User login
Tapinarof effective for AD in patients as young as 2 years
BERLIN – of age, according to results of two pivotal trials presented at the at the annual congress of the European Academy of Dermatology and Venereology.
If approved for AD, one advantage of tapinarof cream relative to topical corticosteroids is potential use “without restrictions on duration, extent, or site of application,” reported Jonathan I. Silverberg, MD, PhD, MPH, director of clinical research, George Washington University, Washington.
Tapinarof cream, 1%, an aryl hydrocarbon receptor agonist, was approved in 2022 for treating plaque psoriasis in adults.
In the two phase 3 trials, ADORING 1 and ADORING 2, which were presented together at the meeting, the primary endpoint was Validated Investigator Global Assessment (vIGA) for AD of 0 (clear) or 1 (almost clear) at 8 weeks. For this endpoint and all secondary endpoints, the relative advantage of the active cream over the vehicle alone was about the same in both studies.
For example, the vIGA clear or almost clear response was met by 45.4% and 46.4% of those in the experimental arm of ADORING 1 and 2, respectively, but only 13.9% and 18.0% in the control arms (P < .0001 for both).
For the secondary endpoint of Eczema Area and Severity Index (EASI75), signifying 75% clearance of skin lesions, the response rates were 55.8% and 59.1% in the two trials, but only 22.9% and 24.1% in the respective control arms (P < .0001 for both).
The two identically designed trials randomized patients with moderate to severe AD in a 2:1 ratio to tapinarof cream or vehicle alone. There were 407 patients ages 2-81 years in ADORING I and 406 in ADORING 2. Patients were instructed to apply the active cream or vehicle once per day.
The safety data for tapinarof in these studies was generally consistent with the experience with this agent in plaque psoriasis. According to Dr. Silverberg, there was a modest increase in reports of headache early in this study, but these were transient. Follicular events were also more common on tapinarof than on its vehicle, but Dr. Silverberg said that the rate of discontinuations for adverse events, although low in both arms, was numerically lower in the active treatment arm in both trials.
“There were reports of contact dermatitis in the psoriasis studies, but we have not seen this in the AD trials,” Dr. Silverberg said.
Itch control evaluated
In a separate presentation of ADORING 1 and 2 results, Eric Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland, provided detailed information about itch control, which was evaluated with the Peak Pruritus–Numerical Rating Scale (PP-NRS).
“The PP-NRS considers a person’s worst itch over the past 24 hours based on an 11-point scale,” explained Dr. Simpson, who said that patients scored itch daily with comparisons made at weeks 1, 2, 4, and 8.
Over time, pruritus scores fell in both groups, but reductions were far steeper among those in the active treatment arms.
“In ADORING 1, there were greater reductions in itch as early as day 1,” Dr. Simpson reported. Although the differences in itch were not detected until day 2 in ADORING 2, the differences were already significant and clinically meaningful in both studies by the end of the first week.
By week 8, the mean reductions in PP-NRS scores were 2.6 and 2.4 in the vehicle arms of ADORING 1 and 2, respectively. In the treatment arm, the reduction was 4.1 points in both arms (P < .0001 for both studies).
Forty-eight–week follow-up planned
More than 90% of patients in both studies have rolled over into the open-label extension ADORING 3 trial, with a planned follow-up of 48 weeks, according to Dr. Silverberg, who said that those in the placebo arm have been crossed over to tapinarof.
The response and the safety appear to be similar in adults and children, although Dr. Silverberg said that further analyses of outcomes by age are planned. He noted that there is also an ongoing study of tapinarof in children with plaque psoriasis.
In AD in particular, Dr. Silverberg said there is “an unmet need” for a topical nonsteroidal anti-inflammatory. While topical corticosteroids are a mainstay of AD therapy in children as well as adults, he noted the limitations of these drugs, including that they can only be applied for limited periods.
Tapinarof binds to the aryl hydrocarbon receptor (AhR), which regulates immune function in the skin and is expressed in many skin cell types. By inhibiting AhR, tapinarof blocks cytokine activation and has an antioxidant effect.
Adelaide A. Hebert, MD, professor and director of pediatric dermatology, McGovern Medical School at UTHealth, Houston, has participated in clinical studies of tapinarof for AD, and said she has been impressed with its efficacy and tolerability in children as well as adults. In the case of children, parents, as well as patients, “valued the rapid onset of disease control, the once-daily application regimen, and the itch control,” she said in an interview after the meeting.
If approved, Dr. Hebert said, “this novel steroid-free medication has the potential to change the management arena for pediatric and adult patients with moderate to severe atopic dermatitis.”
The recent introduction of new systemic therapies for AD, such as JAK inhibitors, has increased options for AD control, but “we still need effective and safe topical therapies, especially in children and young adults,” said Sonja Ständer, MD, head of the Interdisciplinary Center for Chronic Pruritus, University of Münster (Germany). Author of a comprehensive review article on AD in the New England Journal of Medicine 2 years ago, Dr. Ständer said results from the phase 3 topical tapinarof trials, as well as the phase 3 topical ruxolitinib trials, which were also presented as late breakers at the 2023 EADV meeting, provide “hope that an alternative to topical steroids will soon be available.”
Based on their safety and rapid control of itch in children with AD, “these will complement our current portfolio of topical therapies very well and have the potential to replace topical steroids early in therapy or to replace them altogether,” she told this news organization.
Dermavant Sciences, manufacturer of tapinarof, anticipates filing for Food and Drug Administration approval for AD in the first quarter of 2024, according to a company statement.
Dr. Silverberg and Dr. Simpson reported financial relationships with multiple pharmaceutical companies, including Dermavant, which provided funding for the ADORING trials. Dr. Hebert has financial relationship with more than 15 pharmaceutical companies, including Dermavent and other companies that have or are developing therapies for AD. Dr. Ständer reported financial relationships with Beiersdorf, Eli Lilly, Galderma, Kiniksa, Pfizer, and Sanofi.
BERLIN – of age, according to results of two pivotal trials presented at the at the annual congress of the European Academy of Dermatology and Venereology.
If approved for AD, one advantage of tapinarof cream relative to topical corticosteroids is potential use “without restrictions on duration, extent, or site of application,” reported Jonathan I. Silverberg, MD, PhD, MPH, director of clinical research, George Washington University, Washington.
Tapinarof cream, 1%, an aryl hydrocarbon receptor agonist, was approved in 2022 for treating plaque psoriasis in adults.
In the two phase 3 trials, ADORING 1 and ADORING 2, which were presented together at the meeting, the primary endpoint was Validated Investigator Global Assessment (vIGA) for AD of 0 (clear) or 1 (almost clear) at 8 weeks. For this endpoint and all secondary endpoints, the relative advantage of the active cream over the vehicle alone was about the same in both studies.
For example, the vIGA clear or almost clear response was met by 45.4% and 46.4% of those in the experimental arm of ADORING 1 and 2, respectively, but only 13.9% and 18.0% in the control arms (P < .0001 for both).
For the secondary endpoint of Eczema Area and Severity Index (EASI75), signifying 75% clearance of skin lesions, the response rates were 55.8% and 59.1% in the two trials, but only 22.9% and 24.1% in the respective control arms (P < .0001 for both).
The two identically designed trials randomized patients with moderate to severe AD in a 2:1 ratio to tapinarof cream or vehicle alone. There were 407 patients ages 2-81 years in ADORING I and 406 in ADORING 2. Patients were instructed to apply the active cream or vehicle once per day.
The safety data for tapinarof in these studies was generally consistent with the experience with this agent in plaque psoriasis. According to Dr. Silverberg, there was a modest increase in reports of headache early in this study, but these were transient. Follicular events were also more common on tapinarof than on its vehicle, but Dr. Silverberg said that the rate of discontinuations for adverse events, although low in both arms, was numerically lower in the active treatment arm in both trials.
“There were reports of contact dermatitis in the psoriasis studies, but we have not seen this in the AD trials,” Dr. Silverberg said.
Itch control evaluated
In a separate presentation of ADORING 1 and 2 results, Eric Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland, provided detailed information about itch control, which was evaluated with the Peak Pruritus–Numerical Rating Scale (PP-NRS).
“The PP-NRS considers a person’s worst itch over the past 24 hours based on an 11-point scale,” explained Dr. Simpson, who said that patients scored itch daily with comparisons made at weeks 1, 2, 4, and 8.
Over time, pruritus scores fell in both groups, but reductions were far steeper among those in the active treatment arms.
“In ADORING 1, there were greater reductions in itch as early as day 1,” Dr. Simpson reported. Although the differences in itch were not detected until day 2 in ADORING 2, the differences were already significant and clinically meaningful in both studies by the end of the first week.
By week 8, the mean reductions in PP-NRS scores were 2.6 and 2.4 in the vehicle arms of ADORING 1 and 2, respectively. In the treatment arm, the reduction was 4.1 points in both arms (P < .0001 for both studies).
Forty-eight–week follow-up planned
More than 90% of patients in both studies have rolled over into the open-label extension ADORING 3 trial, with a planned follow-up of 48 weeks, according to Dr. Silverberg, who said that those in the placebo arm have been crossed over to tapinarof.
The response and the safety appear to be similar in adults and children, although Dr. Silverberg said that further analyses of outcomes by age are planned. He noted that there is also an ongoing study of tapinarof in children with plaque psoriasis.
In AD in particular, Dr. Silverberg said there is “an unmet need” for a topical nonsteroidal anti-inflammatory. While topical corticosteroids are a mainstay of AD therapy in children as well as adults, he noted the limitations of these drugs, including that they can only be applied for limited periods.
Tapinarof binds to the aryl hydrocarbon receptor (AhR), which regulates immune function in the skin and is expressed in many skin cell types. By inhibiting AhR, tapinarof blocks cytokine activation and has an antioxidant effect.
Adelaide A. Hebert, MD, professor and director of pediatric dermatology, McGovern Medical School at UTHealth, Houston, has participated in clinical studies of tapinarof for AD, and said she has been impressed with its efficacy and tolerability in children as well as adults. In the case of children, parents, as well as patients, “valued the rapid onset of disease control, the once-daily application regimen, and the itch control,” she said in an interview after the meeting.
If approved, Dr. Hebert said, “this novel steroid-free medication has the potential to change the management arena for pediatric and adult patients with moderate to severe atopic dermatitis.”
The recent introduction of new systemic therapies for AD, such as JAK inhibitors, has increased options for AD control, but “we still need effective and safe topical therapies, especially in children and young adults,” said Sonja Ständer, MD, head of the Interdisciplinary Center for Chronic Pruritus, University of Münster (Germany). Author of a comprehensive review article on AD in the New England Journal of Medicine 2 years ago, Dr. Ständer said results from the phase 3 topical tapinarof trials, as well as the phase 3 topical ruxolitinib trials, which were also presented as late breakers at the 2023 EADV meeting, provide “hope that an alternative to topical steroids will soon be available.”
Based on their safety and rapid control of itch in children with AD, “these will complement our current portfolio of topical therapies very well and have the potential to replace topical steroids early in therapy or to replace them altogether,” she told this news organization.
Dermavant Sciences, manufacturer of tapinarof, anticipates filing for Food and Drug Administration approval for AD in the first quarter of 2024, according to a company statement.
Dr. Silverberg and Dr. Simpson reported financial relationships with multiple pharmaceutical companies, including Dermavant, which provided funding for the ADORING trials. Dr. Hebert has financial relationship with more than 15 pharmaceutical companies, including Dermavent and other companies that have or are developing therapies for AD. Dr. Ständer reported financial relationships with Beiersdorf, Eli Lilly, Galderma, Kiniksa, Pfizer, and Sanofi.
BERLIN – of age, according to results of two pivotal trials presented at the at the annual congress of the European Academy of Dermatology and Venereology.
If approved for AD, one advantage of tapinarof cream relative to topical corticosteroids is potential use “without restrictions on duration, extent, or site of application,” reported Jonathan I. Silverberg, MD, PhD, MPH, director of clinical research, George Washington University, Washington.
Tapinarof cream, 1%, an aryl hydrocarbon receptor agonist, was approved in 2022 for treating plaque psoriasis in adults.
In the two phase 3 trials, ADORING 1 and ADORING 2, which were presented together at the meeting, the primary endpoint was Validated Investigator Global Assessment (vIGA) for AD of 0 (clear) or 1 (almost clear) at 8 weeks. For this endpoint and all secondary endpoints, the relative advantage of the active cream over the vehicle alone was about the same in both studies.
For example, the vIGA clear or almost clear response was met by 45.4% and 46.4% of those in the experimental arm of ADORING 1 and 2, respectively, but only 13.9% and 18.0% in the control arms (P < .0001 for both).
For the secondary endpoint of Eczema Area and Severity Index (EASI75), signifying 75% clearance of skin lesions, the response rates were 55.8% and 59.1% in the two trials, but only 22.9% and 24.1% in the respective control arms (P < .0001 for both).
The two identically designed trials randomized patients with moderate to severe AD in a 2:1 ratio to tapinarof cream or vehicle alone. There were 407 patients ages 2-81 years in ADORING I and 406 in ADORING 2. Patients were instructed to apply the active cream or vehicle once per day.
The safety data for tapinarof in these studies was generally consistent with the experience with this agent in plaque psoriasis. According to Dr. Silverberg, there was a modest increase in reports of headache early in this study, but these were transient. Follicular events were also more common on tapinarof than on its vehicle, but Dr. Silverberg said that the rate of discontinuations for adverse events, although low in both arms, was numerically lower in the active treatment arm in both trials.
“There were reports of contact dermatitis in the psoriasis studies, but we have not seen this in the AD trials,” Dr. Silverberg said.
Itch control evaluated
In a separate presentation of ADORING 1 and 2 results, Eric Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland, provided detailed information about itch control, which was evaluated with the Peak Pruritus–Numerical Rating Scale (PP-NRS).
“The PP-NRS considers a person’s worst itch over the past 24 hours based on an 11-point scale,” explained Dr. Simpson, who said that patients scored itch daily with comparisons made at weeks 1, 2, 4, and 8.
Over time, pruritus scores fell in both groups, but reductions were far steeper among those in the active treatment arms.
“In ADORING 1, there were greater reductions in itch as early as day 1,” Dr. Simpson reported. Although the differences in itch were not detected until day 2 in ADORING 2, the differences were already significant and clinically meaningful in both studies by the end of the first week.
By week 8, the mean reductions in PP-NRS scores were 2.6 and 2.4 in the vehicle arms of ADORING 1 and 2, respectively. In the treatment arm, the reduction was 4.1 points in both arms (P < .0001 for both studies).
Forty-eight–week follow-up planned
More than 90% of patients in both studies have rolled over into the open-label extension ADORING 3 trial, with a planned follow-up of 48 weeks, according to Dr. Silverberg, who said that those in the placebo arm have been crossed over to tapinarof.
The response and the safety appear to be similar in adults and children, although Dr. Silverberg said that further analyses of outcomes by age are planned. He noted that there is also an ongoing study of tapinarof in children with plaque psoriasis.
In AD in particular, Dr. Silverberg said there is “an unmet need” for a topical nonsteroidal anti-inflammatory. While topical corticosteroids are a mainstay of AD therapy in children as well as adults, he noted the limitations of these drugs, including that they can only be applied for limited periods.
Tapinarof binds to the aryl hydrocarbon receptor (AhR), which regulates immune function in the skin and is expressed in many skin cell types. By inhibiting AhR, tapinarof blocks cytokine activation and has an antioxidant effect.
Adelaide A. Hebert, MD, professor and director of pediatric dermatology, McGovern Medical School at UTHealth, Houston, has participated in clinical studies of tapinarof for AD, and said she has been impressed with its efficacy and tolerability in children as well as adults. In the case of children, parents, as well as patients, “valued the rapid onset of disease control, the once-daily application regimen, and the itch control,” she said in an interview after the meeting.
If approved, Dr. Hebert said, “this novel steroid-free medication has the potential to change the management arena for pediatric and adult patients with moderate to severe atopic dermatitis.”
The recent introduction of new systemic therapies for AD, such as JAK inhibitors, has increased options for AD control, but “we still need effective and safe topical therapies, especially in children and young adults,” said Sonja Ständer, MD, head of the Interdisciplinary Center for Chronic Pruritus, University of Münster (Germany). Author of a comprehensive review article on AD in the New England Journal of Medicine 2 years ago, Dr. Ständer said results from the phase 3 topical tapinarof trials, as well as the phase 3 topical ruxolitinib trials, which were also presented as late breakers at the 2023 EADV meeting, provide “hope that an alternative to topical steroids will soon be available.”
Based on their safety and rapid control of itch in children with AD, “these will complement our current portfolio of topical therapies very well and have the potential to replace topical steroids early in therapy or to replace them altogether,” she told this news organization.
Dermavant Sciences, manufacturer of tapinarof, anticipates filing for Food and Drug Administration approval for AD in the first quarter of 2024, according to a company statement.
Dr. Silverberg and Dr. Simpson reported financial relationships with multiple pharmaceutical companies, including Dermavant, which provided funding for the ADORING trials. Dr. Hebert has financial relationship with more than 15 pharmaceutical companies, including Dermavent and other companies that have or are developing therapies for AD. Dr. Ständer reported financial relationships with Beiersdorf, Eli Lilly, Galderma, Kiniksa, Pfizer, and Sanofi.
AT THE EADV CONGRESS
Parent concerns a factor when treating eczema in children with darker skin types
NEW YORK –
Skin diseases pose a greater risk of both hyper- and hypopigmentation in patients with darker skin types, but the fear and concern that this raises for permanent disfigurement is not limited to Blacks, Dr. Heath, assistant professor of pediatric dermatology at Temple University, Philadelphia, said at the Skin of Color Update 2023.
“Culturally, pigmentation changes can be huge. For people of Indian descent, for example, pigmentary changes like light spots on the skin might be an obstacle to marriage, so it can really be life changing,” she added.
In patients with darker skin tones presenting with an inflammatory skin disease, such as AD or psoriasis, Dr. Heath advised asking specifically about change in skin tone even if it is not readily apparent. In pediatric patients, it is also appropriate to include parents in this conversation.
Consider the parent’s perspective
“When you are taking care of a child or adolescent, the patient is likely to be concerned about changes in pigmentation, but it is important to remember that the adult in the room might have had their own journey with brown skin and has dealt with the burden of pigment changes,” Dr. Heath said.
For the parent, the pigmentation changes, rather than the inflammation, might be the governing issue and the reason that he or she brought the child to the clinician. Dr. Heath suggested that it is important for caregivers to explicitly recognize their concern, explain that addressing the pigmentary changes is part of the treatment plan, and to create realistic expectations about how long pigmentary changes will take to resolve.
As an example, Dr. Heath recounted a difficult case of a Black infant with disseminated hyperpigmentation and features that did not preclude pathology other than AD. Dr. Heath created a multifaceted treatment plan to address the inflammation in distinct areas of the body that included low-strength topical steroids for the face, stronger steroids for the body, and advice on scalp and skin care.
“I thought this was a great treatment plan out of the gate – I was covering all of the things on my differential list – I thought that the mom would be thinking, this doctor is amazing,” Dr. Heath said.
Pigmentary changes are a priority
However, that was not what the patient’s mother was thinking. Having failed to explicitly recognize her concern about the pigmentation changes and how the treatment would address this issue, the mother was disappointed.
“She had one question: Will my baby ever be one color? That was her main concern,” said Dr. Heath, indicating that other clinicians seeing inflammatory diseases in children with darker skin types can learn from her experience.
“Really, you have to acknowledge that the condition you are treating is causing the pigmentation change, and we do see that and that we have a treatment plan in place,” she said.
Because of differences in how inflammatory skin diseases present in darker skin types, there is plenty of room for a delayed diagnosis for clinicians who do not see many of these patients, according to Dr. Heath. Follicular eczema, which is common in skin of color, often presents with pruritus but differences in the appearance of the underlying disease can threaten a delay in diagnosis.
In cases of follicular eczema with itch in darker skin, the bumps look and feel like goose bumps, which “means that the eczema is really active and inflamed,” Dr. Heath said. When the skin becomes smooth and the itch dissipates, “you know that they are under great control.”
Psoriasis is often missed in children with darker skin types based on the misperception that it is rare. Although it is true that it is less common in Blacks than Whites, it is not rare, according to Dr. Heath. In inspecting the telltale erythematous plaque–like lesions, clinicians might start to consider alternative diagnoses when they do not detect the same erythematous appearance, but the reddish tone is often concealed in darker skin.
She said that predominant involvement in the head and neck and diaper area is often more common in children of color and that nail or scalp involvement, when present, is often a clue that psoriasis is the diagnosis.
Again, because many clinicians do not think immediately of psoriasis in darker skin children with lesions in the scalp, Dr. Heath advised this is another reason to include psoriasis in the differential diagnosis.
“If you have a child that has failed multiple courses of treatment for tinea capitis and they have well-demarcated plaques, it’s time to really start to think about pediatric psoriasis,” she said.
Restoring skin tone can be the priority
Asked to comment on Dr. Heath’s advice about the importance of acknowledging pigmentary changes associated with inflammatory skin diseases in patients of color, Jenna Lester, MD, the founding director of the Skin of Color Clinic at the University of California, San Francisco, called it an “often unspoken concern of patients.”
“Pigmentary changes that occur secondary to an inflammatory condition should be addressed and treated alongside the inciting condition,” she agreed.
Even if changes in skin color or skin tone are not a specific complaint of the patients, Dr. Lester also urged clinicians to raise the topic. If change in skin pigmentation is part of the clinical picture, this should be targeted in the treatment plan.
“In acne, for example, often times I find that patients are as worried about postinflammatory hyperpigmentation as they are about their acne,” she said, reiterating the advice provided by Dr. Heath.
Dr. Heath has financial relationships with Arcutis, Janssen, Johnson & Johnson, Lilly, and Regeneron. Dr. Lester reported no potential conflicts of interest.
NEW YORK –
Skin diseases pose a greater risk of both hyper- and hypopigmentation in patients with darker skin types, but the fear and concern that this raises for permanent disfigurement is not limited to Blacks, Dr. Heath, assistant professor of pediatric dermatology at Temple University, Philadelphia, said at the Skin of Color Update 2023.
“Culturally, pigmentation changes can be huge. For people of Indian descent, for example, pigmentary changes like light spots on the skin might be an obstacle to marriage, so it can really be life changing,” she added.
In patients with darker skin tones presenting with an inflammatory skin disease, such as AD or psoriasis, Dr. Heath advised asking specifically about change in skin tone even if it is not readily apparent. In pediatric patients, it is also appropriate to include parents in this conversation.
Consider the parent’s perspective
“When you are taking care of a child or adolescent, the patient is likely to be concerned about changes in pigmentation, but it is important to remember that the adult in the room might have had their own journey with brown skin and has dealt with the burden of pigment changes,” Dr. Heath said.
For the parent, the pigmentation changes, rather than the inflammation, might be the governing issue and the reason that he or she brought the child to the clinician. Dr. Heath suggested that it is important for caregivers to explicitly recognize their concern, explain that addressing the pigmentary changes is part of the treatment plan, and to create realistic expectations about how long pigmentary changes will take to resolve.
As an example, Dr. Heath recounted a difficult case of a Black infant with disseminated hyperpigmentation and features that did not preclude pathology other than AD. Dr. Heath created a multifaceted treatment plan to address the inflammation in distinct areas of the body that included low-strength topical steroids for the face, stronger steroids for the body, and advice on scalp and skin care.
“I thought this was a great treatment plan out of the gate – I was covering all of the things on my differential list – I thought that the mom would be thinking, this doctor is amazing,” Dr. Heath said.
Pigmentary changes are a priority
However, that was not what the patient’s mother was thinking. Having failed to explicitly recognize her concern about the pigmentation changes and how the treatment would address this issue, the mother was disappointed.
“She had one question: Will my baby ever be one color? That was her main concern,” said Dr. Heath, indicating that other clinicians seeing inflammatory diseases in children with darker skin types can learn from her experience.
“Really, you have to acknowledge that the condition you are treating is causing the pigmentation change, and we do see that and that we have a treatment plan in place,” she said.
Because of differences in how inflammatory skin diseases present in darker skin types, there is plenty of room for a delayed diagnosis for clinicians who do not see many of these patients, according to Dr. Heath. Follicular eczema, which is common in skin of color, often presents with pruritus but differences in the appearance of the underlying disease can threaten a delay in diagnosis.
In cases of follicular eczema with itch in darker skin, the bumps look and feel like goose bumps, which “means that the eczema is really active and inflamed,” Dr. Heath said. When the skin becomes smooth and the itch dissipates, “you know that they are under great control.”
Psoriasis is often missed in children with darker skin types based on the misperception that it is rare. Although it is true that it is less common in Blacks than Whites, it is not rare, according to Dr. Heath. In inspecting the telltale erythematous plaque–like lesions, clinicians might start to consider alternative diagnoses when they do not detect the same erythematous appearance, but the reddish tone is often concealed in darker skin.
She said that predominant involvement in the head and neck and diaper area is often more common in children of color and that nail or scalp involvement, when present, is often a clue that psoriasis is the diagnosis.
Again, because many clinicians do not think immediately of psoriasis in darker skin children with lesions in the scalp, Dr. Heath advised this is another reason to include psoriasis in the differential diagnosis.
“If you have a child that has failed multiple courses of treatment for tinea capitis and they have well-demarcated plaques, it’s time to really start to think about pediatric psoriasis,” she said.
Restoring skin tone can be the priority
Asked to comment on Dr. Heath’s advice about the importance of acknowledging pigmentary changes associated with inflammatory skin diseases in patients of color, Jenna Lester, MD, the founding director of the Skin of Color Clinic at the University of California, San Francisco, called it an “often unspoken concern of patients.”
“Pigmentary changes that occur secondary to an inflammatory condition should be addressed and treated alongside the inciting condition,” she agreed.
Even if changes in skin color or skin tone are not a specific complaint of the patients, Dr. Lester also urged clinicians to raise the topic. If change in skin pigmentation is part of the clinical picture, this should be targeted in the treatment plan.
“In acne, for example, often times I find that patients are as worried about postinflammatory hyperpigmentation as they are about their acne,” she said, reiterating the advice provided by Dr. Heath.
Dr. Heath has financial relationships with Arcutis, Janssen, Johnson & Johnson, Lilly, and Regeneron. Dr. Lester reported no potential conflicts of interest.
NEW YORK –
Skin diseases pose a greater risk of both hyper- and hypopigmentation in patients with darker skin types, but the fear and concern that this raises for permanent disfigurement is not limited to Blacks, Dr. Heath, assistant professor of pediatric dermatology at Temple University, Philadelphia, said at the Skin of Color Update 2023.
“Culturally, pigmentation changes can be huge. For people of Indian descent, for example, pigmentary changes like light spots on the skin might be an obstacle to marriage, so it can really be life changing,” she added.
In patients with darker skin tones presenting with an inflammatory skin disease, such as AD or psoriasis, Dr. Heath advised asking specifically about change in skin tone even if it is not readily apparent. In pediatric patients, it is also appropriate to include parents in this conversation.
Consider the parent’s perspective
“When you are taking care of a child or adolescent, the patient is likely to be concerned about changes in pigmentation, but it is important to remember that the adult in the room might have had their own journey with brown skin and has dealt with the burden of pigment changes,” Dr. Heath said.
For the parent, the pigmentation changes, rather than the inflammation, might be the governing issue and the reason that he or she brought the child to the clinician. Dr. Heath suggested that it is important for caregivers to explicitly recognize their concern, explain that addressing the pigmentary changes is part of the treatment plan, and to create realistic expectations about how long pigmentary changes will take to resolve.
As an example, Dr. Heath recounted a difficult case of a Black infant with disseminated hyperpigmentation and features that did not preclude pathology other than AD. Dr. Heath created a multifaceted treatment plan to address the inflammation in distinct areas of the body that included low-strength topical steroids for the face, stronger steroids for the body, and advice on scalp and skin care.
“I thought this was a great treatment plan out of the gate – I was covering all of the things on my differential list – I thought that the mom would be thinking, this doctor is amazing,” Dr. Heath said.
Pigmentary changes are a priority
However, that was not what the patient’s mother was thinking. Having failed to explicitly recognize her concern about the pigmentation changes and how the treatment would address this issue, the mother was disappointed.
“She had one question: Will my baby ever be one color? That was her main concern,” said Dr. Heath, indicating that other clinicians seeing inflammatory diseases in children with darker skin types can learn from her experience.
“Really, you have to acknowledge that the condition you are treating is causing the pigmentation change, and we do see that and that we have a treatment plan in place,” she said.
Because of differences in how inflammatory skin diseases present in darker skin types, there is plenty of room for a delayed diagnosis for clinicians who do not see many of these patients, according to Dr. Heath. Follicular eczema, which is common in skin of color, often presents with pruritus but differences in the appearance of the underlying disease can threaten a delay in diagnosis.
In cases of follicular eczema with itch in darker skin, the bumps look and feel like goose bumps, which “means that the eczema is really active and inflamed,” Dr. Heath said. When the skin becomes smooth and the itch dissipates, “you know that they are under great control.”
Psoriasis is often missed in children with darker skin types based on the misperception that it is rare. Although it is true that it is less common in Blacks than Whites, it is not rare, according to Dr. Heath. In inspecting the telltale erythematous plaque–like lesions, clinicians might start to consider alternative diagnoses when they do not detect the same erythematous appearance, but the reddish tone is often concealed in darker skin.
She said that predominant involvement in the head and neck and diaper area is often more common in children of color and that nail or scalp involvement, when present, is often a clue that psoriasis is the diagnosis.
Again, because many clinicians do not think immediately of psoriasis in darker skin children with lesions in the scalp, Dr. Heath advised this is another reason to include psoriasis in the differential diagnosis.
“If you have a child that has failed multiple courses of treatment for tinea capitis and they have well-demarcated plaques, it’s time to really start to think about pediatric psoriasis,” she said.
Restoring skin tone can be the priority
Asked to comment on Dr. Heath’s advice about the importance of acknowledging pigmentary changes associated with inflammatory skin diseases in patients of color, Jenna Lester, MD, the founding director of the Skin of Color Clinic at the University of California, San Francisco, called it an “often unspoken concern of patients.”
“Pigmentary changes that occur secondary to an inflammatory condition should be addressed and treated alongside the inciting condition,” she agreed.
Even if changes in skin color or skin tone are not a specific complaint of the patients, Dr. Lester also urged clinicians to raise the topic. If change in skin pigmentation is part of the clinical picture, this should be targeted in the treatment plan.
“In acne, for example, often times I find that patients are as worried about postinflammatory hyperpigmentation as they are about their acne,” she said, reiterating the advice provided by Dr. Heath.
Dr. Heath has financial relationships with Arcutis, Janssen, Johnson & Johnson, Lilly, and Regeneron. Dr. Lester reported no potential conflicts of interest.
AT SOC 2023
Commentary: JAK Inhibitors and Comorbidities in AD, December 2023
Schlösser and colleagues provide a real-world report of 48 patients treated with upadacitinib for atopic dermatitis, many of whom had previously been treated with cyclosporine and dupilumab. The upbeat authors concluded, "Overall, adverse events were mostly well tolerated." Being a cynical, glass-is-half-empty kind of person, I wondered what that meant. Most patients (56%) reported adverse events, the most common being acne (25% of patients treated), nausea (13%), respiratory tract infections (10%), and herpes virus (8%). The herpes virus signal is not just a bit of a concern for me, but it also makes it hard for me to convince patients to take a Janus kinase (JAK) inhibitor, as when I even mention herpes, patients reply, often rather emphatically, "I don't want herpes!" I'll be encouraging patients to get vaccinated for shingles when starting them on JAK inhibitors.
Dupilumab seems to work great in real-life use. In Martinez-Cabriales and colleagues' study of 62 children age < 12 with atopic dermatitis, only four discontinued the treatment. One of these was a nonresponder who took only one injection and had flushing, and one of the other three discontinued because their skin had completely cleared.
When I saw the title of Rand and colleagues' article, "Matching-Adjusted Indirect Comparison of the Long-Term Efficacy Maintenance and Adverse Event Rates of Lebrikizumab Versus Dupilumab in Moderate-to-Severe Atopic Dermatitis," I thought, Oh, this is great — a head-to-head, long-term trial comparing lebrikizumab and dupilumab. I was disappointed to find that this was simply a retrospective analysis of data reported from different studies. The study found little difference in efficacy or safety of the two drugs. Both seem to be excellent medications for atopic dermatitis.
Here's another study (Zhou et al) that reports possible increased risk for a comorbidity (cognitive dysfunction) associated with atopic dermatitis. This study reports that there is an elevated hazard ratio that is statistically significant; the article fails to report what the increased absolute risk is for cognitive dysfunction associated with atopic dermatitis. My guess is that it is small and probably clinically unimportant. The hazard ratio for developing dementia was 1.16. It's hard to know how that translates into absolute risk, but my brilliant friend and former partner, Dr Alan Fleischer, once told me that the odds ratio for smoking and lung cancer is something like 100; the hazard ratio is in the range of 20. On the basis of a hazard ratio of 1.16, I don't think patients with atopic dermatitis need to be any more worried about dementia than those without. (Though, to be honest, I think we can all be worried about developing dementia.)
In this tour de force analysis of 83 trials with over 20,000 participants, Drucker and colleagues determined that high doses of abrocitinib and upadacitinib are more effective than even dupilumab for atopic dermatitis. The standard doses of these JAK inhibitors were similar in efficacy to dupilumab. I think it's safe to say that JAK inhibitors are, at least at their high doses, more effective than dupilumab, but safety remains a critical factor in treatment decision-making. I think JAK inhibitors are a great option for patients who need the most effective treatment or who fail to respond to dupilumab.
The title of the article by Oh and colleagues, "Increased Risk of Renal Malignancy in Patients With Moderate to Severe Atopic Dermatitis," seems like it could terrify patients. The study involved an analysis of an enormous number of people, including tens of thousands with atopic dermatitis and millions of controls. The investigators did find statistically significant differences in the rate of malignancy. The rate of renal cancer was about 1.6 per 10,000 person-years for people without atopic dermatitis or people with mild atopic dermatitis; the rate was about 2.5 per 10,000 people for patients with moderate to severe atopic dermatitis. While the rate of renal cancer was statistically significantly higher in patients with moderate to severe atopic dermatitis (ie, the higher rate was unlikely to be occurring due to chance alone), these patients have very little risk for renal malignancy. The authors' conclusion that regular checkups for renal malignancy are recommended for patients with severe atopic dermatitis seems unnecessary to me.
Schlösser and colleagues provide a real-world report of 48 patients treated with upadacitinib for atopic dermatitis, many of whom had previously been treated with cyclosporine and dupilumab. The upbeat authors concluded, "Overall, adverse events were mostly well tolerated." Being a cynical, glass-is-half-empty kind of person, I wondered what that meant. Most patients (56%) reported adverse events, the most common being acne (25% of patients treated), nausea (13%), respiratory tract infections (10%), and herpes virus (8%). The herpes virus signal is not just a bit of a concern for me, but it also makes it hard for me to convince patients to take a Janus kinase (JAK) inhibitor, as when I even mention herpes, patients reply, often rather emphatically, "I don't want herpes!" I'll be encouraging patients to get vaccinated for shingles when starting them on JAK inhibitors.
Dupilumab seems to work great in real-life use. In Martinez-Cabriales and colleagues' study of 62 children age < 12 with atopic dermatitis, only four discontinued the treatment. One of these was a nonresponder who took only one injection and had flushing, and one of the other three discontinued because their skin had completely cleared.
When I saw the title of Rand and colleagues' article, "Matching-Adjusted Indirect Comparison of the Long-Term Efficacy Maintenance and Adverse Event Rates of Lebrikizumab Versus Dupilumab in Moderate-to-Severe Atopic Dermatitis," I thought, Oh, this is great — a head-to-head, long-term trial comparing lebrikizumab and dupilumab. I was disappointed to find that this was simply a retrospective analysis of data reported from different studies. The study found little difference in efficacy or safety of the two drugs. Both seem to be excellent medications for atopic dermatitis.
Here's another study (Zhou et al) that reports possible increased risk for a comorbidity (cognitive dysfunction) associated with atopic dermatitis. This study reports that there is an elevated hazard ratio that is statistically significant; the article fails to report what the increased absolute risk is for cognitive dysfunction associated with atopic dermatitis. My guess is that it is small and probably clinically unimportant. The hazard ratio for developing dementia was 1.16. It's hard to know how that translates into absolute risk, but my brilliant friend and former partner, Dr Alan Fleischer, once told me that the odds ratio for smoking and lung cancer is something like 100; the hazard ratio is in the range of 20. On the basis of a hazard ratio of 1.16, I don't think patients with atopic dermatitis need to be any more worried about dementia than those without. (Though, to be honest, I think we can all be worried about developing dementia.)
In this tour de force analysis of 83 trials with over 20,000 participants, Drucker and colleagues determined that high doses of abrocitinib and upadacitinib are more effective than even dupilumab for atopic dermatitis. The standard doses of these JAK inhibitors were similar in efficacy to dupilumab. I think it's safe to say that JAK inhibitors are, at least at their high doses, more effective than dupilumab, but safety remains a critical factor in treatment decision-making. I think JAK inhibitors are a great option for patients who need the most effective treatment or who fail to respond to dupilumab.
The title of the article by Oh and colleagues, "Increased Risk of Renal Malignancy in Patients With Moderate to Severe Atopic Dermatitis," seems like it could terrify patients. The study involved an analysis of an enormous number of people, including tens of thousands with atopic dermatitis and millions of controls. The investigators did find statistically significant differences in the rate of malignancy. The rate of renal cancer was about 1.6 per 10,000 person-years for people without atopic dermatitis or people with mild atopic dermatitis; the rate was about 2.5 per 10,000 people for patients with moderate to severe atopic dermatitis. While the rate of renal cancer was statistically significantly higher in patients with moderate to severe atopic dermatitis (ie, the higher rate was unlikely to be occurring due to chance alone), these patients have very little risk for renal malignancy. The authors' conclusion that regular checkups for renal malignancy are recommended for patients with severe atopic dermatitis seems unnecessary to me.
Schlösser and colleagues provide a real-world report of 48 patients treated with upadacitinib for atopic dermatitis, many of whom had previously been treated with cyclosporine and dupilumab. The upbeat authors concluded, "Overall, adverse events were mostly well tolerated." Being a cynical, glass-is-half-empty kind of person, I wondered what that meant. Most patients (56%) reported adverse events, the most common being acne (25% of patients treated), nausea (13%), respiratory tract infections (10%), and herpes virus (8%). The herpes virus signal is not just a bit of a concern for me, but it also makes it hard for me to convince patients to take a Janus kinase (JAK) inhibitor, as when I even mention herpes, patients reply, often rather emphatically, "I don't want herpes!" I'll be encouraging patients to get vaccinated for shingles when starting them on JAK inhibitors.
Dupilumab seems to work great in real-life use. In Martinez-Cabriales and colleagues' study of 62 children age < 12 with atopic dermatitis, only four discontinued the treatment. One of these was a nonresponder who took only one injection and had flushing, and one of the other three discontinued because their skin had completely cleared.
When I saw the title of Rand and colleagues' article, "Matching-Adjusted Indirect Comparison of the Long-Term Efficacy Maintenance and Adverse Event Rates of Lebrikizumab Versus Dupilumab in Moderate-to-Severe Atopic Dermatitis," I thought, Oh, this is great — a head-to-head, long-term trial comparing lebrikizumab and dupilumab. I was disappointed to find that this was simply a retrospective analysis of data reported from different studies. The study found little difference in efficacy or safety of the two drugs. Both seem to be excellent medications for atopic dermatitis.
Here's another study (Zhou et al) that reports possible increased risk for a comorbidity (cognitive dysfunction) associated with atopic dermatitis. This study reports that there is an elevated hazard ratio that is statistically significant; the article fails to report what the increased absolute risk is for cognitive dysfunction associated with atopic dermatitis. My guess is that it is small and probably clinically unimportant. The hazard ratio for developing dementia was 1.16. It's hard to know how that translates into absolute risk, but my brilliant friend and former partner, Dr Alan Fleischer, once told me that the odds ratio for smoking and lung cancer is something like 100; the hazard ratio is in the range of 20. On the basis of a hazard ratio of 1.16, I don't think patients with atopic dermatitis need to be any more worried about dementia than those without. (Though, to be honest, I think we can all be worried about developing dementia.)
In this tour de force analysis of 83 trials with over 20,000 participants, Drucker and colleagues determined that high doses of abrocitinib and upadacitinib are more effective than even dupilumab for atopic dermatitis. The standard doses of these JAK inhibitors were similar in efficacy to dupilumab. I think it's safe to say that JAK inhibitors are, at least at their high doses, more effective than dupilumab, but safety remains a critical factor in treatment decision-making. I think JAK inhibitors are a great option for patients who need the most effective treatment or who fail to respond to dupilumab.
The title of the article by Oh and colleagues, "Increased Risk of Renal Malignancy in Patients With Moderate to Severe Atopic Dermatitis," seems like it could terrify patients. The study involved an analysis of an enormous number of people, including tens of thousands with atopic dermatitis and millions of controls. The investigators did find statistically significant differences in the rate of malignancy. The rate of renal cancer was about 1.6 per 10,000 person-years for people without atopic dermatitis or people with mild atopic dermatitis; the rate was about 2.5 per 10,000 people for patients with moderate to severe atopic dermatitis. While the rate of renal cancer was statistically significantly higher in patients with moderate to severe atopic dermatitis (ie, the higher rate was unlikely to be occurring due to chance alone), these patients have very little risk for renal malignancy. The authors' conclusion that regular checkups for renal malignancy are recommended for patients with severe atopic dermatitis seems unnecessary to me.
Lebrikizumab gets European nod for treating moderate-to-severe atopic dermatitis
The , according to a press release from the manufacturer.
Lebrikizumab, which selectively targets interleukin-13 and inhibits its signaling pathway, will first be available in Germany, with a rollout in other European countries expected through 2024, according to Almirall, the manufacturer.
The European approval of lebrikizumab (Ebglyss) was based on data from a trio of pivotal phase 3 studies including ADvocate1 and ADvocate2, which evaluated lebrikizumab as monotherapy, and ADhere, which evaluated lebrikizumab in combination with topical corticosteroids. All three trials included adult and adolescent patients aged 12 years and older with moderate-to-severe AD.
In the two ADvocate studies, published in the New England Journal of Medicine, participants were randomized to a 250-mg injection of lebrikizumab or placebo every 2 weeks. The primary outcome was a score of clear or almost clear skin based on the Investigator’s Global Assessment with at least a 2-point reduction from baseline to 16 weeks.
Compared with placebo, lebrikizumab showed significant clinical efficacy in both studies. In study 1, 43.1% of 283 patients treated with lebrikizumab versus 12.7% of 141 patients on placebo met the primary endpoint (P < .001), as did 33.2% of the 281 patients on lebrikizumab and 10.8% of 146 patients on placebo in study 2 (P < .001). In addition, 58.8% and 52.1% of patients on lebrikizumab in studies 1 and 2, respectively, met the secondary endpoint of a 75% reduction in the Eczema Area and Severity Index score (EASI-75), versus 16.2% and 18.1% of patients on placebo in study 1 and 2, respectively (P < .001 for both).
In the ADhere study, published in JAMA Dermatology, 41.2% of patients receiving a lebrikizumab/corticosteroid combination and 22.1% of those randomized to a placebo/corticosteroid combination met the primary endpoint of IGA scores of 0 or 1 at 16 weeks, and nearly 70% patients treated with a combination of lebrikizumab and topical corticosteroids achieved EASI-75, compared with 42% of those on the combination.
Nearly 80% of patients who responded at 16 weeks and continued treatment with lebrikizumab as monotherapy or combination therapy showed sustained results up to 52 weeks with maintenance monthly dosing, according to the Almirall press release.
Most adverse events across the studies were mild or moderate and were not associated with treatment discontinuation. The most common adverse reactions were conjunctivitis, injection site reactions, allergic conjunctivitis, and dry eye.
Further research has shown showed clinical efficacy and safety in patients who used lebrikizumab for up to 2 years, either as monotherapy or in combination with topical corticosteroids, according to the manufacturer.
Lebrikizumab remains under review in the United States after the Food and Drug Administration issued a complete response letter in October regarding findings made during an inspection of a third-party contract manufacturer that included the “monoclonal antibody drug substance” for lebrikizumab, although no concerns about clinical data or safety were raised, Eli Lilly announced in October. Eli Lilly has the rights to develop lebrikizumab in the United States and the rest of the world excluding Europe.
The , according to a press release from the manufacturer.
Lebrikizumab, which selectively targets interleukin-13 and inhibits its signaling pathway, will first be available in Germany, with a rollout in other European countries expected through 2024, according to Almirall, the manufacturer.
The European approval of lebrikizumab (Ebglyss) was based on data from a trio of pivotal phase 3 studies including ADvocate1 and ADvocate2, which evaluated lebrikizumab as monotherapy, and ADhere, which evaluated lebrikizumab in combination with topical corticosteroids. All three trials included adult and adolescent patients aged 12 years and older with moderate-to-severe AD.
In the two ADvocate studies, published in the New England Journal of Medicine, participants were randomized to a 250-mg injection of lebrikizumab or placebo every 2 weeks. The primary outcome was a score of clear or almost clear skin based on the Investigator’s Global Assessment with at least a 2-point reduction from baseline to 16 weeks.
Compared with placebo, lebrikizumab showed significant clinical efficacy in both studies. In study 1, 43.1% of 283 patients treated with lebrikizumab versus 12.7% of 141 patients on placebo met the primary endpoint (P < .001), as did 33.2% of the 281 patients on lebrikizumab and 10.8% of 146 patients on placebo in study 2 (P < .001). In addition, 58.8% and 52.1% of patients on lebrikizumab in studies 1 and 2, respectively, met the secondary endpoint of a 75% reduction in the Eczema Area and Severity Index score (EASI-75), versus 16.2% and 18.1% of patients on placebo in study 1 and 2, respectively (P < .001 for both).
In the ADhere study, published in JAMA Dermatology, 41.2% of patients receiving a lebrikizumab/corticosteroid combination and 22.1% of those randomized to a placebo/corticosteroid combination met the primary endpoint of IGA scores of 0 or 1 at 16 weeks, and nearly 70% patients treated with a combination of lebrikizumab and topical corticosteroids achieved EASI-75, compared with 42% of those on the combination.
Nearly 80% of patients who responded at 16 weeks and continued treatment with lebrikizumab as monotherapy or combination therapy showed sustained results up to 52 weeks with maintenance monthly dosing, according to the Almirall press release.
Most adverse events across the studies were mild or moderate and were not associated with treatment discontinuation. The most common adverse reactions were conjunctivitis, injection site reactions, allergic conjunctivitis, and dry eye.
Further research has shown showed clinical efficacy and safety in patients who used lebrikizumab for up to 2 years, either as monotherapy or in combination with topical corticosteroids, according to the manufacturer.
Lebrikizumab remains under review in the United States after the Food and Drug Administration issued a complete response letter in October regarding findings made during an inspection of a third-party contract manufacturer that included the “monoclonal antibody drug substance” for lebrikizumab, although no concerns about clinical data or safety were raised, Eli Lilly announced in October. Eli Lilly has the rights to develop lebrikizumab in the United States and the rest of the world excluding Europe.
The , according to a press release from the manufacturer.
Lebrikizumab, which selectively targets interleukin-13 and inhibits its signaling pathway, will first be available in Germany, with a rollout in other European countries expected through 2024, according to Almirall, the manufacturer.
The European approval of lebrikizumab (Ebglyss) was based on data from a trio of pivotal phase 3 studies including ADvocate1 and ADvocate2, which evaluated lebrikizumab as monotherapy, and ADhere, which evaluated lebrikizumab in combination with topical corticosteroids. All three trials included adult and adolescent patients aged 12 years and older with moderate-to-severe AD.
In the two ADvocate studies, published in the New England Journal of Medicine, participants were randomized to a 250-mg injection of lebrikizumab or placebo every 2 weeks. The primary outcome was a score of clear or almost clear skin based on the Investigator’s Global Assessment with at least a 2-point reduction from baseline to 16 weeks.
Compared with placebo, lebrikizumab showed significant clinical efficacy in both studies. In study 1, 43.1% of 283 patients treated with lebrikizumab versus 12.7% of 141 patients on placebo met the primary endpoint (P < .001), as did 33.2% of the 281 patients on lebrikizumab and 10.8% of 146 patients on placebo in study 2 (P < .001). In addition, 58.8% and 52.1% of patients on lebrikizumab in studies 1 and 2, respectively, met the secondary endpoint of a 75% reduction in the Eczema Area and Severity Index score (EASI-75), versus 16.2% and 18.1% of patients on placebo in study 1 and 2, respectively (P < .001 for both).
In the ADhere study, published in JAMA Dermatology, 41.2% of patients receiving a lebrikizumab/corticosteroid combination and 22.1% of those randomized to a placebo/corticosteroid combination met the primary endpoint of IGA scores of 0 or 1 at 16 weeks, and nearly 70% patients treated with a combination of lebrikizumab and topical corticosteroids achieved EASI-75, compared with 42% of those on the combination.
Nearly 80% of patients who responded at 16 weeks and continued treatment with lebrikizumab as monotherapy or combination therapy showed sustained results up to 52 weeks with maintenance monthly dosing, according to the Almirall press release.
Most adverse events across the studies were mild or moderate and were not associated with treatment discontinuation. The most common adverse reactions were conjunctivitis, injection site reactions, allergic conjunctivitis, and dry eye.
Further research has shown showed clinical efficacy and safety in patients who used lebrikizumab for up to 2 years, either as monotherapy or in combination with topical corticosteroids, according to the manufacturer.
Lebrikizumab remains under review in the United States after the Food and Drug Administration issued a complete response letter in October regarding findings made during an inspection of a third-party contract manufacturer that included the “monoclonal antibody drug substance” for lebrikizumab, although no concerns about clinical data or safety were raised, Eli Lilly announced in October. Eli Lilly has the rights to develop lebrikizumab in the United States and the rest of the world excluding Europe.
Dupilumab for Dyshidrotic Eczema With Secondary Improvement in Eosinophilic Interstitial Lung Disease
To the Editor:
Biologic medications are increasingly utilized in adults with moderate to severe atopic dermatitis (AD) that is inadequately controlled with topical medication. By targeting the IL-4 receptor alpha subunit, dupilumab inhibits the biologic effects of IL-4 and IL-13, resulting in remarkable improvement in disease and quality of life for many patients with refractory AD.1
In 2017, the US Food and Drug Administration approved dupilumab for use in AD, asthma, and chronic rhinosinusitis. However, there is evidence of the drug’s off-label efficacy in conditions such as eosinophilic annular erythema.2 We present a patient with dyshidrotic eczema treated with dupilumab who experienced contemporaneous secondary improvement in chronic eosinophilic pneumonia (CEP) and interstitial lung disease (ILD).
A 45-year-old man was referred to our dermatology clinic for chronic hand dermatitis refractory to increasing strengths of topical corticosteroids. He had a history of progressive shortness of breath of unknown cause, which began 2 years prior, and he was being followed at our institution’s ILD clinic. Earlier pulmonary function testing revealed a restrictive pattern with interstitial infiltrates seen on chest computed tomography. A lung biopsy demonstrated features of fibrotic nonspecific interstitial pneumonitis with superimposed eosinophilic pneumonia. His pulmonary symptoms had progressively worsened; over a period of several months, the supplemental oxygen requirement had increased to 6 L at rest and 12 L upon exertion. Prednisone therapy was initiated, which alleviated respiratory symptoms; however, the patient was unable to tolerate a gradual wean of the medication, which rendered him steroid dependent at 30 mg/d.
Along with respiratory symptoms, the patient reported symptoms consistent with an autoimmune process, including dry eyes. Muscle weakness and tenderness also were noted. Ultimately, a diagnosis of anti–PL-7 (anti-threonyl-transfer RNA synthetase) antisynthetase syndrome was rendered by identification of anti–PL-7 antibodies and an elevated level of creatinine kinase.
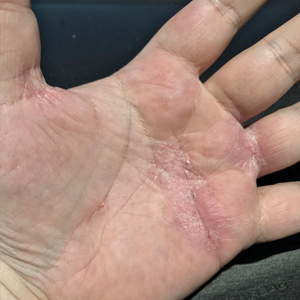
Physical examination at our clinic revealed subtle palmar scaling on the hands and multiple small clear vesicles on the lateral aspects of the digits (Figure, A), consistent with dyshidrotic eczema. He initially was treated with clobetasol propionate ointment 0.05%. Despite adherence to this high-potency topical corticosteroid, he experienced only minimal improvement over a period of 3 months. Dupilumab was started at standard dosing—600 mg at initiation, followed by 300 mg every 2 weeks. The patient reported rapid improvement in dyshidrotic eczema over several months with near-complete resolution (Figure, B).
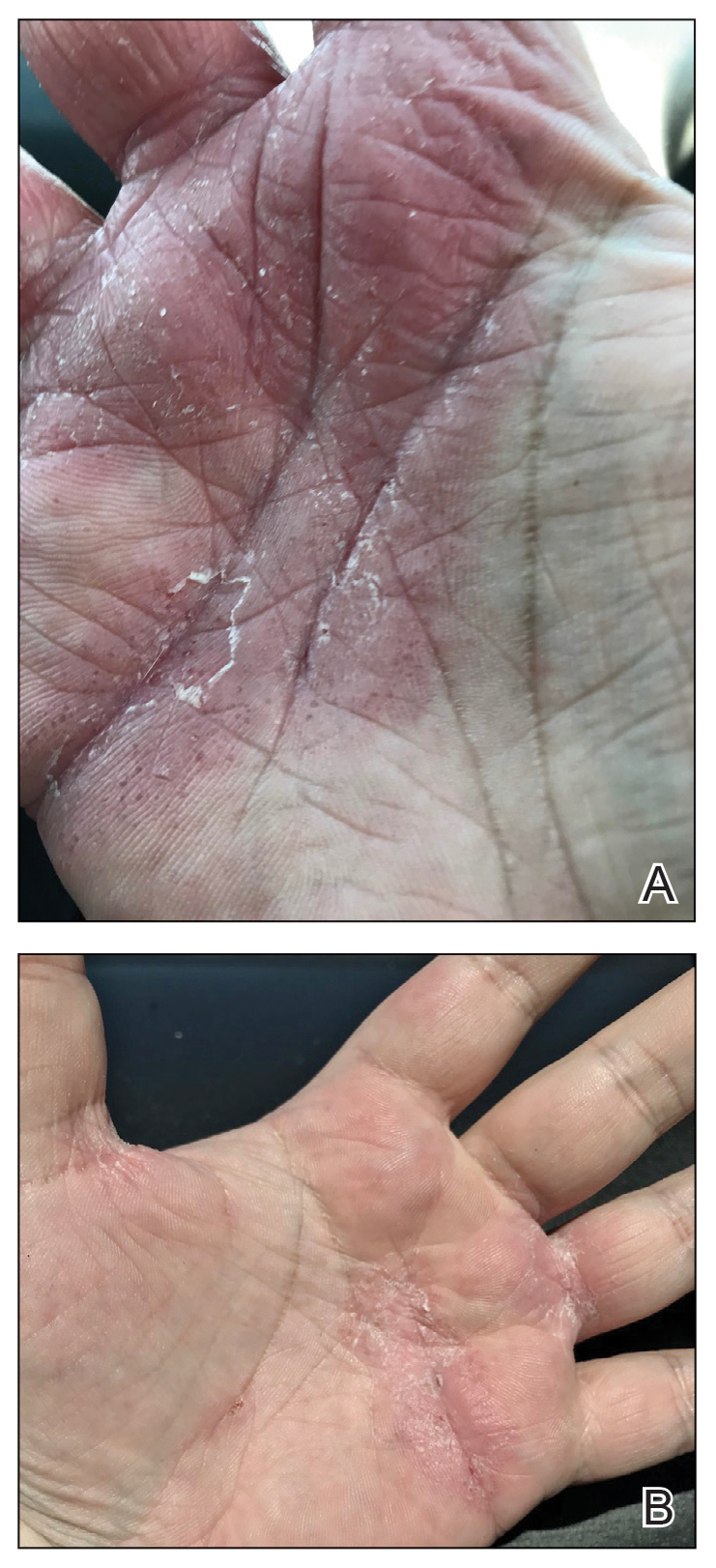
Concurrent with initiation and continued use of dupilumab, without other changes in his medication regimen, the patient noted gradual improvement in respiratory symptoms. At 6-month follow-up he reported notable improvement in respiratory function and quality of life. He then tolerated a gradual wean of prednisone to 10 mg/d, with a similar reduction in supplemental oxygen.
Off-label use of dupilumab for various eosinophilic conditions has shown promising efficacy. Our patient experienced improvement in CEP shortly after initiation of dupilumab, enabling weaning of prednisone, which has a well established adverse effect profile associated with long term use.3,4 In comparison, dupilumab generally is well tolerated, with rare ophthalmologic complications and injection-site reactions.5
One case report suggested that CEP may represent a potential rare adverse effect of dupilumab initiation.6 However, prior to initiation of dupilumab, that patient had poorly controlled asthma requiring frequent oral corticosteroid therapy. It is possible that CEP was subclinical prior to initiation of dupilumab and became more noticeable once the patient was weaned from corticosteroids, which had served as an indirect treatment.6 Nonetheless, more research is needed to definitively establish the efficacy of dupilumab in CEP prior to more widespread use.
Irrespective of the potential efficacy of dupilumab for the treatment of CEP, our case highlights the growing body of evidence that dupilumab should be considered in the treatment of dyshidrotic eczema, particularly in cases refractory to topical treatment.7 When a systemic medication is preferred, dupilumab likely represents an option with a relatively well-tolerated adverse effect profile compared to traditional systemic treatments for dyshidrotic eczema.
1. Barbarot S, Wollenberg A, Silverberg JI, et al. Dupilumab provides rapid and sustained improvement in SCORAD outcomes in adults with moderate-to-severe atopic dermatitis: combined results ofour randomized phase 3 trials. J Dermatolog Treat. 2022;33:266-277. doi:10.1080/09546634.2020.1750550
2. Gordon SC, Robinson SN, Abudu M, et al. Eosinophilic annular erythema treated with dupilumab. Pediatr Dermatol. 2018;35:E255-E256. doi:10.1111/pde.13533
3. Callaghan DJ 3rd. Use of Google Trends to examine interest in Mohs micrographic surgery: 2004 to 2016. Dermatol Surg. 2018;44:186-192. doi:10.1097/DSS.0000000000001270
4. Fowler C, Hoover W. Dupilumab for chronic eosinophilic pneumonia. Pediatr Pulmonol. 2020;55:3229-3230. doi:10.1002/ppul.25096
5. Simpson EL, Akinlade B, Ardeleanu M. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2017;376:1090-1091. doi:10.1056/NEJMc1700366
6. Menzella F, Montanari G, Patricelli G, et al. A case of chronic eosinophilic pneumonia in a patient treated with dupilumab. Ther Clin Risk Manag. 2019;15:869-875. doi:10.2147/TCRM.S207402
7. Waldman RA, DeWane ME, Sloan B, et al. Dupilumab for the treatment of dyshidrotic eczema in 15 consecutive patients. J Am Acad Dermatol. 2020;82:1251-1252. doi:10.1016/j.jaad.2019.12.053
To the Editor:
Biologic medications are increasingly utilized in adults with moderate to severe atopic dermatitis (AD) that is inadequately controlled with topical medication. By targeting the IL-4 receptor alpha subunit, dupilumab inhibits the biologic effects of IL-4 and IL-13, resulting in remarkable improvement in disease and quality of life for many patients with refractory AD.1
In 2017, the US Food and Drug Administration approved dupilumab for use in AD, asthma, and chronic rhinosinusitis. However, there is evidence of the drug’s off-label efficacy in conditions such as eosinophilic annular erythema.2 We present a patient with dyshidrotic eczema treated with dupilumab who experienced contemporaneous secondary improvement in chronic eosinophilic pneumonia (CEP) and interstitial lung disease (ILD).
A 45-year-old man was referred to our dermatology clinic for chronic hand dermatitis refractory to increasing strengths of topical corticosteroids. He had a history of progressive shortness of breath of unknown cause, which began 2 years prior, and he was being followed at our institution’s ILD clinic. Earlier pulmonary function testing revealed a restrictive pattern with interstitial infiltrates seen on chest computed tomography. A lung biopsy demonstrated features of fibrotic nonspecific interstitial pneumonitis with superimposed eosinophilic pneumonia. His pulmonary symptoms had progressively worsened; over a period of several months, the supplemental oxygen requirement had increased to 6 L at rest and 12 L upon exertion. Prednisone therapy was initiated, which alleviated respiratory symptoms; however, the patient was unable to tolerate a gradual wean of the medication, which rendered him steroid dependent at 30 mg/d.
Along with respiratory symptoms, the patient reported symptoms consistent with an autoimmune process, including dry eyes. Muscle weakness and tenderness also were noted. Ultimately, a diagnosis of anti–PL-7 (anti-threonyl-transfer RNA synthetase) antisynthetase syndrome was rendered by identification of anti–PL-7 antibodies and an elevated level of creatinine kinase.
Physical examination at our clinic revealed subtle palmar scaling on the hands and multiple small clear vesicles on the lateral aspects of the digits (Figure, A), consistent with dyshidrotic eczema. He initially was treated with clobetasol propionate ointment 0.05%. Despite adherence to this high-potency topical corticosteroid, he experienced only minimal improvement over a period of 3 months. Dupilumab was started at standard dosing—600 mg at initiation, followed by 300 mg every 2 weeks. The patient reported rapid improvement in dyshidrotic eczema over several months with near-complete resolution (Figure, B).

Concurrent with initiation and continued use of dupilumab, without other changes in his medication regimen, the patient noted gradual improvement in respiratory symptoms. At 6-month follow-up he reported notable improvement in respiratory function and quality of life. He then tolerated a gradual wean of prednisone to 10 mg/d, with a similar reduction in supplemental oxygen.
Off-label use of dupilumab for various eosinophilic conditions has shown promising efficacy. Our patient experienced improvement in CEP shortly after initiation of dupilumab, enabling weaning of prednisone, which has a well established adverse effect profile associated with long term use.3,4 In comparison, dupilumab generally is well tolerated, with rare ophthalmologic complications and injection-site reactions.5
One case report suggested that CEP may represent a potential rare adverse effect of dupilumab initiation.6 However, prior to initiation of dupilumab, that patient had poorly controlled asthma requiring frequent oral corticosteroid therapy. It is possible that CEP was subclinical prior to initiation of dupilumab and became more noticeable once the patient was weaned from corticosteroids, which had served as an indirect treatment.6 Nonetheless, more research is needed to definitively establish the efficacy of dupilumab in CEP prior to more widespread use.
Irrespective of the potential efficacy of dupilumab for the treatment of CEP, our case highlights the growing body of evidence that dupilumab should be considered in the treatment of dyshidrotic eczema, particularly in cases refractory to topical treatment.7 When a systemic medication is preferred, dupilumab likely represents an option with a relatively well-tolerated adverse effect profile compared to traditional systemic treatments for dyshidrotic eczema.
To the Editor:
Biologic medications are increasingly utilized in adults with moderate to severe atopic dermatitis (AD) that is inadequately controlled with topical medication. By targeting the IL-4 receptor alpha subunit, dupilumab inhibits the biologic effects of IL-4 and IL-13, resulting in remarkable improvement in disease and quality of life for many patients with refractory AD.1
In 2017, the US Food and Drug Administration approved dupilumab for use in AD, asthma, and chronic rhinosinusitis. However, there is evidence of the drug’s off-label efficacy in conditions such as eosinophilic annular erythema.2 We present a patient with dyshidrotic eczema treated with dupilumab who experienced contemporaneous secondary improvement in chronic eosinophilic pneumonia (CEP) and interstitial lung disease (ILD).
A 45-year-old man was referred to our dermatology clinic for chronic hand dermatitis refractory to increasing strengths of topical corticosteroids. He had a history of progressive shortness of breath of unknown cause, which began 2 years prior, and he was being followed at our institution’s ILD clinic. Earlier pulmonary function testing revealed a restrictive pattern with interstitial infiltrates seen on chest computed tomography. A lung biopsy demonstrated features of fibrotic nonspecific interstitial pneumonitis with superimposed eosinophilic pneumonia. His pulmonary symptoms had progressively worsened; over a period of several months, the supplemental oxygen requirement had increased to 6 L at rest and 12 L upon exertion. Prednisone therapy was initiated, which alleviated respiratory symptoms; however, the patient was unable to tolerate a gradual wean of the medication, which rendered him steroid dependent at 30 mg/d.
Along with respiratory symptoms, the patient reported symptoms consistent with an autoimmune process, including dry eyes. Muscle weakness and tenderness also were noted. Ultimately, a diagnosis of anti–PL-7 (anti-threonyl-transfer RNA synthetase) antisynthetase syndrome was rendered by identification of anti–PL-7 antibodies and an elevated level of creatinine kinase.
Physical examination at our clinic revealed subtle palmar scaling on the hands and multiple small clear vesicles on the lateral aspects of the digits (Figure, A), consistent with dyshidrotic eczema. He initially was treated with clobetasol propionate ointment 0.05%. Despite adherence to this high-potency topical corticosteroid, he experienced only minimal improvement over a period of 3 months. Dupilumab was started at standard dosing—600 mg at initiation, followed by 300 mg every 2 weeks. The patient reported rapid improvement in dyshidrotic eczema over several months with near-complete resolution (Figure, B).

Concurrent with initiation and continued use of dupilumab, without other changes in his medication regimen, the patient noted gradual improvement in respiratory symptoms. At 6-month follow-up he reported notable improvement in respiratory function and quality of life. He then tolerated a gradual wean of prednisone to 10 mg/d, with a similar reduction in supplemental oxygen.
Off-label use of dupilumab for various eosinophilic conditions has shown promising efficacy. Our patient experienced improvement in CEP shortly after initiation of dupilumab, enabling weaning of prednisone, which has a well established adverse effect profile associated with long term use.3,4 In comparison, dupilumab generally is well tolerated, with rare ophthalmologic complications and injection-site reactions.5
One case report suggested that CEP may represent a potential rare adverse effect of dupilumab initiation.6 However, prior to initiation of dupilumab, that patient had poorly controlled asthma requiring frequent oral corticosteroid therapy. It is possible that CEP was subclinical prior to initiation of dupilumab and became more noticeable once the patient was weaned from corticosteroids, which had served as an indirect treatment.6 Nonetheless, more research is needed to definitively establish the efficacy of dupilumab in CEP prior to more widespread use.
Irrespective of the potential efficacy of dupilumab for the treatment of CEP, our case highlights the growing body of evidence that dupilumab should be considered in the treatment of dyshidrotic eczema, particularly in cases refractory to topical treatment.7 When a systemic medication is preferred, dupilumab likely represents an option with a relatively well-tolerated adverse effect profile compared to traditional systemic treatments for dyshidrotic eczema.
1. Barbarot S, Wollenberg A, Silverberg JI, et al. Dupilumab provides rapid and sustained improvement in SCORAD outcomes in adults with moderate-to-severe atopic dermatitis: combined results ofour randomized phase 3 trials. J Dermatolog Treat. 2022;33:266-277. doi:10.1080/09546634.2020.1750550
2. Gordon SC, Robinson SN, Abudu M, et al. Eosinophilic annular erythema treated with dupilumab. Pediatr Dermatol. 2018;35:E255-E256. doi:10.1111/pde.13533
3. Callaghan DJ 3rd. Use of Google Trends to examine interest in Mohs micrographic surgery: 2004 to 2016. Dermatol Surg. 2018;44:186-192. doi:10.1097/DSS.0000000000001270
4. Fowler C, Hoover W. Dupilumab for chronic eosinophilic pneumonia. Pediatr Pulmonol. 2020;55:3229-3230. doi:10.1002/ppul.25096
5. Simpson EL, Akinlade B, Ardeleanu M. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2017;376:1090-1091. doi:10.1056/NEJMc1700366
6. Menzella F, Montanari G, Patricelli G, et al. A case of chronic eosinophilic pneumonia in a patient treated with dupilumab. Ther Clin Risk Manag. 2019;15:869-875. doi:10.2147/TCRM.S207402
7. Waldman RA, DeWane ME, Sloan B, et al. Dupilumab for the treatment of dyshidrotic eczema in 15 consecutive patients. J Am Acad Dermatol. 2020;82:1251-1252. doi:10.1016/j.jaad.2019.12.053
1. Barbarot S, Wollenberg A, Silverberg JI, et al. Dupilumab provides rapid and sustained improvement in SCORAD outcomes in adults with moderate-to-severe atopic dermatitis: combined results ofour randomized phase 3 trials. J Dermatolog Treat. 2022;33:266-277. doi:10.1080/09546634.2020.1750550
2. Gordon SC, Robinson SN, Abudu M, et al. Eosinophilic annular erythema treated with dupilumab. Pediatr Dermatol. 2018;35:E255-E256. doi:10.1111/pde.13533
3. Callaghan DJ 3rd. Use of Google Trends to examine interest in Mohs micrographic surgery: 2004 to 2016. Dermatol Surg. 2018;44:186-192. doi:10.1097/DSS.0000000000001270
4. Fowler C, Hoover W. Dupilumab for chronic eosinophilic pneumonia. Pediatr Pulmonol. 2020;55:3229-3230. doi:10.1002/ppul.25096
5. Simpson EL, Akinlade B, Ardeleanu M. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2017;376:1090-1091. doi:10.1056/NEJMc1700366
6. Menzella F, Montanari G, Patricelli G, et al. A case of chronic eosinophilic pneumonia in a patient treated with dupilumab. Ther Clin Risk Manag. 2019;15:869-875. doi:10.2147/TCRM.S207402
7. Waldman RA, DeWane ME, Sloan B, et al. Dupilumab for the treatment of dyshidrotic eczema in 15 consecutive patients. J Am Acad Dermatol. 2020;82:1251-1252. doi:10.1016/j.jaad.2019.12.053
Practice Points
- Dupilumab can be considered for treatment of refractory dyshidrotic eczema.
- Dupilumab may provide secondary efficacy in patients with dyshidrotic eczema who also have an eosinophilic condition such as eosinophilic pneumonia.
Impact of the COVID-19 Pandemic on Care for Patients With Atopic Dermatitis
To the Editor:
Atopic dermatitis (AD) is a widely prevalent dermatologic condition that can severely impact a patient’s quality of life.1 Individuals with AD have been substantially affected during the COVID-19 pandemic due to the increased use of irritants, decreased access to care, and rise in psychological stress.1,2 These factors have resulted in lower quality of life and worsening dermatologic symptoms for many AD patients over the last few years.1 One major potential contributory component of these findings is decreased accessibility to in-office care during the pandemic, with a shift to telemedicine instead. Accessibility to care during the COVID-19 pandemic for AD patients compared to those without AD remains unknown. Therefore, we explored the impact of the COVID-19 pandemic on care for patients with AD in a large US population.
Using anonymous survey data from the 2021 National Health Interview Survey,3 we conducted a population-based, cross-sectional study to evaluate access to care during the COVID-19 pandemic for patients with AD compared to those without AD. We assigned the following 3 survey questions as outcome variables to assess access to care: delayed medical care due to COVID-19 pandemic (yes/no), did not get care due to COVID-19 pandemic (yes/no), and virtual medical appointment in the last 12 months (yes/no). In Table 1, numerous categorical survey variables, including sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region, were analyzed using χ2 testing to evaluate for differences among individuals with and without AD. Multivariable logistic regression models evaluating the relationship between AD and access to care were constructed using Stata/MP 17 (StataCorp LLC). In our analysis we controlled for age, sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region.
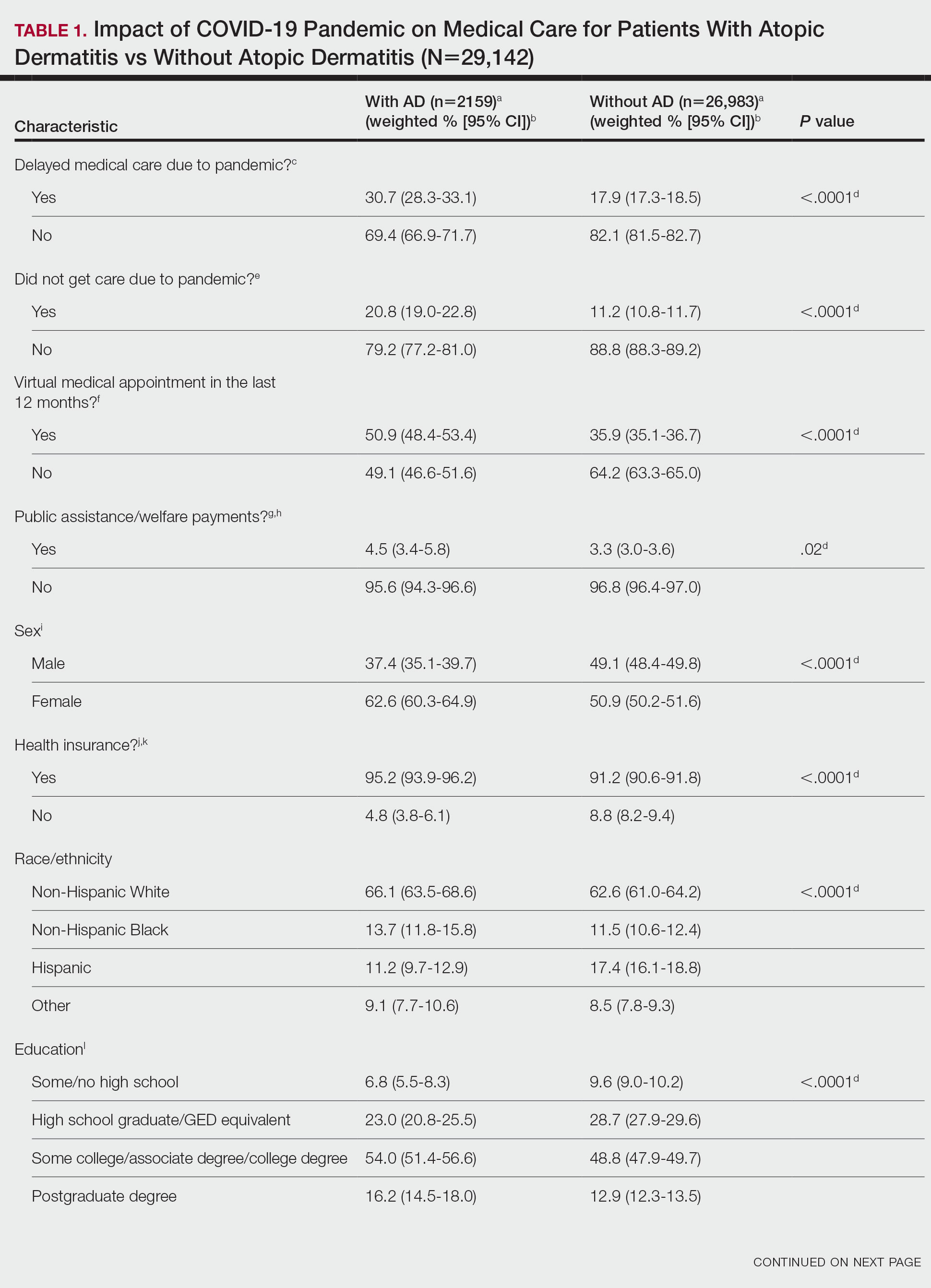
There were 29,142 adult patients (aged ≥18 years) included in our analysis. Approximately 7.4% (weighted) of individuals had AD (Table 1). After adjusting for confounding variables, patients with AD had a higher odds of delaying medical care due to the COVID-19 pandemic (adjusted odds ratio [AOR], 1.91; 95% CI, 1.69-2.16; P<.001), not receiving care due to the COVID-19 pandemic (AOR, 1.94; 95% CI, 1.71-2.22; P<.001), and having a virtual medical visit in the last 12 months (AOR, 1.72; 95% CI, 1.54-1.93; P<.001)(Table 2) compared with patients without AD.
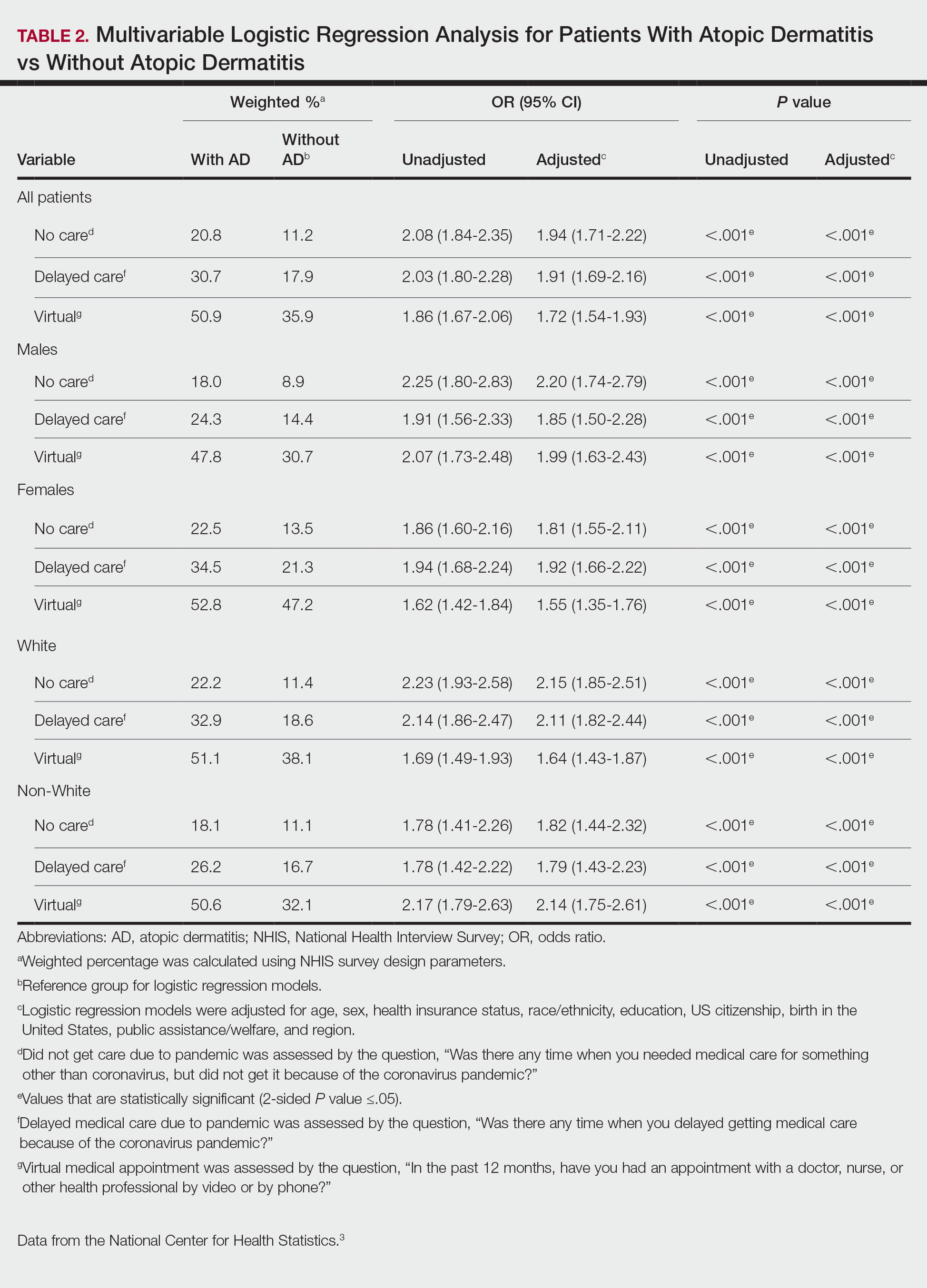
Our findings support the association between AD and decreased access to in-person care due to the COVID-19 pandemic. Moreover, telemedicine was utilized more among individuals with AD, possibly due to the accessibility of diagnostic tools for dermatologic diagnoses, such as high-quality photographs.4 According to Trinidad et al,4 telemedicine became an invaluable tool for dermatology hospitalists during the COVID-19 pandemic, as many physicians were able to comfortably diagnose patients with cutaneous diseases without an in-person visit. Utilizing telemedicine for patient care can help reduce the risk for COVID-19 transmission while also providing quality care for individuals living in rural areas.5 Chiricozzi et al6 discussed the importance of telemedicine in Italy during the pandemic, as many AD patients were able to maintain control of their disease while on systemic treatments.
Limitations of this study include self-reported measures; inability to compare patients with AD to individuals with other cutaneous diseases; and additional potential confounders, such as chronic comorbidities. Future studies should evaluate the use of telemedicine and access to care among individuals with other common skin diseases and help determine why such discrepancies exist. Understanding the difficulties in access to care and the viable alternatives in place may increase awareness and assist clinicians with adequate management of patients with AD.
1. Sieniawska J, Lesiak A, Cia˛z˙yn´ski K, et al. Impact of the COVID-19 pandemic on atopic dermatitis patients. Int J Environ Res Public Health. 2022;19:1734. doi:10.3390/ijerph19031734
2. Pourani MR, Ganji R, Dashti T, et al. Impact of COVID-19 pandemic on patients with atopic dermatitis [in Spanish]. Actas Dermosifiliogr. 2022;113:T286-T293. doi:10.1016/j.ad.2021.08.004
3. National Center for Health Statistics. NHIS Data, Questionnaires and Related Documentation. Centers for Disease Control and Prevention website. Accessed February 1, 2023. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
4. Trinidad J, Gabel CK, Han JJ, et al. Telemedicine and dermatology hospital consultations during the COVID-19 pandemic: a multi-centre observational study on resource utilization and conversion to in-person consultations during the COVID-19 pandemic. J Eur Acad Dermatol Venereol. 2022;36:E323-E325. doi:10.1111/jdv.17898
5. Marasca C, Annunziata MC, Camela E, et al. Teledermatology and inflammatory skin conditions during COVID-19 era: new perspectives and applications. J Clin Med. 2022;11:1511. doi:10.3390/jcm11061511
6. Chiricozzi A, Talamonti M, De Simone C, et al. Management of patients with atopic dermatitis undergoing systemic therapy during COVID-19 pandemic in Italy: data from the DA-COVID-19 registry. Allergy. 2021;76:1813-1824. doi:10.1111/all.14767
To the Editor:
Atopic dermatitis (AD) is a widely prevalent dermatologic condition that can severely impact a patient’s quality of life.1 Individuals with AD have been substantially affected during the COVID-19 pandemic due to the increased use of irritants, decreased access to care, and rise in psychological stress.1,2 These factors have resulted in lower quality of life and worsening dermatologic symptoms for many AD patients over the last few years.1 One major potential contributory component of these findings is decreased accessibility to in-office care during the pandemic, with a shift to telemedicine instead. Accessibility to care during the COVID-19 pandemic for AD patients compared to those without AD remains unknown. Therefore, we explored the impact of the COVID-19 pandemic on care for patients with AD in a large US population.
Using anonymous survey data from the 2021 National Health Interview Survey,3 we conducted a population-based, cross-sectional study to evaluate access to care during the COVID-19 pandemic for patients with AD compared to those without AD. We assigned the following 3 survey questions as outcome variables to assess access to care: delayed medical care due to COVID-19 pandemic (yes/no), did not get care due to COVID-19 pandemic (yes/no), and virtual medical appointment in the last 12 months (yes/no). In Table 1, numerous categorical survey variables, including sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region, were analyzed using χ2 testing to evaluate for differences among individuals with and without AD. Multivariable logistic regression models evaluating the relationship between AD and access to care were constructed using Stata/MP 17 (StataCorp LLC). In our analysis we controlled for age, sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region.


There were 29,142 adult patients (aged ≥18 years) included in our analysis. Approximately 7.4% (weighted) of individuals had AD (Table 1). After adjusting for confounding variables, patients with AD had a higher odds of delaying medical care due to the COVID-19 pandemic (adjusted odds ratio [AOR], 1.91; 95% CI, 1.69-2.16; P<.001), not receiving care due to the COVID-19 pandemic (AOR, 1.94; 95% CI, 1.71-2.22; P<.001), and having a virtual medical visit in the last 12 months (AOR, 1.72; 95% CI, 1.54-1.93; P<.001)(Table 2) compared with patients without AD.

Our findings support the association between AD and decreased access to in-person care due to the COVID-19 pandemic. Moreover, telemedicine was utilized more among individuals with AD, possibly due to the accessibility of diagnostic tools for dermatologic diagnoses, such as high-quality photographs.4 According to Trinidad et al,4 telemedicine became an invaluable tool for dermatology hospitalists during the COVID-19 pandemic, as many physicians were able to comfortably diagnose patients with cutaneous diseases without an in-person visit. Utilizing telemedicine for patient care can help reduce the risk for COVID-19 transmission while also providing quality care for individuals living in rural areas.5 Chiricozzi et al6 discussed the importance of telemedicine in Italy during the pandemic, as many AD patients were able to maintain control of their disease while on systemic treatments.
Limitations of this study include self-reported measures; inability to compare patients with AD to individuals with other cutaneous diseases; and additional potential confounders, such as chronic comorbidities. Future studies should evaluate the use of telemedicine and access to care among individuals with other common skin diseases and help determine why such discrepancies exist. Understanding the difficulties in access to care and the viable alternatives in place may increase awareness and assist clinicians with adequate management of patients with AD.
To the Editor:
Atopic dermatitis (AD) is a widely prevalent dermatologic condition that can severely impact a patient’s quality of life.1 Individuals with AD have been substantially affected during the COVID-19 pandemic due to the increased use of irritants, decreased access to care, and rise in psychological stress.1,2 These factors have resulted in lower quality of life and worsening dermatologic symptoms for many AD patients over the last few years.1 One major potential contributory component of these findings is decreased accessibility to in-office care during the pandemic, with a shift to telemedicine instead. Accessibility to care during the COVID-19 pandemic for AD patients compared to those without AD remains unknown. Therefore, we explored the impact of the COVID-19 pandemic on care for patients with AD in a large US population.
Using anonymous survey data from the 2021 National Health Interview Survey,3 we conducted a population-based, cross-sectional study to evaluate access to care during the COVID-19 pandemic for patients with AD compared to those without AD. We assigned the following 3 survey questions as outcome variables to assess access to care: delayed medical care due to COVID-19 pandemic (yes/no), did not get care due to COVID-19 pandemic (yes/no), and virtual medical appointment in the last 12 months (yes/no). In Table 1, numerous categorical survey variables, including sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region, were analyzed using χ2 testing to evaluate for differences among individuals with and without AD. Multivariable logistic regression models evaluating the relationship between AD and access to care were constructed using Stata/MP 17 (StataCorp LLC). In our analysis we controlled for age, sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region.


There were 29,142 adult patients (aged ≥18 years) included in our analysis. Approximately 7.4% (weighted) of individuals had AD (Table 1). After adjusting for confounding variables, patients with AD had a higher odds of delaying medical care due to the COVID-19 pandemic (adjusted odds ratio [AOR], 1.91; 95% CI, 1.69-2.16; P<.001), not receiving care due to the COVID-19 pandemic (AOR, 1.94; 95% CI, 1.71-2.22; P<.001), and having a virtual medical visit in the last 12 months (AOR, 1.72; 95% CI, 1.54-1.93; P<.001)(Table 2) compared with patients without AD.

Our findings support the association between AD and decreased access to in-person care due to the COVID-19 pandemic. Moreover, telemedicine was utilized more among individuals with AD, possibly due to the accessibility of diagnostic tools for dermatologic diagnoses, such as high-quality photographs.4 According to Trinidad et al,4 telemedicine became an invaluable tool for dermatology hospitalists during the COVID-19 pandemic, as many physicians were able to comfortably diagnose patients with cutaneous diseases without an in-person visit. Utilizing telemedicine for patient care can help reduce the risk for COVID-19 transmission while also providing quality care for individuals living in rural areas.5 Chiricozzi et al6 discussed the importance of telemedicine in Italy during the pandemic, as many AD patients were able to maintain control of their disease while on systemic treatments.
Limitations of this study include self-reported measures; inability to compare patients with AD to individuals with other cutaneous diseases; and additional potential confounders, such as chronic comorbidities. Future studies should evaluate the use of telemedicine and access to care among individuals with other common skin diseases and help determine why such discrepancies exist. Understanding the difficulties in access to care and the viable alternatives in place may increase awareness and assist clinicians with adequate management of patients with AD.
1. Sieniawska J, Lesiak A, Cia˛z˙yn´ski K, et al. Impact of the COVID-19 pandemic on atopic dermatitis patients. Int J Environ Res Public Health. 2022;19:1734. doi:10.3390/ijerph19031734
2. Pourani MR, Ganji R, Dashti T, et al. Impact of COVID-19 pandemic on patients with atopic dermatitis [in Spanish]. Actas Dermosifiliogr. 2022;113:T286-T293. doi:10.1016/j.ad.2021.08.004
3. National Center for Health Statistics. NHIS Data, Questionnaires and Related Documentation. Centers for Disease Control and Prevention website. Accessed February 1, 2023. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
4. Trinidad J, Gabel CK, Han JJ, et al. Telemedicine and dermatology hospital consultations during the COVID-19 pandemic: a multi-centre observational study on resource utilization and conversion to in-person consultations during the COVID-19 pandemic. J Eur Acad Dermatol Venereol. 2022;36:E323-E325. doi:10.1111/jdv.17898
5. Marasca C, Annunziata MC, Camela E, et al. Teledermatology and inflammatory skin conditions during COVID-19 era: new perspectives and applications. J Clin Med. 2022;11:1511. doi:10.3390/jcm11061511
6. Chiricozzi A, Talamonti M, De Simone C, et al. Management of patients with atopic dermatitis undergoing systemic therapy during COVID-19 pandemic in Italy: data from the DA-COVID-19 registry. Allergy. 2021;76:1813-1824. doi:10.1111/all.14767
1. Sieniawska J, Lesiak A, Cia˛z˙yn´ski K, et al. Impact of the COVID-19 pandemic on atopic dermatitis patients. Int J Environ Res Public Health. 2022;19:1734. doi:10.3390/ijerph19031734
2. Pourani MR, Ganji R, Dashti T, et al. Impact of COVID-19 pandemic on patients with atopic dermatitis [in Spanish]. Actas Dermosifiliogr. 2022;113:T286-T293. doi:10.1016/j.ad.2021.08.004
3. National Center for Health Statistics. NHIS Data, Questionnaires and Related Documentation. Centers for Disease Control and Prevention website. Accessed February 1, 2023. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
4. Trinidad J, Gabel CK, Han JJ, et al. Telemedicine and dermatology hospital consultations during the COVID-19 pandemic: a multi-centre observational study on resource utilization and conversion to in-person consultations during the COVID-19 pandemic. J Eur Acad Dermatol Venereol. 2022;36:E323-E325. doi:10.1111/jdv.17898
5. Marasca C, Annunziata MC, Camela E, et al. Teledermatology and inflammatory skin conditions during COVID-19 era: new perspectives and applications. J Clin Med. 2022;11:1511. doi:10.3390/jcm11061511
6. Chiricozzi A, Talamonti M, De Simone C, et al. Management of patients with atopic dermatitis undergoing systemic therapy during COVID-19 pandemic in Italy: data from the DA-COVID-19 registry. Allergy. 2021;76:1813-1824. doi:10.1111/all.14767
Practice Points
- The landscape of dermatology has seen major changes due to the COVID-19 pandemic, as many patients now utilize telemedicine to receive care.
- Understanding accessibility to in-person care for patients with atopic dermatitis during the COVID-19 pandemic can assist with the development of methods to enhance management.
Factors influencing clinical response to dupilumab treatment in AD
Key clinical point: Patients with moderate-to-severe atopic dermatitis (AD) presenting with classic or generalized lichenoid and inflammatory phenotypes vs other non-classic phenotypes and with Eczema Area and Severity Index (EASI) scores < 29 vs ≥ 29 showed an early response to dupilumab by achieving a mild disease state.
Major finding: Factors with a significant predictive value for an early response to dupilumab included the classic phenotype (odds ratio [OR] 6.92; 95% CI 2.04-23.48) or generalized lichenoid and inflammatory phenotypes (OR 4.22; 95% CI 1.22-14.66) vs the nummular eczema phenotype and a baseline EASI score of ≤ 24 (OR 3.13; 95% CI 1.81-5.41) or 24-29 (OR 1.79; 95% CI 1.05-3.07) vs ≥ 29.
Study details: Findings are from a retrospective single-center observational study including 492 patients (age > 12 years) with moderate-to-severe AD treated with dupilumab.
Disclosures: This study did not receive any external funding. S Ferrucci and AV Marzano declared serving as speakers or advisory board members of various organizations. The other authors declared no conflicts of interest.
Source: Ferrucci S et al. Predictive factors of early response to dupilumab in patients with moderate-to-severe atopic dermatitis. J Clin Med. 2023;12(20):6575 (Oct 17). doi: 10.3390/jcm12206575.
Key clinical point: Patients with moderate-to-severe atopic dermatitis (AD) presenting with classic or generalized lichenoid and inflammatory phenotypes vs other non-classic phenotypes and with Eczema Area and Severity Index (EASI) scores < 29 vs ≥ 29 showed an early response to dupilumab by achieving a mild disease state.
Major finding: Factors with a significant predictive value for an early response to dupilumab included the classic phenotype (odds ratio [OR] 6.92; 95% CI 2.04-23.48) or generalized lichenoid and inflammatory phenotypes (OR 4.22; 95% CI 1.22-14.66) vs the nummular eczema phenotype and a baseline EASI score of ≤ 24 (OR 3.13; 95% CI 1.81-5.41) or 24-29 (OR 1.79; 95% CI 1.05-3.07) vs ≥ 29.
Study details: Findings are from a retrospective single-center observational study including 492 patients (age > 12 years) with moderate-to-severe AD treated with dupilumab.
Disclosures: This study did not receive any external funding. S Ferrucci and AV Marzano declared serving as speakers or advisory board members of various organizations. The other authors declared no conflicts of interest.
Source: Ferrucci S et al. Predictive factors of early response to dupilumab in patients with moderate-to-severe atopic dermatitis. J Clin Med. 2023;12(20):6575 (Oct 17). doi: 10.3390/jcm12206575.
Key clinical point: Patients with moderate-to-severe atopic dermatitis (AD) presenting with classic or generalized lichenoid and inflammatory phenotypes vs other non-classic phenotypes and with Eczema Area and Severity Index (EASI) scores < 29 vs ≥ 29 showed an early response to dupilumab by achieving a mild disease state.
Major finding: Factors with a significant predictive value for an early response to dupilumab included the classic phenotype (odds ratio [OR] 6.92; 95% CI 2.04-23.48) or generalized lichenoid and inflammatory phenotypes (OR 4.22; 95% CI 1.22-14.66) vs the nummular eczema phenotype and a baseline EASI score of ≤ 24 (OR 3.13; 95% CI 1.81-5.41) or 24-29 (OR 1.79; 95% CI 1.05-3.07) vs ≥ 29.
Study details: Findings are from a retrospective single-center observational study including 492 patients (age > 12 years) with moderate-to-severe AD treated with dupilumab.
Disclosures: This study did not receive any external funding. S Ferrucci and AV Marzano declared serving as speakers or advisory board members of various organizations. The other authors declared no conflicts of interest.
Source: Ferrucci S et al. Predictive factors of early response to dupilumab in patients with moderate-to-severe atopic dermatitis. J Clin Med. 2023;12(20):6575 (Oct 17). doi: 10.3390/jcm12206575.
Adults with moderate-to-severe AD are prone to renal malignancy
Key clinical point: Adults with moderate-to-severe atopic dermatitis (AD) are at a significantly higher risk for renal malignancy, with the risk for overall malignancy being higher in adults with AD regardless of the disease severity.
Major finding: Compared with adults without AD, those with moderate-to-severe AD had a significantly increased risk for renal malignancy (adjusted hazard ratio [aHR] 1.533; 95% CI 1.209-1.944); moreover, the risk for overall malignancy was higher in adults with mild (aHR 1.061; 95% CI 1.006-1.118) and moderate-to-severe (aHR 1.061; 95% CI 1.014-1.110) AD.
Study details: Findings are from a population-based cohort study including 22,430 adults with mild AD, 34,187 adults with moderate-to-severe AD, and 3,810,530 adults without AD.
Disclosures: This study did not receive any external funding. The authors declared no conflicts of interest.
Source: Oh J et al. Increased risk of renal malignancy in patients with moderate to severe atopic dermatitis. Cancers (Basel). 2023;15(20):5007 (Oct 16). doi: 10.3390/cancers15205007
Key clinical point: Adults with moderate-to-severe atopic dermatitis (AD) are at a significantly higher risk for renal malignancy, with the risk for overall malignancy being higher in adults with AD regardless of the disease severity.
Major finding: Compared with adults without AD, those with moderate-to-severe AD had a significantly increased risk for renal malignancy (adjusted hazard ratio [aHR] 1.533; 95% CI 1.209-1.944); moreover, the risk for overall malignancy was higher in adults with mild (aHR 1.061; 95% CI 1.006-1.118) and moderate-to-severe (aHR 1.061; 95% CI 1.014-1.110) AD.
Study details: Findings are from a population-based cohort study including 22,430 adults with mild AD, 34,187 adults with moderate-to-severe AD, and 3,810,530 adults without AD.
Disclosures: This study did not receive any external funding. The authors declared no conflicts of interest.
Source: Oh J et al. Increased risk of renal malignancy in patients with moderate to severe atopic dermatitis. Cancers (Basel). 2023;15(20):5007 (Oct 16). doi: 10.3390/cancers15205007
Key clinical point: Adults with moderate-to-severe atopic dermatitis (AD) are at a significantly higher risk for renal malignancy, with the risk for overall malignancy being higher in adults with AD regardless of the disease severity.
Major finding: Compared with adults without AD, those with moderate-to-severe AD had a significantly increased risk for renal malignancy (adjusted hazard ratio [aHR] 1.533; 95% CI 1.209-1.944); moreover, the risk for overall malignancy was higher in adults with mild (aHR 1.061; 95% CI 1.006-1.118) and moderate-to-severe (aHR 1.061; 95% CI 1.014-1.110) AD.
Study details: Findings are from a population-based cohort study including 22,430 adults with mild AD, 34,187 adults with moderate-to-severe AD, and 3,810,530 adults without AD.
Disclosures: This study did not receive any external funding. The authors declared no conflicts of interest.
Source: Oh J et al. Increased risk of renal malignancy in patients with moderate to severe atopic dermatitis. Cancers (Basel). 2023;15(20):5007 (Oct 16). doi: 10.3390/cancers15205007
Meta-analysis evaluates the comparative efficacy of systemic immunomodulators against AD
Key clinical point: The binary outcomes of atopic dermatitis (AD) were most effectively improved up to week 16 by 30 mg upadacitinib daily and 200 mg abrocitinib daily, followed by 15 mg upadacitinib daily, and 600 mg dupilumab and subsequently 300 mg dupilumab every 2 weeks.
Major finding: The odds of achieving 50% improvement in the Eczema Area and Severity Index scores were higher with daily doses of 200 mg abrocitinib (odds ratio [OR] 1.5, 95% credible interval [CrI] 1.1-2.2), 30 mg upadacitinib (OR 2.5, 95% CrI 1.3-5.0), and 15 mg upadacitinib (OR 1.7; 95% CrI 0.9-3.3) and lower with 100 mg abrocitinib daily (OR 0.7; 95% CrI 0.5-1.0) and 4 mg baricitinib daily (OR 0.5; 95% CrI 0.3-0.7) compared with dupilumab every 2 weeks.
Study details: This network meta-analysis of 83 trials included 22,122 patients with moderate-to-severe AD receiving systemic immunomodulatory treatment for ≥8 weeks.
Disclosures: This study was sponsored by a UK National Institute for Health Research Career Development Fellowship held by C Flohr and other funds. Seven authors declared ties with various sources.
Source: Drucker AM et al. Comparing binary efficacy outcomes for systemic immunomodulatory treatments for atopic dermatitis in a living systematic review and network meta-analysis. Br J Dermatol. 2023 (Oct 13). doi: 10.1093/bjd/ljad393
Key clinical point: The binary outcomes of atopic dermatitis (AD) were most effectively improved up to week 16 by 30 mg upadacitinib daily and 200 mg abrocitinib daily, followed by 15 mg upadacitinib daily, and 600 mg dupilumab and subsequently 300 mg dupilumab every 2 weeks.
Major finding: The odds of achieving 50% improvement in the Eczema Area and Severity Index scores were higher with daily doses of 200 mg abrocitinib (odds ratio [OR] 1.5, 95% credible interval [CrI] 1.1-2.2), 30 mg upadacitinib (OR 2.5, 95% CrI 1.3-5.0), and 15 mg upadacitinib (OR 1.7; 95% CrI 0.9-3.3) and lower with 100 mg abrocitinib daily (OR 0.7; 95% CrI 0.5-1.0) and 4 mg baricitinib daily (OR 0.5; 95% CrI 0.3-0.7) compared with dupilumab every 2 weeks.
Study details: This network meta-analysis of 83 trials included 22,122 patients with moderate-to-severe AD receiving systemic immunomodulatory treatment for ≥8 weeks.
Disclosures: This study was sponsored by a UK National Institute for Health Research Career Development Fellowship held by C Flohr and other funds. Seven authors declared ties with various sources.
Source: Drucker AM et al. Comparing binary efficacy outcomes for systemic immunomodulatory treatments for atopic dermatitis in a living systematic review and network meta-analysis. Br J Dermatol. 2023 (Oct 13). doi: 10.1093/bjd/ljad393
Key clinical point: The binary outcomes of atopic dermatitis (AD) were most effectively improved up to week 16 by 30 mg upadacitinib daily and 200 mg abrocitinib daily, followed by 15 mg upadacitinib daily, and 600 mg dupilumab and subsequently 300 mg dupilumab every 2 weeks.
Major finding: The odds of achieving 50% improvement in the Eczema Area and Severity Index scores were higher with daily doses of 200 mg abrocitinib (odds ratio [OR] 1.5, 95% credible interval [CrI] 1.1-2.2), 30 mg upadacitinib (OR 2.5, 95% CrI 1.3-5.0), and 15 mg upadacitinib (OR 1.7; 95% CrI 0.9-3.3) and lower with 100 mg abrocitinib daily (OR 0.7; 95% CrI 0.5-1.0) and 4 mg baricitinib daily (OR 0.5; 95% CrI 0.3-0.7) compared with dupilumab every 2 weeks.
Study details: This network meta-analysis of 83 trials included 22,122 patients with moderate-to-severe AD receiving systemic immunomodulatory treatment for ≥8 weeks.
Disclosures: This study was sponsored by a UK National Institute for Health Research Career Development Fellowship held by C Flohr and other funds. Seven authors declared ties with various sources.
Source: Drucker AM et al. Comparing binary efficacy outcomes for systemic immunomodulatory treatments for atopic dermatitis in a living systematic review and network meta-analysis. Br J Dermatol. 2023 (Oct 13). doi: 10.1093/bjd/ljad393
Atopic dermatitis is a potential risk factor for cognitive dysfunction in middle-aged and older adults
Key clinical point: Atopic dermatitis (AD) significantly increases the risk for cognitive dysfunction, particularly that of all-cause dementia and Alzheimer’s disease-related dementia, in middle-aged adults (age 45-59 years) and older adults (age ≥60 years).
Major finding: Patients with AD vs control individuals had a significantly higher risk of developing all-cause dementia (pooled hazard ratio [HR] 1.16; 95% CI 1.10-1.23) and Alzheimer’s disease-related dementia (pooled HR 1.28; 95% CI 1.01-1.63). However, no significant association was observed between AD and vascular dementia (pooled HR 1.42; 95% CI 0.99-2.04).
Study details: Findings are from a meta-analysis of five studies including 8,595,252 patients with AD and a corresponding number of control individuals without AD.
Disclosures: This study did not receive any specific funding. The authors declared no conflicts of interest.
Source: Zhou Q et al. Atopic dermatitis and cognitive dysfunction in middle-aged and older adults: A systematic review and meta-analysis. PLoS One. 2023;18(10):e0292987 (Oct 25). doi: 10.1371/journal.pone.0292987
Key clinical point: Atopic dermatitis (AD) significantly increases the risk for cognitive dysfunction, particularly that of all-cause dementia and Alzheimer’s disease-related dementia, in middle-aged adults (age 45-59 years) and older adults (age ≥60 years).
Major finding: Patients with AD vs control individuals had a significantly higher risk of developing all-cause dementia (pooled hazard ratio [HR] 1.16; 95% CI 1.10-1.23) and Alzheimer’s disease-related dementia (pooled HR 1.28; 95% CI 1.01-1.63). However, no significant association was observed between AD and vascular dementia (pooled HR 1.42; 95% CI 0.99-2.04).
Study details: Findings are from a meta-analysis of five studies including 8,595,252 patients with AD and a corresponding number of control individuals without AD.
Disclosures: This study did not receive any specific funding. The authors declared no conflicts of interest.
Source: Zhou Q et al. Atopic dermatitis and cognitive dysfunction in middle-aged and older adults: A systematic review and meta-analysis. PLoS One. 2023;18(10):e0292987 (Oct 25). doi: 10.1371/journal.pone.0292987
Key clinical point: Atopic dermatitis (AD) significantly increases the risk for cognitive dysfunction, particularly that of all-cause dementia and Alzheimer’s disease-related dementia, in middle-aged adults (age 45-59 years) and older adults (age ≥60 years).
Major finding: Patients with AD vs control individuals had a significantly higher risk of developing all-cause dementia (pooled hazard ratio [HR] 1.16; 95% CI 1.10-1.23) and Alzheimer’s disease-related dementia (pooled HR 1.28; 95% CI 1.01-1.63). However, no significant association was observed between AD and vascular dementia (pooled HR 1.42; 95% CI 0.99-2.04).
Study details: Findings are from a meta-analysis of five studies including 8,595,252 patients with AD and a corresponding number of control individuals without AD.
Disclosures: This study did not receive any specific funding. The authors declared no conflicts of interest.
Source: Zhou Q et al. Atopic dermatitis and cognitive dysfunction in middle-aged and older adults: A systematic review and meta-analysis. PLoS One. 2023;18(10):e0292987 (Oct 25). doi: 10.1371/journal.pone.0292987