User login
Painful Flesh-Colored Nodule on the Shoulder
Painful Flesh-Colored Nodule on the Shoulder
THE DIAGNOSIS: Dermatofibrosarcoma Protuberans
The histologic findings showed fascicular proliferation of relatively monomorphic spindle cells with extensive entrapment of collagen and adipocytes. Immunohistochemical staining showed that the lesional cells were diffusely positive for CD34 and negative for SOX10, S100, desmin, and factor XIIIa. The decision was made to perform cytogenetic testing with fluorescence in situ hybridization to evaluate for the presence of platelet-derived growth factor receptor beta (PDGFB) polypeptide rearrangement, a key biomarker known to be positive in most patients with dermatofibrosarcoma protuberans (DFSP).1 This rearrangement results in overproduction of PDGFB, continuous activation of platelet-derived growth factor receptor beta, cellular proliferation, and tumor formation.2 In our patient, results were positive for the PDGFB polypeptide rearrangement, which confirmed suspected diagnosis of DFSP with fibrous histiocytoma like morphology. The patient was referred for Mohs micrographic surgery for proper management.
Dermatofibrosarcoma protuberans is a rare soft-tissue tumor that involves the dermis, subcutaneous fat, and sometimes muscle and fascia.2 Dermatofibrosarcoma protuberans primarily affects young to middle-aged adults, with a slight predilection for individuals in the third to fifth decades of life.3 Lesions preferentially involve the trunk, particularly the shoulder and chest regions, and manifest as poorly circumscribed, locally aggressive mesenchymal neoplasms with a high local recurrence rate but low metastatic potential.4,5 Clinically, the lesions appear as flesh-colored, rubbery plaques or nodules. A diagnosis of DFSP requires a high index of clinical suspicion, and histologic, immunohistochemical, and molecular testing usually are required for confirmation.
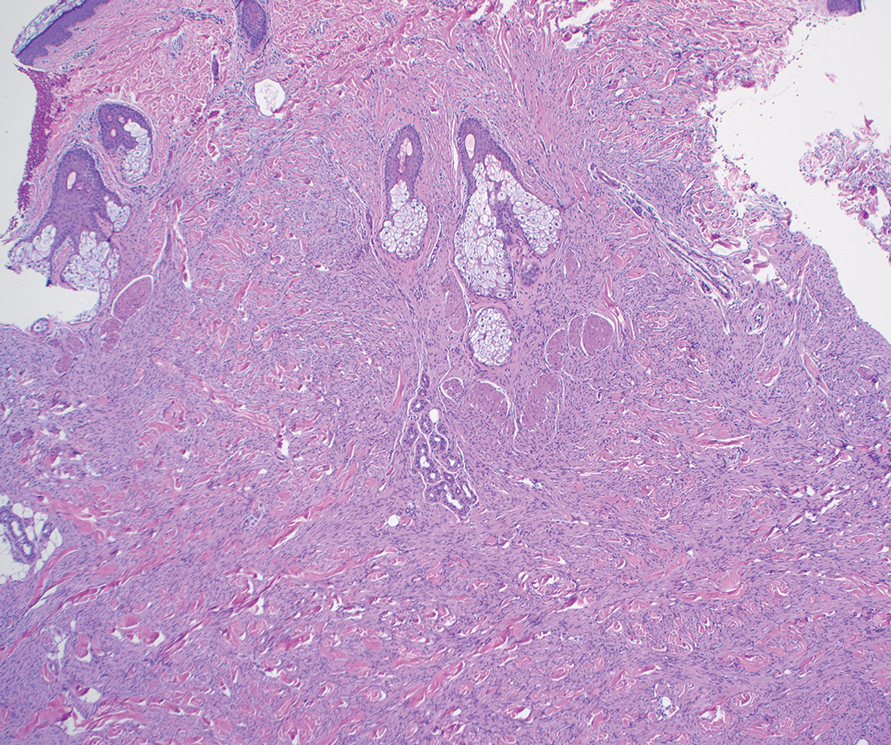
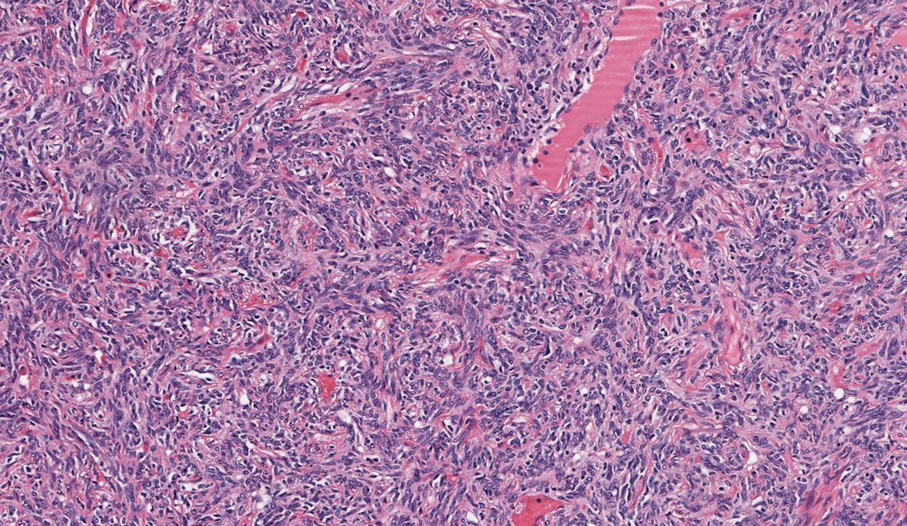
On histopathologic examination, DFSP classically demonstrates uniform, spindle-shaped cells that traditionally are arranged in an intersecting pattern and primarily are based in the dermis (Figure 1).5 Infiltration into the underlying tissue is a common feature, with neoplastic extensions causing a classic honeycomb pattern6 that also can be seen in diffuse neurofibroma and may cause diagnostic challenges; however, the immunohistology staining of neurofibroma differs from DFSP in that it stains positive for CD34, SOX-100, and S100, while DFSP has strong and diffuse CD34 immunoreactivity with negative immunostaining for SOX10, S100, desmin, and factor XIIIa.2,6
Dermatofibrosarcoma protuberans can cause considerable fat infiltration compared to other soft-tissue neoplasms, making this finding suspicious for—if not characteristic of—DFSP. Collagen trapping also can be observed; however, this is more pathognomonic in cellular fibrous histiocytoma, which is a distinct clinical variant of dermatofibromas. Due to its similarity to other lesions, histopathologic examination along with immunostaining can assist in differentiating and accurately diagnosing DFSP.6
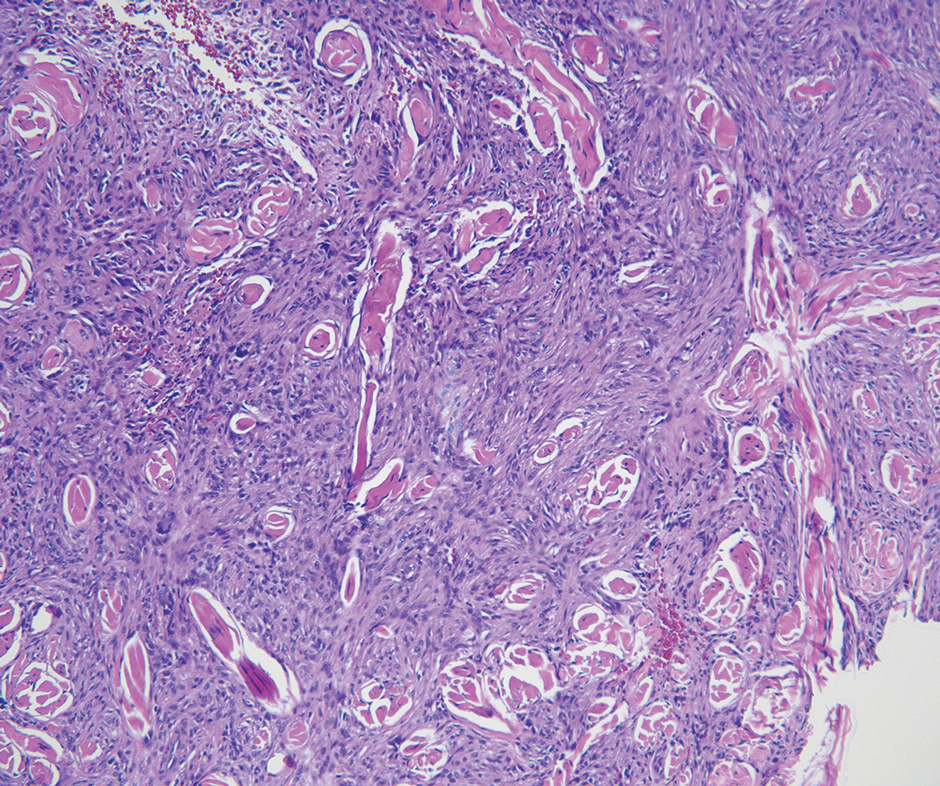
Cellular fibrous histiocytoma (CFH), a distinct clinical variant of dermatofibromas, is a benign tumor of mesenchymal origin that occurs more commonly on the trunk, arms, and legs. On histologic examination, CFH is composed of spindle-shaped cells with variable amounts of eosinophilic cytoplasm and small, oval-shaped eosinophilic nuclei and collagen trapping (Figure 2).7,8 Most CFHs occupy the superficial dermis but can extend into the deep reticular dermis, thus mimicking the honeycomb pattern seen in DFSP. This neoplasm can show a similar architecture to DFSP, which is why further investigation including cytogenetics and immunohistochemical staining can help differentiate the two conditions. Cellular fibrous histiocytoma typically stains negative for CD34 and positive for factor XIIIa.9 However, CD34 can be positive in a subset of CFHs, with a considerable subset showing peripheral CD34 positivity and a smaller subset showing central CD34 the positivity.10 This suggests that CD34 cannot be the only factor differentiating these 2 lesions in making a proper dermatopathologic diagnosis.
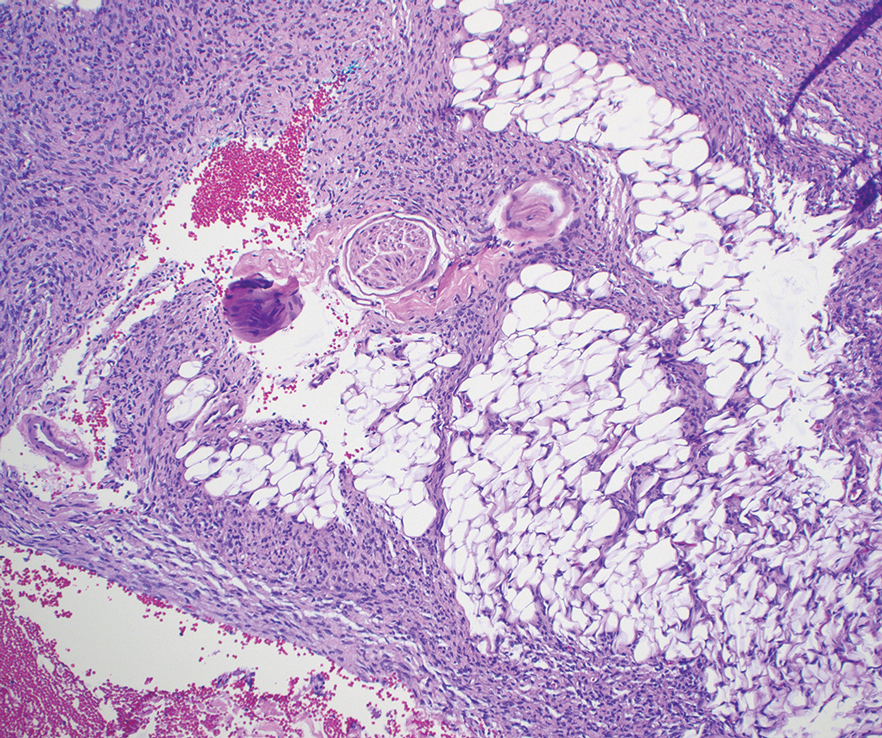
Solitary fibrous tumor (SFT) is a rare mesenchymal tumor that can occur anywhere on the body and typically manifests as a deep, painless, enlarging mass in adults aged 50 to 60 years.11 On histologic examination, SFT consists of randomly arranged cells with a spindle or ovoid shape within a collagenous stroma intermixed with blood vessels with a characteristic staghorn shape (Figure 3).11 Low-grade SFT shows a patternless arrangement with spindle cells, a low number of mitotic figures, and vessels with a staghorn appearance compared to high-grade SFT, which shows hypercellularity with nuclear pleomorphism and a high number of mitotic figures.11 Solitary fibrous tumors are positive for CD34 and STAT-6 and negative for CD31 and typically demonstrate NGFI-A binding protein 2 (NAB2)—signal transducer and activator of transcription 6 (STAT 6) gene fusion.11
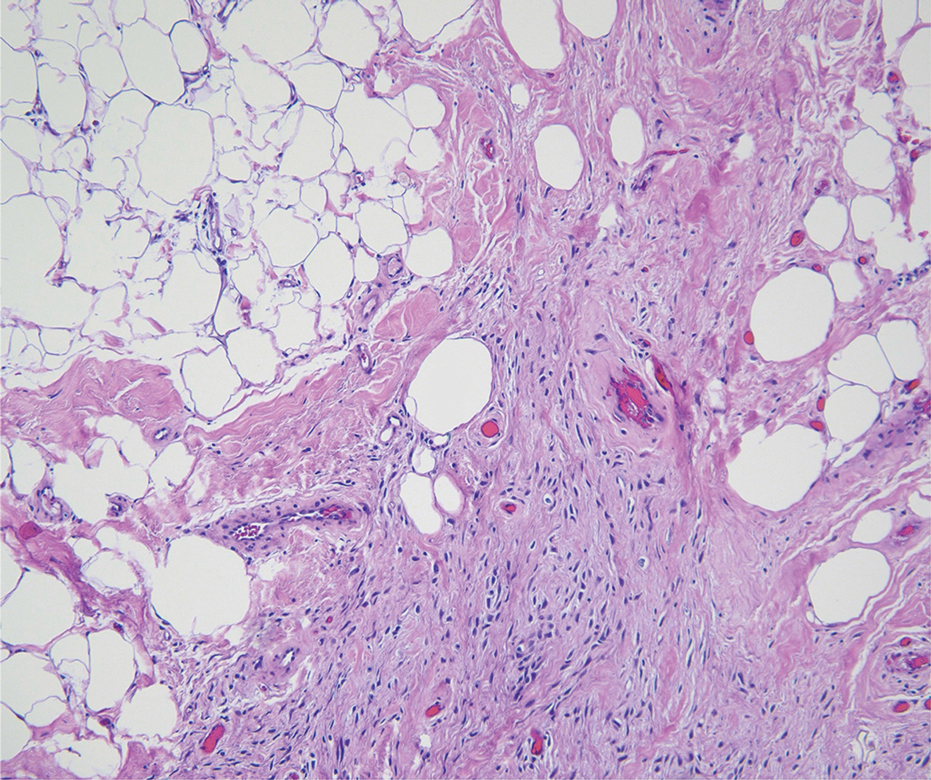
Spindle-cell lipomas are rare, benign, slow-growing, lipomatous tumors that typically manifest in men aged 40 to 70 years.12 These lesions originate most frequently in the subcutaneous tissue of the upper back, posterior neck, and shoulders. The histologic growth pattern of spindle-cell lipomas can mimic other spindle-cell and myxoid tumors, which is why cytogenetic analysis is crucial for differentiating these lesions. On histologic examination, spindle-cell lipomas exhibit a mixture of mature adipocytes, uniform spindle cells, and collagen bundles (eFigure). Spindle-cell lipoma stains positive for CD34 but negative for S100.13 In addition, spindle-cell lipomas tend to show structural rearrangements (mainly deletions) of the long arm of chromosome 13 or even losses of whole chromosome 13, which contains the retinoblastoma (RB1) gene.13
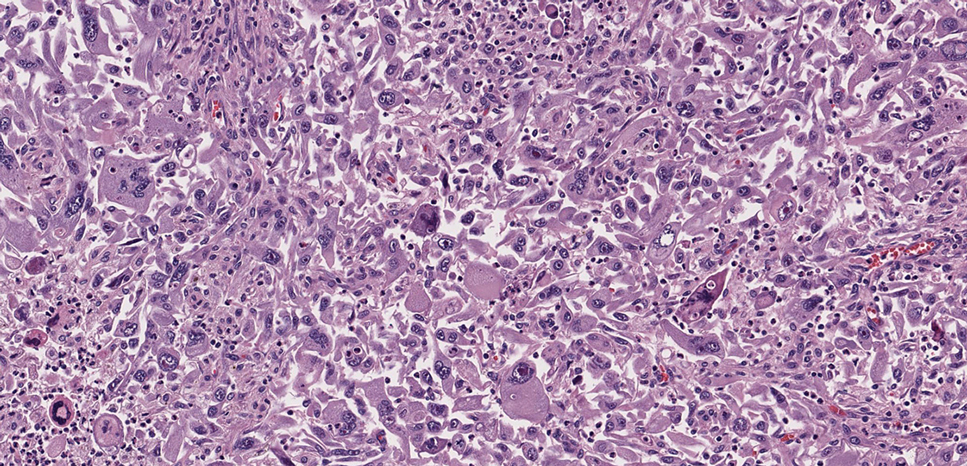
Pleomorphic dermal sarcoma is a rare mesenchymal tumor that can appear clinically and histologically similar to atypical fibroxanthoma.14 This lesion often manifests in elderly patients and is strongly associated with chronic sun exposure.15 Pleomorphic dermal sarcoma is a locally aggressive tumor with metastatic potential to the skin or lymph nodes. On histologic examination, these tumors exhibit pleomorphic atypical epithelioid or spindle cells as well as multinucleated tumor giant cells with possible tumor necrosis, lymphovascular invasion, or perineural infiltration (Figure 4). Pleomorphic dermal sarcoma, typically a diagnosis of exclusion, requires immunohistochemistry to aid in proper identification.16 These lesions stain positive for CD10 and negative for cytokeratins, desmin, HMB45, CD34, p63, p40, SOX10, and S100.15,16
- Ugurel S, Kortmann R, Mohr P, et al. S1 guidelines for dermatofibrosarcoma protuberans (DFSP)—update 2018. J Dtsch Dermatol Ges. 2019;17:663-668. doi:10.1111/ddg.13849
- Brooks J, Ramsey ML. Dermatofibrosarcoma protuberans. StatPearls Publishing; 2024. Updated April 18, 2024. Accessed April 30, 2025.
- Bowne WB, Antonescu CR, Leung DH, et al. Dermatofibrosarcoma protuberans: a clinicopathologic analysis of patients treated and followed at a single institution. Cancer. 2000;88:2711-2720.
- Lim SX, Ramaiya A, Levell NJ, et al. Review of dermatofibrosarcoma protuberans. Clin Exp Dermatol. 2022;48:297-302. doi:10.1093/ced/llac111
- Trinidad CM, Wangsiricharoen S, Prieto VG, et al. Rare variants of dermatofibrosarcoma protuberans: clinical, histologic, and molecular features and diagnostic pitfalls. Dermatopathology. 2023;10:54-62. doi:10.3390/dermatopathology10010008
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752. doi:10.3390/jcm9061752
- Tsunoda K, Oikawa H, Maeda F, et al. A case of cellular fibrous histiocytoma on the right elbow with repeated relapse within a short period. Case Rep Dermatol. 2015;7:10–16. https://doi.org/10.1159/000371790
- Calonje E, Mentzel T, Fletcher CD. Cellular benign fibrous histiocytoma. Clinicopathologic analysis of 74 cases of a distinctive variant of cutaneous fibrous histiocytoma with frequent recurrence. Am J Surg Pathol. 1994;18:668-676.
- Goldblum JR, Tuthill RJ. CD34 and factor-XIIIa immunoreactivity in dermatofibrosarcoma protuberans and dermatofibroma. Am J Dermatopathology. 1997;19:147-153. doi:10.1097/00000372-199704000-00008
- Volpicelli ER, Fletcher CD. Desmin and CD34 positivity in cellular fibrous histiocytoma: an immunohistochemical analysis of 100 cases. J Cutan Pathol. 2012;39:747-752. doi:10.1111/j.1600-0560.2012.01944.x
- Martin-Broto J, Mondaza-Hernandez JL, Moura DS, et al. A comprehensive review on solitary fibrous tumor: new insights for new horizons. Cancers (Basel). 2021;13:2913. doi:10.3390/cancers13122913
- Machol JA, Cusic JG, O’Connor EA, et al. Spindle cell lipoma of the neck: review of the literature and case report. Plast Reconstr Surg Glob Open. 2015;3:E550. doi:10.1097/GOX.0000000000000405
- Domanski HA, Carlén B, Jonsson K, et al. Distinct cytologic features of spindle cell lipoma. a cytologic-histologic study with clinical, radiologic, electron microscopic, and cytogenetic correlations. Cancer. 2001;93:381-389. doi:10.1002/cncr.10142
- Devine RL, Cameron A, Holden AM, et al. The pleomorphic dermal sarcoma: its management, follow-up and the need for more guidance. Adv Oral Maxillofac Surg. 2021;2:100046. doi:10.1016 /j.adoms.2021.100046
- Seretis K, Klaroudas A, Galani V, et al. Pleomorphic dermal sarcoma: it might be rare but it exists [published online August 4, 2023]. J Surg Case Rep. doi:10.1093/jscr/rjad374
- Miller K, Goodlad JR, Brenn T. Pleomorphic dermal sarcoma. Am J Surg Pathol. 2012;36:1317-1326. doi:10.1097/pas.0b013e31825359e1
THE DIAGNOSIS: Dermatofibrosarcoma Protuberans
The histologic findings showed fascicular proliferation of relatively monomorphic spindle cells with extensive entrapment of collagen and adipocytes. Immunohistochemical staining showed that the lesional cells were diffusely positive for CD34 and negative for SOX10, S100, desmin, and factor XIIIa. The decision was made to perform cytogenetic testing with fluorescence in situ hybridization to evaluate for the presence of platelet-derived growth factor receptor beta (PDGFB) polypeptide rearrangement, a key biomarker known to be positive in most patients with dermatofibrosarcoma protuberans (DFSP).1 This rearrangement results in overproduction of PDGFB, continuous activation of platelet-derived growth factor receptor beta, cellular proliferation, and tumor formation.2 In our patient, results were positive for the PDGFB polypeptide rearrangement, which confirmed suspected diagnosis of DFSP with fibrous histiocytoma like morphology. The patient was referred for Mohs micrographic surgery for proper management.
Dermatofibrosarcoma protuberans is a rare soft-tissue tumor that involves the dermis, subcutaneous fat, and sometimes muscle and fascia.2 Dermatofibrosarcoma protuberans primarily affects young to middle-aged adults, with a slight predilection for individuals in the third to fifth decades of life.3 Lesions preferentially involve the trunk, particularly the shoulder and chest regions, and manifest as poorly circumscribed, locally aggressive mesenchymal neoplasms with a high local recurrence rate but low metastatic potential.4,5 Clinically, the lesions appear as flesh-colored, rubbery plaques or nodules. A diagnosis of DFSP requires a high index of clinical suspicion, and histologic, immunohistochemical, and molecular testing usually are required for confirmation.
On histopathologic examination, DFSP classically demonstrates uniform, spindle-shaped cells that traditionally are arranged in an intersecting pattern and primarily are based in the dermis (Figure 1).5 Infiltration into the underlying tissue is a common feature, with neoplastic extensions causing a classic honeycomb pattern6 that also can be seen in diffuse neurofibroma and may cause diagnostic challenges; however, the immunohistology staining of neurofibroma differs from DFSP in that it stains positive for CD34, SOX-100, and S100, while DFSP has strong and diffuse CD34 immunoreactivity with negative immunostaining for SOX10, S100, desmin, and factor XIIIa.2,6

Dermatofibrosarcoma protuberans can cause considerable fat infiltration compared to other soft-tissue neoplasms, making this finding suspicious for—if not characteristic of—DFSP. Collagen trapping also can be observed; however, this is more pathognomonic in cellular fibrous histiocytoma, which is a distinct clinical variant of dermatofibromas. Due to its similarity to other lesions, histopathologic examination along with immunostaining can assist in differentiating and accurately diagnosing DFSP.6
Cellular fibrous histiocytoma (CFH), a distinct clinical variant of dermatofibromas, is a benign tumor of mesenchymal origin that occurs more commonly on the trunk, arms, and legs. On histologic examination, CFH is composed of spindle-shaped cells with variable amounts of eosinophilic cytoplasm and small, oval-shaped eosinophilic nuclei and collagen trapping (Figure 2).7,8 Most CFHs occupy the superficial dermis but can extend into the deep reticular dermis, thus mimicking the honeycomb pattern seen in DFSP. This neoplasm can show a similar architecture to DFSP, which is why further investigation including cytogenetics and immunohistochemical staining can help differentiate the two conditions. Cellular fibrous histiocytoma typically stains negative for CD34 and positive for factor XIIIa.9 However, CD34 can be positive in a subset of CFHs, with a considerable subset showing peripheral CD34 positivity and a smaller subset showing central CD34 the positivity.10 This suggests that CD34 cannot be the only factor differentiating these 2 lesions in making a proper dermatopathologic diagnosis.

Solitary fibrous tumor (SFT) is a rare mesenchymal tumor that can occur anywhere on the body and typically manifests as a deep, painless, enlarging mass in adults aged 50 to 60 years.11 On histologic examination, SFT consists of randomly arranged cells with a spindle or ovoid shape within a collagenous stroma intermixed with blood vessels with a characteristic staghorn shape (Figure 3).11 Low-grade SFT shows a patternless arrangement with spindle cells, a low number of mitotic figures, and vessels with a staghorn appearance compared to high-grade SFT, which shows hypercellularity with nuclear pleomorphism and a high number of mitotic figures.11 Solitary fibrous tumors are positive for CD34 and STAT-6 and negative for CD31 and typically demonstrate NGFI-A binding protein 2 (NAB2)—signal transducer and activator of transcription 6 (STAT 6) gene fusion.11

Spindle-cell lipomas are rare, benign, slow-growing, lipomatous tumors that typically manifest in men aged 40 to 70 years.12 These lesions originate most frequently in the subcutaneous tissue of the upper back, posterior neck, and shoulders. The histologic growth pattern of spindle-cell lipomas can mimic other spindle-cell and myxoid tumors, which is why cytogenetic analysis is crucial for differentiating these lesions. On histologic examination, spindle-cell lipomas exhibit a mixture of mature adipocytes, uniform spindle cells, and collagen bundles (eFigure). Spindle-cell lipoma stains positive for CD34 but negative for S100.13 In addition, spindle-cell lipomas tend to show structural rearrangements (mainly deletions) of the long arm of chromosome 13 or even losses of whole chromosome 13, which contains the retinoblastoma (RB1) gene.13

Pleomorphic dermal sarcoma is a rare mesenchymal tumor that can appear clinically and histologically similar to atypical fibroxanthoma.14 This lesion often manifests in elderly patients and is strongly associated with chronic sun exposure.15 Pleomorphic dermal sarcoma is a locally aggressive tumor with metastatic potential to the skin or lymph nodes. On histologic examination, these tumors exhibit pleomorphic atypical epithelioid or spindle cells as well as multinucleated tumor giant cells with possible tumor necrosis, lymphovascular invasion, or perineural infiltration (Figure 4). Pleomorphic dermal sarcoma, typically a diagnosis of exclusion, requires immunohistochemistry to aid in proper identification.16 These lesions stain positive for CD10 and negative for cytokeratins, desmin, HMB45, CD34, p63, p40, SOX10, and S100.15,16

THE DIAGNOSIS: Dermatofibrosarcoma Protuberans
The histologic findings showed fascicular proliferation of relatively monomorphic spindle cells with extensive entrapment of collagen and adipocytes. Immunohistochemical staining showed that the lesional cells were diffusely positive for CD34 and negative for SOX10, S100, desmin, and factor XIIIa. The decision was made to perform cytogenetic testing with fluorescence in situ hybridization to evaluate for the presence of platelet-derived growth factor receptor beta (PDGFB) polypeptide rearrangement, a key biomarker known to be positive in most patients with dermatofibrosarcoma protuberans (DFSP).1 This rearrangement results in overproduction of PDGFB, continuous activation of platelet-derived growth factor receptor beta, cellular proliferation, and tumor formation.2 In our patient, results were positive for the PDGFB polypeptide rearrangement, which confirmed suspected diagnosis of DFSP with fibrous histiocytoma like morphology. The patient was referred for Mohs micrographic surgery for proper management.
Dermatofibrosarcoma protuberans is a rare soft-tissue tumor that involves the dermis, subcutaneous fat, and sometimes muscle and fascia.2 Dermatofibrosarcoma protuberans primarily affects young to middle-aged adults, with a slight predilection for individuals in the third to fifth decades of life.3 Lesions preferentially involve the trunk, particularly the shoulder and chest regions, and manifest as poorly circumscribed, locally aggressive mesenchymal neoplasms with a high local recurrence rate but low metastatic potential.4,5 Clinically, the lesions appear as flesh-colored, rubbery plaques or nodules. A diagnosis of DFSP requires a high index of clinical suspicion, and histologic, immunohistochemical, and molecular testing usually are required for confirmation.
On histopathologic examination, DFSP classically demonstrates uniform, spindle-shaped cells that traditionally are arranged in an intersecting pattern and primarily are based in the dermis (Figure 1).5 Infiltration into the underlying tissue is a common feature, with neoplastic extensions causing a classic honeycomb pattern6 that also can be seen in diffuse neurofibroma and may cause diagnostic challenges; however, the immunohistology staining of neurofibroma differs from DFSP in that it stains positive for CD34, SOX-100, and S100, while DFSP has strong and diffuse CD34 immunoreactivity with negative immunostaining for SOX10, S100, desmin, and factor XIIIa.2,6

Dermatofibrosarcoma protuberans can cause considerable fat infiltration compared to other soft-tissue neoplasms, making this finding suspicious for—if not characteristic of—DFSP. Collagen trapping also can be observed; however, this is more pathognomonic in cellular fibrous histiocytoma, which is a distinct clinical variant of dermatofibromas. Due to its similarity to other lesions, histopathologic examination along with immunostaining can assist in differentiating and accurately diagnosing DFSP.6
Cellular fibrous histiocytoma (CFH), a distinct clinical variant of dermatofibromas, is a benign tumor of mesenchymal origin that occurs more commonly on the trunk, arms, and legs. On histologic examination, CFH is composed of spindle-shaped cells with variable amounts of eosinophilic cytoplasm and small, oval-shaped eosinophilic nuclei and collagen trapping (Figure 2).7,8 Most CFHs occupy the superficial dermis but can extend into the deep reticular dermis, thus mimicking the honeycomb pattern seen in DFSP. This neoplasm can show a similar architecture to DFSP, which is why further investigation including cytogenetics and immunohistochemical staining can help differentiate the two conditions. Cellular fibrous histiocytoma typically stains negative for CD34 and positive for factor XIIIa.9 However, CD34 can be positive in a subset of CFHs, with a considerable subset showing peripheral CD34 positivity and a smaller subset showing central CD34 the positivity.10 This suggests that CD34 cannot be the only factor differentiating these 2 lesions in making a proper dermatopathologic diagnosis.

Solitary fibrous tumor (SFT) is a rare mesenchymal tumor that can occur anywhere on the body and typically manifests as a deep, painless, enlarging mass in adults aged 50 to 60 years.11 On histologic examination, SFT consists of randomly arranged cells with a spindle or ovoid shape within a collagenous stroma intermixed with blood vessels with a characteristic staghorn shape (Figure 3).11 Low-grade SFT shows a patternless arrangement with spindle cells, a low number of mitotic figures, and vessels with a staghorn appearance compared to high-grade SFT, which shows hypercellularity with nuclear pleomorphism and a high number of mitotic figures.11 Solitary fibrous tumors are positive for CD34 and STAT-6 and negative for CD31 and typically demonstrate NGFI-A binding protein 2 (NAB2)—signal transducer and activator of transcription 6 (STAT 6) gene fusion.11

Spindle-cell lipomas are rare, benign, slow-growing, lipomatous tumors that typically manifest in men aged 40 to 70 years.12 These lesions originate most frequently in the subcutaneous tissue of the upper back, posterior neck, and shoulders. The histologic growth pattern of spindle-cell lipomas can mimic other spindle-cell and myxoid tumors, which is why cytogenetic analysis is crucial for differentiating these lesions. On histologic examination, spindle-cell lipomas exhibit a mixture of mature adipocytes, uniform spindle cells, and collagen bundles (eFigure). Spindle-cell lipoma stains positive for CD34 but negative for S100.13 In addition, spindle-cell lipomas tend to show structural rearrangements (mainly deletions) of the long arm of chromosome 13 or even losses of whole chromosome 13, which contains the retinoblastoma (RB1) gene.13

Pleomorphic dermal sarcoma is a rare mesenchymal tumor that can appear clinically and histologically similar to atypical fibroxanthoma.14 This lesion often manifests in elderly patients and is strongly associated with chronic sun exposure.15 Pleomorphic dermal sarcoma is a locally aggressive tumor with metastatic potential to the skin or lymph nodes. On histologic examination, these tumors exhibit pleomorphic atypical epithelioid or spindle cells as well as multinucleated tumor giant cells with possible tumor necrosis, lymphovascular invasion, or perineural infiltration (Figure 4). Pleomorphic dermal sarcoma, typically a diagnosis of exclusion, requires immunohistochemistry to aid in proper identification.16 These lesions stain positive for CD10 and negative for cytokeratins, desmin, HMB45, CD34, p63, p40, SOX10, and S100.15,16

- Ugurel S, Kortmann R, Mohr P, et al. S1 guidelines for dermatofibrosarcoma protuberans (DFSP)—update 2018. J Dtsch Dermatol Ges. 2019;17:663-668. doi:10.1111/ddg.13849
- Brooks J, Ramsey ML. Dermatofibrosarcoma protuberans. StatPearls Publishing; 2024. Updated April 18, 2024. Accessed April 30, 2025.
- Bowne WB, Antonescu CR, Leung DH, et al. Dermatofibrosarcoma protuberans: a clinicopathologic analysis of patients treated and followed at a single institution. Cancer. 2000;88:2711-2720.
- Lim SX, Ramaiya A, Levell NJ, et al. Review of dermatofibrosarcoma protuberans. Clin Exp Dermatol. 2022;48:297-302. doi:10.1093/ced/llac111
- Trinidad CM, Wangsiricharoen S, Prieto VG, et al. Rare variants of dermatofibrosarcoma protuberans: clinical, histologic, and molecular features and diagnostic pitfalls. Dermatopathology. 2023;10:54-62. doi:10.3390/dermatopathology10010008
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752. doi:10.3390/jcm9061752
- Tsunoda K, Oikawa H, Maeda F, et al. A case of cellular fibrous histiocytoma on the right elbow with repeated relapse within a short period. Case Rep Dermatol. 2015;7:10–16. https://doi.org/10.1159/000371790
- Calonje E, Mentzel T, Fletcher CD. Cellular benign fibrous histiocytoma. Clinicopathologic analysis of 74 cases of a distinctive variant of cutaneous fibrous histiocytoma with frequent recurrence. Am J Surg Pathol. 1994;18:668-676.
- Goldblum JR, Tuthill RJ. CD34 and factor-XIIIa immunoreactivity in dermatofibrosarcoma protuberans and dermatofibroma. Am J Dermatopathology. 1997;19:147-153. doi:10.1097/00000372-199704000-00008
- Volpicelli ER, Fletcher CD. Desmin and CD34 positivity in cellular fibrous histiocytoma: an immunohistochemical analysis of 100 cases. J Cutan Pathol. 2012;39:747-752. doi:10.1111/j.1600-0560.2012.01944.x
- Martin-Broto J, Mondaza-Hernandez JL, Moura DS, et al. A comprehensive review on solitary fibrous tumor: new insights for new horizons. Cancers (Basel). 2021;13:2913. doi:10.3390/cancers13122913
- Machol JA, Cusic JG, O’Connor EA, et al. Spindle cell lipoma of the neck: review of the literature and case report. Plast Reconstr Surg Glob Open. 2015;3:E550. doi:10.1097/GOX.0000000000000405
- Domanski HA, Carlén B, Jonsson K, et al. Distinct cytologic features of spindle cell lipoma. a cytologic-histologic study with clinical, radiologic, electron microscopic, and cytogenetic correlations. Cancer. 2001;93:381-389. doi:10.1002/cncr.10142
- Devine RL, Cameron A, Holden AM, et al. The pleomorphic dermal sarcoma: its management, follow-up and the need for more guidance. Adv Oral Maxillofac Surg. 2021;2:100046. doi:10.1016 /j.adoms.2021.100046
- Seretis K, Klaroudas A, Galani V, et al. Pleomorphic dermal sarcoma: it might be rare but it exists [published online August 4, 2023]. J Surg Case Rep. doi:10.1093/jscr/rjad374
- Miller K, Goodlad JR, Brenn T. Pleomorphic dermal sarcoma. Am J Surg Pathol. 2012;36:1317-1326. doi:10.1097/pas.0b013e31825359e1
- Ugurel S, Kortmann R, Mohr P, et al. S1 guidelines for dermatofibrosarcoma protuberans (DFSP)—update 2018. J Dtsch Dermatol Ges. 2019;17:663-668. doi:10.1111/ddg.13849
- Brooks J, Ramsey ML. Dermatofibrosarcoma protuberans. StatPearls Publishing; 2024. Updated April 18, 2024. Accessed April 30, 2025.
- Bowne WB, Antonescu CR, Leung DH, et al. Dermatofibrosarcoma protuberans: a clinicopathologic analysis of patients treated and followed at a single institution. Cancer. 2000;88:2711-2720.
- Lim SX, Ramaiya A, Levell NJ, et al. Review of dermatofibrosarcoma protuberans. Clin Exp Dermatol. 2022;48:297-302. doi:10.1093/ced/llac111
- Trinidad CM, Wangsiricharoen S, Prieto VG, et al. Rare variants of dermatofibrosarcoma protuberans: clinical, histologic, and molecular features and diagnostic pitfalls. Dermatopathology. 2023;10:54-62. doi:10.3390/dermatopathology10010008
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752. doi:10.3390/jcm9061752
- Tsunoda K, Oikawa H, Maeda F, et al. A case of cellular fibrous histiocytoma on the right elbow with repeated relapse within a short period. Case Rep Dermatol. 2015;7:10–16. https://doi.org/10.1159/000371790
- Calonje E, Mentzel T, Fletcher CD. Cellular benign fibrous histiocytoma. Clinicopathologic analysis of 74 cases of a distinctive variant of cutaneous fibrous histiocytoma with frequent recurrence. Am J Surg Pathol. 1994;18:668-676.
- Goldblum JR, Tuthill RJ. CD34 and factor-XIIIa immunoreactivity in dermatofibrosarcoma protuberans and dermatofibroma. Am J Dermatopathology. 1997;19:147-153. doi:10.1097/00000372-199704000-00008
- Volpicelli ER, Fletcher CD. Desmin and CD34 positivity in cellular fibrous histiocytoma: an immunohistochemical analysis of 100 cases. J Cutan Pathol. 2012;39:747-752. doi:10.1111/j.1600-0560.2012.01944.x
- Martin-Broto J, Mondaza-Hernandez JL, Moura DS, et al. A comprehensive review on solitary fibrous tumor: new insights for new horizons. Cancers (Basel). 2021;13:2913. doi:10.3390/cancers13122913
- Machol JA, Cusic JG, O’Connor EA, et al. Spindle cell lipoma of the neck: review of the literature and case report. Plast Reconstr Surg Glob Open. 2015;3:E550. doi:10.1097/GOX.0000000000000405
- Domanski HA, Carlén B, Jonsson K, et al. Distinct cytologic features of spindle cell lipoma. a cytologic-histologic study with clinical, radiologic, electron microscopic, and cytogenetic correlations. Cancer. 2001;93:381-389. doi:10.1002/cncr.10142
- Devine RL, Cameron A, Holden AM, et al. The pleomorphic dermal sarcoma: its management, follow-up and the need for more guidance. Adv Oral Maxillofac Surg. 2021;2:100046. doi:10.1016 /j.adoms.2021.100046
- Seretis K, Klaroudas A, Galani V, et al. Pleomorphic dermal sarcoma: it might be rare but it exists [published online August 4, 2023]. J Surg Case Rep. doi:10.1093/jscr/rjad374
- Miller K, Goodlad JR, Brenn T. Pleomorphic dermal sarcoma. Am J Surg Pathol. 2012;36:1317-1326. doi:10.1097/pas.0b013e31825359e1
Painful Flesh-Colored Nodule on the Shoulder
Painful Flesh-Colored Nodule on the Shoulder
A 26-year-old man with no notable medical history presented to the dermatology clinic with an inconspicuous, painful, raised lesion on the right posterior shoulder of 6 months’ duration. The patient reported that the lesion was tender to light palpation and bothersome in his daily activities. Physical examination revealed a firm, flesh-colored, 1.8-cm nodule with no erythema or pigmentation on the right shoulder. An elliptical excisional biopsy was performed and submitted for histologic evaluation.