User login
Tumor with central crusting
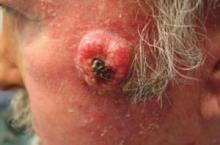
A 63-year-old man came into our dermatologic surgery clinic with a growth on his left cheek just anterior to his sideburn (FIGURE). Our patient indicated that the lesion, which appeared 6 weeks earlier, started as a small, hard papule with a central depression and rapidly grew to reach its current size and shape.
The patient’s face was sun damaged, and he had a prominent 2.1×1.8 cm well-circumscribed, skin-colored tumor on his cheek. The tumor had a central depression covered by a crust that appeared to conceal a deep keratinous plug. The tumor also had a volcano-like shape and was firm in texture, but tender to palpation and pressure. No lymphadenopathy was present.
The patient had a history of extensive sun exposure, and he’d had previous nonmelanoma skin cancers treated with various medical and surgical techniques. The rest of his history and exam were within normal limits. We performed a biopsy to confirm our clinical diagnosis.
FIGURE
Rapidly growing skin-colored tumor
What is your diagnosis?
How would you manage this condition?
Diagnosis: Solitary keratoacanthoma
Our patient had a solitary keratoacanthoma, a unique epidermal tumor that’s characterized by rapid, abundant growth and spontaneous resolution. This tumor goes by many names—molluscum sebaceum, molluscum pseudocarcinomatosum, cutaneous sebaceous neoplasm, and self-healing squamous epithelioma—but keratoacanthoma is the preferred term.1,2
There are several types of keratoacanthoma, but solitary keratoacanthoma remains the most common. It is typically found in light-skinned people in hair-bearing, sun-exposed areas. Peak incidence occurs between the ages of 50 and 69, although the tumors have been reported in patients of all ages. Both sexes are about equally affected, although there is a slight predilection for males. Keratoacanthomas mainly develop on the face (lower lip, cheek, nose, and eyelid), neck, and hands.
The tumors are often considered benign, but they become aggressive in 20% of cases—showing signs of perineural, perivascular, and intravascular invasion and metastases to regional lymph nodes. As a result, some clinicians consider keratoacanthoma to be a pseudomalignancy with self-regressing potential, while others view it as a pseudo-benign tumor progressing into an invasive squamous cell carcinoma (SCC).1,2
The exact etiology of keratoacanthoma is unknown. However, several precipitating factors have been implicated. Primary among them is exposure to ultraviolet light. Other etiological factors include:3-6
- chemical carcinogens (tar, pitch, mineral oil, and cigarette smoking)
- trauma (body peel, carbon dioxide laser resurfacing, megavoltage radiation therapy, and cryosurgery)
- immunosuppression
- surgical scar
- human papilloma virus (HPV).
A tumor with 3 stages
Keratoacanthoma undergoes a proliferative, mature, and involution stage.
In the proliferative stage, there is a rapid increase in tumor size; the tumor can get as big as 10 to 25 mm in diameter in 6 to 8 weeks.1,7
In the mature stage, the tumor stops growing and maintains a typical volcano-like form with a central keratin-filled crater.
In the involution stage, up to 50% of keratoacanthomas undergo spontaneous resolution with expulsion of the keratin plug and resorption of the tumoral mass. The process lasts 4 to 6 weeks, on average, but may take up to 1 year. What’s left behind is a residual atrophic and hypopigmented scar.8
Some lesions persist for a year or more, although the entire process from start to spontaneous resolution usually takes about 4 to 9 months.2,7,8
Is it an SCC or keratoacanthoma?
Histology is the gold standard in diagnosing a keratoacanthoma. A deep biopsy specimen that preferably includes part or full subcutaneous fat with excision of the entire lesion should allow for good histologic interpretation and diagnosis. Keratoacanthoma presents as a downgrowth of well-differentiated squamous epithelium. However, even with a well-performed biopsy, the diagnosis of keratoacanthoma remains challenging due to the lack of sufficient sensitive or specific histological features that can distinguish between keratoacanthomas and SCCs.
As a rule, a normal surface epithelium surrounding the keratin plug with sharp demarcation between tumor and stroma favors keratoacanthoma, whereas ulceration, numerous mitoses, and marked pleomorphism/anaplasia favor SCC. Because of the lack of a universal diagnostic criterion, several experts recommend that all keratoacanthomas be considered potential SCCs and thus treated as such.1,2
When in doubt, cut it out
Although the natural course of a keratoacanthoma is spontaneous regression, the lack of reliable criteria to differentiate it from an SCC with confidence renders therapeutic intervention the safest approach. Solitary keratoacanthomas respond well to surgical excision and may require aggressive procedures if they become too large or invade other structures. Since Mohs’ micrographic surgery is tissue sparing, consider it the treatment of choice if the keratoacanthoma is located in a sensitive area, such as the face.
Cryotherapy with liquid nitrogen, electrodessication and curettage, radiation therapy, and CO2 laser surgery have all been used in small solitary keratoacanthomas with good success.9,10 Other treatment options include intralesional and/or topical treatment using several compounds, such as 5-fluorouracil, corticosteroids, bleomycin, imiquimod, interferon alpha 2b, and methotrexate.7,9-13
Keratoacanthoma patients are often UV light sensitive, so they must avoid excessive sun exposure and use sunscreen with high SPF at all times to prevent recurrence and minimize scarring.
We opted for Mohs’ surgery for our patient
Given the cosmetically sensitive location of our patient’s keratoacanthoma, the size of it, and the patient’s history of skin cancers, we decided to use Mohs’ micrographic surgery for the management of this tumor, with good clinical outcome. There were no new lesions or recurrence on follow-up visit 6 months later.
Correspondence
Amor Khachemoune, MD, CWS, Assistant Professor, Ronald O. Perelman Department of Dermatology, New York University School of Medicine, 530 First Avenue, Suite 7R, New York, NY 10016; amorkh@pol.net.
1. Beham A, Regauer S, Soyer HP, Beham-Schmid C. Keratoacanthoma: a clinically distinct variant of well differentiated squamous cell carcinoma. Ad Anat Pathol. 1998;5:269-280.
2. Schwartz RA. Keratoacanthoma: a clinico-pathologic enigma. Dermatol Surg. 2004;30(2 Pt 2):326-333; discussion 333.
3. Miot HA, Miot LD, da Costa AL, Matsuo CY, Stolf HO, Marques ME. Association between solitary keratoacanthoma and cigarette smoking: a case-control study. Dermatol Online J. 2006;12(2):2.-
4. Pattee SF, Silvis NG. Keratoacanthoma developing in sites of previous trauma: a report of two cases and review of the literature. J Am Acad Dermatol. 2003;48(2 suppl):S35-S38.
5. Goldberg LH, Silapunt S, Beyrau KK, Peterson SR, Friedman PM, Alam M. Keratoacanthoma as a postoperative complication of skin cancer excision. J Am Acad Dermatol. 2004;50:753-758.
6. Stockfleth E, Meinke B, Arndt R, Christophers E, Meyer T. Identification of DNA sequences of both genital and cutaneous HPV types in a small number of keratoacanthomas of nonimmunosuppressed patients. Dermatology. 1999;198:122-125.
7. Oh CK, Son HS, Lee JB, Jang HS, Kwon KS. Intralesional interferon alfa-2b treatment of keratoacanthomas. J Am Acad Dermatol. 2004;51(5 suppl):S177-S180.
8. Griffiths RW. Keratoacanthoma observed. Br J Plast Surg. 2004;57:485-501.
9. Caccialanza M, Sopelana N. Radiation therapy of keratoacanthomas: results in 55 patients. Int J Radiat Oncol Biol Phys. 1989;16:475-477.
10. Gray RJ, Meland NB. Topical 5-fluorouracil as primary therapy for keratoacanthoma. Ann Plast Surg. 2000;44:82-85.
11. Sanders S, Busam KJ, Halpern AC, Nehal KS. Intralesional corticosteroid treatment of multiple eruptive keratoacanthomas: case report and review of a controversial therapy. Dermatol Surg. 2002;28:954-958.
12. Di Lernia V, Ricci C, Albertini G. Spontaneous regression of keratoacanthoma can be promoted by topical treatment with imiquimod cream. J Eur Acad Dermatol Venereol. 2004;18:626-629.
13. Cohen PR, Schulze KE, Teller CF, Nelson BR. Intralesional methotrexate for keratoacanthoma of the nose. Skinmed. 2005;4:393-395.
A 63-year-old man came into our dermatologic surgery clinic with a growth on his left cheek just anterior to his sideburn (FIGURE). Our patient indicated that the lesion, which appeared 6 weeks earlier, started as a small, hard papule with a central depression and rapidly grew to reach its current size and shape.
The patient’s face was sun damaged, and he had a prominent 2.1×1.8 cm well-circumscribed, skin-colored tumor on his cheek. The tumor had a central depression covered by a crust that appeared to conceal a deep keratinous plug. The tumor also had a volcano-like shape and was firm in texture, but tender to palpation and pressure. No lymphadenopathy was present.
The patient had a history of extensive sun exposure, and he’d had previous nonmelanoma skin cancers treated with various medical and surgical techniques. The rest of his history and exam were within normal limits. We performed a biopsy to confirm our clinical diagnosis.
FIGURE
Rapidly growing skin-colored tumor
What is your diagnosis?
How would you manage this condition?
Diagnosis: Solitary keratoacanthoma
Our patient had a solitary keratoacanthoma, a unique epidermal tumor that’s characterized by rapid, abundant growth and spontaneous resolution. This tumor goes by many names—molluscum sebaceum, molluscum pseudocarcinomatosum, cutaneous sebaceous neoplasm, and self-healing squamous epithelioma—but keratoacanthoma is the preferred term.1,2
There are several types of keratoacanthoma, but solitary keratoacanthoma remains the most common. It is typically found in light-skinned people in hair-bearing, sun-exposed areas. Peak incidence occurs between the ages of 50 and 69, although the tumors have been reported in patients of all ages. Both sexes are about equally affected, although there is a slight predilection for males. Keratoacanthomas mainly develop on the face (lower lip, cheek, nose, and eyelid), neck, and hands.
The tumors are often considered benign, but they become aggressive in 20% of cases—showing signs of perineural, perivascular, and intravascular invasion and metastases to regional lymph nodes. As a result, some clinicians consider keratoacanthoma to be a pseudomalignancy with self-regressing potential, while others view it as a pseudo-benign tumor progressing into an invasive squamous cell carcinoma (SCC).1,2
The exact etiology of keratoacanthoma is unknown. However, several precipitating factors have been implicated. Primary among them is exposure to ultraviolet light. Other etiological factors include:3-6
- chemical carcinogens (tar, pitch, mineral oil, and cigarette smoking)
- trauma (body peel, carbon dioxide laser resurfacing, megavoltage radiation therapy, and cryosurgery)
- immunosuppression
- surgical scar
- human papilloma virus (HPV).
A tumor with 3 stages
Keratoacanthoma undergoes a proliferative, mature, and involution stage.
In the proliferative stage, there is a rapid increase in tumor size; the tumor can get as big as 10 to 25 mm in diameter in 6 to 8 weeks.1,7
In the mature stage, the tumor stops growing and maintains a typical volcano-like form with a central keratin-filled crater.
In the involution stage, up to 50% of keratoacanthomas undergo spontaneous resolution with expulsion of the keratin plug and resorption of the tumoral mass. The process lasts 4 to 6 weeks, on average, but may take up to 1 year. What’s left behind is a residual atrophic and hypopigmented scar.8
Some lesions persist for a year or more, although the entire process from start to spontaneous resolution usually takes about 4 to 9 months.2,7,8
Is it an SCC or keratoacanthoma?
Histology is the gold standard in diagnosing a keratoacanthoma. A deep biopsy specimen that preferably includes part or full subcutaneous fat with excision of the entire lesion should allow for good histologic interpretation and diagnosis. Keratoacanthoma presents as a downgrowth of well-differentiated squamous epithelium. However, even with a well-performed biopsy, the diagnosis of keratoacanthoma remains challenging due to the lack of sufficient sensitive or specific histological features that can distinguish between keratoacanthomas and SCCs.
As a rule, a normal surface epithelium surrounding the keratin plug with sharp demarcation between tumor and stroma favors keratoacanthoma, whereas ulceration, numerous mitoses, and marked pleomorphism/anaplasia favor SCC. Because of the lack of a universal diagnostic criterion, several experts recommend that all keratoacanthomas be considered potential SCCs and thus treated as such.1,2
When in doubt, cut it out
Although the natural course of a keratoacanthoma is spontaneous regression, the lack of reliable criteria to differentiate it from an SCC with confidence renders therapeutic intervention the safest approach. Solitary keratoacanthomas respond well to surgical excision and may require aggressive procedures if they become too large or invade other structures. Since Mohs’ micrographic surgery is tissue sparing, consider it the treatment of choice if the keratoacanthoma is located in a sensitive area, such as the face.
Cryotherapy with liquid nitrogen, electrodessication and curettage, radiation therapy, and CO2 laser surgery have all been used in small solitary keratoacanthomas with good success.9,10 Other treatment options include intralesional and/or topical treatment using several compounds, such as 5-fluorouracil, corticosteroids, bleomycin, imiquimod, interferon alpha 2b, and methotrexate.7,9-13
Keratoacanthoma patients are often UV light sensitive, so they must avoid excessive sun exposure and use sunscreen with high SPF at all times to prevent recurrence and minimize scarring.
We opted for Mohs’ surgery for our patient
Given the cosmetically sensitive location of our patient’s keratoacanthoma, the size of it, and the patient’s history of skin cancers, we decided to use Mohs’ micrographic surgery for the management of this tumor, with good clinical outcome. There were no new lesions or recurrence on follow-up visit 6 months later.
Correspondence
Amor Khachemoune, MD, CWS, Assistant Professor, Ronald O. Perelman Department of Dermatology, New York University School of Medicine, 530 First Avenue, Suite 7R, New York, NY 10016; amorkh@pol.net.
A 63-year-old man came into our dermatologic surgery clinic with a growth on his left cheek just anterior to his sideburn (FIGURE). Our patient indicated that the lesion, which appeared 6 weeks earlier, started as a small, hard papule with a central depression and rapidly grew to reach its current size and shape.
The patient’s face was sun damaged, and he had a prominent 2.1×1.8 cm well-circumscribed, skin-colored tumor on his cheek. The tumor had a central depression covered by a crust that appeared to conceal a deep keratinous plug. The tumor also had a volcano-like shape and was firm in texture, but tender to palpation and pressure. No lymphadenopathy was present.
The patient had a history of extensive sun exposure, and he’d had previous nonmelanoma skin cancers treated with various medical and surgical techniques. The rest of his history and exam were within normal limits. We performed a biopsy to confirm our clinical diagnosis.
FIGURE
Rapidly growing skin-colored tumor
What is your diagnosis?
How would you manage this condition?
Diagnosis: Solitary keratoacanthoma
Our patient had a solitary keratoacanthoma, a unique epidermal tumor that’s characterized by rapid, abundant growth and spontaneous resolution. This tumor goes by many names—molluscum sebaceum, molluscum pseudocarcinomatosum, cutaneous sebaceous neoplasm, and self-healing squamous epithelioma—but keratoacanthoma is the preferred term.1,2
There are several types of keratoacanthoma, but solitary keratoacanthoma remains the most common. It is typically found in light-skinned people in hair-bearing, sun-exposed areas. Peak incidence occurs between the ages of 50 and 69, although the tumors have been reported in patients of all ages. Both sexes are about equally affected, although there is a slight predilection for males. Keratoacanthomas mainly develop on the face (lower lip, cheek, nose, and eyelid), neck, and hands.
The tumors are often considered benign, but they become aggressive in 20% of cases—showing signs of perineural, perivascular, and intravascular invasion and metastases to regional lymph nodes. As a result, some clinicians consider keratoacanthoma to be a pseudomalignancy with self-regressing potential, while others view it as a pseudo-benign tumor progressing into an invasive squamous cell carcinoma (SCC).1,2
The exact etiology of keratoacanthoma is unknown. However, several precipitating factors have been implicated. Primary among them is exposure to ultraviolet light. Other etiological factors include:3-6
- chemical carcinogens (tar, pitch, mineral oil, and cigarette smoking)
- trauma (body peel, carbon dioxide laser resurfacing, megavoltage radiation therapy, and cryosurgery)
- immunosuppression
- surgical scar
- human papilloma virus (HPV).
A tumor with 3 stages
Keratoacanthoma undergoes a proliferative, mature, and involution stage.
In the proliferative stage, there is a rapid increase in tumor size; the tumor can get as big as 10 to 25 mm in diameter in 6 to 8 weeks.1,7
In the mature stage, the tumor stops growing and maintains a typical volcano-like form with a central keratin-filled crater.
In the involution stage, up to 50% of keratoacanthomas undergo spontaneous resolution with expulsion of the keratin plug and resorption of the tumoral mass. The process lasts 4 to 6 weeks, on average, but may take up to 1 year. What’s left behind is a residual atrophic and hypopigmented scar.8
Some lesions persist for a year or more, although the entire process from start to spontaneous resolution usually takes about 4 to 9 months.2,7,8
Is it an SCC or keratoacanthoma?
Histology is the gold standard in diagnosing a keratoacanthoma. A deep biopsy specimen that preferably includes part or full subcutaneous fat with excision of the entire lesion should allow for good histologic interpretation and diagnosis. Keratoacanthoma presents as a downgrowth of well-differentiated squamous epithelium. However, even with a well-performed biopsy, the diagnosis of keratoacanthoma remains challenging due to the lack of sufficient sensitive or specific histological features that can distinguish between keratoacanthomas and SCCs.
As a rule, a normal surface epithelium surrounding the keratin plug with sharp demarcation between tumor and stroma favors keratoacanthoma, whereas ulceration, numerous mitoses, and marked pleomorphism/anaplasia favor SCC. Because of the lack of a universal diagnostic criterion, several experts recommend that all keratoacanthomas be considered potential SCCs and thus treated as such.1,2
When in doubt, cut it out
Although the natural course of a keratoacanthoma is spontaneous regression, the lack of reliable criteria to differentiate it from an SCC with confidence renders therapeutic intervention the safest approach. Solitary keratoacanthomas respond well to surgical excision and may require aggressive procedures if they become too large or invade other structures. Since Mohs’ micrographic surgery is tissue sparing, consider it the treatment of choice if the keratoacanthoma is located in a sensitive area, such as the face.
Cryotherapy with liquid nitrogen, electrodessication and curettage, radiation therapy, and CO2 laser surgery have all been used in small solitary keratoacanthomas with good success.9,10 Other treatment options include intralesional and/or topical treatment using several compounds, such as 5-fluorouracil, corticosteroids, bleomycin, imiquimod, interferon alpha 2b, and methotrexate.7,9-13
Keratoacanthoma patients are often UV light sensitive, so they must avoid excessive sun exposure and use sunscreen with high SPF at all times to prevent recurrence and minimize scarring.
We opted for Mohs’ surgery for our patient
Given the cosmetically sensitive location of our patient’s keratoacanthoma, the size of it, and the patient’s history of skin cancers, we decided to use Mohs’ micrographic surgery for the management of this tumor, with good clinical outcome. There were no new lesions or recurrence on follow-up visit 6 months later.
Correspondence
Amor Khachemoune, MD, CWS, Assistant Professor, Ronald O. Perelman Department of Dermatology, New York University School of Medicine, 530 First Avenue, Suite 7R, New York, NY 10016; amorkh@pol.net.
1. Beham A, Regauer S, Soyer HP, Beham-Schmid C. Keratoacanthoma: a clinically distinct variant of well differentiated squamous cell carcinoma. Ad Anat Pathol. 1998;5:269-280.
2. Schwartz RA. Keratoacanthoma: a clinico-pathologic enigma. Dermatol Surg. 2004;30(2 Pt 2):326-333; discussion 333.
3. Miot HA, Miot LD, da Costa AL, Matsuo CY, Stolf HO, Marques ME. Association between solitary keratoacanthoma and cigarette smoking: a case-control study. Dermatol Online J. 2006;12(2):2.-
4. Pattee SF, Silvis NG. Keratoacanthoma developing in sites of previous trauma: a report of two cases and review of the literature. J Am Acad Dermatol. 2003;48(2 suppl):S35-S38.
5. Goldberg LH, Silapunt S, Beyrau KK, Peterson SR, Friedman PM, Alam M. Keratoacanthoma as a postoperative complication of skin cancer excision. J Am Acad Dermatol. 2004;50:753-758.
6. Stockfleth E, Meinke B, Arndt R, Christophers E, Meyer T. Identification of DNA sequences of both genital and cutaneous HPV types in a small number of keratoacanthomas of nonimmunosuppressed patients. Dermatology. 1999;198:122-125.
7. Oh CK, Son HS, Lee JB, Jang HS, Kwon KS. Intralesional interferon alfa-2b treatment of keratoacanthomas. J Am Acad Dermatol. 2004;51(5 suppl):S177-S180.
8. Griffiths RW. Keratoacanthoma observed. Br J Plast Surg. 2004;57:485-501.
9. Caccialanza M, Sopelana N. Radiation therapy of keratoacanthomas: results in 55 patients. Int J Radiat Oncol Biol Phys. 1989;16:475-477.
10. Gray RJ, Meland NB. Topical 5-fluorouracil as primary therapy for keratoacanthoma. Ann Plast Surg. 2000;44:82-85.
11. Sanders S, Busam KJ, Halpern AC, Nehal KS. Intralesional corticosteroid treatment of multiple eruptive keratoacanthomas: case report and review of a controversial therapy. Dermatol Surg. 2002;28:954-958.
12. Di Lernia V, Ricci C, Albertini G. Spontaneous regression of keratoacanthoma can be promoted by topical treatment with imiquimod cream. J Eur Acad Dermatol Venereol. 2004;18:626-629.
13. Cohen PR, Schulze KE, Teller CF, Nelson BR. Intralesional methotrexate for keratoacanthoma of the nose. Skinmed. 2005;4:393-395.
1. Beham A, Regauer S, Soyer HP, Beham-Schmid C. Keratoacanthoma: a clinically distinct variant of well differentiated squamous cell carcinoma. Ad Anat Pathol. 1998;5:269-280.
2. Schwartz RA. Keratoacanthoma: a clinico-pathologic enigma. Dermatol Surg. 2004;30(2 Pt 2):326-333; discussion 333.
3. Miot HA, Miot LD, da Costa AL, Matsuo CY, Stolf HO, Marques ME. Association between solitary keratoacanthoma and cigarette smoking: a case-control study. Dermatol Online J. 2006;12(2):2.-
4. Pattee SF, Silvis NG. Keratoacanthoma developing in sites of previous trauma: a report of two cases and review of the literature. J Am Acad Dermatol. 2003;48(2 suppl):S35-S38.
5. Goldberg LH, Silapunt S, Beyrau KK, Peterson SR, Friedman PM, Alam M. Keratoacanthoma as a postoperative complication of skin cancer excision. J Am Acad Dermatol. 2004;50:753-758.
6. Stockfleth E, Meinke B, Arndt R, Christophers E, Meyer T. Identification of DNA sequences of both genital and cutaneous HPV types in a small number of keratoacanthomas of nonimmunosuppressed patients. Dermatology. 1999;198:122-125.
7. Oh CK, Son HS, Lee JB, Jang HS, Kwon KS. Intralesional interferon alfa-2b treatment of keratoacanthomas. J Am Acad Dermatol. 2004;51(5 suppl):S177-S180.
8. Griffiths RW. Keratoacanthoma observed. Br J Plast Surg. 2004;57:485-501.
9. Caccialanza M, Sopelana N. Radiation therapy of keratoacanthomas: results in 55 patients. Int J Radiat Oncol Biol Phys. 1989;16:475-477.
10. Gray RJ, Meland NB. Topical 5-fluorouracil as primary therapy for keratoacanthoma. Ann Plast Surg. 2000;44:82-85.
11. Sanders S, Busam KJ, Halpern AC, Nehal KS. Intralesional corticosteroid treatment of multiple eruptive keratoacanthomas: case report and review of a controversial therapy. Dermatol Surg. 2002;28:954-958.
12. Di Lernia V, Ricci C, Albertini G. Spontaneous regression of keratoacanthoma can be promoted by topical treatment with imiquimod cream. J Eur Acad Dermatol Venereol. 2004;18:626-629.
13. Cohen PR, Schulze KE, Teller CF, Nelson BR. Intralesional methotrexate for keratoacanthoma of the nose. Skinmed. 2005;4:393-395.