User login
Anticonvulsants for alcohol withdrawal: A review of the evidence
Abrupt cessation or reduction of alcohol consumption may result in alcohol withdrawal syndrome (AWS), which is a medical emergency that can lead to serious complications when unrecognized or treatment is delayed. Symptoms of AWS include tremors, anxiety attacks, cognitive impairment, hallucinations, seizures, delirium tremens (DT), and in severe, untreated cases, death.1 Low to moderate alcohol consumption produces euphoria and excitation via activation of glutamatergic neurotransmission, while higher concentrations produce severe intoxication via GABAergic mechanisms. Acute withdrawal unmasks the hyper-excitatory state of the brain, causing anxiety, agitation, and autonomic activation characteristic of AWS, which typically begins 1 to 3 days after the last drink.2 In the 2012-2013 National Epidemiologic Survey on Alcohol and Related Conditions conducted by the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the 12-month and lifetime prevalences of AWS were 13.9% and 29.1%, respectively.3 Within the general inpatient population, AWS can be present in nearly 30% of patients; if left untreated, AWS has a 15% mortality rate, although when AWS is recognized early and treated, the mortality rate falls dramatically to 2%.4
AWS has most commonly been treated with benzodiazepines.5 However, benzodiazepines have the potential for significant adverse effects when used in older adults and in individuals with complicated medical issues, such as obstructive lung disease and sleep apnea.6 Anticonvulsants have been increasingly used to treat alcohol withdrawal, and their use is supported by several retrospective and prospective studies. In this article, we review the data from randomized control trials (RCTs) on the use of anticonvulsants for the treatment of AWS to see if we can make any recommendations for the use of anticonvulsants for treating AWS.
Our literature search
We searched 5 databases (PubMed, Cochrane, Medline, PsycInfo, and Embase) using the following terms: “alcohol withdrawal syndrome treatment”, “anticonvulsants”, “anti-epileptic”, “gabapentin”, “carbamazepine”, “sodium valproate”, “oxcarbazepine”, “phenytoin”, “levetiracetam”, and “lamotrigine.” We included only double-blind RCTs published between January 1, 1976 and September 30, 2016 in English-language journals or that had an official English translation. There were no restrictions on patient age or location of treatment (inpatient vs outpatient). All RCTs that compared anticonvulsants or a combination of an anticonvulsant and an active pharmacotherapeutic agent with either placebo or gold standard treatment for AWS were included. Database reviews, systematic reviews, and meta-analyses were excluded.
We identified 662 articles that met these criteria. However, most were duplicates, review articles, systematic reviews, meta-analyses, case reports, or open-label or non-randomized trials. Only 16 articles met our inclusion criteria. In the following sections, we discuss these 16 studies by medication type and in chronological order.
Gabapentin
The characteristics of the gabapentin studies included in this review are summarized in Table 1.7-13
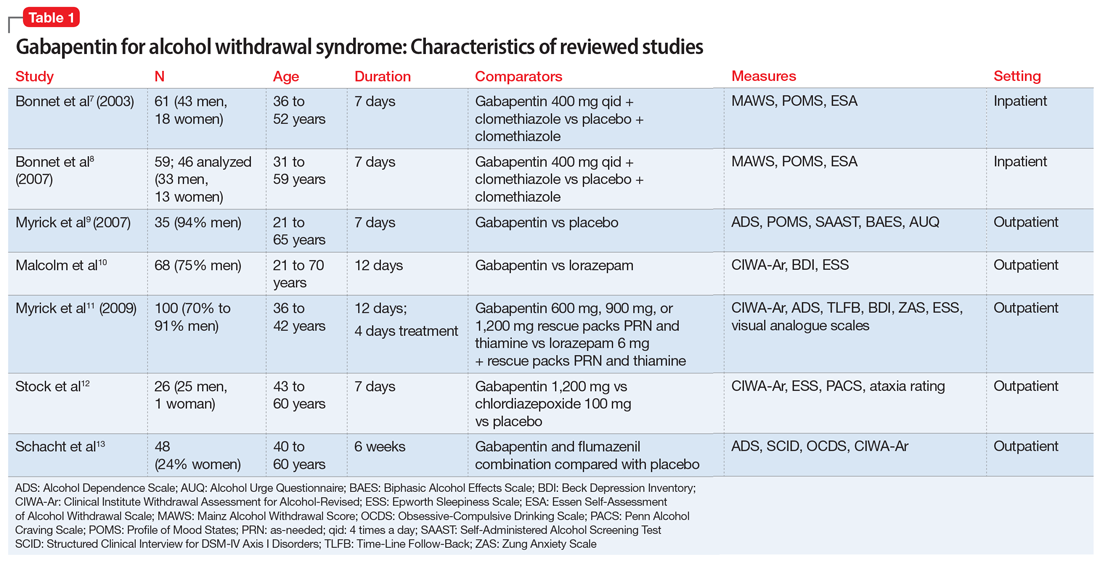
Bonnet et al7 (2003) examined 61 adults who met the clinical criteria for alcohol dependence and displayed moderate or severe AWS according to their Mainz Alcohol Withdrawal Score (MAWS ≥4). They were randomized to receive placebo or gabapentin, 400 mg 4 times a day, along with clomethiazole. The attrition rate was not significantly different between the 2 groups (P = .66). The difference in the number of clomethiazole capsules taken during the first 24 hours between the groups was small and not significant (P = .96). Analysis of MAWS over time revealed no significant main effect for group (P = .26) and a significant effect for the time variable (P < .001). The interaction between group and time was not significant (P =.4). This means that there was a significant decrease in MAWS from baseline over 48 hours, and this decrease in MAWS was considered equal for both study groups. Adverse clinical events were observed in both groups, and there was no significant difference (P = .74) between the groups. Nausea and ataxia, which are specific to gabapentin, were observed more frequently in this group.
Conclusion: The authors concluded that gabapentin, 400 mg 4 times a day, is no better than placebo in reducing the amount of clomethiazole required to treat acute AWS.7
Continue to: Bonnet et al
Bonnet et al8 (2007) also conducted a study examining 59 patients with alcohol dependence who displayed moderate or severe AWS. Participants received placebo or gabapentin, 400 mg, and a rescue medication, clomethiazole, if needed. Subsequently, a capsule of study medication was administered every 6 hours for 2 days and then tapered. During the study, mood was measured by Profile of Mood States (POMS), and subjective complaints of withdrawal were measured using the Essen Self-Assessment of Alcohol Withdrawal Scale (ESA). Of the 59 patients, only 46 were analyzed; 5 patients dropped out, and 8 patients were missing data. Compared with the placebo group, the gabapentin group displayed less dejection, fatigue, and anger, and more vigor. Analysis of variance (ANOVA) measures revealed significant overall changes over time on all 4 scales (all P < .001). A significant (F = 3.62, df 2;43, P = .035) group × time interaction resulted exclusively for vigor. Analysis was repeated using rank-transformed data, resulting in a significant (P = .046) interaction effect. The significant increase in vigor was not apparent after tapering off gabapentin, which suggests gabapentin has a reversible effect on vigor. There was a significant (P < .001) overall decline of subjective withdrawal symptoms complaints, but no group × time interaction (P = .62). Analysis of 11 patients with comorbid mild depression revealed no significant time × group interaction for dejection, fatigue, anger, or subjective withdrawal (all P > .05). However, for vigor, the group × time interaction was significant (P = .022). Throughout the treatment, vigor scores of those mild depressive patients who received gabapentin increased to a level comparable to that of patients without a mood disorder.
Conclusion: The authors authors concluded that gabapentin was markedly more efficacious in improving vigor in the small subgroup of patients with mild depression.8
Myrick et al9 (2007) evaluated the safety and tolerability of gabapentin in patients who abused alcohol, as well as the ability of gabapentin to reduce alcohol craving and consumption. This study included 35 participants randomly assigned to receive gabapentin (n = 17) or placebo (n = 18) for 7 days. All medications were administered in standard gel caps with riboflavin, 25 mg, to assess for compliance via a laboratory-based urinary fluorescence assay. Urine samples were assessed for riboflavin at baseline and Day 6, and a reading of 1,500 ng/mL of riboflavin on Day 6 was interpreted as being compliant. Participants were required to abstain completely from drinking alcohol on Day 6 and the morning of Day 7. At the first session, the following measures were completed: demographic form, alcohol and drug section of the Structured Clinical Interview (SCID), Obsessive-Compulsive Drinking Scale, Self-Administered Alcohol Screening Test (SAAST), and Alcohol Dependence Scale (ADS); there also was collection of a urine sample for detection of abused drugs and a blood sample for liver function and general health screening.
At the second session, patients completed the psychiatric sections of the SCID and the Alcohol Craving Questionnaire, and received a physical exam. To assess the negative clinical effects of gabapentin and alcohol on the CNS, the Epworth Sleepiness Scale (ESS) and POMS were administered at baseline and on Day 6. Also, several other scales were used to identify any impact of gabapentin on acute alcohol effects and craving: the Clinical Institute Withdrawal Assessment of Alcohol, Revised (CIWA-Ar), Biphasic Alcohol Effects Scale (BAES), Subjective High Assessment Scale (SHAS), and Alcohol Urge Questionnaire (AUQ).
Conclusion: Gabapentin was well tolerated, but compared with placebo, gabapentin had no effect on alcohol stimulation (P = .75) or sedation (P = .99) as measured by the BAES. The difference in SHAS scores was also not significant (P = .19). There was also no significant reduction in craving for alcohol as measured on the AUQ scale
Continue to: Malcolm et al
Malcolm et al10 conducted an outpatient treatment study. Patients were men and women age 21 to 70 years from multiple ethnic groups. They were randomized to receive gabapentin or lorazepam; 449 patients were screened and 68 completed the follow-up. Scales used included the CIWA-Ar, Beck Depression Inventory (BDI), and ESS.
Patients receiving lorazepam reported less insomnia and more sleepiness early in treatment than patients receiving gabapentin. However, upon completing treatment and discontinuing medication administration, patients previously treated with lorazepam reported increased insomnia and daytime sleepiness, while patients previously treated with gabapentin continued to report improvements in these self-reported sleep measures. The differences between lorazepam and gabapentin were further evidenced in BDI scores at Day 5, Day 7, and Day 12 in patients who had previously experienced multiple withdrawals. Gabapentin was superior to lorazepam in reducing insomnia as assessed by BDI score, an effect that was sustained throughout the post-treatment week. Participants’ ESS scores indicated less daytime sleepiness in the gabapentin group than in the lorazepam group.
Conclusion: Among patients who abused alcohol and had a history of multiple withdrawals, lorazepam is less effective than gabapentin in reducing insomnia.10 However, this study had several limitations: <25% of individuals who were initially screened were enrolled in the study, and it used subjective tests such as BDI. Objective electrophysiologic measures of sleep and daytime sleepiness would have been very helpful.
Myrick et al11 (2009) also compared gabapentin and lorazepam for treating alcohol withdrawal. One hundred patients were randomized to receive 4 days of fixed-dose taper of gabapentin or lorazepam. Patients could receive 1 of 3 gabapentin dosing regimens (600 mg/d, 900 mg/d, or 1,200 mg/d) for 3 days. Participants who were randomized to receive lorazepam were given 6 mg/d for 3 days and then tapered to 4 mg/d. Also, blinded supplemental medications (rescue packs) were given to each patient on Days 1 to 4 to treat subjective feelings of alcohol withdrawal. All patients also received thiamine for 12 days. Assessment of severity of alcohol withdrawal was measured by the CIWA-Ar. To quantify the severity of alcohol dependence and alcohol use, patients were asked to complete the ADS and Time-Line Follow-Back (TLFB) scales, respectively. Other scales administered included the BDI, Zung Anxiety Scale (ZAS), ESS, and visual analogue scales that assessed craving, ability to perform work, and need for additional medication.
There was a decrease in CIWA-Ar scores over time in all groups. High-dose gabapentin was found to be statistically superior but clinically similar to lorazepam (P = .009). Researchers also found that compared with patients who were treated with lorazepam, patients who were treated with gabapentin experienced reduced craving and anxiety/depressive symptoms, and complained of less subjective discomfort. Compared to patients who were treated with gabapentin, patients who were treated with lorazepam had higher probabilities of drinking on the first day of dose decrease (Day 2) and the second day off medication (Day 6) (P = .0002). During post-treatment, patients who were treated with gabapentin had less probability of drinking during the follow-up post-treatment period (P = .2 for 900 mg/d and P = .3 for 1,200 mg/d) compared with patients who were treated with lorazepam (P = .55).
Continue to: Conclusion
Conclusion: The researchers concluded that gabapentin was well tolerated and effectively diminished the symptoms of alcohol withdrawal, especially at the higher target dose (1,200 mg/d), and that compared with lorazepam, gabapentin decreased the probability of drinking during alcohol withdrawal and in the immediate post-withdrawal week.11
Stock et al12 randomized 26 patients who met criteria for AWS to receive gabapentin or chlordiazepoxide. Gabapentin doses were 1,200 mg/d orally for 3 days, followed by 900 mg/d, 600 mg/d, and 300 mg/d for 1 day each. Chlordiazepoxide doses were 100 mg/d orally for 3 days, followed by 75 mg/d, 50 mg/d, and 25 mg/d for 1 day each. The ESS, Penn Alcohol Craving Scale (PACS), ataxia rating, and CIWA-Ar were administered daily. Thirty-five percent of participants dropped out at the end of the 7-day treatment period. Days 1 to 4 were considered the early treatment period, and Days 5 to 7 were considered the late treatment period. The adjusted mean ESS score did not differ significantly between the randomized groups during the early stage (P = .61) vs the late stage, in which the adjusted mean ESS score was significantly lower with gabapentin compared with chlordiazepoxide (P = .04). The differences in PACS scores between the groups were not statistically significant in either stage (early stage P = .59 vs late stage P = .08), but a trend of lower PACS scores was noted with gabapentin in the later stage. No participant in either group had ataxia during the study. In both groups, CIWA-Ar scores were reduced similarly.
Conclusion: The researchers concluded that gabapentin treatment resulted in a significantly greater reduction in sedation (ESS) and a trend toward reduced alcohol craving (PACS) by the end of treatment compared with chlordiazepoxide treatment.12
Schacht et al13 analyzed functional magnetic resonance imaging data from 48 patients who were alcohol-dependent in a 6-week RCT. Patients were randomized to receive gabapentin up to 1,200 mg/d for 39 days plus flumazenil for 2 days (GP/FMZ group) or an oral placebo and placebo infusions on the same time course. Evaluations included the SCID, ADS, and Obsessive-Compulsive Drinking Scale (OCDS). On Day 1, the CIWA-Ar was administered; it was used to ensure equal distribution of individuals with higher alcohol withdrawal symptoms between medication groups. There were no significant effects of initial alcohol withdrawal symptom status or medication. However, there was a significant interaction between these factors: patients with higher alcohol withdrawal symptoms who received GP/FMZ and those with lower alcohol withdrawal symptoms who received placebo demonstrated greater cue-elicited activation, relative to the other groups, and had less subsequent drinking, which reflected differences in deactivation between alcohol and beverage stimuli, in a cluster that encompassed the dorsal ACC (dACC) (family-wise error-corrected cluster probability of P = .012; 99 voxels; local maxima at [-3, 39, 18] and [6, 33, 9]). In the GP/FMZ group, patients with higher alcohol withdrawal symptoms had significantly greater activation, while in the placebo group, patients with lower alcohol withdrawal symptoms had greater activation.
Conclusion: The researchers concluded that alterations in task-related deactivation of dACC, a component of the default mode network, may predict better alcohol treatment response, while activation of DLPFC, an area associated with selective attention, may predict relapse drinking.13
Continue to: Carbamazepine
Carbamazepine
The characteristics of the carbamazepine studies included in this review are summarized in Table 2.14-19
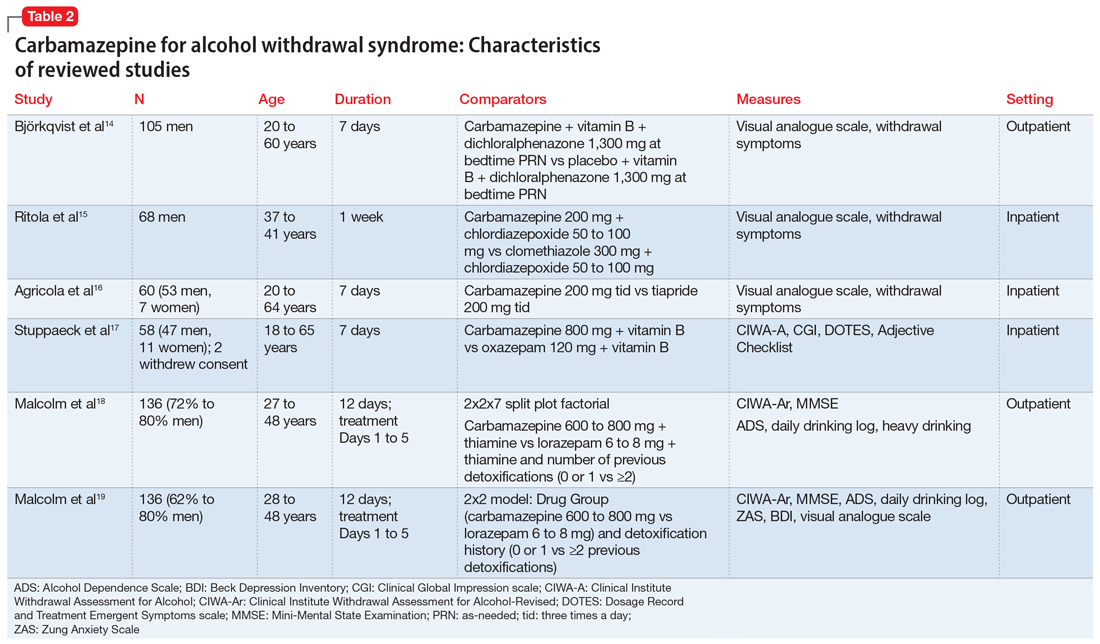
Björkqvist et al14 randomized 105 men with AWS to placebo or carbamazepine. On initial assessment, history, physical examination, relevant labs, and intoxication assessments were recorded. On subsequent visits, nursing staff recorded withdrawal symptoms for patients as 0 to 2 (0 = no specific symptoms, 1 = patient only complained when asked about specific symptoms, 2 = patient complained of withdrawal symptoms without being asked, or if symptoms were severe or obvious to others). Along with the above, vital signs and a visual analogue scale of 0 to 10 (0 = feeling could not be worse, 10 = feeling could not be better) were recorded at each visit. The dose was weight-dependent and administered as follows: on Days 1 and 2, 1+1+2 tablets of carbamazepine, 200 mg, or placebo; Days 3 and 4, 1+1+1 tablets; and Days 5 and 6, 1+0+1 tablets. Every patient received dichloralphenazone as needed. All patients were given vitamin B 3 times a day. Most withdrawal symptoms decreased faster in the carbamazepine group on Day 2 (P = .01) and on Day 4 (P = .1). On the visual analogue scale, scores varied between patients. On Day 1, the mean score was 2.5 times higher in the carbamazepine group compared with the placebo group, and this difference increased to 3 times by Day 7 (P < .01). The patient’s estimated ability to work improved significantly faster in the carbamazepine group than in the placebo group (P < .01).
Conclusion: The authors concluded that compared with placebo, carbamazepine was able to more quickly decrease withdrawal symptoms, especially insomnia and subjective recovery.14
Ritola et al15 randomized 68 hospitalized men with AWS to carbamazepine, 200 mg/d, or clomethiazole, 300 mg/d, for 1 week. The target withdrawal symptoms included gastrointestinal and sleep disturbances; anxiety; aggressiveness; and cardiovascular, depressive, psychotic, and neurologic symptoms. A 4-point rating scale was used for individual symptoms (0 = no symptom, 1 = mild symptom, 2 = moderate symptom, and 3 = severe symptom). On the day of admission (Day 0), all patients were given 50 to 100 mg of chlordiazepoxide IM and 2 tablets and 4 capsules of the trial preparations (either the tablets or capsules were active, and the others were placebos) in the evening. Five patients dropped out of the clomethiazole group and 1 from the carbamazepine group. No significant difference between the 2 treatments were found by the patient, nurse, or physician.
Conclusion: The authors concluded that carbamazepine seemed to be as effective as clomethiazole in the treatment of milder alcohol withdrawal symptoms. Final treatment results were equally good in both groups. Sleep disturbance resolved faster in the carbamazepine group.15
Continue to: Agricola et al
Agricola et al16 compared carbamazepine to tiapride for treatment of acute AWS. In this study, 60 patients were randomized to carbamazepine, 200 mg 3 times a day, or tiapride, 200 mg 3 times a day. All patients were hospitalized with severe AWS preceding DT. The patients were evaluated for withdrawal symptoms (gastrointestinal and cardiovascular symptoms, sleep disturbances, anxiety, aggression, fear, depression, psychotic symptoms, and certain neurologic symptoms). The severity of these symptoms was scored as follows: 0 = no symptoms; 1 = moderate symptoms; and 2 = severe symptoms. At each visit, an overall evaluation of the patient’s clinical condition was made according to a visual analogue scale (100 = worst condition, 0 = best condition). On Day 7, both the doctor and patient evaluated treatment efficacy according to a 4-point scale (1 = no efficacy, 4 = excellent efficacy). There was no significant difference between carbamazepine and tiapride in terms of total symptoms score and visual analogue scale assessment. Carbamazepine was found to have faster relief of symptoms and a significantly greater reduction in symptom score on Day 2 (P < .01). Carbamazepine had a preferential action on fear, nightmares, and hallucinations. The proportion of patients in whom anxiety improved after treatment was 96.2% for carbamazepine and 71.4% for tiapride (P < .05). Aggressiveness and gastrointestinal discomfort resolved faster in the tiapride group. No cases of DT were observed.
Conclusion: The researchers concluded that either carbamazepine or tiapride provides an appropriate alternative in the treatment of inpatients with severe AWS.16
Stuppaeck et al17 compared the efficacy of carbamazepine to oxazepam in 60 inpatients who had symptoms of alcohol withdrawal. Alcohol withdrawal was measured with the CIWA-A, and patients with scores >20 were enrolled in the study. The Clinical Global Impression (CGI) scale and self-rated Adjective Checklist (ACL) were also used. On Days 1 to 3, patients received oxazepam, 120 mg/d, or carbamazepine, 800 mg/d. From Day 4 to 7, doses were decreased to 90 mg/d and 600 mg/d, respectively. After the 7-day trial, all patients were treated with carbamazepine, 200 mg twice a day on Day 8 and 200 mg at night on Day 9. Two patients withdrew consent and 6 dropped out due to adverse effects. During the 7-day trial, when comparing all improvements on CIWA-A, ACL, and CGI scales, carbamazepine was equivalent to oxazepam up to Day 5, and then superior on Days 6 and 7 (P ≤ .05). No decrease in white blood cell count was found in the carbamazepine group.
Conclusion: The authors concluded that carbamazepine is as effective as oxazepam and may be a viable alternative that does not interact with alcohol or cause delirium.17
Malcolm et al18 compared the effects of carbamazepine and lorazepam in patients in an outpatient setting who had single vs multiple previous alcohol withdrawals. The study included 136 patients who satisfied DSM-IV criteria for alcohol dependence and alcohol withdrawal, with a blood alcohol level ≤0.1 g/dL, a Mini-Mental State Examination (MMSE) score ≤26, and a CIWA-Ar score ≤10 on admission. Patients also completed the ADS to quantify the severity of alcohol dependence. Daily drinking was measured by patient report using a daily drinking log and blood alcohol level. Heavy drinking was defined as ≥4 standard drinks per day for women and ≥5 drinks per day for men. On Day 1, patients were randomized to receive carbamazepine, 600 to 800 mg/d,or lorazepam, 6 to 8 mg/d, in divided doses, which was tapered to carbamazepine, 200 mg/d, or lorazepam, 2 mg/d, on Day 5. All patients received thiamine for 12 days. In the immediate post-detoxification period, carbamazepine-treated patients were less likely to relapse, and if they did drink, they drank less than those treated with lorazepam (P = .003). Even in patients who had multiple previous detoxifications, those randomized to carbamazepine drank less than those in lorazepam group (P = .004). Patients in the lorazepam group had significant higher rebound withdrawal symptoms (P = .007).
Continue to: Conclusion
Conclusion: The researchers concluded that carbamazepine and lorazepam were both effective in reducing alcohol withdrawal symptoms. They also concluded that carbamazepine was less likely to cause rebound withdrawal and more likely to reduce post-treatment drinking; among those who did drink, there was less heavy drinking.18
Malcolm et al19 conducted a 5-day double-blind RCT with 136 outpatients who met DSM-IV criteria for alcohol withdrawal. Patients were evaluated by CIWA before getting medications and then daily for 5 days. Patients were randomized to receive carbamazepine, 600 to 800 mg/d on Day 1, 200 mg 3 times a day on Day 2, 200 mg twice a day on Days 3 and 4, and 200 mg once on Day 5. Participants were randomized to receive lorazepam, 6 to 8 mg/d in divided doses on Day 1, 2 mg 3 times a day on Day 2, 2 mg twice a day on Days 3 and 4, and 2 mg once on Day 5. Ability to return to work was self-rated on a 100-mm visual analogue scale, with 0 being “totally unable to return to work’’ and 100 representing “being fully able to return to work.’’ Self-report measures of sleep quality were made using a 100-mm visual analogue scale, with 0 = “the very worst night’s sleep I’ve ever had’’ and 100 = “the very best night’s sleep I’ve ever had.’’ Carbamazepine significantly reduced anxiety (P = .0007). Visual analogue measures of sleep quality indicated a statistically significant main effect of medication on sleep that favored carbamazepine (P = .0186).
Conclusion: The authors concluded that when treating patients with mild to moderate alcohol withdrawal symptoms, carbamazepine was superior to lorazepam in reducing anxiety and improving sleep.19
Sodium valproate
The characteristics of the sodium valproate studies included in this review are summarized in Table 3.20,21
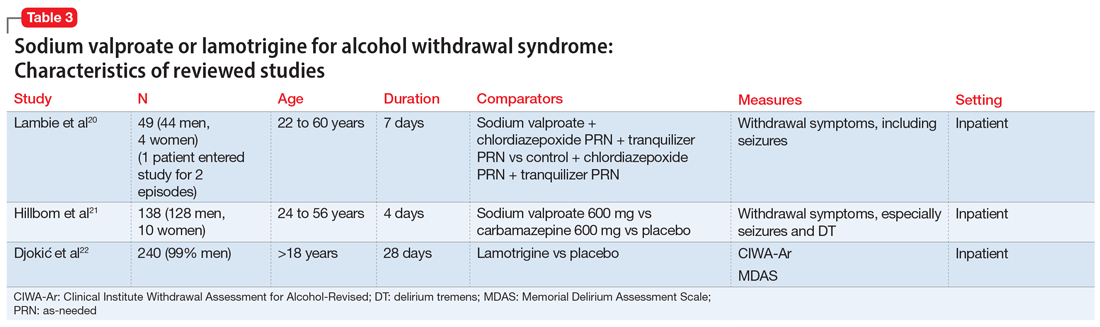
Lambie et al20 evaluated the use of sodium valproate in the treatment of AWS. A total of 49 patients were randomized to a sodium valproate group (n = 22) or a control group (n = 27). All participants were inpatients receiving treatment for alcohol use disorder and substance use disorder for 7 days. Patients in the sodium valproate group received 800 mg every 8 hours for 7 days. Patients were observed daily for occurrence of withdrawal symptoms. Nurses who were blinded to the group assignment graded the degree and severity of symptoms. The trial was initially designed so that chlormethiazole and/or tranquilizers were added to sodium valproate when withdrawal symptoms occurred. However, after treating the first few patients, it became evident that additional medications were not needed. In the treatment group, 13 participants received only sodium valproate, 4 patients needed a tranquilizer, 4 needed chlormethiazole, and 1 needed both. In the control group, 1 received only sodium valproate, 4 received a tranquilizer, 14 received chlormethiazole, and 8 needed both. One patient, who entered the study twice, had a withdrawal seizure when in control group and no seizure on second admission in the sodium valproate group. Physical symptoms disappeared quickly in the sodium valproate group (mean of 2 days vs 2.6 days in the control group). Fourteen patients in the control group received chlormethiazole, compared with only 4 patients in sodium valproate group.
Continue to: Conclusion
Conclusion: The researchers concluded that physical symptoms disappeared quicker in the sodium valproate group than in the control group.20
Hillbom et al21 evaluated the efficacy of sodium valproate vs carbamazepine vs placebo to prevent alcohol withdrawal seizures. A total of 138 participants were studied. Forty-three were assigned to the carbamazepine group, 46 to the sodium valproate group, and 49 to the placebo group. The RCT lasted 4 days. The initial medication doses were 1,200 mg/d. Participants in the carbamazepine group experienced more adverse effects than those in the sodium valproate or placebo groups (P < .001). As a result, approximately one-half of the participants in the carbamazepine group stopped taking the medication. This finding was dependent on the dose of carbamazepine; >800 mg/d resulted in poor tolerance to adverse effects. Seizures occurred among patients in all 3 arms of the study; in the sodium valproate group, 1 participant had a seizure vs 2 participants in the carbamazepine group and 3 in the placebo group. On the other hand, DT occurred only in the sodium valproate and placebo groups.
Conclusion: Researchers concluded that when using sodium valproate or carbamazepine to prevent alcohol withdrawal seizures in an outpatient setting, the adverse effects may outweigh the benefits.21
Lamotrigine
The characteristics of the lamotrigine study included in this review are summarized in Table 3.22
Djokić et al22 evaluated the efficiency of lamotrigine in the treatment of DT. A total of 240 participants who met International Classification of Diseases-10 criteria for DT were randomized to a control group that was treated with anticonvulsants according to an NIAAA protocol (2004), or to an experimental group that was treated with lamotrigine. The CIWA-Ar and the Memorial Delirium Assessment Scale (MDAS) were administered for objective assessment of clinical symptoms, superimposed medical complications, general condition of the patient, adverse effects, and mortality rate. Statistically significant differences between the experimental and control groups were apparent after the third day of therapy, when a drop in the average CIWA-Ar score was observed in the experimental group, while the control group still had high scores (P < .01). After the fifth day of treatment, the differences in scores were more apparent, with the experimental group showing CIWA-Ar scores equal to those of persons with mild/moderate DT, while those in the control group still had high scores. After the tenth day, participants in the experimental group did not have any alcohol withdrawal symptoms, while control group participants were just beginning to get out of life-threatening danger. Death occurred in 4.1% of control group participants and 3.4% of experimental group participants; this difference in mortality rate was not statistically significant.
Continue to: Conclusion
Conclusion: Researchers concluded that lamotrigine is significantly efficacious in the treatment of DT, but does not decrease the mortality rate.22
What to know before you prescribe
AWS is a medical emergency that if left untreated leads to several complications and possibly death. Although benzodiazepines are considered the gold standard for treating AWS, the adverse effects associated with their use advocates for finding alternatives. Anticonvulsants can be an effective alternative for treating AWS. In our literature review, we found 16 double-blind RCTs that used an anticonvulsant medication for the treatment of AWS. Of these, 7 involved gabapentin, 6 involved carbamazepine, 1 involved sodium valproate, 1 involved sodium valproate vs carbamazepine, and 1 involved lamotrigine. Overall, the use of anticonvulsants resulted in significant improvement of mild to moderate symptoms of AWS.
There were more studies of carbamazepine and gabapentin than of other anticonvulsants. All the anticonvulsants offered potential benefits. They decreased the probability of a withdrawal seizure and other complications and effectively reduced alcohol cravings. Anticonvulsants were useful for preventing rebound withdrawal symptoms and reducing post-treatment alcohol consumption, especially in patients who had multiple previous withdrawals. Anticonvulsants were particularly helpful for patients with mood disorders such as depression. In the studies we reviewed, anticonvulsants caused less sedation compared with benzodiazepines, and also decreased the occurrence of relapse.
Dosing recommendations. In the studies included in our review, gabapentin was effective at a dosage of 1,600 mg/d (given as 400 mg 4 times a day). This was tapered as follows: 400 mg 4 times a day on Days 1 to 3, 400 mg 3 times a day on Day 4, 400 mg twice a day on Day 5, and 400 mg once a day on Day 6. Carbamazepine was effective at 600 to 800 mg/d, and was tapered by decreasing by 200 mg as follows: 800 mg/d on Days 1 to 3, 600 mg/d on Day 4, 400 mg on Day 5, and 200 mg/d on Day 6. In the reviewed study, the maximum dose of lamotrigine never exceeded 200 mg/d and was administered for 28 days; the exact dosing and taper plan were not described. The dosing of sodium valproate ranged from 1,200 mg/d to 1600 mg/d for 7 days, followed by decreasing by 200 mg each day. The recommended duration of treatment varied; on average for all anticonvulsants, it was 7 to 12 days, followed by a taper. Carbamazepine was shown to be superior to oxazepam in ameliorating the symptoms of AWS.
Adverse effects. When considering the tolerability, adverse effect profile, duration of action, and effectiveness of the anticonvulsants included in our review, gabapentin appears to be the safest agent to choose. For the other anticonvulsants, the risks might outweigh the benefits. Specifically, in a comparison of sodium valproate and carbamazepine, Hillbom et al21 concluded that in doses >800 mg/d, carbamazepine has potential to cause more adverse effects than benefits. However, Agricola et al16 found that carbamazepine had a preferential action on fear, nightmares, and hallucinations.
Continue to: A few caveats
A few caveats
Our review focused a large collection of data from multiple databases and RCTs only. However, its limitations include:
- there was no measure of heterogeneity
- the studies had short treatment duration
- most studies evaluated predominantly male participants
- some studies were underpowered.
Our review laid a groundwork for future research that includes more well-designed RCTs and/or meta-analyses of recent studies that evaluated the use anticonvulsants for treating AWS.
Bottom Line
Evidence suggests certain anticonvulsants may be an effective alternative to benzodiazepines for the treatment of mild to moderate alcohol withdrawal syndrome. Gabapentin may be the safest anticonvulsant to prescribe. Other anticonvulsants to consider include carbamazepine, sodium valproate, and lamotrigine, but for these agents, the risks might outweigh the benefits.
Related Resources
- Myrick H, Anton RF. Treatment of alcohol withdrawal. Alcohol Health Res World. 1998;22(1):38-43. https://pubs.niaaa.nih.gov/publications/arh22-1/38-43.pdf
- World Health Organization. Management of alcohol withdrawal. Published 2012. https://www.who.int/mental_health/mhgap/evidence/alcohol/q2/en/
Drug Brand Names
Carbamazepine • Tegretol
Gabapentin • Neurontin
Lamotrigine • Lamictal
Levetiracetam • Keppra
Lorazepam • Ativan
Oxcarbazepine • Trileptal
Phenytoin • Dilantin
Sodium valproate • Depakote
Acknowledgments
The authors thank Geetha Manikkara, MD, Madhuri Jakkam Setty, MD, and Elizabeth DeOreo, MD, for their efforts with the systematic review research.
1. Trevisan LA, Boutros N, Petrakis IL, et al. Complications of alcohol withdrawal: pathophysiological insights. Alcohol Health Res World. 1998;22(1):61-66.
2. Borghesani P. Alcohol withdrawal. In: Nordstrom KD, Wilson MP, eds. Quick guide to psychiatric emergencies. Springer: 2018;209-215.
3. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757-766.
4. Ungur LA, Neuner B, John S, et al. Prevention and therapy of alcohol withdrawal on intensive care units: systematic review of controlled trials. Alcohol Clin Exp Res. 2013;37(4):675-686.
5. Sachdeva A, Choudhary M, Chandra M. Alcohol withdrawal syndrome: benzodiazepines and beyond. J Clin Diagn Res. 2015;9(9):VE01-VE07.
6. Ashton H. Toxicity and adverse consequences of benzodiazepine use. Psychiatr Ann. 1995;25:158-165.
7. Bonnet U, Banger M, Leweke FM, et al. Treatment of acute alcohol withdrawal with gabapentin: results from a controlled two-center trial. J Clin Psychopharmacol. 2003;23(5):514-519.
8. Bonnet U, Specka M, Leweke FM, et al. Gabapentin’s acute effect on mood profile--a controlled study on patients with alcohol withdrawal. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(2):434-438.
9. Myrick H, Anton R, Voronin K, et al. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res. 2007;31(2):221-227.
10. Malcolm R, Myrick L, Veatch L, et al. Self-reported sleep, sleepiness, and repeated alcohol withdrawals: a randomized, double blind, controlled comparison of lorazepam vs gabapentin. J Clin Sleep Med. 2007;3(1):24-32.
11. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588.
12. Stock CJ, Carpenter L, Ying J, et al. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother. 2013;47(7-8):961-969.
13. Schacht JP, Anton RF, Randall PK, et al. Effects of a GABA-ergic medication combination and initial alcohol withdrawal severity on cue-elicited brain activation among treatment-seeking alcoholics. Psychopharmacol. 2013;227(4):627-637.
14. Björkqvist SE, Isohanni M, Mäkelä R, et al. Ambulant treatment of alcohol withdrawal symptoms with carbamazepine: a formal multicenter double blind comparison with placebo. Acta Psychiatr Scand. 1976;53(5):333-342.
15. Ritola E, Malinen L. A double-blind comparison of carbamazepine and clomethiazole in the treatment of alcohol withdrawal syndrome. Acta Psychiatr Scand. 1981;64(3):254-259.
16. Agricola R, Mazzarino M, Urani R, et al. Treatment of acute alcohol withdrawal syndrome with carbamazepine: a double-blind comparison with tiapride. J Int Med Res. 1982;10(3):160-165.
17. Stuppaeck CH, Pycha R, Miller C, et al. Carbamazepine versus oxazepam in the treatment of alcohol withdrawal: a double-blind study. Alcohol Alcohol. 1992;27(2):153-158.
18. Malcolm R, Myrick H, Roberts J, et al. The effects of carbamazepine and lorazepam on single vs multiple previous withdrawals in an outpatient randomized trial. J Gen Intern Med. 2002;17(5):349-355.
19. Malcolm R, Myrick H, Roberts J, et al. The differential effects of medications on mood, sleep disturbance, and work ability in outpatient alcohol detoxification. Am J Addict. 2002;11(2):141-150.
20. Lambie D, Johnson R, Vijayasenan M, et al. Sodium valproate in the treatment of the alcohol withdrawal syndrome. Aust N Z J. 1980;14(3):213-215.
21. Hillbom M, Tokola R, Kuusela V, et al. Prevention of alcohol withdrawal seizures with carbamazepine and valproic acid. Alcohol. 1989;6(3):223-226.
22. Djokic
Abrupt cessation or reduction of alcohol consumption may result in alcohol withdrawal syndrome (AWS), which is a medical emergency that can lead to serious complications when unrecognized or treatment is delayed. Symptoms of AWS include tremors, anxiety attacks, cognitive impairment, hallucinations, seizures, delirium tremens (DT), and in severe, untreated cases, death.1 Low to moderate alcohol consumption produces euphoria and excitation via activation of glutamatergic neurotransmission, while higher concentrations produce severe intoxication via GABAergic mechanisms. Acute withdrawal unmasks the hyper-excitatory state of the brain, causing anxiety, agitation, and autonomic activation characteristic of AWS, which typically begins 1 to 3 days after the last drink.2 In the 2012-2013 National Epidemiologic Survey on Alcohol and Related Conditions conducted by the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the 12-month and lifetime prevalences of AWS were 13.9% and 29.1%, respectively.3 Within the general inpatient population, AWS can be present in nearly 30% of patients; if left untreated, AWS has a 15% mortality rate, although when AWS is recognized early and treated, the mortality rate falls dramatically to 2%.4
AWS has most commonly been treated with benzodiazepines.5 However, benzodiazepines have the potential for significant adverse effects when used in older adults and in individuals with complicated medical issues, such as obstructive lung disease and sleep apnea.6 Anticonvulsants have been increasingly used to treat alcohol withdrawal, and their use is supported by several retrospective and prospective studies. In this article, we review the data from randomized control trials (RCTs) on the use of anticonvulsants for the treatment of AWS to see if we can make any recommendations for the use of anticonvulsants for treating AWS.
Our literature search
We searched 5 databases (PubMed, Cochrane, Medline, PsycInfo, and Embase) using the following terms: “alcohol withdrawal syndrome treatment”, “anticonvulsants”, “anti-epileptic”, “gabapentin”, “carbamazepine”, “sodium valproate”, “oxcarbazepine”, “phenytoin”, “levetiracetam”, and “lamotrigine.” We included only double-blind RCTs published between January 1, 1976 and September 30, 2016 in English-language journals or that had an official English translation. There were no restrictions on patient age or location of treatment (inpatient vs outpatient). All RCTs that compared anticonvulsants or a combination of an anticonvulsant and an active pharmacotherapeutic agent with either placebo or gold standard treatment for AWS were included. Database reviews, systematic reviews, and meta-analyses were excluded.
We identified 662 articles that met these criteria. However, most were duplicates, review articles, systematic reviews, meta-analyses, case reports, or open-label or non-randomized trials. Only 16 articles met our inclusion criteria. In the following sections, we discuss these 16 studies by medication type and in chronological order.
Gabapentin
The characteristics of the gabapentin studies included in this review are summarized in Table 1.7-13

Bonnet et al7 (2003) examined 61 adults who met the clinical criteria for alcohol dependence and displayed moderate or severe AWS according to their Mainz Alcohol Withdrawal Score (MAWS ≥4). They were randomized to receive placebo or gabapentin, 400 mg 4 times a day, along with clomethiazole. The attrition rate was not significantly different between the 2 groups (P = .66). The difference in the number of clomethiazole capsules taken during the first 24 hours between the groups was small and not significant (P = .96). Analysis of MAWS over time revealed no significant main effect for group (P = .26) and a significant effect for the time variable (P < .001). The interaction between group and time was not significant (P =.4). This means that there was a significant decrease in MAWS from baseline over 48 hours, and this decrease in MAWS was considered equal for both study groups. Adverse clinical events were observed in both groups, and there was no significant difference (P = .74) between the groups. Nausea and ataxia, which are specific to gabapentin, were observed more frequently in this group.
Conclusion: The authors concluded that gabapentin, 400 mg 4 times a day, is no better than placebo in reducing the amount of clomethiazole required to treat acute AWS.7
Continue to: Bonnet et al
Bonnet et al8 (2007) also conducted a study examining 59 patients with alcohol dependence who displayed moderate or severe AWS. Participants received placebo or gabapentin, 400 mg, and a rescue medication, clomethiazole, if needed. Subsequently, a capsule of study medication was administered every 6 hours for 2 days and then tapered. During the study, mood was measured by Profile of Mood States (POMS), and subjective complaints of withdrawal were measured using the Essen Self-Assessment of Alcohol Withdrawal Scale (ESA). Of the 59 patients, only 46 were analyzed; 5 patients dropped out, and 8 patients were missing data. Compared with the placebo group, the gabapentin group displayed less dejection, fatigue, and anger, and more vigor. Analysis of variance (ANOVA) measures revealed significant overall changes over time on all 4 scales (all P < .001). A significant (F = 3.62, df 2;43, P = .035) group × time interaction resulted exclusively for vigor. Analysis was repeated using rank-transformed data, resulting in a significant (P = .046) interaction effect. The significant increase in vigor was not apparent after tapering off gabapentin, which suggests gabapentin has a reversible effect on vigor. There was a significant (P < .001) overall decline of subjective withdrawal symptoms complaints, but no group × time interaction (P = .62). Analysis of 11 patients with comorbid mild depression revealed no significant time × group interaction for dejection, fatigue, anger, or subjective withdrawal (all P > .05). However, for vigor, the group × time interaction was significant (P = .022). Throughout the treatment, vigor scores of those mild depressive patients who received gabapentin increased to a level comparable to that of patients without a mood disorder.
Conclusion: The authors authors concluded that gabapentin was markedly more efficacious in improving vigor in the small subgroup of patients with mild depression.8
Myrick et al9 (2007) evaluated the safety and tolerability of gabapentin in patients who abused alcohol, as well as the ability of gabapentin to reduce alcohol craving and consumption. This study included 35 participants randomly assigned to receive gabapentin (n = 17) or placebo (n = 18) for 7 days. All medications were administered in standard gel caps with riboflavin, 25 mg, to assess for compliance via a laboratory-based urinary fluorescence assay. Urine samples were assessed for riboflavin at baseline and Day 6, and a reading of 1,500 ng/mL of riboflavin on Day 6 was interpreted as being compliant. Participants were required to abstain completely from drinking alcohol on Day 6 and the morning of Day 7. At the first session, the following measures were completed: demographic form, alcohol and drug section of the Structured Clinical Interview (SCID), Obsessive-Compulsive Drinking Scale, Self-Administered Alcohol Screening Test (SAAST), and Alcohol Dependence Scale (ADS); there also was collection of a urine sample for detection of abused drugs and a blood sample for liver function and general health screening.
At the second session, patients completed the psychiatric sections of the SCID and the Alcohol Craving Questionnaire, and received a physical exam. To assess the negative clinical effects of gabapentin and alcohol on the CNS, the Epworth Sleepiness Scale (ESS) and POMS were administered at baseline and on Day 6. Also, several other scales were used to identify any impact of gabapentin on acute alcohol effects and craving: the Clinical Institute Withdrawal Assessment of Alcohol, Revised (CIWA-Ar), Biphasic Alcohol Effects Scale (BAES), Subjective High Assessment Scale (SHAS), and Alcohol Urge Questionnaire (AUQ).
Conclusion: Gabapentin was well tolerated, but compared with placebo, gabapentin had no effect on alcohol stimulation (P = .75) or sedation (P = .99) as measured by the BAES. The difference in SHAS scores was also not significant (P = .19). There was also no significant reduction in craving for alcohol as measured on the AUQ scale
Continue to: Malcolm et al
Malcolm et al10 conducted an outpatient treatment study. Patients were men and women age 21 to 70 years from multiple ethnic groups. They were randomized to receive gabapentin or lorazepam; 449 patients were screened and 68 completed the follow-up. Scales used included the CIWA-Ar, Beck Depression Inventory (BDI), and ESS.
Patients receiving lorazepam reported less insomnia and more sleepiness early in treatment than patients receiving gabapentin. However, upon completing treatment and discontinuing medication administration, patients previously treated with lorazepam reported increased insomnia and daytime sleepiness, while patients previously treated with gabapentin continued to report improvements in these self-reported sleep measures. The differences between lorazepam and gabapentin were further evidenced in BDI scores at Day 5, Day 7, and Day 12 in patients who had previously experienced multiple withdrawals. Gabapentin was superior to lorazepam in reducing insomnia as assessed by BDI score, an effect that was sustained throughout the post-treatment week. Participants’ ESS scores indicated less daytime sleepiness in the gabapentin group than in the lorazepam group.
Conclusion: Among patients who abused alcohol and had a history of multiple withdrawals, lorazepam is less effective than gabapentin in reducing insomnia.10 However, this study had several limitations: <25% of individuals who were initially screened were enrolled in the study, and it used subjective tests such as BDI. Objective electrophysiologic measures of sleep and daytime sleepiness would have been very helpful.
Myrick et al11 (2009) also compared gabapentin and lorazepam for treating alcohol withdrawal. One hundred patients were randomized to receive 4 days of fixed-dose taper of gabapentin or lorazepam. Patients could receive 1 of 3 gabapentin dosing regimens (600 mg/d, 900 mg/d, or 1,200 mg/d) for 3 days. Participants who were randomized to receive lorazepam were given 6 mg/d for 3 days and then tapered to 4 mg/d. Also, blinded supplemental medications (rescue packs) were given to each patient on Days 1 to 4 to treat subjective feelings of alcohol withdrawal. All patients also received thiamine for 12 days. Assessment of severity of alcohol withdrawal was measured by the CIWA-Ar. To quantify the severity of alcohol dependence and alcohol use, patients were asked to complete the ADS and Time-Line Follow-Back (TLFB) scales, respectively. Other scales administered included the BDI, Zung Anxiety Scale (ZAS), ESS, and visual analogue scales that assessed craving, ability to perform work, and need for additional medication.
There was a decrease in CIWA-Ar scores over time in all groups. High-dose gabapentin was found to be statistically superior but clinically similar to lorazepam (P = .009). Researchers also found that compared with patients who were treated with lorazepam, patients who were treated with gabapentin experienced reduced craving and anxiety/depressive symptoms, and complained of less subjective discomfort. Compared to patients who were treated with gabapentin, patients who were treated with lorazepam had higher probabilities of drinking on the first day of dose decrease (Day 2) and the second day off medication (Day 6) (P = .0002). During post-treatment, patients who were treated with gabapentin had less probability of drinking during the follow-up post-treatment period (P = .2 for 900 mg/d and P = .3 for 1,200 mg/d) compared with patients who were treated with lorazepam (P = .55).
Continue to: Conclusion
Conclusion: The researchers concluded that gabapentin was well tolerated and effectively diminished the symptoms of alcohol withdrawal, especially at the higher target dose (1,200 mg/d), and that compared with lorazepam, gabapentin decreased the probability of drinking during alcohol withdrawal and in the immediate post-withdrawal week.11
Stock et al12 randomized 26 patients who met criteria for AWS to receive gabapentin or chlordiazepoxide. Gabapentin doses were 1,200 mg/d orally for 3 days, followed by 900 mg/d, 600 mg/d, and 300 mg/d for 1 day each. Chlordiazepoxide doses were 100 mg/d orally for 3 days, followed by 75 mg/d, 50 mg/d, and 25 mg/d for 1 day each. The ESS, Penn Alcohol Craving Scale (PACS), ataxia rating, and CIWA-Ar were administered daily. Thirty-five percent of participants dropped out at the end of the 7-day treatment period. Days 1 to 4 were considered the early treatment period, and Days 5 to 7 were considered the late treatment period. The adjusted mean ESS score did not differ significantly between the randomized groups during the early stage (P = .61) vs the late stage, in which the adjusted mean ESS score was significantly lower with gabapentin compared with chlordiazepoxide (P = .04). The differences in PACS scores between the groups were not statistically significant in either stage (early stage P = .59 vs late stage P = .08), but a trend of lower PACS scores was noted with gabapentin in the later stage. No participant in either group had ataxia during the study. In both groups, CIWA-Ar scores were reduced similarly.
Conclusion: The researchers concluded that gabapentin treatment resulted in a significantly greater reduction in sedation (ESS) and a trend toward reduced alcohol craving (PACS) by the end of treatment compared with chlordiazepoxide treatment.12
Schacht et al13 analyzed functional magnetic resonance imaging data from 48 patients who were alcohol-dependent in a 6-week RCT. Patients were randomized to receive gabapentin up to 1,200 mg/d for 39 days plus flumazenil for 2 days (GP/FMZ group) or an oral placebo and placebo infusions on the same time course. Evaluations included the SCID, ADS, and Obsessive-Compulsive Drinking Scale (OCDS). On Day 1, the CIWA-Ar was administered; it was used to ensure equal distribution of individuals with higher alcohol withdrawal symptoms between medication groups. There were no significant effects of initial alcohol withdrawal symptom status or medication. However, there was a significant interaction between these factors: patients with higher alcohol withdrawal symptoms who received GP/FMZ and those with lower alcohol withdrawal symptoms who received placebo demonstrated greater cue-elicited activation, relative to the other groups, and had less subsequent drinking, which reflected differences in deactivation between alcohol and beverage stimuli, in a cluster that encompassed the dorsal ACC (dACC) (family-wise error-corrected cluster probability of P = .012; 99 voxels; local maxima at [-3, 39, 18] and [6, 33, 9]). In the GP/FMZ group, patients with higher alcohol withdrawal symptoms had significantly greater activation, while in the placebo group, patients with lower alcohol withdrawal symptoms had greater activation.
Conclusion: The researchers concluded that alterations in task-related deactivation of dACC, a component of the default mode network, may predict better alcohol treatment response, while activation of DLPFC, an area associated with selective attention, may predict relapse drinking.13
Continue to: Carbamazepine
Carbamazepine
The characteristics of the carbamazepine studies included in this review are summarized in Table 2.14-19

Björkqvist et al14 randomized 105 men with AWS to placebo or carbamazepine. On initial assessment, history, physical examination, relevant labs, and intoxication assessments were recorded. On subsequent visits, nursing staff recorded withdrawal symptoms for patients as 0 to 2 (0 = no specific symptoms, 1 = patient only complained when asked about specific symptoms, 2 = patient complained of withdrawal symptoms without being asked, or if symptoms were severe or obvious to others). Along with the above, vital signs and a visual analogue scale of 0 to 10 (0 = feeling could not be worse, 10 = feeling could not be better) were recorded at each visit. The dose was weight-dependent and administered as follows: on Days 1 and 2, 1+1+2 tablets of carbamazepine, 200 mg, or placebo; Days 3 and 4, 1+1+1 tablets; and Days 5 and 6, 1+0+1 tablets. Every patient received dichloralphenazone as needed. All patients were given vitamin B 3 times a day. Most withdrawal symptoms decreased faster in the carbamazepine group on Day 2 (P = .01) and on Day 4 (P = .1). On the visual analogue scale, scores varied between patients. On Day 1, the mean score was 2.5 times higher in the carbamazepine group compared with the placebo group, and this difference increased to 3 times by Day 7 (P < .01). The patient’s estimated ability to work improved significantly faster in the carbamazepine group than in the placebo group (P < .01).
Conclusion: The authors concluded that compared with placebo, carbamazepine was able to more quickly decrease withdrawal symptoms, especially insomnia and subjective recovery.14
Ritola et al15 randomized 68 hospitalized men with AWS to carbamazepine, 200 mg/d, or clomethiazole, 300 mg/d, for 1 week. The target withdrawal symptoms included gastrointestinal and sleep disturbances; anxiety; aggressiveness; and cardiovascular, depressive, psychotic, and neurologic symptoms. A 4-point rating scale was used for individual symptoms (0 = no symptom, 1 = mild symptom, 2 = moderate symptom, and 3 = severe symptom). On the day of admission (Day 0), all patients were given 50 to 100 mg of chlordiazepoxide IM and 2 tablets and 4 capsules of the trial preparations (either the tablets or capsules were active, and the others were placebos) in the evening. Five patients dropped out of the clomethiazole group and 1 from the carbamazepine group. No significant difference between the 2 treatments were found by the patient, nurse, or physician.
Conclusion: The authors concluded that carbamazepine seemed to be as effective as clomethiazole in the treatment of milder alcohol withdrawal symptoms. Final treatment results were equally good in both groups. Sleep disturbance resolved faster in the carbamazepine group.15
Continue to: Agricola et al
Agricola et al16 compared carbamazepine to tiapride for treatment of acute AWS. In this study, 60 patients were randomized to carbamazepine, 200 mg 3 times a day, or tiapride, 200 mg 3 times a day. All patients were hospitalized with severe AWS preceding DT. The patients were evaluated for withdrawal symptoms (gastrointestinal and cardiovascular symptoms, sleep disturbances, anxiety, aggression, fear, depression, psychotic symptoms, and certain neurologic symptoms). The severity of these symptoms was scored as follows: 0 = no symptoms; 1 = moderate symptoms; and 2 = severe symptoms. At each visit, an overall evaluation of the patient’s clinical condition was made according to a visual analogue scale (100 = worst condition, 0 = best condition). On Day 7, both the doctor and patient evaluated treatment efficacy according to a 4-point scale (1 = no efficacy, 4 = excellent efficacy). There was no significant difference between carbamazepine and tiapride in terms of total symptoms score and visual analogue scale assessment. Carbamazepine was found to have faster relief of symptoms and a significantly greater reduction in symptom score on Day 2 (P < .01). Carbamazepine had a preferential action on fear, nightmares, and hallucinations. The proportion of patients in whom anxiety improved after treatment was 96.2% for carbamazepine and 71.4% for tiapride (P < .05). Aggressiveness and gastrointestinal discomfort resolved faster in the tiapride group. No cases of DT were observed.
Conclusion: The researchers concluded that either carbamazepine or tiapride provides an appropriate alternative in the treatment of inpatients with severe AWS.16
Stuppaeck et al17 compared the efficacy of carbamazepine to oxazepam in 60 inpatients who had symptoms of alcohol withdrawal. Alcohol withdrawal was measured with the CIWA-A, and patients with scores >20 were enrolled in the study. The Clinical Global Impression (CGI) scale and self-rated Adjective Checklist (ACL) were also used. On Days 1 to 3, patients received oxazepam, 120 mg/d, or carbamazepine, 800 mg/d. From Day 4 to 7, doses were decreased to 90 mg/d and 600 mg/d, respectively. After the 7-day trial, all patients were treated with carbamazepine, 200 mg twice a day on Day 8 and 200 mg at night on Day 9. Two patients withdrew consent and 6 dropped out due to adverse effects. During the 7-day trial, when comparing all improvements on CIWA-A, ACL, and CGI scales, carbamazepine was equivalent to oxazepam up to Day 5, and then superior on Days 6 and 7 (P ≤ .05). No decrease in white blood cell count was found in the carbamazepine group.
Conclusion: The authors concluded that carbamazepine is as effective as oxazepam and may be a viable alternative that does not interact with alcohol or cause delirium.17
Malcolm et al18 compared the effects of carbamazepine and lorazepam in patients in an outpatient setting who had single vs multiple previous alcohol withdrawals. The study included 136 patients who satisfied DSM-IV criteria for alcohol dependence and alcohol withdrawal, with a blood alcohol level ≤0.1 g/dL, a Mini-Mental State Examination (MMSE) score ≤26, and a CIWA-Ar score ≤10 on admission. Patients also completed the ADS to quantify the severity of alcohol dependence. Daily drinking was measured by patient report using a daily drinking log and blood alcohol level. Heavy drinking was defined as ≥4 standard drinks per day for women and ≥5 drinks per day for men. On Day 1, patients were randomized to receive carbamazepine, 600 to 800 mg/d,or lorazepam, 6 to 8 mg/d, in divided doses, which was tapered to carbamazepine, 200 mg/d, or lorazepam, 2 mg/d, on Day 5. All patients received thiamine for 12 days. In the immediate post-detoxification period, carbamazepine-treated patients were less likely to relapse, and if they did drink, they drank less than those treated with lorazepam (P = .003). Even in patients who had multiple previous detoxifications, those randomized to carbamazepine drank less than those in lorazepam group (P = .004). Patients in the lorazepam group had significant higher rebound withdrawal symptoms (P = .007).
Continue to: Conclusion
Conclusion: The researchers concluded that carbamazepine and lorazepam were both effective in reducing alcohol withdrawal symptoms. They also concluded that carbamazepine was less likely to cause rebound withdrawal and more likely to reduce post-treatment drinking; among those who did drink, there was less heavy drinking.18
Malcolm et al19 conducted a 5-day double-blind RCT with 136 outpatients who met DSM-IV criteria for alcohol withdrawal. Patients were evaluated by CIWA before getting medications and then daily for 5 days. Patients were randomized to receive carbamazepine, 600 to 800 mg/d on Day 1, 200 mg 3 times a day on Day 2, 200 mg twice a day on Days 3 and 4, and 200 mg once on Day 5. Participants were randomized to receive lorazepam, 6 to 8 mg/d in divided doses on Day 1, 2 mg 3 times a day on Day 2, 2 mg twice a day on Days 3 and 4, and 2 mg once on Day 5. Ability to return to work was self-rated on a 100-mm visual analogue scale, with 0 being “totally unable to return to work’’ and 100 representing “being fully able to return to work.’’ Self-report measures of sleep quality were made using a 100-mm visual analogue scale, with 0 = “the very worst night’s sleep I’ve ever had’’ and 100 = “the very best night’s sleep I’ve ever had.’’ Carbamazepine significantly reduced anxiety (P = .0007). Visual analogue measures of sleep quality indicated a statistically significant main effect of medication on sleep that favored carbamazepine (P = .0186).
Conclusion: The authors concluded that when treating patients with mild to moderate alcohol withdrawal symptoms, carbamazepine was superior to lorazepam in reducing anxiety and improving sleep.19
Sodium valproate
The characteristics of the sodium valproate studies included in this review are summarized in Table 3.20,21

Lambie et al20 evaluated the use of sodium valproate in the treatment of AWS. A total of 49 patients were randomized to a sodium valproate group (n = 22) or a control group (n = 27). All participants were inpatients receiving treatment for alcohol use disorder and substance use disorder for 7 days. Patients in the sodium valproate group received 800 mg every 8 hours for 7 days. Patients were observed daily for occurrence of withdrawal symptoms. Nurses who were blinded to the group assignment graded the degree and severity of symptoms. The trial was initially designed so that chlormethiazole and/or tranquilizers were added to sodium valproate when withdrawal symptoms occurred. However, after treating the first few patients, it became evident that additional medications were not needed. In the treatment group, 13 participants received only sodium valproate, 4 patients needed a tranquilizer, 4 needed chlormethiazole, and 1 needed both. In the control group, 1 received only sodium valproate, 4 received a tranquilizer, 14 received chlormethiazole, and 8 needed both. One patient, who entered the study twice, had a withdrawal seizure when in control group and no seizure on second admission in the sodium valproate group. Physical symptoms disappeared quickly in the sodium valproate group (mean of 2 days vs 2.6 days in the control group). Fourteen patients in the control group received chlormethiazole, compared with only 4 patients in sodium valproate group.
Continue to: Conclusion
Conclusion: The researchers concluded that physical symptoms disappeared quicker in the sodium valproate group than in the control group.20
Hillbom et al21 evaluated the efficacy of sodium valproate vs carbamazepine vs placebo to prevent alcohol withdrawal seizures. A total of 138 participants were studied. Forty-three were assigned to the carbamazepine group, 46 to the sodium valproate group, and 49 to the placebo group. The RCT lasted 4 days. The initial medication doses were 1,200 mg/d. Participants in the carbamazepine group experienced more adverse effects than those in the sodium valproate or placebo groups (P < .001). As a result, approximately one-half of the participants in the carbamazepine group stopped taking the medication. This finding was dependent on the dose of carbamazepine; >800 mg/d resulted in poor tolerance to adverse effects. Seizures occurred among patients in all 3 arms of the study; in the sodium valproate group, 1 participant had a seizure vs 2 participants in the carbamazepine group and 3 in the placebo group. On the other hand, DT occurred only in the sodium valproate and placebo groups.
Conclusion: Researchers concluded that when using sodium valproate or carbamazepine to prevent alcohol withdrawal seizures in an outpatient setting, the adverse effects may outweigh the benefits.21
Lamotrigine
The characteristics of the lamotrigine study included in this review are summarized in Table 3.22
Djokić et al22 evaluated the efficiency of lamotrigine in the treatment of DT. A total of 240 participants who met International Classification of Diseases-10 criteria for DT were randomized to a control group that was treated with anticonvulsants according to an NIAAA protocol (2004), or to an experimental group that was treated with lamotrigine. The CIWA-Ar and the Memorial Delirium Assessment Scale (MDAS) were administered for objective assessment of clinical symptoms, superimposed medical complications, general condition of the patient, adverse effects, and mortality rate. Statistically significant differences between the experimental and control groups were apparent after the third day of therapy, when a drop in the average CIWA-Ar score was observed in the experimental group, while the control group still had high scores (P < .01). After the fifth day of treatment, the differences in scores were more apparent, with the experimental group showing CIWA-Ar scores equal to those of persons with mild/moderate DT, while those in the control group still had high scores. After the tenth day, participants in the experimental group did not have any alcohol withdrawal symptoms, while control group participants were just beginning to get out of life-threatening danger. Death occurred in 4.1% of control group participants and 3.4% of experimental group participants; this difference in mortality rate was not statistically significant.
Continue to: Conclusion
Conclusion: Researchers concluded that lamotrigine is significantly efficacious in the treatment of DT, but does not decrease the mortality rate.22
What to know before you prescribe
AWS is a medical emergency that if left untreated leads to several complications and possibly death. Although benzodiazepines are considered the gold standard for treating AWS, the adverse effects associated with their use advocates for finding alternatives. Anticonvulsants can be an effective alternative for treating AWS. In our literature review, we found 16 double-blind RCTs that used an anticonvulsant medication for the treatment of AWS. Of these, 7 involved gabapentin, 6 involved carbamazepine, 1 involved sodium valproate, 1 involved sodium valproate vs carbamazepine, and 1 involved lamotrigine. Overall, the use of anticonvulsants resulted in significant improvement of mild to moderate symptoms of AWS.
There were more studies of carbamazepine and gabapentin than of other anticonvulsants. All the anticonvulsants offered potential benefits. They decreased the probability of a withdrawal seizure and other complications and effectively reduced alcohol cravings. Anticonvulsants were useful for preventing rebound withdrawal symptoms and reducing post-treatment alcohol consumption, especially in patients who had multiple previous withdrawals. Anticonvulsants were particularly helpful for patients with mood disorders such as depression. In the studies we reviewed, anticonvulsants caused less sedation compared with benzodiazepines, and also decreased the occurrence of relapse.
Dosing recommendations. In the studies included in our review, gabapentin was effective at a dosage of 1,600 mg/d (given as 400 mg 4 times a day). This was tapered as follows: 400 mg 4 times a day on Days 1 to 3, 400 mg 3 times a day on Day 4, 400 mg twice a day on Day 5, and 400 mg once a day on Day 6. Carbamazepine was effective at 600 to 800 mg/d, and was tapered by decreasing by 200 mg as follows: 800 mg/d on Days 1 to 3, 600 mg/d on Day 4, 400 mg on Day 5, and 200 mg/d on Day 6. In the reviewed study, the maximum dose of lamotrigine never exceeded 200 mg/d and was administered for 28 days; the exact dosing and taper plan were not described. The dosing of sodium valproate ranged from 1,200 mg/d to 1600 mg/d for 7 days, followed by decreasing by 200 mg each day. The recommended duration of treatment varied; on average for all anticonvulsants, it was 7 to 12 days, followed by a taper. Carbamazepine was shown to be superior to oxazepam in ameliorating the symptoms of AWS.
Adverse effects. When considering the tolerability, adverse effect profile, duration of action, and effectiveness of the anticonvulsants included in our review, gabapentin appears to be the safest agent to choose. For the other anticonvulsants, the risks might outweigh the benefits. Specifically, in a comparison of sodium valproate and carbamazepine, Hillbom et al21 concluded that in doses >800 mg/d, carbamazepine has potential to cause more adverse effects than benefits. However, Agricola et al16 found that carbamazepine had a preferential action on fear, nightmares, and hallucinations.
Continue to: A few caveats
A few caveats
Our review focused a large collection of data from multiple databases and RCTs only. However, its limitations include:
- there was no measure of heterogeneity
- the studies had short treatment duration
- most studies evaluated predominantly male participants
- some studies were underpowered.
Our review laid a groundwork for future research that includes more well-designed RCTs and/or meta-analyses of recent studies that evaluated the use anticonvulsants for treating AWS.
Bottom Line
Evidence suggests certain anticonvulsants may be an effective alternative to benzodiazepines for the treatment of mild to moderate alcohol withdrawal syndrome. Gabapentin may be the safest anticonvulsant to prescribe. Other anticonvulsants to consider include carbamazepine, sodium valproate, and lamotrigine, but for these agents, the risks might outweigh the benefits.
Related Resources
- Myrick H, Anton RF. Treatment of alcohol withdrawal. Alcohol Health Res World. 1998;22(1):38-43. https://pubs.niaaa.nih.gov/publications/arh22-1/38-43.pdf
- World Health Organization. Management of alcohol withdrawal. Published 2012. https://www.who.int/mental_health/mhgap/evidence/alcohol/q2/en/
Drug Brand Names
Carbamazepine • Tegretol
Gabapentin • Neurontin
Lamotrigine • Lamictal
Levetiracetam • Keppra
Lorazepam • Ativan
Oxcarbazepine • Trileptal
Phenytoin • Dilantin
Sodium valproate • Depakote
Acknowledgments
The authors thank Geetha Manikkara, MD, Madhuri Jakkam Setty, MD, and Elizabeth DeOreo, MD, for their efforts with the systematic review research.
Abrupt cessation or reduction of alcohol consumption may result in alcohol withdrawal syndrome (AWS), which is a medical emergency that can lead to serious complications when unrecognized or treatment is delayed. Symptoms of AWS include tremors, anxiety attacks, cognitive impairment, hallucinations, seizures, delirium tremens (DT), and in severe, untreated cases, death.1 Low to moderate alcohol consumption produces euphoria and excitation via activation of glutamatergic neurotransmission, while higher concentrations produce severe intoxication via GABAergic mechanisms. Acute withdrawal unmasks the hyper-excitatory state of the brain, causing anxiety, agitation, and autonomic activation characteristic of AWS, which typically begins 1 to 3 days after the last drink.2 In the 2012-2013 National Epidemiologic Survey on Alcohol and Related Conditions conducted by the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the 12-month and lifetime prevalences of AWS were 13.9% and 29.1%, respectively.3 Within the general inpatient population, AWS can be present in nearly 30% of patients; if left untreated, AWS has a 15% mortality rate, although when AWS is recognized early and treated, the mortality rate falls dramatically to 2%.4
AWS has most commonly been treated with benzodiazepines.5 However, benzodiazepines have the potential for significant adverse effects when used in older adults and in individuals with complicated medical issues, such as obstructive lung disease and sleep apnea.6 Anticonvulsants have been increasingly used to treat alcohol withdrawal, and their use is supported by several retrospective and prospective studies. In this article, we review the data from randomized control trials (RCTs) on the use of anticonvulsants for the treatment of AWS to see if we can make any recommendations for the use of anticonvulsants for treating AWS.
Our literature search
We searched 5 databases (PubMed, Cochrane, Medline, PsycInfo, and Embase) using the following terms: “alcohol withdrawal syndrome treatment”, “anticonvulsants”, “anti-epileptic”, “gabapentin”, “carbamazepine”, “sodium valproate”, “oxcarbazepine”, “phenytoin”, “levetiracetam”, and “lamotrigine.” We included only double-blind RCTs published between January 1, 1976 and September 30, 2016 in English-language journals or that had an official English translation. There were no restrictions on patient age or location of treatment (inpatient vs outpatient). All RCTs that compared anticonvulsants or a combination of an anticonvulsant and an active pharmacotherapeutic agent with either placebo or gold standard treatment for AWS were included. Database reviews, systematic reviews, and meta-analyses were excluded.
We identified 662 articles that met these criteria. However, most were duplicates, review articles, systematic reviews, meta-analyses, case reports, or open-label or non-randomized trials. Only 16 articles met our inclusion criteria. In the following sections, we discuss these 16 studies by medication type and in chronological order.
Gabapentin
The characteristics of the gabapentin studies included in this review are summarized in Table 1.7-13

Bonnet et al7 (2003) examined 61 adults who met the clinical criteria for alcohol dependence and displayed moderate or severe AWS according to their Mainz Alcohol Withdrawal Score (MAWS ≥4). They were randomized to receive placebo or gabapentin, 400 mg 4 times a day, along with clomethiazole. The attrition rate was not significantly different between the 2 groups (P = .66). The difference in the number of clomethiazole capsules taken during the first 24 hours between the groups was small and not significant (P = .96). Analysis of MAWS over time revealed no significant main effect for group (P = .26) and a significant effect for the time variable (P < .001). The interaction between group and time was not significant (P =.4). This means that there was a significant decrease in MAWS from baseline over 48 hours, and this decrease in MAWS was considered equal for both study groups. Adverse clinical events were observed in both groups, and there was no significant difference (P = .74) between the groups. Nausea and ataxia, which are specific to gabapentin, were observed more frequently in this group.
Conclusion: The authors concluded that gabapentin, 400 mg 4 times a day, is no better than placebo in reducing the amount of clomethiazole required to treat acute AWS.7
Continue to: Bonnet et al
Bonnet et al8 (2007) also conducted a study examining 59 patients with alcohol dependence who displayed moderate or severe AWS. Participants received placebo or gabapentin, 400 mg, and a rescue medication, clomethiazole, if needed. Subsequently, a capsule of study medication was administered every 6 hours for 2 days and then tapered. During the study, mood was measured by Profile of Mood States (POMS), and subjective complaints of withdrawal were measured using the Essen Self-Assessment of Alcohol Withdrawal Scale (ESA). Of the 59 patients, only 46 were analyzed; 5 patients dropped out, and 8 patients were missing data. Compared with the placebo group, the gabapentin group displayed less dejection, fatigue, and anger, and more vigor. Analysis of variance (ANOVA) measures revealed significant overall changes over time on all 4 scales (all P < .001). A significant (F = 3.62, df 2;43, P = .035) group × time interaction resulted exclusively for vigor. Analysis was repeated using rank-transformed data, resulting in a significant (P = .046) interaction effect. The significant increase in vigor was not apparent after tapering off gabapentin, which suggests gabapentin has a reversible effect on vigor. There was a significant (P < .001) overall decline of subjective withdrawal symptoms complaints, but no group × time interaction (P = .62). Analysis of 11 patients with comorbid mild depression revealed no significant time × group interaction for dejection, fatigue, anger, or subjective withdrawal (all P > .05). However, for vigor, the group × time interaction was significant (P = .022). Throughout the treatment, vigor scores of those mild depressive patients who received gabapentin increased to a level comparable to that of patients without a mood disorder.
Conclusion: The authors authors concluded that gabapentin was markedly more efficacious in improving vigor in the small subgroup of patients with mild depression.8
Myrick et al9 (2007) evaluated the safety and tolerability of gabapentin in patients who abused alcohol, as well as the ability of gabapentin to reduce alcohol craving and consumption. This study included 35 participants randomly assigned to receive gabapentin (n = 17) or placebo (n = 18) for 7 days. All medications were administered in standard gel caps with riboflavin, 25 mg, to assess for compliance via a laboratory-based urinary fluorescence assay. Urine samples were assessed for riboflavin at baseline and Day 6, and a reading of 1,500 ng/mL of riboflavin on Day 6 was interpreted as being compliant. Participants were required to abstain completely from drinking alcohol on Day 6 and the morning of Day 7. At the first session, the following measures were completed: demographic form, alcohol and drug section of the Structured Clinical Interview (SCID), Obsessive-Compulsive Drinking Scale, Self-Administered Alcohol Screening Test (SAAST), and Alcohol Dependence Scale (ADS); there also was collection of a urine sample for detection of abused drugs and a blood sample for liver function and general health screening.
At the second session, patients completed the psychiatric sections of the SCID and the Alcohol Craving Questionnaire, and received a physical exam. To assess the negative clinical effects of gabapentin and alcohol on the CNS, the Epworth Sleepiness Scale (ESS) and POMS were administered at baseline and on Day 6. Also, several other scales were used to identify any impact of gabapentin on acute alcohol effects and craving: the Clinical Institute Withdrawal Assessment of Alcohol, Revised (CIWA-Ar), Biphasic Alcohol Effects Scale (BAES), Subjective High Assessment Scale (SHAS), and Alcohol Urge Questionnaire (AUQ).
Conclusion: Gabapentin was well tolerated, but compared with placebo, gabapentin had no effect on alcohol stimulation (P = .75) or sedation (P = .99) as measured by the BAES. The difference in SHAS scores was also not significant (P = .19). There was also no significant reduction in craving for alcohol as measured on the AUQ scale
Continue to: Malcolm et al
Malcolm et al10 conducted an outpatient treatment study. Patients were men and women age 21 to 70 years from multiple ethnic groups. They were randomized to receive gabapentin or lorazepam; 449 patients were screened and 68 completed the follow-up. Scales used included the CIWA-Ar, Beck Depression Inventory (BDI), and ESS.
Patients receiving lorazepam reported less insomnia and more sleepiness early in treatment than patients receiving gabapentin. However, upon completing treatment and discontinuing medication administration, patients previously treated with lorazepam reported increased insomnia and daytime sleepiness, while patients previously treated with gabapentin continued to report improvements in these self-reported sleep measures. The differences between lorazepam and gabapentin were further evidenced in BDI scores at Day 5, Day 7, and Day 12 in patients who had previously experienced multiple withdrawals. Gabapentin was superior to lorazepam in reducing insomnia as assessed by BDI score, an effect that was sustained throughout the post-treatment week. Participants’ ESS scores indicated less daytime sleepiness in the gabapentin group than in the lorazepam group.
Conclusion: Among patients who abused alcohol and had a history of multiple withdrawals, lorazepam is less effective than gabapentin in reducing insomnia.10 However, this study had several limitations: <25% of individuals who were initially screened were enrolled in the study, and it used subjective tests such as BDI. Objective electrophysiologic measures of sleep and daytime sleepiness would have been very helpful.
Myrick et al11 (2009) also compared gabapentin and lorazepam for treating alcohol withdrawal. One hundred patients were randomized to receive 4 days of fixed-dose taper of gabapentin or lorazepam. Patients could receive 1 of 3 gabapentin dosing regimens (600 mg/d, 900 mg/d, or 1,200 mg/d) for 3 days. Participants who were randomized to receive lorazepam were given 6 mg/d for 3 days and then tapered to 4 mg/d. Also, blinded supplemental medications (rescue packs) were given to each patient on Days 1 to 4 to treat subjective feelings of alcohol withdrawal. All patients also received thiamine for 12 days. Assessment of severity of alcohol withdrawal was measured by the CIWA-Ar. To quantify the severity of alcohol dependence and alcohol use, patients were asked to complete the ADS and Time-Line Follow-Back (TLFB) scales, respectively. Other scales administered included the BDI, Zung Anxiety Scale (ZAS), ESS, and visual analogue scales that assessed craving, ability to perform work, and need for additional medication.
There was a decrease in CIWA-Ar scores over time in all groups. High-dose gabapentin was found to be statistically superior but clinically similar to lorazepam (P = .009). Researchers also found that compared with patients who were treated with lorazepam, patients who were treated with gabapentin experienced reduced craving and anxiety/depressive symptoms, and complained of less subjective discomfort. Compared to patients who were treated with gabapentin, patients who were treated with lorazepam had higher probabilities of drinking on the first day of dose decrease (Day 2) and the second day off medication (Day 6) (P = .0002). During post-treatment, patients who were treated with gabapentin had less probability of drinking during the follow-up post-treatment period (P = .2 for 900 mg/d and P = .3 for 1,200 mg/d) compared with patients who were treated with lorazepam (P = .55).
Continue to: Conclusion
Conclusion: The researchers concluded that gabapentin was well tolerated and effectively diminished the symptoms of alcohol withdrawal, especially at the higher target dose (1,200 mg/d), and that compared with lorazepam, gabapentin decreased the probability of drinking during alcohol withdrawal and in the immediate post-withdrawal week.11
Stock et al12 randomized 26 patients who met criteria for AWS to receive gabapentin or chlordiazepoxide. Gabapentin doses were 1,200 mg/d orally for 3 days, followed by 900 mg/d, 600 mg/d, and 300 mg/d for 1 day each. Chlordiazepoxide doses were 100 mg/d orally for 3 days, followed by 75 mg/d, 50 mg/d, and 25 mg/d for 1 day each. The ESS, Penn Alcohol Craving Scale (PACS), ataxia rating, and CIWA-Ar were administered daily. Thirty-five percent of participants dropped out at the end of the 7-day treatment period. Days 1 to 4 were considered the early treatment period, and Days 5 to 7 were considered the late treatment period. The adjusted mean ESS score did not differ significantly between the randomized groups during the early stage (P = .61) vs the late stage, in which the adjusted mean ESS score was significantly lower with gabapentin compared with chlordiazepoxide (P = .04). The differences in PACS scores between the groups were not statistically significant in either stage (early stage P = .59 vs late stage P = .08), but a trend of lower PACS scores was noted with gabapentin in the later stage. No participant in either group had ataxia during the study. In both groups, CIWA-Ar scores were reduced similarly.
Conclusion: The researchers concluded that gabapentin treatment resulted in a significantly greater reduction in sedation (ESS) and a trend toward reduced alcohol craving (PACS) by the end of treatment compared with chlordiazepoxide treatment.12
Schacht et al13 analyzed functional magnetic resonance imaging data from 48 patients who were alcohol-dependent in a 6-week RCT. Patients were randomized to receive gabapentin up to 1,200 mg/d for 39 days plus flumazenil for 2 days (GP/FMZ group) or an oral placebo and placebo infusions on the same time course. Evaluations included the SCID, ADS, and Obsessive-Compulsive Drinking Scale (OCDS). On Day 1, the CIWA-Ar was administered; it was used to ensure equal distribution of individuals with higher alcohol withdrawal symptoms between medication groups. There were no significant effects of initial alcohol withdrawal symptom status or medication. However, there was a significant interaction between these factors: patients with higher alcohol withdrawal symptoms who received GP/FMZ and those with lower alcohol withdrawal symptoms who received placebo demonstrated greater cue-elicited activation, relative to the other groups, and had less subsequent drinking, which reflected differences in deactivation between alcohol and beverage stimuli, in a cluster that encompassed the dorsal ACC (dACC) (family-wise error-corrected cluster probability of P = .012; 99 voxels; local maxima at [-3, 39, 18] and [6, 33, 9]). In the GP/FMZ group, patients with higher alcohol withdrawal symptoms had significantly greater activation, while in the placebo group, patients with lower alcohol withdrawal symptoms had greater activation.
Conclusion: The researchers concluded that alterations in task-related deactivation of dACC, a component of the default mode network, may predict better alcohol treatment response, while activation of DLPFC, an area associated with selective attention, may predict relapse drinking.13
Continue to: Carbamazepine
Carbamazepine
The characteristics of the carbamazepine studies included in this review are summarized in Table 2.14-19

Björkqvist et al14 randomized 105 men with AWS to placebo or carbamazepine. On initial assessment, history, physical examination, relevant labs, and intoxication assessments were recorded. On subsequent visits, nursing staff recorded withdrawal symptoms for patients as 0 to 2 (0 = no specific symptoms, 1 = patient only complained when asked about specific symptoms, 2 = patient complained of withdrawal symptoms without being asked, or if symptoms were severe or obvious to others). Along with the above, vital signs and a visual analogue scale of 0 to 10 (0 = feeling could not be worse, 10 = feeling could not be better) were recorded at each visit. The dose was weight-dependent and administered as follows: on Days 1 and 2, 1+1+2 tablets of carbamazepine, 200 mg, or placebo; Days 3 and 4, 1+1+1 tablets; and Days 5 and 6, 1+0+1 tablets. Every patient received dichloralphenazone as needed. All patients were given vitamin B 3 times a day. Most withdrawal symptoms decreased faster in the carbamazepine group on Day 2 (P = .01) and on Day 4 (P = .1). On the visual analogue scale, scores varied between patients. On Day 1, the mean score was 2.5 times higher in the carbamazepine group compared with the placebo group, and this difference increased to 3 times by Day 7 (P < .01). The patient’s estimated ability to work improved significantly faster in the carbamazepine group than in the placebo group (P < .01).
Conclusion: The authors concluded that compared with placebo, carbamazepine was able to more quickly decrease withdrawal symptoms, especially insomnia and subjective recovery.14
Ritola et al15 randomized 68 hospitalized men with AWS to carbamazepine, 200 mg/d, or clomethiazole, 300 mg/d, for 1 week. The target withdrawal symptoms included gastrointestinal and sleep disturbances; anxiety; aggressiveness; and cardiovascular, depressive, psychotic, and neurologic symptoms. A 4-point rating scale was used for individual symptoms (0 = no symptom, 1 = mild symptom, 2 = moderate symptom, and 3 = severe symptom). On the day of admission (Day 0), all patients were given 50 to 100 mg of chlordiazepoxide IM and 2 tablets and 4 capsules of the trial preparations (either the tablets or capsules were active, and the others were placebos) in the evening. Five patients dropped out of the clomethiazole group and 1 from the carbamazepine group. No significant difference between the 2 treatments were found by the patient, nurse, or physician.
Conclusion: The authors concluded that carbamazepine seemed to be as effective as clomethiazole in the treatment of milder alcohol withdrawal symptoms. Final treatment results were equally good in both groups. Sleep disturbance resolved faster in the carbamazepine group.15
Continue to: Agricola et al
Agricola et al16 compared carbamazepine to tiapride for treatment of acute AWS. In this study, 60 patients were randomized to carbamazepine, 200 mg 3 times a day, or tiapride, 200 mg 3 times a day. All patients were hospitalized with severe AWS preceding DT. The patients were evaluated for withdrawal symptoms (gastrointestinal and cardiovascular symptoms, sleep disturbances, anxiety, aggression, fear, depression, psychotic symptoms, and certain neurologic symptoms). The severity of these symptoms was scored as follows: 0 = no symptoms; 1 = moderate symptoms; and 2 = severe symptoms. At each visit, an overall evaluation of the patient’s clinical condition was made according to a visual analogue scale (100 = worst condition, 0 = best condition). On Day 7, both the doctor and patient evaluated treatment efficacy according to a 4-point scale (1 = no efficacy, 4 = excellent efficacy). There was no significant difference between carbamazepine and tiapride in terms of total symptoms score and visual analogue scale assessment. Carbamazepine was found to have faster relief of symptoms and a significantly greater reduction in symptom score on Day 2 (P < .01). Carbamazepine had a preferential action on fear, nightmares, and hallucinations. The proportion of patients in whom anxiety improved after treatment was 96.2% for carbamazepine and 71.4% for tiapride (P < .05). Aggressiveness and gastrointestinal discomfort resolved faster in the tiapride group. No cases of DT were observed.
Conclusion: The researchers concluded that either carbamazepine or tiapride provides an appropriate alternative in the treatment of inpatients with severe AWS.16
Stuppaeck et al17 compared the efficacy of carbamazepine to oxazepam in 60 inpatients who had symptoms of alcohol withdrawal. Alcohol withdrawal was measured with the CIWA-A, and patients with scores >20 were enrolled in the study. The Clinical Global Impression (CGI) scale and self-rated Adjective Checklist (ACL) were also used. On Days 1 to 3, patients received oxazepam, 120 mg/d, or carbamazepine, 800 mg/d. From Day 4 to 7, doses were decreased to 90 mg/d and 600 mg/d, respectively. After the 7-day trial, all patients were treated with carbamazepine, 200 mg twice a day on Day 8 and 200 mg at night on Day 9. Two patients withdrew consent and 6 dropped out due to adverse effects. During the 7-day trial, when comparing all improvements on CIWA-A, ACL, and CGI scales, carbamazepine was equivalent to oxazepam up to Day 5, and then superior on Days 6 and 7 (P ≤ .05). No decrease in white blood cell count was found in the carbamazepine group.
Conclusion: The authors concluded that carbamazepine is as effective as oxazepam and may be a viable alternative that does not interact with alcohol or cause delirium.17
Malcolm et al18 compared the effects of carbamazepine and lorazepam in patients in an outpatient setting who had single vs multiple previous alcohol withdrawals. The study included 136 patients who satisfied DSM-IV criteria for alcohol dependence and alcohol withdrawal, with a blood alcohol level ≤0.1 g/dL, a Mini-Mental State Examination (MMSE) score ≤26, and a CIWA-Ar score ≤10 on admission. Patients also completed the ADS to quantify the severity of alcohol dependence. Daily drinking was measured by patient report using a daily drinking log and blood alcohol level. Heavy drinking was defined as ≥4 standard drinks per day for women and ≥5 drinks per day for men. On Day 1, patients were randomized to receive carbamazepine, 600 to 800 mg/d,or lorazepam, 6 to 8 mg/d, in divided doses, which was tapered to carbamazepine, 200 mg/d, or lorazepam, 2 mg/d, on Day 5. All patients received thiamine for 12 days. In the immediate post-detoxification period, carbamazepine-treated patients were less likely to relapse, and if they did drink, they drank less than those treated with lorazepam (P = .003). Even in patients who had multiple previous detoxifications, those randomized to carbamazepine drank less than those in lorazepam group (P = .004). Patients in the lorazepam group had significant higher rebound withdrawal symptoms (P = .007).
Continue to: Conclusion
Conclusion: The researchers concluded that carbamazepine and lorazepam were both effective in reducing alcohol withdrawal symptoms. They also concluded that carbamazepine was less likely to cause rebound withdrawal and more likely to reduce post-treatment drinking; among those who did drink, there was less heavy drinking.18
Malcolm et al19 conducted a 5-day double-blind RCT with 136 outpatients who met DSM-IV criteria for alcohol withdrawal. Patients were evaluated by CIWA before getting medications and then daily for 5 days. Patients were randomized to receive carbamazepine, 600 to 800 mg/d on Day 1, 200 mg 3 times a day on Day 2, 200 mg twice a day on Days 3 and 4, and 200 mg once on Day 5. Participants were randomized to receive lorazepam, 6 to 8 mg/d in divided doses on Day 1, 2 mg 3 times a day on Day 2, 2 mg twice a day on Days 3 and 4, and 2 mg once on Day 5. Ability to return to work was self-rated on a 100-mm visual analogue scale, with 0 being “totally unable to return to work’’ and 100 representing “being fully able to return to work.’’ Self-report measures of sleep quality were made using a 100-mm visual analogue scale, with 0 = “the very worst night’s sleep I’ve ever had’’ and 100 = “the very best night’s sleep I’ve ever had.’’ Carbamazepine significantly reduced anxiety (P = .0007). Visual analogue measures of sleep quality indicated a statistically significant main effect of medication on sleep that favored carbamazepine (P = .0186).
Conclusion: The authors concluded that when treating patients with mild to moderate alcohol withdrawal symptoms, carbamazepine was superior to lorazepam in reducing anxiety and improving sleep.19
Sodium valproate
The characteristics of the sodium valproate studies included in this review are summarized in Table 3.20,21

Lambie et al20 evaluated the use of sodium valproate in the treatment of AWS. A total of 49 patients were randomized to a sodium valproate group (n = 22) or a control group (n = 27). All participants were inpatients receiving treatment for alcohol use disorder and substance use disorder for 7 days. Patients in the sodium valproate group received 800 mg every 8 hours for 7 days. Patients were observed daily for occurrence of withdrawal symptoms. Nurses who were blinded to the group assignment graded the degree and severity of symptoms. The trial was initially designed so that chlormethiazole and/or tranquilizers were added to sodium valproate when withdrawal symptoms occurred. However, after treating the first few patients, it became evident that additional medications were not needed. In the treatment group, 13 participants received only sodium valproate, 4 patients needed a tranquilizer, 4 needed chlormethiazole, and 1 needed both. In the control group, 1 received only sodium valproate, 4 received a tranquilizer, 14 received chlormethiazole, and 8 needed both. One patient, who entered the study twice, had a withdrawal seizure when in control group and no seizure on second admission in the sodium valproate group. Physical symptoms disappeared quickly in the sodium valproate group (mean of 2 days vs 2.6 days in the control group). Fourteen patients in the control group received chlormethiazole, compared with only 4 patients in sodium valproate group.
Continue to: Conclusion
Conclusion: The researchers concluded that physical symptoms disappeared quicker in the sodium valproate group than in the control group.20
Hillbom et al21 evaluated the efficacy of sodium valproate vs carbamazepine vs placebo to prevent alcohol withdrawal seizures. A total of 138 participants were studied. Forty-three were assigned to the carbamazepine group, 46 to the sodium valproate group, and 49 to the placebo group. The RCT lasted 4 days. The initial medication doses were 1,200 mg/d. Participants in the carbamazepine group experienced more adverse effects than those in the sodium valproate or placebo groups (P < .001). As a result, approximately one-half of the participants in the carbamazepine group stopped taking the medication. This finding was dependent on the dose of carbamazepine; >800 mg/d resulted in poor tolerance to adverse effects. Seizures occurred among patients in all 3 arms of the study; in the sodium valproate group, 1 participant had a seizure vs 2 participants in the carbamazepine group and 3 in the placebo group. On the other hand, DT occurred only in the sodium valproate and placebo groups.
Conclusion: Researchers concluded that when using sodium valproate or carbamazepine to prevent alcohol withdrawal seizures in an outpatient setting, the adverse effects may outweigh the benefits.21
Lamotrigine
The characteristics of the lamotrigine study included in this review are summarized in Table 3.22
Djokić et al22 evaluated the efficiency of lamotrigine in the treatment of DT. A total of 240 participants who met International Classification of Diseases-10 criteria for DT were randomized to a control group that was treated with anticonvulsants according to an NIAAA protocol (2004), or to an experimental group that was treated with lamotrigine. The CIWA-Ar and the Memorial Delirium Assessment Scale (MDAS) were administered for objective assessment of clinical symptoms, superimposed medical complications, general condition of the patient, adverse effects, and mortality rate. Statistically significant differences between the experimental and control groups were apparent after the third day of therapy, when a drop in the average CIWA-Ar score was observed in the experimental group, while the control group still had high scores (P < .01). After the fifth day of treatment, the differences in scores were more apparent, with the experimental group showing CIWA-Ar scores equal to those of persons with mild/moderate DT, while those in the control group still had high scores. After the tenth day, participants in the experimental group did not have any alcohol withdrawal symptoms, while control group participants were just beginning to get out of life-threatening danger. Death occurred in 4.1% of control group participants and 3.4% of experimental group participants; this difference in mortality rate was not statistically significant.
Continue to: Conclusion
Conclusion: Researchers concluded that lamotrigine is significantly efficacious in the treatment of DT, but does not decrease the mortality rate.22
What to know before you prescribe
AWS is a medical emergency that if left untreated leads to several complications and possibly death. Although benzodiazepines are considered the gold standard for treating AWS, the adverse effects associated with their use advocates for finding alternatives. Anticonvulsants can be an effective alternative for treating AWS. In our literature review, we found 16 double-blind RCTs that used an anticonvulsant medication for the treatment of AWS. Of these, 7 involved gabapentin, 6 involved carbamazepine, 1 involved sodium valproate, 1 involved sodium valproate vs carbamazepine, and 1 involved lamotrigine. Overall, the use of anticonvulsants resulted in significant improvement of mild to moderate symptoms of AWS.
There were more studies of carbamazepine and gabapentin than of other anticonvulsants. All the anticonvulsants offered potential benefits. They decreased the probability of a withdrawal seizure and other complications and effectively reduced alcohol cravings. Anticonvulsants were useful for preventing rebound withdrawal symptoms and reducing post-treatment alcohol consumption, especially in patients who had multiple previous withdrawals. Anticonvulsants were particularly helpful for patients with mood disorders such as depression. In the studies we reviewed, anticonvulsants caused less sedation compared with benzodiazepines, and also decreased the occurrence of relapse.
Dosing recommendations. In the studies included in our review, gabapentin was effective at a dosage of 1,600 mg/d (given as 400 mg 4 times a day). This was tapered as follows: 400 mg 4 times a day on Days 1 to 3, 400 mg 3 times a day on Day 4, 400 mg twice a day on Day 5, and 400 mg once a day on Day 6. Carbamazepine was effective at 600 to 800 mg/d, and was tapered by decreasing by 200 mg as follows: 800 mg/d on Days 1 to 3, 600 mg/d on Day 4, 400 mg on Day 5, and 200 mg/d on Day 6. In the reviewed study, the maximum dose of lamotrigine never exceeded 200 mg/d and was administered for 28 days; the exact dosing and taper plan were not described. The dosing of sodium valproate ranged from 1,200 mg/d to 1600 mg/d for 7 days, followed by decreasing by 200 mg each day. The recommended duration of treatment varied; on average for all anticonvulsants, it was 7 to 12 days, followed by a taper. Carbamazepine was shown to be superior to oxazepam in ameliorating the symptoms of AWS.
Adverse effects. When considering the tolerability, adverse effect profile, duration of action, and effectiveness of the anticonvulsants included in our review, gabapentin appears to be the safest agent to choose. For the other anticonvulsants, the risks might outweigh the benefits. Specifically, in a comparison of sodium valproate and carbamazepine, Hillbom et al21 concluded that in doses >800 mg/d, carbamazepine has potential to cause more adverse effects than benefits. However, Agricola et al16 found that carbamazepine had a preferential action on fear, nightmares, and hallucinations.
Continue to: A few caveats
A few caveats
Our review focused a large collection of data from multiple databases and RCTs only. However, its limitations include:
- there was no measure of heterogeneity
- the studies had short treatment duration
- most studies evaluated predominantly male participants
- some studies were underpowered.
Our review laid a groundwork for future research that includes more well-designed RCTs and/or meta-analyses of recent studies that evaluated the use anticonvulsants for treating AWS.
Bottom Line
Evidence suggests certain anticonvulsants may be an effective alternative to benzodiazepines for the treatment of mild to moderate alcohol withdrawal syndrome. Gabapentin may be the safest anticonvulsant to prescribe. Other anticonvulsants to consider include carbamazepine, sodium valproate, and lamotrigine, but for these agents, the risks might outweigh the benefits.
Related Resources
- Myrick H, Anton RF. Treatment of alcohol withdrawal. Alcohol Health Res World. 1998;22(1):38-43. https://pubs.niaaa.nih.gov/publications/arh22-1/38-43.pdf
- World Health Organization. Management of alcohol withdrawal. Published 2012. https://www.who.int/mental_health/mhgap/evidence/alcohol/q2/en/
Drug Brand Names
Carbamazepine • Tegretol
Gabapentin • Neurontin
Lamotrigine • Lamictal
Levetiracetam • Keppra
Lorazepam • Ativan
Oxcarbazepine • Trileptal
Phenytoin • Dilantin
Sodium valproate • Depakote
Acknowledgments
The authors thank Geetha Manikkara, MD, Madhuri Jakkam Setty, MD, and Elizabeth DeOreo, MD, for their efforts with the systematic review research.
1. Trevisan LA, Boutros N, Petrakis IL, et al. Complications of alcohol withdrawal: pathophysiological insights. Alcohol Health Res World. 1998;22(1):61-66.
2. Borghesani P. Alcohol withdrawal. In: Nordstrom KD, Wilson MP, eds. Quick guide to psychiatric emergencies. Springer: 2018;209-215.
3. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757-766.
4. Ungur LA, Neuner B, John S, et al. Prevention and therapy of alcohol withdrawal on intensive care units: systematic review of controlled trials. Alcohol Clin Exp Res. 2013;37(4):675-686.
5. Sachdeva A, Choudhary M, Chandra M. Alcohol withdrawal syndrome: benzodiazepines and beyond. J Clin Diagn Res. 2015;9(9):VE01-VE07.
6. Ashton H. Toxicity and adverse consequences of benzodiazepine use. Psychiatr Ann. 1995;25:158-165.
7. Bonnet U, Banger M, Leweke FM, et al. Treatment of acute alcohol withdrawal with gabapentin: results from a controlled two-center trial. J Clin Psychopharmacol. 2003;23(5):514-519.
8. Bonnet U, Specka M, Leweke FM, et al. Gabapentin’s acute effect on mood profile--a controlled study on patients with alcohol withdrawal. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(2):434-438.
9. Myrick H, Anton R, Voronin K, et al. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res. 2007;31(2):221-227.
10. Malcolm R, Myrick L, Veatch L, et al. Self-reported sleep, sleepiness, and repeated alcohol withdrawals: a randomized, double blind, controlled comparison of lorazepam vs gabapentin. J Clin Sleep Med. 2007;3(1):24-32.
11. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588.
12. Stock CJ, Carpenter L, Ying J, et al. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother. 2013;47(7-8):961-969.
13. Schacht JP, Anton RF, Randall PK, et al. Effects of a GABA-ergic medication combination and initial alcohol withdrawal severity on cue-elicited brain activation among treatment-seeking alcoholics. Psychopharmacol. 2013;227(4):627-637.
14. Björkqvist SE, Isohanni M, Mäkelä R, et al. Ambulant treatment of alcohol withdrawal symptoms with carbamazepine: a formal multicenter double blind comparison with placebo. Acta Psychiatr Scand. 1976;53(5):333-342.
15. Ritola E, Malinen L. A double-blind comparison of carbamazepine and clomethiazole in the treatment of alcohol withdrawal syndrome. Acta Psychiatr Scand. 1981;64(3):254-259.
16. Agricola R, Mazzarino M, Urani R, et al. Treatment of acute alcohol withdrawal syndrome with carbamazepine: a double-blind comparison with tiapride. J Int Med Res. 1982;10(3):160-165.
17. Stuppaeck CH, Pycha R, Miller C, et al. Carbamazepine versus oxazepam in the treatment of alcohol withdrawal: a double-blind study. Alcohol Alcohol. 1992;27(2):153-158.
18. Malcolm R, Myrick H, Roberts J, et al. The effects of carbamazepine and lorazepam on single vs multiple previous withdrawals in an outpatient randomized trial. J Gen Intern Med. 2002;17(5):349-355.
19. Malcolm R, Myrick H, Roberts J, et al. The differential effects of medications on mood, sleep disturbance, and work ability in outpatient alcohol detoxification. Am J Addict. 2002;11(2):141-150.
20. Lambie D, Johnson R, Vijayasenan M, et al. Sodium valproate in the treatment of the alcohol withdrawal syndrome. Aust N Z J. 1980;14(3):213-215.
21. Hillbom M, Tokola R, Kuusela V, et al. Prevention of alcohol withdrawal seizures with carbamazepine and valproic acid. Alcohol. 1989;6(3):223-226.
22. Djokic
1. Trevisan LA, Boutros N, Petrakis IL, et al. Complications of alcohol withdrawal: pathophysiological insights. Alcohol Health Res World. 1998;22(1):61-66.
2. Borghesani P. Alcohol withdrawal. In: Nordstrom KD, Wilson MP, eds. Quick guide to psychiatric emergencies. Springer: 2018;209-215.
3. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757-766.
4. Ungur LA, Neuner B, John S, et al. Prevention and therapy of alcohol withdrawal on intensive care units: systematic review of controlled trials. Alcohol Clin Exp Res. 2013;37(4):675-686.
5. Sachdeva A, Choudhary M, Chandra M. Alcohol withdrawal syndrome: benzodiazepines and beyond. J Clin Diagn Res. 2015;9(9):VE01-VE07.
6. Ashton H. Toxicity and adverse consequences of benzodiazepine use. Psychiatr Ann. 1995;25:158-165.
7. Bonnet U, Banger M, Leweke FM, et al. Treatment of acute alcohol withdrawal with gabapentin: results from a controlled two-center trial. J Clin Psychopharmacol. 2003;23(5):514-519.
8. Bonnet U, Specka M, Leweke FM, et al. Gabapentin’s acute effect on mood profile--a controlled study on patients with alcohol withdrawal. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(2):434-438.
9. Myrick H, Anton R, Voronin K, et al. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res. 2007;31(2):221-227.
10. Malcolm R, Myrick L, Veatch L, et al. Self-reported sleep, sleepiness, and repeated alcohol withdrawals: a randomized, double blind, controlled comparison of lorazepam vs gabapentin. J Clin Sleep Med. 2007;3(1):24-32.
11. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588.
12. Stock CJ, Carpenter L, Ying J, et al. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother. 2013;47(7-8):961-969.
13. Schacht JP, Anton RF, Randall PK, et al. Effects of a GABA-ergic medication combination and initial alcohol withdrawal severity on cue-elicited brain activation among treatment-seeking alcoholics. Psychopharmacol. 2013;227(4):627-637.
14. Björkqvist SE, Isohanni M, Mäkelä R, et al. Ambulant treatment of alcohol withdrawal symptoms with carbamazepine: a formal multicenter double blind comparison with placebo. Acta Psychiatr Scand. 1976;53(5):333-342.
15. Ritola E, Malinen L. A double-blind comparison of carbamazepine and clomethiazole in the treatment of alcohol withdrawal syndrome. Acta Psychiatr Scand. 1981;64(3):254-259.
16. Agricola R, Mazzarino M, Urani R, et al. Treatment of acute alcohol withdrawal syndrome with carbamazepine: a double-blind comparison with tiapride. J Int Med Res. 1982;10(3):160-165.
17. Stuppaeck CH, Pycha R, Miller C, et al. Carbamazepine versus oxazepam in the treatment of alcohol withdrawal: a double-blind study. Alcohol Alcohol. 1992;27(2):153-158.
18. Malcolm R, Myrick H, Roberts J, et al. The effects of carbamazepine and lorazepam on single vs multiple previous withdrawals in an outpatient randomized trial. J Gen Intern Med. 2002;17(5):349-355.
19. Malcolm R, Myrick H, Roberts J, et al. The differential effects of medications on mood, sleep disturbance, and work ability in outpatient alcohol detoxification. Am J Addict. 2002;11(2):141-150.
20. Lambie D, Johnson R, Vijayasenan M, et al. Sodium valproate in the treatment of the alcohol withdrawal syndrome. Aust N Z J. 1980;14(3):213-215.
21. Hillbom M, Tokola R, Kuusela V, et al. Prevention of alcohol withdrawal seizures with carbamazepine and valproic acid. Alcohol. 1989;6(3):223-226.
22. Djokic
Alcohol use disorder: How best to screen and intervene
THE CASE
Ms. E, a 42-year-old woman, visited her new physician for a physical exam. When asked about alcohol intake, she reported that she drank 3 to 4 beers after work and sometimes 5 to 8 beers a day on the weekends. Occasionally, she exceeded those amounts, but she didn’t feel guilty about her drinking. She was often late to work and said her relationship with her boyfriend was strained. A review of systems was positive for fatigue, poor concentration, abdominal pain, and weight gain. Her body mass index was 41, pulse 100 beats/min, blood pressure 125/75 mm Hg, and she was afebrile. Her physical exam was otherwise within normal limits.
How would you proceed with this patient?
Alcohol use disorder (AUD) is a common and often untreated condition that is increasingly prevalent in the United States.1 The Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) characterizes AUD as a combination of signs and symptoms typifying alcohol abuse and dependence (discussed in a bit).2
Data from the 2015 National Survey on Drug Use and Health (NSDUH) showed 15.7 million Americans with AUD, affecting 6.2% of the population ages 18 years or older and 2.5% of adolescents ages 12 to 17 years.3
Alcohol use and AUD account for an estimated 3.8% of all global deaths and 4.6% of global disability-adjusted life years.4 AUD adversely affects several systems (TABLE 15), and patients with AUD are sicker and more likely to die younger than those without AUD.4 In the United States, prevalence of AUD has increased in recent years among women, older adults, racial minorities, and individuals with a low education level.6
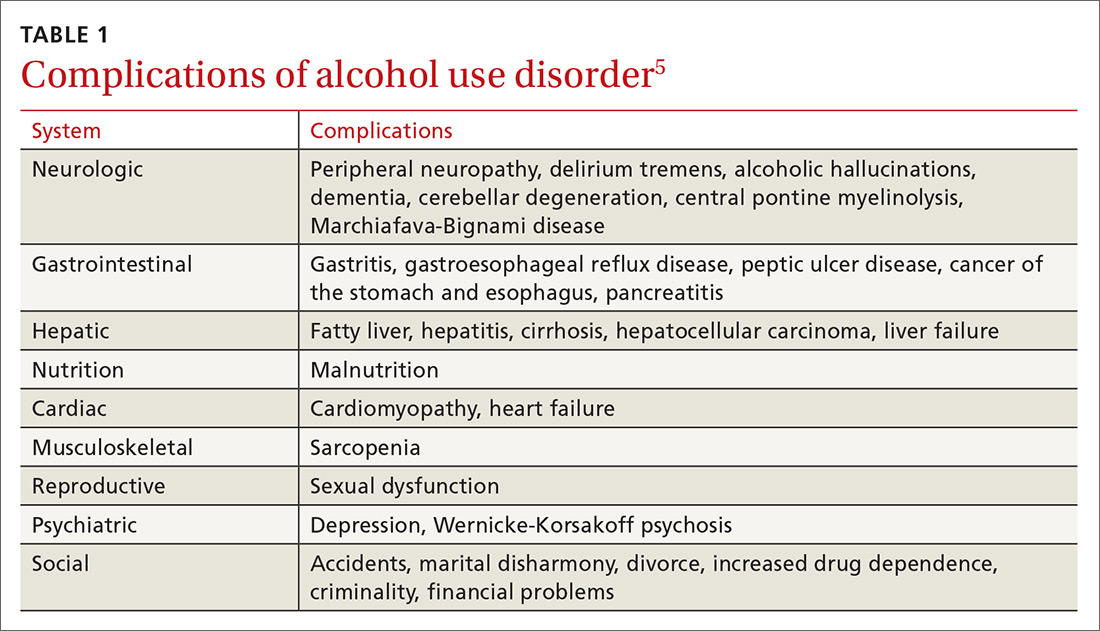
Screening for AUD is reasonable and straightforward, although diagnosis and treatment of AUD in primary care settings may be challenging due to competing clinical priorities; lack of training, resources, and support; and skepticism about the efficacy of behavioral and pharmacologic treatments.7,8 However, family physicians are in an excellent position to diagnose and help address the complex biopsychosocial needs of patients with AUD, often in collaboration with colleagues and community organizations.
Signs and symptoms of AUD
In clinical practice, at least 2 of the following 11 behaviors or symptoms are required to diagnose AUD2:
- consuming larger amounts of alcohol over a longer period than intended
- persistent desire or unsuccessful efforts to cut down or control alcohol use
- making a significant effort to obtain, use, or recover from alcohol
In moderate-to-severe cases:
- cravings or urges to use alcohol
- recurrent failure to fulfill major work, school, or social obligations
- continued alcohol use despite recurrent social and interpersonal problems
- giving up social, occupational, and recreational activities due to alcohol
- using alcohol in physically dangerous situations
- continued alcohol use despite having physical or psychological problems
- tolerance to alcohol’s effects
- withdrawal symptoms.
Continue to: Patients meet criteria for mild AUD severity if...
Patients meet criteria for mild AUD severity if they exhibit 2 or 3 symptoms, moderate AUD with 4 or 5 symptoms, and severe AUD if there are 6 or more symptoms.2
Those who meet criteria for AUD and are able to stop using alcohol are deemed to be in early remission if the criteria have gone unfulfilled for at least 3 months and less than 12 months. Patients are considered to be in sustained remission if they have not met criteria for AUD at any time during a period of 12 months or longer.
How to detect AUD
Several clues in a patient’s history can suggest AUD (TABLE 29,10). Most imbibers are unaware of the dangers and may consider themselves merely “social drinkers.” Binge drinking may be an early indicator of vulnerability to AUD and should be assessed as part of a thorough clinical evaluation.11 The US Preventive Services Task Force (USPSTF) recommends (Grade B) that clinicians screen adults ages 18 years or older for alcohol misuse.12

Studies demonstrate that both genetic and environmental factors play important roles in the development of AUD.13 A family history of excessive alcohol use increases the risk of AUD. Comorbidity of AUD and other mental health conditions is extremely common. For example, high rates of association between major depressive disorder and AUD have been observed.14
Tools to use in screening and diagnosing AUD
Screening for AUD during an office visit can be done fairly quickly. While 96% of primary care physicians screen for alcohol misuse in some way, only 38% use 1 of the 3 tools recommended by the USPSTF15—the Alcohol Use Disorders Identification Test (AUDIT), the abbreviated AUDIT-C, or the National Institute on Alcohol Abuse and Alcoholism (NIAAA) single question screen—which detect the full spectrum of alcohol misuse in adults.12 Although the commonly used CAGE questionnaire is one of the most studied self-report tools, it has lower sensitivity at a lower level of alcohol intake.16
Continue to: The NIAAA single-question screen asks...
The NIAAA single-question screen asks how many times in the past year the patient had ≥4 drinks (women) or ≥5 drinks (men) in a day.15 The sensitivity and specificity of single-question screening are 82% to 87% and 61% to 79%, respectively, and the test has been validated in several different settings.12 The AUDIT screening tool, freely available from the World Health Organization, is a 10-item questionnaire that probes an individual’s alcohol intake, alcohol dependence, and adverse consequences of alcohol use. Administration of the AUDIT typically requires only 2 minutes. AUDIT-C17 is an abbreviated version of the AUDIT questionnaire that asks 3 consumption questions to screen for AUD.
It was found that AUDIT scores in the range of 8 to 15 indicate a medium-level alcohol problem, whereas a score of ≥16 indicates a high-level alcohol problem. The AUDIT-C is scored from 0 to 12, with ≥4 indicating a problem in men and ≥3
THE CASE
The physician had used the NIAAA single- question screen to determine that Ms. E drank more than 4 beers per day during social events and weekends, which occurred 2 to 3 times per month over the past year. She lives alone and said that she’d been seeing less and less of her boyfriend lately. Her score on the Patient Health Questionnaire (PHQ), which screens for depression, was 11, indicating moderate impairment. Her response on the CAGE questionnaire was negative for a problem with alcohol. However, her AUDIT score was 17, indicating a high-level alcohol problem. Based on these findings, her physician expressed concern that her alcohol use might be contributing to her symptoms and difficulties.
Although she did not have a history of increasing usage per day, a persistent desire to cut down, significant effort to obtain alcohol, or cravings, she was having work troubles and continued to drink even though it was straining relationships, promoting weight gain, and causing abdominal pain.
The physician asked her to schedule a return visit and ordered several blood studies. He also offered to connect her with a colleague with whom he collaborated who could speak with her about possible alcohol use disorders and depression.
Continue to: Selecting blood work in screening for AUD
Selecting blood work in screening for AUD
Lab tests used to measure hepatic injury due to alcohol include gamma-glutamyl-transferase, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and macrocytic volume, although the indices of hepatic damage have low specificity. Elevated serum ethanol levels can reveal recent alcohol use, and vitamin deficiencies and other abnormalities can be used to differentiate other causes of hepatic inflammation and co-existing health issues (TABLE 310,18). A number of as-yet-unvalidated biomarkers are being studied to assist in screening, diagnosing, and treating AUD.18
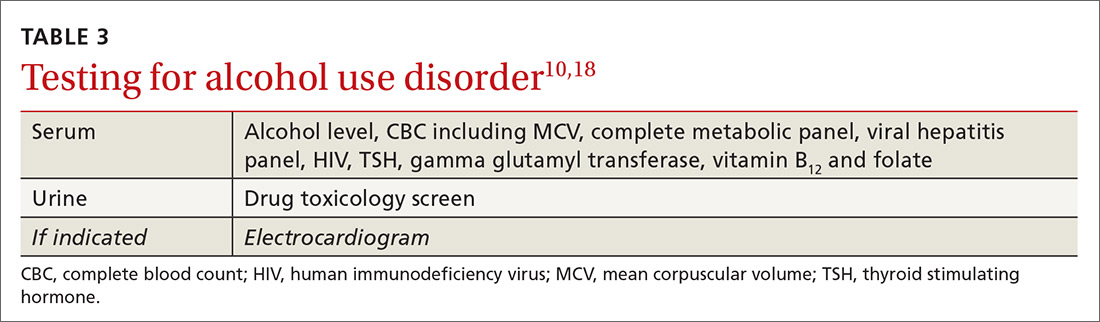
What treatment approaches work for AUD?
Family physicians can efficiently and productively address AUD by using alcohol screening and brief intervention, which have been shown to reduce risky drinking. Reimbursement for this service is covered by such CPT codes as 99408, 99409, or H0049, or with other evaluation and management (E/M) codes by using modifier 25.
Treatment of AUD varies and should be customized to each patient’s needs, readiness, preferences, and resources. Individual and group counseling approaches can be effective, and medications are available for inpatient and outpatient settings. Psychotherapy options include brief interventions, 12-step programs (eg, Alcoholics Anonymous—https://www.aa.org/pages/en_US/find-aa-resources),motivational enhancement therapy, and cognitive behavioral therapy. Although it is beyond the scope of this article to describe these options in detail, resources are available for those who wish to learn more.19-21
Psychopharmacologic management includes US Food and Drug Administration (FDA)-approved medications such as disulfiram, naltrexone, and acamprosate, and off-label uses of other medications (TABLE 49). Not enough empiric evidence is available to judge the effectiveness of these medications in adolescents, and the FDA has not approved them for such use. Evidence from meta-analyses comparing naltrexone and acamprosate have shown naltrexone to be more efficacious in reducing heavy drinking and cravings, while acamprosate is effective in promoting abstinence.22,23 Naltrexone combined with behavioral intervention reduces the heavy drinking days and percentage of abstinence days.24
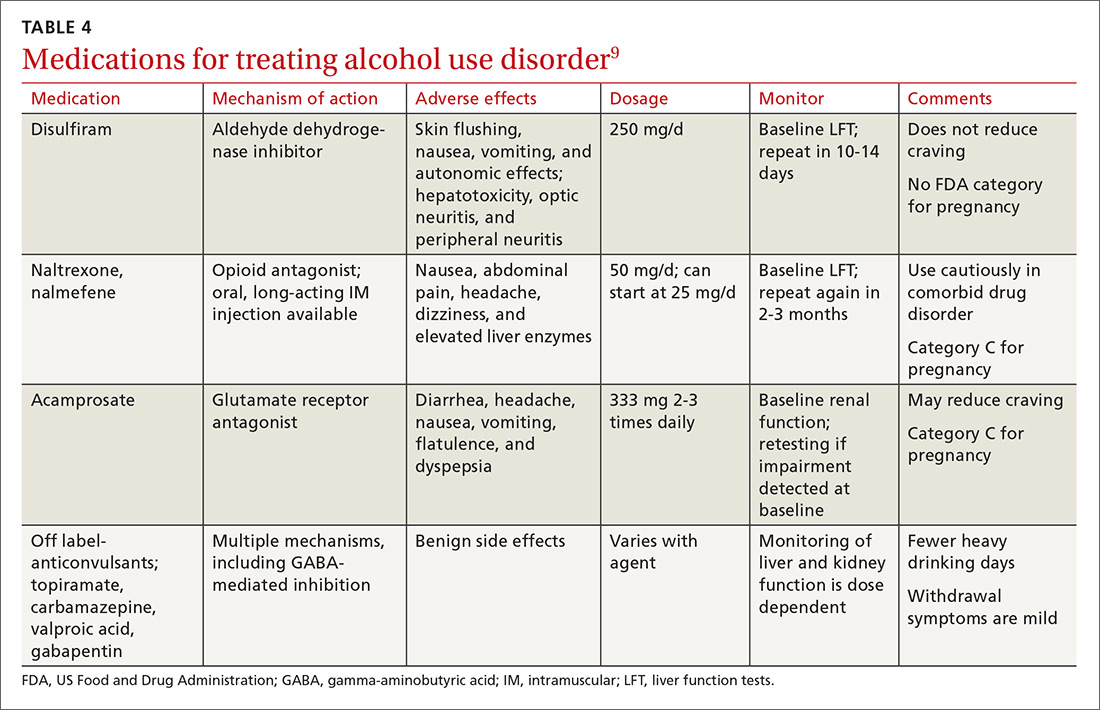
Current guideline recommendations from the American Psychiatric Association25 include:
- Naltrexone and acamprosate are recommended to treat patients with moderate-to-severe AUD in specific circumstances (eg, when nonpharmacologic approaches have failed to produce an effect or when patients prefer to use one of these medications).
- Topiramate and gabapentin are also suggested as medications for patients with moderate-to-severe AUD, but typically after first trying naltrexone and acamprosate.
- Disulfiram generally should not be used as first-line treatment. It produces physical reactions (eg, flushing) if alcohol is consumed within 12 to 24 hours of medication use.
Continue to: THE CASE
THE CASE
Ms. E was open to the idea of decreasing her alcohol use and agreed that she was depressed. Her lab tests at follow-up were normal other than an elevated AST/ALT of 90/80 U/L. S
She continued to get counseling for her AUD and for her comorbid depression in addition to taking a selective serotonin reuptake inhibitor. She is now in early remission for her alcohol use.
CORRESPONDENCE
Jaividhya Dasarathy, MD, Department of Family Medicine, Metro Health Medical Center, 2500 MetroHealth Drive, Cleveland, OH 44109; jdasarathy@metrohealth.org.
1. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757-766.
2. APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington DC; 2013.
3. HHS. Results from the 2015 National Survey on Drug Use and Health: summary of national findings. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.pdf. Accessed November 27, 2018.
4. Rehm J, Mathers C, Popova S, et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223-2233.
5. Chase V, Neild R, Sadler CW, et al. The medical complications of alcohol use: understanding mechanisms to improve management. Drug Alcohol Rev. 2005;24:253-265.
6. Grant BF, Chou SP, Saha TD, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74:911-923.
7. Williams EC, Achtmeyer CE, Young JP, et al. Barriers to and facilitators of alcohol use disorder pharmacotherapy in primary care: a qualitative study in five VA clinics. J Gen Intern Med. 2018;33:258-267.
8. Zhang DX, Li ST, Lee QK, et al. Systematic review of guidelines on managing patients with harmful use of alcohol in primary healthcare settings. Alcohol Alcohol. 2017;52:595-609.
9. Wackernah RC, Minnick MJ, Clapp P. Alcohol use disorder: pathophysiology, effects, and pharmacologic options for treatment. Subst Abuse Rehabil. 2014;5:1-12.
10. Kattimani S, Bharadwaj B. Clinical management of alcohol withdrawal: a systematic review. Ind Psychiatry J. 2013;22:100-108.
11. Gowin JL, Sloan ME, Stangl BL, et al. Vulnerability for alcohol use disorder and rate of alcohol consumption. Am J Psychiatry. 2017;174:1094-1101.
12. Moyer VA; Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:210-218.
13. Tarter RE, Alterman AI, Edwards KL. Vulnerability to alcoholism in men: a behavior-genetic perspective. J Stud Alcohol. 1985;46:329-356.
14. Brière FN, Rohde P, Seeley JR, et al. Comorbidity between major depression and alcohol use disorder from adolescence to adulthood [published online ahead of print, October 22, 2013]. Compr Psychiatry. 2014;55:526-533. doi: 10.1016/j.comppsych.2013.10.007.
15. Tan CH, Hungerford DW, Denny CH, et al. Screening for alcohol misuse: practices among U.S. primary care providers, DocStyles 2016. Am J Prev Med. 2018;54:173-180.
16. Aertgeerts B, Buntinx F, Kester A. The value of the CAGE in screening for alcohol abuse and alcohol dependence in general clinical populations: a diagnostic meta-analysis. J Clin Epidemiol. 2004;57:30-39.
17. Bush K, Kivlahan DR, McDonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789-1795.
18. Nanau RM, Neuman MG. Biomolecules and biomarkers used in diagnosis of alcohol drinking and in monitoring therapeutic interventions. Biomolecules. 2015;5:1339-1385.
19. Raddock M, Martukovich R, Berko E, et al. 7 tools to help patients adopt healthier behaviors. J Fam Pract. 2015;64:97-103.
20. AHRQ. Whitlock EP, Green CA, Polen MR, et al. Behavioral Counseling Interventions in Primary Care to Reduce Risky/Harmful Alcohol Use. 2004. https://www.ncbi.nlm.nih.gov/books/NBK42863/. Accessed November 17, 2018.
21. Miller WR, Baca C, Compton WM, et al. Addressing substance abuse in health care settings. Alcohol Clin Exp Res. 2006;30:292-302.
22. Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108:275-293.
23. Rosner S, Leucht S, Lehert P, et al. Acamprosate supports abstinence, naltrexone prevents excessive drinking: evidence from a meta-analysis with unreported outcomes. J Psychopharmacol. 2008;22:11-23.
24. Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003-2017.
25. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. Am J Psychiatry. 2018;175:86-90.
THE CASE
Ms. E, a 42-year-old woman, visited her new physician for a physical exam. When asked about alcohol intake, she reported that she drank 3 to 4 beers after work and sometimes 5 to 8 beers a day on the weekends. Occasionally, she exceeded those amounts, but she didn’t feel guilty about her drinking. She was often late to work and said her relationship with her boyfriend was strained. A review of systems was positive for fatigue, poor concentration, abdominal pain, and weight gain. Her body mass index was 41, pulse 100 beats/min, blood pressure 125/75 mm Hg, and she was afebrile. Her physical exam was otherwise within normal limits.
How would you proceed with this patient?
Alcohol use disorder (AUD) is a common and often untreated condition that is increasingly prevalent in the United States.1 The Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) characterizes AUD as a combination of signs and symptoms typifying alcohol abuse and dependence (discussed in a bit).2
Data from the 2015 National Survey on Drug Use and Health (NSDUH) showed 15.7 million Americans with AUD, affecting 6.2% of the population ages 18 years or older and 2.5% of adolescents ages 12 to 17 years.3
Alcohol use and AUD account for an estimated 3.8% of all global deaths and 4.6% of global disability-adjusted life years.4 AUD adversely affects several systems (TABLE 15), and patients with AUD are sicker and more likely to die younger than those without AUD.4 In the United States, prevalence of AUD has increased in recent years among women, older adults, racial minorities, and individuals with a low education level.6

Screening for AUD is reasonable and straightforward, although diagnosis and treatment of AUD in primary care settings may be challenging due to competing clinical priorities; lack of training, resources, and support; and skepticism about the efficacy of behavioral and pharmacologic treatments.7,8 However, family physicians are in an excellent position to diagnose and help address the complex biopsychosocial needs of patients with AUD, often in collaboration with colleagues and community organizations.
Signs and symptoms of AUD
In clinical practice, at least 2 of the following 11 behaviors or symptoms are required to diagnose AUD2:
- consuming larger amounts of alcohol over a longer period than intended
- persistent desire or unsuccessful efforts to cut down or control alcohol use
- making a significant effort to obtain, use, or recover from alcohol
In moderate-to-severe cases:
- cravings or urges to use alcohol
- recurrent failure to fulfill major work, school, or social obligations
- continued alcohol use despite recurrent social and interpersonal problems
- giving up social, occupational, and recreational activities due to alcohol
- using alcohol in physically dangerous situations
- continued alcohol use despite having physical or psychological problems
- tolerance to alcohol’s effects
- withdrawal symptoms.
Continue to: Patients meet criteria for mild AUD severity if...
Patients meet criteria for mild AUD severity if they exhibit 2 or 3 symptoms, moderate AUD with 4 or 5 symptoms, and severe AUD if there are 6 or more symptoms.2
Those who meet criteria for AUD and are able to stop using alcohol are deemed to be in early remission if the criteria have gone unfulfilled for at least 3 months and less than 12 months. Patients are considered to be in sustained remission if they have not met criteria for AUD at any time during a period of 12 months or longer.
How to detect AUD
Several clues in a patient’s history can suggest AUD (TABLE 29,10). Most imbibers are unaware of the dangers and may consider themselves merely “social drinkers.” Binge drinking may be an early indicator of vulnerability to AUD and should be assessed as part of a thorough clinical evaluation.11 The US Preventive Services Task Force (USPSTF) recommends (Grade B) that clinicians screen adults ages 18 years or older for alcohol misuse.12

Studies demonstrate that both genetic and environmental factors play important roles in the development of AUD.13 A family history of excessive alcohol use increases the risk of AUD. Comorbidity of AUD and other mental health conditions is extremely common. For example, high rates of association between major depressive disorder and AUD have been observed.14
Tools to use in screening and diagnosing AUD
Screening for AUD during an office visit can be done fairly quickly. While 96% of primary care physicians screen for alcohol misuse in some way, only 38% use 1 of the 3 tools recommended by the USPSTF15—the Alcohol Use Disorders Identification Test (AUDIT), the abbreviated AUDIT-C, or the National Institute on Alcohol Abuse and Alcoholism (NIAAA) single question screen—which detect the full spectrum of alcohol misuse in adults.12 Although the commonly used CAGE questionnaire is one of the most studied self-report tools, it has lower sensitivity at a lower level of alcohol intake.16
Continue to: The NIAAA single-question screen asks...
The NIAAA single-question screen asks how many times in the past year the patient had ≥4 drinks (women) or ≥5 drinks (men) in a day.15 The sensitivity and specificity of single-question screening are 82% to 87% and 61% to 79%, respectively, and the test has been validated in several different settings.12 The AUDIT screening tool, freely available from the World Health Organization, is a 10-item questionnaire that probes an individual’s alcohol intake, alcohol dependence, and adverse consequences of alcohol use. Administration of the AUDIT typically requires only 2 minutes. AUDIT-C17 is an abbreviated version of the AUDIT questionnaire that asks 3 consumption questions to screen for AUD.
It was found that AUDIT scores in the range of 8 to 15 indicate a medium-level alcohol problem, whereas a score of ≥16 indicates a high-level alcohol problem. The AUDIT-C is scored from 0 to 12, with ≥4 indicating a problem in men and ≥3
THE CASE
The physician had used the NIAAA single- question screen to determine that Ms. E drank more than 4 beers per day during social events and weekends, which occurred 2 to 3 times per month over the past year. She lives alone and said that she’d been seeing less and less of her boyfriend lately. Her score on the Patient Health Questionnaire (PHQ), which screens for depression, was 11, indicating moderate impairment. Her response on the CAGE questionnaire was negative for a problem with alcohol. However, her AUDIT score was 17, indicating a high-level alcohol problem. Based on these findings, her physician expressed concern that her alcohol use might be contributing to her symptoms and difficulties.
Although she did not have a history of increasing usage per day, a persistent desire to cut down, significant effort to obtain alcohol, or cravings, she was having work troubles and continued to drink even though it was straining relationships, promoting weight gain, and causing abdominal pain.
The physician asked her to schedule a return visit and ordered several blood studies. He also offered to connect her with a colleague with whom he collaborated who could speak with her about possible alcohol use disorders and depression.
Continue to: Selecting blood work in screening for AUD
Selecting blood work in screening for AUD
Lab tests used to measure hepatic injury due to alcohol include gamma-glutamyl-transferase, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and macrocytic volume, although the indices of hepatic damage have low specificity. Elevated serum ethanol levels can reveal recent alcohol use, and vitamin deficiencies and other abnormalities can be used to differentiate other causes of hepatic inflammation and co-existing health issues (TABLE 310,18). A number of as-yet-unvalidated biomarkers are being studied to assist in screening, diagnosing, and treating AUD.18

What treatment approaches work for AUD?
Family physicians can efficiently and productively address AUD by using alcohol screening and brief intervention, which have been shown to reduce risky drinking. Reimbursement for this service is covered by such CPT codes as 99408, 99409, or H0049, or with other evaluation and management (E/M) codes by using modifier 25.
Treatment of AUD varies and should be customized to each patient’s needs, readiness, preferences, and resources. Individual and group counseling approaches can be effective, and medications are available for inpatient and outpatient settings. Psychotherapy options include brief interventions, 12-step programs (eg, Alcoholics Anonymous—https://www.aa.org/pages/en_US/find-aa-resources),motivational enhancement therapy, and cognitive behavioral therapy. Although it is beyond the scope of this article to describe these options in detail, resources are available for those who wish to learn more.19-21
Psychopharmacologic management includes US Food and Drug Administration (FDA)-approved medications such as disulfiram, naltrexone, and acamprosate, and off-label uses of other medications (TABLE 49). Not enough empiric evidence is available to judge the effectiveness of these medications in adolescents, and the FDA has not approved them for such use. Evidence from meta-analyses comparing naltrexone and acamprosate have shown naltrexone to be more efficacious in reducing heavy drinking and cravings, while acamprosate is effective in promoting abstinence.22,23 Naltrexone combined with behavioral intervention reduces the heavy drinking days and percentage of abstinence days.24

Current guideline recommendations from the American Psychiatric Association25 include:
- Naltrexone and acamprosate are recommended to treat patients with moderate-to-severe AUD in specific circumstances (eg, when nonpharmacologic approaches have failed to produce an effect or when patients prefer to use one of these medications).
- Topiramate and gabapentin are also suggested as medications for patients with moderate-to-severe AUD, but typically after first trying naltrexone and acamprosate.
- Disulfiram generally should not be used as first-line treatment. It produces physical reactions (eg, flushing) if alcohol is consumed within 12 to 24 hours of medication use.
Continue to: THE CASE
THE CASE
Ms. E was open to the idea of decreasing her alcohol use and agreed that she was depressed. Her lab tests at follow-up were normal other than an elevated AST/ALT of 90/80 U/L. S
She continued to get counseling for her AUD and for her comorbid depression in addition to taking a selective serotonin reuptake inhibitor. She is now in early remission for her alcohol use.
CORRESPONDENCE
Jaividhya Dasarathy, MD, Department of Family Medicine, Metro Health Medical Center, 2500 MetroHealth Drive, Cleveland, OH 44109; jdasarathy@metrohealth.org.
THE CASE
Ms. E, a 42-year-old woman, visited her new physician for a physical exam. When asked about alcohol intake, she reported that she drank 3 to 4 beers after work and sometimes 5 to 8 beers a day on the weekends. Occasionally, she exceeded those amounts, but she didn’t feel guilty about her drinking. She was often late to work and said her relationship with her boyfriend was strained. A review of systems was positive for fatigue, poor concentration, abdominal pain, and weight gain. Her body mass index was 41, pulse 100 beats/min, blood pressure 125/75 mm Hg, and she was afebrile. Her physical exam was otherwise within normal limits.
How would you proceed with this patient?
Alcohol use disorder (AUD) is a common and often untreated condition that is increasingly prevalent in the United States.1 The Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) characterizes AUD as a combination of signs and symptoms typifying alcohol abuse and dependence (discussed in a bit).2
Data from the 2015 National Survey on Drug Use and Health (NSDUH) showed 15.7 million Americans with AUD, affecting 6.2% of the population ages 18 years or older and 2.5% of adolescents ages 12 to 17 years.3
Alcohol use and AUD account for an estimated 3.8% of all global deaths and 4.6% of global disability-adjusted life years.4 AUD adversely affects several systems (TABLE 15), and patients with AUD are sicker and more likely to die younger than those without AUD.4 In the United States, prevalence of AUD has increased in recent years among women, older adults, racial minorities, and individuals with a low education level.6

Screening for AUD is reasonable and straightforward, although diagnosis and treatment of AUD in primary care settings may be challenging due to competing clinical priorities; lack of training, resources, and support; and skepticism about the efficacy of behavioral and pharmacologic treatments.7,8 However, family physicians are in an excellent position to diagnose and help address the complex biopsychosocial needs of patients with AUD, often in collaboration with colleagues and community organizations.
Signs and symptoms of AUD
In clinical practice, at least 2 of the following 11 behaviors or symptoms are required to diagnose AUD2:
- consuming larger amounts of alcohol over a longer period than intended
- persistent desire or unsuccessful efforts to cut down or control alcohol use
- making a significant effort to obtain, use, or recover from alcohol
In moderate-to-severe cases:
- cravings or urges to use alcohol
- recurrent failure to fulfill major work, school, or social obligations
- continued alcohol use despite recurrent social and interpersonal problems
- giving up social, occupational, and recreational activities due to alcohol
- using alcohol in physically dangerous situations
- continued alcohol use despite having physical or psychological problems
- tolerance to alcohol’s effects
- withdrawal symptoms.
Continue to: Patients meet criteria for mild AUD severity if...
Patients meet criteria for mild AUD severity if they exhibit 2 or 3 symptoms, moderate AUD with 4 or 5 symptoms, and severe AUD if there are 6 or more symptoms.2
Those who meet criteria for AUD and are able to stop using alcohol are deemed to be in early remission if the criteria have gone unfulfilled for at least 3 months and less than 12 months. Patients are considered to be in sustained remission if they have not met criteria for AUD at any time during a period of 12 months or longer.
How to detect AUD
Several clues in a patient’s history can suggest AUD (TABLE 29,10). Most imbibers are unaware of the dangers and may consider themselves merely “social drinkers.” Binge drinking may be an early indicator of vulnerability to AUD and should be assessed as part of a thorough clinical evaluation.11 The US Preventive Services Task Force (USPSTF) recommends (Grade B) that clinicians screen adults ages 18 years or older for alcohol misuse.12

Studies demonstrate that both genetic and environmental factors play important roles in the development of AUD.13 A family history of excessive alcohol use increases the risk of AUD. Comorbidity of AUD and other mental health conditions is extremely common. For example, high rates of association between major depressive disorder and AUD have been observed.14
Tools to use in screening and diagnosing AUD
Screening for AUD during an office visit can be done fairly quickly. While 96% of primary care physicians screen for alcohol misuse in some way, only 38% use 1 of the 3 tools recommended by the USPSTF15—the Alcohol Use Disorders Identification Test (AUDIT), the abbreviated AUDIT-C, or the National Institute on Alcohol Abuse and Alcoholism (NIAAA) single question screen—which detect the full spectrum of alcohol misuse in adults.12 Although the commonly used CAGE questionnaire is one of the most studied self-report tools, it has lower sensitivity at a lower level of alcohol intake.16
Continue to: The NIAAA single-question screen asks...
The NIAAA single-question screen asks how many times in the past year the patient had ≥4 drinks (women) or ≥5 drinks (men) in a day.15 The sensitivity and specificity of single-question screening are 82% to 87% and 61% to 79%, respectively, and the test has been validated in several different settings.12 The AUDIT screening tool, freely available from the World Health Organization, is a 10-item questionnaire that probes an individual’s alcohol intake, alcohol dependence, and adverse consequences of alcohol use. Administration of the AUDIT typically requires only 2 minutes. AUDIT-C17 is an abbreviated version of the AUDIT questionnaire that asks 3 consumption questions to screen for AUD.
It was found that AUDIT scores in the range of 8 to 15 indicate a medium-level alcohol problem, whereas a score of ≥16 indicates a high-level alcohol problem. The AUDIT-C is scored from 0 to 12, with ≥4 indicating a problem in men and ≥3
THE CASE
The physician had used the NIAAA single- question screen to determine that Ms. E drank more than 4 beers per day during social events and weekends, which occurred 2 to 3 times per month over the past year. She lives alone and said that she’d been seeing less and less of her boyfriend lately. Her score on the Patient Health Questionnaire (PHQ), which screens for depression, was 11, indicating moderate impairment. Her response on the CAGE questionnaire was negative for a problem with alcohol. However, her AUDIT score was 17, indicating a high-level alcohol problem. Based on these findings, her physician expressed concern that her alcohol use might be contributing to her symptoms and difficulties.
Although she did not have a history of increasing usage per day, a persistent desire to cut down, significant effort to obtain alcohol, or cravings, she was having work troubles and continued to drink even though it was straining relationships, promoting weight gain, and causing abdominal pain.
The physician asked her to schedule a return visit and ordered several blood studies. He also offered to connect her with a colleague with whom he collaborated who could speak with her about possible alcohol use disorders and depression.
Continue to: Selecting blood work in screening for AUD
Selecting blood work in screening for AUD
Lab tests used to measure hepatic injury due to alcohol include gamma-glutamyl-transferase, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and macrocytic volume, although the indices of hepatic damage have low specificity. Elevated serum ethanol levels can reveal recent alcohol use, and vitamin deficiencies and other abnormalities can be used to differentiate other causes of hepatic inflammation and co-existing health issues (TABLE 310,18). A number of as-yet-unvalidated biomarkers are being studied to assist in screening, diagnosing, and treating AUD.18

What treatment approaches work for AUD?
Family physicians can efficiently and productively address AUD by using alcohol screening and brief intervention, which have been shown to reduce risky drinking. Reimbursement for this service is covered by such CPT codes as 99408, 99409, or H0049, or with other evaluation and management (E/M) codes by using modifier 25.
Treatment of AUD varies and should be customized to each patient’s needs, readiness, preferences, and resources. Individual and group counseling approaches can be effective, and medications are available for inpatient and outpatient settings. Psychotherapy options include brief interventions, 12-step programs (eg, Alcoholics Anonymous—https://www.aa.org/pages/en_US/find-aa-resources),motivational enhancement therapy, and cognitive behavioral therapy. Although it is beyond the scope of this article to describe these options in detail, resources are available for those who wish to learn more.19-21
Psychopharmacologic management includes US Food and Drug Administration (FDA)-approved medications such as disulfiram, naltrexone, and acamprosate, and off-label uses of other medications (TABLE 49). Not enough empiric evidence is available to judge the effectiveness of these medications in adolescents, and the FDA has not approved them for such use. Evidence from meta-analyses comparing naltrexone and acamprosate have shown naltrexone to be more efficacious in reducing heavy drinking and cravings, while acamprosate is effective in promoting abstinence.22,23 Naltrexone combined with behavioral intervention reduces the heavy drinking days and percentage of abstinence days.24

Current guideline recommendations from the American Psychiatric Association25 include:
- Naltrexone and acamprosate are recommended to treat patients with moderate-to-severe AUD in specific circumstances (eg, when nonpharmacologic approaches have failed to produce an effect or when patients prefer to use one of these medications).
- Topiramate and gabapentin are also suggested as medications for patients with moderate-to-severe AUD, but typically after first trying naltrexone and acamprosate.
- Disulfiram generally should not be used as first-line treatment. It produces physical reactions (eg, flushing) if alcohol is consumed within 12 to 24 hours of medication use.
Continue to: THE CASE
THE CASE
Ms. E was open to the idea of decreasing her alcohol use and agreed that she was depressed. Her lab tests at follow-up were normal other than an elevated AST/ALT of 90/80 U/L. S
She continued to get counseling for her AUD and for her comorbid depression in addition to taking a selective serotonin reuptake inhibitor. She is now in early remission for her alcohol use.
CORRESPONDENCE
Jaividhya Dasarathy, MD, Department of Family Medicine, Metro Health Medical Center, 2500 MetroHealth Drive, Cleveland, OH 44109; jdasarathy@metrohealth.org.
1. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757-766.
2. APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington DC; 2013.
3. HHS. Results from the 2015 National Survey on Drug Use and Health: summary of national findings. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.pdf. Accessed November 27, 2018.
4. Rehm J, Mathers C, Popova S, et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223-2233.
5. Chase V, Neild R, Sadler CW, et al. The medical complications of alcohol use: understanding mechanisms to improve management. Drug Alcohol Rev. 2005;24:253-265.
6. Grant BF, Chou SP, Saha TD, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74:911-923.
7. Williams EC, Achtmeyer CE, Young JP, et al. Barriers to and facilitators of alcohol use disorder pharmacotherapy in primary care: a qualitative study in five VA clinics. J Gen Intern Med. 2018;33:258-267.
8. Zhang DX, Li ST, Lee QK, et al. Systematic review of guidelines on managing patients with harmful use of alcohol in primary healthcare settings. Alcohol Alcohol. 2017;52:595-609.
9. Wackernah RC, Minnick MJ, Clapp P. Alcohol use disorder: pathophysiology, effects, and pharmacologic options for treatment. Subst Abuse Rehabil. 2014;5:1-12.
10. Kattimani S, Bharadwaj B. Clinical management of alcohol withdrawal: a systematic review. Ind Psychiatry J. 2013;22:100-108.
11. Gowin JL, Sloan ME, Stangl BL, et al. Vulnerability for alcohol use disorder and rate of alcohol consumption. Am J Psychiatry. 2017;174:1094-1101.
12. Moyer VA; Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:210-218.
13. Tarter RE, Alterman AI, Edwards KL. Vulnerability to alcoholism in men: a behavior-genetic perspective. J Stud Alcohol. 1985;46:329-356.
14. Brière FN, Rohde P, Seeley JR, et al. Comorbidity between major depression and alcohol use disorder from adolescence to adulthood [published online ahead of print, October 22, 2013]. Compr Psychiatry. 2014;55:526-533. doi: 10.1016/j.comppsych.2013.10.007.
15. Tan CH, Hungerford DW, Denny CH, et al. Screening for alcohol misuse: practices among U.S. primary care providers, DocStyles 2016. Am J Prev Med. 2018;54:173-180.
16. Aertgeerts B, Buntinx F, Kester A. The value of the CAGE in screening for alcohol abuse and alcohol dependence in general clinical populations: a diagnostic meta-analysis. J Clin Epidemiol. 2004;57:30-39.
17. Bush K, Kivlahan DR, McDonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789-1795.
18. Nanau RM, Neuman MG. Biomolecules and biomarkers used in diagnosis of alcohol drinking and in monitoring therapeutic interventions. Biomolecules. 2015;5:1339-1385.
19. Raddock M, Martukovich R, Berko E, et al. 7 tools to help patients adopt healthier behaviors. J Fam Pract. 2015;64:97-103.
20. AHRQ. Whitlock EP, Green CA, Polen MR, et al. Behavioral Counseling Interventions in Primary Care to Reduce Risky/Harmful Alcohol Use. 2004. https://www.ncbi.nlm.nih.gov/books/NBK42863/. Accessed November 17, 2018.
21. Miller WR, Baca C, Compton WM, et al. Addressing substance abuse in health care settings. Alcohol Clin Exp Res. 2006;30:292-302.
22. Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108:275-293.
23. Rosner S, Leucht S, Lehert P, et al. Acamprosate supports abstinence, naltrexone prevents excessive drinking: evidence from a meta-analysis with unreported outcomes. J Psychopharmacol. 2008;22:11-23.
24. Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003-2017.
25. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. Am J Psychiatry. 2018;175:86-90.
1. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757-766.
2. APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington DC; 2013.
3. HHS. Results from the 2015 National Survey on Drug Use and Health: summary of national findings. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.pdf. Accessed November 27, 2018.
4. Rehm J, Mathers C, Popova S, et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223-2233.
5. Chase V, Neild R, Sadler CW, et al. The medical complications of alcohol use: understanding mechanisms to improve management. Drug Alcohol Rev. 2005;24:253-265.
6. Grant BF, Chou SP, Saha TD, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74:911-923.
7. Williams EC, Achtmeyer CE, Young JP, et al. Barriers to and facilitators of alcohol use disorder pharmacotherapy in primary care: a qualitative study in five VA clinics. J Gen Intern Med. 2018;33:258-267.
8. Zhang DX, Li ST, Lee QK, et al. Systematic review of guidelines on managing patients with harmful use of alcohol in primary healthcare settings. Alcohol Alcohol. 2017;52:595-609.
9. Wackernah RC, Minnick MJ, Clapp P. Alcohol use disorder: pathophysiology, effects, and pharmacologic options for treatment. Subst Abuse Rehabil. 2014;5:1-12.
10. Kattimani S, Bharadwaj B. Clinical management of alcohol withdrawal: a systematic review. Ind Psychiatry J. 2013;22:100-108.
11. Gowin JL, Sloan ME, Stangl BL, et al. Vulnerability for alcohol use disorder and rate of alcohol consumption. Am J Psychiatry. 2017;174:1094-1101.
12. Moyer VA; Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:210-218.
13. Tarter RE, Alterman AI, Edwards KL. Vulnerability to alcoholism in men: a behavior-genetic perspective. J Stud Alcohol. 1985;46:329-356.
14. Brière FN, Rohde P, Seeley JR, et al. Comorbidity between major depression and alcohol use disorder from adolescence to adulthood [published online ahead of print, October 22, 2013]. Compr Psychiatry. 2014;55:526-533. doi: 10.1016/j.comppsych.2013.10.007.
15. Tan CH, Hungerford DW, Denny CH, et al. Screening for alcohol misuse: practices among U.S. primary care providers, DocStyles 2016. Am J Prev Med. 2018;54:173-180.
16. Aertgeerts B, Buntinx F, Kester A. The value of the CAGE in screening for alcohol abuse and alcohol dependence in general clinical populations: a diagnostic meta-analysis. J Clin Epidemiol. 2004;57:30-39.
17. Bush K, Kivlahan DR, McDonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789-1795.
18. Nanau RM, Neuman MG. Biomolecules and biomarkers used in diagnosis of alcohol drinking and in monitoring therapeutic interventions. Biomolecules. 2015;5:1339-1385.
19. Raddock M, Martukovich R, Berko E, et al. 7 tools to help patients adopt healthier behaviors. J Fam Pract. 2015;64:97-103.
20. AHRQ. Whitlock EP, Green CA, Polen MR, et al. Behavioral Counseling Interventions in Primary Care to Reduce Risky/Harmful Alcohol Use. 2004. https://www.ncbi.nlm.nih.gov/books/NBK42863/. Accessed November 17, 2018.
21. Miller WR, Baca C, Compton WM, et al. Addressing substance abuse in health care settings. Alcohol Clin Exp Res. 2006;30:292-302.
22. Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108:275-293.
23. Rosner S, Leucht S, Lehert P, et al. Acamprosate supports abstinence, naltrexone prevents excessive drinking: evidence from a meta-analysis with unreported outcomes. J Psychopharmacol. 2008;22:11-23.
24. Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003-2017.
25. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. Am J Psychiatry. 2018;175:86-90.

