with mental illness. The most well-characterized mutation currently is C677T (rsID1801133), which is a single amino acid base pair change (cytosine [C] to thymine [T]) that leads to increased thermolability and instability of the enzyme.5 Carrying 1 or 2 T alleles can lead to a 35% or 70% reduction in enzyme activity, respectively. The T variant allele is most frequent in Hispanics (20% to 25%), Asians (up to 63%), and Caucasians (8% to 20%); however, it is relatively uncommon in African Americans (<2%).5,6 Another variant, A1289C (rs1801131), has also been associated with decreased enzyme function, particularly when analyzed in combination with C677T. However, carrying the 1289C variant allele does not appear to result in as large of a reduction of enzyme function as the 677T variant.7
What is the relationship between MTHFR C677T and depression?
Some researchers have proposed that the C677T mutation in MTHFR may be associated with depression as a result of decreased neurotransmitter synthesis, but studies have not consistently supported this hypothesis. Several studies suggest an association between MTHFR mutations and MDD8-10:
Jiang et al8 performed a meta-analysis of 13 studies including 1,295 Chinese patients and found that having at least 1 C677T variant allele was significantly associated with an increased risk of depression (for T vs C odds ratio 1.52, 95% confidence interval 1.24 to 1.85). The authors noted a stronger association identified in the Northern Chinese population compared with the Southern Chinese population.8
Bousman et al9 found that American patients with MDD and the 677CC genotype had greater Patient Health Questionnaire-9 (PHQ-9) scores at assessments at 24, 36, and 48 months post-baseline compared with those with the 677TT genotype (P = .024), which was unexpected based on previously reported associations.9
Schiepers et al10 also assessed the association between the MTHFR genotype in a Dutch ambulatory care population over 12 years. There was no association identified between scores on the depression subscale of the Symptom Checklist 90 and C677T diplotype.10
Table 16,8-12 provides summaries of these and other selected studies on MTHFR and MDD. Overall, although a pathophysiological basis for depression and decreased MTHFR function has been proposed, the current body of literature does not indicate a consistent link between MTHFR C677T genetic variants alone and depression.

Continue to: Medication changes based on MTHFR: What is the evidence?
Medication changes based on MTHFR: What is the evidence?
Some evidence supports the use of active folate supplementation to improve symptoms of MDD.
Shelton et al3 conducted an observational study that assessed the effects of adding L-methylfolate (brand name: Deplin), 7.5 or 15 mg, to existing antidepressant therapy in 502 patients with MDD who had baseline PHQ-9 scores of at least 5. After an average 95 days of therapy, PHQ-9 scores were reduced by a mean of 8.5 points, with 67.9% of patients achieving at least a 50% reduction in PHQ-9 scores. The study did not take into account patients’ MTHFR genotype or differentiate results between the 2 doses of L-methylfolate.3
Papakostas et al13 performed 2 randomized, double-blind, parallel-sequential, placebo-controlled trials of L-methylfolate for patients with MDD. The first compared L-methylfolate, 7.5 and 15 mg, to placebo, without regard to MTHFR genotype.13 There was no significant difference between the 7.5-mg dose and placebo, or the 15-mg dose and placebo. However, among the group receiving the 15-mg dose, the response rate was 24%, vs 9% in the placebo group, which approached significance (P = .1). Papakostas et al13 followed up with a smaller trial comparing the 15-mg dose alone to placebo, and found the response rate was 32.3% in patients treated with L-methylfolate compared with 14.6% in the placebo group (P = .04).13
Although the Shelton et al3 and Papakostas et al13 studies showed some improvement in depressive symptom scores among patients who received L-methylfolate supplementation, an important consideration is if MTHFR genotype may predict patient response to this therapy.
Papakostas et al14 performed a post hoc analysis of their earlier study to assess potential associations amongst multiple other biomarkers of inflammation and metabolic disturbances hypothesized by the authors to be associated with MDD, as well as body mass index (BMI), with treatment outcome.14 When change in the Hamilton Depression Rating Scale-28 (HDRS-28) was analyzed by C677T and A1298C variant groups (677 CT vs TT and 1298 AC vs CC), no statistically significant improvements were identified (C677T mean change from baseline −3.8 points, P = .087; A1298C mean change from baseline −0.5 points, P = .807).14 However, statistically significant improvements in HDRS-28 scores were observed compared with baseline when the C677T genotype was pooled with other biomarkers, including methionine synthase (MTR 2756 AG/GG, −23.3 points vs baseline, P < .001) and a voltage-dependent calcium channel (CACNAIC AG/AA, −9 points vs baseline, P < .001), as well as with BMI ≥ 30 kg/m2 (−9.9 points vs baseline, P = .001).14
Continue to: Mech and Farah...
Mech and Farah15 performed a randomized, double-blind, placebo-controlled study of the use of EnLyte, a supplement containing 7-mg L-methylfolate, in patients with at least 1 variant of MTHFR (either C677T or A1298C) over an 8-week period. In addition to L-methylfolate, this supplement contains other active ingredients, including leucovorin (or folinic acid), magnesium ascorbate, and ferrous glycine cysteinate. Montgomery-Åsberg Depression Scale (MADRS) scores improved by 12 points in patients who received the supplement and by 1.3 points in patients who received placebo. However, because the supplement contained many ingredients, the response observed in this study cannot be attributed to L-methylfolate alone.15
Table 23,13,15,16 contains summaries of these and other selected studies assessing active folate supplementation in MDD.

CASE CONTINUED
Over the next several weeks, Ms. T experiences some modest improvement in mood while taking L-methylfolate and her antidepressant regimen, and she experiences no notable adverse effects. Unfortunately, after 3 months, Ms. T discontinues the supplement due to the cost.
The value of MTHFR testing
Ms. T’s case is an example of how clinicians may respond to MTHFR pharmacogenetic testing. Although L-methylfolate has shown some benefit in several randomized clinical trials, available data do not confirm the relevance of MTHFR functional status to symptom response. Additionally, there is likely interplay among multiple factors affecting patients’ response to L-methylfolate. Larger randomized trials prospectively assessing other pharmacogenetic and lifestyle factors may shed more light on which patients would benefit.
Based on available data, the decision to prescribe L-methylfolate should not necessarily hinge on MTHFR genetics alone. Both patients and clinicians must be aware of the potentially prohibitive cost if L-methylfolate is recommended, as prescription insurance may not provide coverage (eg, a recent search on GoodRx.com showed that generic L-methylfolate was approximately $40 for 30 tablets; prices may vary). Additionally, clinicians should be aware that L-methylfolate is regulated as a medical food product and is not subject to strict quality standards required for prescription medications. Future prospective studies assessing the use of L-methylfolate specifically in patients with a MTHFR variants while investigating other relevant covariates may help identify which specific patient populations would benefit from supplementation.
Continue to: Related Resources
Related Resources
- Gilbody S, Lewis S, Lightfoot T. Methylenetetrahydrofolate reductase (MTHFR) genetic polymorphisms and psychiatric disorders: a HuGE review. Am J Epidemiol. 2007;165(1):1-13.
- Trimmer E. Methylenetetrahydrofolate reductase: biochemical characterization and medical significance. Current Pharmaceutical Design. 2013;19(4):2574-3595.
Drug Brand Names
Citalopram • Celexa
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
L-methylfolate • Deplin
Mirtazapine • Remeron
Paroxetine • Paxil
Sertraline • Zoloft
Ms. T, age 55, presents to her psychiatrist’s clinic with a chief complaint of ongoing symptoms of anhedonia and lethargy related to her diagnosis of major depressive disorder (MDD). She also has a history of peripheral arterial disease, hypothyroidism, and generalized anxiety disorder. Her current antidepressant regimen is duloxetine, 60 mg/d, and mirtazapine, 15 mg at night. She recently elected to undergo pharmacogenetic testing, which showed that she is heterozygous for the methylenetetrahydrofolate reductase (MTHFR) C677T mutation (MTHFR C677T CT carrier). Her test report states that she may have impaired folate metabolism. Her psychiatrist adds L-methylfolate, 15 mg/d, to her current antidepressant regimen.
What is the relationship between folic acid and MTHFR?
Methylenetetrahydrofolate reductase is an intracellular enzyme responsible for one of several steps involved in converting dietary folic acid to its physiologically active form, L-methylfolate.1 Once active, L-methylfolate can be transported into the CNS, where it participates in one-carbon transfer reactions.2,3 Mutations in the MTHFR gene have been associated with decreased activity of the enzyme, which has been shown to result in accumulation of homocysteine and may lead to decreased synthesis of neurotransmitters.2,4Commercial pharmacogenetic testing panels may offer MTHFR genetic testing to assist with prescribing decisions for patients with mental illness. The most well-characterized mutation currently is C677T (rsID1801133), which is a single amino acid base pair change (cytosine [C] to thymine [T]) that leads to increased thermolability and instability of the enzyme.5 Carrying 1 or 2 T alleles can lead to a 35% or 70% reduction in enzyme activity, respectively. The T variant allele is most frequent in Hispanics (20% to 25%), Asians (up to 63%), and Caucasians (8% to 20%); however, it is relatively uncommon in African Americans (<2%).5,6 Another variant, A1289C (rs1801131), has also been associated with decreased enzyme function, particularly when analyzed in combination with C677T. However, carrying the 1289C variant allele does not appear to result in as large of a reduction of enzyme function as the 677T variant.7
What is the relationship between MTHFR C677T and depression?
Some researchers have proposed that the C677T mutation in MTHFR may be associated with depression as a result of decreased neurotransmitter synthesis, but studies have not consistently supported this hypothesis. Several studies suggest an association between MTHFR mutations and MDD8-10:
Jiang et al8 performed a meta-analysis of 13 studies including 1,295 Chinese patients and found that having at least 1 C677T variant allele was significantly associated with an increased risk of depression (for T vs C odds ratio 1.52, 95% confidence interval 1.24 to 1.85). The authors noted a stronger association identified in the Northern Chinese population compared with the Southern Chinese population.8
Bousman et al9 found that American patients with MDD and the 677CC genotype had greater Patient Health Questionnaire-9 (PHQ-9) scores at assessments at 24, 36, and 48 months post-baseline compared with those with the 677TT genotype (P = .024), which was unexpected based on previously reported associations.9
Schiepers et al10 also assessed the association between the MTHFR genotype in a Dutch ambulatory care population over 12 years. There was no association identified between scores on the depression subscale of the Symptom Checklist 90 and C677T diplotype.10
Table 16,8-12 provides summaries of these and other selected studies on MTHFR and MDD. Overall, although a pathophysiological basis for depression and decreased MTHFR function has been proposed, the current body of literature does not indicate a consistent link between MTHFR C677T genetic variants alone and depression.

Continue to: Medication changes based on MTHFR: What is the evidence?
Medication changes based on MTHFR: What is the evidence?
Some evidence supports the use of active folate supplementation to improve symptoms of MDD.
Shelton et al3 conducted an observational study that assessed the effects of adding L-methylfolate (brand name: Deplin), 7.5 or 15 mg, to existing antidepressant therapy in 502 patients with MDD who had baseline PHQ-9 scores of at least 5. After an average 95 days of therapy, PHQ-9 scores were reduced by a mean of 8.5 points, with 67.9% of patients achieving at least a 50% reduction in PHQ-9 scores. The study did not take into account patients’ MTHFR genotype or differentiate results between the 2 doses of L-methylfolate.3
Papakostas et al13 performed 2 randomized, double-blind, parallel-sequential, placebo-controlled trials of L-methylfolate for patients with MDD. The first compared L-methylfolate, 7.5 and 15 mg, to placebo, without regard to MTHFR genotype.13 There was no significant difference between the 7.5-mg dose and placebo, or the 15-mg dose and placebo. However, among the group receiving the 15-mg dose, the response rate was 24%, vs 9% in the placebo group, which approached significance (P = .1). Papakostas et al13 followed up with a smaller trial comparing the 15-mg dose alone to placebo, and found the response rate was 32.3% in patients treated with L-methylfolate compared with 14.6% in the placebo group (P = .04).13
Although the Shelton et al3 and Papakostas et al13 studies showed some improvement in depressive symptom scores among patients who received L-methylfolate supplementation, an important consideration is if MTHFR genotype may predict patient response to this therapy.
Papakostas et al14 performed a post hoc analysis of their earlier study to assess potential associations amongst multiple other biomarkers of inflammation and metabolic disturbances hypothesized by the authors to be associated with MDD, as well as body mass index (BMI), with treatment outcome.14 When change in the Hamilton Depression Rating Scale-28 (HDRS-28) was analyzed by C677T and A1298C variant groups (677 CT vs TT and 1298 AC vs CC), no statistically significant improvements were identified (C677T mean change from baseline −3.8 points, P = .087; A1298C mean change from baseline −0.5 points, P = .807).14 However, statistically significant improvements in HDRS-28 scores were observed compared with baseline when the C677T genotype was pooled with other biomarkers, including methionine synthase (MTR 2756 AG/GG, −23.3 points vs baseline, P < .001) and a voltage-dependent calcium channel (CACNAIC AG/AA, −9 points vs baseline, P < .001), as well as with BMI ≥ 30 kg/m2 (−9.9 points vs baseline, P = .001).14
Continue to: Mech and Farah...
Mech and Farah15 performed a randomized, double-blind, placebo-controlled study of the use of EnLyte, a supplement containing 7-mg L-methylfolate, in patients with at least 1 variant of MTHFR (either C677T or A1298C) over an 8-week period. In addition to L-methylfolate, this supplement contains other active ingredients, including leucovorin (or folinic acid), magnesium ascorbate, and ferrous glycine cysteinate. Montgomery-Åsberg Depression Scale (MADRS) scores improved by 12 points in patients who received the supplement and by 1.3 points in patients who received placebo. However, because the supplement contained many ingredients, the response observed in this study cannot be attributed to L-methylfolate alone.15
Table 23,13,15,16 contains summaries of these and other selected studies assessing active folate supplementation in MDD.

CASE CONTINUED
Over the next several weeks, Ms. T experiences some modest improvement in mood while taking L-methylfolate and her antidepressant regimen, and she experiences no notable adverse effects. Unfortunately, after 3 months, Ms. T discontinues the supplement due to the cost.
The value of MTHFR testing
Ms. T’s case is an example of how clinicians may respond to MTHFR pharmacogenetic testing. Although L-methylfolate has shown some benefit in several randomized clinical trials, available data do not confirm the relevance of MTHFR functional status to symptom response. Additionally, there is likely interplay among multiple factors affecting patients’ response to L-methylfolate. Larger randomized trials prospectively assessing other pharmacogenetic and lifestyle factors may shed more light on which patients would benefit.
Based on available data, the decision to prescribe L-methylfolate should not necessarily hinge on MTHFR genetics alone. Both patients and clinicians must be aware of the potentially prohibitive cost if L-methylfolate is recommended, as prescription insurance may not provide coverage (eg, a recent search on GoodRx.com showed that generic L-methylfolate was approximately $40 for 30 tablets; prices may vary). Additionally, clinicians should be aware that L-methylfolate is regulated as a medical food product and is not subject to strict quality standards required for prescription medications. Future prospective studies assessing the use of L-methylfolate specifically in patients with a MTHFR variants while investigating other relevant covariates may help identify which specific patient populations would benefit from supplementation.
Continue to: Related Resources
Related Resources
- Gilbody S, Lewis S, Lightfoot T. Methylenetetrahydrofolate reductase (MTHFR) genetic polymorphisms and psychiatric disorders: a HuGE review. Am J Epidemiol. 2007;165(1):1-13.
- Trimmer E. Methylenetetrahydrofolate reductase: biochemical characterization and medical significance. Current Pharmaceutical Design. 2013;19(4):2574-3595.
Drug Brand Names
Citalopram • Celexa
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
L-methylfolate • Deplin
Mirtazapine • Remeron
Paroxetine • Paxil
Sertraline • Zoloft
1. Scaglione F, Panzavolta G. Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica. 2014;44(5):480-488.
2. Jadavji N, Wieske F, Dirnagl U, et al. Methylenetetrahydrofolate reductase deficiency alters levels of glutamate and gamma-aminobutyric acid in brain tissue. Molecular Genetics and Metabolism Reports. 2015;3(Issue C):1-4.
3. Shelton R, Manning J, Barrentine L, et al. Assessing effects of L-methylfolate in depression management: results of a real-world patient experience trial. Prim Care Companion CNS Disord. 2013;15(4):pii:PCC.13m01520. doi: 10.4088/PCC.13m01520.
4. Brustolin S, Giugliani R, Felix T. Genetics of homocysteine metabolism and associated disorders. Braz J Med Biol Res. 2010;43(1):1-7.
5. Blom H, Smulders Y. Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects. J Inherit Metab Dis. 2011;34:75-81.
6. Moorthy D, Peter I, Scott T, et al. Status of vitamins B-12 and B-6 but not of folate, homocysteine, and the methylenetetrahydrofolate reductase C677T polymorphism are associated with impaired cognition and depression in adults. J Nutr. 2012;142:1554-1560.
7. Lievers K, Boers G, Verhoef P, et al. A second common variant in the methylenetetrahydrofolate reductase (MTHFR) gene and its relationship to MTHFR enzyme activity, homocysteine, and cardiovascular disease risk. J Mol Med (Berl). 2001;79(9):522-528.
8. Jiang W, Xu J, Lu X, et al. Association between MTHFR C677T polymorphism and depression: a meta-analysis in the Chinese population. Psychol Health Med. 2015;21(6):675-685.
9. Bousman C, Potiriadis M, Everall I, et al. Methylenetetrahydrofolate reductase (MTHFR) genetic variation and major depressive disorder prognosis: a five-year prospective cohort study of primary care attendees. Am J Med Genet B Neuropsychiatr Genet. 2014;165B(1):68-76.
10. Schiepers O, Van Boxtel M, de Groot R, et al. Genetic variation in folate metabolism is not associated with cognitive functioning or mood in healthy adults. Prog Neuro-Psychopharmacol Biol Psychiatry. 2011;35(7):1682-1688.
11. Lizer M, Bogdan R, Kidd R. Comparison of the frequency of the methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism in depressed versus nondepressed patients. J Psychiatr Pract. 2011;17(6):404-409.
12. Bjelland I, Tell G, Vollset S, et al. Folate, vitamin B12, homocysteine, and the MTHFR 677C->T polymorphism in anxiety and depression: the Hordaland Homocysteine Study. Arch Gen Psychiatry. 2003;60(6):618-626.
13. Papakostas G, Shelton R, Zajecka J, et al. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel sequential trials. Am J Psychiatry. 2012;169(12):1267-1274.
14. Papakostas G, Shelton R, Zajecka J, et al. Effect of adjunctive L-methylfolate 15 mg among inadequate responders to SSRIs in depressed patients who were stratified by biomarker levels and genotype: results from a randomized clinical trial. J Clin Psychiatry. 2014;75(8):855-863.
15. Mech A, Farah A. Correlation of clinical response with homocysteine reduction during therapy with reduced B vitamins in patients with MDD who are positive for MTHFR C677T or A1298C polymorphism: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2016;77(5):668-671.
16. Godfrey P, Toone B, Carney M, et al. Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 1990;336(8712):392-395.
1. Scaglione F, Panzavolta G. Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica. 2014;44(5):480-488.
2. Jadavji N, Wieske F, Dirnagl U, et al. Methylenetetrahydrofolate reductase deficiency alters levels of glutamate and gamma-aminobutyric acid in brain tissue. Molecular Genetics and Metabolism Reports. 2015;3(Issue C):1-4.
3. Shelton R, Manning J, Barrentine L, et al. Assessing effects of L-methylfolate in depression management: results of a real-world patient experience trial. Prim Care Companion CNS Disord. 2013;15(4):pii:PCC.13m01520. doi: 10.4088/PCC.13m01520.
4. Brustolin S, Giugliani R, Felix T. Genetics of homocysteine metabolism and associated disorders. Braz J Med Biol Res. 2010;43(1):1-7.
5. Blom H, Smulders Y. Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects. J Inherit Metab Dis. 2011;34:75-81.
6. Moorthy D, Peter I, Scott T, et al. Status of vitamins B-12 and B-6 but not of folate, homocysteine, and the methylenetetrahydrofolate reductase C677T polymorphism are associated with impaired cognition and depression in adults. J Nutr. 2012;142:1554-1560.
7. Lievers K, Boers G, Verhoef P, et al. A second common variant in the methylenetetrahydrofolate reductase (MTHFR) gene and its relationship to MTHFR enzyme activity, homocysteine, and cardiovascular disease risk. J Mol Med (Berl). 2001;79(9):522-528.
8. Jiang W, Xu J, Lu X, et al. Association between MTHFR C677T polymorphism and depression: a meta-analysis in the Chinese population. Psychol Health Med. 2015;21(6):675-685.
9. Bousman C, Potiriadis M, Everall I, et al. Methylenetetrahydrofolate reductase (MTHFR) genetic variation and major depressive disorder prognosis: a five-year prospective cohort study of primary care attendees. Am J Med Genet B Neuropsychiatr Genet. 2014;165B(1):68-76.
10. Schiepers O, Van Boxtel M, de Groot R, et al. Genetic variation in folate metabolism is not associated with cognitive functioning or mood in healthy adults. Prog Neuro-Psychopharmacol Biol Psychiatry. 2011;35(7):1682-1688.
11. Lizer M, Bogdan R, Kidd R. Comparison of the frequency of the methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism in depressed versus nondepressed patients. J Psychiatr Pract. 2011;17(6):404-409.
12. Bjelland I, Tell G, Vollset S, et al. Folate, vitamin B12, homocysteine, and the MTHFR 677C->T polymorphism in anxiety and depression: the Hordaland Homocysteine Study. Arch Gen Psychiatry. 2003;60(6):618-626.
13. Papakostas G, Shelton R, Zajecka J, et al. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel sequential trials. Am J Psychiatry. 2012;169(12):1267-1274.
14. Papakostas G, Shelton R, Zajecka J, et al. Effect of adjunctive L-methylfolate 15 mg among inadequate responders to SSRIs in depressed patients who were stratified by biomarker levels and genotype: results from a randomized clinical trial. J Clin Psychiatry. 2014;75(8):855-863.
15. Mech A, Farah A. Correlation of clinical response with homocysteine reduction during therapy with reduced B vitamins in patients with MDD who are positive for MTHFR C677T or A1298C polymorphism: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2016;77(5):668-671.
16. Godfrey P, Toone B, Carney M, et al. Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 1990;336(8712):392-395.