User login
Check Out Our New Irritable Bowel Syndrome Clinician Toolkit
AGA’s new irritable bowel syndrome toolkit gathers all our clinical guidance, continuing education materials, patient education resources, and FAQs in one convenient place. Be sure to check it out and bookmark it for easy access!
Curious about our other toolkits? Visit gastro.org/clinical-guidance to explore our toolkits on ulcerative colitis and Crohn’s disease. Keep an eye out for more coming soon!
The toolkit includes clinical guidance on:
- Pharmacological management of irritable bowel syndrome with diarrhea (IBS-D)
- Pharmacological management of irritable bowel syndrome with constipation (IBS-C)
For more resources for ulcerative colitis patients, visit the Patient Center on the AGA website. The AGA Patient Center has a variety of information that can be shared with your patients, including tips on diet, vaccine recommendations, and information on biosimilars.
AGA’s new irritable bowel syndrome toolkit gathers all our clinical guidance, continuing education materials, patient education resources, and FAQs in one convenient place. Be sure to check it out and bookmark it for easy access!
Curious about our other toolkits? Visit gastro.org/clinical-guidance to explore our toolkits on ulcerative colitis and Crohn’s disease. Keep an eye out for more coming soon!
The toolkit includes clinical guidance on:
- Pharmacological management of irritable bowel syndrome with diarrhea (IBS-D)
- Pharmacological management of irritable bowel syndrome with constipation (IBS-C)
For more resources for ulcerative colitis patients, visit the Patient Center on the AGA website. The AGA Patient Center has a variety of information that can be shared with your patients, including tips on diet, vaccine recommendations, and information on biosimilars.
AGA’s new irritable bowel syndrome toolkit gathers all our clinical guidance, continuing education materials, patient education resources, and FAQs in one convenient place. Be sure to check it out and bookmark it for easy access!
Curious about our other toolkits? Visit gastro.org/clinical-guidance to explore our toolkits on ulcerative colitis and Crohn’s disease. Keep an eye out for more coming soon!
The toolkit includes clinical guidance on:
- Pharmacological management of irritable bowel syndrome with diarrhea (IBS-D)
- Pharmacological management of irritable bowel syndrome with constipation (IBS-C)
For more resources for ulcerative colitis patients, visit the Patient Center on the AGA website. The AGA Patient Center has a variety of information that can be shared with your patients, including tips on diet, vaccine recommendations, and information on biosimilars.
Changing the tumor board conversation: Immunotherapy in resectable NSCLC
Without a doubt, immunotherapy has transformed the treatment landscape of non-small cell lung cancer (NSCLC) and enhanced survival rates across the different stages of disease. High recurrence rates following complete surgical resection prompted the study of immune checkpoint inhibitors (ICI) in earlier, operable stages of disease. This shift toward early application of ICI reflects the larger trend toward merging precision oncology with lung cancer staging. The resulting complexity in treatment and decision making creates systemic and logistical challenges that will require health care systems to adapt and improve.
Adjuvant immunotherapy for NSCLC
Prior to recent approvals for adjuvant immunotherapy, it was standard to give chemotherapy following resection of stage IB-IIIA disease, which offered a statistically nonsignificant survival gain. Recurrence in these patients is believed to be related to postsurgical micrometastasis. The utilization of alternative mechanisms to prevent recurrence is increasingly more common.
Atezolizumab, a PD-L1 inhibitor, is currently approved as first-line adjuvant treatment following chemotherapy in post-NSCLC resection patients with PD-L1 scores ≥1%. This category one recommendation by the National Comprehensive Cancer Network (NCCN) is based on results from the IMpower010 trial, which randomized patients to Atezolizumab vs best supportive care. All were early-stage NSCLC, stage IB-IIIA, who underwent resection followed by platinum-based chemotherapy. Statistically significant benefits were found in disease-free survival (DFS) with a trend toward overall survival.1
The PEARLS/KEYNOTE-091 trial evaluated another PD-L1 inhibitor, Pembrolizumab, as adjuvant therapy. Its design largely mirrored the IMPower010 study, but it differed in that the ICI was administered with or without chemotherapy following resection in patients with stage IB-IIIA NSCLC. Improvements in DFS were found in the overall population, leading to FDA approval for adjuvant therapy in 2023.2
These approvals require changes to the management of operable NSCLC. Until recently, it was not routine to send surgical specimens for additional testing because adjuvant treatment meant chemotherapy only. However, it is now essential that all surgically resected malignant tissue be sent for genomic sequencing and PD-L1 testing. Selecting the next form of therapy, whether it is an ICI or targeted drug therapy, depends on it.
From a surgical perspective, quality surgery with accurate nodal staging is crucial. The surgical findings can determine and identify those who are candidates for adjuvant immunotherapy. For these same reasons, it is helpful to advise surgeons preoperatively that targeted adjuvant therapy is being considered after resection.
Neoadjuvant immunotherapy for NSCLC
ICIs have also been used as neoadjuvant treatment for operable NSCLC. In 2021, the Checkmate-816 trial evaluated Nivolumab with platinum doublet chemotherapy prior to resection of stage IB-IIIa NSCLC. When compared with chemotherapy alone, there were significant improvements in EFS, MPR, and time to death or distant metastasis (TTDM) out to 3 years. At a median follow-up time of 41.4 months, only 28% in the nivolumab group had recurrence postsurgery compared with 42% in the chemotherapy-alone group.3 As a result, certain patients who are likely to receive adjuvant chemotherapy may additionally receive neoadjuvant immunotherapy with chemotherapy before surgical resection. In 2023, the KEYNOTE-671 study demonstrated that neoadjuvant Pembrolizumab and chemotherapy in patients with resectable stage II-IIIb (N2 stage) NSCLC improved EFS. At a median follow-up of 25.2 months, the EFS was 62.4% in the Pembrolizumab group vs 40.6% in the placebo group (P < .001).4
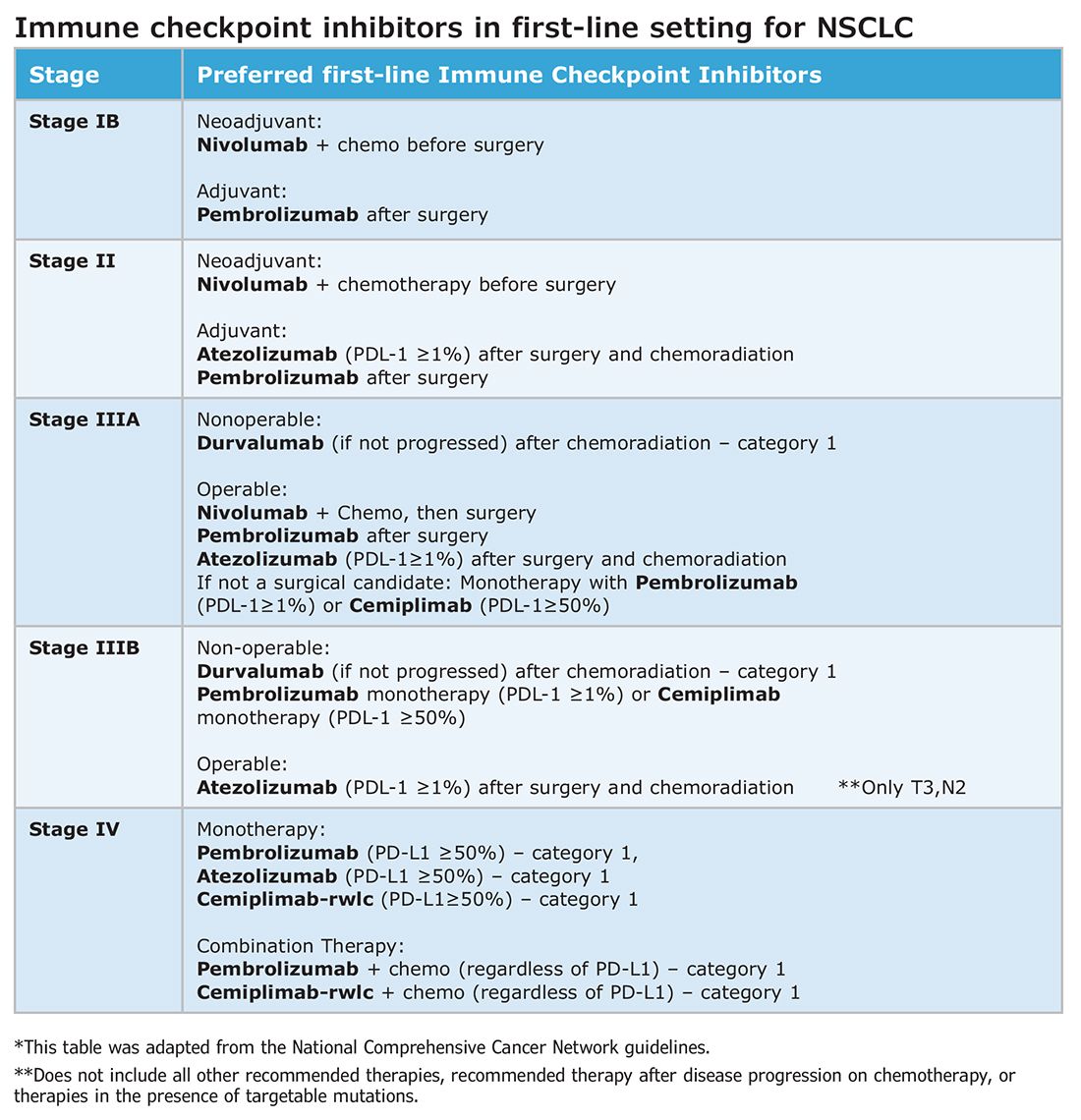
Such changes in treatment options mean patients should be discussed first and simultaneous referrals to oncology and surgery should occur in early-stage NSCLC. Up-front genomic phenotyping and PD-L1 testing may assist in decision making. High PD-L1 levels correlate better with response.
When an ICI-chemotherapy combination is given up front for newly diagnosed NSCLC, there is the potential for large reductions in tumor size and lymph node burden. Although the NCCN does not recommend ICIs to induce resectability, a patient originally deemed inoperable could theoretically become a surgical candidate with neoadjuvant ICI treatment. There is also the potential for toxicity, which could increase the risk of surgery when it does occur. Such scenarios will require frequent tumor board discussions so plans can be adjusted in real time to optimize outcomes as clinical circumstances change.
Perioperative immunotherapy for NSCLC
It is clear that both neoadjuvant and adjuvant immunotherapy can improve outcomes for patients with resectable NSCLC. The combination of neoadjuvant with adjuvant immunotherapy/chemotherapy is currently being studied. Two recent phase III clinical trials, NEOTORCH and AEGAEN, have found statistical improvements in EFS and MPR with this approach.5,6 These studies have not found their way into the NCCN guidelines yet but are sure to be considered in future iterations. Once adopted, the tumor board at each institution will have more options to choose from but many more decisions to make.
References
1. Felip E, Altorki N, Zhou C, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. 2021;398(10308):1344-1357. [Published correction appears in Lancet. 2021 Nov 6;398(10312):1686.]
2. O’Brien M, Paz-Ares L, Marreaud S, et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. 2022;23(10):1274-1286.
3. Forde PM, Spicer J, Lu S, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386(21):1973-1985.
4. Wakelee H, Liberman M, Kato T, et al. Perioperative pembrolizumab for early-stage non-small-cell lung cancer. N Engl J Med. 2023;389(6):491-503.
5. Lu S, Zhang W, Wu L, et al. Perioperative toripalimab plus chemotherapy for patients with resectable non-small cell lung cancer: the neotorch randomized clinical trial. JAMA. 2024;331(3):201-211.
6. Heymach JV, Harpole D, Mitsudomi T, et al. Perioperative durvalumab for resectable non-small-cell lung cancer. N Engl J Med. 2023;389(18):1672-1684.
Without a doubt, immunotherapy has transformed the treatment landscape of non-small cell lung cancer (NSCLC) and enhanced survival rates across the different stages of disease. High recurrence rates following complete surgical resection prompted the study of immune checkpoint inhibitors (ICI) in earlier, operable stages of disease. This shift toward early application of ICI reflects the larger trend toward merging precision oncology with lung cancer staging. The resulting complexity in treatment and decision making creates systemic and logistical challenges that will require health care systems to adapt and improve.
Adjuvant immunotherapy for NSCLC
Prior to recent approvals for adjuvant immunotherapy, it was standard to give chemotherapy following resection of stage IB-IIIA disease, which offered a statistically nonsignificant survival gain. Recurrence in these patients is believed to be related to postsurgical micrometastasis. The utilization of alternative mechanisms to prevent recurrence is increasingly more common.
Atezolizumab, a PD-L1 inhibitor, is currently approved as first-line adjuvant treatment following chemotherapy in post-NSCLC resection patients with PD-L1 scores ≥1%. This category one recommendation by the National Comprehensive Cancer Network (NCCN) is based on results from the IMpower010 trial, which randomized patients to Atezolizumab vs best supportive care. All were early-stage NSCLC, stage IB-IIIA, who underwent resection followed by platinum-based chemotherapy. Statistically significant benefits were found in disease-free survival (DFS) with a trend toward overall survival.1
The PEARLS/KEYNOTE-091 trial evaluated another PD-L1 inhibitor, Pembrolizumab, as adjuvant therapy. Its design largely mirrored the IMPower010 study, but it differed in that the ICI was administered with or without chemotherapy following resection in patients with stage IB-IIIA NSCLC. Improvements in DFS were found in the overall population, leading to FDA approval for adjuvant therapy in 2023.2
These approvals require changes to the management of operable NSCLC. Until recently, it was not routine to send surgical specimens for additional testing because adjuvant treatment meant chemotherapy only. However, it is now essential that all surgically resected malignant tissue be sent for genomic sequencing and PD-L1 testing. Selecting the next form of therapy, whether it is an ICI or targeted drug therapy, depends on it.
From a surgical perspective, quality surgery with accurate nodal staging is crucial. The surgical findings can determine and identify those who are candidates for adjuvant immunotherapy. For these same reasons, it is helpful to advise surgeons preoperatively that targeted adjuvant therapy is being considered after resection.
Neoadjuvant immunotherapy for NSCLC
ICIs have also been used as neoadjuvant treatment for operable NSCLC. In 2021, the Checkmate-816 trial evaluated Nivolumab with platinum doublet chemotherapy prior to resection of stage IB-IIIa NSCLC. When compared with chemotherapy alone, there were significant improvements in EFS, MPR, and time to death or distant metastasis (TTDM) out to 3 years. At a median follow-up time of 41.4 months, only 28% in the nivolumab group had recurrence postsurgery compared with 42% in the chemotherapy-alone group.3 As a result, certain patients who are likely to receive adjuvant chemotherapy may additionally receive neoadjuvant immunotherapy with chemotherapy before surgical resection. In 2023, the KEYNOTE-671 study demonstrated that neoadjuvant Pembrolizumab and chemotherapy in patients with resectable stage II-IIIb (N2 stage) NSCLC improved EFS. At a median follow-up of 25.2 months, the EFS was 62.4% in the Pembrolizumab group vs 40.6% in the placebo group (P < .001).4

Such changes in treatment options mean patients should be discussed first and simultaneous referrals to oncology and surgery should occur in early-stage NSCLC. Up-front genomic phenotyping and PD-L1 testing may assist in decision making. High PD-L1 levels correlate better with response.
When an ICI-chemotherapy combination is given up front for newly diagnosed NSCLC, there is the potential for large reductions in tumor size and lymph node burden. Although the NCCN does not recommend ICIs to induce resectability, a patient originally deemed inoperable could theoretically become a surgical candidate with neoadjuvant ICI treatment. There is also the potential for toxicity, which could increase the risk of surgery when it does occur. Such scenarios will require frequent tumor board discussions so plans can be adjusted in real time to optimize outcomes as clinical circumstances change.
Perioperative immunotherapy for NSCLC
It is clear that both neoadjuvant and adjuvant immunotherapy can improve outcomes for patients with resectable NSCLC. The combination of neoadjuvant with adjuvant immunotherapy/chemotherapy is currently being studied. Two recent phase III clinical trials, NEOTORCH and AEGAEN, have found statistical improvements in EFS and MPR with this approach.5,6 These studies have not found their way into the NCCN guidelines yet but are sure to be considered in future iterations. Once adopted, the tumor board at each institution will have more options to choose from but many more decisions to make.
References
1. Felip E, Altorki N, Zhou C, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. 2021;398(10308):1344-1357. [Published correction appears in Lancet. 2021 Nov 6;398(10312):1686.]
2. O’Brien M, Paz-Ares L, Marreaud S, et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. 2022;23(10):1274-1286.
3. Forde PM, Spicer J, Lu S, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386(21):1973-1985.
4. Wakelee H, Liberman M, Kato T, et al. Perioperative pembrolizumab for early-stage non-small-cell lung cancer. N Engl J Med. 2023;389(6):491-503.
5. Lu S, Zhang W, Wu L, et al. Perioperative toripalimab plus chemotherapy for patients with resectable non-small cell lung cancer: the neotorch randomized clinical trial. JAMA. 2024;331(3):201-211.
6. Heymach JV, Harpole D, Mitsudomi T, et al. Perioperative durvalumab for resectable non-small-cell lung cancer. N Engl J Med. 2023;389(18):1672-1684.
Without a doubt, immunotherapy has transformed the treatment landscape of non-small cell lung cancer (NSCLC) and enhanced survival rates across the different stages of disease. High recurrence rates following complete surgical resection prompted the study of immune checkpoint inhibitors (ICI) in earlier, operable stages of disease. This shift toward early application of ICI reflects the larger trend toward merging precision oncology with lung cancer staging. The resulting complexity in treatment and decision making creates systemic and logistical challenges that will require health care systems to adapt and improve.
Adjuvant immunotherapy for NSCLC
Prior to recent approvals for adjuvant immunotherapy, it was standard to give chemotherapy following resection of stage IB-IIIA disease, which offered a statistically nonsignificant survival gain. Recurrence in these patients is believed to be related to postsurgical micrometastasis. The utilization of alternative mechanisms to prevent recurrence is increasingly more common.
Atezolizumab, a PD-L1 inhibitor, is currently approved as first-line adjuvant treatment following chemotherapy in post-NSCLC resection patients with PD-L1 scores ≥1%. This category one recommendation by the National Comprehensive Cancer Network (NCCN) is based on results from the IMpower010 trial, which randomized patients to Atezolizumab vs best supportive care. All were early-stage NSCLC, stage IB-IIIA, who underwent resection followed by platinum-based chemotherapy. Statistically significant benefits were found in disease-free survival (DFS) with a trend toward overall survival.1
The PEARLS/KEYNOTE-091 trial evaluated another PD-L1 inhibitor, Pembrolizumab, as adjuvant therapy. Its design largely mirrored the IMPower010 study, but it differed in that the ICI was administered with or without chemotherapy following resection in patients with stage IB-IIIA NSCLC. Improvements in DFS were found in the overall population, leading to FDA approval for adjuvant therapy in 2023.2
These approvals require changes to the management of operable NSCLC. Until recently, it was not routine to send surgical specimens for additional testing because adjuvant treatment meant chemotherapy only. However, it is now essential that all surgically resected malignant tissue be sent for genomic sequencing and PD-L1 testing. Selecting the next form of therapy, whether it is an ICI or targeted drug therapy, depends on it.
From a surgical perspective, quality surgery with accurate nodal staging is crucial. The surgical findings can determine and identify those who are candidates for adjuvant immunotherapy. For these same reasons, it is helpful to advise surgeons preoperatively that targeted adjuvant therapy is being considered after resection.
Neoadjuvant immunotherapy for NSCLC
ICIs have also been used as neoadjuvant treatment for operable NSCLC. In 2021, the Checkmate-816 trial evaluated Nivolumab with platinum doublet chemotherapy prior to resection of stage IB-IIIa NSCLC. When compared with chemotherapy alone, there were significant improvements in EFS, MPR, and time to death or distant metastasis (TTDM) out to 3 years. At a median follow-up time of 41.4 months, only 28% in the nivolumab group had recurrence postsurgery compared with 42% in the chemotherapy-alone group.3 As a result, certain patients who are likely to receive adjuvant chemotherapy may additionally receive neoadjuvant immunotherapy with chemotherapy before surgical resection. In 2023, the KEYNOTE-671 study demonstrated that neoadjuvant Pembrolizumab and chemotherapy in patients with resectable stage II-IIIb (N2 stage) NSCLC improved EFS. At a median follow-up of 25.2 months, the EFS was 62.4% in the Pembrolizumab group vs 40.6% in the placebo group (P < .001).4

Such changes in treatment options mean patients should be discussed first and simultaneous referrals to oncology and surgery should occur in early-stage NSCLC. Up-front genomic phenotyping and PD-L1 testing may assist in decision making. High PD-L1 levels correlate better with response.
When an ICI-chemotherapy combination is given up front for newly diagnosed NSCLC, there is the potential for large reductions in tumor size and lymph node burden. Although the NCCN does not recommend ICIs to induce resectability, a patient originally deemed inoperable could theoretically become a surgical candidate with neoadjuvant ICI treatment. There is also the potential for toxicity, which could increase the risk of surgery when it does occur. Such scenarios will require frequent tumor board discussions so plans can be adjusted in real time to optimize outcomes as clinical circumstances change.
Perioperative immunotherapy for NSCLC
It is clear that both neoadjuvant and adjuvant immunotherapy can improve outcomes for patients with resectable NSCLC. The combination of neoadjuvant with adjuvant immunotherapy/chemotherapy is currently being studied. Two recent phase III clinical trials, NEOTORCH and AEGAEN, have found statistical improvements in EFS and MPR with this approach.5,6 These studies have not found their way into the NCCN guidelines yet but are sure to be considered in future iterations. Once adopted, the tumor board at each institution will have more options to choose from but many more decisions to make.
References
1. Felip E, Altorki N, Zhou C, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. 2021;398(10308):1344-1357. [Published correction appears in Lancet. 2021 Nov 6;398(10312):1686.]
2. O’Brien M, Paz-Ares L, Marreaud S, et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. 2022;23(10):1274-1286.
3. Forde PM, Spicer J, Lu S, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386(21):1973-1985.
4. Wakelee H, Liberman M, Kato T, et al. Perioperative pembrolizumab for early-stage non-small-cell lung cancer. N Engl J Med. 2023;389(6):491-503.
5. Lu S, Zhang W, Wu L, et al. Perioperative toripalimab plus chemotherapy for patients with resectable non-small cell lung cancer: the neotorch randomized clinical trial. JAMA. 2024;331(3):201-211.
6. Heymach JV, Harpole D, Mitsudomi T, et al. Perioperative durvalumab for resectable non-small-cell lung cancer. N Engl J Med. 2023;389(18):1672-1684.
Trauma epidemiology and the organization of trauma care in the US
CHEST INFECTIONS AND DISASTER RESPONSE NETWORK
Disaster Response and Global Health Section
Patients who are injured do better when treated at trauma centers.
During CHEST 2023 in Honolulu last year, the Disaster Response and Global Health Section hosted a presentation to a packed audience highlighting the history of the trauma model system in America. Attendees learned about the emergence of trauma systems in the US and the survival advantages that trauma centers offer to patients who are injured.
Inception of the first “trauma manual” was during President Lincoln’s term to address the need for a process to care for patients who were injured during the Civil War. By the time World War II occurred, hospitals had established the idea of emergency departments, and the foundations for an emergency medical system (EMS) emerged on the world scene. Eventually, the Hill-Burton Act of 1946 required hospitals to have emergency departments by incentivizing them with federal funding.
The Committee on Trauma was founded in the 1950s as an adaptation of the American College of Surgeons’ 1922 Committee on Fractures. Then, in 1966, the National Highway Traffic Safety Administration rolled out a federal initiative for all states to develop EMS programs. Additionally, the National Academy of Sciences published Accidental Death and Disability: The Neglected Disease of Modern Society, paving the way for the Emergency Medical Services Systems Act of 1973, along with the Wedworth-Townsend Paramedic Act in California.
The concept of today’s trauma centers started in 1966 at Cook County Hospital in Chicago and Shock Trauma Center in Baltimore.
CHEST INFECTIONS AND DISASTER RESPONSE NETWORK
Disaster Response and Global Health Section
Patients who are injured do better when treated at trauma centers.
During CHEST 2023 in Honolulu last year, the Disaster Response and Global Health Section hosted a presentation to a packed audience highlighting the history of the trauma model system in America. Attendees learned about the emergence of trauma systems in the US and the survival advantages that trauma centers offer to patients who are injured.
Inception of the first “trauma manual” was during President Lincoln’s term to address the need for a process to care for patients who were injured during the Civil War. By the time World War II occurred, hospitals had established the idea of emergency departments, and the foundations for an emergency medical system (EMS) emerged on the world scene. Eventually, the Hill-Burton Act of 1946 required hospitals to have emergency departments by incentivizing them with federal funding.
The Committee on Trauma was founded in the 1950s as an adaptation of the American College of Surgeons’ 1922 Committee on Fractures. Then, in 1966, the National Highway Traffic Safety Administration rolled out a federal initiative for all states to develop EMS programs. Additionally, the National Academy of Sciences published Accidental Death and Disability: The Neglected Disease of Modern Society, paving the way for the Emergency Medical Services Systems Act of 1973, along with the Wedworth-Townsend Paramedic Act in California.
The concept of today’s trauma centers started in 1966 at Cook County Hospital in Chicago and Shock Trauma Center in Baltimore.
CHEST INFECTIONS AND DISASTER RESPONSE NETWORK
Disaster Response and Global Health Section
Patients who are injured do better when treated at trauma centers.
During CHEST 2023 in Honolulu last year, the Disaster Response and Global Health Section hosted a presentation to a packed audience highlighting the history of the trauma model system in America. Attendees learned about the emergence of trauma systems in the US and the survival advantages that trauma centers offer to patients who are injured.
Inception of the first “trauma manual” was during President Lincoln’s term to address the need for a process to care for patients who were injured during the Civil War. By the time World War II occurred, hospitals had established the idea of emergency departments, and the foundations for an emergency medical system (EMS) emerged on the world scene. Eventually, the Hill-Burton Act of 1946 required hospitals to have emergency departments by incentivizing them with federal funding.
The Committee on Trauma was founded in the 1950s as an adaptation of the American College of Surgeons’ 1922 Committee on Fractures. Then, in 1966, the National Highway Traffic Safety Administration rolled out a federal initiative for all states to develop EMS programs. Additionally, the National Academy of Sciences published Accidental Death and Disability: The Neglected Disease of Modern Society, paving the way for the Emergency Medical Services Systems Act of 1973, along with the Wedworth-Townsend Paramedic Act in California.
The concept of today’s trauma centers started in 1966 at Cook County Hospital in Chicago and Shock Trauma Center in Baltimore.
On 5 Ps: PSG, PM, PPG, PulseOx, and PAT
OSA is a very prevalent condition in the general population, but still many patients remain undiagnosed and untreated. Prolonged, untreated OSA is an independent risk factor for major cardiovascular morbidity and mortality. Therefore, timely diagnosis and treatment are required.
Polysomnography (PSG) remains to this day the gold standard for diagnosing sleep apnea. A standard PSG (type I) is performed in a sleep laboratory in the presence of specialized sleep technicians and utilizes EEG, electrooculogram (EOG), and electromyogram (EMG) to determine sleep stages, oronasal thermal and pressure transducer sensors to monitor airflow, respiratory inductance plethysmography to record respiratory effort, EMG for limb movements, pulse oximetry (PulseOx), ECG, and video or body sensor devices to confirm body position. Rising rates of sleep testing have created demand for an alternative to cumbersome, costly, and resource-intensive in-lab PSGs. As such, home sleep apnea testing (HSAT) has emerged as a simpler, more accessible, and cost-effective alternative diagnostic tool.
In 2007, the American Academy of Sleep Medicine (AASM) endorsed Portable Monitoring (PM) as an alternative to standard PSG, with the caveat that it should be used only in patients with a high pretest probability of sleep apnea, without respiratory or cardiovascular disorders and comorbid sleep disorders. All HSAT devices (type II-IV) are required to have a minimum of an oronasal thermal sensor/nasal pressure transducer, respiratory inductance plethysmography, and PulseOx. A major limitation of most HSAT devices is the lack of EEG, preventing detection of cortical arousals and wake time, forcing the use of total recording time as a surrogate for total sleep time.
Peripheral arterial tonometry (PAT)-based HSAT devices are unique in this respect, as their proprietary algorithms allow estimates of total sleep time by monitoring changes in peripheral vascular tone. Anyone who has seen a PAT-based HSAT may have noticed very different outputs from traditional HSATs.
PAT is based on the concept that airflow obstruction may lead to a surge in sympathetic tone, causing vasoconstriction and reduced blood volume in the peripheral vascular bed. A PAT-based device measures relative changes in blood volume and combines this information with actigraphy signals, PulseOx, and heart rate to diagnose the presence of respiratory events. Sleep apnea severity stratification is accomplished by the use of pAHI or pRDI (PAT-based apnea-hypopnea index and respiratory disturbance index, respectively).
PAT-based technology was first approved by the FDA in 2001 as a diagnostic tool for sleep apnea. The 2 best-known medical devices are WatchPAT® and NightOwl®, both of which have been FDA-approved and studied against PSG. To obtain an accurate and sustainable PAT signal, WatchPAT has a pneumo-optic finger probe designed to generate a uniform, subdiastolic pressure on the finger that minimizes venous blood pooling, prevents uncontrolled venous backflow, and effectively unloads the arterial wall tension without blocking digital arterial flow. NightOwl is a smaller device, with a single fingertip sensor that acquires actigraphy and PPG data to measure heart rate, Pulse Ox, and PAT.
The physiological basis of PAT relies on photoplethysmography (PPG), a noninvasive optical monitoring technique that generates a waveform, which ultimately correlates with the circulatory volume of the respective tissue.1 The PPG technology relies on the fact that when a specific tissue is exposed to light signal of a specific wavelength, its absorbance by tissue fluctuates with arterial pulsations. Pulse oximetry represents the most used application of PPG. Recent advances in PPG signal analysis have fueled its use in clinical and consumer sleep technologies and allowed new capabilities, including capturing heart rate and rhythm, pulse rate variability, arterial stiffness, and even—with somewhat less accuracy—energy expenditure, maximum O2 consumption, and blood pressure. Combining actigraphy monitoring with PPG technology took both consumer and medical-grade sleep technologies further, allowing the estimation of parameters such as sleep stages, sleep times, and respiratory events. With myriad new sleep trackers claiming to assess total sleep time, wake time, light or deep sleep, and even respiratory events, the obvious clinical question is centered on their comparative accuracy, as well against more traditional PM and the gold standard PSG.
Numerous studies have evaluated the efficacy of PAT-based devices for diagnosing sleep apnea, with variable findings. Many have shown good correlations between mean AHI and pAHI. Others have highlighted significant discrepancies in the measurements between PSG and PAT, questioning the reliability of PAT-based devices in the diagnosis and severity stratification of OSA. One meta-analysis of 14 studies showed a high degree of correlation between pAHI and AHI.2 Another study reported a concordance of 80% between PAT-based testing and consecutive PSG, with an increase to 86% at a higher AHI (>15/h).3 A subsequent meta-analysis showed that PAT was significantly less sensitive for diagnosing OSA than PSG, particularly for mild or moderate severity disease, emphasizing the need for further confirmation with PSG when faced with inconclusive or negative results.4 A large sleep clinic-based cohort study of 500 patients with OSA showed that WatchPAT devices misclassify OSA in a sizeable proportion of patients (30%-50%), leading to both over- and under-estimation of severity.5 Van Pee, et al, found that their PAT-based HSAT NightOwl performed better, using both the 3% and 4% hypopnea scoring rules and a novel near-border zone labeling.6
Some of the discordance in AHI between PAT and PSG appears to be related to age and sex. In our large sample comparing PAT to PSG, we found that using PAT-based data in concert with demographic (age, gender) and anthropometric (neck circumference, body mass index) variables improved the diagnostic accuracy of PAT-based testing.7 Another study concluded that manual scoring of WatchPAT automated results improved concordance with PSG, particularly in older participants and women. Several studies on WatchPAT recordings have demonstrated significant artifacts and inaccuracies in the PulseOx data. Although WatchPAT employs automated algorithms to remove erroneous data, a thorough visual inspection and manual correction of study data is still essential to derive accurate results.
Recent studies have found that PAT-based tests can also differentiate between central and obstructive respiratory events by using pulse signal upstroke variations caused by changes in intrathoracic pressure and respiratory/chest wall movement recorded by body position sensors, but large-scale studies are needed to confirm these findings. Korkalainen, et al, recently employed a deep-learning model to perform sleep staging on the PPG PulseOx signals from nearly 900 PSGs in patients with suspected OSA.8 The deep learning approach enabled the differentiation of sleep stages and accurate estimation of the total sleep time. Going forward, this could easily enhance the diagnostic yield of PM recordings and enable cost-efficient, long-term monitoring of sleep.
Although PAT-based home sleep tests have emerged as a simple and convenient option for the evaluation of sleep apnea, several studies have highlighted their limited sensitivity as a screening tool for mild and moderate cases of sleep apnea. Furthermore, the scope of these tests remains limited, rendering them rather unsuitable for assessment of more complex sleep disorders like narcolepsy or restless leg syndrome. Therefore, when OSA is suspected, the PAT-based sleep study is a good screening tool, but negative tests should not preclude further investigation. Where a high probability of sleep apnea exists but PAT-based testing shows no or mild OSA, an in-lab sleep study should be performed.
References
1. Ryals S, Chiang A, Schutte-Rodin S, et al. Photoplethysmography -- new applications for an old technology: a sleep technology review. J Clin Sleep Med. 2023;19(1):189-195.
2. Yalamanchali S, Farajian V, Hamilton C, Pott TR, Samuelson CG, Friedman M. Diagnosis of obstructive sleep apnea by peripheral arterial tonometry: meta-analysis. JAMA Otolaryngol Head Neck Surg. 2013;139(12):1343-1350.
3. Röcken J, Schumann DM, Herrmann MJ, et al. Peripheral arterial tonometry versus polysomnography in suspected obstructive sleep apnoea. Eur J Med Res. 2023;28(1):251.
4. Iftikhar IH, Finch CE, Shah AS, Augunstein CA, Ioachimescu OC. A meta-analysis of diagnostic test performance of peripheral arterial tonometry studies. J Clin Sleep Med. 2022;18(4):1093-1102.
5. Ioachimescu OC, Allam JS, Samarghandi A, et al. Performance of peripheral arterial tonometry-based testing for the diagnosis of obstructive sleep apnea in a large sleep clinic cohort. J Clin Sleep Med. 2020;16(10):1663-1674.
6. Van Pee B, Massie F, Vits S, et al. A multicentric validation study of a novel home sleep apnea test based on peripheral arterial tonometry. Sleep. 2022;45(5).
7. Ioachimescu OC, Dholakia SA, Venkateshiah SB, et al. Improving the performance of peripheral arterial tonometry-based testing for the diagnosis of obstructive sleep apnea. J Investig Med. 2020;68(8):1370-1378.
8. Korkalainen H, Aakko J, Duce B, et al. Deep learning enables sleep staging from photoplethysmogram for patients with suspected sleep apnea. Sleep. 2020;43(11).
OSA is a very prevalent condition in the general population, but still many patients remain undiagnosed and untreated. Prolonged, untreated OSA is an independent risk factor for major cardiovascular morbidity and mortality. Therefore, timely diagnosis and treatment are required.
Polysomnography (PSG) remains to this day the gold standard for diagnosing sleep apnea. A standard PSG (type I) is performed in a sleep laboratory in the presence of specialized sleep technicians and utilizes EEG, electrooculogram (EOG), and electromyogram (EMG) to determine sleep stages, oronasal thermal and pressure transducer sensors to monitor airflow, respiratory inductance plethysmography to record respiratory effort, EMG for limb movements, pulse oximetry (PulseOx), ECG, and video or body sensor devices to confirm body position. Rising rates of sleep testing have created demand for an alternative to cumbersome, costly, and resource-intensive in-lab PSGs. As such, home sleep apnea testing (HSAT) has emerged as a simpler, more accessible, and cost-effective alternative diagnostic tool.
In 2007, the American Academy of Sleep Medicine (AASM) endorsed Portable Monitoring (PM) as an alternative to standard PSG, with the caveat that it should be used only in patients with a high pretest probability of sleep apnea, without respiratory or cardiovascular disorders and comorbid sleep disorders. All HSAT devices (type II-IV) are required to have a minimum of an oronasal thermal sensor/nasal pressure transducer, respiratory inductance plethysmography, and PulseOx. A major limitation of most HSAT devices is the lack of EEG, preventing detection of cortical arousals and wake time, forcing the use of total recording time as a surrogate for total sleep time.
Peripheral arterial tonometry (PAT)-based HSAT devices are unique in this respect, as their proprietary algorithms allow estimates of total sleep time by monitoring changes in peripheral vascular tone. Anyone who has seen a PAT-based HSAT may have noticed very different outputs from traditional HSATs.
PAT is based on the concept that airflow obstruction may lead to a surge in sympathetic tone, causing vasoconstriction and reduced blood volume in the peripheral vascular bed. A PAT-based device measures relative changes in blood volume and combines this information with actigraphy signals, PulseOx, and heart rate to diagnose the presence of respiratory events. Sleep apnea severity stratification is accomplished by the use of pAHI or pRDI (PAT-based apnea-hypopnea index and respiratory disturbance index, respectively).
PAT-based technology was first approved by the FDA in 2001 as a diagnostic tool for sleep apnea. The 2 best-known medical devices are WatchPAT® and NightOwl®, both of which have been FDA-approved and studied against PSG. To obtain an accurate and sustainable PAT signal, WatchPAT has a pneumo-optic finger probe designed to generate a uniform, subdiastolic pressure on the finger that minimizes venous blood pooling, prevents uncontrolled venous backflow, and effectively unloads the arterial wall tension without blocking digital arterial flow. NightOwl is a smaller device, with a single fingertip sensor that acquires actigraphy and PPG data to measure heart rate, Pulse Ox, and PAT.
The physiological basis of PAT relies on photoplethysmography (PPG), a noninvasive optical monitoring technique that generates a waveform, which ultimately correlates with the circulatory volume of the respective tissue.1 The PPG technology relies on the fact that when a specific tissue is exposed to light signal of a specific wavelength, its absorbance by tissue fluctuates with arterial pulsations. Pulse oximetry represents the most used application of PPG. Recent advances in PPG signal analysis have fueled its use in clinical and consumer sleep technologies and allowed new capabilities, including capturing heart rate and rhythm, pulse rate variability, arterial stiffness, and even—with somewhat less accuracy—energy expenditure, maximum O2 consumption, and blood pressure. Combining actigraphy monitoring with PPG technology took both consumer and medical-grade sleep technologies further, allowing the estimation of parameters such as sleep stages, sleep times, and respiratory events. With myriad new sleep trackers claiming to assess total sleep time, wake time, light or deep sleep, and even respiratory events, the obvious clinical question is centered on their comparative accuracy, as well against more traditional PM and the gold standard PSG.
Numerous studies have evaluated the efficacy of PAT-based devices for diagnosing sleep apnea, with variable findings. Many have shown good correlations between mean AHI and pAHI. Others have highlighted significant discrepancies in the measurements between PSG and PAT, questioning the reliability of PAT-based devices in the diagnosis and severity stratification of OSA. One meta-analysis of 14 studies showed a high degree of correlation between pAHI and AHI.2 Another study reported a concordance of 80% between PAT-based testing and consecutive PSG, with an increase to 86% at a higher AHI (>15/h).3 A subsequent meta-analysis showed that PAT was significantly less sensitive for diagnosing OSA than PSG, particularly for mild or moderate severity disease, emphasizing the need for further confirmation with PSG when faced with inconclusive or negative results.4 A large sleep clinic-based cohort study of 500 patients with OSA showed that WatchPAT devices misclassify OSA in a sizeable proportion of patients (30%-50%), leading to both over- and under-estimation of severity.5 Van Pee, et al, found that their PAT-based HSAT NightOwl performed better, using both the 3% and 4% hypopnea scoring rules and a novel near-border zone labeling.6
Some of the discordance in AHI between PAT and PSG appears to be related to age and sex. In our large sample comparing PAT to PSG, we found that using PAT-based data in concert with demographic (age, gender) and anthropometric (neck circumference, body mass index) variables improved the diagnostic accuracy of PAT-based testing.7 Another study concluded that manual scoring of WatchPAT automated results improved concordance with PSG, particularly in older participants and women. Several studies on WatchPAT recordings have demonstrated significant artifacts and inaccuracies in the PulseOx data. Although WatchPAT employs automated algorithms to remove erroneous data, a thorough visual inspection and manual correction of study data is still essential to derive accurate results.
Recent studies have found that PAT-based tests can also differentiate between central and obstructive respiratory events by using pulse signal upstroke variations caused by changes in intrathoracic pressure and respiratory/chest wall movement recorded by body position sensors, but large-scale studies are needed to confirm these findings. Korkalainen, et al, recently employed a deep-learning model to perform sleep staging on the PPG PulseOx signals from nearly 900 PSGs in patients with suspected OSA.8 The deep learning approach enabled the differentiation of sleep stages and accurate estimation of the total sleep time. Going forward, this could easily enhance the diagnostic yield of PM recordings and enable cost-efficient, long-term monitoring of sleep.
Although PAT-based home sleep tests have emerged as a simple and convenient option for the evaluation of sleep apnea, several studies have highlighted their limited sensitivity as a screening tool for mild and moderate cases of sleep apnea. Furthermore, the scope of these tests remains limited, rendering them rather unsuitable for assessment of more complex sleep disorders like narcolepsy or restless leg syndrome. Therefore, when OSA is suspected, the PAT-based sleep study is a good screening tool, but negative tests should not preclude further investigation. Where a high probability of sleep apnea exists but PAT-based testing shows no or mild OSA, an in-lab sleep study should be performed.
References
1. Ryals S, Chiang A, Schutte-Rodin S, et al. Photoplethysmography -- new applications for an old technology: a sleep technology review. J Clin Sleep Med. 2023;19(1):189-195.
2. Yalamanchali S, Farajian V, Hamilton C, Pott TR, Samuelson CG, Friedman M. Diagnosis of obstructive sleep apnea by peripheral arterial tonometry: meta-analysis. JAMA Otolaryngol Head Neck Surg. 2013;139(12):1343-1350.
3. Röcken J, Schumann DM, Herrmann MJ, et al. Peripheral arterial tonometry versus polysomnography in suspected obstructive sleep apnoea. Eur J Med Res. 2023;28(1):251.
4. Iftikhar IH, Finch CE, Shah AS, Augunstein CA, Ioachimescu OC. A meta-analysis of diagnostic test performance of peripheral arterial tonometry studies. J Clin Sleep Med. 2022;18(4):1093-1102.
5. Ioachimescu OC, Allam JS, Samarghandi A, et al. Performance of peripheral arterial tonometry-based testing for the diagnosis of obstructive sleep apnea in a large sleep clinic cohort. J Clin Sleep Med. 2020;16(10):1663-1674.
6. Van Pee B, Massie F, Vits S, et al. A multicentric validation study of a novel home sleep apnea test based on peripheral arterial tonometry. Sleep. 2022;45(5).
7. Ioachimescu OC, Dholakia SA, Venkateshiah SB, et al. Improving the performance of peripheral arterial tonometry-based testing for the diagnosis of obstructive sleep apnea. J Investig Med. 2020;68(8):1370-1378.
8. Korkalainen H, Aakko J, Duce B, et al. Deep learning enables sleep staging from photoplethysmogram for patients with suspected sleep apnea. Sleep. 2020;43(11).
OSA is a very prevalent condition in the general population, but still many patients remain undiagnosed and untreated. Prolonged, untreated OSA is an independent risk factor for major cardiovascular morbidity and mortality. Therefore, timely diagnosis and treatment are required.
Polysomnography (PSG) remains to this day the gold standard for diagnosing sleep apnea. A standard PSG (type I) is performed in a sleep laboratory in the presence of specialized sleep technicians and utilizes EEG, electrooculogram (EOG), and electromyogram (EMG) to determine sleep stages, oronasal thermal and pressure transducer sensors to monitor airflow, respiratory inductance plethysmography to record respiratory effort, EMG for limb movements, pulse oximetry (PulseOx), ECG, and video or body sensor devices to confirm body position. Rising rates of sleep testing have created demand for an alternative to cumbersome, costly, and resource-intensive in-lab PSGs. As such, home sleep apnea testing (HSAT) has emerged as a simpler, more accessible, and cost-effective alternative diagnostic tool.
In 2007, the American Academy of Sleep Medicine (AASM) endorsed Portable Monitoring (PM) as an alternative to standard PSG, with the caveat that it should be used only in patients with a high pretest probability of sleep apnea, without respiratory or cardiovascular disorders and comorbid sleep disorders. All HSAT devices (type II-IV) are required to have a minimum of an oronasal thermal sensor/nasal pressure transducer, respiratory inductance plethysmography, and PulseOx. A major limitation of most HSAT devices is the lack of EEG, preventing detection of cortical arousals and wake time, forcing the use of total recording time as a surrogate for total sleep time.
Peripheral arterial tonometry (PAT)-based HSAT devices are unique in this respect, as their proprietary algorithms allow estimates of total sleep time by monitoring changes in peripheral vascular tone. Anyone who has seen a PAT-based HSAT may have noticed very different outputs from traditional HSATs.
PAT is based on the concept that airflow obstruction may lead to a surge in sympathetic tone, causing vasoconstriction and reduced blood volume in the peripheral vascular bed. A PAT-based device measures relative changes in blood volume and combines this information with actigraphy signals, PulseOx, and heart rate to diagnose the presence of respiratory events. Sleep apnea severity stratification is accomplished by the use of pAHI or pRDI (PAT-based apnea-hypopnea index and respiratory disturbance index, respectively).
PAT-based technology was first approved by the FDA in 2001 as a diagnostic tool for sleep apnea. The 2 best-known medical devices are WatchPAT® and NightOwl®, both of which have been FDA-approved and studied against PSG. To obtain an accurate and sustainable PAT signal, WatchPAT has a pneumo-optic finger probe designed to generate a uniform, subdiastolic pressure on the finger that minimizes venous blood pooling, prevents uncontrolled venous backflow, and effectively unloads the arterial wall tension without blocking digital arterial flow. NightOwl is a smaller device, with a single fingertip sensor that acquires actigraphy and PPG data to measure heart rate, Pulse Ox, and PAT.
The physiological basis of PAT relies on photoplethysmography (PPG), a noninvasive optical monitoring technique that generates a waveform, which ultimately correlates with the circulatory volume of the respective tissue.1 The PPG technology relies on the fact that when a specific tissue is exposed to light signal of a specific wavelength, its absorbance by tissue fluctuates with arterial pulsations. Pulse oximetry represents the most used application of PPG. Recent advances in PPG signal analysis have fueled its use in clinical and consumer sleep technologies and allowed new capabilities, including capturing heart rate and rhythm, pulse rate variability, arterial stiffness, and even—with somewhat less accuracy—energy expenditure, maximum O2 consumption, and blood pressure. Combining actigraphy monitoring with PPG technology took both consumer and medical-grade sleep technologies further, allowing the estimation of parameters such as sleep stages, sleep times, and respiratory events. With myriad new sleep trackers claiming to assess total sleep time, wake time, light or deep sleep, and even respiratory events, the obvious clinical question is centered on their comparative accuracy, as well against more traditional PM and the gold standard PSG.
Numerous studies have evaluated the efficacy of PAT-based devices for diagnosing sleep apnea, with variable findings. Many have shown good correlations between mean AHI and pAHI. Others have highlighted significant discrepancies in the measurements between PSG and PAT, questioning the reliability of PAT-based devices in the diagnosis and severity stratification of OSA. One meta-analysis of 14 studies showed a high degree of correlation between pAHI and AHI.2 Another study reported a concordance of 80% between PAT-based testing and consecutive PSG, with an increase to 86% at a higher AHI (>15/h).3 A subsequent meta-analysis showed that PAT was significantly less sensitive for diagnosing OSA than PSG, particularly for mild or moderate severity disease, emphasizing the need for further confirmation with PSG when faced with inconclusive or negative results.4 A large sleep clinic-based cohort study of 500 patients with OSA showed that WatchPAT devices misclassify OSA in a sizeable proportion of patients (30%-50%), leading to both over- and under-estimation of severity.5 Van Pee, et al, found that their PAT-based HSAT NightOwl performed better, using both the 3% and 4% hypopnea scoring rules and a novel near-border zone labeling.6
Some of the discordance in AHI between PAT and PSG appears to be related to age and sex. In our large sample comparing PAT to PSG, we found that using PAT-based data in concert with demographic (age, gender) and anthropometric (neck circumference, body mass index) variables improved the diagnostic accuracy of PAT-based testing.7 Another study concluded that manual scoring of WatchPAT automated results improved concordance with PSG, particularly in older participants and women. Several studies on WatchPAT recordings have demonstrated significant artifacts and inaccuracies in the PulseOx data. Although WatchPAT employs automated algorithms to remove erroneous data, a thorough visual inspection and manual correction of study data is still essential to derive accurate results.
Recent studies have found that PAT-based tests can also differentiate between central and obstructive respiratory events by using pulse signal upstroke variations caused by changes in intrathoracic pressure and respiratory/chest wall movement recorded by body position sensors, but large-scale studies are needed to confirm these findings. Korkalainen, et al, recently employed a deep-learning model to perform sleep staging on the PPG PulseOx signals from nearly 900 PSGs in patients with suspected OSA.8 The deep learning approach enabled the differentiation of sleep stages and accurate estimation of the total sleep time. Going forward, this could easily enhance the diagnostic yield of PM recordings and enable cost-efficient, long-term monitoring of sleep.
Although PAT-based home sleep tests have emerged as a simple and convenient option for the evaluation of sleep apnea, several studies have highlighted their limited sensitivity as a screening tool for mild and moderate cases of sleep apnea. Furthermore, the scope of these tests remains limited, rendering them rather unsuitable for assessment of more complex sleep disorders like narcolepsy or restless leg syndrome. Therefore, when OSA is suspected, the PAT-based sleep study is a good screening tool, but negative tests should not preclude further investigation. Where a high probability of sleep apnea exists but PAT-based testing shows no or mild OSA, an in-lab sleep study should be performed.
References
1. Ryals S, Chiang A, Schutte-Rodin S, et al. Photoplethysmography -- new applications for an old technology: a sleep technology review. J Clin Sleep Med. 2023;19(1):189-195.
2. Yalamanchali S, Farajian V, Hamilton C, Pott TR, Samuelson CG, Friedman M. Diagnosis of obstructive sleep apnea by peripheral arterial tonometry: meta-analysis. JAMA Otolaryngol Head Neck Surg. 2013;139(12):1343-1350.
3. Röcken J, Schumann DM, Herrmann MJ, et al. Peripheral arterial tonometry versus polysomnography in suspected obstructive sleep apnoea. Eur J Med Res. 2023;28(1):251.
4. Iftikhar IH, Finch CE, Shah AS, Augunstein CA, Ioachimescu OC. A meta-analysis of diagnostic test performance of peripheral arterial tonometry studies. J Clin Sleep Med. 2022;18(4):1093-1102.
5. Ioachimescu OC, Allam JS, Samarghandi A, et al. Performance of peripheral arterial tonometry-based testing for the diagnosis of obstructive sleep apnea in a large sleep clinic cohort. J Clin Sleep Med. 2020;16(10):1663-1674.
6. Van Pee B, Massie F, Vits S, et al. A multicentric validation study of a novel home sleep apnea test based on peripheral arterial tonometry. Sleep. 2022;45(5).
7. Ioachimescu OC, Dholakia SA, Venkateshiah SB, et al. Improving the performance of peripheral arterial tonometry-based testing for the diagnosis of obstructive sleep apnea. J Investig Med. 2020;68(8):1370-1378.
8. Korkalainen H, Aakko J, Duce B, et al. Deep learning enables sleep staging from photoplethysmogram for patients with suspected sleep apnea. Sleep. 2020;43(11).
On thoughtful selection of medications in the acute critical care setting
CRITICAL CARE NETWORK
Palliative and End-of-Life Care Section
As critical care medicine continues to advance understanding of ICU survivorship, thoughtful selection of medications in the acute setting can potentially mitigate long-term cognitive, physical, and affective effects.
As of yet, no significant studies have linked opioid use in critical care to new diagnoses of opioid use disorder, but the opioid epidemic has taught us that profligate use of opioids can have devastating effects despite best intentions. Continuous infusions of full agonist opioids for sedation remain an important tool in management of sedation. For acute pain, buprenorphine represents an attractive alternative for patients who are both intubated and nonintubated. It provides equal pain relief as full agonist opioids while causing less respiratory depression, less delirium, less nausea, less constipation, less euphoria, and less misuse potential. Its unique partial mu-opioid agonism is responsible for the improved nausea, constipation, and respiratory depression, while the kappa and delta receptor antagonisms are responsible for antidepressant effects as well as lessened opioid craving, sedation, and dysphoria. Given the variety of doses and routes for buprenorphine, palliative medicine consults can help navigate preventing precipitated withdrawal in patients who are opioid-tolerant and the variety of available dosing and routes.
It is a testament to the growth of critical care medicine that we now have the privilege and responsibility to account for long-term sequelae of our lifesaving interventions, rather than the old model of “prevent death at all costs.” Continued integration of high-quality symptom management into critical care offers an opportunity to better balance life-prolonging treatment and optimize quality of life.
References
1. Neale KJ, Weimer MB, Davis MP, et al. Top ten tips palliative care clinicians should know about buprenorphine. J Palliat Med. 2023;26(1):120-130. doi: 10.1089/jpm.2022.0399
CRITICAL CARE NETWORK
Palliative and End-of-Life Care Section
As critical care medicine continues to advance understanding of ICU survivorship, thoughtful selection of medications in the acute setting can potentially mitigate long-term cognitive, physical, and affective effects.
As of yet, no significant studies have linked opioid use in critical care to new diagnoses of opioid use disorder, but the opioid epidemic has taught us that profligate use of opioids can have devastating effects despite best intentions. Continuous infusions of full agonist opioids for sedation remain an important tool in management of sedation. For acute pain, buprenorphine represents an attractive alternative for patients who are both intubated and nonintubated. It provides equal pain relief as full agonist opioids while causing less respiratory depression, less delirium, less nausea, less constipation, less euphoria, and less misuse potential. Its unique partial mu-opioid agonism is responsible for the improved nausea, constipation, and respiratory depression, while the kappa and delta receptor antagonisms are responsible for antidepressant effects as well as lessened opioid craving, sedation, and dysphoria. Given the variety of doses and routes for buprenorphine, palliative medicine consults can help navigate preventing precipitated withdrawal in patients who are opioid-tolerant and the variety of available dosing and routes.
It is a testament to the growth of critical care medicine that we now have the privilege and responsibility to account for long-term sequelae of our lifesaving interventions, rather than the old model of “prevent death at all costs.” Continued integration of high-quality symptom management into critical care offers an opportunity to better balance life-prolonging treatment and optimize quality of life.
References
1. Neale KJ, Weimer MB, Davis MP, et al. Top ten tips palliative care clinicians should know about buprenorphine. J Palliat Med. 2023;26(1):120-130. doi: 10.1089/jpm.2022.0399
CRITICAL CARE NETWORK
Palliative and End-of-Life Care Section
As critical care medicine continues to advance understanding of ICU survivorship, thoughtful selection of medications in the acute setting can potentially mitigate long-term cognitive, physical, and affective effects.
As of yet, no significant studies have linked opioid use in critical care to new diagnoses of opioid use disorder, but the opioid epidemic has taught us that profligate use of opioids can have devastating effects despite best intentions. Continuous infusions of full agonist opioids for sedation remain an important tool in management of sedation. For acute pain, buprenorphine represents an attractive alternative for patients who are both intubated and nonintubated. It provides equal pain relief as full agonist opioids while causing less respiratory depression, less delirium, less nausea, less constipation, less euphoria, and less misuse potential. Its unique partial mu-opioid agonism is responsible for the improved nausea, constipation, and respiratory depression, while the kappa and delta receptor antagonisms are responsible for antidepressant effects as well as lessened opioid craving, sedation, and dysphoria. Given the variety of doses and routes for buprenorphine, palliative medicine consults can help navigate preventing precipitated withdrawal in patients who are opioid-tolerant and the variety of available dosing and routes.
It is a testament to the growth of critical care medicine that we now have the privilege and responsibility to account for long-term sequelae of our lifesaving interventions, rather than the old model of “prevent death at all costs.” Continued integration of high-quality symptom management into critical care offers an opportunity to better balance life-prolonging treatment and optimize quality of life.
References
1. Neale KJ, Weimer MB, Davis MP, et al. Top ten tips palliative care clinicians should know about buprenorphine. J Palliat Med. 2023;26(1):120-130. doi: 10.1089/jpm.2022.0399
Atypical pulmonary cysts: Why to care
THORACIC ONCOLOGY AND CHEST PROCEDURES NETWORK
Lung Cancer Section
Since the American College of Radiology (ACR) updated its Lung CT Screening Reporting & Data System (Lung-RADS) to include atypical pulmonary cysts in 2022, there has been little discussion among chest physicians regarding the significance of pulmonary cysts and why these changes were made.
Lung-RADS 2022 defined atypical pulmonary cysts as single, unilocular cysts with a wall thickness greater than 2 mm or any multilocular cysts. These can be uniform, asymmetric, or have a focal nodularity. This change was prompted by data derived from multiple studies. First, a finding that 3.6% of lung cancers were associated with cysts at baseline.1 This was followed by a reanalysis of the NELSON trial’s missed cancers showing 22% of those overlooked during initial screening had findings of cystic disease, reaffirming the significance of atypical pulmonary cysts.2 Though the number is low, we now know 1.1% of all cancers present as an atypical cyst, with 4.7% of them being malignant.3
Based on these studies, cysts are a baseline Lung-RADS 4A—a finding that correlates to a higher risk and needs to be followed with a short-term CT scan in 3 months vs a PET. ACR does recommend reserving PET scans for wall thickness > 8 mm. If the repeat CT scan is stable, then the Lung-RADS designation is dropped to a 3 for follow-up.
References
1. Farooqi AO, Cham M, Zhang L, et al. Lung cancer associated with cystic airspaces. AJR Am J Roentgenol. 2012;199(4):781-786.
2. Scholten ET, Horeweg N, Koning HJ, et al. Computed tomographic characteristics of interval and post screen carcinomas in lung cancer screening. Eur Radiol. 2015;25(1):81-88.
3. Mascalchi M, Attinà D, Bertelli E, et al. Lung cancer associated with cystic airspaces. J Comput Assist Tomogr. 2015;39(1):102-108.
THORACIC ONCOLOGY AND CHEST PROCEDURES NETWORK
Lung Cancer Section
Since the American College of Radiology (ACR) updated its Lung CT Screening Reporting & Data System (Lung-RADS) to include atypical pulmonary cysts in 2022, there has been little discussion among chest physicians regarding the significance of pulmonary cysts and why these changes were made.
Lung-RADS 2022 defined atypical pulmonary cysts as single, unilocular cysts with a wall thickness greater than 2 mm or any multilocular cysts. These can be uniform, asymmetric, or have a focal nodularity. This change was prompted by data derived from multiple studies. First, a finding that 3.6% of lung cancers were associated with cysts at baseline.1 This was followed by a reanalysis of the NELSON trial’s missed cancers showing 22% of those overlooked during initial screening had findings of cystic disease, reaffirming the significance of atypical pulmonary cysts.2 Though the number is low, we now know 1.1% of all cancers present as an atypical cyst, with 4.7% of them being malignant.3
Based on these studies, cysts are a baseline Lung-RADS 4A—a finding that correlates to a higher risk and needs to be followed with a short-term CT scan in 3 months vs a PET. ACR does recommend reserving PET scans for wall thickness > 8 mm. If the repeat CT scan is stable, then the Lung-RADS designation is dropped to a 3 for follow-up.
References
1. Farooqi AO, Cham M, Zhang L, et al. Lung cancer associated with cystic airspaces. AJR Am J Roentgenol. 2012;199(4):781-786.
2. Scholten ET, Horeweg N, Koning HJ, et al. Computed tomographic characteristics of interval and post screen carcinomas in lung cancer screening. Eur Radiol. 2015;25(1):81-88.
3. Mascalchi M, Attinà D, Bertelli E, et al. Lung cancer associated with cystic airspaces. J Comput Assist Tomogr. 2015;39(1):102-108.
THORACIC ONCOLOGY AND CHEST PROCEDURES NETWORK
Lung Cancer Section
Since the American College of Radiology (ACR) updated its Lung CT Screening Reporting & Data System (Lung-RADS) to include atypical pulmonary cysts in 2022, there has been little discussion among chest physicians regarding the significance of pulmonary cysts and why these changes were made.
Lung-RADS 2022 defined atypical pulmonary cysts as single, unilocular cysts with a wall thickness greater than 2 mm or any multilocular cysts. These can be uniform, asymmetric, or have a focal nodularity. This change was prompted by data derived from multiple studies. First, a finding that 3.6% of lung cancers were associated with cysts at baseline.1 This was followed by a reanalysis of the NELSON trial’s missed cancers showing 22% of those overlooked during initial screening had findings of cystic disease, reaffirming the significance of atypical pulmonary cysts.2 Though the number is low, we now know 1.1% of all cancers present as an atypical cyst, with 4.7% of them being malignant.3
Based on these studies, cysts are a baseline Lung-RADS 4A—a finding that correlates to a higher risk and needs to be followed with a short-term CT scan in 3 months vs a PET. ACR does recommend reserving PET scans for wall thickness > 8 mm. If the repeat CT scan is stable, then the Lung-RADS designation is dropped to a 3 for follow-up.
References
1. Farooqi AO, Cham M, Zhang L, et al. Lung cancer associated with cystic airspaces. AJR Am J Roentgenol. 2012;199(4):781-786.
2. Scholten ET, Horeweg N, Koning HJ, et al. Computed tomographic characteristics of interval and post screen carcinomas in lung cancer screening. Eur Radiol. 2015;25(1):81-88.
3. Mascalchi M, Attinà D, Bertelli E, et al. Lung cancer associated with cystic airspaces. J Comput Assist Tomogr. 2015;39(1):102-108.
Improving ILD diagnosis in primary care settings
Interstitial lung diseases (ILDs), with their many ubiquitous symptoms, are often hard to diagnose in patients. That’s why Amirahwaty Abdullah, MBBS, and Kavitha Selvan, MD, see value in educating clinicians on how to identify and diagnose ILD.
Both Dr. Abdullah and Dr. Selvan received quality improvement grants from CHEST in October 2023 to do just that. Their projects put the ILD Clinician Toolkit into practice, created as a part of CHEST’s Bridging Specialties™: Timely Diagnosis for ILD initiative aimed at shortening the time to diagnosis.
Recently, CHEST caught up with the two grant recipients to see how their projects were progressing.
Combating the prevalence of ILDs in West Virginia
Dr. Abdullah and Co-Principal Investigator, Haroon Ahmed, MD, are two of the staff members supporting West Virginia University ILD Clinic, where the ILD Clinician Toolkit is being utilized.
“ILD is so prevalent here, so we thought it would be an excellent opportunity to do the quality improvement project here because we really do need the resources to improve the care of our [patients with ILD],” Dr. Abdullah said. “For the entire state of West Virginia, we’re the only ILD center.”
In West Virginia, many factors are at play making ILD prevalent, with a recent study showing that the state has the second highest rate of interstitial pulmonary fibrosis, Dr. Abdullah said. This is all due to the economy of the state, the rurality of the population, and the occupational hazards with common jobs like coal mining and farming.
“This topic about bridging the gap and early diagnosis really resonated with us because we see all these patients who end up seeing us after they’ve been on oxygen for years, when they can’t do anything else,” Dr. Ahmed said. “There was a big gap here, and we saw that every day in our clinical practice.”
Since implementing the ILD Clinician Toolkit into their program, the two have started providing these resources to primary care physicians in an effort to help expedite their workups when they see patients with common ILD symptoms. This was done through grand rounds and educational conferences for those practicing in family medicine, internal medicine, and pediatric medicine. And more education is planned for the future.
They have also created working relationships with these departments and have encouraged them to send patients to the ILD Clinic, so patients don’t have to be referred to multiple different physicians.
The next step for the project is to implement telemedicine capabilities, which will allow the team to roll out the patient questionnaire from the toolkit. The questionnaire helps physicians gather a detailed history about their patients, including their past and current medications, surgeries, occupational and environmental exposures, and known comorbidities.
“We definitely want to reach out via telemedicine to patients because, at this point in time, some of our patients travel 3, 4 hours one way just to come see us. So, if we can make it more accessible, we will,” Dr. Abdullah said.
Their plan is to provide iPads that are equipped with the questionnaire and other toolkit resources to the providers. Through this method, the team will be able to see how often the questionnaire is used.
“We are very thankful for this grant because I do think we are saving lives,” Dr. Abdullah said. “Any little thing that we can do to improve the outcomes of these patients who have a rare but difficult-to-treat disease—it’s crucial. This gives us the ability to reach out and help patients who are out of our physical bounds.”
Diagnosing ILD among underrepresented minority populations in Chicago
Dr. Kavitha Selvan is conducting her quality improvement research project at the University of Chicago, alongside her team.
“The community we serve in South Chicago houses a significant number of underrepresented [minority populations],” she said.
“We know that Black patients with ILD experience higher rates of hospitalization, compared with White patients,” she said. “And they are hospitalized, require lung transplants, and die at a younger age too.”
In clinical practice, Dr. Selvan has noticed a trend of patients being referred for evaluation of possible ILD later and later. In some cases, several years go by before the appropriate work for ILD is initiated, and that valuable time lost leads to irreversible loss of lung function.
Dr. Selvan’s plan is to partner with primary care providers who are seeing these patients first in order to expedite the work-up and referral of ILD when appropriate and, ultimately, reduce the amount of time that passes between symptom onset and definitive diagnosis.
“To me, this grant and the resources it provided represented an important opportunity to improve outcomes in the high-risk patients we care for through early disease recognition and treatment,” she said.
Dr. Selvan’s project began with educating members of the Primary Care Group at UChicago on risk factors and exam findings that may suggest ILD in patients coming to clinic with nonspecific respiratory complaints. Then they had to equip providers with the ILD Clinician Toolkit and reach patients by handing out the patient questionnaire in primary care clinic.
The next step for this project is going to be dissecting the answers to a postintervention survey that was sent out to understand the practices and comfort of primary care providers in evaluating suspected ILD and the utility of the additional resources created by Dr. Selvan’s team.
“My hope is that we can utilize this partnership with the Primary Care Group to provide the education that’s needed both on the patient side and the provider side, like knowing not to ignore respiratory symptoms, knowing which patients warrant ILD-specific testing, and knowing what the appropriate tests are. In doing so, we can get patients into ILD clinic earlier, confirm their diagnosis, and get them initiated on the appropriate therapies sooner,” she said.
Dr. Selvan credits the quality improvement grant as being fundamental in her success as a fellow and early faculty at UChicago Medicine and believes that this project has created a culture of awareness that wouldn’t have been possible without funding.
“I truly believe that early detection and risk factor modification is the most critical aspect of interstitial lung disease care, and the mechanism for how we’re going to actually improve patient outcomes in our community,” Dr. Selvan said. “Building the infrastructure to accomplish that requires time, resources, and support from institutions and philanthropic foundations that believe in that mission too."
Opportunities like this are made possible by generous contributions from our donors. Make a gift to CHEST and select “Clinical Research” to help aid future research into interstitial lung disease and much more.
Interstitial lung diseases (ILDs), with their many ubiquitous symptoms, are often hard to diagnose in patients. That’s why Amirahwaty Abdullah, MBBS, and Kavitha Selvan, MD, see value in educating clinicians on how to identify and diagnose ILD.
Both Dr. Abdullah and Dr. Selvan received quality improvement grants from CHEST in October 2023 to do just that. Their projects put the ILD Clinician Toolkit into practice, created as a part of CHEST’s Bridging Specialties™: Timely Diagnosis for ILD initiative aimed at shortening the time to diagnosis.
Recently, CHEST caught up with the two grant recipients to see how their projects were progressing.
Combating the prevalence of ILDs in West Virginia
Dr. Abdullah and Co-Principal Investigator, Haroon Ahmed, MD, are two of the staff members supporting West Virginia University ILD Clinic, where the ILD Clinician Toolkit is being utilized.
“ILD is so prevalent here, so we thought it would be an excellent opportunity to do the quality improvement project here because we really do need the resources to improve the care of our [patients with ILD],” Dr. Abdullah said. “For the entire state of West Virginia, we’re the only ILD center.”
In West Virginia, many factors are at play making ILD prevalent, with a recent study showing that the state has the second highest rate of interstitial pulmonary fibrosis, Dr. Abdullah said. This is all due to the economy of the state, the rurality of the population, and the occupational hazards with common jobs like coal mining and farming.
“This topic about bridging the gap and early diagnosis really resonated with us because we see all these patients who end up seeing us after they’ve been on oxygen for years, when they can’t do anything else,” Dr. Ahmed said. “There was a big gap here, and we saw that every day in our clinical practice.”
Since implementing the ILD Clinician Toolkit into their program, the two have started providing these resources to primary care physicians in an effort to help expedite their workups when they see patients with common ILD symptoms. This was done through grand rounds and educational conferences for those practicing in family medicine, internal medicine, and pediatric medicine. And more education is planned for the future.
They have also created working relationships with these departments and have encouraged them to send patients to the ILD Clinic, so patients don’t have to be referred to multiple different physicians.
The next step for the project is to implement telemedicine capabilities, which will allow the team to roll out the patient questionnaire from the toolkit. The questionnaire helps physicians gather a detailed history about their patients, including their past and current medications, surgeries, occupational and environmental exposures, and known comorbidities.
“We definitely want to reach out via telemedicine to patients because, at this point in time, some of our patients travel 3, 4 hours one way just to come see us. So, if we can make it more accessible, we will,” Dr. Abdullah said.
Their plan is to provide iPads that are equipped with the questionnaire and other toolkit resources to the providers. Through this method, the team will be able to see how often the questionnaire is used.
“We are very thankful for this grant because I do think we are saving lives,” Dr. Abdullah said. “Any little thing that we can do to improve the outcomes of these patients who have a rare but difficult-to-treat disease—it’s crucial. This gives us the ability to reach out and help patients who are out of our physical bounds.”
Diagnosing ILD among underrepresented minority populations in Chicago
Dr. Kavitha Selvan is conducting her quality improvement research project at the University of Chicago, alongside her team.
“The community we serve in South Chicago houses a significant number of underrepresented [minority populations],” she said.
“We know that Black patients with ILD experience higher rates of hospitalization, compared with White patients,” she said. “And they are hospitalized, require lung transplants, and die at a younger age too.”
In clinical practice, Dr. Selvan has noticed a trend of patients being referred for evaluation of possible ILD later and later. In some cases, several years go by before the appropriate work for ILD is initiated, and that valuable time lost leads to irreversible loss of lung function.
Dr. Selvan’s plan is to partner with primary care providers who are seeing these patients first in order to expedite the work-up and referral of ILD when appropriate and, ultimately, reduce the amount of time that passes between symptom onset and definitive diagnosis.
“To me, this grant and the resources it provided represented an important opportunity to improve outcomes in the high-risk patients we care for through early disease recognition and treatment,” she said.
Dr. Selvan’s project began with educating members of the Primary Care Group at UChicago on risk factors and exam findings that may suggest ILD in patients coming to clinic with nonspecific respiratory complaints. Then they had to equip providers with the ILD Clinician Toolkit and reach patients by handing out the patient questionnaire in primary care clinic.
The next step for this project is going to be dissecting the answers to a postintervention survey that was sent out to understand the practices and comfort of primary care providers in evaluating suspected ILD and the utility of the additional resources created by Dr. Selvan’s team.
“My hope is that we can utilize this partnership with the Primary Care Group to provide the education that’s needed both on the patient side and the provider side, like knowing not to ignore respiratory symptoms, knowing which patients warrant ILD-specific testing, and knowing what the appropriate tests are. In doing so, we can get patients into ILD clinic earlier, confirm their diagnosis, and get them initiated on the appropriate therapies sooner,” she said.
Dr. Selvan credits the quality improvement grant as being fundamental in her success as a fellow and early faculty at UChicago Medicine and believes that this project has created a culture of awareness that wouldn’t have been possible without funding.
“I truly believe that early detection and risk factor modification is the most critical aspect of interstitial lung disease care, and the mechanism for how we’re going to actually improve patient outcomes in our community,” Dr. Selvan said. “Building the infrastructure to accomplish that requires time, resources, and support from institutions and philanthropic foundations that believe in that mission too."
Opportunities like this are made possible by generous contributions from our donors. Make a gift to CHEST and select “Clinical Research” to help aid future research into interstitial lung disease and much more.
Interstitial lung diseases (ILDs), with their many ubiquitous symptoms, are often hard to diagnose in patients. That’s why Amirahwaty Abdullah, MBBS, and Kavitha Selvan, MD, see value in educating clinicians on how to identify and diagnose ILD.
Both Dr. Abdullah and Dr. Selvan received quality improvement grants from CHEST in October 2023 to do just that. Their projects put the ILD Clinician Toolkit into practice, created as a part of CHEST’s Bridging Specialties™: Timely Diagnosis for ILD initiative aimed at shortening the time to diagnosis.
Recently, CHEST caught up with the two grant recipients to see how their projects were progressing.
Combating the prevalence of ILDs in West Virginia
Dr. Abdullah and Co-Principal Investigator, Haroon Ahmed, MD, are two of the staff members supporting West Virginia University ILD Clinic, where the ILD Clinician Toolkit is being utilized.
“ILD is so prevalent here, so we thought it would be an excellent opportunity to do the quality improvement project here because we really do need the resources to improve the care of our [patients with ILD],” Dr. Abdullah said. “For the entire state of West Virginia, we’re the only ILD center.”
In West Virginia, many factors are at play making ILD prevalent, with a recent study showing that the state has the second highest rate of interstitial pulmonary fibrosis, Dr. Abdullah said. This is all due to the economy of the state, the rurality of the population, and the occupational hazards with common jobs like coal mining and farming.
“This topic about bridging the gap and early diagnosis really resonated with us because we see all these patients who end up seeing us after they’ve been on oxygen for years, when they can’t do anything else,” Dr. Ahmed said. “There was a big gap here, and we saw that every day in our clinical practice.”
Since implementing the ILD Clinician Toolkit into their program, the two have started providing these resources to primary care physicians in an effort to help expedite their workups when they see patients with common ILD symptoms. This was done through grand rounds and educational conferences for those practicing in family medicine, internal medicine, and pediatric medicine. And more education is planned for the future.
They have also created working relationships with these departments and have encouraged them to send patients to the ILD Clinic, so patients don’t have to be referred to multiple different physicians.
The next step for the project is to implement telemedicine capabilities, which will allow the team to roll out the patient questionnaire from the toolkit. The questionnaire helps physicians gather a detailed history about their patients, including their past and current medications, surgeries, occupational and environmental exposures, and known comorbidities.
“We definitely want to reach out via telemedicine to patients because, at this point in time, some of our patients travel 3, 4 hours one way just to come see us. So, if we can make it more accessible, we will,” Dr. Abdullah said.
Their plan is to provide iPads that are equipped with the questionnaire and other toolkit resources to the providers. Through this method, the team will be able to see how often the questionnaire is used.
“We are very thankful for this grant because I do think we are saving lives,” Dr. Abdullah said. “Any little thing that we can do to improve the outcomes of these patients who have a rare but difficult-to-treat disease—it’s crucial. This gives us the ability to reach out and help patients who are out of our physical bounds.”
Diagnosing ILD among underrepresented minority populations in Chicago
Dr. Kavitha Selvan is conducting her quality improvement research project at the University of Chicago, alongside her team.
“The community we serve in South Chicago houses a significant number of underrepresented [minority populations],” she said.
“We know that Black patients with ILD experience higher rates of hospitalization, compared with White patients,” she said. “And they are hospitalized, require lung transplants, and die at a younger age too.”
In clinical practice, Dr. Selvan has noticed a trend of patients being referred for evaluation of possible ILD later and later. In some cases, several years go by before the appropriate work for ILD is initiated, and that valuable time lost leads to irreversible loss of lung function.
Dr. Selvan’s plan is to partner with primary care providers who are seeing these patients first in order to expedite the work-up and referral of ILD when appropriate and, ultimately, reduce the amount of time that passes between symptom onset and definitive diagnosis.
“To me, this grant and the resources it provided represented an important opportunity to improve outcomes in the high-risk patients we care for through early disease recognition and treatment,” she said.
Dr. Selvan’s project began with educating members of the Primary Care Group at UChicago on risk factors and exam findings that may suggest ILD in patients coming to clinic with nonspecific respiratory complaints. Then they had to equip providers with the ILD Clinician Toolkit and reach patients by handing out the patient questionnaire in primary care clinic.
The next step for this project is going to be dissecting the answers to a postintervention survey that was sent out to understand the practices and comfort of primary care providers in evaluating suspected ILD and the utility of the additional resources created by Dr. Selvan’s team.
“My hope is that we can utilize this partnership with the Primary Care Group to provide the education that’s needed both on the patient side and the provider side, like knowing not to ignore respiratory symptoms, knowing which patients warrant ILD-specific testing, and knowing what the appropriate tests are. In doing so, we can get patients into ILD clinic earlier, confirm their diagnosis, and get them initiated on the appropriate therapies sooner,” she said.
Dr. Selvan credits the quality improvement grant as being fundamental in her success as a fellow and early faculty at UChicago Medicine and believes that this project has created a culture of awareness that wouldn’t have been possible without funding.
“I truly believe that early detection and risk factor modification is the most critical aspect of interstitial lung disease care, and the mechanism for how we’re going to actually improve patient outcomes in our community,” Dr. Selvan said. “Building the infrastructure to accomplish that requires time, resources, and support from institutions and philanthropic foundations that believe in that mission too."
Opportunities like this are made possible by generous contributions from our donors. Make a gift to CHEST and select “Clinical Research” to help aid future research into interstitial lung disease and much more.
Top reads from the CHEST journal portfolio
Studying endotypes in sleep apnea, diagnostic testing in ILD, and inhaled corticosteroid use in children
Journal CHEST®
By Xiaoting Wang, MM, and colleagues
This study from the Shanghai Sleep Health Study cohort enhances our understanding of physiologic endotypes in sleep apnea—nonpositional OSA (NPOSA) and positional OSA (POSA). The study found that nonanatomic traits play a more significant role in POSA severity. Patients with POSA exhibited lower arousal thresholds and greater ventilatory compensation, while anatomical abnormalities were more prominent in NPOSA. This research underscores the need for personalized treatment strategies based on specific endotypes, especially with the increasing emergence of alternatives to CPAP therapy, such as mandibular advancement therapy and hypoglossal nerve stimulation. Patients with obesity with NPOSA were found to have elevated loop gain, which is known to be impacted by weight loss. This is particularly relevant in light of recent studies showing that new GLP-1 agonists can dramatically reduce sleep apnea severity. Future research should focus on refining these personalized approaches and integrating alternative treatments to provide a more individualized approach to managing sleep apnea.
– Commentary by Shyam Subramanian, MD, FCCP, Member of the CHEST Physician Editorial Board
CHEST® Pulmonary
By Fayez Kheir, MD, and colleagues
The novel mRNA genomic array, Envisia Genomic Classifier (EGC), used in this study has been shown to be highly specific for a histopathologic diagnosis of usual interstitial pneumonia (UIP). A diagnosis of UIP carries significant prognostic import as patients exhibit a higher risk for progressive fibrosis and death compared with other interstitial lung diseases (ILDs). In this retrospective study, the combination of bronchoscopic lung cryobiopsy and EGC testing was associated with a significant change in clinical management of those living with an indeterminate ILD. Participants were more likely to be started on antifibrotic therapy following this intervention. This study emphasizes the need for prospective trials on the diagnostic yield of molecular phenotyping with bronchoscopic lung biopsy compared with the traditional surgical approach for the undifferentiated ILD.
– Commentary by Michael Marll, MD, Member of the CHEST Physician Editorial Board
CHEST® Critical Care
By Elizabeth Landzberg, MD, and colleagues
Respiratory illness is the most common reason for both hospitalization and ICU admission in children. Studies suggest inhaled corticosteroids (ICSs) may protect adults with direct lung injury (DLI) from developing respiratory failure. However, there is a paucity of literature on this therapeutic intervention within the pediatric population. This retrospective, large, single-center study identified children seeking treatment at the emergency department (ED) with DLI before hospitalization. ICS use before hospitalization was associated with one-half the odds of escalation to intubation and noninvasive respiratory support (NRS) in pediatric patients seeking treatment in the ED with DLI. The protective effect was greatest in patients with a history of asthma. According to the authors, this is the first study to assess ICS exposure before hospitalization and progression to respiratory failure in all-cause pediatric DLI. It highlights the need for future studies to evaluate whether a role exists for early prophylactic ICS use in pediatric DLI without status asthmaticus, stratified by history of asthma.
– Commentary by Anne C. Coates, MD, FCCP, Member of the CHEST Physician Editorial Board
Studying endotypes in sleep apnea, diagnostic testing in ILD, and inhaled corticosteroid use in children
Studying endotypes in sleep apnea, diagnostic testing in ILD, and inhaled corticosteroid use in children
Journal CHEST®
By Xiaoting Wang, MM, and colleagues
This study from the Shanghai Sleep Health Study cohort enhances our understanding of physiologic endotypes in sleep apnea—nonpositional OSA (NPOSA) and positional OSA (POSA). The study found that nonanatomic traits play a more significant role in POSA severity. Patients with POSA exhibited lower arousal thresholds and greater ventilatory compensation, while anatomical abnormalities were more prominent in NPOSA. This research underscores the need for personalized treatment strategies based on specific endotypes, especially with the increasing emergence of alternatives to CPAP therapy, such as mandibular advancement therapy and hypoglossal nerve stimulation. Patients with obesity with NPOSA were found to have elevated loop gain, which is known to be impacted by weight loss. This is particularly relevant in light of recent studies showing that new GLP-1 agonists can dramatically reduce sleep apnea severity. Future research should focus on refining these personalized approaches and integrating alternative treatments to provide a more individualized approach to managing sleep apnea.
– Commentary by Shyam Subramanian, MD, FCCP, Member of the CHEST Physician Editorial Board
CHEST® Pulmonary
By Fayez Kheir, MD, and colleagues
The novel mRNA genomic array, Envisia Genomic Classifier (EGC), used in this study has been shown to be highly specific for a histopathologic diagnosis of usual interstitial pneumonia (UIP). A diagnosis of UIP carries significant prognostic import as patients exhibit a higher risk for progressive fibrosis and death compared with other interstitial lung diseases (ILDs). In this retrospective study, the combination of bronchoscopic lung cryobiopsy and EGC testing was associated with a significant change in clinical management of those living with an indeterminate ILD. Participants were more likely to be started on antifibrotic therapy following this intervention. This study emphasizes the need for prospective trials on the diagnostic yield of molecular phenotyping with bronchoscopic lung biopsy compared with the traditional surgical approach for the undifferentiated ILD.
– Commentary by Michael Marll, MD, Member of the CHEST Physician Editorial Board
CHEST® Critical Care
By Elizabeth Landzberg, MD, and colleagues
Respiratory illness is the most common reason for both hospitalization and ICU admission in children. Studies suggest inhaled corticosteroids (ICSs) may protect adults with direct lung injury (DLI) from developing respiratory failure. However, there is a paucity of literature on this therapeutic intervention within the pediatric population. This retrospective, large, single-center study identified children seeking treatment at the emergency department (ED) with DLI before hospitalization. ICS use before hospitalization was associated with one-half the odds of escalation to intubation and noninvasive respiratory support (NRS) in pediatric patients seeking treatment in the ED with DLI. The protective effect was greatest in patients with a history of asthma. According to the authors, this is the first study to assess ICS exposure before hospitalization and progression to respiratory failure in all-cause pediatric DLI. It highlights the need for future studies to evaluate whether a role exists for early prophylactic ICS use in pediatric DLI without status asthmaticus, stratified by history of asthma.
– Commentary by Anne C. Coates, MD, FCCP, Member of the CHEST Physician Editorial Board
Journal CHEST®
By Xiaoting Wang, MM, and colleagues
This study from the Shanghai Sleep Health Study cohort enhances our understanding of physiologic endotypes in sleep apnea—nonpositional OSA (NPOSA) and positional OSA (POSA). The study found that nonanatomic traits play a more significant role in POSA severity. Patients with POSA exhibited lower arousal thresholds and greater ventilatory compensation, while anatomical abnormalities were more prominent in NPOSA. This research underscores the need for personalized treatment strategies based on specific endotypes, especially with the increasing emergence of alternatives to CPAP therapy, such as mandibular advancement therapy and hypoglossal nerve stimulation. Patients with obesity with NPOSA were found to have elevated loop gain, which is known to be impacted by weight loss. This is particularly relevant in light of recent studies showing that new GLP-1 agonists can dramatically reduce sleep apnea severity. Future research should focus on refining these personalized approaches and integrating alternative treatments to provide a more individualized approach to managing sleep apnea.
– Commentary by Shyam Subramanian, MD, FCCP, Member of the CHEST Physician Editorial Board
CHEST® Pulmonary
By Fayez Kheir, MD, and colleagues
The novel mRNA genomic array, Envisia Genomic Classifier (EGC), used in this study has been shown to be highly specific for a histopathologic diagnosis of usual interstitial pneumonia (UIP). A diagnosis of UIP carries significant prognostic import as patients exhibit a higher risk for progressive fibrosis and death compared with other interstitial lung diseases (ILDs). In this retrospective study, the combination of bronchoscopic lung cryobiopsy and EGC testing was associated with a significant change in clinical management of those living with an indeterminate ILD. Participants were more likely to be started on antifibrotic therapy following this intervention. This study emphasizes the need for prospective trials on the diagnostic yield of molecular phenotyping with bronchoscopic lung biopsy compared with the traditional surgical approach for the undifferentiated ILD.
– Commentary by Michael Marll, MD, Member of the CHEST Physician Editorial Board
CHEST® Critical Care
By Elizabeth Landzberg, MD, and colleagues
Respiratory illness is the most common reason for both hospitalization and ICU admission in children. Studies suggest inhaled corticosteroids (ICSs) may protect adults with direct lung injury (DLI) from developing respiratory failure. However, there is a paucity of literature on this therapeutic intervention within the pediatric population. This retrospective, large, single-center study identified children seeking treatment at the emergency department (ED) with DLI before hospitalization. ICS use before hospitalization was associated with one-half the odds of escalation to intubation and noninvasive respiratory support (NRS) in pediatric patients seeking treatment in the ED with DLI. The protective effect was greatest in patients with a history of asthma. According to the authors, this is the first study to assess ICS exposure before hospitalization and progression to respiratory failure in all-cause pediatric DLI. It highlights the need for future studies to evaluate whether a role exists for early prophylactic ICS use in pediatric DLI without status asthmaticus, stratified by history of asthma.
– Commentary by Anne C. Coates, MD, FCCP, Member of the CHEST Physician Editorial Board
APPs and POCUS: Overcoming credentialing challenges
APP INTERSECTION
Advanced practice providers (APPs) play an integral role in the care and management of patients both in the ICU and across the spectrum of health care. Due to reduced residency hours and the coming physician shortage, APPs are playing, and will continue to play, a greater role in the care and management of critically ill patients.
Point of care ultrasound (POCUS) is a key diagnostic modality that promotes rapid diagnosis, shortens time to key interventions, and allows for interval reassessment. However, the benefit relies heavily on operator skill to obtain good quality images, interpret this within the context of the patient, and know what interventions, if any, to make. It is imperative that APPs, given their role within critical care, become not only familiar with POCUS but demonstrate proficiency in practice.
Before this can become standard, there are a number of challenges to overcome. Educational institutions are behind on integrating POCUS into the APP curriculum.1 Additionally, APPs have few opportunities to develop these skills as residencies and fellowships are not required prior to entering the workforce. Instead, for a majority of APPs, POCUS training relies on nonstandardized on-the-job training or an (typically expensive) off-site training, for which they may not receive reimbursement. Other barriers include cultural practices that limit POCUS use by APPs; inability to upload and document images obtained; lack of willing, skilled mentors with time to provide feedback and assess for clinical competence; and even accessibility of ultrasound machines.2
Despite these challenges, the clinical benefits of utilizing ultrasound in practice necessitates overcoming these barriers. Institutionally, when APPs perform POCUS, it can lead to more rapid diagnosis and triage of patients, provide billing opportunities, and may reduce overall costs of care. From an APP perspective, having formalized training and credentialing can lead to more consistent POCUS assessment between providers, comprehensive patient care, security in one’s skills and knowledge base, and a tangible way of communicating one’s credibility between institutions.
While some institutions have overcome these barriers and APPs are trained and credentialed in POCUS, this is not always the norm. For this to come to fruition, we need educators to incorporate this into curriculum, colleagues who are willing to mentor learners, cultural changes to allow APPs to develop and utilize necessary skills for practice, clear structure for obtaining and maintaining competency, physicians to champion these efforts, and institutions to credential skilled APPs. Until then, it is important that we as APPs seek to facilitate these institutional changes and continue to set and maintain high quality standards for ourselves.
CHEST 2024 is for APPs too
With more than 300 educational sessions on the CHEST 2024 program, it can be overwhelming to figure out which ones you should put on your schedule. That’s why CHEST asked Danielle McCamey, DNP, CRNP, ACNP-BC, FCCP, and LaDonna Brown, DNP, CRNA, of DNPs of Color (DOC), as well as Corinne Young, MSN, FNP-C, FCCP, of the Association of Pulmonary Advanced Practice Providers (APAPP), for their recommendations on sessions that advanced practice providers shouldn’t miss. Visit chestnet.org/annual-meeting-apps for all of their recommendations.
References
1. Rudy S, Widmar B, Wilbeck J. Ultrasound education for nurse practitioner students: strategies for curricular integration. J Nurse Pract. 2024;20(6):104986.
2. Resnyk J, Weichold A. Barriers to learning and performing point-of-care ultrasound (POCUS): An integrative review. J Prof Nursing. 2024;54:54-62.
APP INTERSECTION
Advanced practice providers (APPs) play an integral role in the care and management of patients both in the ICU and across the spectrum of health care. Due to reduced residency hours and the coming physician shortage, APPs are playing, and will continue to play, a greater role in the care and management of critically ill patients.
Point of care ultrasound (POCUS) is a key diagnostic modality that promotes rapid diagnosis, shortens time to key interventions, and allows for interval reassessment. However, the benefit relies heavily on operator skill to obtain good quality images, interpret this within the context of the patient, and know what interventions, if any, to make. It is imperative that APPs, given their role within critical care, become not only familiar with POCUS but demonstrate proficiency in practice.
Before this can become standard, there are a number of challenges to overcome. Educational institutions are behind on integrating POCUS into the APP curriculum.1 Additionally, APPs have few opportunities to develop these skills as residencies and fellowships are not required prior to entering the workforce. Instead, for a majority of APPs, POCUS training relies on nonstandardized on-the-job training or an (typically expensive) off-site training, for which they may not receive reimbursement. Other barriers include cultural practices that limit POCUS use by APPs; inability to upload and document images obtained; lack of willing, skilled mentors with time to provide feedback and assess for clinical competence; and even accessibility of ultrasound machines.2
Despite these challenges, the clinical benefits of utilizing ultrasound in practice necessitates overcoming these barriers. Institutionally, when APPs perform POCUS, it can lead to more rapid diagnosis and triage of patients, provide billing opportunities, and may reduce overall costs of care. From an APP perspective, having formalized training and credentialing can lead to more consistent POCUS assessment between providers, comprehensive patient care, security in one’s skills and knowledge base, and a tangible way of communicating one’s credibility between institutions.
While some institutions have overcome these barriers and APPs are trained and credentialed in POCUS, this is not always the norm. For this to come to fruition, we need educators to incorporate this into curriculum, colleagues who are willing to mentor learners, cultural changes to allow APPs to develop and utilize necessary skills for practice, clear structure for obtaining and maintaining competency, physicians to champion these efforts, and institutions to credential skilled APPs. Until then, it is important that we as APPs seek to facilitate these institutional changes and continue to set and maintain high quality standards for ourselves.
CHEST 2024 is for APPs too
With more than 300 educational sessions on the CHEST 2024 program, it can be overwhelming to figure out which ones you should put on your schedule. That’s why CHEST asked Danielle McCamey, DNP, CRNP, ACNP-BC, FCCP, and LaDonna Brown, DNP, CRNA, of DNPs of Color (DOC), as well as Corinne Young, MSN, FNP-C, FCCP, of the Association of Pulmonary Advanced Practice Providers (APAPP), for their recommendations on sessions that advanced practice providers shouldn’t miss. Visit chestnet.org/annual-meeting-apps for all of their recommendations.
References
1. Rudy S, Widmar B, Wilbeck J. Ultrasound education for nurse practitioner students: strategies for curricular integration. J Nurse Pract. 2024;20(6):104986.
2. Resnyk J, Weichold A. Barriers to learning and performing point-of-care ultrasound (POCUS): An integrative review. J Prof Nursing. 2024;54:54-62.
APP INTERSECTION
Advanced practice providers (APPs) play an integral role in the care and management of patients both in the ICU and across the spectrum of health care. Due to reduced residency hours and the coming physician shortage, APPs are playing, and will continue to play, a greater role in the care and management of critically ill patients.
Point of care ultrasound (POCUS) is a key diagnostic modality that promotes rapid diagnosis, shortens time to key interventions, and allows for interval reassessment. However, the benefit relies heavily on operator skill to obtain good quality images, interpret this within the context of the patient, and know what interventions, if any, to make. It is imperative that APPs, given their role within critical care, become not only familiar with POCUS but demonstrate proficiency in practice.
Before this can become standard, there are a number of challenges to overcome. Educational institutions are behind on integrating POCUS into the APP curriculum.1 Additionally, APPs have few opportunities to develop these skills as residencies and fellowships are not required prior to entering the workforce. Instead, for a majority of APPs, POCUS training relies on nonstandardized on-the-job training or an (typically expensive) off-site training, for which they may not receive reimbursement. Other barriers include cultural practices that limit POCUS use by APPs; inability to upload and document images obtained; lack of willing, skilled mentors with time to provide feedback and assess for clinical competence; and even accessibility of ultrasound machines.2
Despite these challenges, the clinical benefits of utilizing ultrasound in practice necessitates overcoming these barriers. Institutionally, when APPs perform POCUS, it can lead to more rapid diagnosis and triage of patients, provide billing opportunities, and may reduce overall costs of care. From an APP perspective, having formalized training and credentialing can lead to more consistent POCUS assessment between providers, comprehensive patient care, security in one’s skills and knowledge base, and a tangible way of communicating one’s credibility between institutions.
While some institutions have overcome these barriers and APPs are trained and credentialed in POCUS, this is not always the norm. For this to come to fruition, we need educators to incorporate this into curriculum, colleagues who are willing to mentor learners, cultural changes to allow APPs to develop and utilize necessary skills for practice, clear structure for obtaining and maintaining competency, physicians to champion these efforts, and institutions to credential skilled APPs. Until then, it is important that we as APPs seek to facilitate these institutional changes and continue to set and maintain high quality standards for ourselves.
CHEST 2024 is for APPs too
With more than 300 educational sessions on the CHEST 2024 program, it can be overwhelming to figure out which ones you should put on your schedule. That’s why CHEST asked Danielle McCamey, DNP, CRNP, ACNP-BC, FCCP, and LaDonna Brown, DNP, CRNA, of DNPs of Color (DOC), as well as Corinne Young, MSN, FNP-C, FCCP, of the Association of Pulmonary Advanced Practice Providers (APAPP), for their recommendations on sessions that advanced practice providers shouldn’t miss. Visit chestnet.org/annual-meeting-apps for all of their recommendations.
References
1. Rudy S, Widmar B, Wilbeck J. Ultrasound education for nurse practitioner students: strategies for curricular integration. J Nurse Pract. 2024;20(6):104986.
2. Resnyk J, Weichold A. Barriers to learning and performing point-of-care ultrasound (POCUS): An integrative review. J Prof Nursing. 2024;54:54-62.
In memoriam: Dr. James E. Dalen
CHEST was recently informed of the death of former CHEST President, James E. Dalen, MD, MPH, Master FCCP. He served as CHEST President from 1985 to 1986.
Dr. Dalen was a graduate of Washington State University. (His undergraduate education was temporarily interrupted when he served as a Corpsman in the Navy and Marines.) He held a master’s degree from the University of Michigan, an MD from the University of Washington, an MPH from Harvard University, and an honorary Doctor of Science degree from the University of Massachusetts. He was internationally recognized as a medical visionary and esteemed cardiologist.
Dr. Dalen’s academic career spanned 3 medical schools. During the time he spent at each, he achieved great things and nurtured great ideas that helped transform medicine. From 1967 to 1975, he was on the faculty of Harvard Medical School (and the Peter Bent Brigham Hospital). From 1975 to 1988, he was a faculty member at the University of Massachusetts Medical School where he served as Chairman of Cardiovascular Medicine from 1975 to 1977 and as Chairman of Medicine from 1977 to 1988. From 1986 to 1987, he served as Interim Chancellor of the University of Massachusetts at the Worcester Campus. Dr. Dalen left Massachusetts in 1988 to become the Dean of the University of Arizona College of Medicine.
During his tenure as Dean and Vice-President at the University of Arizona, Dr. Dalen oversaw the establishment of the Zuckerman College of Public Health, the Arizona Center for Integrative Medicine, and the Arizona Telemedicine Program. Successful fundraising under his leadership led to the establishment of new research facilities, including the Children’s Research Center, the Sarver Heart Center, the Arizona Arthritis Center, and a major expansion of the Arizona Cancer Center. While at the University of Arizona College of Medicine, Dr. Dalen recognized the importance of integrative medicine and the need to promote integrative medicine in the academic environment. With the active participation of Dr. Andrew Weil, this led to the creation of the Academic Consortium for Integrative Medicine and Health and the Arizona Center for Integrative Medicine.
Dr. Dalen received many teaching awards. In 1987, he received the Distinguished Public Service Award from the University of Massachusetts. In 1988, he was named the University of Washington Distinguished Medical Alumnus of the Year and received the Alumni Achievement Award from Washington State University. In 2000, he received the College Medal from CHEST and was named a Master Fellow. In 2010, he was awarded the Harvard School of Public Health’s highest honor for its alumni: the 2010 Alumni Award of Merit. In 2012, he was named a Master Fellow of the American College of Physicians and was awarded an honorary Doctor of Science degree by the University of Massachusetts in 2013. In 2015, he received the Bravewell Distinguished Service Award from the Academic Consortium for Integrative Medicine and Health for his role as one of the founders of the consortium and as one of the founders of the Arizona Center for Integrative Medicine at the University of Arizona.
In his spare time, he enjoyed reading, gardening, sailing, art, and music and was a lifelong student with a passion for the pursuit of knowledge. Dr. Dalen enjoyed watching basketball, particularly the Arizona Wildcats. His favorite place to spend time was by the ocean in Coronado, California. Dr. Dalen was an advocate for public health, social justice, and health care reform throughout his life and career. He mentored thousands of medical students and championed access to health care for populations who were underserved. He touched many lives as a leading physician and caring friend.
Dr. Dalen is survived by his devoted wife, Priscilla M. Dalen (Dunton); loving children, James E. Dalen, Jr, and Angela M. Snodgrass (Dalen); daughter-in-law, Suzan M. Dalen; son-in-law, Daniel N. Snodgrass; and many step-grandchildren. We remember our colleague and extend our sincere condolences.
CHEST was recently informed of the death of former CHEST President, James E. Dalen, MD, MPH, Master FCCP. He served as CHEST President from 1985 to 1986.
Dr. Dalen was a graduate of Washington State University. (His undergraduate education was temporarily interrupted when he served as a Corpsman in the Navy and Marines.) He held a master’s degree from the University of Michigan, an MD from the University of Washington, an MPH from Harvard University, and an honorary Doctor of Science degree from the University of Massachusetts. He was internationally recognized as a medical visionary and esteemed cardiologist.
Dr. Dalen’s academic career spanned 3 medical schools. During the time he spent at each, he achieved great things and nurtured great ideas that helped transform medicine. From 1967 to 1975, he was on the faculty of Harvard Medical School (and the Peter Bent Brigham Hospital). From 1975 to 1988, he was a faculty member at the University of Massachusetts Medical School where he served as Chairman of Cardiovascular Medicine from 1975 to 1977 and as Chairman of Medicine from 1977 to 1988. From 1986 to 1987, he served as Interim Chancellor of the University of Massachusetts at the Worcester Campus. Dr. Dalen left Massachusetts in 1988 to become the Dean of the University of Arizona College of Medicine.
During his tenure as Dean and Vice-President at the University of Arizona, Dr. Dalen oversaw the establishment of the Zuckerman College of Public Health, the Arizona Center for Integrative Medicine, and the Arizona Telemedicine Program. Successful fundraising under his leadership led to the establishment of new research facilities, including the Children’s Research Center, the Sarver Heart Center, the Arizona Arthritis Center, and a major expansion of the Arizona Cancer Center. While at the University of Arizona College of Medicine, Dr. Dalen recognized the importance of integrative medicine and the need to promote integrative medicine in the academic environment. With the active participation of Dr. Andrew Weil, this led to the creation of the Academic Consortium for Integrative Medicine and Health and the Arizona Center for Integrative Medicine.
Dr. Dalen received many teaching awards. In 1987, he received the Distinguished Public Service Award from the University of Massachusetts. In 1988, he was named the University of Washington Distinguished Medical Alumnus of the Year and received the Alumni Achievement Award from Washington State University. In 2000, he received the College Medal from CHEST and was named a Master Fellow. In 2010, he was awarded the Harvard School of Public Health’s highest honor for its alumni: the 2010 Alumni Award of Merit. In 2012, he was named a Master Fellow of the American College of Physicians and was awarded an honorary Doctor of Science degree by the University of Massachusetts in 2013. In 2015, he received the Bravewell Distinguished Service Award from the Academic Consortium for Integrative Medicine and Health for his role as one of the founders of the consortium and as one of the founders of the Arizona Center for Integrative Medicine at the University of Arizona.
In his spare time, he enjoyed reading, gardening, sailing, art, and music and was a lifelong student with a passion for the pursuit of knowledge. Dr. Dalen enjoyed watching basketball, particularly the Arizona Wildcats. His favorite place to spend time was by the ocean in Coronado, California. Dr. Dalen was an advocate for public health, social justice, and health care reform throughout his life and career. He mentored thousands of medical students and championed access to health care for populations who were underserved. He touched many lives as a leading physician and caring friend.
Dr. Dalen is survived by his devoted wife, Priscilla M. Dalen (Dunton); loving children, James E. Dalen, Jr, and Angela M. Snodgrass (Dalen); daughter-in-law, Suzan M. Dalen; son-in-law, Daniel N. Snodgrass; and many step-grandchildren. We remember our colleague and extend our sincere condolences.
CHEST was recently informed of the death of former CHEST President, James E. Dalen, MD, MPH, Master FCCP. He served as CHEST President from 1985 to 1986.
Dr. Dalen was a graduate of Washington State University. (His undergraduate education was temporarily interrupted when he served as a Corpsman in the Navy and Marines.) He held a master’s degree from the University of Michigan, an MD from the University of Washington, an MPH from Harvard University, and an honorary Doctor of Science degree from the University of Massachusetts. He was internationally recognized as a medical visionary and esteemed cardiologist.
Dr. Dalen’s academic career spanned 3 medical schools. During the time he spent at each, he achieved great things and nurtured great ideas that helped transform medicine. From 1967 to 1975, he was on the faculty of Harvard Medical School (and the Peter Bent Brigham Hospital). From 1975 to 1988, he was a faculty member at the University of Massachusetts Medical School where he served as Chairman of Cardiovascular Medicine from 1975 to 1977 and as Chairman of Medicine from 1977 to 1988. From 1986 to 1987, he served as Interim Chancellor of the University of Massachusetts at the Worcester Campus. Dr. Dalen left Massachusetts in 1988 to become the Dean of the University of Arizona College of Medicine.
During his tenure as Dean and Vice-President at the University of Arizona, Dr. Dalen oversaw the establishment of the Zuckerman College of Public Health, the Arizona Center for Integrative Medicine, and the Arizona Telemedicine Program. Successful fundraising under his leadership led to the establishment of new research facilities, including the Children’s Research Center, the Sarver Heart Center, the Arizona Arthritis Center, and a major expansion of the Arizona Cancer Center. While at the University of Arizona College of Medicine, Dr. Dalen recognized the importance of integrative medicine and the need to promote integrative medicine in the academic environment. With the active participation of Dr. Andrew Weil, this led to the creation of the Academic Consortium for Integrative Medicine and Health and the Arizona Center for Integrative Medicine.
Dr. Dalen received many teaching awards. In 1987, he received the Distinguished Public Service Award from the University of Massachusetts. In 1988, he was named the University of Washington Distinguished Medical Alumnus of the Year and received the Alumni Achievement Award from Washington State University. In 2000, he received the College Medal from CHEST and was named a Master Fellow. In 2010, he was awarded the Harvard School of Public Health’s highest honor for its alumni: the 2010 Alumni Award of Merit. In 2012, he was named a Master Fellow of the American College of Physicians and was awarded an honorary Doctor of Science degree by the University of Massachusetts in 2013. In 2015, he received the Bravewell Distinguished Service Award from the Academic Consortium for Integrative Medicine and Health for his role as one of the founders of the consortium and as one of the founders of the Arizona Center for Integrative Medicine at the University of Arizona.
In his spare time, he enjoyed reading, gardening, sailing, art, and music and was a lifelong student with a passion for the pursuit of knowledge. Dr. Dalen enjoyed watching basketball, particularly the Arizona Wildcats. His favorite place to spend time was by the ocean in Coronado, California. Dr. Dalen was an advocate for public health, social justice, and health care reform throughout his life and career. He mentored thousands of medical students and championed access to health care for populations who were underserved. He touched many lives as a leading physician and caring friend.
Dr. Dalen is survived by his devoted wife, Priscilla M. Dalen (Dunton); loving children, James E. Dalen, Jr, and Angela M. Snodgrass (Dalen); daughter-in-law, Suzan M. Dalen; son-in-law, Daniel N. Snodgrass; and many step-grandchildren. We remember our colleague and extend our sincere condolences.























