User login
Semaglutide compares well with sitagliptin
NEW ORLEANS – Semaglutide, a GLP-1 agonist for type 2 diabetes that’s dosed weekly, was superior to daily sitagliptin in improving glycemic control and reducing body weight in people who are also on metformin and/or thiazolidinediones (TZDs), based on results from a phase III trial. But while the serious adverse event profile was similar for both treatments, far more patients on semaglutide discontinued treatment because of adverse events.
The SUSTAIN study includes more than 8,000 patients with type 2 diabetes. The results are the basis for a new drug application filed in December with the Food and Drug Administration by the investigational drug’s manufacturer, Novo Nordisk, which made the announcement in a press release.
“The SUSTAIN 2 trial has shown that semaglutide at both doses, 0.5 and 1 mg, is superior at improving glycemic control in subjects with type 2 diabetes, compared with sitagliptin, and showed a reduction of 1.3% and 1.6%, respectively, from the baseline HbA1c of 8.1%,” Dr. Ahrén said. For comparison, the sitagliptin group showed an average HbA1c reduction of 0.5%, he said.
The treatments were well tolerated with no new safety concerns, Dr. Ahrén said. “As expected, semaglutide caused more gastrointestinal adverse events, but the frequency was similar to those reported with other GLP-1 receptor agonists,” he said.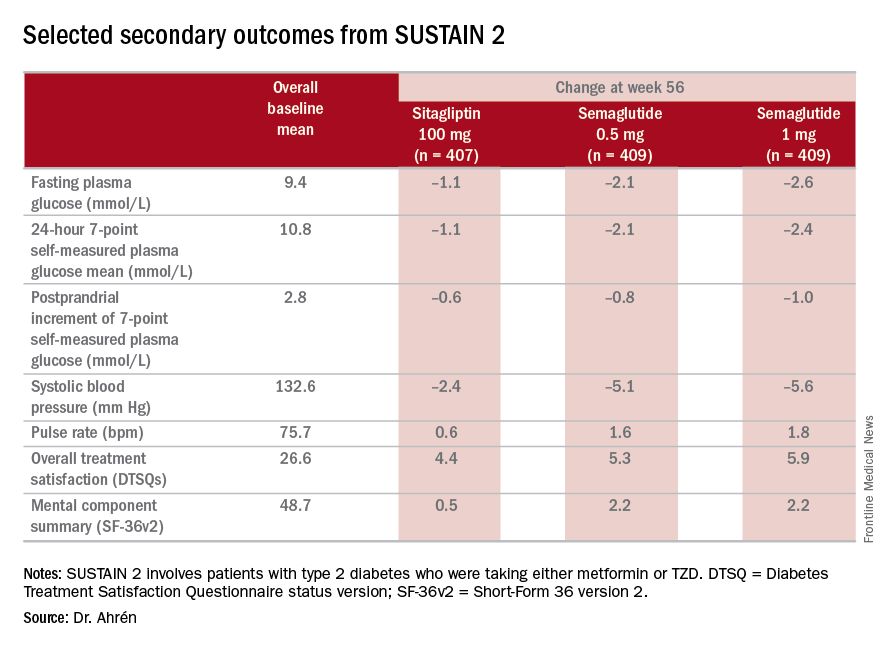
The study’s investigators also looked at a composite endpoint of HbA1c less than 7% without symptomatic hypoglycemia and no weight gain, Dr. Ahrén said, achieved by 63% on 0.5 mg and 74% on 1 mg of semaglutide vs. 27% of the sitagliptin group.
The serious adverse event (AE) profile was similar in all three groups: 7.3% in both semaglutide groups and 7.1% in the sitagliptin group. However, far more patients on semaglutide discontinued treatment because of AEs: 8.1% and 9.5% on 0.5 and 1 mg, respectively, vs. 2.9% on sitagliptin. Gastrointestinal AEs in all groups were 43.5% and 39.9% in the 0.5- and 1-mg semaglutide groups, respectively, and 23.6% in the sitagliptin group.
Six deaths were reported in the study population, Dr. Ahrén said: two on 0.5-mg semaglutide dosing, one on the 1-mg dosing, and three on sitagliptin.
Hypoglycemia rates were also “very low,” he said, with 14 patients overall having reported it; seven on 0.5-mg semaglutide therapy and two in the 1-mg group, and five on sitagliptin, “So there were no increased risks for hypoglycemia with semaglutide.”
Dr. Ahrén disclosed relationships with Novo Nordisk and several other drug companies.
NEW ORLEANS – Semaglutide, a GLP-1 agonist for type 2 diabetes that’s dosed weekly, was superior to daily sitagliptin in improving glycemic control and reducing body weight in people who are also on metformin and/or thiazolidinediones (TZDs), based on results from a phase III trial. But while the serious adverse event profile was similar for both treatments, far more patients on semaglutide discontinued treatment because of adverse events.
The SUSTAIN study includes more than 8,000 patients with type 2 diabetes. The results are the basis for a new drug application filed in December with the Food and Drug Administration by the investigational drug’s manufacturer, Novo Nordisk, which made the announcement in a press release.
“The SUSTAIN 2 trial has shown that semaglutide at both doses, 0.5 and 1 mg, is superior at improving glycemic control in subjects with type 2 diabetes, compared with sitagliptin, and showed a reduction of 1.3% and 1.6%, respectively, from the baseline HbA1c of 8.1%,” Dr. Ahrén said. For comparison, the sitagliptin group showed an average HbA1c reduction of 0.5%, he said.
The treatments were well tolerated with no new safety concerns, Dr. Ahrén said. “As expected, semaglutide caused more gastrointestinal adverse events, but the frequency was similar to those reported with other GLP-1 receptor agonists,” he said.
The study’s investigators also looked at a composite endpoint of HbA1c less than 7% without symptomatic hypoglycemia and no weight gain, Dr. Ahrén said, achieved by 63% on 0.5 mg and 74% on 1 mg of semaglutide vs. 27% of the sitagliptin group.
The serious adverse event (AE) profile was similar in all three groups: 7.3% in both semaglutide groups and 7.1% in the sitagliptin group. However, far more patients on semaglutide discontinued treatment because of AEs: 8.1% and 9.5% on 0.5 and 1 mg, respectively, vs. 2.9% on sitagliptin. Gastrointestinal AEs in all groups were 43.5% and 39.9% in the 0.5- and 1-mg semaglutide groups, respectively, and 23.6% in the sitagliptin group.
Six deaths were reported in the study population, Dr. Ahrén said: two on 0.5-mg semaglutide dosing, one on the 1-mg dosing, and three on sitagliptin.
Hypoglycemia rates were also “very low,” he said, with 14 patients overall having reported it; seven on 0.5-mg semaglutide therapy and two in the 1-mg group, and five on sitagliptin, “So there were no increased risks for hypoglycemia with semaglutide.”
Dr. Ahrén disclosed relationships with Novo Nordisk and several other drug companies.
NEW ORLEANS – Semaglutide, a GLP-1 agonist for type 2 diabetes that’s dosed weekly, was superior to daily sitagliptin in improving glycemic control and reducing body weight in people who are also on metformin and/or thiazolidinediones (TZDs), based on results from a phase III trial. But while the serious adverse event profile was similar for both treatments, far more patients on semaglutide discontinued treatment because of adverse events.
The SUSTAIN study includes more than 8,000 patients with type 2 diabetes. The results are the basis for a new drug application filed in December with the Food and Drug Administration by the investigational drug’s manufacturer, Novo Nordisk, which made the announcement in a press release.
“The SUSTAIN 2 trial has shown that semaglutide at both doses, 0.5 and 1 mg, is superior at improving glycemic control in subjects with type 2 diabetes, compared with sitagliptin, and showed a reduction of 1.3% and 1.6%, respectively, from the baseline HbA1c of 8.1%,” Dr. Ahrén said. For comparison, the sitagliptin group showed an average HbA1c reduction of 0.5%, he said.
The treatments were well tolerated with no new safety concerns, Dr. Ahrén said. “As expected, semaglutide caused more gastrointestinal adverse events, but the frequency was similar to those reported with other GLP-1 receptor agonists,” he said.
The study’s investigators also looked at a composite endpoint of HbA1c less than 7% without symptomatic hypoglycemia and no weight gain, Dr. Ahrén said, achieved by 63% on 0.5 mg and 74% on 1 mg of semaglutide vs. 27% of the sitagliptin group.
The serious adverse event (AE) profile was similar in all three groups: 7.3% in both semaglutide groups and 7.1% in the sitagliptin group. However, far more patients on semaglutide discontinued treatment because of AEs: 8.1% and 9.5% on 0.5 and 1 mg, respectively, vs. 2.9% on sitagliptin. Gastrointestinal AEs in all groups were 43.5% and 39.9% in the 0.5- and 1-mg semaglutide groups, respectively, and 23.6% in the sitagliptin group.
Six deaths were reported in the study population, Dr. Ahrén said: two on 0.5-mg semaglutide dosing, one on the 1-mg dosing, and three on sitagliptin.
Hypoglycemia rates were also “very low,” he said, with 14 patients overall having reported it; seven on 0.5-mg semaglutide therapy and two in the 1-mg group, and five on sitagliptin, “So there were no increased risks for hypoglycemia with semaglutide.”
Dr. Ahrén disclosed relationships with Novo Nordisk and several other drug companies.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point: Investigators for a phase III trial have found weekly semaglutide superior to daily sitagliptin as add-on therapy for improving glycemic control and reducing body weight in type 2 diabetes.
Major finding: Semaglutide 0.5 and 1 mg showed a reduction of 1.3% and 1.6%, respectively, from the baseline HbA1c, compared with an average reduction of 0.5% for sitagliptin.
Data source: SUSTAIN 2 double-blind, randomized trial of 1,231 patients with type 2 diabetes taking either metformin or thiazolidinediones.
Disclosures: Dr. Ahrén disclosed relationships with Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Merck, Novartis, Novo Nordisk, and Sanofi-Aventis Deutschland.
Type 2 diabetes in youth needs new treatment options
NEW ORLEANS – For adolescents and children with type 2 diabetes, there aren’t a lot of therapeutic options other than insulin and metformin. And the situation isn’t likely to change without extraordinary collaboration, Kristen J. Nadeau, MD, research director of the department of pediatric endocrinology at Children’s Hospital Colorado, Colorado Springs, said at the annual scientific sessions of the American Diabetes Association.
“Type 2 diabetes in youth appears to differ not only from pediatric type 1 diabetes, but also from adult type 2 diabetes, and current treatment options are limited,” Dr. Nadeau said. The estimated number of type 2 diabetes cases in the United States per year stands at 1,469,000 cases (12.3 per 100) in adults, compared with 5,100 (0.5 per 100) in youth. In adults there is a slight male predominance, whereas in kids girls are almost twice as likely as boys to be affected. Moreover, beta cell function declines faster in youth with type 2 diabetes.
The majority of insulins used by adults with type 2 diabetes are also approved for use in children and adolescents, but the only non-insulin medication approved for youth is metformin. According to Dr. Nadeau, 11 clinical safety and efficacy studies and 3 pharmacokinetic studies are ongoing for four DPP-4 inhibitors, two GLP-1 analogs, three SGLT2 inhibitors, colesevelam, bromocriptine, and insulins. A total of 5,000 youth are needed to complete current and planned trials, which “would require 100% participation from every child diagnosed in the next year, which is not feasible,” she said.
The required safety and efficacy studies are too difficult “because of the combination of unique challenges of the target population, study design concerns, and a lack of collaboration between agencies,” Dr. Nadeau said during a session that focused on the conclusions of the American Diabetes Association’s consensus conference on youth with type 2 diabetes, which took place on Oct. 20, 2015 in Alexandria, Va.
The consensus report was published online in Diabetes Care, and addresses the current status of type 2 diabetes in youth, the challenges of treatment, and priorities for research. Dr. Nadeau co-chaired the effort along with Dr. Philip Zeitler, section head of pediatric endocrinology at Children’s Hospital Colorado and medical director of the Children’s Hospital Colorado Clinical and Translational Research Center, Denver. Collaborators included the American Academy of Pediatrics, the International Society for Pediatric and Adolescent Diabetes, and the Pediatric Endocrine Society.
One example of the research challenges is evident in data from the Today trial, which found that only about 39% of kids with type 2 diabetes live with both parents (J Clin Endocrinol Metab. 2011 Jan; 96[1]:159-67). “Whenever you have only one parent in the home, there are difficulties with transportation by definition, so it’s a lot harder for these kids to participate in studies,” Dr. Nadeau said. “In addition, only 17% of their parents had a college or advanced education and 41% had a household income of less than $25,000 per year.”
The social environment is critical, she continued, because the lifestyle factors associated with type 2 diabetes often result in poor outcomes. “It’s very hard to make lifestyle changes if there is a socioeconomic challenge,” she said. “We can’t make change without understanding the community and culture that these youth live in. It’s also critical that we have participation of minorities and other research participants with diverse backgrounds in order for [clinical] trials to be effective for the population that this disease is affecting.”
Another issue keeping drug trials of youth with type 2 diabetes from being completed is the entry criteria. Some studies require youth to be drug naive and have a hemoglobin A1c greater than 7%. “This is difficult, because many youth that are referred to our diabetes center already come in on metformin, leaving only about 7% of subjects available for this criteria,” Dr. Nadeau explained. Another common study entry criterion is being on metformin and having a hemoglobin A1c of about 7%, “so basically being a metformin failure,” she said. “This is difficult to meet because metformin is relatively effective in the early stages of diabetes.”
“We need clear strategies for research, prevention, and treatment. Clarifying unique pathophysiology, complications, and psychosocial impact will enable industry, academia, funding agencies, advocacy groups, and regulators to collectively evaluate the best approaches to research, treatment, and prevention,” Dr. Nadeau said.
The consensus conference participants recommended the following objectives: clarify the biology of type 2 diabetes in youth, obtain new pediatric information on drugs, encourage the use of appropriate medications, and inform clinical decision-making. “We have a desperate need to understand the actions of drugs in type 2 diabetes youth,” Dr. Nadeau said. “Our current approach is not working. Potential solutions include considering efficacy outcomes besides A1c, potentially looking at improvement in insulin sensitivity, preservation of beta-cell function, trying to prevent the A1c increase instead of looking for an A1c reduction, and trying to extrapolate from effects in adults, if we can understand enough to do that.”
The conference participants also called for infrastructure changes, such as creating a resource for patients with type 2 diabetes in the model of the Type 1 Diabetes Exchange. “We need to have collaborations internationally,” she said. “We also need support for teams and clinical groups to work together to be able to accomplish these collaboratively.”
Dr. Nadeau reported having no financial disclosures.
NEW ORLEANS – For adolescents and children with type 2 diabetes, there aren’t a lot of therapeutic options other than insulin and metformin. And the situation isn’t likely to change without extraordinary collaboration, Kristen J. Nadeau, MD, research director of the department of pediatric endocrinology at Children’s Hospital Colorado, Colorado Springs, said at the annual scientific sessions of the American Diabetes Association.
“Type 2 diabetes in youth appears to differ not only from pediatric type 1 diabetes, but also from adult type 2 diabetes, and current treatment options are limited,” Dr. Nadeau said. The estimated number of type 2 diabetes cases in the United States per year stands at 1,469,000 cases (12.3 per 100) in adults, compared with 5,100 (0.5 per 100) in youth. In adults there is a slight male predominance, whereas in kids girls are almost twice as likely as boys to be affected. Moreover, beta cell function declines faster in youth with type 2 diabetes.
The majority of insulins used by adults with type 2 diabetes are also approved for use in children and adolescents, but the only non-insulin medication approved for youth is metformin. According to Dr. Nadeau, 11 clinical safety and efficacy studies and 3 pharmacokinetic studies are ongoing for four DPP-4 inhibitors, two GLP-1 analogs, three SGLT2 inhibitors, colesevelam, bromocriptine, and insulins. A total of 5,000 youth are needed to complete current and planned trials, which “would require 100% participation from every child diagnosed in the next year, which is not feasible,” she said.
The required safety and efficacy studies are too difficult “because of the combination of unique challenges of the target population, study design concerns, and a lack of collaboration between agencies,” Dr. Nadeau said during a session that focused on the conclusions of the American Diabetes Association’s consensus conference on youth with type 2 diabetes, which took place on Oct. 20, 2015 in Alexandria, Va.
The consensus report was published online in Diabetes Care, and addresses the current status of type 2 diabetes in youth, the challenges of treatment, and priorities for research. Dr. Nadeau co-chaired the effort along with Dr. Philip Zeitler, section head of pediatric endocrinology at Children’s Hospital Colorado and medical director of the Children’s Hospital Colorado Clinical and Translational Research Center, Denver. Collaborators included the American Academy of Pediatrics, the International Society for Pediatric and Adolescent Diabetes, and the Pediatric Endocrine Society.
One example of the research challenges is evident in data from the Today trial, which found that only about 39% of kids with type 2 diabetes live with both parents (J Clin Endocrinol Metab. 2011 Jan; 96[1]:159-67). “Whenever you have only one parent in the home, there are difficulties with transportation by definition, so it’s a lot harder for these kids to participate in studies,” Dr. Nadeau said. “In addition, only 17% of their parents had a college or advanced education and 41% had a household income of less than $25,000 per year.”
The social environment is critical, she continued, because the lifestyle factors associated with type 2 diabetes often result in poor outcomes. “It’s very hard to make lifestyle changes if there is a socioeconomic challenge,” she said. “We can’t make change without understanding the community and culture that these youth live in. It’s also critical that we have participation of minorities and other research participants with diverse backgrounds in order for [clinical] trials to be effective for the population that this disease is affecting.”
Another issue keeping drug trials of youth with type 2 diabetes from being completed is the entry criteria. Some studies require youth to be drug naive and have a hemoglobin A1c greater than 7%. “This is difficult, because many youth that are referred to our diabetes center already come in on metformin, leaving only about 7% of subjects available for this criteria,” Dr. Nadeau explained. Another common study entry criterion is being on metformin and having a hemoglobin A1c of about 7%, “so basically being a metformin failure,” she said. “This is difficult to meet because metformin is relatively effective in the early stages of diabetes.”
“We need clear strategies for research, prevention, and treatment. Clarifying unique pathophysiology, complications, and psychosocial impact will enable industry, academia, funding agencies, advocacy groups, and regulators to collectively evaluate the best approaches to research, treatment, and prevention,” Dr. Nadeau said.
The consensus conference participants recommended the following objectives: clarify the biology of type 2 diabetes in youth, obtain new pediatric information on drugs, encourage the use of appropriate medications, and inform clinical decision-making. “We have a desperate need to understand the actions of drugs in type 2 diabetes youth,” Dr. Nadeau said. “Our current approach is not working. Potential solutions include considering efficacy outcomes besides A1c, potentially looking at improvement in insulin sensitivity, preservation of beta-cell function, trying to prevent the A1c increase instead of looking for an A1c reduction, and trying to extrapolate from effects in adults, if we can understand enough to do that.”
The conference participants also called for infrastructure changes, such as creating a resource for patients with type 2 diabetes in the model of the Type 1 Diabetes Exchange. “We need to have collaborations internationally,” she said. “We also need support for teams and clinical groups to work together to be able to accomplish these collaboratively.”
Dr. Nadeau reported having no financial disclosures.
NEW ORLEANS – For adolescents and children with type 2 diabetes, there aren’t a lot of therapeutic options other than insulin and metformin. And the situation isn’t likely to change without extraordinary collaboration, Kristen J. Nadeau, MD, research director of the department of pediatric endocrinology at Children’s Hospital Colorado, Colorado Springs, said at the annual scientific sessions of the American Diabetes Association.
“Type 2 diabetes in youth appears to differ not only from pediatric type 1 diabetes, but also from adult type 2 diabetes, and current treatment options are limited,” Dr. Nadeau said. The estimated number of type 2 diabetes cases in the United States per year stands at 1,469,000 cases (12.3 per 100) in adults, compared with 5,100 (0.5 per 100) in youth. In adults there is a slight male predominance, whereas in kids girls are almost twice as likely as boys to be affected. Moreover, beta cell function declines faster in youth with type 2 diabetes.
The majority of insulins used by adults with type 2 diabetes are also approved for use in children and adolescents, but the only non-insulin medication approved for youth is metformin. According to Dr. Nadeau, 11 clinical safety and efficacy studies and 3 pharmacokinetic studies are ongoing for four DPP-4 inhibitors, two GLP-1 analogs, three SGLT2 inhibitors, colesevelam, bromocriptine, and insulins. A total of 5,000 youth are needed to complete current and planned trials, which “would require 100% participation from every child diagnosed in the next year, which is not feasible,” she said.
The required safety and efficacy studies are too difficult “because of the combination of unique challenges of the target population, study design concerns, and a lack of collaboration between agencies,” Dr. Nadeau said during a session that focused on the conclusions of the American Diabetes Association’s consensus conference on youth with type 2 diabetes, which took place on Oct. 20, 2015 in Alexandria, Va.
The consensus report was published online in Diabetes Care, and addresses the current status of type 2 diabetes in youth, the challenges of treatment, and priorities for research. Dr. Nadeau co-chaired the effort along with Dr. Philip Zeitler, section head of pediatric endocrinology at Children’s Hospital Colorado and medical director of the Children’s Hospital Colorado Clinical and Translational Research Center, Denver. Collaborators included the American Academy of Pediatrics, the International Society for Pediatric and Adolescent Diabetes, and the Pediatric Endocrine Society.
One example of the research challenges is evident in data from the Today trial, which found that only about 39% of kids with type 2 diabetes live with both parents (J Clin Endocrinol Metab. 2011 Jan; 96[1]:159-67). “Whenever you have only one parent in the home, there are difficulties with transportation by definition, so it’s a lot harder for these kids to participate in studies,” Dr. Nadeau said. “In addition, only 17% of their parents had a college or advanced education and 41% had a household income of less than $25,000 per year.”
The social environment is critical, she continued, because the lifestyle factors associated with type 2 diabetes often result in poor outcomes. “It’s very hard to make lifestyle changes if there is a socioeconomic challenge,” she said. “We can’t make change without understanding the community and culture that these youth live in. It’s also critical that we have participation of minorities and other research participants with diverse backgrounds in order for [clinical] trials to be effective for the population that this disease is affecting.”
Another issue keeping drug trials of youth with type 2 diabetes from being completed is the entry criteria. Some studies require youth to be drug naive and have a hemoglobin A1c greater than 7%. “This is difficult, because many youth that are referred to our diabetes center already come in on metformin, leaving only about 7% of subjects available for this criteria,” Dr. Nadeau explained. Another common study entry criterion is being on metformin and having a hemoglobin A1c of about 7%, “so basically being a metformin failure,” she said. “This is difficult to meet because metformin is relatively effective in the early stages of diabetes.”
“We need clear strategies for research, prevention, and treatment. Clarifying unique pathophysiology, complications, and psychosocial impact will enable industry, academia, funding agencies, advocacy groups, and regulators to collectively evaluate the best approaches to research, treatment, and prevention,” Dr. Nadeau said.
The consensus conference participants recommended the following objectives: clarify the biology of type 2 diabetes in youth, obtain new pediatric information on drugs, encourage the use of appropriate medications, and inform clinical decision-making. “We have a desperate need to understand the actions of drugs in type 2 diabetes youth,” Dr. Nadeau said. “Our current approach is not working. Potential solutions include considering efficacy outcomes besides A1c, potentially looking at improvement in insulin sensitivity, preservation of beta-cell function, trying to prevent the A1c increase instead of looking for an A1c reduction, and trying to extrapolate from effects in adults, if we can understand enough to do that.”
The conference participants also called for infrastructure changes, such as creating a resource for patients with type 2 diabetes in the model of the Type 1 Diabetes Exchange. “We need to have collaborations internationally,” she said. “We also need support for teams and clinical groups to work together to be able to accomplish these collaboratively.”
Dr. Nadeau reported having no financial disclosures.
Study Examines Long-term Trends in Type 2 Diabetes Medication Use
NEW ORLEANS – Treatment options for patients with type 2 diabetes mellitus have increased markedly post metformin therapy, results from a long-term study suggests. However, the proportion of patients who have maintained a hemoglobin A1c level of less than 7% has remained steady since 2008.
“It would seem that further research and guidance for personalized treatment pathways is needed to help patients achieve optimal diabetes control,” lead study author Victoria Higgins said at the annual scientific sessions of the American Diabetes Association.
Ms. Higgins, franchise director at Adelphi Real World, Cheshire, United Kingdom, presented data from the Adelphi Real World Diabetes Disease Specific Program, a cross-sectional, observational study of patients with type 2 diabetes in France, Germany, Italy, Spain, the United Kingdom, and the United States. Patients were older than 18 years of age with a confirmed diagnosis of type 2 diabetes and were prescribed at least one antidiabetic drug and/or insulin. Data were collected from the second quarter of 2000 to the second quarter of 2015, gleaned from face-to-face interviews with 3,555 diabetes specialists and 5,109 primary care physicians (PCPs) and completion of physician-reported forms from consultations with patients with type 2 diabetes. Ms. Higgins reported data from 70,657 patients. Of these, 38,489 consulted with a PCP, while 32,168 consulted with a diabetes specialist.
The researchers found that between 2000 and 2015, the number of PCPs who indicated that they would introduce insulin at an HbA1c level less than 8% fell from 24% to 7%, while among diabetes specialists, it fell from 34% to 7%. In addition, a similar proportion of respondents said they would introduce insulin at an HbA1c of 9% or higher in 2015 (42% of PCPs and 39% of diabetes specialists), than in 2004 (36% of PCPs and 24% of diabetes specialists). The introduction of new therapies – such as DPP-4, and more recently GLP-1 and SGLT2 agents – affected treatment patterns over the time period studied. “The main treatment is noninsulin only, but among specialists, a higher prevalence of patients are on noninsulin plus insulin, as well as insulin only,” Ms. Higgins said. “Also interesting to see is there are still some type 2 diabetics who are still on diet and exercise only.” (In 2015, the proportion on a diet and exercise only–regimen was 10% of patients who consulted with primary care physicians and 6% of patients who consulted with diabetes specialists.)
Between 2000 and 2015, the mean number of drugs per patient rose from 1.4 to 1.7 among those who consulted with PCPs, while the mean number of drugs per patient rose from 1.6 to 2.1 among those who consulted with diabetes specialists. A metformin-only regimen is used more often by PCPs than by diabetes specialists, moving toward a higher polypharmacy among the specialists.
Ms. Higgins and her colleagues also found that while there were improvements in HbA1c levels between 2000 and 2008, there has not been any substantial improvement in HbA1c since that time. In 2008, 40% of patients who consulted with PCPs achieved an HbA1c level of less than 7%, compared with 39% of those who consulted with diabetes care specialists. In 2015, those percentages were 50% and 36%, respectively. Ms. Higgins reported having no financial disclosures.
NEW ORLEANS – Treatment options for patients with type 2 diabetes mellitus have increased markedly post metformin therapy, results from a long-term study suggests. However, the proportion of patients who have maintained a hemoglobin A1c level of less than 7% has remained steady since 2008.
“It would seem that further research and guidance for personalized treatment pathways is needed to help patients achieve optimal diabetes control,” lead study author Victoria Higgins said at the annual scientific sessions of the American Diabetes Association.
Ms. Higgins, franchise director at Adelphi Real World, Cheshire, United Kingdom, presented data from the Adelphi Real World Diabetes Disease Specific Program, a cross-sectional, observational study of patients with type 2 diabetes in France, Germany, Italy, Spain, the United Kingdom, and the United States. Patients were older than 18 years of age with a confirmed diagnosis of type 2 diabetes and were prescribed at least one antidiabetic drug and/or insulin. Data were collected from the second quarter of 2000 to the second quarter of 2015, gleaned from face-to-face interviews with 3,555 diabetes specialists and 5,109 primary care physicians (PCPs) and completion of physician-reported forms from consultations with patients with type 2 diabetes. Ms. Higgins reported data from 70,657 patients. Of these, 38,489 consulted with a PCP, while 32,168 consulted with a diabetes specialist.
The researchers found that between 2000 and 2015, the number of PCPs who indicated that they would introduce insulin at an HbA1c level less than 8% fell from 24% to 7%, while among diabetes specialists, it fell from 34% to 7%. In addition, a similar proportion of respondents said they would introduce insulin at an HbA1c of 9% or higher in 2015 (42% of PCPs and 39% of diabetes specialists), than in 2004 (36% of PCPs and 24% of diabetes specialists). The introduction of new therapies – such as DPP-4, and more recently GLP-1 and SGLT2 agents – affected treatment patterns over the time period studied. “The main treatment is noninsulin only, but among specialists, a higher prevalence of patients are on noninsulin plus insulin, as well as insulin only,” Ms. Higgins said. “Also interesting to see is there are still some type 2 diabetics who are still on diet and exercise only.” (In 2015, the proportion on a diet and exercise only–regimen was 10% of patients who consulted with primary care physicians and 6% of patients who consulted with diabetes specialists.)
Between 2000 and 2015, the mean number of drugs per patient rose from 1.4 to 1.7 among those who consulted with PCPs, while the mean number of drugs per patient rose from 1.6 to 2.1 among those who consulted with diabetes specialists. A metformin-only regimen is used more often by PCPs than by diabetes specialists, moving toward a higher polypharmacy among the specialists.
Ms. Higgins and her colleagues also found that while there were improvements in HbA1c levels between 2000 and 2008, there has not been any substantial improvement in HbA1c since that time. In 2008, 40% of patients who consulted with PCPs achieved an HbA1c level of less than 7%, compared with 39% of those who consulted with diabetes care specialists. In 2015, those percentages were 50% and 36%, respectively. Ms. Higgins reported having no financial disclosures.
NEW ORLEANS – Treatment options for patients with type 2 diabetes mellitus have increased markedly post metformin therapy, results from a long-term study suggests. However, the proportion of patients who have maintained a hemoglobin A1c level of less than 7% has remained steady since 2008.
“It would seem that further research and guidance for personalized treatment pathways is needed to help patients achieve optimal diabetes control,” lead study author Victoria Higgins said at the annual scientific sessions of the American Diabetes Association.
Ms. Higgins, franchise director at Adelphi Real World, Cheshire, United Kingdom, presented data from the Adelphi Real World Diabetes Disease Specific Program, a cross-sectional, observational study of patients with type 2 diabetes in France, Germany, Italy, Spain, the United Kingdom, and the United States. Patients were older than 18 years of age with a confirmed diagnosis of type 2 diabetes and were prescribed at least one antidiabetic drug and/or insulin. Data were collected from the second quarter of 2000 to the second quarter of 2015, gleaned from face-to-face interviews with 3,555 diabetes specialists and 5,109 primary care physicians (PCPs) and completion of physician-reported forms from consultations with patients with type 2 diabetes. Ms. Higgins reported data from 70,657 patients. Of these, 38,489 consulted with a PCP, while 32,168 consulted with a diabetes specialist.
The researchers found that between 2000 and 2015, the number of PCPs who indicated that they would introduce insulin at an HbA1c level less than 8% fell from 24% to 7%, while among diabetes specialists, it fell from 34% to 7%. In addition, a similar proportion of respondents said they would introduce insulin at an HbA1c of 9% or higher in 2015 (42% of PCPs and 39% of diabetes specialists), than in 2004 (36% of PCPs and 24% of diabetes specialists). The introduction of new therapies – such as DPP-4, and more recently GLP-1 and SGLT2 agents – affected treatment patterns over the time period studied. “The main treatment is noninsulin only, but among specialists, a higher prevalence of patients are on noninsulin plus insulin, as well as insulin only,” Ms. Higgins said. “Also interesting to see is there are still some type 2 diabetics who are still on diet and exercise only.” (In 2015, the proportion on a diet and exercise only–regimen was 10% of patients who consulted with primary care physicians and 6% of patients who consulted with diabetes specialists.)
Between 2000 and 2015, the mean number of drugs per patient rose from 1.4 to 1.7 among those who consulted with PCPs, while the mean number of drugs per patient rose from 1.6 to 2.1 among those who consulted with diabetes specialists. A metformin-only regimen is used more often by PCPs than by diabetes specialists, moving toward a higher polypharmacy among the specialists.
Ms. Higgins and her colleagues also found that while there were improvements in HbA1c levels between 2000 and 2008, there has not been any substantial improvement in HbA1c since that time. In 2008, 40% of patients who consulted with PCPs achieved an HbA1c level of less than 7%, compared with 39% of those who consulted with diabetes care specialists. In 2015, those percentages were 50% and 36%, respectively. Ms. Higgins reported having no financial disclosures.
AT THE ADA SCIENTIFIC SESSIONS
Study examines long-term trends in type 2 diabetes medication use
NEW ORLEANS – Treatment options for patients with type 2 diabetes mellitus have increased markedly post metformin therapy, results from a long-term study suggests. However, the proportion of patients who have maintained a hemoglobin A1c level of less than 7% has remained steady since 2008.
“It would seem that further research and guidance for personalized treatment pathways is needed to help patients achieve optimal diabetes control,” lead study author Victoria Higgins said at the annual scientific sessions of the American Diabetes Association.
Ms. Higgins, franchise director at Adelphi Real World, Cheshire, United Kingdom, presented data from the Adelphi Real World Diabetes Disease Specific Program, a cross-sectional, observational study of patients with type 2 diabetes in France, Germany, Italy, Spain, the United Kingdom, and the United States. Patients were older than 18 years of age with a confirmed diagnosis of type 2 diabetes and were prescribed at least one antidiabetic drug and/or insulin. Data were collected from the second quarter of 2000 to the second quarter of 2015, gleaned from face-to-face interviews with 3,555 diabetes specialists and 5,109 primary care physicians (PCPs) and completion of physician-reported forms from consultations with patients with type 2 diabetes. Ms. Higgins reported data from 70,657 patients. Of these, 38,489 consulted with a PCP, while 32,168 consulted with a diabetes specialist.
The researchers found that between 2000 and 2015, the number of PCPs who indicated that they would introduce insulin at an HbA1c level less than 8% fell from 24% to 7%, while among diabetes specialists, it fell from 34% to 7%. In addition, a similar proportion of respondents said they would introduce insulin at an HbA1c of 9% or higher in 2015 (42% of PCPs and 39% of diabetes specialists), than in 2004 (36% of PCPs and 24% of diabetes specialists). The introduction of new therapies – such as DPP-4, and more recently GLP-1 and SGLT2 agents – affected treatment patterns over the time period studied. “The main treatment is noninsulin only, but among specialists, a higher prevalence of patients are on noninsulin plus insulin, as well as insulin only,” Ms. Higgins said. “Also interesting to see is there are still some type 2 diabetics who are still on diet and exercise only.” (In 2015, the proportion on a diet and exercise only–regimen was 10% of patients who consulted with primary care physicians and 6% of patients who consulted with diabetes specialists.)
Between 2000 and 2015, the mean number of drugs per patient rose from 1.4 to 1.7 among those who consulted with PCPs, while the mean number of drugs per patient rose from 1.6 to 2.1 among those who consulted with diabetes specialists. A metformin-only regimen is used more often by PCPs than by diabetes specialists, moving toward a higher polypharmacy among the specialists.
Ms. Higgins and her colleagues also found that while there were improvements in HbA1c levels between 2000 and 2008, there has not been any substantial improvement in HbA1c since that time. In 2008, 48% of patients who consulted with PCPs achieved an HbA1c level of less than 7%, compared with 39% of those who consulted with diabetes care specialists. In 2015, those percentages were 50% and 36%, respectively.* Ms. Higgins reported having no financial disclosures.
*CORRECTION 11/7/16: An earlier version of this article misstated the percentage of patients who consulted with PCPs and achieved an HbA1c level of less than 7%.
NEW ORLEANS – Treatment options for patients with type 2 diabetes mellitus have increased markedly post metformin therapy, results from a long-term study suggests. However, the proportion of patients who have maintained a hemoglobin A1c level of less than 7% has remained steady since 2008.
“It would seem that further research and guidance for personalized treatment pathways is needed to help patients achieve optimal diabetes control,” lead study author Victoria Higgins said at the annual scientific sessions of the American Diabetes Association.
Ms. Higgins, franchise director at Adelphi Real World, Cheshire, United Kingdom, presented data from the Adelphi Real World Diabetes Disease Specific Program, a cross-sectional, observational study of patients with type 2 diabetes in France, Germany, Italy, Spain, the United Kingdom, and the United States. Patients were older than 18 years of age with a confirmed diagnosis of type 2 diabetes and were prescribed at least one antidiabetic drug and/or insulin. Data were collected from the second quarter of 2000 to the second quarter of 2015, gleaned from face-to-face interviews with 3,555 diabetes specialists and 5,109 primary care physicians (PCPs) and completion of physician-reported forms from consultations with patients with type 2 diabetes. Ms. Higgins reported data from 70,657 patients. Of these, 38,489 consulted with a PCP, while 32,168 consulted with a diabetes specialist.
The researchers found that between 2000 and 2015, the number of PCPs who indicated that they would introduce insulin at an HbA1c level less than 8% fell from 24% to 7%, while among diabetes specialists, it fell from 34% to 7%. In addition, a similar proportion of respondents said they would introduce insulin at an HbA1c of 9% or higher in 2015 (42% of PCPs and 39% of diabetes specialists), than in 2004 (36% of PCPs and 24% of diabetes specialists). The introduction of new therapies – such as DPP-4, and more recently GLP-1 and SGLT2 agents – affected treatment patterns over the time period studied. “The main treatment is noninsulin only, but among specialists, a higher prevalence of patients are on noninsulin plus insulin, as well as insulin only,” Ms. Higgins said. “Also interesting to see is there are still some type 2 diabetics who are still on diet and exercise only.” (In 2015, the proportion on a diet and exercise only–regimen was 10% of patients who consulted with primary care physicians and 6% of patients who consulted with diabetes specialists.)
Between 2000 and 2015, the mean number of drugs per patient rose from 1.4 to 1.7 among those who consulted with PCPs, while the mean number of drugs per patient rose from 1.6 to 2.1 among those who consulted with diabetes specialists. A metformin-only regimen is used more often by PCPs than by diabetes specialists, moving toward a higher polypharmacy among the specialists.
Ms. Higgins and her colleagues also found that while there were improvements in HbA1c levels between 2000 and 2008, there has not been any substantial improvement in HbA1c since that time. In 2008, 48% of patients who consulted with PCPs achieved an HbA1c level of less than 7%, compared with 39% of those who consulted with diabetes care specialists. In 2015, those percentages were 50% and 36%, respectively.* Ms. Higgins reported having no financial disclosures.
*CORRECTION 11/7/16: An earlier version of this article misstated the percentage of patients who consulted with PCPs and achieved an HbA1c level of less than 7%.
NEW ORLEANS – Treatment options for patients with type 2 diabetes mellitus have increased markedly post metformin therapy, results from a long-term study suggests. However, the proportion of patients who have maintained a hemoglobin A1c level of less than 7% has remained steady since 2008.
“It would seem that further research and guidance for personalized treatment pathways is needed to help patients achieve optimal diabetes control,” lead study author Victoria Higgins said at the annual scientific sessions of the American Diabetes Association.
Ms. Higgins, franchise director at Adelphi Real World, Cheshire, United Kingdom, presented data from the Adelphi Real World Diabetes Disease Specific Program, a cross-sectional, observational study of patients with type 2 diabetes in France, Germany, Italy, Spain, the United Kingdom, and the United States. Patients were older than 18 years of age with a confirmed diagnosis of type 2 diabetes and were prescribed at least one antidiabetic drug and/or insulin. Data were collected from the second quarter of 2000 to the second quarter of 2015, gleaned from face-to-face interviews with 3,555 diabetes specialists and 5,109 primary care physicians (PCPs) and completion of physician-reported forms from consultations with patients with type 2 diabetes. Ms. Higgins reported data from 70,657 patients. Of these, 38,489 consulted with a PCP, while 32,168 consulted with a diabetes specialist.
The researchers found that between 2000 and 2015, the number of PCPs who indicated that they would introduce insulin at an HbA1c level less than 8% fell from 24% to 7%, while among diabetes specialists, it fell from 34% to 7%. In addition, a similar proportion of respondents said they would introduce insulin at an HbA1c of 9% or higher in 2015 (42% of PCPs and 39% of diabetes specialists), than in 2004 (36% of PCPs and 24% of diabetes specialists). The introduction of new therapies – such as DPP-4, and more recently GLP-1 and SGLT2 agents – affected treatment patterns over the time period studied. “The main treatment is noninsulin only, but among specialists, a higher prevalence of patients are on noninsulin plus insulin, as well as insulin only,” Ms. Higgins said. “Also interesting to see is there are still some type 2 diabetics who are still on diet and exercise only.” (In 2015, the proportion on a diet and exercise only–regimen was 10% of patients who consulted with primary care physicians and 6% of patients who consulted with diabetes specialists.)
Between 2000 and 2015, the mean number of drugs per patient rose from 1.4 to 1.7 among those who consulted with PCPs, while the mean number of drugs per patient rose from 1.6 to 2.1 among those who consulted with diabetes specialists. A metformin-only regimen is used more often by PCPs than by diabetes specialists, moving toward a higher polypharmacy among the specialists.
Ms. Higgins and her colleagues also found that while there were improvements in HbA1c levels between 2000 and 2008, there has not been any substantial improvement in HbA1c since that time. In 2008, 48% of patients who consulted with PCPs achieved an HbA1c level of less than 7%, compared with 39% of those who consulted with diabetes care specialists. In 2015, those percentages were 50% and 36%, respectively.* Ms. Higgins reported having no financial disclosures.
*CORRECTION 11/7/16: An earlier version of this article misstated the percentage of patients who consulted with PCPs and achieved an HbA1c level of less than 7%.
AT THE ADA SCIENTIFIC SESSIONS
Key clinical point: Treatment options for patients with type 2 diabetes have increased markedly since 2000.
Major finding: Between 2000 and 2015, the mean number of drugs per patient rose from 1.4 to 1.7 among those who consulted with primary care physicians, while the mean number of drugs per patient rose from 1.6 to 2.1 among those who consulted with diabetes specialists.
Data source: A cross-sectional study of 38,489 type 2 diabetes patients who consulted with a PCP and 32,168 who consulted with a diabetes specialist between 2000 and 2015.
Disclosures: Ms. Higgins reported having no financial disclosures.
Case report: Insulin pump therapy feasible in legally blind patients
NEW ORLEANS – Insulin pump therapy may be feasible and beneficial for select low-vision diabetes patients, results from a case report demonstrated.
“A significant complication of poorly controlled diabetes is visual impairment,” Anna Simos, MPH, said at the annual scientific sessions of the American Diabetes Association. “Insulin dosing and diabetes self-management are challenging for visually impaired patients. Despite the known benefits of insulin pumps, the use is low in patients with visual impairment due to inherent limitations. As a result, there is little published data about insulin pump therapy in visually impaired patients.”
Ms. Simos, a certified diabetes educator at Stanford (Calif.) Health Care, discussed the case of a 49-year-old legally blind patient with type 1 diabetes since who presented to Stanford’s endocrine clinic in early 2015, complaining of increasingly poor glycemic control. He lives with his wife and son, works full time from home, and provides his own self-care with occasional help from family members. Ms. Simos described his medication regimen as “complex due to the diabetes and related comborbidities.” The man’s medical history includes coronary artery disease, obesity, hypertension, hyperlipidemia, and Gaucher disease. His diabetes-related complications include retinopathy, end-stage renal disease, neuropathy, osteomyelitis, and gastroparesis.
Upon presentation at the endocrine clinic the patient’s hemoglobin A1c was 9.8%, his diabetes-related comorbidities were rapidly advancing, and there was a loss of integrity at his injection sites. “There was also a concern about incomplete insulin delivery,” Ms. Simos said. “In addition, there were challenges with his glucose meter and his record keeping. After analysis of all the barriers, we decided to transition the patient to insulin pump therapy.”
The patient reviewed all available existing pumps and selected the OmniPod Insulin Management System, a small, adhesive, tubeless insulin patch pump that features an automated cannula insertion. The device is paired with a wireless, handheld personal diabetes manager controller that programs the pod. The controller “is responsible for the insulin delivery instructions and controls the insulin delivery,” she explained. “It also contains an integrated blood glucose meter.”
After a series of training sessions, the patient began using the patch pump in April of 2015. At the same time, he used a smart phone app called the KNFB Reader for iOS, which translates the controller’s personal diabetes manager screen text and written instructions into speech. “Smartphone reminders were integrated with his basic pump functions as a safety mechanism and his caregiver support team was trained to confirm that the pod was placed correctly,” Ms. Simos said.
After the patient used the device for 6 months, the treatment team noticed a significant decrease in his total daily dose of insulin. “His insulin to carb rate decreased, his glucose correction factor decreased, and his target blood glucoses decreased,” she said. “In addition, we noticed increased compliance of his monitoring.”
Patch pump use was associated with a total daily insulin dose (TDD) of 70-75 units, compared with a TDD of 110-112 units he experienced while using a metered dose inhaler (MDI), a reduction of nearly 40%. Average A1c levels also fell to 6.9% after use of the patch pump, down from the 9.8% he experienced while using a MDI.
According to Ms. Simos, factors to consider when initializing insulin pump therapy with a visually impaired patient include motivation to collaborate, willingness to dedicate time to train on a device, and having a committed support team at home. “We found it beneficial to perform a site visit at the patient’s home and alleviate any potential risks in his environment,” she said.
She reported having no financial disclosures.
NEW ORLEANS – Insulin pump therapy may be feasible and beneficial for select low-vision diabetes patients, results from a case report demonstrated.
“A significant complication of poorly controlled diabetes is visual impairment,” Anna Simos, MPH, said at the annual scientific sessions of the American Diabetes Association. “Insulin dosing and diabetes self-management are challenging for visually impaired patients. Despite the known benefits of insulin pumps, the use is low in patients with visual impairment due to inherent limitations. As a result, there is little published data about insulin pump therapy in visually impaired patients.”
Ms. Simos, a certified diabetes educator at Stanford (Calif.) Health Care, discussed the case of a 49-year-old legally blind patient with type 1 diabetes since who presented to Stanford’s endocrine clinic in early 2015, complaining of increasingly poor glycemic control. He lives with his wife and son, works full time from home, and provides his own self-care with occasional help from family members. Ms. Simos described his medication regimen as “complex due to the diabetes and related comborbidities.” The man’s medical history includes coronary artery disease, obesity, hypertension, hyperlipidemia, and Gaucher disease. His diabetes-related complications include retinopathy, end-stage renal disease, neuropathy, osteomyelitis, and gastroparesis.
Upon presentation at the endocrine clinic the patient’s hemoglobin A1c was 9.8%, his diabetes-related comorbidities were rapidly advancing, and there was a loss of integrity at his injection sites. “There was also a concern about incomplete insulin delivery,” Ms. Simos said. “In addition, there were challenges with his glucose meter and his record keeping. After analysis of all the barriers, we decided to transition the patient to insulin pump therapy.”
The patient reviewed all available existing pumps and selected the OmniPod Insulin Management System, a small, adhesive, tubeless insulin patch pump that features an automated cannula insertion. The device is paired with a wireless, handheld personal diabetes manager controller that programs the pod. The controller “is responsible for the insulin delivery instructions and controls the insulin delivery,” she explained. “It also contains an integrated blood glucose meter.”
After a series of training sessions, the patient began using the patch pump in April of 2015. At the same time, he used a smart phone app called the KNFB Reader for iOS, which translates the controller’s personal diabetes manager screen text and written instructions into speech. “Smartphone reminders were integrated with his basic pump functions as a safety mechanism and his caregiver support team was trained to confirm that the pod was placed correctly,” Ms. Simos said.
After the patient used the device for 6 months, the treatment team noticed a significant decrease in his total daily dose of insulin. “His insulin to carb rate decreased, his glucose correction factor decreased, and his target blood glucoses decreased,” she said. “In addition, we noticed increased compliance of his monitoring.”
Patch pump use was associated with a total daily insulin dose (TDD) of 70-75 units, compared with a TDD of 110-112 units he experienced while using a metered dose inhaler (MDI), a reduction of nearly 40%. Average A1c levels also fell to 6.9% after use of the patch pump, down from the 9.8% he experienced while using a MDI.
According to Ms. Simos, factors to consider when initializing insulin pump therapy with a visually impaired patient include motivation to collaborate, willingness to dedicate time to train on a device, and having a committed support team at home. “We found it beneficial to perform a site visit at the patient’s home and alleviate any potential risks in his environment,” she said.
She reported having no financial disclosures.
NEW ORLEANS – Insulin pump therapy may be feasible and beneficial for select low-vision diabetes patients, results from a case report demonstrated.
“A significant complication of poorly controlled diabetes is visual impairment,” Anna Simos, MPH, said at the annual scientific sessions of the American Diabetes Association. “Insulin dosing and diabetes self-management are challenging for visually impaired patients. Despite the known benefits of insulin pumps, the use is low in patients with visual impairment due to inherent limitations. As a result, there is little published data about insulin pump therapy in visually impaired patients.”
Ms. Simos, a certified diabetes educator at Stanford (Calif.) Health Care, discussed the case of a 49-year-old legally blind patient with type 1 diabetes since who presented to Stanford’s endocrine clinic in early 2015, complaining of increasingly poor glycemic control. He lives with his wife and son, works full time from home, and provides his own self-care with occasional help from family members. Ms. Simos described his medication regimen as “complex due to the diabetes and related comborbidities.” The man’s medical history includes coronary artery disease, obesity, hypertension, hyperlipidemia, and Gaucher disease. His diabetes-related complications include retinopathy, end-stage renal disease, neuropathy, osteomyelitis, and gastroparesis.
Upon presentation at the endocrine clinic the patient’s hemoglobin A1c was 9.8%, his diabetes-related comorbidities were rapidly advancing, and there was a loss of integrity at his injection sites. “There was also a concern about incomplete insulin delivery,” Ms. Simos said. “In addition, there were challenges with his glucose meter and his record keeping. After analysis of all the barriers, we decided to transition the patient to insulin pump therapy.”
The patient reviewed all available existing pumps and selected the OmniPod Insulin Management System, a small, adhesive, tubeless insulin patch pump that features an automated cannula insertion. The device is paired with a wireless, handheld personal diabetes manager controller that programs the pod. The controller “is responsible for the insulin delivery instructions and controls the insulin delivery,” she explained. “It also contains an integrated blood glucose meter.”
After a series of training sessions, the patient began using the patch pump in April of 2015. At the same time, he used a smart phone app called the KNFB Reader for iOS, which translates the controller’s personal diabetes manager screen text and written instructions into speech. “Smartphone reminders were integrated with his basic pump functions as a safety mechanism and his caregiver support team was trained to confirm that the pod was placed correctly,” Ms. Simos said.
After the patient used the device for 6 months, the treatment team noticed a significant decrease in his total daily dose of insulin. “His insulin to carb rate decreased, his glucose correction factor decreased, and his target blood glucoses decreased,” she said. “In addition, we noticed increased compliance of his monitoring.”
Patch pump use was associated with a total daily insulin dose (TDD) of 70-75 units, compared with a TDD of 110-112 units he experienced while using a metered dose inhaler (MDI), a reduction of nearly 40%. Average A1c levels also fell to 6.9% after use of the patch pump, down from the 9.8% he experienced while using a MDI.
According to Ms. Simos, factors to consider when initializing insulin pump therapy with a visually impaired patient include motivation to collaborate, willingness to dedicate time to train on a device, and having a committed support team at home. “We found it beneficial to perform a site visit at the patient’s home and alleviate any potential risks in his environment,” she said.
She reported having no financial disclosures.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point: Insulin pump therapy was found to benefit a legally blind patient with type 1 diabetes.
Major finding: Use of the patch insulin pump use was associated with a total daily insulin dose (TDD) of 70-75 units, compared with a TDD of 110-112 units the patient experienced while using a metered dose inhaler (MDI), a reduction of nearly 40%.
Data source: A case report of a 49-year-old legally blind male with type 1 diabetes who presented to an endocrine clinic with increasingly poor glycemic control.
Disclosures: The researchers reported having no financial conflicts.
Newer Insulin Glargine Formula Curbs Nocturnal Hypoglycemia
NEW ORLEANS – Insulin glargine 300 U/mL provided comparable glycemic control to that seen with insulin glargine 100 U/mL and consistently reduced the risk of nocturnal hypoglycemia in patients with type 2 diabetes, regardless of their renal function, results from a large post hoc meta-analysis showed.
The EDITION I, II, and III studies showed that over a period of 6 months, Gla-300 provided comparable glycemic control to Gla-100 with less hypoglycemia in patients with type 2 diabetes. However, “renal impairment increases the risk of hypoglycemia in people with type 2 diabetes, and may limit glucose-lowering therapy options,” Javier Escalada, M.D., said at the annual scientific sessions of the American Diabetes Association. “Therefore, it may be more challenging to manage diabetes in this population than in people with normal renal function.”
Dr. Escalada of the department of endocrinology and nutrition at Clinic University of Navarra, Pamplona, Spain, and his associates set out to investigate the impact of renal function on hemoglobin A1c reduction and hypoglycemia in a post hoc meta-analysis of 2,468 patients aged 18 years and older with type 2 diabetes who were treated with Gla-300 or Gla-100 for 6 months in the EDITION I, II, and III studies. Treatment consisted of once-daily evening doses of Gla-300 or Gla-100 titrated to a fasting self-measured plasma glucose of 80-100 mg/dL. Patients were classified by their renal function as having moderate loss (30 to less than 60 mL/min per 1.73 m3; 399 patients), mild loss (60 to less than 90; 1,386 patients), or normal function (at least 90; 683 patients).
Outcomes of interest were change in HbA1c from baseline to month 6, and the percentages of patients achieving an HbA1c target of lower than 7.0% and lower than 7.5% at month 6. The researchers also assessed the cumulative number of hypoglycemic events, the relative risk of at least one confirmed or severe hypoglycemic event, and the nocturnal and at any time event rate per participant year.
Slightly more than half of participants (56%) had a baseline estimated glomerular filtration rate of 60 to less than 90 mL/min per 1.73 m3. Dr. Escalada reported that noninferiority for HbA1c reduction was shown for Gla-300 and Gla-100 regardless of renal function, and that evidence of heterogeneity of treatment effect across subgroups was observed (P = .46). However, the risk of confirmed or severe hypoglycemia was significantly lower for nocturnal events in the Gla-300 group, compared with the Gla-100 group (30% vs. 40% overall, respectively), while the risk of anytime hypoglycemia events in a 24-hour period was comparable to or lower in the Gla-300 group, compared with the Gla-100 group. Renal function did not affect the lower rate of nocturnal or anytime hypoglycemia. “Severe hypoglycemia was rare, and renal function did not affect the rate of severe events,” he said.
The trial was sponsored by Sanofi. Dr. Escalada disclosed that he is a member of the advisory panel for Sanofi and for Merck Sharp & Dohme. He is also a member of the speakers bureau for both companies as well as for AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Novo Nordisk.
NEW ORLEANS – Insulin glargine 300 U/mL provided comparable glycemic control to that seen with insulin glargine 100 U/mL and consistently reduced the risk of nocturnal hypoglycemia in patients with type 2 diabetes, regardless of their renal function, results from a large post hoc meta-analysis showed.
The EDITION I, II, and III studies showed that over a period of 6 months, Gla-300 provided comparable glycemic control to Gla-100 with less hypoglycemia in patients with type 2 diabetes. However, “renal impairment increases the risk of hypoglycemia in people with type 2 diabetes, and may limit glucose-lowering therapy options,” Javier Escalada, M.D., said at the annual scientific sessions of the American Diabetes Association. “Therefore, it may be more challenging to manage diabetes in this population than in people with normal renal function.”
Dr. Escalada of the department of endocrinology and nutrition at Clinic University of Navarra, Pamplona, Spain, and his associates set out to investigate the impact of renal function on hemoglobin A1c reduction and hypoglycemia in a post hoc meta-analysis of 2,468 patients aged 18 years and older with type 2 diabetes who were treated with Gla-300 or Gla-100 for 6 months in the EDITION I, II, and III studies. Treatment consisted of once-daily evening doses of Gla-300 or Gla-100 titrated to a fasting self-measured plasma glucose of 80-100 mg/dL. Patients were classified by their renal function as having moderate loss (30 to less than 60 mL/min per 1.73 m3; 399 patients), mild loss (60 to less than 90; 1,386 patients), or normal function (at least 90; 683 patients).
Outcomes of interest were change in HbA1c from baseline to month 6, and the percentages of patients achieving an HbA1c target of lower than 7.0% and lower than 7.5% at month 6. The researchers also assessed the cumulative number of hypoglycemic events, the relative risk of at least one confirmed or severe hypoglycemic event, and the nocturnal and at any time event rate per participant year.
Slightly more than half of participants (56%) had a baseline estimated glomerular filtration rate of 60 to less than 90 mL/min per 1.73 m3. Dr. Escalada reported that noninferiority for HbA1c reduction was shown for Gla-300 and Gla-100 regardless of renal function, and that evidence of heterogeneity of treatment effect across subgroups was observed (P = .46). However, the risk of confirmed or severe hypoglycemia was significantly lower for nocturnal events in the Gla-300 group, compared with the Gla-100 group (30% vs. 40% overall, respectively), while the risk of anytime hypoglycemia events in a 24-hour period was comparable to or lower in the Gla-300 group, compared with the Gla-100 group. Renal function did not affect the lower rate of nocturnal or anytime hypoglycemia. “Severe hypoglycemia was rare, and renal function did not affect the rate of severe events,” he said.
The trial was sponsored by Sanofi. Dr. Escalada disclosed that he is a member of the advisory panel for Sanofi and for Merck Sharp & Dohme. He is also a member of the speakers bureau for both companies as well as for AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Novo Nordisk.
NEW ORLEANS – Insulin glargine 300 U/mL provided comparable glycemic control to that seen with insulin glargine 100 U/mL and consistently reduced the risk of nocturnal hypoglycemia in patients with type 2 diabetes, regardless of their renal function, results from a large post hoc meta-analysis showed.
The EDITION I, II, and III studies showed that over a period of 6 months, Gla-300 provided comparable glycemic control to Gla-100 with less hypoglycemia in patients with type 2 diabetes. However, “renal impairment increases the risk of hypoglycemia in people with type 2 diabetes, and may limit glucose-lowering therapy options,” Javier Escalada, M.D., said at the annual scientific sessions of the American Diabetes Association. “Therefore, it may be more challenging to manage diabetes in this population than in people with normal renal function.”
Dr. Escalada of the department of endocrinology and nutrition at Clinic University of Navarra, Pamplona, Spain, and his associates set out to investigate the impact of renal function on hemoglobin A1c reduction and hypoglycemia in a post hoc meta-analysis of 2,468 patients aged 18 years and older with type 2 diabetes who were treated with Gla-300 or Gla-100 for 6 months in the EDITION I, II, and III studies. Treatment consisted of once-daily evening doses of Gla-300 or Gla-100 titrated to a fasting self-measured plasma glucose of 80-100 mg/dL. Patients were classified by their renal function as having moderate loss (30 to less than 60 mL/min per 1.73 m3; 399 patients), mild loss (60 to less than 90; 1,386 patients), or normal function (at least 90; 683 patients).
Outcomes of interest were change in HbA1c from baseline to month 6, and the percentages of patients achieving an HbA1c target of lower than 7.0% and lower than 7.5% at month 6. The researchers also assessed the cumulative number of hypoglycemic events, the relative risk of at least one confirmed or severe hypoglycemic event, and the nocturnal and at any time event rate per participant year.
Slightly more than half of participants (56%) had a baseline estimated glomerular filtration rate of 60 to less than 90 mL/min per 1.73 m3. Dr. Escalada reported that noninferiority for HbA1c reduction was shown for Gla-300 and Gla-100 regardless of renal function, and that evidence of heterogeneity of treatment effect across subgroups was observed (P = .46). However, the risk of confirmed or severe hypoglycemia was significantly lower for nocturnal events in the Gla-300 group, compared with the Gla-100 group (30% vs. 40% overall, respectively), while the risk of anytime hypoglycemia events in a 24-hour period was comparable to or lower in the Gla-300 group, compared with the Gla-100 group. Renal function did not affect the lower rate of nocturnal or anytime hypoglycemia. “Severe hypoglycemia was rare, and renal function did not affect the rate of severe events,” he said.
The trial was sponsored by Sanofi. Dr. Escalada disclosed that he is a member of the advisory panel for Sanofi and for Merck Sharp & Dohme. He is also a member of the speakers bureau for both companies as well as for AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Novo Nordisk.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Newer insulin glargine formula curbs nocturnal hypoglycemia
NEW ORLEANS – Insulin glargine 300 U/mL provided comparable glycemic control to that seen with insulin glargine 100 U/mL and consistently reduced the risk of nocturnal hypoglycemia in patients with type 2 diabetes, regardless of their renal function, results from a large post hoc meta-analysis showed.
The EDITION I, II, and III studies showed that over a period of 6 months, Gla-300 provided comparable glycemic control to Gla-100 with less hypoglycemia in patients with type 2 diabetes. However, “renal impairment increases the risk of hypoglycemia in people with type 2 diabetes, and may limit glucose-lowering therapy options,” Javier Escalada, M.D., said at the annual scientific sessions of the American Diabetes Association. “Therefore, it may be more challenging to manage diabetes in this population than in people with normal renal function.”
Dr. Escalada of the department of endocrinology and nutrition at Clinic University of Navarra, Pamplona, Spain, and his associates set out to investigate the impact of renal function on hemoglobin A1c reduction and hypoglycemia in a post hoc meta-analysis of 2,468 patients aged 18 years and older with type 2 diabetes who were treated with Gla-300 or Gla-100 for 6 months in the EDITION I, II, and III studies. Treatment consisted of once-daily evening doses of Gla-300 or Gla-100 titrated to a fasting self-measured plasma glucose of 80-100 mg/dL. Patients were classified by their renal function as having moderate loss (30 to less than 60 mL/min per 1.73 m3; 399 patients), mild loss (60 to less than 90; 1,386 patients), or normal function (at least 90; 683 patients).
Outcomes of interest were change in HbA1c from baseline to month 6, and the percentages of patients achieving an HbA1c target of lower than 7.0% and lower than 7.5% at month 6. The researchers also assessed the cumulative number of hypoglycemic events, the relative risk of at least one confirmed or severe hypoglycemic event, and the nocturnal and at any time event rate per participant year.
Slightly more than half of participants (56%) had a baseline estimated glomerular filtration rate of 60 to less than 90 mL/min per 1.73 m3. Dr. Escalada reported that noninferiority for HbA1c reduction was shown for Gla-300 and Gla-100 regardless of renal function, and that evidence of heterogeneity of treatment effect across subgroups was observed (P = .46). However, the risk of confirmed or severe hypoglycemia was significantly lower for nocturnal events in the Gla-300 group, compared with the Gla-100 group (30% vs. 40% overall, respectively), while the risk of anytime hypoglycemia events in a 24-hour period was comparable to or lower in the Gla-300 group, compared with the Gla-100 group. Renal function did not affect the lower rate of nocturnal or anytime hypoglycemia. “Severe hypoglycemia was rare, and renal function did not affect the rate of severe events,” he said.
The trial was sponsored by Sanofi. Dr. Escalada disclosed that he is a member of the advisory panel for Sanofi and for Merck Sharp & Dohme. He is also a member of the speakers bureau for both companies as well as for AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Novo Nordisk.
NEW ORLEANS – Insulin glargine 300 U/mL provided comparable glycemic control to that seen with insulin glargine 100 U/mL and consistently reduced the risk of nocturnal hypoglycemia in patients with type 2 diabetes, regardless of their renal function, results from a large post hoc meta-analysis showed.
The EDITION I, II, and III studies showed that over a period of 6 months, Gla-300 provided comparable glycemic control to Gla-100 with less hypoglycemia in patients with type 2 diabetes. However, “renal impairment increases the risk of hypoglycemia in people with type 2 diabetes, and may limit glucose-lowering therapy options,” Javier Escalada, M.D., said at the annual scientific sessions of the American Diabetes Association. “Therefore, it may be more challenging to manage diabetes in this population than in people with normal renal function.”
Dr. Escalada of the department of endocrinology and nutrition at Clinic University of Navarra, Pamplona, Spain, and his associates set out to investigate the impact of renal function on hemoglobin A1c reduction and hypoglycemia in a post hoc meta-analysis of 2,468 patients aged 18 years and older with type 2 diabetes who were treated with Gla-300 or Gla-100 for 6 months in the EDITION I, II, and III studies. Treatment consisted of once-daily evening doses of Gla-300 or Gla-100 titrated to a fasting self-measured plasma glucose of 80-100 mg/dL. Patients were classified by their renal function as having moderate loss (30 to less than 60 mL/min per 1.73 m3; 399 patients), mild loss (60 to less than 90; 1,386 patients), or normal function (at least 90; 683 patients).
Outcomes of interest were change in HbA1c from baseline to month 6, and the percentages of patients achieving an HbA1c target of lower than 7.0% and lower than 7.5% at month 6. The researchers also assessed the cumulative number of hypoglycemic events, the relative risk of at least one confirmed or severe hypoglycemic event, and the nocturnal and at any time event rate per participant year.
Slightly more than half of participants (56%) had a baseline estimated glomerular filtration rate of 60 to less than 90 mL/min per 1.73 m3. Dr. Escalada reported that noninferiority for HbA1c reduction was shown for Gla-300 and Gla-100 regardless of renal function, and that evidence of heterogeneity of treatment effect across subgroups was observed (P = .46). However, the risk of confirmed or severe hypoglycemia was significantly lower for nocturnal events in the Gla-300 group, compared with the Gla-100 group (30% vs. 40% overall, respectively), while the risk of anytime hypoglycemia events in a 24-hour period was comparable to or lower in the Gla-300 group, compared with the Gla-100 group. Renal function did not affect the lower rate of nocturnal or anytime hypoglycemia. “Severe hypoglycemia was rare, and renal function did not affect the rate of severe events,” he said.
The trial was sponsored by Sanofi. Dr. Escalada disclosed that he is a member of the advisory panel for Sanofi and for Merck Sharp & Dohme. He is also a member of the speakers bureau for both companies as well as for AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Novo Nordisk.
NEW ORLEANS – Insulin glargine 300 U/mL provided comparable glycemic control to that seen with insulin glargine 100 U/mL and consistently reduced the risk of nocturnal hypoglycemia in patients with type 2 diabetes, regardless of their renal function, results from a large post hoc meta-analysis showed.
The EDITION I, II, and III studies showed that over a period of 6 months, Gla-300 provided comparable glycemic control to Gla-100 with less hypoglycemia in patients with type 2 diabetes. However, “renal impairment increases the risk of hypoglycemia in people with type 2 diabetes, and may limit glucose-lowering therapy options,” Javier Escalada, M.D., said at the annual scientific sessions of the American Diabetes Association. “Therefore, it may be more challenging to manage diabetes in this population than in people with normal renal function.”
Dr. Escalada of the department of endocrinology and nutrition at Clinic University of Navarra, Pamplona, Spain, and his associates set out to investigate the impact of renal function on hemoglobin A1c reduction and hypoglycemia in a post hoc meta-analysis of 2,468 patients aged 18 years and older with type 2 diabetes who were treated with Gla-300 or Gla-100 for 6 months in the EDITION I, II, and III studies. Treatment consisted of once-daily evening doses of Gla-300 or Gla-100 titrated to a fasting self-measured plasma glucose of 80-100 mg/dL. Patients were classified by their renal function as having moderate loss (30 to less than 60 mL/min per 1.73 m3; 399 patients), mild loss (60 to less than 90; 1,386 patients), or normal function (at least 90; 683 patients).
Outcomes of interest were change in HbA1c from baseline to month 6, and the percentages of patients achieving an HbA1c target of lower than 7.0% and lower than 7.5% at month 6. The researchers also assessed the cumulative number of hypoglycemic events, the relative risk of at least one confirmed or severe hypoglycemic event, and the nocturnal and at any time event rate per participant year.
Slightly more than half of participants (56%) had a baseline estimated glomerular filtration rate of 60 to less than 90 mL/min per 1.73 m3. Dr. Escalada reported that noninferiority for HbA1c reduction was shown for Gla-300 and Gla-100 regardless of renal function, and that evidence of heterogeneity of treatment effect across subgroups was observed (P = .46). However, the risk of confirmed or severe hypoglycemia was significantly lower for nocturnal events in the Gla-300 group, compared with the Gla-100 group (30% vs. 40% overall, respectively), while the risk of anytime hypoglycemia events in a 24-hour period was comparable to or lower in the Gla-300 group, compared with the Gla-100 group. Renal function did not affect the lower rate of nocturnal or anytime hypoglycemia. “Severe hypoglycemia was rare, and renal function did not affect the rate of severe events,” he said.
The trial was sponsored by Sanofi. Dr. Escalada disclosed that he is a member of the advisory panel for Sanofi and for Merck Sharp & Dohme. He is also a member of the speakers bureau for both companies as well as for AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Novo Nordisk.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point: Insulin glargine 300 U/mL provided glycemic control comparable to insulin glargine 100 U/mL, but it reduced the risk of nocturnal hypoglycemia by a greater margin, regardless of renal function.
Major finding: The risk of confirmed or severe hypoglycemia was significantly lower for nocturnal events in the Gla-300 group, compared with the Gla-100 group (30% vs. 40% overall, respectively), while the risk of anytime hypoglycemia events in a 24-hour period was comparable to or lower in the Gla-300 group, compared with the Gla-100 group.
Data source: A post hoc meta-analysis of 2,468 patients aged 18 years and older with type 2 diabetes who were treated with Gla-300 or Gla-100 for 6 months in the EDITION I, II, and III studies.
Disclosures: The trial was sponsored by Sanofi. Dr. Escalada disclosed that he is a member of the advisory panel for Sanofi and for Merck Sharp & Dohme. He is also a member of the speakers bureau for both companies as well as for AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Novo Nordisk.
Triple Therapy Helped Type 1 Patients Improve Glycemia
NEW ORLEANS – The addition of dapagliflozin to insulin and liraglutide in patients with type 1 diabetes resulted in a significant improvement in glycemia, results from a single-center randomized trial showed.
“Because liraglutide suppresses glucagon and lowers free fatty acids while SGLT-2 inhibitors increase glucagon and risk of diabetic ketoacidosis, it’s possible that the combination of the two agents may neutralize the diabetic ketoacidosis risk,” Paresh Dandona, MD, said at the annual scientific sessions of the American Diabetes Association.
In a recent study Dr. Dandona, chief of endocrinology at the University of Buffalo (N.Y.), and his associates showed that the addition of liraglutide to insulin significantly improved glycemic control in patients with type 1 diabetes (Diabetes Care 2016 Jun; 39[6]:1027-35). The purpose of the current study was to investigate whether the addition of dapagliflozin, a sodium-glucose cotransporter–2 inhibitor, to the combination of insulin and liraglutide would further improve glycemic control.
A total of 30 type 1 diabetes patients on insulin and liraglutide therapy for a minimum of 6 months were randomized to receive either dapagliflozin 10 mg or placebo daily for 12 weeks. Dapagliflozin was initiated at 5 mg daily for 1 week and increased to 10 mg daily thereafter. All patients had type 1 diabetes for at least 1 year, were on insulin therapy, had no detectable C-peptide in plasma, and were on 1.8 mg of liraglutide for 7 months. These patients were evaluated every week for the first and last 2 weeks and then every 2 weeks for 12 weeks. Blood and 24-hour urine samples were collected before and after intervention.
At baseline, the mean age of patients was 54 years and both groups were similar in terms of mean hemoglobin A1c, glucose levels, and insulin dosages. After 12 weeks, the mean HbA1c did not change in the placebo group, but it dropped significantly in the triple-therapy group, by .66% (P less than .01). In addition, the average weekly glucose concentration fell in the triple-therapy group by 13 mg/dL (P = .35) and there was a reduction in standard deviations of glycemic excursions. The triple-therapy group also experienced a small reduction in the total insulin dose, compared with the placebo group (–3.5 vs. –.4 units, respectively; P = .29)
Dr. Dandona went on to note that the episodes of hypoglycemia did not increase after triple therapy but body weight fell by 1.9 kg after 12 weeks, while there was no real change in the placebo arm. Two patients had to withdraw from the study because they developed diabetic ketoacidosis within a day after increasing the dose of dapagliflozin to 10 mg. “One of these patients had euglycemic DKA with blood glucose concentrations of less than 160 mg/dL, while the other had marked hyperglycemia with unchanged insulin dose at 26 units,” Dr. Dandona said. “This patient had experienced a marked reduction in insulin dose during the time she was on liraglutide prior to starting on dapagliflozin.”
Dr. Dandona disclosed that he has received research support from AstraZeneca and Novo Nordisk.
NEW ORLEANS – The addition of dapagliflozin to insulin and liraglutide in patients with type 1 diabetes resulted in a significant improvement in glycemia, results from a single-center randomized trial showed.
“Because liraglutide suppresses glucagon and lowers free fatty acids while SGLT-2 inhibitors increase glucagon and risk of diabetic ketoacidosis, it’s possible that the combination of the two agents may neutralize the diabetic ketoacidosis risk,” Paresh Dandona, MD, said at the annual scientific sessions of the American Diabetes Association.
In a recent study Dr. Dandona, chief of endocrinology at the University of Buffalo (N.Y.), and his associates showed that the addition of liraglutide to insulin significantly improved glycemic control in patients with type 1 diabetes (Diabetes Care 2016 Jun; 39[6]:1027-35). The purpose of the current study was to investigate whether the addition of dapagliflozin, a sodium-glucose cotransporter–2 inhibitor, to the combination of insulin and liraglutide would further improve glycemic control.
A total of 30 type 1 diabetes patients on insulin and liraglutide therapy for a minimum of 6 months were randomized to receive either dapagliflozin 10 mg or placebo daily for 12 weeks. Dapagliflozin was initiated at 5 mg daily for 1 week and increased to 10 mg daily thereafter. All patients had type 1 diabetes for at least 1 year, were on insulin therapy, had no detectable C-peptide in plasma, and were on 1.8 mg of liraglutide for 7 months. These patients were evaluated every week for the first and last 2 weeks and then every 2 weeks for 12 weeks. Blood and 24-hour urine samples were collected before and after intervention.
At baseline, the mean age of patients was 54 years and both groups were similar in terms of mean hemoglobin A1c, glucose levels, and insulin dosages. After 12 weeks, the mean HbA1c did not change in the placebo group, but it dropped significantly in the triple-therapy group, by .66% (P less than .01). In addition, the average weekly glucose concentration fell in the triple-therapy group by 13 mg/dL (P = .35) and there was a reduction in standard deviations of glycemic excursions. The triple-therapy group also experienced a small reduction in the total insulin dose, compared with the placebo group (–3.5 vs. –.4 units, respectively; P = .29)
Dr. Dandona went on to note that the episodes of hypoglycemia did not increase after triple therapy but body weight fell by 1.9 kg after 12 weeks, while there was no real change in the placebo arm. Two patients had to withdraw from the study because they developed diabetic ketoacidosis within a day after increasing the dose of dapagliflozin to 10 mg. “One of these patients had euglycemic DKA with blood glucose concentrations of less than 160 mg/dL, while the other had marked hyperglycemia with unchanged insulin dose at 26 units,” Dr. Dandona said. “This patient had experienced a marked reduction in insulin dose during the time she was on liraglutide prior to starting on dapagliflozin.”
Dr. Dandona disclosed that he has received research support from AstraZeneca and Novo Nordisk.
NEW ORLEANS – The addition of dapagliflozin to insulin and liraglutide in patients with type 1 diabetes resulted in a significant improvement in glycemia, results from a single-center randomized trial showed.
“Because liraglutide suppresses glucagon and lowers free fatty acids while SGLT-2 inhibitors increase glucagon and risk of diabetic ketoacidosis, it’s possible that the combination of the two agents may neutralize the diabetic ketoacidosis risk,” Paresh Dandona, MD, said at the annual scientific sessions of the American Diabetes Association.
In a recent study Dr. Dandona, chief of endocrinology at the University of Buffalo (N.Y.), and his associates showed that the addition of liraglutide to insulin significantly improved glycemic control in patients with type 1 diabetes (Diabetes Care 2016 Jun; 39[6]:1027-35). The purpose of the current study was to investigate whether the addition of dapagliflozin, a sodium-glucose cotransporter–2 inhibitor, to the combination of insulin and liraglutide would further improve glycemic control.
A total of 30 type 1 diabetes patients on insulin and liraglutide therapy for a minimum of 6 months were randomized to receive either dapagliflozin 10 mg or placebo daily for 12 weeks. Dapagliflozin was initiated at 5 mg daily for 1 week and increased to 10 mg daily thereafter. All patients had type 1 diabetes for at least 1 year, were on insulin therapy, had no detectable C-peptide in plasma, and were on 1.8 mg of liraglutide for 7 months. These patients were evaluated every week for the first and last 2 weeks and then every 2 weeks for 12 weeks. Blood and 24-hour urine samples were collected before and after intervention.
At baseline, the mean age of patients was 54 years and both groups were similar in terms of mean hemoglobin A1c, glucose levels, and insulin dosages. After 12 weeks, the mean HbA1c did not change in the placebo group, but it dropped significantly in the triple-therapy group, by .66% (P less than .01). In addition, the average weekly glucose concentration fell in the triple-therapy group by 13 mg/dL (P = .35) and there was a reduction in standard deviations of glycemic excursions. The triple-therapy group also experienced a small reduction in the total insulin dose, compared with the placebo group (–3.5 vs. –.4 units, respectively; P = .29)
Dr. Dandona went on to note that the episodes of hypoglycemia did not increase after triple therapy but body weight fell by 1.9 kg after 12 weeks, while there was no real change in the placebo arm. Two patients had to withdraw from the study because they developed diabetic ketoacidosis within a day after increasing the dose of dapagliflozin to 10 mg. “One of these patients had euglycemic DKA with blood glucose concentrations of less than 160 mg/dL, while the other had marked hyperglycemia with unchanged insulin dose at 26 units,” Dr. Dandona said. “This patient had experienced a marked reduction in insulin dose during the time she was on liraglutide prior to starting on dapagliflozin.”
Dr. Dandona disclosed that he has received research support from AstraZeneca and Novo Nordisk.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Triple therapy helped type 1 patients improve glycemia
NEW ORLEANS – The addition of dapagliflozin to insulin and liraglutide in patients with type 1 diabetes resulted in a significant improvement in glycemia, results from a single-center randomized trial showed.
“Because liraglutide suppresses glucagon and lowers free fatty acids while SGLT-2 inhibitors increase glucagon and risk of diabetic ketoacidosis, it’s possible that the combination of the two agents may neutralize the diabetic ketoacidosis risk,” Paresh Dandona, MD, said at the annual scientific sessions of the American Diabetes Association.
In a recent study Dr. Dandona, chief of endocrinology at the University of Buffalo (N.Y.), and his associates showed that the addition of liraglutide to insulin significantly improved glycemic control in patients with type 1 diabetes (Diabetes Care 2016 Jun; 39[6]:1027-35). The purpose of the current study was to investigate whether the addition of dapagliflozin, a sodium-glucose cotransporter–2 inhibitor, to the combination of insulin and liraglutide would further improve glycemic control.
A total of 30 type 1 diabetes patients on insulin and liraglutide therapy for a minimum of 6 months were randomized to receive either dapagliflozin 10 mg or placebo daily for 12 weeks. Dapagliflozin was initiated at 5 mg daily for 1 week and increased to 10 mg daily thereafter. All patients had type 1 diabetes for at least 1 year, were on insulin therapy, had no detectable C-peptide in plasma, and were on 1.8 mg of liraglutide for 7 months. These patients were evaluated every week for the first and last 2 weeks and then every 2 weeks for 12 weeks. Blood and 24-hour urine samples were collected before and after intervention.
At baseline, the mean age of patients was 54 years and both groups were similar in terms of mean hemoglobin A1c, glucose levels, and insulin dosages. After 12 weeks, the mean HbA1c did not change in the placebo group, but it dropped significantly in the triple-therapy group, by .66% (P less than .01). In addition, the average weekly glucose concentration fell in the triple-therapy group by 13 mg/dL (P = .35) and there was a reduction in standard deviations of glycemic excursions. The triple-therapy group also experienced a small reduction in the total insulin dose, compared with the placebo group (–3.5 vs. –.4 units, respectively; P = .29)
Dr. Dandona went on to note that the episodes of hypoglycemia did not increase after triple therapy but body weight fell by 1.9 kg after 12 weeks, while there was no real change in the placebo arm. Two patients had to withdraw from the study because they developed diabetic ketoacidosis within a day after increasing the dose of dapagliflozin to 10 mg. “One of these patients had euglycemic DKA with blood glucose concentrations of less than 160 mg/dL, while the other had marked hyperglycemia with unchanged insulin dose at 26 units,” Dr. Dandona said. “This patient had experienced a marked reduction in insulin dose during the time she was on liraglutide prior to starting on dapagliflozin.”
Dr. Dandona disclosed that he has received research support from AstraZeneca and Novo Nordisk.
NEW ORLEANS – The addition of dapagliflozin to insulin and liraglutide in patients with type 1 diabetes resulted in a significant improvement in glycemia, results from a single-center randomized trial showed.
“Because liraglutide suppresses glucagon and lowers free fatty acids while SGLT-2 inhibitors increase glucagon and risk of diabetic ketoacidosis, it’s possible that the combination of the two agents may neutralize the diabetic ketoacidosis risk,” Paresh Dandona, MD, said at the annual scientific sessions of the American Diabetes Association.
In a recent study Dr. Dandona, chief of endocrinology at the University of Buffalo (N.Y.), and his associates showed that the addition of liraglutide to insulin significantly improved glycemic control in patients with type 1 diabetes (Diabetes Care 2016 Jun; 39[6]:1027-35). The purpose of the current study was to investigate whether the addition of dapagliflozin, a sodium-glucose cotransporter–2 inhibitor, to the combination of insulin and liraglutide would further improve glycemic control.
A total of 30 type 1 diabetes patients on insulin and liraglutide therapy for a minimum of 6 months were randomized to receive either dapagliflozin 10 mg or placebo daily for 12 weeks. Dapagliflozin was initiated at 5 mg daily for 1 week and increased to 10 mg daily thereafter. All patients had type 1 diabetes for at least 1 year, were on insulin therapy, had no detectable C-peptide in plasma, and were on 1.8 mg of liraglutide for 7 months. These patients were evaluated every week for the first and last 2 weeks and then every 2 weeks for 12 weeks. Blood and 24-hour urine samples were collected before and after intervention.
At baseline, the mean age of patients was 54 years and both groups were similar in terms of mean hemoglobin A1c, glucose levels, and insulin dosages. After 12 weeks, the mean HbA1c did not change in the placebo group, but it dropped significantly in the triple-therapy group, by .66% (P less than .01). In addition, the average weekly glucose concentration fell in the triple-therapy group by 13 mg/dL (P = .35) and there was a reduction in standard deviations of glycemic excursions. The triple-therapy group also experienced a small reduction in the total insulin dose, compared with the placebo group (–3.5 vs. –.4 units, respectively; P = .29)
Dr. Dandona went on to note that the episodes of hypoglycemia did not increase after triple therapy but body weight fell by 1.9 kg after 12 weeks, while there was no real change in the placebo arm. Two patients had to withdraw from the study because they developed diabetic ketoacidosis within a day after increasing the dose of dapagliflozin to 10 mg. “One of these patients had euglycemic DKA with blood glucose concentrations of less than 160 mg/dL, while the other had marked hyperglycemia with unchanged insulin dose at 26 units,” Dr. Dandona said. “This patient had experienced a marked reduction in insulin dose during the time she was on liraglutide prior to starting on dapagliflozin.”
Dr. Dandona disclosed that he has received research support from AstraZeneca and Novo Nordisk.
NEW ORLEANS – The addition of dapagliflozin to insulin and liraglutide in patients with type 1 diabetes resulted in a significant improvement in glycemia, results from a single-center randomized trial showed.
“Because liraglutide suppresses glucagon and lowers free fatty acids while SGLT-2 inhibitors increase glucagon and risk of diabetic ketoacidosis, it’s possible that the combination of the two agents may neutralize the diabetic ketoacidosis risk,” Paresh Dandona, MD, said at the annual scientific sessions of the American Diabetes Association.
In a recent study Dr. Dandona, chief of endocrinology at the University of Buffalo (N.Y.), and his associates showed that the addition of liraglutide to insulin significantly improved glycemic control in patients with type 1 diabetes (Diabetes Care 2016 Jun; 39[6]:1027-35). The purpose of the current study was to investigate whether the addition of dapagliflozin, a sodium-glucose cotransporter–2 inhibitor, to the combination of insulin and liraglutide would further improve glycemic control.
A total of 30 type 1 diabetes patients on insulin and liraglutide therapy for a minimum of 6 months were randomized to receive either dapagliflozin 10 mg or placebo daily for 12 weeks. Dapagliflozin was initiated at 5 mg daily for 1 week and increased to 10 mg daily thereafter. All patients had type 1 diabetes for at least 1 year, were on insulin therapy, had no detectable C-peptide in plasma, and were on 1.8 mg of liraglutide for 7 months. These patients were evaluated every week for the first and last 2 weeks and then every 2 weeks for 12 weeks. Blood and 24-hour urine samples were collected before and after intervention.
At baseline, the mean age of patients was 54 years and both groups were similar in terms of mean hemoglobin A1c, glucose levels, and insulin dosages. After 12 weeks, the mean HbA1c did not change in the placebo group, but it dropped significantly in the triple-therapy group, by .66% (P less than .01). In addition, the average weekly glucose concentration fell in the triple-therapy group by 13 mg/dL (P = .35) and there was a reduction in standard deviations of glycemic excursions. The triple-therapy group also experienced a small reduction in the total insulin dose, compared with the placebo group (–3.5 vs. –.4 units, respectively; P = .29)
Dr. Dandona went on to note that the episodes of hypoglycemia did not increase after triple therapy but body weight fell by 1.9 kg after 12 weeks, while there was no real change in the placebo arm. Two patients had to withdraw from the study because they developed diabetic ketoacidosis within a day after increasing the dose of dapagliflozin to 10 mg. “One of these patients had euglycemic DKA with blood glucose concentrations of less than 160 mg/dL, while the other had marked hyperglycemia with unchanged insulin dose at 26 units,” Dr. Dandona said. “This patient had experienced a marked reduction in insulin dose during the time she was on liraglutide prior to starting on dapagliflozin.”
Dr. Dandona disclosed that he has received research support from AstraZeneca and Novo Nordisk.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point: Adding dapagliflozin to insulin and liraglutide in patients with type 1 diabetes led to a significant improvement in glycemia.
Major finding: After 12 weeks, the mean HbA1c did not change in the placebo group, but it dropped in the triple-therapy group by 0.66% (P less than .01).
Data source: A single-center study in which 30 type 1 diabetes patients on insulin and liraglutide therapy for a minimum of 6 months were randomized to receive either dapagliflozin 10 mg or placebo daily for 12 weeks.
Disclosures: Dr. Dandona disclosed that he has received research support from AstraZeneca and Novo Nordisk.
Canagliflozin benefits cohort of type 1 diabetes patients
NEW ORLEANS – Compared with placebo, canagliflozin improved glycemic control and reduced glycemic variability over 18 weeks in adults with type 1 diabetes that had been inadequately controlled with insulin, according to results from a randomized trial.
“Patients with type 1 diabetes can experience acute and profound glucose fluctuations, which may be related to variability in insulin activity and changes in day-to-day activities,” Maria Alba, MD, said at the annual scientific sessions of the American Diabetes Association. “A major challenge in the treatment of type 1 diabetes is achieving patient-specific glycemic control while avoiding episodes of hyperglycemia and hypoglycemia.”
Canagliflozin is a sodium glucose cotransporter 2 inhibitor approved for the treatment of type 2 diabetes; it lowers blood glucose through an insulin-dependent mechanism by lowering the renal threshold for glucose and increasing urinary glucose excretion. It is not approved for the treatment of type 1 diabetes. In a phase II study, canagliflozin improved glycemic control and provided reductions in body weight and insulin dose in patients with type 1 diabetes who were on background insulin over 18 weeks (Diabetes Care 2015;39[12]:2258-65).
The purpose of the current study was to assess the effects of canagliflozin on daily mean glucose, glycemic variability, and time spent in glycemic ranges (greater than 70-180 mg/dL) in adults with type 1 diabetes inadequately controlled with insulin therapy, said Dr. Alba of Janssen Research & Development, Raritan, N.J. In all, 351 patients were randomized to placebo or 100 mg or 300 mg of canagliflozin. All had had the disease for at least 1 year, had a baseline hemoglobin A1c level between 7% and 9%, and had to be on a stable insulin regimen for at least 8 weeks. “On the day before randomization, patients were advised to reduce their basal insulin by 10% or 20%, depending on their baseline A1c,” she said. “Once randomized, they were instructed to titrate their insulin to reach specific glycemic goals.”
All patients were to record 9-point self-monitoring blood glucose (SMBG) measurements throughout the study. Continuous glucose monitoring (CGM) assessments were performed at selected study centers in a substudy of 89 patients. Efficacy endpoints assessed by 9-point SMBG at week 18 were change from baseline in mean daily glucose and daily glucose standard deviation. Efficacy endpoints in the CGM substudy at week 18 were change from baseline in mean glucose, patterns of glucose variability, and time spent within target glucose (greater than 70-180 mg/dL), above target (greater than 180 mg/dL), or below target (70 mg/dL or less). The mean age of study participants was 43 years, 55% were male, and their mean baseline A1c was 7.9%.
At 18 weeks, reductions in daily mean glucose were observed in the canagliflozin 100-mg and 300-mg groups, compared with placebo (–22.4 and –19.4 mg/dL, respectively, vs. a rise of 3 mg/dL in the placebo group), as well as reductions in daily glucose standard deviation (–16.4 and –18.1 mg/dL, vs. –1.9 mg/dL), Dr. Alba reported.
The researchers also found that the percentage of time spent within the glycemic target range was greater in the canagliflozin 100- and 300-mg groups, compared with placebo (a change from baseline of 11.6% and 10.1%, respectively, compared with a decrease of 3.5%), while the percentage of time spent above target was lower in both canagliflozin doses, compared with placebo. Canagliflozin was generally well tolerated, with a dose-dependent increase in ketone-related adverse events (5.1% in the 100-mg group and 9.4% in the 300-mg group).
Janssen Research & Development supported the study.
NEW ORLEANS – Compared with placebo, canagliflozin improved glycemic control and reduced glycemic variability over 18 weeks in adults with type 1 diabetes that had been inadequately controlled with insulin, according to results from a randomized trial.
“Patients with type 1 diabetes can experience acute and profound glucose fluctuations, which may be related to variability in insulin activity and changes in day-to-day activities,” Maria Alba, MD, said at the annual scientific sessions of the American Diabetes Association. “A major challenge in the treatment of type 1 diabetes is achieving patient-specific glycemic control while avoiding episodes of hyperglycemia and hypoglycemia.”
Canagliflozin is a sodium glucose cotransporter 2 inhibitor approved for the treatment of type 2 diabetes; it lowers blood glucose through an insulin-dependent mechanism by lowering the renal threshold for glucose and increasing urinary glucose excretion. It is not approved for the treatment of type 1 diabetes. In a phase II study, canagliflozin improved glycemic control and provided reductions in body weight and insulin dose in patients with type 1 diabetes who were on background insulin over 18 weeks (Diabetes Care 2015;39[12]:2258-65).
The purpose of the current study was to assess the effects of canagliflozin on daily mean glucose, glycemic variability, and time spent in glycemic ranges (greater than 70-180 mg/dL) in adults with type 1 diabetes inadequately controlled with insulin therapy, said Dr. Alba of Janssen Research & Development, Raritan, N.J. In all, 351 patients were randomized to placebo or 100 mg or 300 mg of canagliflozin. All had had the disease for at least 1 year, had a baseline hemoglobin A1c level between 7% and 9%, and had to be on a stable insulin regimen for at least 8 weeks. “On the day before randomization, patients were advised to reduce their basal insulin by 10% or 20%, depending on their baseline A1c,” she said. “Once randomized, they were instructed to titrate their insulin to reach specific glycemic goals.”
All patients were to record 9-point self-monitoring blood glucose (SMBG) measurements throughout the study. Continuous glucose monitoring (CGM) assessments were performed at selected study centers in a substudy of 89 patients. Efficacy endpoints assessed by 9-point SMBG at week 18 were change from baseline in mean daily glucose and daily glucose standard deviation. Efficacy endpoints in the CGM substudy at week 18 were change from baseline in mean glucose, patterns of glucose variability, and time spent within target glucose (greater than 70-180 mg/dL), above target (greater than 180 mg/dL), or below target (70 mg/dL or less). The mean age of study participants was 43 years, 55% were male, and their mean baseline A1c was 7.9%.
At 18 weeks, reductions in daily mean glucose were observed in the canagliflozin 100-mg and 300-mg groups, compared with placebo (–22.4 and –19.4 mg/dL, respectively, vs. a rise of 3 mg/dL in the placebo group), as well as reductions in daily glucose standard deviation (–16.4 and –18.1 mg/dL, vs. –1.9 mg/dL), Dr. Alba reported.
The researchers also found that the percentage of time spent within the glycemic target range was greater in the canagliflozin 100- and 300-mg groups, compared with placebo (a change from baseline of 11.6% and 10.1%, respectively, compared with a decrease of 3.5%), while the percentage of time spent above target was lower in both canagliflozin doses, compared with placebo. Canagliflozin was generally well tolerated, with a dose-dependent increase in ketone-related adverse events (5.1% in the 100-mg group and 9.4% in the 300-mg group).
Janssen Research & Development supported the study.
NEW ORLEANS – Compared with placebo, canagliflozin improved glycemic control and reduced glycemic variability over 18 weeks in adults with type 1 diabetes that had been inadequately controlled with insulin, according to results from a randomized trial.
“Patients with type 1 diabetes can experience acute and profound glucose fluctuations, which may be related to variability in insulin activity and changes in day-to-day activities,” Maria Alba, MD, said at the annual scientific sessions of the American Diabetes Association. “A major challenge in the treatment of type 1 diabetes is achieving patient-specific glycemic control while avoiding episodes of hyperglycemia and hypoglycemia.”
Canagliflozin is a sodium glucose cotransporter 2 inhibitor approved for the treatment of type 2 diabetes; it lowers blood glucose through an insulin-dependent mechanism by lowering the renal threshold for glucose and increasing urinary glucose excretion. It is not approved for the treatment of type 1 diabetes. In a phase II study, canagliflozin improved glycemic control and provided reductions in body weight and insulin dose in patients with type 1 diabetes who were on background insulin over 18 weeks (Diabetes Care 2015;39[12]:2258-65).
The purpose of the current study was to assess the effects of canagliflozin on daily mean glucose, glycemic variability, and time spent in glycemic ranges (greater than 70-180 mg/dL) in adults with type 1 diabetes inadequately controlled with insulin therapy, said Dr. Alba of Janssen Research & Development, Raritan, N.J. In all, 351 patients were randomized to placebo or 100 mg or 300 mg of canagliflozin. All had had the disease for at least 1 year, had a baseline hemoglobin A1c level between 7% and 9%, and had to be on a stable insulin regimen for at least 8 weeks. “On the day before randomization, patients were advised to reduce their basal insulin by 10% or 20%, depending on their baseline A1c,” she said. “Once randomized, they were instructed to titrate their insulin to reach specific glycemic goals.”
All patients were to record 9-point self-monitoring blood glucose (SMBG) measurements throughout the study. Continuous glucose monitoring (CGM) assessments were performed at selected study centers in a substudy of 89 patients. Efficacy endpoints assessed by 9-point SMBG at week 18 were change from baseline in mean daily glucose and daily glucose standard deviation. Efficacy endpoints in the CGM substudy at week 18 were change from baseline in mean glucose, patterns of glucose variability, and time spent within target glucose (greater than 70-180 mg/dL), above target (greater than 180 mg/dL), or below target (70 mg/dL or less). The mean age of study participants was 43 years, 55% were male, and their mean baseline A1c was 7.9%.
At 18 weeks, reductions in daily mean glucose were observed in the canagliflozin 100-mg and 300-mg groups, compared with placebo (–22.4 and –19.4 mg/dL, respectively, vs. a rise of 3 mg/dL in the placebo group), as well as reductions in daily glucose standard deviation (–16.4 and –18.1 mg/dL, vs. –1.9 mg/dL), Dr. Alba reported.
The researchers also found that the percentage of time spent within the glycemic target range was greater in the canagliflozin 100- and 300-mg groups, compared with placebo (a change from baseline of 11.6% and 10.1%, respectively, compared with a decrease of 3.5%), while the percentage of time spent above target was lower in both canagliflozin doses, compared with placebo. Canagliflozin was generally well tolerated, with a dose-dependent increase in ketone-related adverse events (5.1% in the 100-mg group and 9.4% in the 300-mg group).
Janssen Research & Development supported the study.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point: Canagliflozin improved glycemic control in patients with type 1 diabetes inadequately controlled with insulin.
Major finding: At 18 weeks, reductions in daily mean glucose were observed in the canagliflozin 100-mg and 300-mg groups, compared with placebo (–22.4 and –19.4 mg/dL, respectively, vs. a rise of 3 mg/dL).
Data source: A study of 351 patients with type 1 diabetes that was inadequately controlled with insulin who were randomized to placebo or 100 mg or 300 mg of canagliflozin.
Disclosures: Ms. Alba is an employee of Janssen Research and Development, which supported the study.










