User login
Psoriasis: Biologics bring potential for long-term remission off treatment
GENEVA – Can potent, highly efficacious biologic therapy induce longstanding remission of moderate to severe plaque psoriasis following discontinuation of all treatment?
The answer appears to be yes, in a minority of patients, based on results of a long-term extension study of two pivotal phase 3 clinical trials of secukinumab (Cosentyx), Mark G. Lebwohl, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.

This 2-year extension study provides the first double-blind, long-term evidence regarding the natural history of psoriasis following discontinuation of a contemporary potent targeted therapy.
The results are exciting because spontaneous remission of moderate to severe psoriasis is rare; psoriasis typically reverts to baseline severity following discontinuation of treatment. In the extension study, though, 21% of patients who achieved a 75% reduction in the Psoriasis Area Severity Index (PASI 75) response on secukinumab at the 300-mg dose for 52 weeks and were then crossed over double-blind to placebo remained continuously relapse-free after 12 months on placebo, and 10% remained relapse-free at 2 years, at which point they were unblinded and put back on secukinumab. No psoriasis therapies were permitted during the placebo phase.
Those findings are particularly impressive because participants in the two pivotal trials of secukinumab combined for the treatment withdrawal extension study – ERASURE and FIXTURE – had a mean 14- and 16-year history of psoriasis at baseline.
“Secukinumab appears to have modified the course of chronic moderate to severe psoriasis, despite intervening late in its course. Treating patients early is expected to have an even greater potential for disease modification,” commented Dr. Lebwohl, the Sol and Clara Kest Professor and chair of the department of dermatology at Icahn School of Medicine at Mount Sinai, New York.
That hypothesis is under investigation in the ongoing multinational STEPin trial (Study of the Efficacy of Early Intervention With Secukinumab 300 mg s.c. [subcutaneous] Compared to Narrow-band UVB in Patients With New-onset Moderate to Severe Plaque Psoriasis). STEPin involves about 200 patients with newly diagnosed disease not previously treated with systemic agents or phototherapy.
Dr. Lebwohl focused on the 120 patients in the extension study who had a PASI 75 response to secukinumab at the 300-mg dose and the 100 patients with a PASI 75 response to the 150-mg dose who, after 52 weeks on the fully human anti-interleukin-17A monoclonal antibody, were crossed over double-blind to placebo for 2 years or until relapse occurred. Patients were assessed monthly during the extension study.
Relapse was defined as loss of 50% of the maximum PASI improvement achieved while on secukinumab. The relapse-free rate was better in patients who had been on secukinumab 300 mg than in those on 150 mg. At 1 year on placebo, 21% of patients formerly on secukinumab at the higher dose remained free of relapse, as did 10% at 2 years. In contrast, the 1- and 2-year relapse-free rates in the group formerly on secukinumab 150 mg were lower at 14% and 6%, respectively, even though everyone in the extension study had a PASI 75 response when they went off treatment.
The mean baseline PASI score in the ERASURE and FIXTURE trials was 23. Among patients who remained relapse-free for 1 year, the mean PASI score was dramatically lower, at 0.7; among those in remission for 2 years, it was 0.4.
Importantly, the longer a patient’s history of psoriasis, the less likely a long-term relapse-free experience, the dermatologist observed.
A separate mechanistic study in a different group of psoriasis patients provided a likely explanation for the long relapse-free period seen off therapy in some patients in the extension study. The mechanistic study entailed transcriptional analysis of psoriasis-related genes in lesional and nonlesional skin before and after 1 year of treatment with secukinumab 300 mg. The major finding: 85% of the upregulated genes and 75% of downregulated genes in lesional skin pretreatment reverted to normal levels in healed lesional skin post treatment.
Moreover, global mRNA expression for key genes involved in the interleukin-23/interleukin-17 inflammatory cytokine pathway closely resembled healthy skin following treatment with secukinumab. This is evidence of profound molecular normalization, Dr. Lebwohl said.
These secukinumab studies were sponsored by Novartis. Dr. Lebwohl reported having no personal financial conflicts of interest, although his department receives research funding from Novartis and numerous other pharmaceutical companies.
GENEVA – Can potent, highly efficacious biologic therapy induce longstanding remission of moderate to severe plaque psoriasis following discontinuation of all treatment?
The answer appears to be yes, in a minority of patients, based on results of a long-term extension study of two pivotal phase 3 clinical trials of secukinumab (Cosentyx), Mark G. Lebwohl, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.

This 2-year extension study provides the first double-blind, long-term evidence regarding the natural history of psoriasis following discontinuation of a contemporary potent targeted therapy.
The results are exciting because spontaneous remission of moderate to severe psoriasis is rare; psoriasis typically reverts to baseline severity following discontinuation of treatment. In the extension study, though, 21% of patients who achieved a 75% reduction in the Psoriasis Area Severity Index (PASI 75) response on secukinumab at the 300-mg dose for 52 weeks and were then crossed over double-blind to placebo remained continuously relapse-free after 12 months on placebo, and 10% remained relapse-free at 2 years, at which point they were unblinded and put back on secukinumab. No psoriasis therapies were permitted during the placebo phase.
Those findings are particularly impressive because participants in the two pivotal trials of secukinumab combined for the treatment withdrawal extension study – ERASURE and FIXTURE – had a mean 14- and 16-year history of psoriasis at baseline.
“Secukinumab appears to have modified the course of chronic moderate to severe psoriasis, despite intervening late in its course. Treating patients early is expected to have an even greater potential for disease modification,” commented Dr. Lebwohl, the Sol and Clara Kest Professor and chair of the department of dermatology at Icahn School of Medicine at Mount Sinai, New York.
That hypothesis is under investigation in the ongoing multinational STEPin trial (Study of the Efficacy of Early Intervention With Secukinumab 300 mg s.c. [subcutaneous] Compared to Narrow-band UVB in Patients With New-onset Moderate to Severe Plaque Psoriasis). STEPin involves about 200 patients with newly diagnosed disease not previously treated with systemic agents or phototherapy.
Dr. Lebwohl focused on the 120 patients in the extension study who had a PASI 75 response to secukinumab at the 300-mg dose and the 100 patients with a PASI 75 response to the 150-mg dose who, after 52 weeks on the fully human anti-interleukin-17A monoclonal antibody, were crossed over double-blind to placebo for 2 years or until relapse occurred. Patients were assessed monthly during the extension study.
Relapse was defined as loss of 50% of the maximum PASI improvement achieved while on secukinumab. The relapse-free rate was better in patients who had been on secukinumab 300 mg than in those on 150 mg. At 1 year on placebo, 21% of patients formerly on secukinumab at the higher dose remained free of relapse, as did 10% at 2 years. In contrast, the 1- and 2-year relapse-free rates in the group formerly on secukinumab 150 mg were lower at 14% and 6%, respectively, even though everyone in the extension study had a PASI 75 response when they went off treatment.
The mean baseline PASI score in the ERASURE and FIXTURE trials was 23. Among patients who remained relapse-free for 1 year, the mean PASI score was dramatically lower, at 0.7; among those in remission for 2 years, it was 0.4.
Importantly, the longer a patient’s history of psoriasis, the less likely a long-term relapse-free experience, the dermatologist observed.
A separate mechanistic study in a different group of psoriasis patients provided a likely explanation for the long relapse-free period seen off therapy in some patients in the extension study. The mechanistic study entailed transcriptional analysis of psoriasis-related genes in lesional and nonlesional skin before and after 1 year of treatment with secukinumab 300 mg. The major finding: 85% of the upregulated genes and 75% of downregulated genes in lesional skin pretreatment reverted to normal levels in healed lesional skin post treatment.
Moreover, global mRNA expression for key genes involved in the interleukin-23/interleukin-17 inflammatory cytokine pathway closely resembled healthy skin following treatment with secukinumab. This is evidence of profound molecular normalization, Dr. Lebwohl said.
These secukinumab studies were sponsored by Novartis. Dr. Lebwohl reported having no personal financial conflicts of interest, although his department receives research funding from Novartis and numerous other pharmaceutical companies.
GENEVA – Can potent, highly efficacious biologic therapy induce longstanding remission of moderate to severe plaque psoriasis following discontinuation of all treatment?
The answer appears to be yes, in a minority of patients, based on results of a long-term extension study of two pivotal phase 3 clinical trials of secukinumab (Cosentyx), Mark G. Lebwohl, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.

This 2-year extension study provides the first double-blind, long-term evidence regarding the natural history of psoriasis following discontinuation of a contemporary potent targeted therapy.
The results are exciting because spontaneous remission of moderate to severe psoriasis is rare; psoriasis typically reverts to baseline severity following discontinuation of treatment. In the extension study, though, 21% of patients who achieved a 75% reduction in the Psoriasis Area Severity Index (PASI 75) response on secukinumab at the 300-mg dose for 52 weeks and were then crossed over double-blind to placebo remained continuously relapse-free after 12 months on placebo, and 10% remained relapse-free at 2 years, at which point they were unblinded and put back on secukinumab. No psoriasis therapies were permitted during the placebo phase.
Those findings are particularly impressive because participants in the two pivotal trials of secukinumab combined for the treatment withdrawal extension study – ERASURE and FIXTURE – had a mean 14- and 16-year history of psoriasis at baseline.
“Secukinumab appears to have modified the course of chronic moderate to severe psoriasis, despite intervening late in its course. Treating patients early is expected to have an even greater potential for disease modification,” commented Dr. Lebwohl, the Sol and Clara Kest Professor and chair of the department of dermatology at Icahn School of Medicine at Mount Sinai, New York.
That hypothesis is under investigation in the ongoing multinational STEPin trial (Study of the Efficacy of Early Intervention With Secukinumab 300 mg s.c. [subcutaneous] Compared to Narrow-band UVB in Patients With New-onset Moderate to Severe Plaque Psoriasis). STEPin involves about 200 patients with newly diagnosed disease not previously treated with systemic agents or phototherapy.
Dr. Lebwohl focused on the 120 patients in the extension study who had a PASI 75 response to secukinumab at the 300-mg dose and the 100 patients with a PASI 75 response to the 150-mg dose who, after 52 weeks on the fully human anti-interleukin-17A monoclonal antibody, were crossed over double-blind to placebo for 2 years or until relapse occurred. Patients were assessed monthly during the extension study.
Relapse was defined as loss of 50% of the maximum PASI improvement achieved while on secukinumab. The relapse-free rate was better in patients who had been on secukinumab 300 mg than in those on 150 mg. At 1 year on placebo, 21% of patients formerly on secukinumab at the higher dose remained free of relapse, as did 10% at 2 years. In contrast, the 1- and 2-year relapse-free rates in the group formerly on secukinumab 150 mg were lower at 14% and 6%, respectively, even though everyone in the extension study had a PASI 75 response when they went off treatment.
The mean baseline PASI score in the ERASURE and FIXTURE trials was 23. Among patients who remained relapse-free for 1 year, the mean PASI score was dramatically lower, at 0.7; among those in remission for 2 years, it was 0.4.
Importantly, the longer a patient’s history of psoriasis, the less likely a long-term relapse-free experience, the dermatologist observed.
A separate mechanistic study in a different group of psoriasis patients provided a likely explanation for the long relapse-free period seen off therapy in some patients in the extension study. The mechanistic study entailed transcriptional analysis of psoriasis-related genes in lesional and nonlesional skin before and after 1 year of treatment with secukinumab 300 mg. The major finding: 85% of the upregulated genes and 75% of downregulated genes in lesional skin pretreatment reverted to normal levels in healed lesional skin post treatment.
Moreover, global mRNA expression for key genes involved in the interleukin-23/interleukin-17 inflammatory cytokine pathway closely resembled healthy skin following treatment with secukinumab. This is evidence of profound molecular normalization, Dr. Lebwohl said.
These secukinumab studies were sponsored by Novartis. Dr. Lebwohl reported having no personal financial conflicts of interest, although his department receives research funding from Novartis and numerous other pharmaceutical companies.
AT THE EADV CONGRESS
Key clinical point: , enabling some patients to experience long-term remission off therapy.
Major finding: After 1 and 2 years without any psoriasis therapy following successful treatment with secukinumab for 52 weeks, 21% and 10% of patients, respectively, remained relapse-free.
Data source: This prospective extension of two phase 3 pivotal trials of secukinumab included 220 psoriasis patients who were taken off the biologic after 52 weeks and crossed over double blind to placebo for up to 2 years.
Disclosures: The study was sponsored by Novartis. Dr. Lebwohl reported having no financial conflicts.
Pediatric psoriasis carries sharply increased risk of selected autoimmune comorbidities
GENEVA – Pediatric psoriasis is associated with sharply increased risks of selected autoimmune diseases, according to a cross-sectional study encompassing every child and adolescent in Denmark.
"Even though the absolute risk of many of these conditions remains rare in childhood, clinicians should keep these associations in mind because they can greatly add to the total disease burden. In particular, we advise focusing on extracutaneous symptoms when treating psoriasis in children,” Christoffer Blegvad, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
He presented a cross-sectional study in which Denmark’s vaunted system of comprehensive national registries was harnessed to obtain health information on all individuals under age 18 years living in Denmark as of the end of 2012. The study population comprised 1,925 children and adolescents with dermatologist-diagnosed psoriasis, including those with psoriasis mild enough to be managed with topical therapies, and 1,194,712 youths without psoriasis.
In a first-pass unadjusted analysis, the psoriasis patients were at significantly increased risk for all nine of the autoimmune diseases examined. When the investigators adjusted for age, sex, and an individual’s health care utilization as reflected in his or her number of dermatology visits, the children and adolescents with psoriasis remained at tenfold increased risk for comorbid psoriatic arthritis, 6.6-fold risk for rheumatoid arthritis, 4.8-fold risk for vitiligo, and smaller yet significantly increased risks for several other autoimmune diseases, compared with individuals without psoriasis.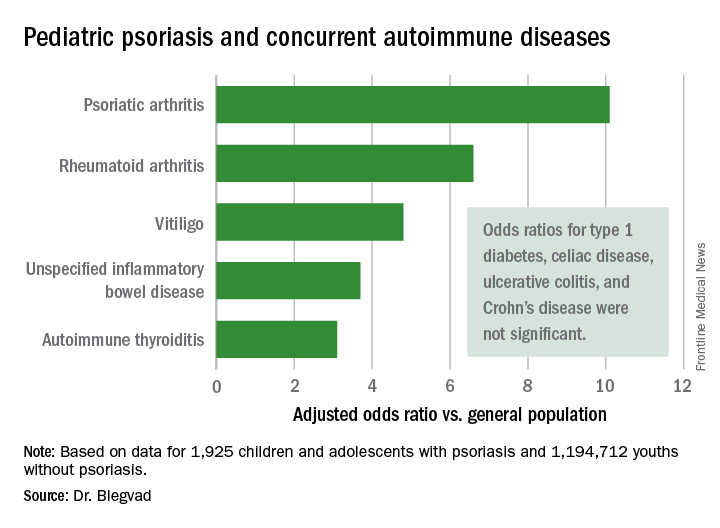
Indeed, while the presence of pediatric psoriasis was associated with an adjusted 4.4-fold increased risk of having at least one autoimmune disease, psoriasis patients with one of the selected autoimmune diseases were at 7.3-fold greater risk of having two or more autoimmune diseases, compared with individuals with one autoimmune disease who didn’t have psoriasis.
This is the first study to show such a clustering effect in either pediatric or adult psoriasis patients. The finding highlights the complex genetic underpinnings of psoriasis, which has previously been shown to share genetic susceptibility loci with inflammatory bowel disease and various other autoimmune diseases, Dr. Blegvad noted.
A small caveat: The psoriatic arthritis category also included individuals with juvenile idiopathic arthritis and juvenile psoriatic arthritis, because the clinical signs and symptoms of the three disorders often are difficult to distinguish in young patients.
Dr. Blegvad reported having no financial conflicts regarding the study, funded by Herlev and Gentofte Hospital and the LEO Foundation.
GENEVA – Pediatric psoriasis is associated with sharply increased risks of selected autoimmune diseases, according to a cross-sectional study encompassing every child and adolescent in Denmark.
"Even though the absolute risk of many of these conditions remains rare in childhood, clinicians should keep these associations in mind because they can greatly add to the total disease burden. In particular, we advise focusing on extracutaneous symptoms when treating psoriasis in children,” Christoffer Blegvad, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
He presented a cross-sectional study in which Denmark’s vaunted system of comprehensive national registries was harnessed to obtain health information on all individuals under age 18 years living in Denmark as of the end of 2012. The study population comprised 1,925 children and adolescents with dermatologist-diagnosed psoriasis, including those with psoriasis mild enough to be managed with topical therapies, and 1,194,712 youths without psoriasis.
In a first-pass unadjusted analysis, the psoriasis patients were at significantly increased risk for all nine of the autoimmune diseases examined. When the investigators adjusted for age, sex, and an individual’s health care utilization as reflected in his or her number of dermatology visits, the children and adolescents with psoriasis remained at tenfold increased risk for comorbid psoriatic arthritis, 6.6-fold risk for rheumatoid arthritis, 4.8-fold risk for vitiligo, and smaller yet significantly increased risks for several other autoimmune diseases, compared with individuals without psoriasis.
Indeed, while the presence of pediatric psoriasis was associated with an adjusted 4.4-fold increased risk of having at least one autoimmune disease, psoriasis patients with one of the selected autoimmune diseases were at 7.3-fold greater risk of having two or more autoimmune diseases, compared with individuals with one autoimmune disease who didn’t have psoriasis.
This is the first study to show such a clustering effect in either pediatric or adult psoriasis patients. The finding highlights the complex genetic underpinnings of psoriasis, which has previously been shown to share genetic susceptibility loci with inflammatory bowel disease and various other autoimmune diseases, Dr. Blegvad noted.
A small caveat: The psoriatic arthritis category also included individuals with juvenile idiopathic arthritis and juvenile psoriatic arthritis, because the clinical signs and symptoms of the three disorders often are difficult to distinguish in young patients.
Dr. Blegvad reported having no financial conflicts regarding the study, funded by Herlev and Gentofte Hospital and the LEO Foundation.
GENEVA – Pediatric psoriasis is associated with sharply increased risks of selected autoimmune diseases, according to a cross-sectional study encompassing every child and adolescent in Denmark.
"Even though the absolute risk of many of these conditions remains rare in childhood, clinicians should keep these associations in mind because they can greatly add to the total disease burden. In particular, we advise focusing on extracutaneous symptoms when treating psoriasis in children,” Christoffer Blegvad, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
He presented a cross-sectional study in which Denmark’s vaunted system of comprehensive national registries was harnessed to obtain health information on all individuals under age 18 years living in Denmark as of the end of 2012. The study population comprised 1,925 children and adolescents with dermatologist-diagnosed psoriasis, including those with psoriasis mild enough to be managed with topical therapies, and 1,194,712 youths without psoriasis.
In a first-pass unadjusted analysis, the psoriasis patients were at significantly increased risk for all nine of the autoimmune diseases examined. When the investigators adjusted for age, sex, and an individual’s health care utilization as reflected in his or her number of dermatology visits, the children and adolescents with psoriasis remained at tenfold increased risk for comorbid psoriatic arthritis, 6.6-fold risk for rheumatoid arthritis, 4.8-fold risk for vitiligo, and smaller yet significantly increased risks for several other autoimmune diseases, compared with individuals without psoriasis.
Indeed, while the presence of pediatric psoriasis was associated with an adjusted 4.4-fold increased risk of having at least one autoimmune disease, psoriasis patients with one of the selected autoimmune diseases were at 7.3-fold greater risk of having two or more autoimmune diseases, compared with individuals with one autoimmune disease who didn’t have psoriasis.
This is the first study to show such a clustering effect in either pediatric or adult psoriasis patients. The finding highlights the complex genetic underpinnings of psoriasis, which has previously been shown to share genetic susceptibility loci with inflammatory bowel disease and various other autoimmune diseases, Dr. Blegvad noted.
A small caveat: The psoriatic arthritis category also included individuals with juvenile idiopathic arthritis and juvenile psoriatic arthritis, because the clinical signs and symptoms of the three disorders often are difficult to distinguish in young patients.
Dr. Blegvad reported having no financial conflicts regarding the study, funded by Herlev and Gentofte Hospital and the LEO Foundation.
AT THE EADV CONGRESS
Key clinical point:
Major finding: Pediatric psoriasis patients were at an adjusted 6.6-fold increased risk of comorbid rheumatoid arthritis, 4.8-fold risk of vitiligo, and significantly increased risks of several other autoimmune diseases, compared with matched youths without psoriasis.
Data source: A cross-sectional study of all children and adolescents living in Denmark at the end of 2012.
Disclosures: The presenter reported having no financial conflicts regarding the study, funded by Herlev and Gentofte Hospital and the LEO Foundation.
Genital psoriasis is the worst: Patients sound off
GENEVA – The great majority of patients with genital psoriasis say their symptoms in the genital area are worse than elsewhere on the body, Kim A. Meeuwis, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
She presented a qualitative study in which 20 patients with longstanding genital psoriasis sounded off, sharing their perspectives on the disease in one-on-one, semistructured, face-to-face interviews.
Genital psoriasis is common. Epidemiologic studies show 30%-60% of psoriasis patients experience genital involvement at some point in the course of their disease. Yet patients seldom discuss their genital psoriasis with their physicians, and the patient perspective on how the experience of genital psoriasis differs from that of having psoriasis at other locations has been addressed only sparsely in the literature. This lack of attention was the impetus for the current study, she explained.
The 20 participants in the study had an average 18-year history of plaque psoriasis, with an average 7.5-year history of genital involvement. The genital psoriasis was rated moderate or severe in 70% of subjects at the time of the study.
The most commonly reported symptoms of genital psoriasis were itch and discomfort, each of which was cited by all study participants. This was followed by erythema, cited by 95%; stinging and burning, also cited by 95%; pain, cited by 85%; scaling, by 75%; and cracking, by 30%.
Of the patients in the study, 85% reported that their pain and/or discomfort were worse in the genital area than at other sites, and 10% said they were highly self-conscious about their genital psoriasis because others had misidentified them as having a sexually transmitted infection.
Since this was a qualitative study, Dr. Meeuwis provided representative quotes from several patients, including one who asserted, “I really only have discomfort on my psoriasis on the rest of my body ... in my genitals is the only place that actually has pain, or the itching is ... really, really bad.”
Dr. Meeuwis said the study results hold an important lesson for physicians who treat psoriasis: “Due to differences in patient experiences between genital and nongenital skin, it’s really important to make time for the specific evaluation of genital involvement in taking care of patients with psoriasis – and to be sure to ask about it.”
Dr. Meeuwis reported serving as a consultant to Eli Lilly, which sponsored the study, as well as being on an advisory board to Beiersdorf.
GENEVA – The great majority of patients with genital psoriasis say their symptoms in the genital area are worse than elsewhere on the body, Kim A. Meeuwis, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
She presented a qualitative study in which 20 patients with longstanding genital psoriasis sounded off, sharing their perspectives on the disease in one-on-one, semistructured, face-to-face interviews.
Genital psoriasis is common. Epidemiologic studies show 30%-60% of psoriasis patients experience genital involvement at some point in the course of their disease. Yet patients seldom discuss their genital psoriasis with their physicians, and the patient perspective on how the experience of genital psoriasis differs from that of having psoriasis at other locations has been addressed only sparsely in the literature. This lack of attention was the impetus for the current study, she explained.
The 20 participants in the study had an average 18-year history of plaque psoriasis, with an average 7.5-year history of genital involvement. The genital psoriasis was rated moderate or severe in 70% of subjects at the time of the study.
The most commonly reported symptoms of genital psoriasis were itch and discomfort, each of which was cited by all study participants. This was followed by erythema, cited by 95%; stinging and burning, also cited by 95%; pain, cited by 85%; scaling, by 75%; and cracking, by 30%.
Of the patients in the study, 85% reported that their pain and/or discomfort were worse in the genital area than at other sites, and 10% said they were highly self-conscious about their genital psoriasis because others had misidentified them as having a sexually transmitted infection.
Since this was a qualitative study, Dr. Meeuwis provided representative quotes from several patients, including one who asserted, “I really only have discomfort on my psoriasis on the rest of my body ... in my genitals is the only place that actually has pain, or the itching is ... really, really bad.”
Dr. Meeuwis said the study results hold an important lesson for physicians who treat psoriasis: “Due to differences in patient experiences between genital and nongenital skin, it’s really important to make time for the specific evaluation of genital involvement in taking care of patients with psoriasis – and to be sure to ask about it.”
Dr. Meeuwis reported serving as a consultant to Eli Lilly, which sponsored the study, as well as being on an advisory board to Beiersdorf.
GENEVA – The great majority of patients with genital psoriasis say their symptoms in the genital area are worse than elsewhere on the body, Kim A. Meeuwis, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
She presented a qualitative study in which 20 patients with longstanding genital psoriasis sounded off, sharing their perspectives on the disease in one-on-one, semistructured, face-to-face interviews.
Genital psoriasis is common. Epidemiologic studies show 30%-60% of psoriasis patients experience genital involvement at some point in the course of their disease. Yet patients seldom discuss their genital psoriasis with their physicians, and the patient perspective on how the experience of genital psoriasis differs from that of having psoriasis at other locations has been addressed only sparsely in the literature. This lack of attention was the impetus for the current study, she explained.
The 20 participants in the study had an average 18-year history of plaque psoriasis, with an average 7.5-year history of genital involvement. The genital psoriasis was rated moderate or severe in 70% of subjects at the time of the study.
The most commonly reported symptoms of genital psoriasis were itch and discomfort, each of which was cited by all study participants. This was followed by erythema, cited by 95%; stinging and burning, also cited by 95%; pain, cited by 85%; scaling, by 75%; and cracking, by 30%.
Of the patients in the study, 85% reported that their pain and/or discomfort were worse in the genital area than at other sites, and 10% said they were highly self-conscious about their genital psoriasis because others had misidentified them as having a sexually transmitted infection.
Since this was a qualitative study, Dr. Meeuwis provided representative quotes from several patients, including one who asserted, “I really only have discomfort on my psoriasis on the rest of my body ... in my genitals is the only place that actually has pain, or the itching is ... really, really bad.”
Dr. Meeuwis said the study results hold an important lesson for physicians who treat psoriasis: “Due to differences in patient experiences between genital and nongenital skin, it’s really important to make time for the specific evaluation of genital involvement in taking care of patients with psoriasis – and to be sure to ask about it.”
Dr. Meeuwis reported serving as a consultant to Eli Lilly, which sponsored the study, as well as being on an advisory board to Beiersdorf.
AT THE EADV CONGRESS
Key clinical point:
Major finding: Of the participants in a study of genital psoriasis, 100% reported that a hallmark of their genital disease was itching and discomfort.
Data source: A qualitative study that involved one-on-one interviews with 20 patients with genital psoriasis, who shared their experiences as to how genital involvement differs from their psoriasis elsewhere.
Disclosures: The study was sponsored by Eli Lilly. The presenter reported serving as a consultant to the company.
Ixekizumab has profound impact on genital psoriasis
GENEVA – The interleukin-17A inhibitor ixekizumab provided rapid clearance of genital psoriasis in a phase 3b clinical trial, with significant improvement seen as early as week 1, Caitriona Ryan, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
The highly targeted monoclonal antibody also improved the intense itching that’s a particularly prominent feature of genital psoriasis.
“Genital psoriasis is a hidden part of psoriasis. Unfortunately, as dermatologists we do a bad job of evaluating our patients for it. They are ashamed and embarrassed to bring up the topic with their dermatologists. Hopefully, this study will create some awareness around the topic,” she said.
This was the first-ever randomized trial to evaluate the effect of a biologic agent specifically on genital psoriasis. It was also the first study of a biologic in psoriasis patients with less than 10% body surface area involved.
“That’s a very important thing,” according to the dermatologist. “There are lots of patients with genital psoriasis who have less than 10% body surface area involved and therefore don’t qualify for biologic therapy, even though their genital psoriasis can be incredibly debilitating.”
The 12-week, multicenter, double-blind trial included 149 patients with a baseline static Physician’s Global Assessment of Genitalia (sPGA-G) score of at least 3 on a 0-5 scale. All participants had failed to respond to at least one topical therapy for their genital psoriasis, such as a corticosteroid, a calcineurin inhibitor, or a vitamin D analog. The subjects averaged a 16-year history of psoriasis and a 9-year history of genital psoriasis. Thirty-eight percent of participants had an involved body surface area of at least 1% but less than 10%.
Patients were randomized to ixekizumab (Taltz) given in the usual way – a subcutaneous loading dose of 160 mg, followed by repeat 80-mg injections every 2 weeks – or placebo.
The primary study endpoint was achievement of an sPGA-G score of 0 or 1, meaning clear or almost clear, as assessed by blinded investigators. At the 12-week mark, the rate was 73% in the ixekizumab group and 8% in controls. The sPGA-G score already differed significantly between the two study arms at the first assessment, after 1 week. The treatment success rate was closely similar in patients with or without at least 10% total body surface area involved.
A key secondary endpoint concerned sexual health. Among patients who at baseline indicated that in the past week, their genital psoriasis “sometimes,” “often,” or “always” limited the frequency of their sexual activity, at week 12, 78% of those in the ixekizumab group answered the same question on the Sexual Frequency Questionnaire “never” or “rarely,” compared with 21% of controls.
“This is huge. It’s such an important part of our patients’ lives, and there was a big difference by week 1,” Dr. Ryan noted.
On another secondary endpoint, 60% of the ixekizumab group reported at least a 3-point improvement in the 0-10 Genital Itch Numeric Rating Scale at week 12, compared with 8% of controls, with a statistically significant difference apparent at week 2.
“Itch is the most frequently reported symptom in our patients with genital psoriasis, and it seems to be much more impactful than itch from psoriasis elsewhere,” Dr. Ryan commented.
The side effect profile of ixekizumab was the same as has been seen in larger, longer-term studies. There were no serious ixekizumab-related adverse events, and no cases of candidiasis.
The study was sponsored by Eli Lilly. Dr. Ryan reported serving as an advisory board member to and/or receiving honoraria from that company and more than half a dozen other pharmaceutical companies.
GENEVA – The interleukin-17A inhibitor ixekizumab provided rapid clearance of genital psoriasis in a phase 3b clinical trial, with significant improvement seen as early as week 1, Caitriona Ryan, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
The highly targeted monoclonal antibody also improved the intense itching that’s a particularly prominent feature of genital psoriasis.
“Genital psoriasis is a hidden part of psoriasis. Unfortunately, as dermatologists we do a bad job of evaluating our patients for it. They are ashamed and embarrassed to bring up the topic with their dermatologists. Hopefully, this study will create some awareness around the topic,” she said.
This was the first-ever randomized trial to evaluate the effect of a biologic agent specifically on genital psoriasis. It was also the first study of a biologic in psoriasis patients with less than 10% body surface area involved.
“That’s a very important thing,” according to the dermatologist. “There are lots of patients with genital psoriasis who have less than 10% body surface area involved and therefore don’t qualify for biologic therapy, even though their genital psoriasis can be incredibly debilitating.”
The 12-week, multicenter, double-blind trial included 149 patients with a baseline static Physician’s Global Assessment of Genitalia (sPGA-G) score of at least 3 on a 0-5 scale. All participants had failed to respond to at least one topical therapy for their genital psoriasis, such as a corticosteroid, a calcineurin inhibitor, or a vitamin D analog. The subjects averaged a 16-year history of psoriasis and a 9-year history of genital psoriasis. Thirty-eight percent of participants had an involved body surface area of at least 1% but less than 10%.
Patients were randomized to ixekizumab (Taltz) given in the usual way – a subcutaneous loading dose of 160 mg, followed by repeat 80-mg injections every 2 weeks – or placebo.
The primary study endpoint was achievement of an sPGA-G score of 0 or 1, meaning clear or almost clear, as assessed by blinded investigators. At the 12-week mark, the rate was 73% in the ixekizumab group and 8% in controls. The sPGA-G score already differed significantly between the two study arms at the first assessment, after 1 week. The treatment success rate was closely similar in patients with or without at least 10% total body surface area involved.
A key secondary endpoint concerned sexual health. Among patients who at baseline indicated that in the past week, their genital psoriasis “sometimes,” “often,” or “always” limited the frequency of their sexual activity, at week 12, 78% of those in the ixekizumab group answered the same question on the Sexual Frequency Questionnaire “never” or “rarely,” compared with 21% of controls.
“This is huge. It’s such an important part of our patients’ lives, and there was a big difference by week 1,” Dr. Ryan noted.
On another secondary endpoint, 60% of the ixekizumab group reported at least a 3-point improvement in the 0-10 Genital Itch Numeric Rating Scale at week 12, compared with 8% of controls, with a statistically significant difference apparent at week 2.
“Itch is the most frequently reported symptom in our patients with genital psoriasis, and it seems to be much more impactful than itch from psoriasis elsewhere,” Dr. Ryan commented.
The side effect profile of ixekizumab was the same as has been seen in larger, longer-term studies. There were no serious ixekizumab-related adverse events, and no cases of candidiasis.
The study was sponsored by Eli Lilly. Dr. Ryan reported serving as an advisory board member to and/or receiving honoraria from that company and more than half a dozen other pharmaceutical companies.
GENEVA – The interleukin-17A inhibitor ixekizumab provided rapid clearance of genital psoriasis in a phase 3b clinical trial, with significant improvement seen as early as week 1, Caitriona Ryan, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
The highly targeted monoclonal antibody also improved the intense itching that’s a particularly prominent feature of genital psoriasis.
“Genital psoriasis is a hidden part of psoriasis. Unfortunately, as dermatologists we do a bad job of evaluating our patients for it. They are ashamed and embarrassed to bring up the topic with their dermatologists. Hopefully, this study will create some awareness around the topic,” she said.
This was the first-ever randomized trial to evaluate the effect of a biologic agent specifically on genital psoriasis. It was also the first study of a biologic in psoriasis patients with less than 10% body surface area involved.
“That’s a very important thing,” according to the dermatologist. “There are lots of patients with genital psoriasis who have less than 10% body surface area involved and therefore don’t qualify for biologic therapy, even though their genital psoriasis can be incredibly debilitating.”
The 12-week, multicenter, double-blind trial included 149 patients with a baseline static Physician’s Global Assessment of Genitalia (sPGA-G) score of at least 3 on a 0-5 scale. All participants had failed to respond to at least one topical therapy for their genital psoriasis, such as a corticosteroid, a calcineurin inhibitor, or a vitamin D analog. The subjects averaged a 16-year history of psoriasis and a 9-year history of genital psoriasis. Thirty-eight percent of participants had an involved body surface area of at least 1% but less than 10%.
Patients were randomized to ixekizumab (Taltz) given in the usual way – a subcutaneous loading dose of 160 mg, followed by repeat 80-mg injections every 2 weeks – or placebo.
The primary study endpoint was achievement of an sPGA-G score of 0 or 1, meaning clear or almost clear, as assessed by blinded investigators. At the 12-week mark, the rate was 73% in the ixekizumab group and 8% in controls. The sPGA-G score already differed significantly between the two study arms at the first assessment, after 1 week. The treatment success rate was closely similar in patients with or without at least 10% total body surface area involved.
A key secondary endpoint concerned sexual health. Among patients who at baseline indicated that in the past week, their genital psoriasis “sometimes,” “often,” or “always” limited the frequency of their sexual activity, at week 12, 78% of those in the ixekizumab group answered the same question on the Sexual Frequency Questionnaire “never” or “rarely,” compared with 21% of controls.
“This is huge. It’s such an important part of our patients’ lives, and there was a big difference by week 1,” Dr. Ryan noted.
On another secondary endpoint, 60% of the ixekizumab group reported at least a 3-point improvement in the 0-10 Genital Itch Numeric Rating Scale at week 12, compared with 8% of controls, with a statistically significant difference apparent at week 2.
“Itch is the most frequently reported symptom in our patients with genital psoriasis, and it seems to be much more impactful than itch from psoriasis elsewhere,” Dr. Ryan commented.
The side effect profile of ixekizumab was the same as has been seen in larger, longer-term studies. There were no serious ixekizumab-related adverse events, and no cases of candidiasis.
The study was sponsored by Eli Lilly. Dr. Ryan reported serving as an advisory board member to and/or receiving honoraria from that company and more than half a dozen other pharmaceutical companies.
AT THE EADV CONGRESS
Key clinical point: First-ever trial of a biologic agent in genital psoriasis shows heartening results.
Major finding: 73% of patients with moderate to severe genital psoriasis were clear or almost clear of their genital disease after 12 weeks of ixekizumab, vs. 8% of controls.
Data source: This was a randomized, double-blind, placebo-controlled, multicenter, 12-week clinical trial in 149 patients with moderate to severe genital psoriasis.
Disclosures: The study was sponsored by Eli Lilly. The presenter reported serving as an advisory board member to and/or receiving honoraria from that company and more than half a dozen other pharmaceutical companies.
Latest on dupilumab for atopic dermatitis: The who and how
GENEVA – The novel interleukin-4 and IL-13 signaling blocker dupilumab displayed consistently strong efficacy across all patient subgroups in a new analysis from the landmark 52-week CHRONOS trial, Andrew Blauvelt, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“Dupilumab with concomitant topical corticosteroids improved signs and symptoms of atopic dermatitis, compared with placebo injections regardless of age, sex, BMI, or prior history of asthma, allergic rhinitis, or food allergies,” he said. Indeed, he quipped that the biologic proved to be “boring” in its broad effectiveness.
CHRONOS was a 14-country, 1-year, randomized, double-blind, placebo-controlled, phase 3 clinical trial in which 740 adults with moderate to very severe AD were assigned to mid-potency topical corticosteroids along with topical calcineurin inhibitors as needed in steroid-sensitive locations, then randomized 3:1:3 to either subcutaneous dupilumab (Dupixent) at 300 mg once weekly, placebo injections, or dupilumab at 300 mg every 2 weeks on top of the topical therapy.
Dupilumab, a fully human monoclonal antibody, was approved by the Food and Drug Administration in March 2017 at a loading dose of 600 mg, followed by 300 mg every 2 weeks for treatment of adults with moderate to severe AD. The biologic agent is soon expected to be approved across the European Union as well. CHRONOS was the first major trial designed to replicate how the biologic would likely be utilized in real-world clinical practice: that is, in conjunction with rather than as an alternative to topical therapy. Earlier pivotal phase 3 trials were required by regulatory agencies to pit dupilumab monotherapy against placebo.
CHRONOS participants were “a very tough crowd, so to speak,” the dermatologist recalled. Their median body surface area of involvement was a whopping 55%, 60% were men, the median baseline EASI (Eczema Area and Severity Index) score was 30, and fully half of patients had an IGA (Investigator’s Global Assessment) score of 4, indicative of severe disease. Plus, comorbid atopic/allergic diseases were common: half of subjects had a history of asthma, nearly half had a history of allergic rhinitis, and one-third had a history of food allergies.
Dr. Blauvelt previously presented the primary results of CHRONOS without the new subgroup analysis at the American Academy of Dermatology 2017 meeting. The primary results were subsequently published (Lancet. 2017 Jun 10;389[10086]:2287-303). To recap, the bottom line: 39% of dupilumab-treated patients achieved an IGA score of 0/1 – that is, clear or almost clear – coupled with at least a 2-point improvement from baseline at week 16, versus 12% of controls on topical steroids plus placebo injections. That’s a strikingly impressive performance, according to the dermatologist.
“An IGA of 0/1 in AD is a very high bar. It tends to correlated with an EASI 90,” Dr. Blauvelt observed.
Rates of EASI 75 – meaning at least a 75% improvement from baseline EASI scores – were achieved in 64% of patients on weekly dupilumab plus topical steroids, 69% with dupilumab every 2 weeks plus topicals, and 23% on topical steroids plus placebo. These efficacy results were essentially mirrored at week 52, with no significant changes in response rates from week 16 to week 52.
In another new finding from CHRONOS presented by Dr. Blauvelt at the EADV congress, itch also improved sharply across the board in dupilumab-treated patients, regardless of their baseline demographic and other characteristics, meaning there’s no point for clinicians to reserve the biologic for selected subgroups of patients with moderate or severe AD. From a mean baseline score of 7.6 on the 10-point peak pruritus numerical rating scale, at least a 3-point improvement was achieved in 56% of patients on biweekly dupilumab plus topical steroids, 43% on weekly dupilumab and topical steroids, and in 16% on topical steroids plus placebo injections.
Of note, 84% of patients in the two dupilumab arms remained in the CHRONOS trial through the full 52 weeks. That’s a high retention rate for a 1-year study involving a disease with a major adverse quality of life impact. In contrast, only about two-thirds of controls on topical therapy plus placebo injections stuck with the study for the duration.
The high retention rate may have had much to do with dupilumab’s generally favorable safely profile, as seen not only in CHRONOS but in earlier trials. No new safety concerns arose during the 52-week study. The dupilumab and control groups had similar rates of most side effects, with just a few exceptions: nonherpetic skin infections and worsening AD occurred more frequently in controls on topical steroids alone, while mild injection site reactions were twice as common in patients who got dupilumab than in those who received placebo injections.
Also, conjunctivitis occurred in 14% of patients on weekly dupilumab and 19% with biweekly dupilumab, compared with 8% of controls. “To me, conjunctivitis is the only significant side effect for this drug that we’ve seen thus far,” Dr. Blauvelt commented. The mechanism of the conjunctivitis is unknown. It’s clear, however, that the rate goes up with duration on therapy.
“Most of the cases have been mild to moderate and have not interfered with therapy. I’ve treated probably 80 patients with dupilumab, and I’ve had only 1 who had to stop due to her eyes,” the dermatologist recalled. The condition often responds to wetting eye drops, although topical corticosteroid eye drops are required sometimes, he added.
CAFE was a double-blind, randomized, placebo-controlled trial including 325 European patients with moderate or severe AD, all of whom were on a mid-potency topical corticosteroid with or without a topical calcineurin inhibitor. Two-thirds had previously been on cyclosporine with poor results; the drug was contraindicated in the others. Participants were randomized to subcutaneous injections of dupilumab at 300 mg weekly or biweekly or to placebo injections.
The primary outcome – an EASI-75 response at 16 weeks – was achieved in 59% of patients on weekly dupilumab, 63% of those on biweekly dupilumab, and 30% of controls on topical steroids plus placebo injections, according to Dr. De Bruin-Weller of the University Medical Center at Utrecht, the Netherlands.
A clinically meaningful 4-point or greater improvement from baseline in the Dermatology Life Quality Index occurred at 16 weeks in 79% and 88% of patients on dupilumab weekly or biweekly, respectively, compared with 44% of controls.
And among the three-quarters of CAFE participants reporting baseline moderate or severe pain or discomfort from AD, 55% of those on weekly dupilumab had none at all as assessed by the EQ-5D Pain/Discomfort Questionnaire at week 16, as did 64% on dupilumab every 2 weeks and 28% of controls on topical steroids.
At the prestigious joint EADV/AAD session of the European congress, Lawrence F. Eichenfield, MD, hailed dupilumab as evidence that “the systemic therapy revolution in atopic dermatitis is certainly happening,” with a plethora of additional agents targeting various key disease pathways now working their way through the developmental pipeline.
A key remaining question about dupilumab is, what about the safety and efficacy of the drug in the pediatric population, for whom the drug is not currently approved? Separate phase 3 randomized trials addressing this issue are now enrolling 6- to 11-year-olds and 12- to 17-year-olds.
Dr. Eichenfield is optimistic about the outcomes of these ongoing pediatric trials on the basis of an open-label, phase 2a, proof of concept study in 6- to 17-year-olds presented in a late-breaking research session at the 2017 annual meeting of the AAD. The pharmacokinetics were very similar to those seen in adults. Moreover, EASI scores improved by 32%-51% after just a single, weight-based injection of dupilumab and by up to 70% following four consecutive weekly injections.
“This is very, very exciting information. Whether the drug could actually be disease modifying is obviously a big question for us in pediatric dermatology,” observed Dr. Eichenfield, who is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego and professor of dermatology and pediatrics at the University of California, San Diego.
He highlighted two important safety considerations regarding dupilumab: patients on the biologic should not be given any live vaccines, and the same IL-4 and IL-13 cytokine pathways targeted by dupilumab in patients with AD also are key in other atopic and allergic diseases. Indeed, the biologic showed positive outcomes in a recent pivotal phase 3 clinical trial for patients with uncontrolled persistent asthma, and an application for an expanded indication for dupilumab in such patients is expected to be filed with the FDA later in 2017. Dupilumab is also in phase 3 for patients with nasal polyps and in phase 2 studies for eosinophilic esophagitis.
“One of the interesting things about dupilumab is that we in dermatology are going to be in a situation where we’re able to impact diseases other than the single disease that the medication has been approved for,” Dr. Eichenfield said.
However, until such time as dupilumab actually receives an expanded indication for asthma and the details of how best to use the biologic in AD patients with that comorbidity have been worked out, it’s important for dermatologists prescribing dupilumab in those dual-diagnosis patients to discuss with them the necessity of staying on their asthma medications even though their skin is much improved, they’re feeling good, and their asthma seems to be doing better. During the dupilumab clinical trials program for AD, a fatal asthma exacerbation occurred in a patient with comorbid asthma who stopped taking asthma medications, he noted.
The CHRONOS and CAFE trials were funded by Sanofi and Regeneron Pharmaceuticals. Dr. Blauvelt, Dr. De Bruin-Weller, and Dr. Eichenfield reported serving as consultants to and researchers for those pharmaceutical companies and numerous others.
GENEVA – The novel interleukin-4 and IL-13 signaling blocker dupilumab displayed consistently strong efficacy across all patient subgroups in a new analysis from the landmark 52-week CHRONOS trial, Andrew Blauvelt, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“Dupilumab with concomitant topical corticosteroids improved signs and symptoms of atopic dermatitis, compared with placebo injections regardless of age, sex, BMI, or prior history of asthma, allergic rhinitis, or food allergies,” he said. Indeed, he quipped that the biologic proved to be “boring” in its broad effectiveness.
CHRONOS was a 14-country, 1-year, randomized, double-blind, placebo-controlled, phase 3 clinical trial in which 740 adults with moderate to very severe AD were assigned to mid-potency topical corticosteroids along with topical calcineurin inhibitors as needed in steroid-sensitive locations, then randomized 3:1:3 to either subcutaneous dupilumab (Dupixent) at 300 mg once weekly, placebo injections, or dupilumab at 300 mg every 2 weeks on top of the topical therapy.
Dupilumab, a fully human monoclonal antibody, was approved by the Food and Drug Administration in March 2017 at a loading dose of 600 mg, followed by 300 mg every 2 weeks for treatment of adults with moderate to severe AD. The biologic agent is soon expected to be approved across the European Union as well. CHRONOS was the first major trial designed to replicate how the biologic would likely be utilized in real-world clinical practice: that is, in conjunction with rather than as an alternative to topical therapy. Earlier pivotal phase 3 trials were required by regulatory agencies to pit dupilumab monotherapy against placebo.
CHRONOS participants were “a very tough crowd, so to speak,” the dermatologist recalled. Their median body surface area of involvement was a whopping 55%, 60% were men, the median baseline EASI (Eczema Area and Severity Index) score was 30, and fully half of patients had an IGA (Investigator’s Global Assessment) score of 4, indicative of severe disease. Plus, comorbid atopic/allergic diseases were common: half of subjects had a history of asthma, nearly half had a history of allergic rhinitis, and one-third had a history of food allergies.
Dr. Blauvelt previously presented the primary results of CHRONOS without the new subgroup analysis at the American Academy of Dermatology 2017 meeting. The primary results were subsequently published (Lancet. 2017 Jun 10;389[10086]:2287-303). To recap, the bottom line: 39% of dupilumab-treated patients achieved an IGA score of 0/1 – that is, clear or almost clear – coupled with at least a 2-point improvement from baseline at week 16, versus 12% of controls on topical steroids plus placebo injections. That’s a strikingly impressive performance, according to the dermatologist.
“An IGA of 0/1 in AD is a very high bar. It tends to correlated with an EASI 90,” Dr. Blauvelt observed.
Rates of EASI 75 – meaning at least a 75% improvement from baseline EASI scores – were achieved in 64% of patients on weekly dupilumab plus topical steroids, 69% with dupilumab every 2 weeks plus topicals, and 23% on topical steroids plus placebo. These efficacy results were essentially mirrored at week 52, with no significant changes in response rates from week 16 to week 52.
In another new finding from CHRONOS presented by Dr. Blauvelt at the EADV congress, itch also improved sharply across the board in dupilumab-treated patients, regardless of their baseline demographic and other characteristics, meaning there’s no point for clinicians to reserve the biologic for selected subgroups of patients with moderate or severe AD. From a mean baseline score of 7.6 on the 10-point peak pruritus numerical rating scale, at least a 3-point improvement was achieved in 56% of patients on biweekly dupilumab plus topical steroids, 43% on weekly dupilumab and topical steroids, and in 16% on topical steroids plus placebo injections.
Of note, 84% of patients in the two dupilumab arms remained in the CHRONOS trial through the full 52 weeks. That’s a high retention rate for a 1-year study involving a disease with a major adverse quality of life impact. In contrast, only about two-thirds of controls on topical therapy plus placebo injections stuck with the study for the duration.
The high retention rate may have had much to do with dupilumab’s generally favorable safely profile, as seen not only in CHRONOS but in earlier trials. No new safety concerns arose during the 52-week study. The dupilumab and control groups had similar rates of most side effects, with just a few exceptions: nonherpetic skin infections and worsening AD occurred more frequently in controls on topical steroids alone, while mild injection site reactions were twice as common in patients who got dupilumab than in those who received placebo injections.
Also, conjunctivitis occurred in 14% of patients on weekly dupilumab and 19% with biweekly dupilumab, compared with 8% of controls. “To me, conjunctivitis is the only significant side effect for this drug that we’ve seen thus far,” Dr. Blauvelt commented. The mechanism of the conjunctivitis is unknown. It’s clear, however, that the rate goes up with duration on therapy.
“Most of the cases have been mild to moderate and have not interfered with therapy. I’ve treated probably 80 patients with dupilumab, and I’ve had only 1 who had to stop due to her eyes,” the dermatologist recalled. The condition often responds to wetting eye drops, although topical corticosteroid eye drops are required sometimes, he added.
CAFE was a double-blind, randomized, placebo-controlled trial including 325 European patients with moderate or severe AD, all of whom were on a mid-potency topical corticosteroid with or without a topical calcineurin inhibitor. Two-thirds had previously been on cyclosporine with poor results; the drug was contraindicated in the others. Participants were randomized to subcutaneous injections of dupilumab at 300 mg weekly or biweekly or to placebo injections.
The primary outcome – an EASI-75 response at 16 weeks – was achieved in 59% of patients on weekly dupilumab, 63% of those on biweekly dupilumab, and 30% of controls on topical steroids plus placebo injections, according to Dr. De Bruin-Weller of the University Medical Center at Utrecht, the Netherlands.
A clinically meaningful 4-point or greater improvement from baseline in the Dermatology Life Quality Index occurred at 16 weeks in 79% and 88% of patients on dupilumab weekly or biweekly, respectively, compared with 44% of controls.
And among the three-quarters of CAFE participants reporting baseline moderate or severe pain or discomfort from AD, 55% of those on weekly dupilumab had none at all as assessed by the EQ-5D Pain/Discomfort Questionnaire at week 16, as did 64% on dupilumab every 2 weeks and 28% of controls on topical steroids.
At the prestigious joint EADV/AAD session of the European congress, Lawrence F. Eichenfield, MD, hailed dupilumab as evidence that “the systemic therapy revolution in atopic dermatitis is certainly happening,” with a plethora of additional agents targeting various key disease pathways now working their way through the developmental pipeline.
A key remaining question about dupilumab is, what about the safety and efficacy of the drug in the pediatric population, for whom the drug is not currently approved? Separate phase 3 randomized trials addressing this issue are now enrolling 6- to 11-year-olds and 12- to 17-year-olds.
Dr. Eichenfield is optimistic about the outcomes of these ongoing pediatric trials on the basis of an open-label, phase 2a, proof of concept study in 6- to 17-year-olds presented in a late-breaking research session at the 2017 annual meeting of the AAD. The pharmacokinetics were very similar to those seen in adults. Moreover, EASI scores improved by 32%-51% after just a single, weight-based injection of dupilumab and by up to 70% following four consecutive weekly injections.
“This is very, very exciting information. Whether the drug could actually be disease modifying is obviously a big question for us in pediatric dermatology,” observed Dr. Eichenfield, who is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego and professor of dermatology and pediatrics at the University of California, San Diego.
He highlighted two important safety considerations regarding dupilumab: patients on the biologic should not be given any live vaccines, and the same IL-4 and IL-13 cytokine pathways targeted by dupilumab in patients with AD also are key in other atopic and allergic diseases. Indeed, the biologic showed positive outcomes in a recent pivotal phase 3 clinical trial for patients with uncontrolled persistent asthma, and an application for an expanded indication for dupilumab in such patients is expected to be filed with the FDA later in 2017. Dupilumab is also in phase 3 for patients with nasal polyps and in phase 2 studies for eosinophilic esophagitis.
“One of the interesting things about dupilumab is that we in dermatology are going to be in a situation where we’re able to impact diseases other than the single disease that the medication has been approved for,” Dr. Eichenfield said.
However, until such time as dupilumab actually receives an expanded indication for asthma and the details of how best to use the biologic in AD patients with that comorbidity have been worked out, it’s important for dermatologists prescribing dupilumab in those dual-diagnosis patients to discuss with them the necessity of staying on their asthma medications even though their skin is much improved, they’re feeling good, and their asthma seems to be doing better. During the dupilumab clinical trials program for AD, a fatal asthma exacerbation occurred in a patient with comorbid asthma who stopped taking asthma medications, he noted.
The CHRONOS and CAFE trials were funded by Sanofi and Regeneron Pharmaceuticals. Dr. Blauvelt, Dr. De Bruin-Weller, and Dr. Eichenfield reported serving as consultants to and researchers for those pharmaceutical companies and numerous others.
GENEVA – The novel interleukin-4 and IL-13 signaling blocker dupilumab displayed consistently strong efficacy across all patient subgroups in a new analysis from the landmark 52-week CHRONOS trial, Andrew Blauvelt, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“Dupilumab with concomitant topical corticosteroids improved signs and symptoms of atopic dermatitis, compared with placebo injections regardless of age, sex, BMI, or prior history of asthma, allergic rhinitis, or food allergies,” he said. Indeed, he quipped that the biologic proved to be “boring” in its broad effectiveness.
CHRONOS was a 14-country, 1-year, randomized, double-blind, placebo-controlled, phase 3 clinical trial in which 740 adults with moderate to very severe AD were assigned to mid-potency topical corticosteroids along with topical calcineurin inhibitors as needed in steroid-sensitive locations, then randomized 3:1:3 to either subcutaneous dupilumab (Dupixent) at 300 mg once weekly, placebo injections, or dupilumab at 300 mg every 2 weeks on top of the topical therapy.
Dupilumab, a fully human monoclonal antibody, was approved by the Food and Drug Administration in March 2017 at a loading dose of 600 mg, followed by 300 mg every 2 weeks for treatment of adults with moderate to severe AD. The biologic agent is soon expected to be approved across the European Union as well. CHRONOS was the first major trial designed to replicate how the biologic would likely be utilized in real-world clinical practice: that is, in conjunction with rather than as an alternative to topical therapy. Earlier pivotal phase 3 trials were required by regulatory agencies to pit dupilumab monotherapy against placebo.
CHRONOS participants were “a very tough crowd, so to speak,” the dermatologist recalled. Their median body surface area of involvement was a whopping 55%, 60% were men, the median baseline EASI (Eczema Area and Severity Index) score was 30, and fully half of patients had an IGA (Investigator’s Global Assessment) score of 4, indicative of severe disease. Plus, comorbid atopic/allergic diseases were common: half of subjects had a history of asthma, nearly half had a history of allergic rhinitis, and one-third had a history of food allergies.
Dr. Blauvelt previously presented the primary results of CHRONOS without the new subgroup analysis at the American Academy of Dermatology 2017 meeting. The primary results were subsequently published (Lancet. 2017 Jun 10;389[10086]:2287-303). To recap, the bottom line: 39% of dupilumab-treated patients achieved an IGA score of 0/1 – that is, clear or almost clear – coupled with at least a 2-point improvement from baseline at week 16, versus 12% of controls on topical steroids plus placebo injections. That’s a strikingly impressive performance, according to the dermatologist.
“An IGA of 0/1 in AD is a very high bar. It tends to correlated with an EASI 90,” Dr. Blauvelt observed.
Rates of EASI 75 – meaning at least a 75% improvement from baseline EASI scores – were achieved in 64% of patients on weekly dupilumab plus topical steroids, 69% with dupilumab every 2 weeks plus topicals, and 23% on topical steroids plus placebo. These efficacy results were essentially mirrored at week 52, with no significant changes in response rates from week 16 to week 52.
In another new finding from CHRONOS presented by Dr. Blauvelt at the EADV congress, itch also improved sharply across the board in dupilumab-treated patients, regardless of their baseline demographic and other characteristics, meaning there’s no point for clinicians to reserve the biologic for selected subgroups of patients with moderate or severe AD. From a mean baseline score of 7.6 on the 10-point peak pruritus numerical rating scale, at least a 3-point improvement was achieved in 56% of patients on biweekly dupilumab plus topical steroids, 43% on weekly dupilumab and topical steroids, and in 16% on topical steroids plus placebo injections.
Of note, 84% of patients in the two dupilumab arms remained in the CHRONOS trial through the full 52 weeks. That’s a high retention rate for a 1-year study involving a disease with a major adverse quality of life impact. In contrast, only about two-thirds of controls on topical therapy plus placebo injections stuck with the study for the duration.
The high retention rate may have had much to do with dupilumab’s generally favorable safely profile, as seen not only in CHRONOS but in earlier trials. No new safety concerns arose during the 52-week study. The dupilumab and control groups had similar rates of most side effects, with just a few exceptions: nonherpetic skin infections and worsening AD occurred more frequently in controls on topical steroids alone, while mild injection site reactions were twice as common in patients who got dupilumab than in those who received placebo injections.
Also, conjunctivitis occurred in 14% of patients on weekly dupilumab and 19% with biweekly dupilumab, compared with 8% of controls. “To me, conjunctivitis is the only significant side effect for this drug that we’ve seen thus far,” Dr. Blauvelt commented. The mechanism of the conjunctivitis is unknown. It’s clear, however, that the rate goes up with duration on therapy.
“Most of the cases have been mild to moderate and have not interfered with therapy. I’ve treated probably 80 patients with dupilumab, and I’ve had only 1 who had to stop due to her eyes,” the dermatologist recalled. The condition often responds to wetting eye drops, although topical corticosteroid eye drops are required sometimes, he added.
CAFE was a double-blind, randomized, placebo-controlled trial including 325 European patients with moderate or severe AD, all of whom were on a mid-potency topical corticosteroid with or without a topical calcineurin inhibitor. Two-thirds had previously been on cyclosporine with poor results; the drug was contraindicated in the others. Participants were randomized to subcutaneous injections of dupilumab at 300 mg weekly or biweekly or to placebo injections.
The primary outcome – an EASI-75 response at 16 weeks – was achieved in 59% of patients on weekly dupilumab, 63% of those on biweekly dupilumab, and 30% of controls on topical steroids plus placebo injections, according to Dr. De Bruin-Weller of the University Medical Center at Utrecht, the Netherlands.
A clinically meaningful 4-point or greater improvement from baseline in the Dermatology Life Quality Index occurred at 16 weeks in 79% and 88% of patients on dupilumab weekly or biweekly, respectively, compared with 44% of controls.
And among the three-quarters of CAFE participants reporting baseline moderate or severe pain or discomfort from AD, 55% of those on weekly dupilumab had none at all as assessed by the EQ-5D Pain/Discomfort Questionnaire at week 16, as did 64% on dupilumab every 2 weeks and 28% of controls on topical steroids.
At the prestigious joint EADV/AAD session of the European congress, Lawrence F. Eichenfield, MD, hailed dupilumab as evidence that “the systemic therapy revolution in atopic dermatitis is certainly happening,” with a plethora of additional agents targeting various key disease pathways now working their way through the developmental pipeline.
A key remaining question about dupilumab is, what about the safety and efficacy of the drug in the pediatric population, for whom the drug is not currently approved? Separate phase 3 randomized trials addressing this issue are now enrolling 6- to 11-year-olds and 12- to 17-year-olds.
Dr. Eichenfield is optimistic about the outcomes of these ongoing pediatric trials on the basis of an open-label, phase 2a, proof of concept study in 6- to 17-year-olds presented in a late-breaking research session at the 2017 annual meeting of the AAD. The pharmacokinetics were very similar to those seen in adults. Moreover, EASI scores improved by 32%-51% after just a single, weight-based injection of dupilumab and by up to 70% following four consecutive weekly injections.
“This is very, very exciting information. Whether the drug could actually be disease modifying is obviously a big question for us in pediatric dermatology,” observed Dr. Eichenfield, who is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego and professor of dermatology and pediatrics at the University of California, San Diego.
He highlighted two important safety considerations regarding dupilumab: patients on the biologic should not be given any live vaccines, and the same IL-4 and IL-13 cytokine pathways targeted by dupilumab in patients with AD also are key in other atopic and allergic diseases. Indeed, the biologic showed positive outcomes in a recent pivotal phase 3 clinical trial for patients with uncontrolled persistent asthma, and an application for an expanded indication for dupilumab in such patients is expected to be filed with the FDA later in 2017. Dupilumab is also in phase 3 for patients with nasal polyps and in phase 2 studies for eosinophilic esophagitis.
“One of the interesting things about dupilumab is that we in dermatology are going to be in a situation where we’re able to impact diseases other than the single disease that the medication has been approved for,” Dr. Eichenfield said.
However, until such time as dupilumab actually receives an expanded indication for asthma and the details of how best to use the biologic in AD patients with that comorbidity have been worked out, it’s important for dermatologists prescribing dupilumab in those dual-diagnosis patients to discuss with them the necessity of staying on their asthma medications even though their skin is much improved, they’re feeling good, and their asthma seems to be doing better. During the dupilumab clinical trials program for AD, a fatal asthma exacerbation occurred in a patient with comorbid asthma who stopped taking asthma medications, he noted.
The CHRONOS and CAFE trials were funded by Sanofi and Regeneron Pharmaceuticals. Dr. Blauvelt, Dr. De Bruin-Weller, and Dr. Eichenfield reported serving as consultants to and researchers for those pharmaceutical companies and numerous others.
EXPERT ANALYSIS FROM THE EADV CONGRESS






