User login
Pyogenic Hepatic Abscess in an Immunocompetent Patient With Poor Oral Health and COVID-19 Infection
Pyogenic hepatic abscess (PHA) is a collection of pus in the liver caused by bacterial infection of the liver parenchyma. This potentially life-threatening condition has a mortality rate reported to be as high as 47%.1 The incidence of PHA is reported to be 2.3 per 100,000 individuals and is more common in immunosuppressed individuals and those with diabetes mellitus, cancer, and liver transplant.2,3 PHA infections are usually polymicrobial and most commonly include enteric organisms like Escherichia coli and Klebsiella pneumoniae.4
We present a rare cause of PHA with Fusobacterium nucleatum (F nucleatum) in an immunocompetent patient with poor oral health, history of diverticulitis, and recent COVID-19 infection whose only symptoms were chest pain and a 4-week history of fever and malaise.
Case Presentation
A 52-year-old man initially presented to the C.W. Bill Young Veterans Affairs Medical Center (CWBYVAMC) emergency department in Bay Pines, Florida, for fever, malaise, and right-sided chest pain on inspiration. The fever and malaise began while he was on vacation 4 weeks prior. He originally presented to an outside hospital where he tested positive for COVID-19 and was recommended ibuprofen and rest. His symptoms did not improve, and he returned a second time to the outside hospital 2 weeks later and was diagnosed with pneumonia and placed on outpatient antibiotics. The patient subsequently returned to CWBYVAMC 2 weeks after starting antibiotics when he began to develop right-sided inspiratory chest pain. He reported no other recent travel and no abdominal pain. The patient’s history was significant for diverticulitis 2 years before. A colonoscopy was performed during that time and showed no masses.
On presentation, the patient was febrile with a temperature of 100.8 °F; otherwise, his vital signs were stable. Physical examinations, including abdominal, respiratory, and cardiovascular, were unremarkable. The initial laboratory workup revealed a white blood cell (WBC) count of 18.7 K/μL (reference range, 5-10 K/μL) and microcytic anemia with a hemoglobin level of 8.8 g/dL. The comprehensive metabolic panel revealed normal aspartate transaminase, alanine transaminase, and total bilirubin levels and elevated alkaline phosphatase of 215 U/L (reference range, 44-147 U/L), revealing possible mild intrahepatic cholestasis. Urinalysis showed trace proteinuria and urobilinogen. Coagulation studies showed elevated D-dimer and procalcitonin levels at 1.9 ng/mL (reference range, < 0.1 ng/mL) and 1.21 ng/mL (reference range, < 0.5 ng/mL), respectively, with normal prothrombin and partial thromboplastin times. The patient had a normal troponin, fecal, and blood culture; entamoeba serology was negative.
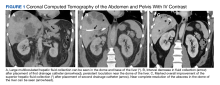
A computed tomograph (CT) angiography of the chest was performed to rule out pulmonary embolism, revealing liver lesions suspicious for abscess or metastatic disease. Minimal pleural effusion was detected bilaterally. A subsequent CT
Following the procedure, the patient developed shaking chills, hypertension, fever, and acute hypoxic respiratory failure. He improved with oxygen and was transferred to the intensive care unit (ICU) where he had an increase in temperature and became septic without shock. A repeat blood culture was negative. An echocardiogram revealed no vegetation. Vancomycin was added for empiric coverage of potentially resistant organisms. The patient clinically improved and was able to leave the ICU 2 days later on hospital day 4.
The patient’s renal function worsened on day 5, and piperacillin-tazobactam and vancomycin were discontinued due to possible acute interstitial nephritis and renal toxicity. He started cefepime and continued metronidazole, and his renal function returned to normal 2 days later. Vancomycin was then re-administered. The results of the culture taken from the abscess came back positive for monomicrobial growth of F nucleatum on hospital day 9.
Due to the patient’s persisting fever and WBC count, a repeat CT of the abdomen on hospital day 10 revealed a partial decrease in the abscess with a persistent collection superior to the location of the initial pigtail catheter placement. A second pigtail catheter was then placed near the dome of the liver 1 day later on hospital day 11. Following the procedure, the patient improved significantly. The repeat CT after 1 week showed marked overall resolution of the abscess, and the repeat culture of the abscess did not reveal any organism growth. Vancomycin was discontinued on day 19, and the drains were removed on hospital day 20. He was discharged home in stable condition on metronidazole and cefdinir for 21 days with follow-up appointments for CT of the abdomen and with primary care, infectious disease, and a dental specialist.
Discussion
F nucleatum is a gram-negative, nonmotile, spindle-shaped rod found in dental plaques.5 The incidence of F nucleatum bacteremia is 0.34 per 100,000 people and increases with age, with the median age being 53.5 years.6 Although our patient did not present with F nucleatum bacteremia, it is possible that bacteremia was present before hospitalization but resolved by the time the sample was drawn for culture. F nucleatum bacteremia can lead to a variety of presentations. The most common primary diagnoses are intra-abdominal infections (eg, PHA, respiratory tract infections, and hematological disorders).1,6
PHA Presentation
The most common presenting symptoms of PHA are fever (88%), abdominal pain (79%), and vomiting (50%).4 The patient’s presentation of inspiratory right-sided chest pain is likely due to irritation of the diaphragmatic pleura of the right lung secondary to the abscess formation. The patient did not experience abdominal pain throughout the course of this disease or on palpation of his right upper quadrant. To our knowledge, this is the only case of PHA in the literature of a patient with inspiratory chest pain without respiratory infection, abdominal pain, and cardiac abnormalities. There was no radiologic evidence or signs of hypoxia on admission to CWBYVAMC, which makes respiratory infection an unlikely cause of the chest pain. Moreover, the patient presented with new-onset chest pain 2 weeks after the diagnosis of pneumonia.
Common laboratory findings of PHA include transaminitis, leukocytosis, and bilirubinemia.4 Of note, increased procalcitonin has also been associated with PHA and extreme elevation (> 200 μg/L) may be a useful biomarker to identify F nucleatum infections before the presence of leukocytosis.3 CT of PHA usually reveals right lobe involvement, and F nucleatum infection usually demonstrates multiple abscesses.4,7
Contributing Factors in F nucleatum PHA
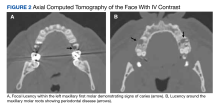
F nucleatum is associated with several oral diseases, such as periodontitis and gingivitis.8 It is important to do an oral inspection on patients with F nucleatum infections because it can spread from oral cavities to different body parts.
F nucleatum is also found in the gut.9 Any disease that can cause a break in the gastrointestinal mucosa may result in F nucleatum bacteremia and PHA. This may be why F nucleatum has been associated with a variety of different diseases, such as diverticulitis, inflammatory bowel disease, appendicitis, and colorectal cancer.10,11 Our patient had a history of diverticulosis with diverticulitis. Bawa and colleagues described a patient with recurrent diverticulitis who developed F nucleatum bacteremia and PHA.11 Our patient did not have any signs of diverticulitis.
Our patient’s COVID-19 infection also had a role in delaying the appropriate treatment of PHA. Without any symptoms of PHA, a diagnosis is difficult in a patient with a positive COVID-19 test, and treatment was delayed 1 month. Moreover, COVID-19 has been reported to delay the diagnosis of PHA even in the absence of a positive COVID-19 test. Collins and Diamond presented a patient during the COVID-19 pandemic who developed a periodontal abscess, which resulted in F nucleatum bacteremia and PHA due to delayed hospital presentation after the patient’s practitioners recommended self-isolation, despite a negative COVID-19 test.12 This highlights the impact that COVID-19 may have on the timely diagnosis and treatment of patients with PHA.
Malignancy has been associated with F nucleatum bacteremia.1,13 Possibly the association is due to gastrointestinal mucosa malignancy’s ability to cause micro-abrasions, resulting in F nucleatum bacteremia.10 Additionally, F nucleatum may promote the development of colorectal neoplasms.8 Due to this association, screening for colorectal cancer in patients with F nucleatum infection is important. In our patient, a colonoscopy was performed during the patient’s hospitalization for diverticulitis 2 years prior. No signs of colorectal neoplasm were noted
Conclusions
PHA due to F nucleatum is a rare but potentially life-threatening condition that must be diagnosed and treated promptly. It usually presents with fever, abdominal pain, and vomiting but can present with chest pain in the absence of a respiratory infection, cardiac abnormalities, and abdominal pain, as in our patient. A wide spectrum of infections can occur with F nucleatum, including PHA.
Suspicion for infection with this organism should be kept high in middle-aged and older individuals who present with an indolent disease course and have risk factors, such as poor oral health and comorbidities. Suspicion should be kept high even in the event of COVID-19 infection, especially in individuals with prolonged fever without other signs indicating respiratory infection. We believe that the most likely causes of this patient’s infection were his dental caries and periodontal disease. The timing of his symptoms is not consistent with his previous episode of diverticulitis. Due to the mortality of PHA, diagnosis and treatment must be prompt. Initial treatment with drainage and empiric anaerobic coverage is recommended, followed by a tailored antibiotic regiment if indicated by culture, and further drainage if suggested by imaging.
1. Yang CC, Ye JJ, Hsu PC, et al. Characteristics and outcomes of Fusobacterium nucleatum bacteremia—a 6-year experience at a tertiary care hospital in northern Taiwan. Diagn Microbiol Infect Dis. 2011;70(2):167-174. doi:10.1016/j.diagmicrobio.2010.12.017
2. Kaplan GG, Gregson DB, Laupland KB. Population-based study of the epidemiology of and the risk factors for pyogenic liver abscess. Clin Gastroenterol Hepatol. 2004;2(11):1032-1038. doi:10.1016/s1542-3565(04)00459-8
3. Cao SA, Hinchey S. Identification and management of fusobacterium nucleatum liver abscess and bacteremia in a young healthy man. Cureus. 2020;12(12):e12303. doi:10.7759/cureus.12303
4. Abbas MT, Khan FY, Muhsin SA, Al-Dehwe B, Abukamar M, Elzouki AN. Epidemiology, clinical features and outcome of liver abscess: a single reference center experience in Qatar. Oman Med J. 2014;29(4):260-263. doi:10.5001/omj.2014.69
5. Bolstad AI, Jensen HB, Bakken V. Taxonomy, biology, and periodontal aspects of Fusobacterium nucleatum. Clin Microbiol Rev. 1996;9(1):55-71. doi:10.1128/CMR.9.1.55
6. Afra K, Laupland K, Leal J, Lloyd T, Gregson D. Incidence, risk factors, and outcomes of Fusobacterium species bacteremia. BMC Infect Dis. 2013;13:264. doi:10.1186/1471-2334-13-264
7. Crippin JS, Wang KK. An unrecognized etiology for pyogenic hepatic abscesses in normal hosts: dental disease. Am J Gastroenterol. 1992;87(12):1740-1743.
8. Shang FM, Liu HL. Fusobacterium nucleatum and colorectal cancer: a review. World J Gastrointest Oncol. 2018;10(3):71-81. doi:10.4251/wjgo.v10.i3.71
9. Allen-Vercoe E, Strauss J, Chadee K. Fusobacterium nucleatum: an emerging gut pathogen? Gut Microbes. 2011;2(5):294-298. doi:10.4161/gmic.2.5.18603
10. Han YW. Fusobacterium nucleatum: a commensal-turned pathogen. Curr Opin Microbiol. 2015;23:141-147. doi:10.1016/j.mib.2014.11.013
11. Bawa A, Kainat A, Raza H, George TB, Omer H, Pillai AC. Fusobacterium bacteremia causing hepatic abscess in a patient with diverticulitis. Cureus. 2022;14(7):e26938. doi:10.7759/cureus.26938
12. Collins L, Diamond T. Fusobacterium nucleatum causing a pyogenic liver abscess: a rare complication of periodontal disease that occurred during the COVID-19 pandemic. BMJ Case Rep. 2021;14(1):e240080. doi:10.1136/bcr-2020-240080
13. Nohrstrom E, Mattila T, Pettila V, et al. Clinical spectrum of bacteraemic Fusobacterium infections: from septic shock to nosocomial bacteraemia. Scand J Infect Dis. 2011;43(6-7):463-470. doi:10.3109/00365548.2011.565071
Pyogenic hepatic abscess (PHA) is a collection of pus in the liver caused by bacterial infection of the liver parenchyma. This potentially life-threatening condition has a mortality rate reported to be as high as 47%.1 The incidence of PHA is reported to be 2.3 per 100,000 individuals and is more common in immunosuppressed individuals and those with diabetes mellitus, cancer, and liver transplant.2,3 PHA infections are usually polymicrobial and most commonly include enteric organisms like Escherichia coli and Klebsiella pneumoniae.4
We present a rare cause of PHA with Fusobacterium nucleatum (F nucleatum) in an immunocompetent patient with poor oral health, history of diverticulitis, and recent COVID-19 infection whose only symptoms were chest pain and a 4-week history of fever and malaise.
Case Presentation
A 52-year-old man initially presented to the C.W. Bill Young Veterans Affairs Medical Center (CWBYVAMC) emergency department in Bay Pines, Florida, for fever, malaise, and right-sided chest pain on inspiration. The fever and malaise began while he was on vacation 4 weeks prior. He originally presented to an outside hospital where he tested positive for COVID-19 and was recommended ibuprofen and rest. His symptoms did not improve, and he returned a second time to the outside hospital 2 weeks later and was diagnosed with pneumonia and placed on outpatient antibiotics. The patient subsequently returned to CWBYVAMC 2 weeks after starting antibiotics when he began to develop right-sided inspiratory chest pain. He reported no other recent travel and no abdominal pain. The patient’s history was significant for diverticulitis 2 years before. A colonoscopy was performed during that time and showed no masses.
On presentation, the patient was febrile with a temperature of 100.8 °F; otherwise, his vital signs were stable. Physical examinations, including abdominal, respiratory, and cardiovascular, were unremarkable. The initial laboratory workup revealed a white blood cell (WBC) count of 18.7 K/μL (reference range, 5-10 K/μL) and microcytic anemia with a hemoglobin level of 8.8 g/dL. The comprehensive metabolic panel revealed normal aspartate transaminase, alanine transaminase, and total bilirubin levels and elevated alkaline phosphatase of 215 U/L (reference range, 44-147 U/L), revealing possible mild intrahepatic cholestasis. Urinalysis showed trace proteinuria and urobilinogen. Coagulation studies showed elevated D-dimer and procalcitonin levels at 1.9 ng/mL (reference range, < 0.1 ng/mL) and 1.21 ng/mL (reference range, < 0.5 ng/mL), respectively, with normal prothrombin and partial thromboplastin times. The patient had a normal troponin, fecal, and blood culture; entamoeba serology was negative.
A computed tomograph (CT) angiography of the chest was performed to rule out pulmonary embolism, revealing liver lesions suspicious for abscess or metastatic disease. Minimal pleural effusion was detected bilaterally. A subsequent CT
Following the procedure, the patient developed shaking chills, hypertension, fever, and acute hypoxic respiratory failure. He improved with oxygen and was transferred to the intensive care unit (ICU) where he had an increase in temperature and became septic without shock. A repeat blood culture was negative. An echocardiogram revealed no vegetation. Vancomycin was added for empiric coverage of potentially resistant organisms. The patient clinically improved and was able to leave the ICU 2 days later on hospital day 4.
The patient’s renal function worsened on day 5, and piperacillin-tazobactam and vancomycin were discontinued due to possible acute interstitial nephritis and renal toxicity. He started cefepime and continued metronidazole, and his renal function returned to normal 2 days later. Vancomycin was then re-administered. The results of the culture taken from the abscess came back positive for monomicrobial growth of F nucleatum on hospital day 9.
Due to the patient’s persisting fever and WBC count, a repeat CT of the abdomen on hospital day 10 revealed a partial decrease in the abscess with a persistent collection superior to the location of the initial pigtail catheter placement. A second pigtail catheter was then placed near the dome of the liver 1 day later on hospital day 11. Following the procedure, the patient improved significantly. The repeat CT after 1 week showed marked overall resolution of the abscess, and the repeat culture of the abscess did not reveal any organism growth. Vancomycin was discontinued on day 19, and the drains were removed on hospital day 20. He was discharged home in stable condition on metronidazole and cefdinir for 21 days with follow-up appointments for CT of the abdomen and with primary care, infectious disease, and a dental specialist.
Discussion
F nucleatum is a gram-negative, nonmotile, spindle-shaped rod found in dental plaques.5 The incidence of F nucleatum bacteremia is 0.34 per 100,000 people and increases with age, with the median age being 53.5 years.6 Although our patient did not present with F nucleatum bacteremia, it is possible that bacteremia was present before hospitalization but resolved by the time the sample was drawn for culture. F nucleatum bacteremia can lead to a variety of presentations. The most common primary diagnoses are intra-abdominal infections (eg, PHA, respiratory tract infections, and hematological disorders).1,6
PHA Presentation
The most common presenting symptoms of PHA are fever (88%), abdominal pain (79%), and vomiting (50%).4 The patient’s presentation of inspiratory right-sided chest pain is likely due to irritation of the diaphragmatic pleura of the right lung secondary to the abscess formation. The patient did not experience abdominal pain throughout the course of this disease or on palpation of his right upper quadrant. To our knowledge, this is the only case of PHA in the literature of a patient with inspiratory chest pain without respiratory infection, abdominal pain, and cardiac abnormalities. There was no radiologic evidence or signs of hypoxia on admission to CWBYVAMC, which makes respiratory infection an unlikely cause of the chest pain. Moreover, the patient presented with new-onset chest pain 2 weeks after the diagnosis of pneumonia.
Common laboratory findings of PHA include transaminitis, leukocytosis, and bilirubinemia.4 Of note, increased procalcitonin has also been associated with PHA and extreme elevation (> 200 μg/L) may be a useful biomarker to identify F nucleatum infections before the presence of leukocytosis.3 CT of PHA usually reveals right lobe involvement, and F nucleatum infection usually demonstrates multiple abscesses.4,7
Contributing Factors in F nucleatum PHA
F nucleatum is associated with several oral diseases, such as periodontitis and gingivitis.8 It is important to do an oral inspection on patients with F nucleatum infections because it can spread from oral cavities to different body parts.
F nucleatum is also found in the gut.9 Any disease that can cause a break in the gastrointestinal mucosa may result in F nucleatum bacteremia and PHA. This may be why F nucleatum has been associated with a variety of different diseases, such as diverticulitis, inflammatory bowel disease, appendicitis, and colorectal cancer.10,11 Our patient had a history of diverticulosis with diverticulitis. Bawa and colleagues described a patient with recurrent diverticulitis who developed F nucleatum bacteremia and PHA.11 Our patient did not have any signs of diverticulitis.
Our patient’s COVID-19 infection also had a role in delaying the appropriate treatment of PHA. Without any symptoms of PHA, a diagnosis is difficult in a patient with a positive COVID-19 test, and treatment was delayed 1 month. Moreover, COVID-19 has been reported to delay the diagnosis of PHA even in the absence of a positive COVID-19 test. Collins and Diamond presented a patient during the COVID-19 pandemic who developed a periodontal abscess, which resulted in F nucleatum bacteremia and PHA due to delayed hospital presentation after the patient’s practitioners recommended self-isolation, despite a negative COVID-19 test.12 This highlights the impact that COVID-19 may have on the timely diagnosis and treatment of patients with PHA.
Malignancy has been associated with F nucleatum bacteremia.1,13 Possibly the association is due to gastrointestinal mucosa malignancy’s ability to cause micro-abrasions, resulting in F nucleatum bacteremia.10 Additionally, F nucleatum may promote the development of colorectal neoplasms.8 Due to this association, screening for colorectal cancer in patients with F nucleatum infection is important. In our patient, a colonoscopy was performed during the patient’s hospitalization for diverticulitis 2 years prior. No signs of colorectal neoplasm were noted
Conclusions
PHA due to F nucleatum is a rare but potentially life-threatening condition that must be diagnosed and treated promptly. It usually presents with fever, abdominal pain, and vomiting but can present with chest pain in the absence of a respiratory infection, cardiac abnormalities, and abdominal pain, as in our patient. A wide spectrum of infections can occur with F nucleatum, including PHA.
Suspicion for infection with this organism should be kept high in middle-aged and older individuals who present with an indolent disease course and have risk factors, such as poor oral health and comorbidities. Suspicion should be kept high even in the event of COVID-19 infection, especially in individuals with prolonged fever without other signs indicating respiratory infection. We believe that the most likely causes of this patient’s infection were his dental caries and periodontal disease. The timing of his symptoms is not consistent with his previous episode of diverticulitis. Due to the mortality of PHA, diagnosis and treatment must be prompt. Initial treatment with drainage and empiric anaerobic coverage is recommended, followed by a tailored antibiotic regiment if indicated by culture, and further drainage if suggested by imaging.
Pyogenic hepatic abscess (PHA) is a collection of pus in the liver caused by bacterial infection of the liver parenchyma. This potentially life-threatening condition has a mortality rate reported to be as high as 47%.1 The incidence of PHA is reported to be 2.3 per 100,000 individuals and is more common in immunosuppressed individuals and those with diabetes mellitus, cancer, and liver transplant.2,3 PHA infections are usually polymicrobial and most commonly include enteric organisms like Escherichia coli and Klebsiella pneumoniae.4
We present a rare cause of PHA with Fusobacterium nucleatum (F nucleatum) in an immunocompetent patient with poor oral health, history of diverticulitis, and recent COVID-19 infection whose only symptoms were chest pain and a 4-week history of fever and malaise.
Case Presentation
A 52-year-old man initially presented to the C.W. Bill Young Veterans Affairs Medical Center (CWBYVAMC) emergency department in Bay Pines, Florida, for fever, malaise, and right-sided chest pain on inspiration. The fever and malaise began while he was on vacation 4 weeks prior. He originally presented to an outside hospital where he tested positive for COVID-19 and was recommended ibuprofen and rest. His symptoms did not improve, and he returned a second time to the outside hospital 2 weeks later and was diagnosed with pneumonia and placed on outpatient antibiotics. The patient subsequently returned to CWBYVAMC 2 weeks after starting antibiotics when he began to develop right-sided inspiratory chest pain. He reported no other recent travel and no abdominal pain. The patient’s history was significant for diverticulitis 2 years before. A colonoscopy was performed during that time and showed no masses.
On presentation, the patient was febrile with a temperature of 100.8 °F; otherwise, his vital signs were stable. Physical examinations, including abdominal, respiratory, and cardiovascular, were unremarkable. The initial laboratory workup revealed a white blood cell (WBC) count of 18.7 K/μL (reference range, 5-10 K/μL) and microcytic anemia with a hemoglobin level of 8.8 g/dL. The comprehensive metabolic panel revealed normal aspartate transaminase, alanine transaminase, and total bilirubin levels and elevated alkaline phosphatase of 215 U/L (reference range, 44-147 U/L), revealing possible mild intrahepatic cholestasis. Urinalysis showed trace proteinuria and urobilinogen. Coagulation studies showed elevated D-dimer and procalcitonin levels at 1.9 ng/mL (reference range, < 0.1 ng/mL) and 1.21 ng/mL (reference range, < 0.5 ng/mL), respectively, with normal prothrombin and partial thromboplastin times. The patient had a normal troponin, fecal, and blood culture; entamoeba serology was negative.
A computed tomograph (CT) angiography of the chest was performed to rule out pulmonary embolism, revealing liver lesions suspicious for abscess or metastatic disease. Minimal pleural effusion was detected bilaterally. A subsequent CT
Following the procedure, the patient developed shaking chills, hypertension, fever, and acute hypoxic respiratory failure. He improved with oxygen and was transferred to the intensive care unit (ICU) where he had an increase in temperature and became septic without shock. A repeat blood culture was negative. An echocardiogram revealed no vegetation. Vancomycin was added for empiric coverage of potentially resistant organisms. The patient clinically improved and was able to leave the ICU 2 days later on hospital day 4.
The patient’s renal function worsened on day 5, and piperacillin-tazobactam and vancomycin were discontinued due to possible acute interstitial nephritis and renal toxicity. He started cefepime and continued metronidazole, and his renal function returned to normal 2 days later. Vancomycin was then re-administered. The results of the culture taken from the abscess came back positive for monomicrobial growth of F nucleatum on hospital day 9.
Due to the patient’s persisting fever and WBC count, a repeat CT of the abdomen on hospital day 10 revealed a partial decrease in the abscess with a persistent collection superior to the location of the initial pigtail catheter placement. A second pigtail catheter was then placed near the dome of the liver 1 day later on hospital day 11. Following the procedure, the patient improved significantly. The repeat CT after 1 week showed marked overall resolution of the abscess, and the repeat culture of the abscess did not reveal any organism growth. Vancomycin was discontinued on day 19, and the drains were removed on hospital day 20. He was discharged home in stable condition on metronidazole and cefdinir for 21 days with follow-up appointments for CT of the abdomen and with primary care, infectious disease, and a dental specialist.
Discussion
F nucleatum is a gram-negative, nonmotile, spindle-shaped rod found in dental plaques.5 The incidence of F nucleatum bacteremia is 0.34 per 100,000 people and increases with age, with the median age being 53.5 years.6 Although our patient did not present with F nucleatum bacteremia, it is possible that bacteremia was present before hospitalization but resolved by the time the sample was drawn for culture. F nucleatum bacteremia can lead to a variety of presentations. The most common primary diagnoses are intra-abdominal infections (eg, PHA, respiratory tract infections, and hematological disorders).1,6
PHA Presentation
The most common presenting symptoms of PHA are fever (88%), abdominal pain (79%), and vomiting (50%).4 The patient’s presentation of inspiratory right-sided chest pain is likely due to irritation of the diaphragmatic pleura of the right lung secondary to the abscess formation. The patient did not experience abdominal pain throughout the course of this disease or on palpation of his right upper quadrant. To our knowledge, this is the only case of PHA in the literature of a patient with inspiratory chest pain without respiratory infection, abdominal pain, and cardiac abnormalities. There was no radiologic evidence or signs of hypoxia on admission to CWBYVAMC, which makes respiratory infection an unlikely cause of the chest pain. Moreover, the patient presented with new-onset chest pain 2 weeks after the diagnosis of pneumonia.
Common laboratory findings of PHA include transaminitis, leukocytosis, and bilirubinemia.4 Of note, increased procalcitonin has also been associated with PHA and extreme elevation (> 200 μg/L) may be a useful biomarker to identify F nucleatum infections before the presence of leukocytosis.3 CT of PHA usually reveals right lobe involvement, and F nucleatum infection usually demonstrates multiple abscesses.4,7
Contributing Factors in F nucleatum PHA
F nucleatum is associated with several oral diseases, such as periodontitis and gingivitis.8 It is important to do an oral inspection on patients with F nucleatum infections because it can spread from oral cavities to different body parts.
F nucleatum is also found in the gut.9 Any disease that can cause a break in the gastrointestinal mucosa may result in F nucleatum bacteremia and PHA. This may be why F nucleatum has been associated with a variety of different diseases, such as diverticulitis, inflammatory bowel disease, appendicitis, and colorectal cancer.10,11 Our patient had a history of diverticulosis with diverticulitis. Bawa and colleagues described a patient with recurrent diverticulitis who developed F nucleatum bacteremia and PHA.11 Our patient did not have any signs of diverticulitis.
Our patient’s COVID-19 infection also had a role in delaying the appropriate treatment of PHA. Without any symptoms of PHA, a diagnosis is difficult in a patient with a positive COVID-19 test, and treatment was delayed 1 month. Moreover, COVID-19 has been reported to delay the diagnosis of PHA even in the absence of a positive COVID-19 test. Collins and Diamond presented a patient during the COVID-19 pandemic who developed a periodontal abscess, which resulted in F nucleatum bacteremia and PHA due to delayed hospital presentation after the patient’s practitioners recommended self-isolation, despite a negative COVID-19 test.12 This highlights the impact that COVID-19 may have on the timely diagnosis and treatment of patients with PHA.
Malignancy has been associated with F nucleatum bacteremia.1,13 Possibly the association is due to gastrointestinal mucosa malignancy’s ability to cause micro-abrasions, resulting in F nucleatum bacteremia.10 Additionally, F nucleatum may promote the development of colorectal neoplasms.8 Due to this association, screening for colorectal cancer in patients with F nucleatum infection is important. In our patient, a colonoscopy was performed during the patient’s hospitalization for diverticulitis 2 years prior. No signs of colorectal neoplasm were noted
Conclusions
PHA due to F nucleatum is a rare but potentially life-threatening condition that must be diagnosed and treated promptly. It usually presents with fever, abdominal pain, and vomiting but can present with chest pain in the absence of a respiratory infection, cardiac abnormalities, and abdominal pain, as in our patient. A wide spectrum of infections can occur with F nucleatum, including PHA.
Suspicion for infection with this organism should be kept high in middle-aged and older individuals who present with an indolent disease course and have risk factors, such as poor oral health and comorbidities. Suspicion should be kept high even in the event of COVID-19 infection, especially in individuals with prolonged fever without other signs indicating respiratory infection. We believe that the most likely causes of this patient’s infection were his dental caries and periodontal disease. The timing of his symptoms is not consistent with his previous episode of diverticulitis. Due to the mortality of PHA, diagnosis and treatment must be prompt. Initial treatment with drainage and empiric anaerobic coverage is recommended, followed by a tailored antibiotic regiment if indicated by culture, and further drainage if suggested by imaging.
1. Yang CC, Ye JJ, Hsu PC, et al. Characteristics and outcomes of Fusobacterium nucleatum bacteremia—a 6-year experience at a tertiary care hospital in northern Taiwan. Diagn Microbiol Infect Dis. 2011;70(2):167-174. doi:10.1016/j.diagmicrobio.2010.12.017
2. Kaplan GG, Gregson DB, Laupland KB. Population-based study of the epidemiology of and the risk factors for pyogenic liver abscess. Clin Gastroenterol Hepatol. 2004;2(11):1032-1038. doi:10.1016/s1542-3565(04)00459-8
3. Cao SA, Hinchey S. Identification and management of fusobacterium nucleatum liver abscess and bacteremia in a young healthy man. Cureus. 2020;12(12):e12303. doi:10.7759/cureus.12303
4. Abbas MT, Khan FY, Muhsin SA, Al-Dehwe B, Abukamar M, Elzouki AN. Epidemiology, clinical features and outcome of liver abscess: a single reference center experience in Qatar. Oman Med J. 2014;29(4):260-263. doi:10.5001/omj.2014.69
5. Bolstad AI, Jensen HB, Bakken V. Taxonomy, biology, and periodontal aspects of Fusobacterium nucleatum. Clin Microbiol Rev. 1996;9(1):55-71. doi:10.1128/CMR.9.1.55
6. Afra K, Laupland K, Leal J, Lloyd T, Gregson D. Incidence, risk factors, and outcomes of Fusobacterium species bacteremia. BMC Infect Dis. 2013;13:264. doi:10.1186/1471-2334-13-264
7. Crippin JS, Wang KK. An unrecognized etiology for pyogenic hepatic abscesses in normal hosts: dental disease. Am J Gastroenterol. 1992;87(12):1740-1743.
8. Shang FM, Liu HL. Fusobacterium nucleatum and colorectal cancer: a review. World J Gastrointest Oncol. 2018;10(3):71-81. doi:10.4251/wjgo.v10.i3.71
9. Allen-Vercoe E, Strauss J, Chadee K. Fusobacterium nucleatum: an emerging gut pathogen? Gut Microbes. 2011;2(5):294-298. doi:10.4161/gmic.2.5.18603
10. Han YW. Fusobacterium nucleatum: a commensal-turned pathogen. Curr Opin Microbiol. 2015;23:141-147. doi:10.1016/j.mib.2014.11.013
11. Bawa A, Kainat A, Raza H, George TB, Omer H, Pillai AC. Fusobacterium bacteremia causing hepatic abscess in a patient with diverticulitis. Cureus. 2022;14(7):e26938. doi:10.7759/cureus.26938
12. Collins L, Diamond T. Fusobacterium nucleatum causing a pyogenic liver abscess: a rare complication of periodontal disease that occurred during the COVID-19 pandemic. BMJ Case Rep. 2021;14(1):e240080. doi:10.1136/bcr-2020-240080
13. Nohrstrom E, Mattila T, Pettila V, et al. Clinical spectrum of bacteraemic Fusobacterium infections: from septic shock to nosocomial bacteraemia. Scand J Infect Dis. 2011;43(6-7):463-470. doi:10.3109/00365548.2011.565071
1. Yang CC, Ye JJ, Hsu PC, et al. Characteristics and outcomes of Fusobacterium nucleatum bacteremia—a 6-year experience at a tertiary care hospital in northern Taiwan. Diagn Microbiol Infect Dis. 2011;70(2):167-174. doi:10.1016/j.diagmicrobio.2010.12.017
2. Kaplan GG, Gregson DB, Laupland KB. Population-based study of the epidemiology of and the risk factors for pyogenic liver abscess. Clin Gastroenterol Hepatol. 2004;2(11):1032-1038. doi:10.1016/s1542-3565(04)00459-8
3. Cao SA, Hinchey S. Identification and management of fusobacterium nucleatum liver abscess and bacteremia in a young healthy man. Cureus. 2020;12(12):e12303. doi:10.7759/cureus.12303
4. Abbas MT, Khan FY, Muhsin SA, Al-Dehwe B, Abukamar M, Elzouki AN. Epidemiology, clinical features and outcome of liver abscess: a single reference center experience in Qatar. Oman Med J. 2014;29(4):260-263. doi:10.5001/omj.2014.69
5. Bolstad AI, Jensen HB, Bakken V. Taxonomy, biology, and periodontal aspects of Fusobacterium nucleatum. Clin Microbiol Rev. 1996;9(1):55-71. doi:10.1128/CMR.9.1.55
6. Afra K, Laupland K, Leal J, Lloyd T, Gregson D. Incidence, risk factors, and outcomes of Fusobacterium species bacteremia. BMC Infect Dis. 2013;13:264. doi:10.1186/1471-2334-13-264
7. Crippin JS, Wang KK. An unrecognized etiology for pyogenic hepatic abscesses in normal hosts: dental disease. Am J Gastroenterol. 1992;87(12):1740-1743.
8. Shang FM, Liu HL. Fusobacterium nucleatum and colorectal cancer: a review. World J Gastrointest Oncol. 2018;10(3):71-81. doi:10.4251/wjgo.v10.i3.71
9. Allen-Vercoe E, Strauss J, Chadee K. Fusobacterium nucleatum: an emerging gut pathogen? Gut Microbes. 2011;2(5):294-298. doi:10.4161/gmic.2.5.18603
10. Han YW. Fusobacterium nucleatum: a commensal-turned pathogen. Curr Opin Microbiol. 2015;23:141-147. doi:10.1016/j.mib.2014.11.013
11. Bawa A, Kainat A, Raza H, George TB, Omer H, Pillai AC. Fusobacterium bacteremia causing hepatic abscess in a patient with diverticulitis. Cureus. 2022;14(7):e26938. doi:10.7759/cureus.26938
12. Collins L, Diamond T. Fusobacterium nucleatum causing a pyogenic liver abscess: a rare complication of periodontal disease that occurred during the COVID-19 pandemic. BMJ Case Rep. 2021;14(1):e240080. doi:10.1136/bcr-2020-240080
13. Nohrstrom E, Mattila T, Pettila V, et al. Clinical spectrum of bacteraemic Fusobacterium infections: from septic shock to nosocomial bacteraemia. Scand J Infect Dis. 2011;43(6-7):463-470. doi:10.3109/00365548.2011.565071