User login
European Academy of Dermatology & Venereology (EADV): Annual Congress
Dupilumab advances for severe atopic dermatitis
AMSTERDAM – The novel biologic agent dupilumab showed strong dose-dependent efficacy in adults with moderate to severe atopic dermatitis not adequately controlled with topical medications in a 380-patient phase IIb dose-ranging study.
“Based on the results of this study, we’ll take the top two doses further to our phase III program. We’re also planning to conduct a maintenance study. At the end of 16 weeks of treatment, we’ll investigate lower-dose regimens that may be capable of maintaining clinical response,” Dr. Marius Ardeleanu said at the annual congress of the European Academy of Dermatology and Venereology.
Dupilumab is an investigational fully human monoclonal antibody that addresses a novel target: It is directed against the interleukin-4 receptor alpha subunit (IL-4Ra). Through this effect it blocks IL-4 and IL-13, the drivers of the type 2 helper T-cell–mediated inflammation responsible for the hallmark symptoms of atopic dermatitis (AD), explained Dr. Ardeleanu of Regeneron Pharmaceuticals in Tarrytown, N.Y.
Participants in this 16-week, double-blind, international phase IIb study were randomized to placebo or one of five dupilumab dosing regimens ranging from a low of 100 mg given subcutaneously every 4 weeks to a maximum of 300 mg once weekly or every 2 weeks.
These patients had a significant disease burden. They were typically in their mid- to late 30s and had a 27-year disease history, a mean baseline SCORAD of 67 on a 0-100 scale, a baseline Eczema Area and Severity Index (EASI) score of 32, an Investigator’s Global Assessment of disease severity score of 3.5 on a 0-4 scale, and 50% body surface area involvement. Their mean average weekly self-rated itching score was 6.8 on a 0-10 scale.
The primary study endpoint was change in the EASI score from baseline to 16 weeks. The score dropped by 20% in placebo-treated controls and by significantly greater margins in all five dupilumab arms. The largest reduction in EASI score – nearly 80% – occurred in the group on 300 mg/wk, with the 300 mg every 2 weeks group showing about a 70% reduction.
Roughly 80% of patients on 300 mg/wk or every 2 weeks showed an EASI 50 response at week 16, meaning a 50% reduction from baseline in their score, which is considered clinically meaningful improvement. One-third of patients on either of these top two–performing regimens achieved an Investigator’s Global Assessment score of 0 or 1, which is virtual remission; none of the controls did. Overall weekly average pruritus scores dropped by more than 60% with weekly treatment at 300 mg and by slightly less with biweekly therapy at 300 mg.
Safety data were similar to those from a recently published earlier phase IIa study (N. Engl. J. Med. 2014; 371:130-9). There were no dose-limiting toxicities. Headache and injection site reactions were the only adverse events more common with dupilumab than with placebo in the phase IIb trial, with the incidence of injection site reactions showing a possible dose-response relationship.
In addition to the large phase III studies now being planned, which will also evaluate step-down maintenance therapy, another study has been scheduled to investigate the use of dupilumab in combination with topical corticosteroid therapy. The earlier phase IIa study provided evidence to suggest this combination has even greater efficacy than dupilumab alone, and with modest use of the topical agent, according to Dr. Ardeleanu.
Dupilumab is also being developed as a treatment for tough-to-control moderate to severe asthma. It showed positive results in a phase II study, with reduced asthma exacerbations and improved lung function, compared with placebo (N. Engl. J. Med. 2013; 368:2455-66).
AMSTERDAM – The novel biologic agent dupilumab showed strong dose-dependent efficacy in adults with moderate to severe atopic dermatitis not adequately controlled with topical medications in a 380-patient phase IIb dose-ranging study.
“Based on the results of this study, we’ll take the top two doses further to our phase III program. We’re also planning to conduct a maintenance study. At the end of 16 weeks of treatment, we’ll investigate lower-dose regimens that may be capable of maintaining clinical response,” Dr. Marius Ardeleanu said at the annual congress of the European Academy of Dermatology and Venereology.
Dupilumab is an investigational fully human monoclonal antibody that addresses a novel target: It is directed against the interleukin-4 receptor alpha subunit (IL-4Ra). Through this effect it blocks IL-4 and IL-13, the drivers of the type 2 helper T-cell–mediated inflammation responsible for the hallmark symptoms of atopic dermatitis (AD), explained Dr. Ardeleanu of Regeneron Pharmaceuticals in Tarrytown, N.Y.
Participants in this 16-week, double-blind, international phase IIb study were randomized to placebo or one of five dupilumab dosing regimens ranging from a low of 100 mg given subcutaneously every 4 weeks to a maximum of 300 mg once weekly or every 2 weeks.
These patients had a significant disease burden. They were typically in their mid- to late 30s and had a 27-year disease history, a mean baseline SCORAD of 67 on a 0-100 scale, a baseline Eczema Area and Severity Index (EASI) score of 32, an Investigator’s Global Assessment of disease severity score of 3.5 on a 0-4 scale, and 50% body surface area involvement. Their mean average weekly self-rated itching score was 6.8 on a 0-10 scale.
The primary study endpoint was change in the EASI score from baseline to 16 weeks. The score dropped by 20% in placebo-treated controls and by significantly greater margins in all five dupilumab arms. The largest reduction in EASI score – nearly 80% – occurred in the group on 300 mg/wk, with the 300 mg every 2 weeks group showing about a 70% reduction.
Roughly 80% of patients on 300 mg/wk or every 2 weeks showed an EASI 50 response at week 16, meaning a 50% reduction from baseline in their score, which is considered clinically meaningful improvement. One-third of patients on either of these top two–performing regimens achieved an Investigator’s Global Assessment score of 0 or 1, which is virtual remission; none of the controls did. Overall weekly average pruritus scores dropped by more than 60% with weekly treatment at 300 mg and by slightly less with biweekly therapy at 300 mg.
Safety data were similar to those from a recently published earlier phase IIa study (N. Engl. J. Med. 2014; 371:130-9). There were no dose-limiting toxicities. Headache and injection site reactions were the only adverse events more common with dupilumab than with placebo in the phase IIb trial, with the incidence of injection site reactions showing a possible dose-response relationship.
In addition to the large phase III studies now being planned, which will also evaluate step-down maintenance therapy, another study has been scheduled to investigate the use of dupilumab in combination with topical corticosteroid therapy. The earlier phase IIa study provided evidence to suggest this combination has even greater efficacy than dupilumab alone, and with modest use of the topical agent, according to Dr. Ardeleanu.
Dupilumab is also being developed as a treatment for tough-to-control moderate to severe asthma. It showed positive results in a phase II study, with reduced asthma exacerbations and improved lung function, compared with placebo (N. Engl. J. Med. 2013; 368:2455-66).
AMSTERDAM – The novel biologic agent dupilumab showed strong dose-dependent efficacy in adults with moderate to severe atopic dermatitis not adequately controlled with topical medications in a 380-patient phase IIb dose-ranging study.
“Based on the results of this study, we’ll take the top two doses further to our phase III program. We’re also planning to conduct a maintenance study. At the end of 16 weeks of treatment, we’ll investigate lower-dose regimens that may be capable of maintaining clinical response,” Dr. Marius Ardeleanu said at the annual congress of the European Academy of Dermatology and Venereology.
Dupilumab is an investigational fully human monoclonal antibody that addresses a novel target: It is directed against the interleukin-4 receptor alpha subunit (IL-4Ra). Through this effect it blocks IL-4 and IL-13, the drivers of the type 2 helper T-cell–mediated inflammation responsible for the hallmark symptoms of atopic dermatitis (AD), explained Dr. Ardeleanu of Regeneron Pharmaceuticals in Tarrytown, N.Y.
Participants in this 16-week, double-blind, international phase IIb study were randomized to placebo or one of five dupilumab dosing regimens ranging from a low of 100 mg given subcutaneously every 4 weeks to a maximum of 300 mg once weekly or every 2 weeks.
These patients had a significant disease burden. They were typically in their mid- to late 30s and had a 27-year disease history, a mean baseline SCORAD of 67 on a 0-100 scale, a baseline Eczema Area and Severity Index (EASI) score of 32, an Investigator’s Global Assessment of disease severity score of 3.5 on a 0-4 scale, and 50% body surface area involvement. Their mean average weekly self-rated itching score was 6.8 on a 0-10 scale.
The primary study endpoint was change in the EASI score from baseline to 16 weeks. The score dropped by 20% in placebo-treated controls and by significantly greater margins in all five dupilumab arms. The largest reduction in EASI score – nearly 80% – occurred in the group on 300 mg/wk, with the 300 mg every 2 weeks group showing about a 70% reduction.
Roughly 80% of patients on 300 mg/wk or every 2 weeks showed an EASI 50 response at week 16, meaning a 50% reduction from baseline in their score, which is considered clinically meaningful improvement. One-third of patients on either of these top two–performing regimens achieved an Investigator’s Global Assessment score of 0 or 1, which is virtual remission; none of the controls did. Overall weekly average pruritus scores dropped by more than 60% with weekly treatment at 300 mg and by slightly less with biweekly therapy at 300 mg.
Safety data were similar to those from a recently published earlier phase IIa study (N. Engl. J. Med. 2014; 371:130-9). There were no dose-limiting toxicities. Headache and injection site reactions were the only adverse events more common with dupilumab than with placebo in the phase IIb trial, with the incidence of injection site reactions showing a possible dose-response relationship.
In addition to the large phase III studies now being planned, which will also evaluate step-down maintenance therapy, another study has been scheduled to investigate the use of dupilumab in combination with topical corticosteroid therapy. The earlier phase IIa study provided evidence to suggest this combination has even greater efficacy than dupilumab alone, and with modest use of the topical agent, according to Dr. Ardeleanu.
Dupilumab is also being developed as a treatment for tough-to-control moderate to severe asthma. It showed positive results in a phase II study, with reduced asthma exacerbations and improved lung function, compared with placebo (N. Engl. J. Med. 2013; 368:2455-66).
AT THE EADV CONGRESS
Key clinical point: A promising new therapy with a novel mechanism of action is progressing through the pipeline for adults with refractory atopic dermatitis.
Major finding: Patients on subcutaneous dupilumab at 300 mg once weekly showed nearly an 80% reduction from baseline in Eczema Area and Severity Index scores after 16 weeks of treatment.
Data source: A randomized, double-blind, placebo-controlled, international, 16-week phase IIb study of 380 adults with moderate to severe atopic dermatitis inadequately controlled by topical therapies.
Disclosures: The study was sponsored by Regeneron Pharmaceuticals and presented by the company’s senior director of clinical sciences.
Certolizumab achieves sustained skin improvement in psoriatic arthritis
AMSTERDAM – Certolizumab pegol maintained significant improvement in dermatologic outcomes in psoriatic arthritis patients through 96 weeks of treatment in the phase III RAPID-PsA trial.
Moreover, the safety profile of this tumor necrosis factor (TNF) inhibitor was in line with findings from shorter-term studies, including the week 24 report from RAPID-PsA. Treatment-emergent adverse events were similar in type and frequency to those in placebo-treated controls, with the exception of an increased rate of minor upper respiratory tract infections. No cases of tuberculosis occurred.
“There were no new safety issues despite the increased exposure time out to 96 weeks,” Dr. Owen Davies reported at the annual congress of the European Academy of Dermatology and Venereology.
RAPID-PsA is an ongoing 216-week phase III study. It was double-blind and placebo-controlled through the first 24 weeks. The study started out with 409 psoriatic arthritis patients, half of whom had previously failed to response to one nonbiologic disease-modifying antirheumatic drug (DMARD), while the other half had been nonresponders to two or more. Of the 273 patients placed on certolizumab, 80% completed both 48 and 96 weeks of the study, explained Dr. Davies of UCB Pharma in Slough, England.
He focused on the dermatologic outcomes because the arthritis outcomes have previously been reported and served to support certolizumab’s regulatory approval for the treatment of psoriatic arthritis. The biologic is also approved for treatment of rheumatoid arthritis, ankylosing spondylitis, and Crohn’s disease. However, certolizumab’s durability of effect on the psoriatic skin manifestations of psoriatic arthritis hasn’t previously been addressed.
Briefly, at week 12 – the primary endpoint for the joint-related outcomes – 55% of patients achieved an ACR 20 response, compared with 24% on placebo. Moreover, 35% of certolizumab-treated patients had an ACR 50 response at that point, and 20% had an ACR 70 response. The ACR response rate in certolizumab-treated patients was similar regardless of whether or not they had previously been on another anti-TNF biologic.
Dr. Davies addressed in detail the dermatologic outcomes in the 166 psoriatic arthritis patients with at least 3% psoriasis body surface area involvement at baseline. They had an average 10-year disease duration, 24% body surface area involvement, and a baseline Psoriasis Area Severity Index (PASI) score of 12.0.
Dermatologic responses to certolizumab were comparable regardless of whether patients had been randomized to the biologic at 200 mg subcutaneously every 2 weeks or 400 mg once every 4 weeks. As was true for the joint-related responses to certolizumab, the skin responses were similar both in anti-TNF–naive and anti-TNF–experienced patients, he noted.
The PASI 75 response rate in this group of psoriatic arthritis patients with significant skin involvement was 61% at week 24, 65% at week 48, and 53% at week 96. The improvement was even greater in the 71 patients with more severe skin involvement as defined by a baseline PASI score of 10 or more. Certolizumab-treated patients also showed important improvements on the Physician Global Assessment and Dermatology Life Quality Index.
Certolizumab is a pegylated Fab’ fragment of a humanized TNF inhibitor monoclonal antibody.
“Certolizumab is structurally different from other currently available anti-TNF agents, which are either IgG1 monoclonal antibodies or, in the case of etanercept, a receptor fusion protein. Whether or not these structural differences will translate into clinical differences is a question being addressed in ongoing clinical trials,” Dr. Davies said.
The RAPID-PsA study is sponsored by UCB Pharma, where Dr. Davies is employed.
AMSTERDAM – Certolizumab pegol maintained significant improvement in dermatologic outcomes in psoriatic arthritis patients through 96 weeks of treatment in the phase III RAPID-PsA trial.
Moreover, the safety profile of this tumor necrosis factor (TNF) inhibitor was in line with findings from shorter-term studies, including the week 24 report from RAPID-PsA. Treatment-emergent adverse events were similar in type and frequency to those in placebo-treated controls, with the exception of an increased rate of minor upper respiratory tract infections. No cases of tuberculosis occurred.
“There were no new safety issues despite the increased exposure time out to 96 weeks,” Dr. Owen Davies reported at the annual congress of the European Academy of Dermatology and Venereology.
RAPID-PsA is an ongoing 216-week phase III study. It was double-blind and placebo-controlled through the first 24 weeks. The study started out with 409 psoriatic arthritis patients, half of whom had previously failed to response to one nonbiologic disease-modifying antirheumatic drug (DMARD), while the other half had been nonresponders to two or more. Of the 273 patients placed on certolizumab, 80% completed both 48 and 96 weeks of the study, explained Dr. Davies of UCB Pharma in Slough, England.
He focused on the dermatologic outcomes because the arthritis outcomes have previously been reported and served to support certolizumab’s regulatory approval for the treatment of psoriatic arthritis. The biologic is also approved for treatment of rheumatoid arthritis, ankylosing spondylitis, and Crohn’s disease. However, certolizumab’s durability of effect on the psoriatic skin manifestations of psoriatic arthritis hasn’t previously been addressed.
Briefly, at week 12 – the primary endpoint for the joint-related outcomes – 55% of patients achieved an ACR 20 response, compared with 24% on placebo. Moreover, 35% of certolizumab-treated patients had an ACR 50 response at that point, and 20% had an ACR 70 response. The ACR response rate in certolizumab-treated patients was similar regardless of whether or not they had previously been on another anti-TNF biologic.
Dr. Davies addressed in detail the dermatologic outcomes in the 166 psoriatic arthritis patients with at least 3% psoriasis body surface area involvement at baseline. They had an average 10-year disease duration, 24% body surface area involvement, and a baseline Psoriasis Area Severity Index (PASI) score of 12.0.
Dermatologic responses to certolizumab were comparable regardless of whether patients had been randomized to the biologic at 200 mg subcutaneously every 2 weeks or 400 mg once every 4 weeks. As was true for the joint-related responses to certolizumab, the skin responses were similar both in anti-TNF–naive and anti-TNF–experienced patients, he noted.
The PASI 75 response rate in this group of psoriatic arthritis patients with significant skin involvement was 61% at week 24, 65% at week 48, and 53% at week 96. The improvement was even greater in the 71 patients with more severe skin involvement as defined by a baseline PASI score of 10 or more. Certolizumab-treated patients also showed important improvements on the Physician Global Assessment and Dermatology Life Quality Index.
Certolizumab is a pegylated Fab’ fragment of a humanized TNF inhibitor monoclonal antibody.
“Certolizumab is structurally different from other currently available anti-TNF agents, which are either IgG1 monoclonal antibodies or, in the case of etanercept, a receptor fusion protein. Whether or not these structural differences will translate into clinical differences is a question being addressed in ongoing clinical trials,” Dr. Davies said.
The RAPID-PsA study is sponsored by UCB Pharma, where Dr. Davies is employed.
AMSTERDAM – Certolizumab pegol maintained significant improvement in dermatologic outcomes in psoriatic arthritis patients through 96 weeks of treatment in the phase III RAPID-PsA trial.
Moreover, the safety profile of this tumor necrosis factor (TNF) inhibitor was in line with findings from shorter-term studies, including the week 24 report from RAPID-PsA. Treatment-emergent adverse events were similar in type and frequency to those in placebo-treated controls, with the exception of an increased rate of minor upper respiratory tract infections. No cases of tuberculosis occurred.
“There were no new safety issues despite the increased exposure time out to 96 weeks,” Dr. Owen Davies reported at the annual congress of the European Academy of Dermatology and Venereology.
RAPID-PsA is an ongoing 216-week phase III study. It was double-blind and placebo-controlled through the first 24 weeks. The study started out with 409 psoriatic arthritis patients, half of whom had previously failed to response to one nonbiologic disease-modifying antirheumatic drug (DMARD), while the other half had been nonresponders to two or more. Of the 273 patients placed on certolizumab, 80% completed both 48 and 96 weeks of the study, explained Dr. Davies of UCB Pharma in Slough, England.
He focused on the dermatologic outcomes because the arthritis outcomes have previously been reported and served to support certolizumab’s regulatory approval for the treatment of psoriatic arthritis. The biologic is also approved for treatment of rheumatoid arthritis, ankylosing spondylitis, and Crohn’s disease. However, certolizumab’s durability of effect on the psoriatic skin manifestations of psoriatic arthritis hasn’t previously been addressed.
Briefly, at week 12 – the primary endpoint for the joint-related outcomes – 55% of patients achieved an ACR 20 response, compared with 24% on placebo. Moreover, 35% of certolizumab-treated patients had an ACR 50 response at that point, and 20% had an ACR 70 response. The ACR response rate in certolizumab-treated patients was similar regardless of whether or not they had previously been on another anti-TNF biologic.
Dr. Davies addressed in detail the dermatologic outcomes in the 166 psoriatic arthritis patients with at least 3% psoriasis body surface area involvement at baseline. They had an average 10-year disease duration, 24% body surface area involvement, and a baseline Psoriasis Area Severity Index (PASI) score of 12.0.
Dermatologic responses to certolizumab were comparable regardless of whether patients had been randomized to the biologic at 200 mg subcutaneously every 2 weeks or 400 mg once every 4 weeks. As was true for the joint-related responses to certolizumab, the skin responses were similar both in anti-TNF–naive and anti-TNF–experienced patients, he noted.
The PASI 75 response rate in this group of psoriatic arthritis patients with significant skin involvement was 61% at week 24, 65% at week 48, and 53% at week 96. The improvement was even greater in the 71 patients with more severe skin involvement as defined by a baseline PASI score of 10 or more. Certolizumab-treated patients also showed important improvements on the Physician Global Assessment and Dermatology Life Quality Index.
Certolizumab is a pegylated Fab’ fragment of a humanized TNF inhibitor monoclonal antibody.
“Certolizumab is structurally different from other currently available anti-TNF agents, which are either IgG1 monoclonal antibodies or, in the case of etanercept, a receptor fusion protein. Whether or not these structural differences will translate into clinical differences is a question being addressed in ongoing clinical trials,” Dr. Davies said.
The RAPID-PsA study is sponsored by UCB Pharma, where Dr. Davies is employed.
AT THE EADV CONGRESS
Key clinical point: Certolizumab pegol maintains sustained improvement in the dermatologic manifestations of psoriatic arthritis through 96 weeks.
Major finding: Sixty-two percent of psoriatic arthritis patients with at least 3% body surface area involvement at a baseline PASI score of 10 or more still had a PASI 75 dermatologic response after 96 weeks on certolizumab.
Data source: The RAPID-PsA study is an ongoing 216-week, prospective, randomized, multicenter trial involving 409 psoriatic arthritis patients, including 166 with significant skin involvement at baseline.
Disclosures: The study is sponsored by UCB Pharma. The presenter is a full-time company employee.
Low-risk actinic keratosis? No such thing
AMSTERDAM – The majority of cutaneous infiltrating squamous cell carcinomas arise via direct transformation of grade 1 actinic keratoses – that is, differentiated dysplasia of the basal one-third of the epidermis – via extension of atypical keratinocytes and ultimately of tumor cells along the adnexal epithelium rather than through the classical pathway most dermatologists know, Dr. Maria Teresa Fernandez-Figueras said at the annual congress of the European Academy of Dermatology and Venereology.
This direct transformation by means of what pathologists call the differentiated pathway has important implications for clinical practice, she added.
“For me, this is something that should change our way of seeing these lesions. When you’re facing an actinic keratosis-1 (AK 1), I think you can no longer call it a low-grade lesion, an early lesion, or a low-risk lesion, because you don’t know if it’s going to follow the differentiated pathway. It can infiltrate at any moment. So from this point of view, all AKs carry a risk of immediate transformation – a low risk, it’s true – and thus maybe all of them require treatment,” explained Dr. Fernandez-Figueras, a pathologist at the University of Barcelona.
The classical pathway by which an AK 1 transforms into an infiltrating squamous cell carcinoma (SCC) is as follows: Before an AK 1 can become a malignant lesion, it first must transition to an AK 2, marked by progression of dysplasia to the lower two-thirds of the epidermis, and then to AK 3, with full-thickness epidermal dysplasia. That’s how most dermatologists understand the process. But there are precedents elsewhere in the body for the existence of a separate differentiated pathway.
The most notable example is vulvar carcinoma. Human papillomavirus–related vulvar malignancy is known to arise from two different pathways. In the classical pathway, vulvar intraepithelial neoplasia 1 (VIN 1) must transition to VIN 2 and then VIN 3 before transformation to SCC. But the cancer can also emerge directly from areas of differentiated dysplastic VIN 1 epidermis, bypassing VINs 2 and 3 altogether.
Dr. Fernandez-Figueras sought to study the relative importance of the two pathways in the formation of cutaneous SCC. To do so, she and two other pathologists examined pathologic specimens and reports for 503 consecutive cases of cutaneous infiltrating SCC. The majority were unsuitable for thorough evaluation because they were fragmented or curettaged, leaving a final study sample of 196 cases.
Two-thirds of all the SCCs had only AK 1 overlying the cutaneous malignancy. Another 15% had overlying AK 2, and 18% had overlying AK 3. A total of 79% of the SCCs had only AK 1 in the epidermis adjacent to the SCC, while 7% had AK 2, and 8% had AK 3; the remaining 6% were unevaluable on this score.
Proliferative growth extending along the sweat ducts and hair follicles of the adnexal epithelium over the SCC was identified in 32% of the cancerous lesions with overlying AK 1, compared with 23% of cancers with overlying AK 2 and 14% with overlying AK 3. Thirty-eight percent of SCCs with AK 1 in the adjacent epithelium had differentiated dysplastic growth migrating along the adnexal epithelium, as did 25% of SCCs with adjacent AK 2 and 11% with neighboring AK 2.
These observations suggest that the differentiated pathway is by far the more common of the two mechanisms of malignant transformation, according to Dr. Fernandez-Figueras.
“Another interesting observation was that very often the areas of invasion were coincident with the focus of adnexal extension,” she continued. “We believe that this tumor advance along the adnexal structures plays a pivotal role in transformation and inversion, especially in AK 1.”
One dermatologist in the audience challenged her. “What do you want us to do – aggressively excise all AKs?”
“I’m a pathologist; I don’t have a suggestion regarding treatment,” Dr. Fernandez-Figueras replied. “I’m just saying, don’t think that AK 1 is better than AK 3, and don’t think that making this distinction gives you any security that you can tell the patient, ‘Don’t worry, nothing is going to happen, we’ll wait until it’s AK 3.’ In my own case, I know that if I had an AK 1, I would treat it. I don’t want to wait to see if it’s going to follow the classical or the differentiated pathway. All lesions can be high grade.”
Session cochair Dr. Michael Reusch pronounced her findings and interpretation of them “completely consistent” with his own experience.
“I think from a histologic point of view, I completely share your view. Still, for the dermatologist part of us, it creates a problem as to what to do. It’s a major issue. The number of AKs out there is incredible,” commented Dr. Reusch of the University Clinics of Hamburg-Eppendorf, Germany.
Dr. Fernandez-Figueras reported that her study received financial support from Almirall.
AMSTERDAM – The majority of cutaneous infiltrating squamous cell carcinomas arise via direct transformation of grade 1 actinic keratoses – that is, differentiated dysplasia of the basal one-third of the epidermis – via extension of atypical keratinocytes and ultimately of tumor cells along the adnexal epithelium rather than through the classical pathway most dermatologists know, Dr. Maria Teresa Fernandez-Figueras said at the annual congress of the European Academy of Dermatology and Venereology.
This direct transformation by means of what pathologists call the differentiated pathway has important implications for clinical practice, she added.
“For me, this is something that should change our way of seeing these lesions. When you’re facing an actinic keratosis-1 (AK 1), I think you can no longer call it a low-grade lesion, an early lesion, or a low-risk lesion, because you don’t know if it’s going to follow the differentiated pathway. It can infiltrate at any moment. So from this point of view, all AKs carry a risk of immediate transformation – a low risk, it’s true – and thus maybe all of them require treatment,” explained Dr. Fernandez-Figueras, a pathologist at the University of Barcelona.
The classical pathway by which an AK 1 transforms into an infiltrating squamous cell carcinoma (SCC) is as follows: Before an AK 1 can become a malignant lesion, it first must transition to an AK 2, marked by progression of dysplasia to the lower two-thirds of the epidermis, and then to AK 3, with full-thickness epidermal dysplasia. That’s how most dermatologists understand the process. But there are precedents elsewhere in the body for the existence of a separate differentiated pathway.
The most notable example is vulvar carcinoma. Human papillomavirus–related vulvar malignancy is known to arise from two different pathways. In the classical pathway, vulvar intraepithelial neoplasia 1 (VIN 1) must transition to VIN 2 and then VIN 3 before transformation to SCC. But the cancer can also emerge directly from areas of differentiated dysplastic VIN 1 epidermis, bypassing VINs 2 and 3 altogether.
Dr. Fernandez-Figueras sought to study the relative importance of the two pathways in the formation of cutaneous SCC. To do so, she and two other pathologists examined pathologic specimens and reports for 503 consecutive cases of cutaneous infiltrating SCC. The majority were unsuitable for thorough evaluation because they were fragmented or curettaged, leaving a final study sample of 196 cases.
Two-thirds of all the SCCs had only AK 1 overlying the cutaneous malignancy. Another 15% had overlying AK 2, and 18% had overlying AK 3. A total of 79% of the SCCs had only AK 1 in the epidermis adjacent to the SCC, while 7% had AK 2, and 8% had AK 3; the remaining 6% were unevaluable on this score.
Proliferative growth extending along the sweat ducts and hair follicles of the adnexal epithelium over the SCC was identified in 32% of the cancerous lesions with overlying AK 1, compared with 23% of cancers with overlying AK 2 and 14% with overlying AK 3. Thirty-eight percent of SCCs with AK 1 in the adjacent epithelium had differentiated dysplastic growth migrating along the adnexal epithelium, as did 25% of SCCs with adjacent AK 2 and 11% with neighboring AK 2.
These observations suggest that the differentiated pathway is by far the more common of the two mechanisms of malignant transformation, according to Dr. Fernandez-Figueras.
“Another interesting observation was that very often the areas of invasion were coincident with the focus of adnexal extension,” she continued. “We believe that this tumor advance along the adnexal structures plays a pivotal role in transformation and inversion, especially in AK 1.”
One dermatologist in the audience challenged her. “What do you want us to do – aggressively excise all AKs?”
“I’m a pathologist; I don’t have a suggestion regarding treatment,” Dr. Fernandez-Figueras replied. “I’m just saying, don’t think that AK 1 is better than AK 3, and don’t think that making this distinction gives you any security that you can tell the patient, ‘Don’t worry, nothing is going to happen, we’ll wait until it’s AK 3.’ In my own case, I know that if I had an AK 1, I would treat it. I don’t want to wait to see if it’s going to follow the classical or the differentiated pathway. All lesions can be high grade.”
Session cochair Dr. Michael Reusch pronounced her findings and interpretation of them “completely consistent” with his own experience.
“I think from a histologic point of view, I completely share your view. Still, for the dermatologist part of us, it creates a problem as to what to do. It’s a major issue. The number of AKs out there is incredible,” commented Dr. Reusch of the University Clinics of Hamburg-Eppendorf, Germany.
Dr. Fernandez-Figueras reported that her study received financial support from Almirall.
AMSTERDAM – The majority of cutaneous infiltrating squamous cell carcinomas arise via direct transformation of grade 1 actinic keratoses – that is, differentiated dysplasia of the basal one-third of the epidermis – via extension of atypical keratinocytes and ultimately of tumor cells along the adnexal epithelium rather than through the classical pathway most dermatologists know, Dr. Maria Teresa Fernandez-Figueras said at the annual congress of the European Academy of Dermatology and Venereology.
This direct transformation by means of what pathologists call the differentiated pathway has important implications for clinical practice, she added.
“For me, this is something that should change our way of seeing these lesions. When you’re facing an actinic keratosis-1 (AK 1), I think you can no longer call it a low-grade lesion, an early lesion, or a low-risk lesion, because you don’t know if it’s going to follow the differentiated pathway. It can infiltrate at any moment. So from this point of view, all AKs carry a risk of immediate transformation – a low risk, it’s true – and thus maybe all of them require treatment,” explained Dr. Fernandez-Figueras, a pathologist at the University of Barcelona.
The classical pathway by which an AK 1 transforms into an infiltrating squamous cell carcinoma (SCC) is as follows: Before an AK 1 can become a malignant lesion, it first must transition to an AK 2, marked by progression of dysplasia to the lower two-thirds of the epidermis, and then to AK 3, with full-thickness epidermal dysplasia. That’s how most dermatologists understand the process. But there are precedents elsewhere in the body for the existence of a separate differentiated pathway.
The most notable example is vulvar carcinoma. Human papillomavirus–related vulvar malignancy is known to arise from two different pathways. In the classical pathway, vulvar intraepithelial neoplasia 1 (VIN 1) must transition to VIN 2 and then VIN 3 before transformation to SCC. But the cancer can also emerge directly from areas of differentiated dysplastic VIN 1 epidermis, bypassing VINs 2 and 3 altogether.
Dr. Fernandez-Figueras sought to study the relative importance of the two pathways in the formation of cutaneous SCC. To do so, she and two other pathologists examined pathologic specimens and reports for 503 consecutive cases of cutaneous infiltrating SCC. The majority were unsuitable for thorough evaluation because they were fragmented or curettaged, leaving a final study sample of 196 cases.
Two-thirds of all the SCCs had only AK 1 overlying the cutaneous malignancy. Another 15% had overlying AK 2, and 18% had overlying AK 3. A total of 79% of the SCCs had only AK 1 in the epidermis adjacent to the SCC, while 7% had AK 2, and 8% had AK 3; the remaining 6% were unevaluable on this score.
Proliferative growth extending along the sweat ducts and hair follicles of the adnexal epithelium over the SCC was identified in 32% of the cancerous lesions with overlying AK 1, compared with 23% of cancers with overlying AK 2 and 14% with overlying AK 3. Thirty-eight percent of SCCs with AK 1 in the adjacent epithelium had differentiated dysplastic growth migrating along the adnexal epithelium, as did 25% of SCCs with adjacent AK 2 and 11% with neighboring AK 2.
These observations suggest that the differentiated pathway is by far the more common of the two mechanisms of malignant transformation, according to Dr. Fernandez-Figueras.
“Another interesting observation was that very often the areas of invasion were coincident with the focus of adnexal extension,” she continued. “We believe that this tumor advance along the adnexal structures plays a pivotal role in transformation and inversion, especially in AK 1.”
One dermatologist in the audience challenged her. “What do you want us to do – aggressively excise all AKs?”
“I’m a pathologist; I don’t have a suggestion regarding treatment,” Dr. Fernandez-Figueras replied. “I’m just saying, don’t think that AK 1 is better than AK 3, and don’t think that making this distinction gives you any security that you can tell the patient, ‘Don’t worry, nothing is going to happen, we’ll wait until it’s AK 3.’ In my own case, I know that if I had an AK 1, I would treat it. I don’t want to wait to see if it’s going to follow the classical or the differentiated pathway. All lesions can be high grade.”
Session cochair Dr. Michael Reusch pronounced her findings and interpretation of them “completely consistent” with his own experience.
“I think from a histologic point of view, I completely share your view. Still, for the dermatologist part of us, it creates a problem as to what to do. It’s a major issue. The number of AKs out there is incredible,” commented Dr. Reusch of the University Clinics of Hamburg-Eppendorf, Germany.
Dr. Fernandez-Figueras reported that her study received financial support from Almirall.
AT THE EADV CONGRESS
Key clinical point: Actinic keratoses traditionally considered low-risk AK 1s with dysplasia confined to the basal one-third of the epidermis can undergo immediate transformation to infiltrating squamous cell carcinoma without mandatory sequential passage through AK 2 and 3.
Major finding: Two-thirds of SCCs had only AK 1 overlying the malignancy, 15% had overlying AK 2, and only 8% had AK 3.
Data source: A retrospective study, in which three pathologists examined the pathologic specimens and supporting reports for 196 cutaneous infiltrating SCCs.
Disclosures: The study received funding support from Almirall.
Oral curcumin shown effective in psoriasis
AMSTERDAM – Oral curcumin proved safe and effective as adjunctive therapy in patients on topical corticosteroids for mild to moderate psoriasis vulgaris in a 12-week, randomized, placebo-controlled clinical trial.
This agent helps fill an unmet need in psoriasis, Dr. Emiliano Antiga observed in presenting the study findings at the annual congress of the European Academy of Dermatology and Venereology.
That’s because by far most of the action in the development of new treatments for psoriasis focuses on biologics and other extremely costly agents targeting patients at the moderate to severe end of the disease spectrum. But psoriasis is a chronic condition, and the many patients with milder disease require long-term therapies that are nontoxic and won’t break the bank. Enter curcumin.
“Oral curcumin is effective, safe, and it is cheap,” declared Dr. Antiga, a dermatologist at the University of Florence (Italy).
Moreover, it has a biologically plausible mechanism of benefit in psoriasis, as was demonstrated in his 60-patient randomized trial. Serum levels of the proinflammatory cytokine interleukin-22 were cut in half in the group assigned to 12 weeks of daily oral curcumin while remaining unchanged in the control group.
Curcumin is derived from turmeric, the dried rhizome of a plant, Curcuma longa. Turmeric is a yellowish Indian spice used in curries. But curcumin has long been used therapeutically in traditional Indian and Chinese medicine. Studies have shown that curcumin has antiproliferative, antiangiogenic, and anti-inflammatory effects. In a small study by other investigators, topical turmeric not only successfully cleared psoriasis lesions, it also suppressed phosphorylase kinase activity, which is important to keratinocyte proliferation (Br. J. Dermatol. 2000;143:937-49).
Dr. Antiga presented a study of 60 patients with mild to moderate psoriasis vulgaris as defined by a baseline median Psoriasis Area and Severity Index (PASI) score of 5.5 who were randomly assigned to 12 weeks of treatment with topical corticosteroids plus 3 g per day of oral curcumin or topical steroids plus placebo. The active-treatment capsules contained curcumin embedded in nanoparticle liposomes to enhance bioavailability.
Forty-nine patients completed the study. The primary endpoint was reduction in PASI values over 12 weeks. Both groups showed improvement – after all, the controls were on active treatment with topical steroids – but the change in PASI scores was significantly greater in the curcumin-treated patients.
Adverse events in the curcumin group were limited to one case of diarrhea. There was one case of nausea and one papular eruption in the control group.
Although IL-22 levels at 12 weeks were halved in the curcumin group and unchanged in controls, levels of the inflammatory cytokines IL-10 and -17 and transforming growth factor–beta remained unchanged in both groups over time.
Dr. Antiga reported having no financial conflicts regarding this study.
AMSTERDAM – Oral curcumin proved safe and effective as adjunctive therapy in patients on topical corticosteroids for mild to moderate psoriasis vulgaris in a 12-week, randomized, placebo-controlled clinical trial.
This agent helps fill an unmet need in psoriasis, Dr. Emiliano Antiga observed in presenting the study findings at the annual congress of the European Academy of Dermatology and Venereology.
That’s because by far most of the action in the development of new treatments for psoriasis focuses on biologics and other extremely costly agents targeting patients at the moderate to severe end of the disease spectrum. But psoriasis is a chronic condition, and the many patients with milder disease require long-term therapies that are nontoxic and won’t break the bank. Enter curcumin.
“Oral curcumin is effective, safe, and it is cheap,” declared Dr. Antiga, a dermatologist at the University of Florence (Italy).
Moreover, it has a biologically plausible mechanism of benefit in psoriasis, as was demonstrated in his 60-patient randomized trial. Serum levels of the proinflammatory cytokine interleukin-22 were cut in half in the group assigned to 12 weeks of daily oral curcumin while remaining unchanged in the control group.
Curcumin is derived from turmeric, the dried rhizome of a plant, Curcuma longa. Turmeric is a yellowish Indian spice used in curries. But curcumin has long been used therapeutically in traditional Indian and Chinese medicine. Studies have shown that curcumin has antiproliferative, antiangiogenic, and anti-inflammatory effects. In a small study by other investigators, topical turmeric not only successfully cleared psoriasis lesions, it also suppressed phosphorylase kinase activity, which is important to keratinocyte proliferation (Br. J. Dermatol. 2000;143:937-49).
Dr. Antiga presented a study of 60 patients with mild to moderate psoriasis vulgaris as defined by a baseline median Psoriasis Area and Severity Index (PASI) score of 5.5 who were randomly assigned to 12 weeks of treatment with topical corticosteroids plus 3 g per day of oral curcumin or topical steroids plus placebo. The active-treatment capsules contained curcumin embedded in nanoparticle liposomes to enhance bioavailability.
Forty-nine patients completed the study. The primary endpoint was reduction in PASI values over 12 weeks. Both groups showed improvement – after all, the controls were on active treatment with topical steroids – but the change in PASI scores was significantly greater in the curcumin-treated patients.
Adverse events in the curcumin group were limited to one case of diarrhea. There was one case of nausea and one papular eruption in the control group.
Although IL-22 levels at 12 weeks were halved in the curcumin group and unchanged in controls, levels of the inflammatory cytokines IL-10 and -17 and transforming growth factor–beta remained unchanged in both groups over time.
Dr. Antiga reported having no financial conflicts regarding this study.
AMSTERDAM – Oral curcumin proved safe and effective as adjunctive therapy in patients on topical corticosteroids for mild to moderate psoriasis vulgaris in a 12-week, randomized, placebo-controlled clinical trial.
This agent helps fill an unmet need in psoriasis, Dr. Emiliano Antiga observed in presenting the study findings at the annual congress of the European Academy of Dermatology and Venereology.
That’s because by far most of the action in the development of new treatments for psoriasis focuses on biologics and other extremely costly agents targeting patients at the moderate to severe end of the disease spectrum. But psoriasis is a chronic condition, and the many patients with milder disease require long-term therapies that are nontoxic and won’t break the bank. Enter curcumin.
“Oral curcumin is effective, safe, and it is cheap,” declared Dr. Antiga, a dermatologist at the University of Florence (Italy).
Moreover, it has a biologically plausible mechanism of benefit in psoriasis, as was demonstrated in his 60-patient randomized trial. Serum levels of the proinflammatory cytokine interleukin-22 were cut in half in the group assigned to 12 weeks of daily oral curcumin while remaining unchanged in the control group.
Curcumin is derived from turmeric, the dried rhizome of a plant, Curcuma longa. Turmeric is a yellowish Indian spice used in curries. But curcumin has long been used therapeutically in traditional Indian and Chinese medicine. Studies have shown that curcumin has antiproliferative, antiangiogenic, and anti-inflammatory effects. In a small study by other investigators, topical turmeric not only successfully cleared psoriasis lesions, it also suppressed phosphorylase kinase activity, which is important to keratinocyte proliferation (Br. J. Dermatol. 2000;143:937-49).
Dr. Antiga presented a study of 60 patients with mild to moderate psoriasis vulgaris as defined by a baseline median Psoriasis Area and Severity Index (PASI) score of 5.5 who were randomly assigned to 12 weeks of treatment with topical corticosteroids plus 3 g per day of oral curcumin or topical steroids plus placebo. The active-treatment capsules contained curcumin embedded in nanoparticle liposomes to enhance bioavailability.
Forty-nine patients completed the study. The primary endpoint was reduction in PASI values over 12 weeks. Both groups showed improvement – after all, the controls were on active treatment with topical steroids – but the change in PASI scores was significantly greater in the curcumin-treated patients.
Adverse events in the curcumin group were limited to one case of diarrhea. There was one case of nausea and one papular eruption in the control group.
Although IL-22 levels at 12 weeks were halved in the curcumin group and unchanged in controls, levels of the inflammatory cytokines IL-10 and -17 and transforming growth factor–beta remained unchanged in both groups over time.
Dr. Antiga reported having no financial conflicts regarding this study.
AT THE EADV CONGRESS
Key clinical point: A common ingredient in Indian curry spice mixes is safe and effective as adjuvant therapy for mild-to-moderate psoriasis.
Major finding: Daily oral curcumin capsules plus topical corticosteroids resulted in a PASI 75 improvement rate of 48% compared with a 12% rate in patients who got topical steroids plus placebo.
Data source: A prospective randomized 12-week clinical trial involving 60 patients with mild to moderate psoriasis vulgaris.
Disclosures: The presenter reported no financial conflicts regarding this study, conducted free of commercial support.
Novel psoriasis biologic wows with jaw-dropping results
AMSTERDAM– The spectacular long-term efficacy achieved with a novel biologic agent for psoriasis in a first-in-humans, proof-of-concept study has raised the prospect of clinical outcomes continuing to ratchet higher in the treatment of moderate-to-severe chronic plaque psoriasis.
How much higher? Six of nine treated patients followed long-term have maintained a PASI 100 response – that is, completely clear – for up to 66 months after a single subcutaneous injection of the agent known for now as BI 655066, Dr. James G. Krueger reported at the annual congress of the European Academy of Dermatology and Venereology.
“For me, this is one of the most interesting features of this proof-of-concept study,” he added. “If this kind of activity is confirmed in the ongoing phase IIb trial, I think this represents the potential for very long-term disease modification. This could become an important agent in the future to treat psoriasis.”
BI 655066 is a monoclonal antibody that specifically targets the p19 subunit of interleukin (IL)-23. Unlike ustekinumab (Stelara), which blocks both IL-23 and IL-12, BI 655066 selectively blocks only IL-23, which Dr. Krueger believes is the central driving force in activating and sustaining the T-cell subsets responsible for the hyperproliferative and inflammatory reactions that define psoriasis.
“This study is all about testing for the specific pathogenic contribution of IL-23 to psoriasis in a first-in-humans study. Our findings really emphasize the importance of IL-23 in driving the key pathways of psoriasis,” observed Dr. Krueger, professor of investigative dermatology and director of the Milstein Medical Research Program at Rockefeller University, New York.
The study included 39 patients with moderate to severe plaque psoriasis. Their baseline PASI was 18, and they averaged more than a 20-year history of psoriasis. Twenty-four patients were randomized 3:1 to a single intravenous injection of BI 655066 at various doses ranging from 0.01 mg/kg to 5 mg/kg or to placebo in order to get an initial sense of the agent’s safety and tolerability.
In the second part of the study, 15 other participants received a single subcutaneous injection: two got placebo and the rest were randomized to BI 655066 at either 0.25 mg/kg or 1.0 mg/kg. Safety and efficacy were assessed at weeks 0, 2, 4, 12, and 24. In addition, skin biopsies were obtained at weeks 0 and 8 for immunohistochemistry studies and RNA sequencing analysis.
By week 12, the PASI 75 response rate in subcutaneous BI 655066 recipients was 87% and the PASI 90 rate was 58%. At week 24, nine patients elected to continue structured prospective follow-up while remaining off treatment, including six PASI 100 responders. Those six PASI 100 responders remained PASI 100 at ongoing follow-up 48-66 weeks after receiving their single dose of the agent.
Biopsy specimens obtained at week 8 showed normalization of the epidermal psoriasiform hyperplasia which had been present at baseline. A normal-looking granular layer had been reestablished. “This looks essentially like the pattern of normal or nonlesional skin,” according to the dermatologist.
RNA sequencing analysis and gene profiling showed normalized production of the IL-23/IL-17-induced proteins that had been strongly overexpressed at baseline, including lipocalin, beta-defensin, and psoriasin.
“The immune axis is turned down. The number of immune cells is way down, although they’re not completely eliminated. With placebo, you still see a psoriasislike pattern of the disease. With blockade of IL-23, most cases have a gene profile like nonlesional skin. This represents a profound cellular and disease modulation,” Dr. Krueger said.
Among all 39 participants, the only serious adverse event deemed possibly treatment related was a 5-minute transient ischemic attack (TIA) episode in a patient on BI 655066. This caught Dr. Krueger’s attention as a possible red flag; however, he noted that more than 200 patients have since received the biologic agent in the ongoing phase IIb trial, with no reported major adverse cardiovascular events.
“I think that TIA may just be bad luck with small numbers,” he added.
Asked what he thinks might explain the remarkably lengthy disease remission seen following a single dose of the biologic, Dr. Krueger offered two possibilities.
“It may be that IL-23 is necessary to sustain pathogenic clones of memory cells in the skin, and as we get rid of it those clones most likely apoptose. And if you’ve sufficiently removed the clones, then you don’t get the expansion. That’s guess one. Guess two would be that we’ve renormalized tolerance mechanisms in some way. Both of these hypotheses can be tested,” according to Dr. Krueger.
The study was funded by Boehringer Ingelheim. Dr. Krueger reported receiving funding from that pharmaceutical company and nearly two dozen others.
AMSTERDAM– The spectacular long-term efficacy achieved with a novel biologic agent for psoriasis in a first-in-humans, proof-of-concept study has raised the prospect of clinical outcomes continuing to ratchet higher in the treatment of moderate-to-severe chronic plaque psoriasis.
How much higher? Six of nine treated patients followed long-term have maintained a PASI 100 response – that is, completely clear – for up to 66 months after a single subcutaneous injection of the agent known for now as BI 655066, Dr. James G. Krueger reported at the annual congress of the European Academy of Dermatology and Venereology.
“For me, this is one of the most interesting features of this proof-of-concept study,” he added. “If this kind of activity is confirmed in the ongoing phase IIb trial, I think this represents the potential for very long-term disease modification. This could become an important agent in the future to treat psoriasis.”
BI 655066 is a monoclonal antibody that specifically targets the p19 subunit of interleukin (IL)-23. Unlike ustekinumab (Stelara), which blocks both IL-23 and IL-12, BI 655066 selectively blocks only IL-23, which Dr. Krueger believes is the central driving force in activating and sustaining the T-cell subsets responsible for the hyperproliferative and inflammatory reactions that define psoriasis.
“This study is all about testing for the specific pathogenic contribution of IL-23 to psoriasis in a first-in-humans study. Our findings really emphasize the importance of IL-23 in driving the key pathways of psoriasis,” observed Dr. Krueger, professor of investigative dermatology and director of the Milstein Medical Research Program at Rockefeller University, New York.
The study included 39 patients with moderate to severe plaque psoriasis. Their baseline PASI was 18, and they averaged more than a 20-year history of psoriasis. Twenty-four patients were randomized 3:1 to a single intravenous injection of BI 655066 at various doses ranging from 0.01 mg/kg to 5 mg/kg or to placebo in order to get an initial sense of the agent’s safety and tolerability.
In the second part of the study, 15 other participants received a single subcutaneous injection: two got placebo and the rest were randomized to BI 655066 at either 0.25 mg/kg or 1.0 mg/kg. Safety and efficacy were assessed at weeks 0, 2, 4, 12, and 24. In addition, skin biopsies were obtained at weeks 0 and 8 for immunohistochemistry studies and RNA sequencing analysis.
By week 12, the PASI 75 response rate in subcutaneous BI 655066 recipients was 87% and the PASI 90 rate was 58%. At week 24, nine patients elected to continue structured prospective follow-up while remaining off treatment, including six PASI 100 responders. Those six PASI 100 responders remained PASI 100 at ongoing follow-up 48-66 weeks after receiving their single dose of the agent.
Biopsy specimens obtained at week 8 showed normalization of the epidermal psoriasiform hyperplasia which had been present at baseline. A normal-looking granular layer had been reestablished. “This looks essentially like the pattern of normal or nonlesional skin,” according to the dermatologist.
RNA sequencing analysis and gene profiling showed normalized production of the IL-23/IL-17-induced proteins that had been strongly overexpressed at baseline, including lipocalin, beta-defensin, and psoriasin.
“The immune axis is turned down. The number of immune cells is way down, although they’re not completely eliminated. With placebo, you still see a psoriasislike pattern of the disease. With blockade of IL-23, most cases have a gene profile like nonlesional skin. This represents a profound cellular and disease modulation,” Dr. Krueger said.
Among all 39 participants, the only serious adverse event deemed possibly treatment related was a 5-minute transient ischemic attack (TIA) episode in a patient on BI 655066. This caught Dr. Krueger’s attention as a possible red flag; however, he noted that more than 200 patients have since received the biologic agent in the ongoing phase IIb trial, with no reported major adverse cardiovascular events.
“I think that TIA may just be bad luck with small numbers,” he added.
Asked what he thinks might explain the remarkably lengthy disease remission seen following a single dose of the biologic, Dr. Krueger offered two possibilities.
“It may be that IL-23 is necessary to sustain pathogenic clones of memory cells in the skin, and as we get rid of it those clones most likely apoptose. And if you’ve sufficiently removed the clones, then you don’t get the expansion. That’s guess one. Guess two would be that we’ve renormalized tolerance mechanisms in some way. Both of these hypotheses can be tested,” according to Dr. Krueger.
The study was funded by Boehringer Ingelheim. Dr. Krueger reported receiving funding from that pharmaceutical company and nearly two dozen others.
AMSTERDAM– The spectacular long-term efficacy achieved with a novel biologic agent for psoriasis in a first-in-humans, proof-of-concept study has raised the prospect of clinical outcomes continuing to ratchet higher in the treatment of moderate-to-severe chronic plaque psoriasis.
How much higher? Six of nine treated patients followed long-term have maintained a PASI 100 response – that is, completely clear – for up to 66 months after a single subcutaneous injection of the agent known for now as BI 655066, Dr. James G. Krueger reported at the annual congress of the European Academy of Dermatology and Venereology.
“For me, this is one of the most interesting features of this proof-of-concept study,” he added. “If this kind of activity is confirmed in the ongoing phase IIb trial, I think this represents the potential for very long-term disease modification. This could become an important agent in the future to treat psoriasis.”
BI 655066 is a monoclonal antibody that specifically targets the p19 subunit of interleukin (IL)-23. Unlike ustekinumab (Stelara), which blocks both IL-23 and IL-12, BI 655066 selectively blocks only IL-23, which Dr. Krueger believes is the central driving force in activating and sustaining the T-cell subsets responsible for the hyperproliferative and inflammatory reactions that define psoriasis.
“This study is all about testing for the specific pathogenic contribution of IL-23 to psoriasis in a first-in-humans study. Our findings really emphasize the importance of IL-23 in driving the key pathways of psoriasis,” observed Dr. Krueger, professor of investigative dermatology and director of the Milstein Medical Research Program at Rockefeller University, New York.
The study included 39 patients with moderate to severe plaque psoriasis. Their baseline PASI was 18, and they averaged more than a 20-year history of psoriasis. Twenty-four patients were randomized 3:1 to a single intravenous injection of BI 655066 at various doses ranging from 0.01 mg/kg to 5 mg/kg or to placebo in order to get an initial sense of the agent’s safety and tolerability.
In the second part of the study, 15 other participants received a single subcutaneous injection: two got placebo and the rest were randomized to BI 655066 at either 0.25 mg/kg or 1.0 mg/kg. Safety and efficacy were assessed at weeks 0, 2, 4, 12, and 24. In addition, skin biopsies were obtained at weeks 0 and 8 for immunohistochemistry studies and RNA sequencing analysis.
By week 12, the PASI 75 response rate in subcutaneous BI 655066 recipients was 87% and the PASI 90 rate was 58%. At week 24, nine patients elected to continue structured prospective follow-up while remaining off treatment, including six PASI 100 responders. Those six PASI 100 responders remained PASI 100 at ongoing follow-up 48-66 weeks after receiving their single dose of the agent.
Biopsy specimens obtained at week 8 showed normalization of the epidermal psoriasiform hyperplasia which had been present at baseline. A normal-looking granular layer had been reestablished. “This looks essentially like the pattern of normal or nonlesional skin,” according to the dermatologist.
RNA sequencing analysis and gene profiling showed normalized production of the IL-23/IL-17-induced proteins that had been strongly overexpressed at baseline, including lipocalin, beta-defensin, and psoriasin.
“The immune axis is turned down. The number of immune cells is way down, although they’re not completely eliminated. With placebo, you still see a psoriasislike pattern of the disease. With blockade of IL-23, most cases have a gene profile like nonlesional skin. This represents a profound cellular and disease modulation,” Dr. Krueger said.
Among all 39 participants, the only serious adverse event deemed possibly treatment related was a 5-minute transient ischemic attack (TIA) episode in a patient on BI 655066. This caught Dr. Krueger’s attention as a possible red flag; however, he noted that more than 200 patients have since received the biologic agent in the ongoing phase IIb trial, with no reported major adverse cardiovascular events.
“I think that TIA may just be bad luck with small numbers,” he added.
Asked what he thinks might explain the remarkably lengthy disease remission seen following a single dose of the biologic, Dr. Krueger offered two possibilities.
“It may be that IL-23 is necessary to sustain pathogenic clones of memory cells in the skin, and as we get rid of it those clones most likely apoptose. And if you’ve sufficiently removed the clones, then you don’t get the expansion. That’s guess one. Guess two would be that we’ve renormalized tolerance mechanisms in some way. Both of these hypotheses can be tested,” according to Dr. Krueger.
The study was funded by Boehringer Ingelheim. Dr. Krueger reported receiving funding from that pharmaceutical company and nearly two dozen others.
AT THE EADV CONGRESS
Key clinical point: Up to 66 months after receiving a single subcutaneous injection of a biologic agent that selectively blocks interleukin-23, six patients with moderate to severe chronic plaque psoriasis at baseline remained PASI 100 responders with clear skin.
Major finding: The PASI 75 response rate 12 weeks after receiving a single dose of the investigational agent BI 655066 was 87%, and the PASI 90 rate was 58%.
Data source: This was a first-in-humans, proof-of-concept study involving 39 psoriasis patients.
Disclosures: The study was sponsored by Boehringer Ingelheim. The presenter reported receiving research funding from that pharmaceutical company and nearly two dozen others.
Secukinumab showed sustained efficacy in psoriasis
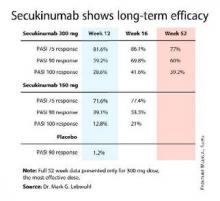
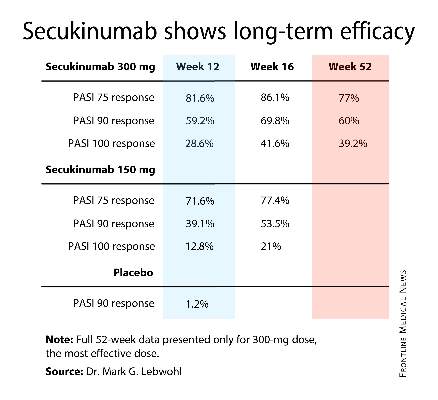
AMSTERDAM – The investigational biologic agent secukinumab continued to show strong efficacy through 52 weeks of treatment in a new secondary analysis of the pivotal phase III ERASURE trial.
For example, 60% of patients treated for moderate-to-severe chronic plaque psoriasis at the 300-mg dose of secukinumab continued to maintain a PASI 90 response through 52 weeks of therapy, Dr. Mark G. Lebwohl reported at the annual congress of the European Academy of Dermatology and Venereology.
Secukinumab is a fully human monoclonal antibody directed against interleukin-17A. While the Food and Drug Administration requested that the primary outcome in ERASURE (Efficacy of Response and Safety of Two Fixed Secukinumab Regimens in Psoriasis) should be the degree of skin clearing at week 12, the efficacy actually peaked at 16 weeks and was then sustained with only modest tailoff through the remainder of the 52-week study (see graphic).
This is important new information, Dr. Lebwohl noted, because 12-week outcomes don’t provide a full picture regarding potent therapies. Psoriasis is a chronic disease requiring long-term therapy, and some biologic agents now marketed for psoriasis tend to show a loss of effect over time. Reassuringly, the long-term ERASURE data show that’s not the case for secukinumab, explained Dr. Lebwohl, professor and chairman of the department of dermatology at Mt. Sinai Medical Center in New York.
ERASURE involved 738 patients randomized double-blind to secukinumab at either 150 mg or 300 mg, or to placebo. Following an initial loading-dose phase when the biologic was given subcutaneously once weekly for 5 weeks, it was then administered every 4 weeks for the remainder of the year-long trial.
The safety profile of secukinumab was similar to placebo with one exception: Upper respiratory tract infections were three- to fourfold more common in patients on the IL-17A inhibitor.
Novartis funded the ERASURE trial, whose primary results were recently published (N. Engl. J. Med. 2014;371:326-38). Dr. Lebwohl reported serving as a consultant to Novartis and more than a dozen other pharmaceutical companies.
AMSTERDAM – The investigational biologic agent secukinumab continued to show strong efficacy through 52 weeks of treatment in a new secondary analysis of the pivotal phase III ERASURE trial.
For example, 60% of patients treated for moderate-to-severe chronic plaque psoriasis at the 300-mg dose of secukinumab continued to maintain a PASI 90 response through 52 weeks of therapy, Dr. Mark G. Lebwohl reported at the annual congress of the European Academy of Dermatology and Venereology.
Secukinumab is a fully human monoclonal antibody directed against interleukin-17A. While the Food and Drug Administration requested that the primary outcome in ERASURE (Efficacy of Response and Safety of Two Fixed Secukinumab Regimens in Psoriasis) should be the degree of skin clearing at week 12, the efficacy actually peaked at 16 weeks and was then sustained with only modest tailoff through the remainder of the 52-week study (see graphic).
This is important new information, Dr. Lebwohl noted, because 12-week outcomes don’t provide a full picture regarding potent therapies. Psoriasis is a chronic disease requiring long-term therapy, and some biologic agents now marketed for psoriasis tend to show a loss of effect over time. Reassuringly, the long-term ERASURE data show that’s not the case for secukinumab, explained Dr. Lebwohl, professor and chairman of the department of dermatology at Mt. Sinai Medical Center in New York.
ERASURE involved 738 patients randomized double-blind to secukinumab at either 150 mg or 300 mg, or to placebo. Following an initial loading-dose phase when the biologic was given subcutaneously once weekly for 5 weeks, it was then administered every 4 weeks for the remainder of the year-long trial.
The safety profile of secukinumab was similar to placebo with one exception: Upper respiratory tract infections were three- to fourfold more common in patients on the IL-17A inhibitor.
Novartis funded the ERASURE trial, whose primary results were recently published (N. Engl. J. Med. 2014;371:326-38). Dr. Lebwohl reported serving as a consultant to Novartis and more than a dozen other pharmaceutical companies.
AMSTERDAM – The investigational biologic agent secukinumab continued to show strong efficacy through 52 weeks of treatment in a new secondary analysis of the pivotal phase III ERASURE trial.
For example, 60% of patients treated for moderate-to-severe chronic plaque psoriasis at the 300-mg dose of secukinumab continued to maintain a PASI 90 response through 52 weeks of therapy, Dr. Mark G. Lebwohl reported at the annual congress of the European Academy of Dermatology and Venereology.
Secukinumab is a fully human monoclonal antibody directed against interleukin-17A. While the Food and Drug Administration requested that the primary outcome in ERASURE (Efficacy of Response and Safety of Two Fixed Secukinumab Regimens in Psoriasis) should be the degree of skin clearing at week 12, the efficacy actually peaked at 16 weeks and was then sustained with only modest tailoff through the remainder of the 52-week study (see graphic).
This is important new information, Dr. Lebwohl noted, because 12-week outcomes don’t provide a full picture regarding potent therapies. Psoriasis is a chronic disease requiring long-term therapy, and some biologic agents now marketed for psoriasis tend to show a loss of effect over time. Reassuringly, the long-term ERASURE data show that’s not the case for secukinumab, explained Dr. Lebwohl, professor and chairman of the department of dermatology at Mt. Sinai Medical Center in New York.
ERASURE involved 738 patients randomized double-blind to secukinumab at either 150 mg or 300 mg, or to placebo. Following an initial loading-dose phase when the biologic was given subcutaneously once weekly for 5 weeks, it was then administered every 4 weeks for the remainder of the year-long trial.
The safety profile of secukinumab was similar to placebo with one exception: Upper respiratory tract infections were three- to fourfold more common in patients on the IL-17A inhibitor.
Novartis funded the ERASURE trial, whose primary results were recently published (N. Engl. J. Med. 2014;371:326-38). Dr. Lebwohl reported serving as a consultant to Novartis and more than a dozen other pharmaceutical companies.
AT THE EADV CONGRESS
Key clinical point: Psoriasis patients’ initial strong clinical response to the interleukin-17A inhibitor secukinumab is sustained through 52 weeks of therapy.
Major finding: Sixty percent of patients with moderate-to-severe chronic plaque psoriasis had a PASI 90 response sustained through a full year of treatment.
Data source: The 52-week long, double-blind, multicenter ERASURE trial randomized 738 patients with moderate-to-severe chronic plaque psoriasis to secukinumab at 150 mg or 300 mg, or to placebo.
Disclosures: Novartis funded the study. Dr. Lebwohl reported serving as a consultant to the company.
In psoriasis, is pushing for PASI 90 really worthwhile?
AMSTERDAM – Does pushing for a PASI 90 response instead of settling for a PASI 75 matter to patients being treated for moderate-to-severe chronic plaque psoriasis?
You bet it does, Dr. Mark G. Lebwohl asserted at the annual congress of the European Academy of Dermatology and Venereology.
He presented a pooled analysis of data from two large pivotal phase III randomized trials of secukinumab for psoriasis. The primary endpoints in the analysis were how often and how soon patients who achieved a PASI 75 or PASI 90 response at 12 weeks reported obtaining a Dermatology Life Quality Index (DLQI) response, defined as a score of 0 or 1.
The answer: More patients who had a PASI 90 response (meaning almost clear at 12 weeks) had a DLQI response, and it occurred a full 4 weeks faster than in PASI 75 responders – at a median of 8 weeks, compared with 12 weeks, reported Dr. Lebwohl, professor and chairman of the department of dermatology at Mt. Sinai Medical Center in New York.
Scores on the DLQI can range from 0, meaning no psoriasis-related impairment of the patient’s quality of life, up to 30. The average baseline score in this study population was 13.5, so a DLQI response dropping the score down to 0 or 1 represents a dramatic improvement in this patient-reported outcome.
Study participants completed the DLQI questionnaire at weeks 4, 8, 12, 24, 36, and again at week 52. The subjects’ mean baseline PASI score was 23.2.
The two double-blind, randomized, placebo-controlled clinical trials that formed the basis for this analysis were the recently published 52-week ERASURE (Efficacy of Response and Safety of Two Fixed Secukinumab Regimens in Psoriasis) and FIXTURE (Full Year Investigative Examination of Secukinumab vs. Etanercept Using Two Dosing Regimens to Determine Efficacy in Psoriasis) studies (N. Engl. J. Med. 2014;371:326-38), in which patients were assigned to secukinumab at a dose of either 150 mg or 300 mg, placebo, or in the case of FIXTURE, to etanercept. The PASI 75 and 90 response rates at 12 weeks were markedly higher at both doses of secukinumab than with etanercept.
Dr. Lebwohl’s pooled analysis was restricted to the 1,470 study participants in the two studies who were randomized to active therapy. A total of 612 patients achieved a PASI 90 response by week 12. Another 365 had a PASI 75 response. Fully 89% of PASI 90 responders also had a DLQI response maintained out to week 52, as did 77% of PASI 75 responders.
The key finding: The median time to a DLQI response in the PASI 90 responders was 8 weeks, compared with 12 weeks in the PASI 75 responders. Thus, patients with a PASI 90 response obtained virtually total relief from what had previously been a debilitating disease a full month sooner than PASI 75 responders. And that, as reported by the patients themselves, constitutes a meaningful advantage, Dr. Lebwohl stated.
Secukinumab is a fully human monoclonal antibody directed against a novel target: interleukin-17A. Novartis has filed for marketing approval of the biologic agent with an indication for psoriasis both with the Food and Drug Administration and European regulators. Secukinumab is also being developed as a treatment for psoriatic arthritis, rheumatoid arthritis, and ankylosing spondylitis.
Novartis funded the analysis. Dr. Lebwohl reported serving as a consultant to Novartis and more than a dozen other pharmaceutical companies.
AMSTERDAM – Does pushing for a PASI 90 response instead of settling for a PASI 75 matter to patients being treated for moderate-to-severe chronic plaque psoriasis?
You bet it does, Dr. Mark G. Lebwohl asserted at the annual congress of the European Academy of Dermatology and Venereology.
He presented a pooled analysis of data from two large pivotal phase III randomized trials of secukinumab for psoriasis. The primary endpoints in the analysis were how often and how soon patients who achieved a PASI 75 or PASI 90 response at 12 weeks reported obtaining a Dermatology Life Quality Index (DLQI) response, defined as a score of 0 or 1.
The answer: More patients who had a PASI 90 response (meaning almost clear at 12 weeks) had a DLQI response, and it occurred a full 4 weeks faster than in PASI 75 responders – at a median of 8 weeks, compared with 12 weeks, reported Dr. Lebwohl, professor and chairman of the department of dermatology at Mt. Sinai Medical Center in New York.
Scores on the DLQI can range from 0, meaning no psoriasis-related impairment of the patient’s quality of life, up to 30. The average baseline score in this study population was 13.5, so a DLQI response dropping the score down to 0 or 1 represents a dramatic improvement in this patient-reported outcome.
Study participants completed the DLQI questionnaire at weeks 4, 8, 12, 24, 36, and again at week 52. The subjects’ mean baseline PASI score was 23.2.
The two double-blind, randomized, placebo-controlled clinical trials that formed the basis for this analysis were the recently published 52-week ERASURE (Efficacy of Response and Safety of Two Fixed Secukinumab Regimens in Psoriasis) and FIXTURE (Full Year Investigative Examination of Secukinumab vs. Etanercept Using Two Dosing Regimens to Determine Efficacy in Psoriasis) studies (N. Engl. J. Med. 2014;371:326-38), in which patients were assigned to secukinumab at a dose of either 150 mg or 300 mg, placebo, or in the case of FIXTURE, to etanercept. The PASI 75 and 90 response rates at 12 weeks were markedly higher at both doses of secukinumab than with etanercept.
Dr. Lebwohl’s pooled analysis was restricted to the 1,470 study participants in the two studies who were randomized to active therapy. A total of 612 patients achieved a PASI 90 response by week 12. Another 365 had a PASI 75 response. Fully 89% of PASI 90 responders also had a DLQI response maintained out to week 52, as did 77% of PASI 75 responders.
The key finding: The median time to a DLQI response in the PASI 90 responders was 8 weeks, compared with 12 weeks in the PASI 75 responders. Thus, patients with a PASI 90 response obtained virtually total relief from what had previously been a debilitating disease a full month sooner than PASI 75 responders. And that, as reported by the patients themselves, constitutes a meaningful advantage, Dr. Lebwohl stated.
Secukinumab is a fully human monoclonal antibody directed against a novel target: interleukin-17A. Novartis has filed for marketing approval of the biologic agent with an indication for psoriasis both with the Food and Drug Administration and European regulators. Secukinumab is also being developed as a treatment for psoriatic arthritis, rheumatoid arthritis, and ankylosing spondylitis.
Novartis funded the analysis. Dr. Lebwohl reported serving as a consultant to Novartis and more than a dozen other pharmaceutical companies.
AMSTERDAM – Does pushing for a PASI 90 response instead of settling for a PASI 75 matter to patients being treated for moderate-to-severe chronic plaque psoriasis?
You bet it does, Dr. Mark G. Lebwohl asserted at the annual congress of the European Academy of Dermatology and Venereology.
He presented a pooled analysis of data from two large pivotal phase III randomized trials of secukinumab for psoriasis. The primary endpoints in the analysis were how often and how soon patients who achieved a PASI 75 or PASI 90 response at 12 weeks reported obtaining a Dermatology Life Quality Index (DLQI) response, defined as a score of 0 or 1.
The answer: More patients who had a PASI 90 response (meaning almost clear at 12 weeks) had a DLQI response, and it occurred a full 4 weeks faster than in PASI 75 responders – at a median of 8 weeks, compared with 12 weeks, reported Dr. Lebwohl, professor and chairman of the department of dermatology at Mt. Sinai Medical Center in New York.
Scores on the DLQI can range from 0, meaning no psoriasis-related impairment of the patient’s quality of life, up to 30. The average baseline score in this study population was 13.5, so a DLQI response dropping the score down to 0 or 1 represents a dramatic improvement in this patient-reported outcome.
Study participants completed the DLQI questionnaire at weeks 4, 8, 12, 24, 36, and again at week 52. The subjects’ mean baseline PASI score was 23.2.
The two double-blind, randomized, placebo-controlled clinical trials that formed the basis for this analysis were the recently published 52-week ERASURE (Efficacy of Response and Safety of Two Fixed Secukinumab Regimens in Psoriasis) and FIXTURE (Full Year Investigative Examination of Secukinumab vs. Etanercept Using Two Dosing Regimens to Determine Efficacy in Psoriasis) studies (N. Engl. J. Med. 2014;371:326-38), in which patients were assigned to secukinumab at a dose of either 150 mg or 300 mg, placebo, or in the case of FIXTURE, to etanercept. The PASI 75 and 90 response rates at 12 weeks were markedly higher at both doses of secukinumab than with etanercept.
Dr. Lebwohl’s pooled analysis was restricted to the 1,470 study participants in the two studies who were randomized to active therapy. A total of 612 patients achieved a PASI 90 response by week 12. Another 365 had a PASI 75 response. Fully 89% of PASI 90 responders also had a DLQI response maintained out to week 52, as did 77% of PASI 75 responders.
The key finding: The median time to a DLQI response in the PASI 90 responders was 8 weeks, compared with 12 weeks in the PASI 75 responders. Thus, patients with a PASI 90 response obtained virtually total relief from what had previously been a debilitating disease a full month sooner than PASI 75 responders. And that, as reported by the patients themselves, constitutes a meaningful advantage, Dr. Lebwohl stated.
Secukinumab is a fully human monoclonal antibody directed against a novel target: interleukin-17A. Novartis has filed for marketing approval of the biologic agent with an indication for psoriasis both with the Food and Drug Administration and European regulators. Secukinumab is also being developed as a treatment for psoriatic arthritis, rheumatoid arthritis, and ankylosing spondylitis.
Novartis funded the analysis. Dr. Lebwohl reported serving as a consultant to Novartis and more than a dozen other pharmaceutical companies.
AT THE EADV CONGRESS
Key clinical point: Psoriasis patients who obtain a PASI 90 response report the quality of life burden imposed by the disease is lifted a full month sooner than in those with a PASI 75 response.
Major finding: The median time to a patient-reported Dermatology Life Quality Index score of 0 or 1 on the 0-30 scale was 8 weeks in PASI 90 responders, compared with 12 weeks in PASI 75 responders.
Data source: A pooled analysis of the 1,470 patients with moderate-to-severe chronic plaque psoriasis who were assigned to active therapy with either secukinumab or etanercept in two pivotal phase III randomized trials.
Disclosures: Novartis funded the analysis. Dr. Lebwohl reported serving as a consultant to the company. <caps/>
Hard water linked to infant eczema
AMSTERDAM – Living in a locale with hard water – defined as a calcium carbonate concentration of 258 mg/L or more in the domestic water supply – was associated with significantly increased risk of eczema at 3 months of age in a large study conducted across England and Wales.
The study findings have potentially far-reaching implications for the prevention of childhood eczema, as well as halting what is often called the “atopic march” from eczema to food allergies to asthma. A national follow-up study is now being prepared in which families will be given an effective water softener machine when a child is born, with randomization to the machine being switched on or off.
“We want to see whether we might be able to prevent eczema by removing calcium carbonate from domestic water,” Dr. Carsten Flohr explained at a meeting of the European Task Force on Atopic Dermatitis held in conjunction with the annual congress of the European Academy of Dermatology and Venereology.
He reported on 1,303 infants who at 3 months of age underwent a structured skin examination with SCORAD (SCORing Atopic Dermatitis) measurement. Twenty-four percent were diagnosed as having eczema, which was typically mild.
Calcium carbonate concentrations in the local water supply vary enormously across England and Wales because rich limestone beds are found in some areas, notably the south and east, Dr. Flohr noted.
Babies living in areas with a calcium carbonate water concentration above the median level had a 45% increase in eczema risk. This risk was mediated by filaggrin loss-of-function (FLG) mutations. All study participants underwent testing for the six most common such mutations. In children without an FLG mutation, water hardness made little difference in eczema risk.
But FLG carriers living in the third quartile for water hardness – defined as a calcium carbonate concentration of 258-285 mg/L – had a 4.9-fold greater risk of eczema than did the general study population. Those in the highest quartile were at 2.67-fold increased risk, which just missed achieving statistical significance.
Looking at calcium carbonate concentration as a continuous variable, FLG carriers had an impressive 1% increase in the risk of eczema for each 1 mg/L rise in domestic water hardness, added Dr. Flohr, a pediatric dermatologist at St. Thomas’ Hospital, London.
The investigators also measured transepidermal water loss (TEWL) as an indicator of skin barrier function. FLG carriers without eczema living in hard-water areas had a high rate of excessive TEWL, defined as at least 15 g/m2 per hour. Those in the third quartile for calcium carbonate were at a 4.63-fold greater risk of abnormal TEWL, compared with FLG carriers living in areas in the two lowest quartiles of water hardness. The risk of excessive TEWL among FLG carriers without eczema rose by 1% for every 1 mg/L increase in calcium carbonate concentration.
“This suggests that the interaction between filaggrin mutation, water hardness, and eczema is related to a direct skin barrier effect,” Dr. Flohr said.
He and his coinvestigators have found supporting evidence in an adult population for their findings of a link between water hardness and infant eczema and TEWL. In a large national United Kingdom cohort of individuals born in 1958, who are part of an ongoing epidemiologic study, data show that those living in areas of high water hardness have increased rates of atopic disease.
As to the possible mechanism involved, Dr. Flohr said it’s known that as calcium carbonate concentration increases, it alters the skin surface pH. This could potentially have a detrimental effect on skin barrier function, although such an effect remains speculative. The attenuated risks of eczema and TEWL seen in FLG-carrying infants in the fourth water hardness quartile as compared to the third water hardness quartile warrants further study, but it might be the result of a calcium-scavenging effect, perhaps because of magnesium in the water, he added.
“I really think that the skin pH is involved in this,” Dr. Flohr emphasized.
Session cochair Dr. Ulf Darsow of Technical University, Munich, noted that Dr. Flohr’s countrymen at the University of Nottingham, England, have already performed an interventional study involving the use of water-softening devices. In the Softened Water Eczema Trial (SWET), the Nottingham group found no beneficial effect on pediatric eczema (PLoS Med. 2011; doi: 10.1371/journal.pmed.1000395).
“That is true, but it was a 12-week trial in children with established moderate to severe eczema,” Dr. Flohr replied. “What I’m suggesting is that potentially calcium carbonate concentration could be involved in the initiation of the skin inflammation, even if fitting a water softener once you’ve got established skin inflammation doesn’t seem to make much of a difference.”
“So this interesting story continues,” Dr. Darsow observed.
Dr. Flohr’s study was funded by the UK National Institute for Health Research. He reported having no financial conflicts.
AMSTERDAM – Living in a locale with hard water – defined as a calcium carbonate concentration of 258 mg/L or more in the domestic water supply – was associated with significantly increased risk of eczema at 3 months of age in a large study conducted across England and Wales.
The study findings have potentially far-reaching implications for the prevention of childhood eczema, as well as halting what is often called the “atopic march” from eczema to food allergies to asthma. A national follow-up study is now being prepared in which families will be given an effective water softener machine when a child is born, with randomization to the machine being switched on or off.
“We want to see whether we might be able to prevent eczema by removing calcium carbonate from domestic water,” Dr. Carsten Flohr explained at a meeting of the European Task Force on Atopic Dermatitis held in conjunction with the annual congress of the European Academy of Dermatology and Venereology.
He reported on 1,303 infants who at 3 months of age underwent a structured skin examination with SCORAD (SCORing Atopic Dermatitis) measurement. Twenty-four percent were diagnosed as having eczema, which was typically mild.
Calcium carbonate concentrations in the local water supply vary enormously across England and Wales because rich limestone beds are found in some areas, notably the south and east, Dr. Flohr noted.
Babies living in areas with a calcium carbonate water concentration above the median level had a 45% increase in eczema risk. This risk was mediated by filaggrin loss-of-function (FLG) mutations. All study participants underwent testing for the six most common such mutations. In children without an FLG mutation, water hardness made little difference in eczema risk.
But FLG carriers living in the third quartile for water hardness – defined as a calcium carbonate concentration of 258-285 mg/L – had a 4.9-fold greater risk of eczema than did the general study population. Those in the highest quartile were at 2.67-fold increased risk, which just missed achieving statistical significance.
Looking at calcium carbonate concentration as a continuous variable, FLG carriers had an impressive 1% increase in the risk of eczema for each 1 mg/L rise in domestic water hardness, added Dr. Flohr, a pediatric dermatologist at St. Thomas’ Hospital, London.
The investigators also measured transepidermal water loss (TEWL) as an indicator of skin barrier function. FLG carriers without eczema living in hard-water areas had a high rate of excessive TEWL, defined as at least 15 g/m2 per hour. Those in the third quartile for calcium carbonate were at a 4.63-fold greater risk of abnormal TEWL, compared with FLG carriers living in areas in the two lowest quartiles of water hardness. The risk of excessive TEWL among FLG carriers without eczema rose by 1% for every 1 mg/L increase in calcium carbonate concentration.
“This suggests that the interaction between filaggrin mutation, water hardness, and eczema is related to a direct skin barrier effect,” Dr. Flohr said.
He and his coinvestigators have found supporting evidence in an adult population for their findings of a link between water hardness and infant eczema and TEWL. In a large national United Kingdom cohort of individuals born in 1958, who are part of an ongoing epidemiologic study, data show that those living in areas of high water hardness have increased rates of atopic disease.
As to the possible mechanism involved, Dr. Flohr said it’s known that as calcium carbonate concentration increases, it alters the skin surface pH. This could potentially have a detrimental effect on skin barrier function, although such an effect remains speculative. The attenuated risks of eczema and TEWL seen in FLG-carrying infants in the fourth water hardness quartile as compared to the third water hardness quartile warrants further study, but it might be the result of a calcium-scavenging effect, perhaps because of magnesium in the water, he added.
“I really think that the skin pH is involved in this,” Dr. Flohr emphasized.
Session cochair Dr. Ulf Darsow of Technical University, Munich, noted that Dr. Flohr’s countrymen at the University of Nottingham, England, have already performed an interventional study involving the use of water-softening devices. In the Softened Water Eczema Trial (SWET), the Nottingham group found no beneficial effect on pediatric eczema (PLoS Med. 2011; doi: 10.1371/journal.pmed.1000395).
“That is true, but it was a 12-week trial in children with established moderate to severe eczema,” Dr. Flohr replied. “What I’m suggesting is that potentially calcium carbonate concentration could be involved in the initiation of the skin inflammation, even if fitting a water softener once you’ve got established skin inflammation doesn’t seem to make much of a difference.”
“So this interesting story continues,” Dr. Darsow observed.
Dr. Flohr’s study was funded by the UK National Institute for Health Research. He reported having no financial conflicts.
AMSTERDAM – Living in a locale with hard water – defined as a calcium carbonate concentration of 258 mg/L or more in the domestic water supply – was associated with significantly increased risk of eczema at 3 months of age in a large study conducted across England and Wales.
The study findings have potentially far-reaching implications for the prevention of childhood eczema, as well as halting what is often called the “atopic march” from eczema to food allergies to asthma. A national follow-up study is now being prepared in which families will be given an effective water softener machine when a child is born, with randomization to the machine being switched on or off.
“We want to see whether we might be able to prevent eczema by removing calcium carbonate from domestic water,” Dr. Carsten Flohr explained at a meeting of the European Task Force on Atopic Dermatitis held in conjunction with the annual congress of the European Academy of Dermatology and Venereology.
He reported on 1,303 infants who at 3 months of age underwent a structured skin examination with SCORAD (SCORing Atopic Dermatitis) measurement. Twenty-four percent were diagnosed as having eczema, which was typically mild.
Calcium carbonate concentrations in the local water supply vary enormously across England and Wales because rich limestone beds are found in some areas, notably the south and east, Dr. Flohr noted.
Babies living in areas with a calcium carbonate water concentration above the median level had a 45% increase in eczema risk. This risk was mediated by filaggrin loss-of-function (FLG) mutations. All study participants underwent testing for the six most common such mutations. In children without an FLG mutation, water hardness made little difference in eczema risk.
But FLG carriers living in the third quartile for water hardness – defined as a calcium carbonate concentration of 258-285 mg/L – had a 4.9-fold greater risk of eczema than did the general study population. Those in the highest quartile were at 2.67-fold increased risk, which just missed achieving statistical significance.
Looking at calcium carbonate concentration as a continuous variable, FLG carriers had an impressive 1% increase in the risk of eczema for each 1 mg/L rise in domestic water hardness, added Dr. Flohr, a pediatric dermatologist at St. Thomas’ Hospital, London.
The investigators also measured transepidermal water loss (TEWL) as an indicator of skin barrier function. FLG carriers without eczema living in hard-water areas had a high rate of excessive TEWL, defined as at least 15 g/m2 per hour. Those in the third quartile for calcium carbonate were at a 4.63-fold greater risk of abnormal TEWL, compared with FLG carriers living in areas in the two lowest quartiles of water hardness. The risk of excessive TEWL among FLG carriers without eczema rose by 1% for every 1 mg/L increase in calcium carbonate concentration.
“This suggests that the interaction between filaggrin mutation, water hardness, and eczema is related to a direct skin barrier effect,” Dr. Flohr said.
He and his coinvestigators have found supporting evidence in an adult population for their findings of a link between water hardness and infant eczema and TEWL. In a large national United Kingdom cohort of individuals born in 1958, who are part of an ongoing epidemiologic study, data show that those living in areas of high water hardness have increased rates of atopic disease.
As to the possible mechanism involved, Dr. Flohr said it’s known that as calcium carbonate concentration increases, it alters the skin surface pH. This could potentially have a detrimental effect on skin barrier function, although such an effect remains speculative. The attenuated risks of eczema and TEWL seen in FLG-carrying infants in the fourth water hardness quartile as compared to the third water hardness quartile warrants further study, but it might be the result of a calcium-scavenging effect, perhaps because of magnesium in the water, he added.
“I really think that the skin pH is involved in this,” Dr. Flohr emphasized.
Session cochair Dr. Ulf Darsow of Technical University, Munich, noted that Dr. Flohr’s countrymen at the University of Nottingham, England, have already performed an interventional study involving the use of water-softening devices. In the Softened Water Eczema Trial (SWET), the Nottingham group found no beneficial effect on pediatric eczema (PLoS Med. 2011; doi: 10.1371/journal.pmed.1000395).
“That is true, but it was a 12-week trial in children with established moderate to severe eczema,” Dr. Flohr replied. “What I’m suggesting is that potentially calcium carbonate concentration could be involved in the initiation of the skin inflammation, even if fitting a water softener once you’ve got established skin inflammation doesn’t seem to make much of a difference.”
“So this interesting story continues,” Dr. Darsow observed.
Dr. Flohr’s study was funded by the UK National Institute for Health Research. He reported having no financial conflicts.
AT THE EADV CONGRESS
Key clinical point: Infants living in areas with a high calcium carbonate concentration in the water supply are at increased risk for eczema, especially if they carry a filaggrin loss-of-function gene mutation.
Major finding: British 3-month-olds with a filaggrin mutation are at up to nearly fivefold increased likelihood of having eczema if they live in an area with above-average water hardness, compared with those in the lowest quartile in terms of water hardness.
Data source: A cross-sectional study of more than 1,300 infants across England and Wales who underwent a structured skin examination looking for eczema at age 3 months.
Disclosures: The study was funded by the UK National Institute for Health Research. The presenter reported having no financial conflicts.
Rosacea’s Comorbidities Are More Than Skin Deep
AMSTERDAM – Rosacea is associated with increased risk of a range of chronic systemic diseases, including allergies and urogential disorders, a case-control study showed.
The common denominator among this linked diverse collection of diseases is probably underlying systemic inflammation, Dr. Barbara M. Rainer explained at the annual congress of the European Academy of Dermatology and Venereology. But regardless of the pathophysiologic mechanisms at work, the important thing is that physicians be on the lookout for these comorbid conditions in their patients with rosacea.
Dr. Rainer of Johns Hopkins University in Baltimore presented a case-control study involving 130 subjects: 65 rosacea patients and an equal number of controls matched for age, sex, and race.
The most common comorbidity was food allergies (odds ratio, 10), followed by urogenital disorders (OR, 7.5).
The rosacea patients averaged 50 years of age and had a mean 11.8-year history of their skin disease. Body mass index, smoking status, alcohol intake, and coffee consumption were similar in cases and controls. Two-thirds of subjects were women. Relative risks for comorbid conditions were calculated using logistic regression analysis.
Dr. Rainer reported no relevant financial conflicts.
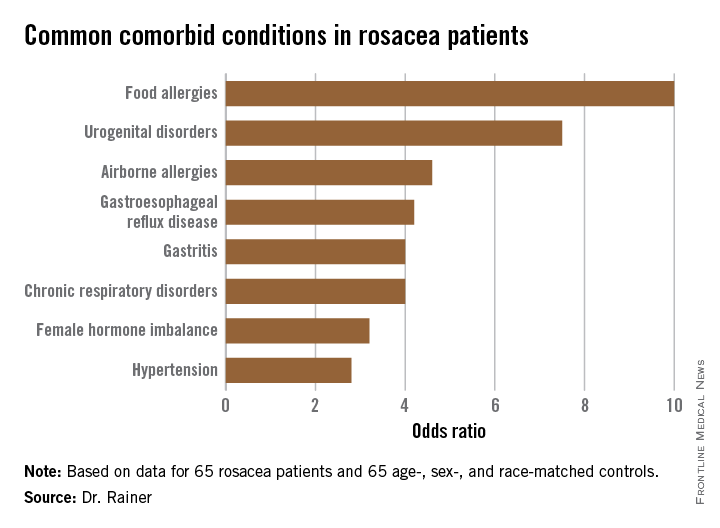
AMSTERDAM – Rosacea is associated with increased risk of a range of chronic systemic diseases, including allergies and urogential disorders, a case-control study showed.
The common denominator among this linked diverse collection of diseases is probably underlying systemic inflammation, Dr. Barbara M. Rainer explained at the annual congress of the European Academy of Dermatology and Venereology. But regardless of the pathophysiologic mechanisms at work, the important thing is that physicians be on the lookout for these comorbid conditions in their patients with rosacea.
Dr. Rainer of Johns Hopkins University in Baltimore presented a case-control study involving 130 subjects: 65 rosacea patients and an equal number of controls matched for age, sex, and race.
The most common comorbidity was food allergies (odds ratio, 10), followed by urogenital disorders (OR, 7.5).
The rosacea patients averaged 50 years of age and had a mean 11.8-year history of their skin disease. Body mass index, smoking status, alcohol intake, and coffee consumption were similar in cases and controls. Two-thirds of subjects were women. Relative risks for comorbid conditions were calculated using logistic regression analysis.
Dr. Rainer reported no relevant financial conflicts.

AMSTERDAM – Rosacea is associated with increased risk of a range of chronic systemic diseases, including allergies and urogential disorders, a case-control study showed.
The common denominator among this linked diverse collection of diseases is probably underlying systemic inflammation, Dr. Barbara M. Rainer explained at the annual congress of the European Academy of Dermatology and Venereology. But regardless of the pathophysiologic mechanisms at work, the important thing is that physicians be on the lookout for these comorbid conditions in their patients with rosacea.
Dr. Rainer of Johns Hopkins University in Baltimore presented a case-control study involving 130 subjects: 65 rosacea patients and an equal number of controls matched for age, sex, and race.
The most common comorbidity was food allergies (odds ratio, 10), followed by urogenital disorders (OR, 7.5).
The rosacea patients averaged 50 years of age and had a mean 11.8-year history of their skin disease. Body mass index, smoking status, alcohol intake, and coffee consumption were similar in cases and controls. Two-thirds of subjects were women. Relative risks for comorbid conditions were calculated using logistic regression analysis.
Dr. Rainer reported no relevant financial conflicts.

AT THE EADV CONGRESS
Rosacea’s comorbidities are more than skin deep
AMSTERDAM – Rosacea is associated with increased risk of a range of chronic systemic diseases, including allergies and urogential disorders, a case-control study showed.
The common denominator among this linked diverse collection of diseases is probably underlying systemic inflammation, Dr. Barbara M. Rainer explained at the annual congress of the European Academy of Dermatology and Venereology. But regardless of the pathophysiologic mechanisms at work, the important thing is that physicians be on the lookout for these comorbid conditions in their patients with rosacea.
Dr. Rainer of Johns Hopkins University in Baltimore presented a case-control study involving 130 subjects: 65 rosacea patients and an equal number of controls matched for age, sex, and race.
The most common comorbidity was food allergies (odds ratio, 10), followed by urogenital disorders (OR, 7.5).
The rosacea patients averaged 50 years of age and had a mean 11.8-year history of their skin disease. Body mass index, smoking status, alcohol intake, and coffee consumption were similar in cases and controls. Two-thirds of subjects were women. Relative risks for comorbid conditions were calculated using logistic regression analysis.
Dr. Rainer reported no relevant financial conflicts.
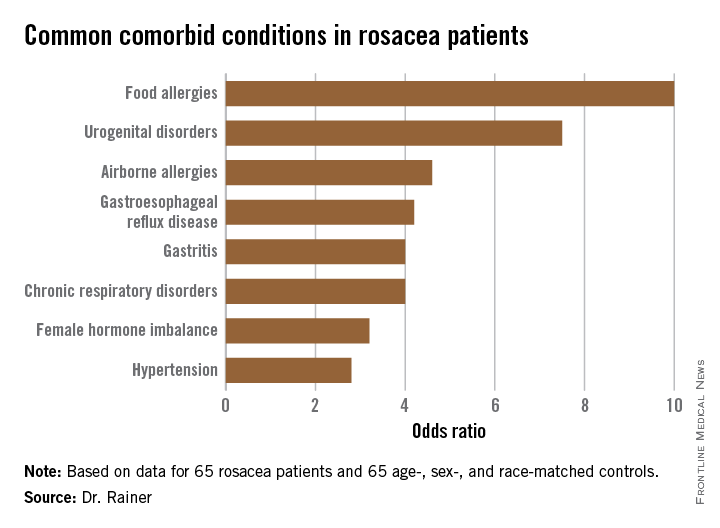
AMSTERDAM – Rosacea is associated with increased risk of a range of chronic systemic diseases, including allergies and urogential disorders, a case-control study showed.
The common denominator among this linked diverse collection of diseases is probably underlying systemic inflammation, Dr. Barbara M. Rainer explained at the annual congress of the European Academy of Dermatology and Venereology. But regardless of the pathophysiologic mechanisms at work, the important thing is that physicians be on the lookout for these comorbid conditions in their patients with rosacea.
Dr. Rainer of Johns Hopkins University in Baltimore presented a case-control study involving 130 subjects: 65 rosacea patients and an equal number of controls matched for age, sex, and race.
The most common comorbidity was food allergies (odds ratio, 10), followed by urogenital disorders (OR, 7.5).
The rosacea patients averaged 50 years of age and had a mean 11.8-year history of their skin disease. Body mass index, smoking status, alcohol intake, and coffee consumption were similar in cases and controls. Two-thirds of subjects were women. Relative risks for comorbid conditions were calculated using logistic regression analysis.
Dr. Rainer reported no relevant financial conflicts.

AMSTERDAM – Rosacea is associated with increased risk of a range of chronic systemic diseases, including allergies and urogential disorders, a case-control study showed.
The common denominator among this linked diverse collection of diseases is probably underlying systemic inflammation, Dr. Barbara M. Rainer explained at the annual congress of the European Academy of Dermatology and Venereology. But regardless of the pathophysiologic mechanisms at work, the important thing is that physicians be on the lookout for these comorbid conditions in their patients with rosacea.
Dr. Rainer of Johns Hopkins University in Baltimore presented a case-control study involving 130 subjects: 65 rosacea patients and an equal number of controls matched for age, sex, and race.
The most common comorbidity was food allergies (odds ratio, 10), followed by urogenital disorders (OR, 7.5).
The rosacea patients averaged 50 years of age and had a mean 11.8-year history of their skin disease. Body mass index, smoking status, alcohol intake, and coffee consumption were similar in cases and controls. Two-thirds of subjects were women. Relative risks for comorbid conditions were calculated using logistic regression analysis.
Dr. Rainer reported no relevant financial conflicts.

AT THE EADV CONGRESS
Key clinical point: Rosacea patients are at increased risk for an eclectic variety of chronic systemic comorbid conditions.
Major finding: The strongest associations seen with rosacea were for food allergies, with a 10-fold increased risk, and urogenital disorders, with a 7.5-fold relative risk.
Data source: A case-control study including 65 rosacea patients and an equal number of matched controls.
Disclosures: Dr. Rainer reported no relevant financial conflicts.