User login
For MD-IQ only
Access to Germline Genetic Testing through Clinical Pathways in Veterans With Prostate Cancer
Background
Germline genetic testing (GGT) is essential in prostate cancer care, informing clinical decisions. The Veterans Affairs National Oncology Program (VA NOP) recommends GGT for patients with specific risk factors in non-metastatic prostate cancer and all patients with metastatic disease. Understanding GGT access helps evaluate care quality and guide improvements. Since 2021, VA NOP has implemented pathway health factor (HF) templates to standardize cancer care documentation, including GGT status, enabling data extraction from the Corporate Data Warehouse (CDW) rather than requiring manual review of clinical notes. This work aims to evaluate Veterans’ access to GGT in prostate cancer care by leveraging pathway HF templates, and to assess the feasibility of using structured electronic health record (EHR) data to monitor adherence to GGT recommendations.
Methods
Process delivery diagrams (PDDs) were used to map data flow from prostate cancer clinical pathways to the VA CDW. We identified and categorized HFs related to prostate cancer GGT through the computerized patient record system (CPRS). Descriptive statistics were used to summarize access, ordering, and consent rates.
Results
We identified 5,744 Veterans with at least one prostate cancer GGT-relevant HF entered between 02/01/2021 and 12/31/2024. Of these, 5,125 (89.2%) had access to GGT, with 4,569 (89.2%) consenting to or having GGT ordered, while 556 (10.8%) declined testing. Among the 619 (10.8%) Veterans without GGT access, providers reported plans to discuss GGT in the future for 528 (85.3%) patients, while 91 (14.7%) were off pathway.
Conclusions
NOP-developed HF templates enabled extraction of GGT information from structured EHR data, eliminating manual extraction from clinical notes. We observed high GGT utilization among Veterans with pathway-entered HFs. However, low overall HF utilization may introduce selection bias. Future work includes developing a Natural Language Processing pipeline using large language models to automatically extract GGT information from clinical notes, with HF data serving as ground truth.
Background
Germline genetic testing (GGT) is essential in prostate cancer care, informing clinical decisions. The Veterans Affairs National Oncology Program (VA NOP) recommends GGT for patients with specific risk factors in non-metastatic prostate cancer and all patients with metastatic disease. Understanding GGT access helps evaluate care quality and guide improvements. Since 2021, VA NOP has implemented pathway health factor (HF) templates to standardize cancer care documentation, including GGT status, enabling data extraction from the Corporate Data Warehouse (CDW) rather than requiring manual review of clinical notes. This work aims to evaluate Veterans’ access to GGT in prostate cancer care by leveraging pathway HF templates, and to assess the feasibility of using structured electronic health record (EHR) data to monitor adherence to GGT recommendations.
Methods
Process delivery diagrams (PDDs) were used to map data flow from prostate cancer clinical pathways to the VA CDW. We identified and categorized HFs related to prostate cancer GGT through the computerized patient record system (CPRS). Descriptive statistics were used to summarize access, ordering, and consent rates.
Results
We identified 5,744 Veterans with at least one prostate cancer GGT-relevant HF entered between 02/01/2021 and 12/31/2024. Of these, 5,125 (89.2%) had access to GGT, with 4,569 (89.2%) consenting to or having GGT ordered, while 556 (10.8%) declined testing. Among the 619 (10.8%) Veterans without GGT access, providers reported plans to discuss GGT in the future for 528 (85.3%) patients, while 91 (14.7%) were off pathway.
Conclusions
NOP-developed HF templates enabled extraction of GGT information from structured EHR data, eliminating manual extraction from clinical notes. We observed high GGT utilization among Veterans with pathway-entered HFs. However, low overall HF utilization may introduce selection bias. Future work includes developing a Natural Language Processing pipeline using large language models to automatically extract GGT information from clinical notes, with HF data serving as ground truth.
Background
Germline genetic testing (GGT) is essential in prostate cancer care, informing clinical decisions. The Veterans Affairs National Oncology Program (VA NOP) recommends GGT for patients with specific risk factors in non-metastatic prostate cancer and all patients with metastatic disease. Understanding GGT access helps evaluate care quality and guide improvements. Since 2021, VA NOP has implemented pathway health factor (HF) templates to standardize cancer care documentation, including GGT status, enabling data extraction from the Corporate Data Warehouse (CDW) rather than requiring manual review of clinical notes. This work aims to evaluate Veterans’ access to GGT in prostate cancer care by leveraging pathway HF templates, and to assess the feasibility of using structured electronic health record (EHR) data to monitor adherence to GGT recommendations.
Methods
Process delivery diagrams (PDDs) were used to map data flow from prostate cancer clinical pathways to the VA CDW. We identified and categorized HFs related to prostate cancer GGT through the computerized patient record system (CPRS). Descriptive statistics were used to summarize access, ordering, and consent rates.
Results
We identified 5,744 Veterans with at least one prostate cancer GGT-relevant HF entered between 02/01/2021 and 12/31/2024. Of these, 5,125 (89.2%) had access to GGT, with 4,569 (89.2%) consenting to or having GGT ordered, while 556 (10.8%) declined testing. Among the 619 (10.8%) Veterans without GGT access, providers reported plans to discuss GGT in the future for 528 (85.3%) patients, while 91 (14.7%) were off pathway.
Conclusions
NOP-developed HF templates enabled extraction of GGT information from structured EHR data, eliminating manual extraction from clinical notes. We observed high GGT utilization among Veterans with pathway-entered HFs. However, low overall HF utilization may introduce selection bias. Future work includes developing a Natural Language Processing pipeline using large language models to automatically extract GGT information from clinical notes, with HF data serving as ground truth.
Genomic Testing Reveals Distinct Mutation Patterns in Black and White Veterans With Metastatic Prostate Cancer
TOPLINE: Next-generation sequencing (NGS) analysis of 5015 veterans with metastatic prostate cancer reveals distinct genomic patterns between non-Hispanic Black and White patients, with Black veterans showing higher odds of immunotherapy targets but lower odds of androgen receptor axis alterations. However, the rates of survival were similar despite the differences.
METHODOLOGY:
Researchers conducted a retrospective cohort study comparing alteration frequencies between 1784 non-Hispanic Black (35.6%) and 3,231 non-Hispanic White (64.4%) veterans who underwent NGS testing from January 23, 2019, to November 2, 2023.
- Analysis included DNA sequencing data from tissue or plasma biospecimens, including prostate biopsy specimens, radical prostatectomy specimens, and prostate cancer metastases, all sequenced with FoundationOne CDx or FoundationOne Liquid CDx platforms.
- Investigators examined pathogenic alterations in individual genes, actionable targets, and canonical prostate cancer pathways, while adjusting for NGS analyte and clinicopathologic covariates.
- Researchers evaluated associations between alteration frequency and race as well as survival through Cox proportional hazards modeling, stratified by race and adjusted for clinical factors.
TAKEAWAY:
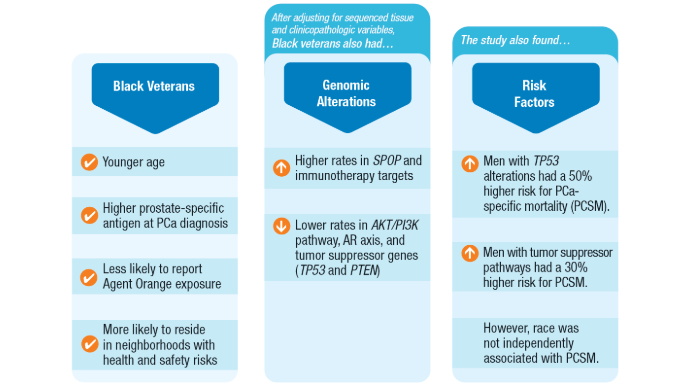
Non-Hispanic Black race and ethnicity was associated with higher odds of genomic alterations in SPOP (odds ratio [OR], 1.7; 95% confidence interval [CI], 1.2-2.6) and immunotherapy targets (OR, 1.7; 95% CI, 1.1-2.5), including high microsatellite instability status (OR, 3.1; 95% CI, 1.1-9.4).
- Non-Hispanic Black veterans showed lower odds of genomic alterations in the AKT/PI3K pathway (OR, 0.6; 95% CI, 0.4-0.7), androgen receptor axis (OR, 0.7; 95% CI, 0.5-0.9), and tumor suppressor genes (OR, 0.7; 95% CI, 0.5-0.8).
- Tumor suppressor alterations were associated with shorter overall survival in both non-Hispanic Black (hazard ratio [HR], 1.54; 95% CI, 1.13-2.11) and non-Hispanic White (HR, 1.52; 95% CI, 1.25-1.85) veterans.
- CDK12 alterations significantly increased the hazard of death in non-Hispanic Black veterans (HR, 2.04; 95% CI, 1.13-3.67), while immunotherapy targets were associated with increased mortality in non-Hispanic White veterans (HR, 1.44; 95% CI, 1.02-2.02).
IN PRACTICE: " we did not identify any genomic alterations or biomarkers that should not be tested in PCa based on patient self-identified race. Ultimately, this work emphasizes that precision oncology enables the individualization of treatment decisions without having to rely on imprecise characteristics such as self-identified race.," wrote the study authors.
SOURCE: Isla P. Garraway, MD, PhD; Kosj Yamoah, MD, PhD; and Kara N. Maxwell, MD, PhD were co-senior authors. The article was published online on May 12 in JAMA Network Open.
LIMITATIONS: According to the authors, a lack of matched germline data for patients, complicated the interpretation of plasma results. In addition, survivorship bias may have inadvertently excluded the most aggressive metastatic prostate cancer phenotypes, as patients who did not live long enough to undergo NGS testing were not included. Results seen in the veteran population served by the Veterans Health Administration may not be generalizable to the broader population.
DISCLOSURES: The study received support from Challenge Award PCF22CHALO2 from the Prostate Cancer Foundation and the Veterans Affairs National Precision Oncology Program. Luca F. Valle, MD, reported receiving grant support from the Bristol Myers Squibb Foundation during the conduct of the study. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
TOPLINE: Next-generation sequencing (NGS) analysis of 5015 veterans with metastatic prostate cancer reveals distinct genomic patterns between non-Hispanic Black and White patients, with Black veterans showing higher odds of immunotherapy targets but lower odds of androgen receptor axis alterations. However, the rates of survival were similar despite the differences.
METHODOLOGY:
Researchers conducted a retrospective cohort study comparing alteration frequencies between 1784 non-Hispanic Black (35.6%) and 3,231 non-Hispanic White (64.4%) veterans who underwent NGS testing from January 23, 2019, to November 2, 2023.
- Analysis included DNA sequencing data from tissue or plasma biospecimens, including prostate biopsy specimens, radical prostatectomy specimens, and prostate cancer metastases, all sequenced with FoundationOne CDx or FoundationOne Liquid CDx platforms.
- Investigators examined pathogenic alterations in individual genes, actionable targets, and canonical prostate cancer pathways, while adjusting for NGS analyte and clinicopathologic covariates.
- Researchers evaluated associations between alteration frequency and race as well as survival through Cox proportional hazards modeling, stratified by race and adjusted for clinical factors.
TAKEAWAY:
Non-Hispanic Black race and ethnicity was associated with higher odds of genomic alterations in SPOP (odds ratio [OR], 1.7; 95% confidence interval [CI], 1.2-2.6) and immunotherapy targets (OR, 1.7; 95% CI, 1.1-2.5), including high microsatellite instability status (OR, 3.1; 95% CI, 1.1-9.4).
- Non-Hispanic Black veterans showed lower odds of genomic alterations in the AKT/PI3K pathway (OR, 0.6; 95% CI, 0.4-0.7), androgen receptor axis (OR, 0.7; 95% CI, 0.5-0.9), and tumor suppressor genes (OR, 0.7; 95% CI, 0.5-0.8).
- Tumor suppressor alterations were associated with shorter overall survival in both non-Hispanic Black (hazard ratio [HR], 1.54; 95% CI, 1.13-2.11) and non-Hispanic White (HR, 1.52; 95% CI, 1.25-1.85) veterans.
- CDK12 alterations significantly increased the hazard of death in non-Hispanic Black veterans (HR, 2.04; 95% CI, 1.13-3.67), while immunotherapy targets were associated with increased mortality in non-Hispanic White veterans (HR, 1.44; 95% CI, 1.02-2.02).
IN PRACTICE: " we did not identify any genomic alterations or biomarkers that should not be tested in PCa based on patient self-identified race. Ultimately, this work emphasizes that precision oncology enables the individualization of treatment decisions without having to rely on imprecise characteristics such as self-identified race.," wrote the study authors.
SOURCE: Isla P. Garraway, MD, PhD; Kosj Yamoah, MD, PhD; and Kara N. Maxwell, MD, PhD were co-senior authors. The article was published online on May 12 in JAMA Network Open.
LIMITATIONS: According to the authors, a lack of matched germline data for patients, complicated the interpretation of plasma results. In addition, survivorship bias may have inadvertently excluded the most aggressive metastatic prostate cancer phenotypes, as patients who did not live long enough to undergo NGS testing were not included. Results seen in the veteran population served by the Veterans Health Administration may not be generalizable to the broader population.
DISCLOSURES: The study received support from Challenge Award PCF22CHALO2 from the Prostate Cancer Foundation and the Veterans Affairs National Precision Oncology Program. Luca F. Valle, MD, reported receiving grant support from the Bristol Myers Squibb Foundation during the conduct of the study. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
TOPLINE: Next-generation sequencing (NGS) analysis of 5015 veterans with metastatic prostate cancer reveals distinct genomic patterns between non-Hispanic Black and White patients, with Black veterans showing higher odds of immunotherapy targets but lower odds of androgen receptor axis alterations. However, the rates of survival were similar despite the differences.
METHODOLOGY:
Researchers conducted a retrospective cohort study comparing alteration frequencies between 1784 non-Hispanic Black (35.6%) and 3,231 non-Hispanic White (64.4%) veterans who underwent NGS testing from January 23, 2019, to November 2, 2023.
- Analysis included DNA sequencing data from tissue or plasma biospecimens, including prostate biopsy specimens, radical prostatectomy specimens, and prostate cancer metastases, all sequenced with FoundationOne CDx or FoundationOne Liquid CDx platforms.
- Investigators examined pathogenic alterations in individual genes, actionable targets, and canonical prostate cancer pathways, while adjusting for NGS analyte and clinicopathologic covariates.
- Researchers evaluated associations between alteration frequency and race as well as survival through Cox proportional hazards modeling, stratified by race and adjusted for clinical factors.
TAKEAWAY:
Non-Hispanic Black race and ethnicity was associated with higher odds of genomic alterations in SPOP (odds ratio [OR], 1.7; 95% confidence interval [CI], 1.2-2.6) and immunotherapy targets (OR, 1.7; 95% CI, 1.1-2.5), including high microsatellite instability status (OR, 3.1; 95% CI, 1.1-9.4).
- Non-Hispanic Black veterans showed lower odds of genomic alterations in the AKT/PI3K pathway (OR, 0.6; 95% CI, 0.4-0.7), androgen receptor axis (OR, 0.7; 95% CI, 0.5-0.9), and tumor suppressor genes (OR, 0.7; 95% CI, 0.5-0.8).
- Tumor suppressor alterations were associated with shorter overall survival in both non-Hispanic Black (hazard ratio [HR], 1.54; 95% CI, 1.13-2.11) and non-Hispanic White (HR, 1.52; 95% CI, 1.25-1.85) veterans.
- CDK12 alterations significantly increased the hazard of death in non-Hispanic Black veterans (HR, 2.04; 95% CI, 1.13-3.67), while immunotherapy targets were associated with increased mortality in non-Hispanic White veterans (HR, 1.44; 95% CI, 1.02-2.02).
IN PRACTICE: " we did not identify any genomic alterations or biomarkers that should not be tested in PCa based on patient self-identified race. Ultimately, this work emphasizes that precision oncology enables the individualization of treatment decisions without having to rely on imprecise characteristics such as self-identified race.," wrote the study authors.
SOURCE: Isla P. Garraway, MD, PhD; Kosj Yamoah, MD, PhD; and Kara N. Maxwell, MD, PhD were co-senior authors. The article was published online on May 12 in JAMA Network Open.
LIMITATIONS: According to the authors, a lack of matched germline data for patients, complicated the interpretation of plasma results. In addition, survivorship bias may have inadvertently excluded the most aggressive metastatic prostate cancer phenotypes, as patients who did not live long enough to undergo NGS testing were not included. Results seen in the veteran population served by the Veterans Health Administration may not be generalizable to the broader population.
DISCLOSURES: The study received support from Challenge Award PCF22CHALO2 from the Prostate Cancer Foundation and the Veterans Affairs National Precision Oncology Program. Luca F. Valle, MD, reported receiving grant support from the Bristol Myers Squibb Foundation during the conduct of the study. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
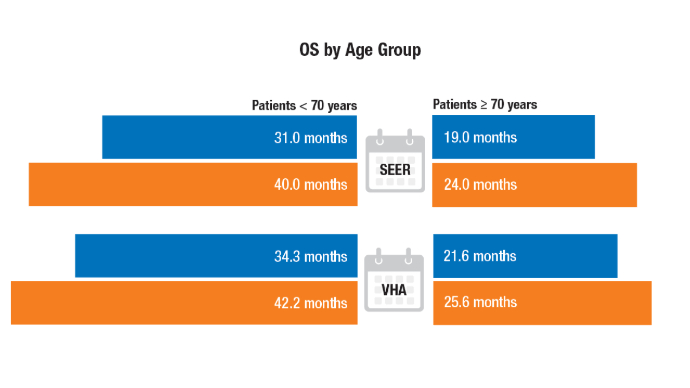
Racial Disparities, Germline Testing, and Improved Overall Survival in Prostate Cancer
Racial Disparities, Germline Testing, and Improved Overall Survival in Prostate Cancer
Click here to view more from Cancer Data Trends 2025.
References
Lillard JW Jr, Moses KA, Mahal BA, George DJ. Racial disparities in Black men with prostate cancer: A literature review. Cancer. 2022 Nov 1;128(21):3787-3795. doi:10.1002/cncr.34433
Wang BR, Chen Y-A, Kao W-H, Lai C-H, Lin H, Hsieh J-T. Developing New Treatment Options for Castration-Resistant Prostate Cancer and Recurrent Disease. Biomedicines. 2022 Aug 3;10(8):1872. doi:10.3390/biomedicines10081872
Valle LF, Li J, Desai H, Hausler R, et al. Oncogenic Alterations, Race, and Survival in US Veterans with Metastatic Prostate Cancer Undergoing Somatic Tumor Next Generation Sequencing. bioRxiv [Preprint]. 2024 Oct 25:2024.10.24.620071. doi:10.1101/2024.10.24.620071
Kwon DH, Scheuner MT, McPhaul M, et al. Germline testing for veterans with advanced prostate cancer: concerns about service-connected benefits. JNCI Cancer Spectr. 2024 Sep 2;8(5):pkae079. doi:10.1093/jncics/pkae079
Kwon DH, McPhaul M, Sumra S, et al. Informed decision-making about germline testing among Veterans with advanced prostate cancer (APC): A mixed-methods study. J Clin Oncol. 2024;42(16_suppl):5105. doi:10.1200/JCO.2024.42.16_suppl.5105
Schoen MW, Montgomery RB, Owens L, Khan S, Sanfilippo KM, Etzioni RB. Survival in Patients With De Novo Metastatic Prostate Cancer. JAMA Netw Open. 2024 Mar 4;7(3):e241970. doi: 10.1001/jamanetworkopen.2024.1970
Schafer EJ, Jemal A, Wiese D, et al. Disparities and Trends in Genitourinary Cancer Incidence and Mortality in the USA. Eur Urol. 2023 Jul;84(1):117-126. doi:10.1016/j.eururo.2022.11.023
U.S. Department of Veterans Affairs. Hines VA Hospital & Loyola University Chicago Physician Awarded $8.6M VA Research Grant. November 8, 2021. https://www.va.gov/hines-health-care/news-releases/hines-va-hospital-loyola-university-chicago-physician-awarded-86m-va-research-grant/ Accessed December 31, 2024.
U.S. Department of Veterans Affairs. National Oncology Program. How VA is Advancing Prostate Cancer Care. https://www.cancer.va.gov/prostate.html Accessed December 31, 2024.
Click here to view more from Cancer Data Trends 2025.
Click here to view more from Cancer Data Trends 2025.
References
Lillard JW Jr, Moses KA, Mahal BA, George DJ. Racial disparities in Black men with prostate cancer: A literature review. Cancer. 2022 Nov 1;128(21):3787-3795. doi:10.1002/cncr.34433
Wang BR, Chen Y-A, Kao W-H, Lai C-H, Lin H, Hsieh J-T. Developing New Treatment Options for Castration-Resistant Prostate Cancer and Recurrent Disease. Biomedicines. 2022 Aug 3;10(8):1872. doi:10.3390/biomedicines10081872
Valle LF, Li J, Desai H, Hausler R, et al. Oncogenic Alterations, Race, and Survival in US Veterans with Metastatic Prostate Cancer Undergoing Somatic Tumor Next Generation Sequencing. bioRxiv [Preprint]. 2024 Oct 25:2024.10.24.620071. doi:10.1101/2024.10.24.620071
Kwon DH, Scheuner MT, McPhaul M, et al. Germline testing for veterans with advanced prostate cancer: concerns about service-connected benefits. JNCI Cancer Spectr. 2024 Sep 2;8(5):pkae079. doi:10.1093/jncics/pkae079
Kwon DH, McPhaul M, Sumra S, et al. Informed decision-making about germline testing among Veterans with advanced prostate cancer (APC): A mixed-methods study. J Clin Oncol. 2024;42(16_suppl):5105. doi:10.1200/JCO.2024.42.16_suppl.5105
Schoen MW, Montgomery RB, Owens L, Khan S, Sanfilippo KM, Etzioni RB. Survival in Patients With De Novo Metastatic Prostate Cancer. JAMA Netw Open. 2024 Mar 4;7(3):e241970. doi: 10.1001/jamanetworkopen.2024.1970
Schafer EJ, Jemal A, Wiese D, et al. Disparities and Trends in Genitourinary Cancer Incidence and Mortality in the USA. Eur Urol. 2023 Jul;84(1):117-126. doi:10.1016/j.eururo.2022.11.023
U.S. Department of Veterans Affairs. Hines VA Hospital & Loyola University Chicago Physician Awarded $8.6M VA Research Grant. November 8, 2021. https://www.va.gov/hines-health-care/news-releases/hines-va-hospital-loyola-university-chicago-physician-awarded-86m-va-research-grant/ Accessed December 31, 2024.
U.S. Department of Veterans Affairs. National Oncology Program. How VA is Advancing Prostate Cancer Care. https://www.cancer.va.gov/prostate.html Accessed December 31, 2024.
References
Lillard JW Jr, Moses KA, Mahal BA, George DJ. Racial disparities in Black men with prostate cancer: A literature review. Cancer. 2022 Nov 1;128(21):3787-3795. doi:10.1002/cncr.34433
Wang BR, Chen Y-A, Kao W-H, Lai C-H, Lin H, Hsieh J-T. Developing New Treatment Options for Castration-Resistant Prostate Cancer and Recurrent Disease. Biomedicines. 2022 Aug 3;10(8):1872. doi:10.3390/biomedicines10081872
Valle LF, Li J, Desai H, Hausler R, et al. Oncogenic Alterations, Race, and Survival in US Veterans with Metastatic Prostate Cancer Undergoing Somatic Tumor Next Generation Sequencing. bioRxiv [Preprint]. 2024 Oct 25:2024.10.24.620071. doi:10.1101/2024.10.24.620071
Kwon DH, Scheuner MT, McPhaul M, et al. Germline testing for veterans with advanced prostate cancer: concerns about service-connected benefits. JNCI Cancer Spectr. 2024 Sep 2;8(5):pkae079. doi:10.1093/jncics/pkae079
Kwon DH, McPhaul M, Sumra S, et al. Informed decision-making about germline testing among Veterans with advanced prostate cancer (APC): A mixed-methods study. J Clin Oncol. 2024;42(16_suppl):5105. doi:10.1200/JCO.2024.42.16_suppl.5105
Schoen MW, Montgomery RB, Owens L, Khan S, Sanfilippo KM, Etzioni RB. Survival in Patients With De Novo Metastatic Prostate Cancer. JAMA Netw Open. 2024 Mar 4;7(3):e241970. doi: 10.1001/jamanetworkopen.2024.1970
Schafer EJ, Jemal A, Wiese D, et al. Disparities and Trends in Genitourinary Cancer Incidence and Mortality in the USA. Eur Urol. 2023 Jul;84(1):117-126. doi:10.1016/j.eururo.2022.11.023
U.S. Department of Veterans Affairs. Hines VA Hospital & Loyola University Chicago Physician Awarded $8.6M VA Research Grant. November 8, 2021. https://www.va.gov/hines-health-care/news-releases/hines-va-hospital-loyola-university-chicago-physician-awarded-86m-va-research-grant/ Accessed December 31, 2024.
U.S. Department of Veterans Affairs. National Oncology Program. How VA is Advancing Prostate Cancer Care. https://www.cancer.va.gov/prostate.html Accessed December 31, 2024.
Racial Disparities, Germline Testing, and Improved Overall Survival in Prostate Cancer
Racial Disparities, Germline Testing, and Improved Overall Survival in Prostate Cancer
Study Gives Clinical Edge to Transperineal Prostate Biopsies
In the largest head-to-head randomized trial of its kind, UK researchers found transperineal prostate biopsies using local anesthesia (LATP) superior to the transrectal approach in detecting clinically significant cancers.
The TRANSLATE study, with more than 1100 patients, found LATP identified 5.7% more cases of clinically significant prostate cancer, defined as Grade Group 2 or higher, than biopsies using transrectal ultrasonography (TRUS).
Previous research comparing the two techniques has focused mainly on rates of infection rather than cancer detection, said Richard Bryant, PhD, a consultant urologist at Nuffield Department of Surgical Sciences, University of Oxford in Oxford, England, who led the trial.
“We decided that the most important thing to look at is the detection rate of clinically significant prostate cancer, because that is why the man is having the biopsy in the first place, rather than to avoid infection, although avoiding infection is of course also important,” Bryant said.
Bryant presented the findings at the 2025 annual congress of the European Association of Urology and his group published the results in The Lancet Oncology.
The TRANSLATE trial was powered to identify a difference in the rate of cancer detection but not factors such as pain and sepsis. Hospitalization after biopsies served as a proxy for sepsis.
Men in the trial were nearly twice as likely to report LATP to be problematic immediately after the procedure than those who underwent transrectal biopsies. These issues included pain, discomfort, and embarrassment.
Two of the 562 men in the LATP group were hospitalized within 35 days of the procedure compared with nine of the 564 in the TRUS group. Bryant said this trend favored LATP, but the difference did not reach statistical significance.
The data on infection and other secondary outcomes were encouraging, but not conclusive, Bryant’s group reported.
Richard Szabo, MD, a prostate biopsy researcher at University of California Irvine, said the reduction in post-biopsy sepsis has been “an additional major advantage” of transperineal over transrectal prostate biopsy.
Almost 90% of men who received LATP had the biopsies without antibiotics — a “bonus,” Bryant said.
Antibiotic stewardship is a major factor in research and policies regarding biopsies in Europe. Transperineal biopsies avoid the rectum and pass needles through the perineum between the anus and the testicles, reducing risk for infection.
Jim Hu, MD, a urologic oncology researcher at Weill Cornell in New York City and the principal investigator on the 2024 PREVENT trial, said three of four randomized trials reported in the past year, including PREVENT and TRANSLATE, have found transperineal biopsies resulted in fewer infections than the transrectal method.
European guidelines call transperineal biopsy the preferred approach based on infection concerns, whereas guidance from the American Urological Association gives equal weight to transperineal and transrectal biopsies.
Badar Mian, MD, a urologist at Albany Med Health System in Albany, New York, said the field should “shift our focus from picking a winner to instead focus on whether prostate biopsy procedures are safe and effective. Patients should be reassured that, while there are trade-offs, both procedures can be performed safely and with a high degree of accuracy.”
The UK’s National Institute for Health and Care Research funded TRANSLATE. Bryant received support from BXTAccelyon to attend biopsy training provided by Guys’ Hospital, in London, England.
Howard Wolinsky is a Chicago-based freelance writer.
A version of this article appeared at Medscape.com.
In the largest head-to-head randomized trial of its kind, UK researchers found transperineal prostate biopsies using local anesthesia (LATP) superior to the transrectal approach in detecting clinically significant cancers.
The TRANSLATE study, with more than 1100 patients, found LATP identified 5.7% more cases of clinically significant prostate cancer, defined as Grade Group 2 or higher, than biopsies using transrectal ultrasonography (TRUS).
Previous research comparing the two techniques has focused mainly on rates of infection rather than cancer detection, said Richard Bryant, PhD, a consultant urologist at Nuffield Department of Surgical Sciences, University of Oxford in Oxford, England, who led the trial.
“We decided that the most important thing to look at is the detection rate of clinically significant prostate cancer, because that is why the man is having the biopsy in the first place, rather than to avoid infection, although avoiding infection is of course also important,” Bryant said.
Bryant presented the findings at the 2025 annual congress of the European Association of Urology and his group published the results in The Lancet Oncology.
The TRANSLATE trial was powered to identify a difference in the rate of cancer detection but not factors such as pain and sepsis. Hospitalization after biopsies served as a proxy for sepsis.
Men in the trial were nearly twice as likely to report LATP to be problematic immediately after the procedure than those who underwent transrectal biopsies. These issues included pain, discomfort, and embarrassment.
Two of the 562 men in the LATP group were hospitalized within 35 days of the procedure compared with nine of the 564 in the TRUS group. Bryant said this trend favored LATP, but the difference did not reach statistical significance.
The data on infection and other secondary outcomes were encouraging, but not conclusive, Bryant’s group reported.
Richard Szabo, MD, a prostate biopsy researcher at University of California Irvine, said the reduction in post-biopsy sepsis has been “an additional major advantage” of transperineal over transrectal prostate biopsy.
Almost 90% of men who received LATP had the biopsies without antibiotics — a “bonus,” Bryant said.
Antibiotic stewardship is a major factor in research and policies regarding biopsies in Europe. Transperineal biopsies avoid the rectum and pass needles through the perineum between the anus and the testicles, reducing risk for infection.
Jim Hu, MD, a urologic oncology researcher at Weill Cornell in New York City and the principal investigator on the 2024 PREVENT trial, said three of four randomized trials reported in the past year, including PREVENT and TRANSLATE, have found transperineal biopsies resulted in fewer infections than the transrectal method.
European guidelines call transperineal biopsy the preferred approach based on infection concerns, whereas guidance from the American Urological Association gives equal weight to transperineal and transrectal biopsies.
Badar Mian, MD, a urologist at Albany Med Health System in Albany, New York, said the field should “shift our focus from picking a winner to instead focus on whether prostate biopsy procedures are safe and effective. Patients should be reassured that, while there are trade-offs, both procedures can be performed safely and with a high degree of accuracy.”
The UK’s National Institute for Health and Care Research funded TRANSLATE. Bryant received support from BXTAccelyon to attend biopsy training provided by Guys’ Hospital, in London, England.
Howard Wolinsky is a Chicago-based freelance writer.
A version of this article appeared at Medscape.com.
In the largest head-to-head randomized trial of its kind, UK researchers found transperineal prostate biopsies using local anesthesia (LATP) superior to the transrectal approach in detecting clinically significant cancers.
The TRANSLATE study, with more than 1100 patients, found LATP identified 5.7% more cases of clinically significant prostate cancer, defined as Grade Group 2 or higher, than biopsies using transrectal ultrasonography (TRUS).
Previous research comparing the two techniques has focused mainly on rates of infection rather than cancer detection, said Richard Bryant, PhD, a consultant urologist at Nuffield Department of Surgical Sciences, University of Oxford in Oxford, England, who led the trial.
“We decided that the most important thing to look at is the detection rate of clinically significant prostate cancer, because that is why the man is having the biopsy in the first place, rather than to avoid infection, although avoiding infection is of course also important,” Bryant said.
Bryant presented the findings at the 2025 annual congress of the European Association of Urology and his group published the results in The Lancet Oncology.
The TRANSLATE trial was powered to identify a difference in the rate of cancer detection but not factors such as pain and sepsis. Hospitalization after biopsies served as a proxy for sepsis.
Men in the trial were nearly twice as likely to report LATP to be problematic immediately after the procedure than those who underwent transrectal biopsies. These issues included pain, discomfort, and embarrassment.
Two of the 562 men in the LATP group were hospitalized within 35 days of the procedure compared with nine of the 564 in the TRUS group. Bryant said this trend favored LATP, but the difference did not reach statistical significance.
The data on infection and other secondary outcomes were encouraging, but not conclusive, Bryant’s group reported.
Richard Szabo, MD, a prostate biopsy researcher at University of California Irvine, said the reduction in post-biopsy sepsis has been “an additional major advantage” of transperineal over transrectal prostate biopsy.
Almost 90% of men who received LATP had the biopsies without antibiotics — a “bonus,” Bryant said.
Antibiotic stewardship is a major factor in research and policies regarding biopsies in Europe. Transperineal biopsies avoid the rectum and pass needles through the perineum between the anus and the testicles, reducing risk for infection.
Jim Hu, MD, a urologic oncology researcher at Weill Cornell in New York City and the principal investigator on the 2024 PREVENT trial, said three of four randomized trials reported in the past year, including PREVENT and TRANSLATE, have found transperineal biopsies resulted in fewer infections than the transrectal method.
European guidelines call transperineal biopsy the preferred approach based on infection concerns, whereas guidance from the American Urological Association gives equal weight to transperineal and transrectal biopsies.
Badar Mian, MD, a urologist at Albany Med Health System in Albany, New York, said the field should “shift our focus from picking a winner to instead focus on whether prostate biopsy procedures are safe and effective. Patients should be reassured that, while there are trade-offs, both procedures can be performed safely and with a high degree of accuracy.”
The UK’s National Institute for Health and Care Research funded TRANSLATE. Bryant received support from BXTAccelyon to attend biopsy training provided by Guys’ Hospital, in London, England.
Howard Wolinsky is a Chicago-based freelance writer.
A version of this article appeared at Medscape.com.
A Better Biopsy for Prostate Cancer?
Micro-ultrasound–guided biopsies were found for the first time to be “noninferior” to MRI-guided procedures, according to new research presented at the 2025 annual congress of the European Association of Urology.
The OPTIMUM study found 4.5% more clinically significant cancers among men who underwent micro-ultrasound–guided biopsies of the prostate than in those scanned using MRI.
“The take-home message is that men being worked up for an elevated PSA [prostate-specific antigen] or an abnormal digital rectal examination who are at increased risk of prostate cancer may safely undergo a micro-ultrasound–guided biopsy rather than an MRI-guided biopsy,” said Adam Kinnaird, MD, PhD, the Frank and Carla Sojonky Chair in Prostate Cancer Research at the University of Alberta, Edmonton, Alberta, Canada, and principal investigator of the study.
Micro-ultrasound can image to as small as 70 μm, ie, the width of a human hair.
OPTIMUM was an international, open-label, randomized, noninferiority trial in 20 centers in eight countries of men with clinical suspicion of prostate cancer, elevated PSAs, abnormal digital rectal exams, or a combination of these risk factors. None of the men previously had undergone biopsies.
The study had three arms to which men were assigned randomly: Micro-ultrasound–guided biopsy (n = 121); biopsies guided by micro-ultrasound and fusion MRI (n = 226), and MRI plus conventional ultrasound–guided biopsy (n = 331).
Subjects had a median age of 65 years and a median PSA level of 6.9 ng/mL; 83% self-identified as White individuals.
“Micro-ultrasound was found to be no worse than MRI at the detection of clinically significant prostate cancer. We don’t show it is equivalent. We don’t show it better. We show it is not worse,” Kinnaird said.
The study, funded by Exact Imaging, which makes the ExactVu micro-ultrasound platform, appeared simultaneously in JAMA.
Laurence Klotz, MD, the Sunnybrook Chair of Prostate Cancer Research at the University of Toronto Sunnybrook Health Sciences Centre, and senior researcher on the OPTIMUM trial, said as the incidence of prostate cancer rises globally, micro-ultrasound may be of particular value in low-income and middle-income countries where MRI is not widely available.
“It’s extremely appealing in places that can’t offer MRI to everyone, but I think it also will have a role going forward in regions where there is no problem about getting access to MRI,” Klotz told Medscape Medical News.
This group is next studying the financial aspects of the technology, he added.
Gerald Andriole, MD, then urology chief at Washington University in St. Louis, St. Louis, Missouri, designed the original studies of the ExactVu system, which the US Food and Drug Administration approved in 2017.
Andriole, now chief medical officer of Prostatype Genomics, said MRIs are costly, subjective, and uncomfortable for many patients, due to claustrophobia and obesity, requiring complicated co-registration procedures to perform an accurate targeted biopsy into the most worrisome regions of the prostate. “Proceeding directly to a micro-ultrasound study avoids these impediments to discovering whether the patient has clinically significant cancer,” he said.
Micro-ultrasound testing involves a single visit to a urologist whereas MRI requires two trips for the patient — one to the urologist and the other to a radiologist, Klotz said. “It’s one-stop shopping,” he said. “So, the patient has his micro-ultrasound. If there’s a target found, he then does the targeted biopsy.”
Klotz said micro-ultrasound helps patients avoid the expense and health risks of gadolinium in contrast with MRIs.
“I don’t think micro-ultrasound is going to replace MRIs,” he said. “I think they’re somewhat complementary. You get cases where they’re visible on MRI and not visible on micro-ultrasound and vice versa.”
The researchers received a grant from Exact Imaging.
A version of this article appeared at Medscape.com.
Howard Wolinsky is a Chicago-based freelance writer.
Micro-ultrasound–guided biopsies were found for the first time to be “noninferior” to MRI-guided procedures, according to new research presented at the 2025 annual congress of the European Association of Urology.
The OPTIMUM study found 4.5% more clinically significant cancers among men who underwent micro-ultrasound–guided biopsies of the prostate than in those scanned using MRI.
“The take-home message is that men being worked up for an elevated PSA [prostate-specific antigen] or an abnormal digital rectal examination who are at increased risk of prostate cancer may safely undergo a micro-ultrasound–guided biopsy rather than an MRI-guided biopsy,” said Adam Kinnaird, MD, PhD, the Frank and Carla Sojonky Chair in Prostate Cancer Research at the University of Alberta, Edmonton, Alberta, Canada, and principal investigator of the study.
Micro-ultrasound can image to as small as 70 μm, ie, the width of a human hair.
OPTIMUM was an international, open-label, randomized, noninferiority trial in 20 centers in eight countries of men with clinical suspicion of prostate cancer, elevated PSAs, abnormal digital rectal exams, or a combination of these risk factors. None of the men previously had undergone biopsies.
The study had three arms to which men were assigned randomly: Micro-ultrasound–guided biopsy (n = 121); biopsies guided by micro-ultrasound and fusion MRI (n = 226), and MRI plus conventional ultrasound–guided biopsy (n = 331).
Subjects had a median age of 65 years and a median PSA level of 6.9 ng/mL; 83% self-identified as White individuals.
“Micro-ultrasound was found to be no worse than MRI at the detection of clinically significant prostate cancer. We don’t show it is equivalent. We don’t show it better. We show it is not worse,” Kinnaird said.
The study, funded by Exact Imaging, which makes the ExactVu micro-ultrasound platform, appeared simultaneously in JAMA.
Laurence Klotz, MD, the Sunnybrook Chair of Prostate Cancer Research at the University of Toronto Sunnybrook Health Sciences Centre, and senior researcher on the OPTIMUM trial, said as the incidence of prostate cancer rises globally, micro-ultrasound may be of particular value in low-income and middle-income countries where MRI is not widely available.
“It’s extremely appealing in places that can’t offer MRI to everyone, but I think it also will have a role going forward in regions where there is no problem about getting access to MRI,” Klotz told Medscape Medical News.
This group is next studying the financial aspects of the technology, he added.
Gerald Andriole, MD, then urology chief at Washington University in St. Louis, St. Louis, Missouri, designed the original studies of the ExactVu system, which the US Food and Drug Administration approved in 2017.
Andriole, now chief medical officer of Prostatype Genomics, said MRIs are costly, subjective, and uncomfortable for many patients, due to claustrophobia and obesity, requiring complicated co-registration procedures to perform an accurate targeted biopsy into the most worrisome regions of the prostate. “Proceeding directly to a micro-ultrasound study avoids these impediments to discovering whether the patient has clinically significant cancer,” he said.
Micro-ultrasound testing involves a single visit to a urologist whereas MRI requires two trips for the patient — one to the urologist and the other to a radiologist, Klotz said. “It’s one-stop shopping,” he said. “So, the patient has his micro-ultrasound. If there’s a target found, he then does the targeted biopsy.”
Klotz said micro-ultrasound helps patients avoid the expense and health risks of gadolinium in contrast with MRIs.
“I don’t think micro-ultrasound is going to replace MRIs,” he said. “I think they’re somewhat complementary. You get cases where they’re visible on MRI and not visible on micro-ultrasound and vice versa.”
The researchers received a grant from Exact Imaging.
A version of this article appeared at Medscape.com.
Howard Wolinsky is a Chicago-based freelance writer.
Micro-ultrasound–guided biopsies were found for the first time to be “noninferior” to MRI-guided procedures, according to new research presented at the 2025 annual congress of the European Association of Urology.
The OPTIMUM study found 4.5% more clinically significant cancers among men who underwent micro-ultrasound–guided biopsies of the prostate than in those scanned using MRI.
“The take-home message is that men being worked up for an elevated PSA [prostate-specific antigen] or an abnormal digital rectal examination who are at increased risk of prostate cancer may safely undergo a micro-ultrasound–guided biopsy rather than an MRI-guided biopsy,” said Adam Kinnaird, MD, PhD, the Frank and Carla Sojonky Chair in Prostate Cancer Research at the University of Alberta, Edmonton, Alberta, Canada, and principal investigator of the study.
Micro-ultrasound can image to as small as 70 μm, ie, the width of a human hair.
OPTIMUM was an international, open-label, randomized, noninferiority trial in 20 centers in eight countries of men with clinical suspicion of prostate cancer, elevated PSAs, abnormal digital rectal exams, or a combination of these risk factors. None of the men previously had undergone biopsies.
The study had three arms to which men were assigned randomly: Micro-ultrasound–guided biopsy (n = 121); biopsies guided by micro-ultrasound and fusion MRI (n = 226), and MRI plus conventional ultrasound–guided biopsy (n = 331).
Subjects had a median age of 65 years and a median PSA level of 6.9 ng/mL; 83% self-identified as White individuals.
“Micro-ultrasound was found to be no worse than MRI at the detection of clinically significant prostate cancer. We don’t show it is equivalent. We don’t show it better. We show it is not worse,” Kinnaird said.
The study, funded by Exact Imaging, which makes the ExactVu micro-ultrasound platform, appeared simultaneously in JAMA.
Laurence Klotz, MD, the Sunnybrook Chair of Prostate Cancer Research at the University of Toronto Sunnybrook Health Sciences Centre, and senior researcher on the OPTIMUM trial, said as the incidence of prostate cancer rises globally, micro-ultrasound may be of particular value in low-income and middle-income countries where MRI is not widely available.
“It’s extremely appealing in places that can’t offer MRI to everyone, but I think it also will have a role going forward in regions where there is no problem about getting access to MRI,” Klotz told Medscape Medical News.
This group is next studying the financial aspects of the technology, he added.
Gerald Andriole, MD, then urology chief at Washington University in St. Louis, St. Louis, Missouri, designed the original studies of the ExactVu system, which the US Food and Drug Administration approved in 2017.
Andriole, now chief medical officer of Prostatype Genomics, said MRIs are costly, subjective, and uncomfortable for many patients, due to claustrophobia and obesity, requiring complicated co-registration procedures to perform an accurate targeted biopsy into the most worrisome regions of the prostate. “Proceeding directly to a micro-ultrasound study avoids these impediments to discovering whether the patient has clinically significant cancer,” he said.
Micro-ultrasound testing involves a single visit to a urologist whereas MRI requires two trips for the patient — one to the urologist and the other to a radiologist, Klotz said. “It’s one-stop shopping,” he said. “So, the patient has his micro-ultrasound. If there’s a target found, he then does the targeted biopsy.”
Klotz said micro-ultrasound helps patients avoid the expense and health risks of gadolinium in contrast with MRIs.
“I don’t think micro-ultrasound is going to replace MRIs,” he said. “I think they’re somewhat complementary. You get cases where they’re visible on MRI and not visible on micro-ultrasound and vice versa.”
The researchers received a grant from Exact Imaging.
A version of this article appeared at Medscape.com.
Howard Wolinsky is a Chicago-based freelance writer.
Inadequate Grading of Intraductal Carcinoma of the Prostate
BOSTON — Solid intraductal carcinoma of the prostate (IDC-P) is associated with significantly worse outcomes compared with conventional Gleason grade 5 prostate cancers and is more commonly present in metastatic than nonmetastatic cancers, according to two studies presented this week at the United States and Canadian Academy of Pathology (USCAP) 2025 Annual Meeting.
“Our findings suggest that solid IDC-P is more aggressive than Gleason grade 5 conventional prostate adenocarcinoma or cribriform IDC-P,” and it may therefore be better not to consider it as a grade 5 pattern, said first author of one of the studies, Hangchuan Shi, MD, PhD, of the University of Rochester Medical Center, in Rochester, New York.
Although IDC-P — reported in about 20% of men with prostate cancer — is known to be associated with poorer response to treatment, there is a debate over whether to grade the entity with Gleason scoring or not.
The International Society of Urological Pathology recommends incorporating IDC-P into the Gleason score, while the Genitourinary Pathology Society does not.
To evaluate the prognostic significance of solid IDC-P compared with Gleason grade 5 conventional prostate cancer, Shi and his colleagues identified 115 cases in the surgical pathology database at the University of Rochester Medical Center between 2008 and 2015 involving Gleason grade 5 conventional prostatic adenocarcinoma as a primary, secondary, or tertiary pattern, as well as cribriform IDC-P.
The researchers excluded cases showing comedonecrosis within IDC-P, due to the potential for worse outcomes.
Of the grade 5 conventional prostate cancer cases with cribriform carcinoma, 28 (24.3%) had solid nest pattern IDC-P. Patients with and without solid IDC-P had a matching mean age of about 64 years, and their mean preoperative PSA was about 12.27 ng/mL.
Adjuvant therapy prior to recurrence was significantly more common in those who had solid IDC-P (60.7% vs 34.5%; P = .016).
Compared with the conventional prostate cancer cases, those with solid IDC-P had a significantly higher incidence of lymph node metastasis (P = .014) and had larger estimated tumor volume (P = .011).
There were no significant differences in other clinicopathologic features, such as preoperative prostate-specific antigen, grade group, pT stage, and surgical margin status.
After adjustment for key factors in a multivariable analysis, solid IDC-P was significantly associated with poorer recurrence-free survival (P = .007), and poorer cancer-specific survival (P = .004).
Finally, solid IDC-P was an independent predictor of recurrence (hazard ratio [HR] 1.960; P = .031), whereas other measures, including prostate-specific antigen (PSA), cancer grade, pT, lymph node metastasis, surgical and tumor volume were not significant factors.
“We found the solid IDC-P patients had almost two-times the risk of recurrence compared with the patients without solid IDC-P in our study,” Shi said.
The findings underscore the importance of accurately identifying IDC-P, senior author Hiroshi Miyamoto, MD, PhD, director of Genitourinary Pathology at School of Medicine and Dentistry, University of Rochester, Rochester, New York, told Medscape Medical News.
“It may be difficult for some pathologists, especially those who have no specific training in genitourinary pathology, to adequately recognize” this form of cancer, he said.
Although it is recognized as an aggressive form of prostate cancer, “based on our studies, we believe that it is inadequate to grade IDC-P” as a Gleason grade 5 cancer, Miyamoto added.
IDC More Common in Metastases
Poorer outcomes associated with IDC-P were further described in a post hoc sub-analysis of the phase 3, prospective PATRON clinical trial that is evaluating prostate-specific membrane antigen (PSMA) PET-CT–guided intensification of therapy.
In the multicenter trial, 825 patients were stratified into three cohorts: High-risk patients receiving radiation therapy (45%), high-risk patients receiving salvage radiation therapy post-radical prostatectomy (47%), and those receiving a radical prostatectomy (8%).
The patients in all three cohorts were randomized 1:1 to receive imaging with or without PSMA PET-CT.
IDC-P and/or cribriform carcinoma were present among 342 patients in the PSMA PET-CT group including 48% of high-risk patients receiving radiotherapy, 42% of high-risk patients receiving salvage radiation therapy post-radical prostatectomy, and 40% of those receiving a radical prostatectomy.
IDC-P was reported in 64% of cases with metastases detected by PSMA PET-CT compared with just 36% of cases without metastasis (P = .008), with the ratios being similar in each individual patient cohort.
Of note, the association between the presence of IDC-P and metastases was not observed when IDC-P and cribriform carcinoma were combined — IDC-P and/or cribriform carcinoma was detected in 54% of cases with PSMA PET-CT–detectable metastasis and in 46% of cases without metastasis (P = .362).
The first author Dominique Trudel, MD, PhD, of the Centre Hospitalier de l’Université de Montréal, Montreal, Quebec, Canada, said the findings add to understanding of IDC-P’s relationship with poorer outcomes.
“As pathologists, we know that IDC is associated with poor outcomes and that men with IDC who are treated with standard therapies do benefit from them, but they never benefit as much as men without IDC,” she told Medscape Medical News.
As the study is ongoing, “in approximately 4-5 years, we will know how much of a difference IDC-P makes in outcomes after treatment,” Trudel noted.
The take-home message from the collective research should be that “IDC-P matters,” she said.
“I think that if your patient has IDC-P and [cribriform carcinoma], it is worth at least asking someone from an academic center to see what the treatment options are. We know that some radiation oncologists are increasing doses for IDC-P. It is empiric, but they’re doing it,” she explained.
The authors had no disclosures to report.
The article first appeared in Medscape.com.
BOSTON — Solid intraductal carcinoma of the prostate (IDC-P) is associated with significantly worse outcomes compared with conventional Gleason grade 5 prostate cancers and is more commonly present in metastatic than nonmetastatic cancers, according to two studies presented this week at the United States and Canadian Academy of Pathology (USCAP) 2025 Annual Meeting.
“Our findings suggest that solid IDC-P is more aggressive than Gleason grade 5 conventional prostate adenocarcinoma or cribriform IDC-P,” and it may therefore be better not to consider it as a grade 5 pattern, said first author of one of the studies, Hangchuan Shi, MD, PhD, of the University of Rochester Medical Center, in Rochester, New York.
Although IDC-P — reported in about 20% of men with prostate cancer — is known to be associated with poorer response to treatment, there is a debate over whether to grade the entity with Gleason scoring or not.
The International Society of Urological Pathology recommends incorporating IDC-P into the Gleason score, while the Genitourinary Pathology Society does not.
To evaluate the prognostic significance of solid IDC-P compared with Gleason grade 5 conventional prostate cancer, Shi and his colleagues identified 115 cases in the surgical pathology database at the University of Rochester Medical Center between 2008 and 2015 involving Gleason grade 5 conventional prostatic adenocarcinoma as a primary, secondary, or tertiary pattern, as well as cribriform IDC-P.
The researchers excluded cases showing comedonecrosis within IDC-P, due to the potential for worse outcomes.
Of the grade 5 conventional prostate cancer cases with cribriform carcinoma, 28 (24.3%) had solid nest pattern IDC-P. Patients with and without solid IDC-P had a matching mean age of about 64 years, and their mean preoperative PSA was about 12.27 ng/mL.
Adjuvant therapy prior to recurrence was significantly more common in those who had solid IDC-P (60.7% vs 34.5%; P = .016).
Compared with the conventional prostate cancer cases, those with solid IDC-P had a significantly higher incidence of lymph node metastasis (P = .014) and had larger estimated tumor volume (P = .011).
There were no significant differences in other clinicopathologic features, such as preoperative prostate-specific antigen, grade group, pT stage, and surgical margin status.
After adjustment for key factors in a multivariable analysis, solid IDC-P was significantly associated with poorer recurrence-free survival (P = .007), and poorer cancer-specific survival (P = .004).
Finally, solid IDC-P was an independent predictor of recurrence (hazard ratio [HR] 1.960; P = .031), whereas other measures, including prostate-specific antigen (PSA), cancer grade, pT, lymph node metastasis, surgical and tumor volume were not significant factors.
“We found the solid IDC-P patients had almost two-times the risk of recurrence compared with the patients without solid IDC-P in our study,” Shi said.
The findings underscore the importance of accurately identifying IDC-P, senior author Hiroshi Miyamoto, MD, PhD, director of Genitourinary Pathology at School of Medicine and Dentistry, University of Rochester, Rochester, New York, told Medscape Medical News.
“It may be difficult for some pathologists, especially those who have no specific training in genitourinary pathology, to adequately recognize” this form of cancer, he said.
Although it is recognized as an aggressive form of prostate cancer, “based on our studies, we believe that it is inadequate to grade IDC-P” as a Gleason grade 5 cancer, Miyamoto added.
IDC More Common in Metastases
Poorer outcomes associated with IDC-P were further described in a post hoc sub-analysis of the phase 3, prospective PATRON clinical trial that is evaluating prostate-specific membrane antigen (PSMA) PET-CT–guided intensification of therapy.
In the multicenter trial, 825 patients were stratified into three cohorts: High-risk patients receiving radiation therapy (45%), high-risk patients receiving salvage radiation therapy post-radical prostatectomy (47%), and those receiving a radical prostatectomy (8%).
The patients in all three cohorts were randomized 1:1 to receive imaging with or without PSMA PET-CT.
IDC-P and/or cribriform carcinoma were present among 342 patients in the PSMA PET-CT group including 48% of high-risk patients receiving radiotherapy, 42% of high-risk patients receiving salvage radiation therapy post-radical prostatectomy, and 40% of those receiving a radical prostatectomy.
IDC-P was reported in 64% of cases with metastases detected by PSMA PET-CT compared with just 36% of cases without metastasis (P = .008), with the ratios being similar in each individual patient cohort.
Of note, the association between the presence of IDC-P and metastases was not observed when IDC-P and cribriform carcinoma were combined — IDC-P and/or cribriform carcinoma was detected in 54% of cases with PSMA PET-CT–detectable metastasis and in 46% of cases without metastasis (P = .362).
The first author Dominique Trudel, MD, PhD, of the Centre Hospitalier de l’Université de Montréal, Montreal, Quebec, Canada, said the findings add to understanding of IDC-P’s relationship with poorer outcomes.
“As pathologists, we know that IDC is associated with poor outcomes and that men with IDC who are treated with standard therapies do benefit from them, but they never benefit as much as men without IDC,” she told Medscape Medical News.
As the study is ongoing, “in approximately 4-5 years, we will know how much of a difference IDC-P makes in outcomes after treatment,” Trudel noted.
The take-home message from the collective research should be that “IDC-P matters,” she said.
“I think that if your patient has IDC-P and [cribriform carcinoma], it is worth at least asking someone from an academic center to see what the treatment options are. We know that some radiation oncologists are increasing doses for IDC-P. It is empiric, but they’re doing it,” she explained.
The authors had no disclosures to report.
The article first appeared in Medscape.com.
BOSTON — Solid intraductal carcinoma of the prostate (IDC-P) is associated with significantly worse outcomes compared with conventional Gleason grade 5 prostate cancers and is more commonly present in metastatic than nonmetastatic cancers, according to two studies presented this week at the United States and Canadian Academy of Pathology (USCAP) 2025 Annual Meeting.
“Our findings suggest that solid IDC-P is more aggressive than Gleason grade 5 conventional prostate adenocarcinoma or cribriform IDC-P,” and it may therefore be better not to consider it as a grade 5 pattern, said first author of one of the studies, Hangchuan Shi, MD, PhD, of the University of Rochester Medical Center, in Rochester, New York.
Although IDC-P — reported in about 20% of men with prostate cancer — is known to be associated with poorer response to treatment, there is a debate over whether to grade the entity with Gleason scoring or not.
The International Society of Urological Pathology recommends incorporating IDC-P into the Gleason score, while the Genitourinary Pathology Society does not.
To evaluate the prognostic significance of solid IDC-P compared with Gleason grade 5 conventional prostate cancer, Shi and his colleagues identified 115 cases in the surgical pathology database at the University of Rochester Medical Center between 2008 and 2015 involving Gleason grade 5 conventional prostatic adenocarcinoma as a primary, secondary, or tertiary pattern, as well as cribriform IDC-P.
The researchers excluded cases showing comedonecrosis within IDC-P, due to the potential for worse outcomes.
Of the grade 5 conventional prostate cancer cases with cribriform carcinoma, 28 (24.3%) had solid nest pattern IDC-P. Patients with and without solid IDC-P had a matching mean age of about 64 years, and their mean preoperative PSA was about 12.27 ng/mL.
Adjuvant therapy prior to recurrence was significantly more common in those who had solid IDC-P (60.7% vs 34.5%; P = .016).
Compared with the conventional prostate cancer cases, those with solid IDC-P had a significantly higher incidence of lymph node metastasis (P = .014) and had larger estimated tumor volume (P = .011).
There were no significant differences in other clinicopathologic features, such as preoperative prostate-specific antigen, grade group, pT stage, and surgical margin status.
After adjustment for key factors in a multivariable analysis, solid IDC-P was significantly associated with poorer recurrence-free survival (P = .007), and poorer cancer-specific survival (P = .004).
Finally, solid IDC-P was an independent predictor of recurrence (hazard ratio [HR] 1.960; P = .031), whereas other measures, including prostate-specific antigen (PSA), cancer grade, pT, lymph node metastasis, surgical and tumor volume were not significant factors.
“We found the solid IDC-P patients had almost two-times the risk of recurrence compared with the patients without solid IDC-P in our study,” Shi said.
The findings underscore the importance of accurately identifying IDC-P, senior author Hiroshi Miyamoto, MD, PhD, director of Genitourinary Pathology at School of Medicine and Dentistry, University of Rochester, Rochester, New York, told Medscape Medical News.
“It may be difficult for some pathologists, especially those who have no specific training in genitourinary pathology, to adequately recognize” this form of cancer, he said.
Although it is recognized as an aggressive form of prostate cancer, “based on our studies, we believe that it is inadequate to grade IDC-P” as a Gleason grade 5 cancer, Miyamoto added.
IDC More Common in Metastases
Poorer outcomes associated with IDC-P were further described in a post hoc sub-analysis of the phase 3, prospective PATRON clinical trial that is evaluating prostate-specific membrane antigen (PSMA) PET-CT–guided intensification of therapy.
In the multicenter trial, 825 patients were stratified into three cohorts: High-risk patients receiving radiation therapy (45%), high-risk patients receiving salvage radiation therapy post-radical prostatectomy (47%), and those receiving a radical prostatectomy (8%).
The patients in all three cohorts were randomized 1:1 to receive imaging with or without PSMA PET-CT.
IDC-P and/or cribriform carcinoma were present among 342 patients in the PSMA PET-CT group including 48% of high-risk patients receiving radiotherapy, 42% of high-risk patients receiving salvage radiation therapy post-radical prostatectomy, and 40% of those receiving a radical prostatectomy.
IDC-P was reported in 64% of cases with metastases detected by PSMA PET-CT compared with just 36% of cases without metastasis (P = .008), with the ratios being similar in each individual patient cohort.
Of note, the association between the presence of IDC-P and metastases was not observed when IDC-P and cribriform carcinoma were combined — IDC-P and/or cribriform carcinoma was detected in 54% of cases with PSMA PET-CT–detectable metastasis and in 46% of cases without metastasis (P = .362).
The first author Dominique Trudel, MD, PhD, of the Centre Hospitalier de l’Université de Montréal, Montreal, Quebec, Canada, said the findings add to understanding of IDC-P’s relationship with poorer outcomes.
“As pathologists, we know that IDC is associated with poor outcomes and that men with IDC who are treated with standard therapies do benefit from them, but they never benefit as much as men without IDC,” she told Medscape Medical News.
As the study is ongoing, “in approximately 4-5 years, we will know how much of a difference IDC-P makes in outcomes after treatment,” Trudel noted.
The take-home message from the collective research should be that “IDC-P matters,” she said.
“I think that if your patient has IDC-P and [cribriform carcinoma], it is worth at least asking someone from an academic center to see what the treatment options are. We know that some radiation oncologists are increasing doses for IDC-P. It is empiric, but they’re doing it,” she explained.
The authors had no disclosures to report.
The article first appeared in Medscape.com.
PSA Screening in VA Patients After Age 70 Years
TOPLINE: Most men receiving care through the Veterans Health Administration (VHA) continue prostate-specific antigen (PSA) screening after aged 70 years despite low absolute risk for prostate cancer-specific mortality (PCSM), even among Black men in the healthiest quintile.
METHODOLOGY:
Researchers conducted a cohort study of 921,609 men aged 70 years receiving VHA care between 2008 and 2020, who had normal screening PSA values (< 4 ng/mL) between ages 65-69 years.
- Analysis included electronic health record data from VHA Corporate Data Warehouse, linked Medicare claims data, and VHA community care data.
- Investigators examined the value of PSA levels, race and ethnicity, and competing mortality in risk stratification for PCSM and mPCa using regression modeling.
TAKEAWAY:
The 10-year cumulative incidence of PCSM was 0.26% overall, with 95% of men having a 10-year risk < 0.73%, and higher baseline PSA levels associated with increased risk (0.79% for 3.00-3.99 ng/mL vs 0.10% for 0.20-0.99 ng/mL).
- Race and ethnicity showed modest association with PCSM risk: Black patients had a 0.79% risk of mPCa vs 0.38% for White patients. The risk of PCSM was 0.36% for Black patients vs 0.25% for White patients.
- Most patients (87%) continued PSA screening after age 70 years, with little variation by competing mortality risk or race and ethnicity.
- Low PSA (0.20-0.99 ng/mL) identified very low-risk populations with < 1% 10-year risk for prostate biopsy, clinically significant prostate cancer diagnosis, and treatment.
IN PRACTICE: "Our data suggest that a simple assessment of personal risk based on PSA values before age 70 years captures a large proportion of relevant prognostic information with respect to mPCa and PCSM risk ... Low PSA (0.20-0.99 ng/mL) was associated with very low PCSM and mPCa risk, even among the healthiest Black men," wrote the authors of the study.
SOURCE: The study was led by Alex K. Bryant,MD, MAS and the Veterans Affairs Center for Clinical Management Research in Ann Arbor. It was published online on February 14 in JAMA Network Open.
LIMITATIONS: According to the authors, any potential PCSM or mPCa reduction from continued PSA screening > age 70 years remains unproven due to lack of randomized trial data. The study relied on death certificates to define PCSM, which may have introduced misclassification error. Family history of prostate cancer was not included due to unreliable electronic medical record data availability. Additionally, veterans have higher comorbidity burdens than the general population and unique military-related environmental exposures, potentially limiting result generalizability.
DISCLOSURES: The study was supported by grants U01CA253915, PSOCA097186, R35CA274442, and R50CA221836 from the National Cancer Institute. Matthew J. Schipper, MD, reported receiving consulting fees from Innovative Analytics. Phoebe A. Tsao, MD, disclosed receiving grants from the Prostate Cancer Foundation outside the submitted work. Kristian D. Stensland, MD, reported receiving a grant from the National Institutes of Health during the conduct of the study.
TOPLINE: Most men receiving care through the Veterans Health Administration (VHA) continue prostate-specific antigen (PSA) screening after aged 70 years despite low absolute risk for prostate cancer-specific mortality (PCSM), even among Black men in the healthiest quintile.
METHODOLOGY:
Researchers conducted a cohort study of 921,609 men aged 70 years receiving VHA care between 2008 and 2020, who had normal screening PSA values (< 4 ng/mL) between ages 65-69 years.
- Analysis included electronic health record data from VHA Corporate Data Warehouse, linked Medicare claims data, and VHA community care data.
- Investigators examined the value of PSA levels, race and ethnicity, and competing mortality in risk stratification for PCSM and mPCa using regression modeling.
TAKEAWAY:
The 10-year cumulative incidence of PCSM was 0.26% overall, with 95% of men having a 10-year risk < 0.73%, and higher baseline PSA levels associated with increased risk (0.79% for 3.00-3.99 ng/mL vs 0.10% for 0.20-0.99 ng/mL).
- Race and ethnicity showed modest association with PCSM risk: Black patients had a 0.79% risk of mPCa vs 0.38% for White patients. The risk of PCSM was 0.36% for Black patients vs 0.25% for White patients.
- Most patients (87%) continued PSA screening after age 70 years, with little variation by competing mortality risk or race and ethnicity.
- Low PSA (0.20-0.99 ng/mL) identified very low-risk populations with < 1% 10-year risk for prostate biopsy, clinically significant prostate cancer diagnosis, and treatment.
IN PRACTICE: "Our data suggest that a simple assessment of personal risk based on PSA values before age 70 years captures a large proportion of relevant prognostic information with respect to mPCa and PCSM risk ... Low PSA (0.20-0.99 ng/mL) was associated with very low PCSM and mPCa risk, even among the healthiest Black men," wrote the authors of the study.
SOURCE: The study was led by Alex K. Bryant,MD, MAS and the Veterans Affairs Center for Clinical Management Research in Ann Arbor. It was published online on February 14 in JAMA Network Open.
LIMITATIONS: According to the authors, any potential PCSM or mPCa reduction from continued PSA screening > age 70 years remains unproven due to lack of randomized trial data. The study relied on death certificates to define PCSM, which may have introduced misclassification error. Family history of prostate cancer was not included due to unreliable electronic medical record data availability. Additionally, veterans have higher comorbidity burdens than the general population and unique military-related environmental exposures, potentially limiting result generalizability.
DISCLOSURES: The study was supported by grants U01CA253915, PSOCA097186, R35CA274442, and R50CA221836 from the National Cancer Institute. Matthew J. Schipper, MD, reported receiving consulting fees from Innovative Analytics. Phoebe A. Tsao, MD, disclosed receiving grants from the Prostate Cancer Foundation outside the submitted work. Kristian D. Stensland, MD, reported receiving a grant from the National Institutes of Health during the conduct of the study.
TOPLINE: Most men receiving care through the Veterans Health Administration (VHA) continue prostate-specific antigen (PSA) screening after aged 70 years despite low absolute risk for prostate cancer-specific mortality (PCSM), even among Black men in the healthiest quintile.
METHODOLOGY:
Researchers conducted a cohort study of 921,609 men aged 70 years receiving VHA care between 2008 and 2020, who had normal screening PSA values (< 4 ng/mL) between ages 65-69 years.
- Analysis included electronic health record data from VHA Corporate Data Warehouse, linked Medicare claims data, and VHA community care data.
- Investigators examined the value of PSA levels, race and ethnicity, and competing mortality in risk stratification for PCSM and mPCa using regression modeling.
TAKEAWAY:
The 10-year cumulative incidence of PCSM was 0.26% overall, with 95% of men having a 10-year risk < 0.73%, and higher baseline PSA levels associated with increased risk (0.79% for 3.00-3.99 ng/mL vs 0.10% for 0.20-0.99 ng/mL).
- Race and ethnicity showed modest association with PCSM risk: Black patients had a 0.79% risk of mPCa vs 0.38% for White patients. The risk of PCSM was 0.36% for Black patients vs 0.25% for White patients.
- Most patients (87%) continued PSA screening after age 70 years, with little variation by competing mortality risk or race and ethnicity.
- Low PSA (0.20-0.99 ng/mL) identified very low-risk populations with < 1% 10-year risk for prostate biopsy, clinically significant prostate cancer diagnosis, and treatment.
IN PRACTICE: "Our data suggest that a simple assessment of personal risk based on PSA values before age 70 years captures a large proportion of relevant prognostic information with respect to mPCa and PCSM risk ... Low PSA (0.20-0.99 ng/mL) was associated with very low PCSM and mPCa risk, even among the healthiest Black men," wrote the authors of the study.
SOURCE: The study was led by Alex K. Bryant,MD, MAS and the Veterans Affairs Center for Clinical Management Research in Ann Arbor. It was published online on February 14 in JAMA Network Open.
LIMITATIONS: According to the authors, any potential PCSM or mPCa reduction from continued PSA screening > age 70 years remains unproven due to lack of randomized trial data. The study relied on death certificates to define PCSM, which may have introduced misclassification error. Family history of prostate cancer was not included due to unreliable electronic medical record data availability. Additionally, veterans have higher comorbidity burdens than the general population and unique military-related environmental exposures, potentially limiting result generalizability.
DISCLOSURES: The study was supported by grants U01CA253915, PSOCA097186, R35CA274442, and R50CA221836 from the National Cancer Institute. Matthew J. Schipper, MD, reported receiving consulting fees from Innovative Analytics. Phoebe A. Tsao, MD, disclosed receiving grants from the Prostate Cancer Foundation outside the submitted work. Kristian D. Stensland, MD, reported receiving a grant from the National Institutes of Health during the conduct of the study.
No Racial Disparities in CVD Outcomes For VA Patients with Prostate Cancer Receiving ADT
TOPLINE: Veterans treated in the Veterans Health Administration (VHA) who had preexisting cardiometabolic disease and received androgen deprivation therapy (ADT) with radiation therapy developed major adverse cardiovascular events (MACE) at 4 times the rate compared to those without cardiometabolic disease. Black and White veterans showed similar cardiovascular outcomes regardless of treatment type.
METHODOLOGY:
Researchers conducted a retrospective cohort study examining 39,580 veterans in the VHA system diagnosed with prostate cancer between 2000 and 2015, following them for a median of 9.6 years to assess time to MACE diagnosis.
- Analysis utilized a 1:1 propensity score matching process to compare outcomes between treatment types (ADT with radiation therapy vs radiation therapy alone) and racial groups (Black vs White men).
- Participants had a mean age of 65.9 years at diagnosis; 68% identified as White and 32% as Black, and 83% had stage 2 disease classified with 43.1% intermediate risk. Most lived in nonrural zip codes
- Primary outcome measure was time to MACE, defined as a composite of cardiovascular death, myocardial infarction, or ischemic stroke, with patients censored at non-cardiovascular death or study end.
TAKEAWAY:
Compared to those without CMD, the hazard ratio (HR) for MACE for men with preexisting CMD who received ADT was 4.2. Those receiving radiation alone had an HR of 2.5.
- Patients diagnosed between 2010 and 2015 showed significantly lower MACE rates compared to those diagnosed in 2000 to 2005: HR, 0.23; 95% CI, 0.08-0.71 for White patients; and HR, 0.23; 95% CI, 0.07-0.77 for Black patients.
- Multiple comorbidities were associated with doubled MACE risk (HR, 2.22; 95% CI, 1.08-4.59) compared to those without comorbidities.
- No significant differences in MACE rates were observed between Black and White veterans, regardless of treatment type.
IN PRACTICE: “Within the VHA, men treated with ADT + radiation therapy for prostate cancer do not appear to be at greater risk for MACE than those receiving radiation therapy alone. Black men have similar risk of MACE as White men, whether receiving radiation therapy alone or in combination with ADT," the authors wrote.
SOURCE: The study was led by Alexander R. Lucas, Virginia Commonwealth University School of Public Health in Richmond. It was published online on February 6 in Cardio-Oncology.
LIMITATIONS: According to the authors, the retrospective nature of the data may have limited their ability to detect MACE events occurring outside the VHA. Additionally, the study was limited to men who initiated ADT prior to radiation therapy, excluding those who had surgery or radiation before ADT. The researchers also note that the analysis did not compare outcomes between different types of ADT treatments, such as GnRH agonists vs antagonists, which may have different cardiovascular risk profiles.
DISCLOSURES: Alexander R. Lucas’s work was partly funded by grants 1KO1HL161419 and NRG FP00019789. Ashit K. Paul disclosed receiving honorarium for serving on scientific consultancy panels of SANOFI-Genzyme, Bayer, and Tempus & Cardinal Health. Additional disclosures are noted but not specified in the article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication
TOPLINE: Veterans treated in the Veterans Health Administration (VHA) who had preexisting cardiometabolic disease and received androgen deprivation therapy (ADT) with radiation therapy developed major adverse cardiovascular events (MACE) at 4 times the rate compared to those without cardiometabolic disease. Black and White veterans showed similar cardiovascular outcomes regardless of treatment type.
METHODOLOGY:
Researchers conducted a retrospective cohort study examining 39,580 veterans in the VHA system diagnosed with prostate cancer between 2000 and 2015, following them for a median of 9.6 years to assess time to MACE diagnosis.
- Analysis utilized a 1:1 propensity score matching process to compare outcomes between treatment types (ADT with radiation therapy vs radiation therapy alone) and racial groups (Black vs White men).
- Participants had a mean age of 65.9 years at diagnosis; 68% identified as White and 32% as Black, and 83% had stage 2 disease classified with 43.1% intermediate risk. Most lived in nonrural zip codes
- Primary outcome measure was time to MACE, defined as a composite of cardiovascular death, myocardial infarction, or ischemic stroke, with patients censored at non-cardiovascular death or study end.
TAKEAWAY:
Compared to those without CMD, the hazard ratio (HR) for MACE for men with preexisting CMD who received ADT was 4.2. Those receiving radiation alone had an HR of 2.5.
- Patients diagnosed between 2010 and 2015 showed significantly lower MACE rates compared to those diagnosed in 2000 to 2005: HR, 0.23; 95% CI, 0.08-0.71 for White patients; and HR, 0.23; 95% CI, 0.07-0.77 for Black patients.
- Multiple comorbidities were associated with doubled MACE risk (HR, 2.22; 95% CI, 1.08-4.59) compared to those without comorbidities.
- No significant differences in MACE rates were observed between Black and White veterans, regardless of treatment type.
IN PRACTICE: “Within the VHA, men treated with ADT + radiation therapy for prostate cancer do not appear to be at greater risk for MACE than those receiving radiation therapy alone. Black men have similar risk of MACE as White men, whether receiving radiation therapy alone or in combination with ADT," the authors wrote.
SOURCE: The study was led by Alexander R. Lucas, Virginia Commonwealth University School of Public Health in Richmond. It was published online on February 6 in Cardio-Oncology.
LIMITATIONS: According to the authors, the retrospective nature of the data may have limited their ability to detect MACE events occurring outside the VHA. Additionally, the study was limited to men who initiated ADT prior to radiation therapy, excluding those who had surgery or radiation before ADT. The researchers also note that the analysis did not compare outcomes between different types of ADT treatments, such as GnRH agonists vs antagonists, which may have different cardiovascular risk profiles.
DISCLOSURES: Alexander R. Lucas’s work was partly funded by grants 1KO1HL161419 and NRG FP00019789. Ashit K. Paul disclosed receiving honorarium for serving on scientific consultancy panels of SANOFI-Genzyme, Bayer, and Tempus & Cardinal Health. Additional disclosures are noted but not specified in the article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication
TOPLINE: Veterans treated in the Veterans Health Administration (VHA) who had preexisting cardiometabolic disease and received androgen deprivation therapy (ADT) with radiation therapy developed major adverse cardiovascular events (MACE) at 4 times the rate compared to those without cardiometabolic disease. Black and White veterans showed similar cardiovascular outcomes regardless of treatment type.
METHODOLOGY:
Researchers conducted a retrospective cohort study examining 39,580 veterans in the VHA system diagnosed with prostate cancer between 2000 and 2015, following them for a median of 9.6 years to assess time to MACE diagnosis.
- Analysis utilized a 1:1 propensity score matching process to compare outcomes between treatment types (ADT with radiation therapy vs radiation therapy alone) and racial groups (Black vs White men).
- Participants had a mean age of 65.9 years at diagnosis; 68% identified as White and 32% as Black, and 83% had stage 2 disease classified with 43.1% intermediate risk. Most lived in nonrural zip codes
- Primary outcome measure was time to MACE, defined as a composite of cardiovascular death, myocardial infarction, or ischemic stroke, with patients censored at non-cardiovascular death or study end.
TAKEAWAY:
Compared to those without CMD, the hazard ratio (HR) for MACE for men with preexisting CMD who received ADT was 4.2. Those receiving radiation alone had an HR of 2.5.
- Patients diagnosed between 2010 and 2015 showed significantly lower MACE rates compared to those diagnosed in 2000 to 2005: HR, 0.23; 95% CI, 0.08-0.71 for White patients; and HR, 0.23; 95% CI, 0.07-0.77 for Black patients.
- Multiple comorbidities were associated with doubled MACE risk (HR, 2.22; 95% CI, 1.08-4.59) compared to those without comorbidities.
- No significant differences in MACE rates were observed between Black and White veterans, regardless of treatment type.
IN PRACTICE: “Within the VHA, men treated with ADT + radiation therapy for prostate cancer do not appear to be at greater risk for MACE than those receiving radiation therapy alone. Black men have similar risk of MACE as White men, whether receiving radiation therapy alone or in combination with ADT," the authors wrote.
SOURCE: The study was led by Alexander R. Lucas, Virginia Commonwealth University School of Public Health in Richmond. It was published online on February 6 in Cardio-Oncology.
LIMITATIONS: According to the authors, the retrospective nature of the data may have limited their ability to detect MACE events occurring outside the VHA. Additionally, the study was limited to men who initiated ADT prior to radiation therapy, excluding those who had surgery or radiation before ADT. The researchers also note that the analysis did not compare outcomes between different types of ADT treatments, such as GnRH agonists vs antagonists, which may have different cardiovascular risk profiles.
DISCLOSURES: Alexander R. Lucas’s work was partly funded by grants 1KO1HL161419 and NRG FP00019789. Ashit K. Paul disclosed receiving honorarium for serving on scientific consultancy panels of SANOFI-Genzyme, Bayer, and Tempus & Cardinal Health. Additional disclosures are noted but not specified in the article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication
Open Clinical Trials for Patients With Prostate Cancer
The clinical trials listed below are open as of March 10, 2025; have ≥ 1 US Department of Veterans Affairs (VA) medical center (VAMC) or US Department of Defense (DoD) military treatment facility location recruiting patients; and are focused on treatments for chronic obstructive pulmonary disease (COPD). For additional information and full inclusion/exclusion criteria, please consult clinicaltrials.gov.
Actively Recruiting
Patient Decision-making About Precision Oncology in Veterans With Advanced Prostate Cancer
This clinical trial explores and implements methods to improve informed decision making (IDM) regarding precision oncology tests amongst veterans with prostate cancer that may have spread from where it first started to nearby tissue, lymph nodes, or distant parts of the body (advanced). Precision oncology, the use of germline genetic testing and tumor-based molecular assays to inform cancer care, has become an important aspect of evidence-based care for men with advanced prostate cancer. Veterans with metastatic castrate-resistant prostate cancer may not be carrying out IDM due to unmet decisional needs. An informed decision is a choice based on complete and accurate information. The information gained from this study will help researchers develop a decision support intervention (DSI) and implement the intervention. A DSI may serve as a valuable tool to reduce ongoing racial disparities in genetic testing and encourage enrollment to precision oncology trials.
ID: NCT05396872
Sponsor; Investigator; Collaborator: University of California, San Francisco; Daniel Kwon, MD; US Department of Defense VA Office of Research and Development; Madalina Macrea, MD, PhD
Location: San Francisco Veterans Affairs Medical Center, CA
Veterans Affairs Seamless Phase II/III Randomized Trial of STAndard Systemic theRapy With or Without PET-directed Local Therapy for Oligometastatic pRosTate Cancer (VA STARPORT)
This is a prospective, open-label, multi-center seamless phase II to phase III randomized clinical trial designed to compare SST with or without PET-directed local therapy in improving the castration-resistant prostate cancer-free survival (CRPC-free survival) for veterans with oligometastatic prostate cancer. Oligometastasis will be defined as 1-10 sites of metastatic disease based on the clinical determination of the LSI which incorporates all imaging, clinical, and pathologic data available.
ID: NCT04787744
Sponsor; Collaborator: VA Office of Research and Development; Abhishek Solanki, MD, MS VA Office of Research and Development; Madalina Macrea, MD, PhD
Location: 19 locations, including Edwards Hines Jr. VA Hospital, Hines, IL
The Prostate Cancer, Genetic Risk, and Equitable Screening Study (ProGRESS) (ProGRESS)
Prostate cancer is the most common non-skin cancer among veterans and the second leading cause of male cancer death. Current methods of screening men for prostate cancer are inaccurate and cannot identify which men do not have prostate cancer or have low-grade cases that will not cause harm and which men have significant prostate cancer needing treatment. False-positive screening tests can result in unnecessary prostate biopsies for men who do not need them. However, new genetic testing might help identify which men are at highest risk for prostate cancer. This study will examine whether a genetic test helps identify men at risk for significant prostate cancer while helping men who are at low risk for prostate cancer avoid unnecessary biopsies. If this genetic test proves beneficial, it will improve the way that health care providers screen male veterans for prostate cancer.
ID: NCT05926102
Sponsor; Collaborator: VA Office of Research and Development; Jason L. Vassy, MD, MPH VA Office of Research and Development; Madalina Macrea, MD, PhD
Location: VA Boston Healthcare System Jamaica Plain Campus, MA
A Single-Arm Phase II Study of Neoadjuvant Intensified Androgen Deprivation (Leuprolide and Abiraterone Acetate) in Combination With AKT Inhibition (Capivasertib) for High-Risk Localized Prostate Cancer With PTEN Loss (SNARE)
The purpose of this study is to learn about how an investigational drug intervention completed before doing prostate surgery (specifically, radical prostatectomy with lymph node dissection) may help in treatment of high risk localized prostate cancers that are most resistant to standard treatments. This is a phase II research study. For this study, capivasertib, the study drug, will be taken with intensified androgen deprivation drugs (iADT; abiraterone and leuprolide) prior to radical prostatectomy. This study drug treatment will be evaluated to see if it is effective in shrinking and destroying prostate cancer tumors prior to surgery and to further evaluate its safety prior to prostate cancer surgery.
ID: NCT05593497
Sponsor; Collaborator: VA Office of Research and Development; Ryan P. Kopp, MD VA Office of Research and Development; Madalina Macrea, MD, PhD
Location: 5 locations, including VA Portland Health Care System, OR
18F-Fluciclovine PET/CT Impact on Predicting Clinical Outcome of 177Lu-PSMA-617 Therapy in Patients With Prostate Cancer
This is a single-center, prospective, exploratory study. Patients with metastatic castration-resistant prostate cancer (mCRPC) scheduled to undergo Lutetium labelled prostate-specific membrane antigen radioligand therapy (LuPSMA RLT) at the West Los Angeles VA (WLA-VA) will be imaged with a baseline F-18 fluorodeoxyglucose positron emission tomography/computed tomography 18F-FDG PET/CT and a 18F-DCFPyL PET/CT (18F-DCFPyL (2-(3-{1-carboxy-5-[(6-18F-fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid)positron emission tomography/computed tomography , as per standard of care in our institution. All patients further undergo eventual follow-up prostate-specific membrane antigen positron emission tomography (PSMA PET) after the 2nd, 4th, and 6th LuPSMA RLT cycle. In this prospective study, an18F-Fluciclovine positron emission tomography/computed tomography (Axumin PET/CT) will be additionally obtained at baseline (pre-LuPSMA RLT), and after the 2nd, 4th, 6th LuPSMA RLT cycles. Axumin PET/CT will be acquired within 7 days from the PSMA PET.
This study is open to veterans only.
ID: NCT06706921
Sponsor; Collaborator: VA Greater Los Angeles Healthcare System; Gholam Berenji, MD, Janake Wijesuriya, BS VA Office of Research and Development; Madalina Macrea, MD, PhD
Location: VA Greater Los Angeles Healthcare System, CA
High Dose Testosterone for ATM, CDK12 or CHEK2 Altered Prostate Cancers (VA-BAT)
This study will determine whether the presence of DNA repair deficiency in the form of alterations in the genes ATM, CDK12 or CHEK2 predicts for a high likelihood of responding to the use of intermittent high dose testosterone. This therapy may result in responses in tumors which are genetically unstable because of DNA repair deficiency and this is a prospective study to test that hypothesis.
ID: NCT05011383
Sponsor; Collaborator: VA Office of Research and Development; Robert B. Montgomery, MD VA Office of Research and Development; Madalina Macrea, MD, PhD
Location: 17 locations, including VA Puget Sound Health Care System, Seattle, WA
A Study of CHeckpoint Inhibitors in Men With prOgressive Metastatic Castrate Resistant Prostate Cancer Characterized by a Mismatch Repair Deficiency or Biallelic CDK12 Inactivation (CHOMP)
The primary objective is to assess the activity and efficacy of pembrolizumab, a checkpoint inhibitor, in veterans with metastatic castration-resistant prostate cancer (mCRPC) characterized by either mismatch repair deficiency (dMMR) or biallelic inactivation of CDK12 (CDK12-/-). The secondary objectives involve determining the frequency with which dMMR and CDK12-/- occur in this patient population, as well as the effects of pembrolizumab on various clinical endpoints (time to PSA progression, maximal PSA response, time to initiation of alternative anti-neoplastic therapy, time to radiographic progression, overall survival, and safety and tolerability). Lastly, the study will compare the pre-treatment and at-progression metastatic tumor biopsies to investigate the molecular correlates of resistance and sensitivity to pembrolizumab via RNA-sequencing, exome-sequencing, selected protein analyses, and multiplexed immunofluorescence.
ID: NCT04104893
Sponsor; Investigator; Collaborator: VA Office of Research and Development; Matthew B. Rettig, MD; Merck Sharp & Dohme LLC VA Office of Research and Development; Madalina Macrea, MD, PhD
Location: 12 locations, including VA Greater Los Angeles Healthcare System, CA
Carboplatin or Olaparib for BRcA Deficient Prostate Cancer (COBRA)
This is an unblinded, randomized clinical study comparing the efficacy of DNA damaging chemotherapy using carboplatin, to standard of care therapy for patients who have metastatic castrate resistant prostate cancer. This trial will use olaparib or carboplatin as initial therapy with crossover to the alternate or second-line drug after first progression for patients with tumors containing BARD1, BRCA1, BRCA2, BRIP1, CHEK1, FANCL, PALB2, RAD51B, RAD51C, RAD51D, or RAD54L inactivating mutations.
Participants are randomized (1:1) and receive either carboplatin (AUC 5, IV) every 21 days, first or olaparib taken orally (300 mg), twice daily in 28-day cycles, until intolerance, complete response, or progression by Prostate Cancer Working Group 3 (PCWG3) criteria.
Participants then cross over from the first-line therapy to the second-line therapy with the opposite study medication and receive treatment to intolerance or progression (whichever is first). Enrolled participants will be allowed to crossover to second line therapy if they continue to meet initial eligibility criteria, and at least three weeks have elapsed since last administration of either carboplatin or olaparib. Throughout the study, safety and tolerability will be assessed. Progression will be evaluated with bone scan, CT of the abdomen/pelvis, or MRI and PSA as per PCWG3 criteria.
ID: NCT04038502
Sponsor; Collaborator: VA Office of Research and Development; Robert B. Montgomery, MD; Ryan Burri, MD; Phoebe Tsao, MD, MSc; Maneesh Jain, MD VA Office of Research and Development; Madalina Macrea, MD, PhD
Location: 17 locations, including VA Puget Sound Health Care System, Seattle, WA
The clinical trials listed below are open as of March 10, 2025; have ≥ 1 US Department of Veterans Affairs (VA) medical center (VAMC) or US Department of Defense (DoD) military treatment facility location recruiting patients; and are focused on treatments for chronic obstructive pulmonary disease (COPD). For additional information and full inclusion/exclusion criteria, please consult clinicaltrials.gov.
Actively Recruiting
Patient Decision-making About Precision Oncology in Veterans With Advanced Prostate Cancer
This clinical trial explores and implements methods to improve informed decision making (IDM) regarding precision oncology tests amongst veterans with prostate cancer that may have spread from where it first started to nearby tissue, lymph nodes, or distant parts of the body (advanced). Precision oncology, the use of germline genetic testing and tumor-based molecular assays to inform cancer care, has become an important aspect of evidence-based care for men with advanced prostate cancer. Veterans with metastatic castrate-resistant prostate cancer may not be carrying out IDM due to unmet decisional needs. An informed decision is a choice based on complete and accurate information. The information gained from this study will help researchers develop a decision support intervention (DSI) and implement the intervention. A DSI may serve as a valuable tool to reduce ongoing racial disparities in genetic testing and encourage enrollment to precision oncology trials.
ID: NCT05396872
Sponsor; Investigator; Collaborator: University of California, San Francisco; Daniel Kwon, MD; US Department of Defense VA Office of Research and Development; Madalina Macrea, MD, PhD
Location: San Francisco Veterans Affairs Medical Center, CA
Veterans Affairs Seamless Phase II/III Randomized Trial of STAndard Systemic theRapy With or Without PET-directed Local Therapy for Oligometastatic pRosTate Cancer (VA STARPORT)
This is a prospective, open-label, multi-center seamless phase II to phase III randomized clinical trial designed to compare SST with or without PET-directed local therapy in improving the castration-resistant prostate cancer-free survival (CRPC-free survival) for veterans with oligometastatic prostate cancer. Oligometastasis will be defined as 1-10 sites of metastatic disease based on the clinical determination of the LSI which incorporates all imaging, clinical, and pathologic data available.
ID: NCT04787744
Sponsor; Collaborator: VA Office of Research and Development; Abhishek Solanki, MD, MS VA Office of Research and Development; Madalina Macrea, MD, PhD
Location: 19 locations, including Edwards Hines Jr. VA Hospital, Hines, IL
The Prostate Cancer, Genetic Risk, and Equitable Screening Study (ProGRESS) (ProGRESS)
Prostate cancer is the most common non-skin cancer among veterans and the second leading cause of male cancer death. Current methods of screening men for prostate cancer are inaccurate and cannot identify which men do not have prostate cancer or have low-grade cases that will not cause harm and which men have significant prostate cancer needing treatment. False-positive screening tests can result in unnecessary prostate biopsies for men who do not need them. However, new genetic testing might help identify which men are at highest risk for prostate cancer. This study will examine whether a genetic test helps identify men at risk for significant prostate cancer while helping men who are at low risk for prostate cancer avoid unnecessary biopsies. If this genetic test proves beneficial, it will improve the way that health care providers screen male veterans for prostate cancer.
ID: NCT05926102
Sponsor; Collaborator: VA Office of Research and Development; Jason L. Vassy, MD, MPH VA Office of Research and Development; Madalina Macrea, MD, PhD
Location: VA Boston Healthcare System Jamaica Plain Campus, MA
A Single-Arm Phase II Study of Neoadjuvant Intensified Androgen Deprivation (Leuprolide and Abiraterone Acetate) in Combination With AKT Inhibition (Capivasertib) for High-Risk Localized Prostate Cancer With PTEN Loss (SNARE)
The purpose of this study is to learn about how an investigational drug intervention completed before doing prostate surgery (specifically, radical prostatectomy with lymph node dissection) may help in treatment of high risk localized prostate cancers that are most resistant to standard treatments. This is a phase II research study. For this study, capivasertib, the study drug, will be taken with intensified androgen deprivation drugs (iADT; abiraterone and leuprolide) prior to radical prostatectomy. This study drug treatment will be evaluated to see if it is effective in shrinking and destroying prostate cancer tumors prior to surgery and to further evaluate its safety prior to prostate cancer surgery.
ID: NCT05593497
Sponsor; Collaborator: VA Office of Research and Development; Ryan P. Kopp, MD VA Office of Research and Development; Madalina Macrea, MD, PhD
Location: 5 locations, including VA Portland Health Care System, OR
18F-Fluciclovine PET/CT Impact on Predicting Clinical Outcome of 177Lu-PSMA-617 Therapy in Patients With Prostate Cancer
This is a single-center, prospective, exploratory study. Patients with metastatic castration-resistant prostate cancer (mCRPC) scheduled to undergo Lutetium labelled prostate-specific membrane antigen radioligand therapy (LuPSMA RLT) at the West Los Angeles VA (WLA-VA) will be imaged with a baseline F-18 fluorodeoxyglucose positron emission tomography/computed tomography 18F-FDG PET/CT and a 18F-DCFPyL PET/CT (18F-DCFPyL (2-(3-{1-carboxy-5-[(6-18F-fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid)positron emission tomography/computed tomography , as per standard of care in our institution. All patients further undergo eventual follow-up prostate-specific membrane antigen positron emission tomography (PSMA PET) after the 2nd, 4th, and 6th LuPSMA RLT cycle. In this prospective study, an18F-Fluciclovine positron emission tomography/computed tomography (Axumin PET/CT) will be additionally obtained at baseline (pre-LuPSMA RLT), and after the 2nd, 4th, 6th LuPSMA RLT cycles. Axumin PET/CT will be acquired within 7 days from the PSMA PET.
This study is open to veterans only.
ID: NCT06706921
Sponsor; Collaborator: VA Greater Los Angeles Healthcare System; Gholam Berenji, MD, Janake Wijesuriya, BS VA Office of Research and Development; Madalina Macrea, MD, PhD
Location: VA Greater Los Angeles Healthcare System, CA
High Dose Testosterone for ATM, CDK12 or CHEK2 Altered Prostate Cancers (VA-BAT)
This study will determine whether the presence of DNA repair deficiency in the form of alterations in the genes ATM, CDK12 or CHEK2 predicts for a high likelihood of responding to the use of intermittent high dose testosterone. This therapy may result in responses in tumors which are genetically unstable because of DNA repair deficiency and this is a prospective study to test that hypothesis.
ID: NCT05011383
Sponsor; Collaborator: VA Office of Research and Development; Robert B. Montgomery, MD VA Office of Research and Development; Madalina Macrea, MD, PhD
Location: 17 locations, including VA Puget Sound Health Care System, Seattle, WA
A Study of CHeckpoint Inhibitors in Men With prOgressive Metastatic Castrate Resistant Prostate Cancer Characterized by a Mismatch Repair Deficiency or Biallelic CDK12 Inactivation (CHOMP)
The primary objective is to assess the activity and efficacy of pembrolizumab, a checkpoint inhibitor, in veterans with metastatic castration-resistant prostate cancer (mCRPC) characterized by either mismatch repair deficiency (dMMR) or biallelic inactivation of CDK12 (CDK12-/-). The secondary objectives involve determining the frequency with which dMMR and CDK12-/- occur in this patient population, as well as the effects of pembrolizumab on various clinical endpoints (time to PSA progression, maximal PSA response, time to initiation of alternative anti-neoplastic therapy, time to radiographic progression, overall survival, and safety and tolerability). Lastly, the study will compare the pre-treatment and at-progression metastatic tumor biopsies to investigate the molecular correlates of resistance and sensitivity to pembrolizumab via RNA-sequencing, exome-sequencing, selected protein analyses, and multiplexed immunofluorescence.
ID: NCT04104893
Sponsor; Investigator; Collaborator: VA Office of Research and Development; Matthew B. Rettig, MD; Merck Sharp & Dohme LLC VA Office of Research and Development; Madalina Macrea, MD, PhD
Location: 12 locations, including VA Greater Los Angeles Healthcare System, CA
Carboplatin or Olaparib for BRcA Deficient Prostate Cancer (COBRA)
This is an unblinded, randomized clinical study comparing the efficacy of DNA damaging chemotherapy using carboplatin, to standard of care therapy for patients who have metastatic castrate resistant prostate cancer. This trial will use olaparib or carboplatin as initial therapy with crossover to the alternate or second-line drug after first progression for patients with tumors containing BARD1, BRCA1, BRCA2, BRIP1, CHEK1, FANCL, PALB2, RAD51B, RAD51C, RAD51D, or RAD54L inactivating mutations.
Participants are randomized (1:1) and receive either carboplatin (AUC 5, IV) every 21 days, first or olaparib taken orally (300 mg), twice daily in 28-day cycles, until intolerance, complete response, or progression by Prostate Cancer Working Group 3 (PCWG3) criteria.
Participants then cross over from the first-line therapy to the second-line therapy with the opposite study medication and receive treatment to intolerance or progression (whichever is first). Enrolled participants will be allowed to crossover to second line therapy if they continue to meet initial eligibility criteria, and at least three weeks have elapsed since last administration of either carboplatin or olaparib. Throughout the study, safety and tolerability will be assessed. Progression will be evaluated with bone scan, CT of the abdomen/pelvis, or MRI and PSA as per PCWG3 criteria.
ID: NCT04038502
Sponsor; Collaborator: VA Office of Research and Development; Robert B. Montgomery, MD; Ryan Burri, MD; Phoebe Tsao, MD, MSc; Maneesh Jain, MD VA Office of Research and Development; Madalina Macrea, MD, PhD
Location: 17 locations, including VA Puget Sound Health Care System, Seattle, WA
The clinical trials listed below are open as of March 10, 2025; have ≥ 1 US Department of Veterans Affairs (VA) medical center (VAMC) or US Department of Defense (DoD) military treatment facility location recruiting patients; and are focused on treatments for chronic obstructive pulmonary disease (COPD). For additional information and full inclusion/exclusion criteria, please consult clinicaltrials.gov.
Actively Recruiting
Patient Decision-making About Precision Oncology in Veterans With Advanced Prostate Cancer
This clinical trial explores and implements methods to improve informed decision making (IDM) regarding precision oncology tests amongst veterans with prostate cancer that may have spread from where it first started to nearby tissue, lymph nodes, or distant parts of the body (advanced). Precision oncology, the use of germline genetic testing and tumor-based molecular assays to inform cancer care, has become an important aspect of evidence-based care for men with advanced prostate cancer. Veterans with metastatic castrate-resistant prostate cancer may not be carrying out IDM due to unmet decisional needs. An informed decision is a choice based on complete and accurate information. The information gained from this study will help researchers develop a decision support intervention (DSI) and implement the intervention. A DSI may serve as a valuable tool to reduce ongoing racial disparities in genetic testing and encourage enrollment to precision oncology trials.
ID: NCT05396872
Sponsor; Investigator; Collaborator: University of California, San Francisco; Daniel Kwon, MD; US Department of Defense VA Office of Research and Development; Madalina Macrea, MD, PhD
Location: San Francisco Veterans Affairs Medical Center, CA
Veterans Affairs Seamless Phase II/III Randomized Trial of STAndard Systemic theRapy With or Without PET-directed Local Therapy for Oligometastatic pRosTate Cancer (VA STARPORT)
This is a prospective, open-label, multi-center seamless phase II to phase III randomized clinical trial designed to compare SST with or without PET-directed local therapy in improving the castration-resistant prostate cancer-free survival (CRPC-free survival) for veterans with oligometastatic prostate cancer. Oligometastasis will be defined as 1-10 sites of metastatic disease based on the clinical determination of the LSI which incorporates all imaging, clinical, and pathologic data available.
ID: NCT04787744
Sponsor; Collaborator: VA Office of Research and Development; Abhishek Solanki, MD, MS VA Office of Research and Development; Madalina Macrea, MD, PhD
Location: 19 locations, including Edwards Hines Jr. VA Hospital, Hines, IL
The Prostate Cancer, Genetic Risk, and Equitable Screening Study (ProGRESS) (ProGRESS)
Prostate cancer is the most common non-skin cancer among veterans and the second leading cause of male cancer death. Current methods of screening men for prostate cancer are inaccurate and cannot identify which men do not have prostate cancer or have low-grade cases that will not cause harm and which men have significant prostate cancer needing treatment. False-positive screening tests can result in unnecessary prostate biopsies for men who do not need them. However, new genetic testing might help identify which men are at highest risk for prostate cancer. This study will examine whether a genetic test helps identify men at risk for significant prostate cancer while helping men who are at low risk for prostate cancer avoid unnecessary biopsies. If this genetic test proves beneficial, it will improve the way that health care providers screen male veterans for prostate cancer.
ID: NCT05926102
Sponsor; Collaborator: VA Office of Research and Development; Jason L. Vassy, MD, MPH VA Office of Research and Development; Madalina Macrea, MD, PhD
Location: VA Boston Healthcare System Jamaica Plain Campus, MA
A Single-Arm Phase II Study of Neoadjuvant Intensified Androgen Deprivation (Leuprolide and Abiraterone Acetate) in Combination With AKT Inhibition (Capivasertib) for High-Risk Localized Prostate Cancer With PTEN Loss (SNARE)
The purpose of this study is to learn about how an investigational drug intervention completed before doing prostate surgery (specifically, radical prostatectomy with lymph node dissection) may help in treatment of high risk localized prostate cancers that are most resistant to standard treatments. This is a phase II research study. For this study, capivasertib, the study drug, will be taken with intensified androgen deprivation drugs (iADT; abiraterone and leuprolide) prior to radical prostatectomy. This study drug treatment will be evaluated to see if it is effective in shrinking and destroying prostate cancer tumors prior to surgery and to further evaluate its safety prior to prostate cancer surgery.
ID: NCT05593497
Sponsor; Collaborator: VA Office of Research and Development; Ryan P. Kopp, MD VA Office of Research and Development; Madalina Macrea, MD, PhD
Location: 5 locations, including VA Portland Health Care System, OR
18F-Fluciclovine PET/CT Impact on Predicting Clinical Outcome of 177Lu-PSMA-617 Therapy in Patients With Prostate Cancer
This is a single-center, prospective, exploratory study. Patients with metastatic castration-resistant prostate cancer (mCRPC) scheduled to undergo Lutetium labelled prostate-specific membrane antigen radioligand therapy (LuPSMA RLT) at the West Los Angeles VA (WLA-VA) will be imaged with a baseline F-18 fluorodeoxyglucose positron emission tomography/computed tomography 18F-FDG PET/CT and a 18F-DCFPyL PET/CT (18F-DCFPyL (2-(3-{1-carboxy-5-[(6-18F-fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid)positron emission tomography/computed tomography , as per standard of care in our institution. All patients further undergo eventual follow-up prostate-specific membrane antigen positron emission tomography (PSMA PET) after the 2nd, 4th, and 6th LuPSMA RLT cycle. In this prospective study, an18F-Fluciclovine positron emission tomography/computed tomography (Axumin PET/CT) will be additionally obtained at baseline (pre-LuPSMA RLT), and after the 2nd, 4th, 6th LuPSMA RLT cycles. Axumin PET/CT will be acquired within 7 days from the PSMA PET.
This study is open to veterans only.
ID: NCT06706921
Sponsor; Collaborator: VA Greater Los Angeles Healthcare System; Gholam Berenji, MD, Janake Wijesuriya, BS VA Office of Research and Development; Madalina Macrea, MD, PhD
Location: VA Greater Los Angeles Healthcare System, CA
High Dose Testosterone for ATM, CDK12 or CHEK2 Altered Prostate Cancers (VA-BAT)
This study will determine whether the presence of DNA repair deficiency in the form of alterations in the genes ATM, CDK12 or CHEK2 predicts for a high likelihood of responding to the use of intermittent high dose testosterone. This therapy may result in responses in tumors which are genetically unstable because of DNA repair deficiency and this is a prospective study to test that hypothesis.
ID: NCT05011383
Sponsor; Collaborator: VA Office of Research and Development; Robert B. Montgomery, MD VA Office of Research and Development; Madalina Macrea, MD, PhD
Location: 17 locations, including VA Puget Sound Health Care System, Seattle, WA
A Study of CHeckpoint Inhibitors in Men With prOgressive Metastatic Castrate Resistant Prostate Cancer Characterized by a Mismatch Repair Deficiency or Biallelic CDK12 Inactivation (CHOMP)
The primary objective is to assess the activity and efficacy of pembrolizumab, a checkpoint inhibitor, in veterans with metastatic castration-resistant prostate cancer (mCRPC) characterized by either mismatch repair deficiency (dMMR) or biallelic inactivation of CDK12 (CDK12-/-). The secondary objectives involve determining the frequency with which dMMR and CDK12-/- occur in this patient population, as well as the effects of pembrolizumab on various clinical endpoints (time to PSA progression, maximal PSA response, time to initiation of alternative anti-neoplastic therapy, time to radiographic progression, overall survival, and safety and tolerability). Lastly, the study will compare the pre-treatment and at-progression metastatic tumor biopsies to investigate the molecular correlates of resistance and sensitivity to pembrolizumab via RNA-sequencing, exome-sequencing, selected protein analyses, and multiplexed immunofluorescence.
ID: NCT04104893
Sponsor; Investigator; Collaborator: VA Office of Research and Development; Matthew B. Rettig, MD; Merck Sharp & Dohme LLC VA Office of Research and Development; Madalina Macrea, MD, PhD
Location: 12 locations, including VA Greater Los Angeles Healthcare System, CA
Carboplatin or Olaparib for BRcA Deficient Prostate Cancer (COBRA)
This is an unblinded, randomized clinical study comparing the efficacy of DNA damaging chemotherapy using carboplatin, to standard of care therapy for patients who have metastatic castrate resistant prostate cancer. This trial will use olaparib or carboplatin as initial therapy with crossover to the alternate or second-line drug after first progression for patients with tumors containing BARD1, BRCA1, BRCA2, BRIP1, CHEK1, FANCL, PALB2, RAD51B, RAD51C, RAD51D, or RAD54L inactivating mutations.
Participants are randomized (1:1) and receive either carboplatin (AUC 5, IV) every 21 days, first or olaparib taken orally (300 mg), twice daily in 28-day cycles, until intolerance, complete response, or progression by Prostate Cancer Working Group 3 (PCWG3) criteria.
Participants then cross over from the first-line therapy to the second-line therapy with the opposite study medication and receive treatment to intolerance or progression (whichever is first). Enrolled participants will be allowed to crossover to second line therapy if they continue to meet initial eligibility criteria, and at least three weeks have elapsed since last administration of either carboplatin or olaparib. Throughout the study, safety and tolerability will be assessed. Progression will be evaluated with bone scan, CT of the abdomen/pelvis, or MRI and PSA as per PCWG3 criteria.
ID: NCT04038502
Sponsor; Collaborator: VA Office of Research and Development; Robert B. Montgomery, MD; Ryan Burri, MD; Phoebe Tsao, MD, MSc; Maneesh Jain, MD VA Office of Research and Development; Madalina Macrea, MD, PhD
Location: 17 locations, including VA Puget Sound Health Care System, Seattle, WA
Landmark VA Study Uncovers Gene Variant Linked to Prostate Cancer
Only about 5% of hereditary prostate cancer (HPC) cases can be explained by known genetic variants, but a groundbreaking US Department of Veterans Affairs (VA) study could revolutionize the diagnosis, prevention, and treatment of HPC in a similar fashion that the discovery of the BRAC2 gene did in breast cancer.
The study, conducted at the VA Tennessee Valley Healthcare System in accordance with Vanderbilt University Medical Center and the VA Million Veteran Program (MVP), linked variants of the WNT9B gene with a greater risk of prostate cancer.
About 15,000 veterans are diagnosed with prostate cancer and treated at the VA annually, and > 200,000 veterans are prostate cancer survivors. According to Bruce Montgomery, MD, an oncologist with VA Puget Sound Health Care System, “Veterans are unique in that those men exposed to Agent Orange during the Vietnam War are at elevated risk for prostate cancer.” Montgomery added that germline pathogenic variants in genes such as BRCA2 and HOXB13 are other risk factors.
This genome-wide study searched for recurrently observed variants that carried the most risk. The study gathered data from a familial case-control population in the Nashville Familial Prostate Cancer Study (NFPCS) and International Consortium for Prostate Cancer Genetics (ICPCG). For evidence of replication, the study turned to 4 biobanks: the MVP, All of Us, the UK Biobank, and FinnGen.
The NFPCS is a case-control study based on family history. Patients included those undergoing treatment for prostate cancer and controls undergoing routine screening at Vanderbilt University Medical Center and the Nashville VA Medical Center between 2003 and 2009. Patients were included in the analysis if they had also had a first- or second-degree relative with prostate cancer.
The ICPCG dataset encompasses unrelated HPC cases aggregated from 12 study sites across Finland, France, Germany, the UK, and the US. The MVP is the nation’s largest biorepository of veteran data and has one of the world’s most diverse cohorts of any genetic research program. More than 1 million veterans are enrolled, and 800-plus researchers are working on > 100 projects.
Pathogenic variants of only 2 genes met the replication requirement with genome-wide significance: HOXB13 and WNT9B. HOXB13 has been reported on in the literature, but this is the first study to investigate WNT9B.
Researchers identified 2 variants of the WNT9B gene: WNT9B E152K carried 2.5-fold risk and reached genome-wide significance under meta-analysis, collectively encompassing one-half million patients. The association of WNT9B E152K with prostate cancer was supported by the familial study populations and each biobank, with genome-wide significance. Variant WNT9B Q47R reached genome-wide significance in the Finnish study. The Q47R founder haplotype was also carried by familial prostate cancer cases in the US and UK.
Autosomal dominant WNT9B pathogenic variants are already known to cause embryonic developmental sequence defects, leading later to prostatic cysts, enlarged prostate, and seminal vesicle cysts. Seminal vesicle adenocarcinoma (or squamous cell carcinoma) and clear cell carcinoma of the prostate have also been reported.
The study found that HOXB13 and WNT9B “share an unexpected commonality.” Both genes function in embryonic genitourinary development. WNT9B pathogenic variants cause the autosomal dominant Mayer-Rokitansky-Küster-Hauser syndrome, featuring genitourinary developmental defects. The study concluded: “Collectively, our observations implicate inherited variation in pathways guiding embryonic genitourinary development in the development of prostate cancer.”
“Significant investments” in VA-specific clinical trials recently have been pursued through a joint agreement between the VA and the Prostate Cancer Foundation, Montgomery said: “The Prostate Cancer Foundation is supporting tumor and germline sequencing of prostate cancer for veterans with advanced disease and providing resources to set up research infrastructure at 10 centers nationwide.”
The VA has also published a prostate cancer clinical pathway and is in the process of creating a national prostate cancer registry. Such a database, as well as the MVP are both unique to the VA and key to research such as the Predicting Metastatic Progression of High Risk Localized Prostate Cancer study, which began in 2023. Five VA medical centers are collaborating on an artificial intelligence algorithm that will detect patterns indicative of aggressive prostate cancer.
“A digital repository for data will allow for development, testing, and validation of prognostic classifiers that could positively impact clinical management of veterans with high-risk prostate cancer,” said Matthew Rettig, MD, chief of oncology and hematology at the Greater Los Angeles VA Medical Center who was coprincipal investigator for the study. “The infrastructure developed by this research will serve as a valuable hub for future discovery.”
About 12% of men with metastatic prostate cancer carry a pathogenic germline alteration that could warrant the use of PARP (poly [ADP-ribose] polymerase) inhibitors or platinum chemotherapy, neither of which is part of standard care. National Comprehensive Cancer Network guidelines recommend germline testing in men with metastatic prostate cancer. In addition, “the family members of veterans who carry these alterations could benefit from undergoing testing and taking advantage of potentially life-saving interventions and surveillance strategies if they are also carriers,” Montgomery wrote.
The VA is committed to improving access to germline testing for men with metastatic prostate cancer in several ways. Montgomery pointed to the system-wide VA genetic counseling and testing resource, the Genomic Medicine Service, and said somatic testing is available across the VA through the National Precision Oncology Program. Both programs can be extremely important to veterans because they provide access to precision oncology studies, along with off-label use of effective treatments.
Precision oncology is the most rapidly moving area in prostate cancer, according to Montgomery. “In the VA, this has been embraced as a very specific need to find these therapeutic options for all veterans as quickly as possible. I am most excited by how the enthusiasm for these approaches is supported at all levels, both nationally and locally, because it makes implementing very significant changes to research and treatment possible.”
Only about 5% of hereditary prostate cancer (HPC) cases can be explained by known genetic variants, but a groundbreaking US Department of Veterans Affairs (VA) study could revolutionize the diagnosis, prevention, and treatment of HPC in a similar fashion that the discovery of the BRAC2 gene did in breast cancer.
The study, conducted at the VA Tennessee Valley Healthcare System in accordance with Vanderbilt University Medical Center and the VA Million Veteran Program (MVP), linked variants of the WNT9B gene with a greater risk of prostate cancer.
About 15,000 veterans are diagnosed with prostate cancer and treated at the VA annually, and > 200,000 veterans are prostate cancer survivors. According to Bruce Montgomery, MD, an oncologist with VA Puget Sound Health Care System, “Veterans are unique in that those men exposed to Agent Orange during the Vietnam War are at elevated risk for prostate cancer.” Montgomery added that germline pathogenic variants in genes such as BRCA2 and HOXB13 are other risk factors.
This genome-wide study searched for recurrently observed variants that carried the most risk. The study gathered data from a familial case-control population in the Nashville Familial Prostate Cancer Study (NFPCS) and International Consortium for Prostate Cancer Genetics (ICPCG). For evidence of replication, the study turned to 4 biobanks: the MVP, All of Us, the UK Biobank, and FinnGen.
The NFPCS is a case-control study based on family history. Patients included those undergoing treatment for prostate cancer and controls undergoing routine screening at Vanderbilt University Medical Center and the Nashville VA Medical Center between 2003 and 2009. Patients were included in the analysis if they had also had a first- or second-degree relative with prostate cancer.
The ICPCG dataset encompasses unrelated HPC cases aggregated from 12 study sites across Finland, France, Germany, the UK, and the US. The MVP is the nation’s largest biorepository of veteran data and has one of the world’s most diverse cohorts of any genetic research program. More than 1 million veterans are enrolled, and 800-plus researchers are working on > 100 projects.
Pathogenic variants of only 2 genes met the replication requirement with genome-wide significance: HOXB13 and WNT9B. HOXB13 has been reported on in the literature, but this is the first study to investigate WNT9B.
Researchers identified 2 variants of the WNT9B gene: WNT9B E152K carried 2.5-fold risk and reached genome-wide significance under meta-analysis, collectively encompassing one-half million patients. The association of WNT9B E152K with prostate cancer was supported by the familial study populations and each biobank, with genome-wide significance. Variant WNT9B Q47R reached genome-wide significance in the Finnish study. The Q47R founder haplotype was also carried by familial prostate cancer cases in the US and UK.
Autosomal dominant WNT9B pathogenic variants are already known to cause embryonic developmental sequence defects, leading later to prostatic cysts, enlarged prostate, and seminal vesicle cysts. Seminal vesicle adenocarcinoma (or squamous cell carcinoma) and clear cell carcinoma of the prostate have also been reported.
The study found that HOXB13 and WNT9B “share an unexpected commonality.” Both genes function in embryonic genitourinary development. WNT9B pathogenic variants cause the autosomal dominant Mayer-Rokitansky-Küster-Hauser syndrome, featuring genitourinary developmental defects. The study concluded: “Collectively, our observations implicate inherited variation in pathways guiding embryonic genitourinary development in the development of prostate cancer.”
“Significant investments” in VA-specific clinical trials recently have been pursued through a joint agreement between the VA and the Prostate Cancer Foundation, Montgomery said: “The Prostate Cancer Foundation is supporting tumor and germline sequencing of prostate cancer for veterans with advanced disease and providing resources to set up research infrastructure at 10 centers nationwide.”
The VA has also published a prostate cancer clinical pathway and is in the process of creating a national prostate cancer registry. Such a database, as well as the MVP are both unique to the VA and key to research such as the Predicting Metastatic Progression of High Risk Localized Prostate Cancer study, which began in 2023. Five VA medical centers are collaborating on an artificial intelligence algorithm that will detect patterns indicative of aggressive prostate cancer.
“A digital repository for data will allow for development, testing, and validation of prognostic classifiers that could positively impact clinical management of veterans with high-risk prostate cancer,” said Matthew Rettig, MD, chief of oncology and hematology at the Greater Los Angeles VA Medical Center who was coprincipal investigator for the study. “The infrastructure developed by this research will serve as a valuable hub for future discovery.”
About 12% of men with metastatic prostate cancer carry a pathogenic germline alteration that could warrant the use of PARP (poly [ADP-ribose] polymerase) inhibitors or platinum chemotherapy, neither of which is part of standard care. National Comprehensive Cancer Network guidelines recommend germline testing in men with metastatic prostate cancer. In addition, “the family members of veterans who carry these alterations could benefit from undergoing testing and taking advantage of potentially life-saving interventions and surveillance strategies if they are also carriers,” Montgomery wrote.
The VA is committed to improving access to germline testing for men with metastatic prostate cancer in several ways. Montgomery pointed to the system-wide VA genetic counseling and testing resource, the Genomic Medicine Service, and said somatic testing is available across the VA through the National Precision Oncology Program. Both programs can be extremely important to veterans because they provide access to precision oncology studies, along with off-label use of effective treatments.
Precision oncology is the most rapidly moving area in prostate cancer, according to Montgomery. “In the VA, this has been embraced as a very specific need to find these therapeutic options for all veterans as quickly as possible. I am most excited by how the enthusiasm for these approaches is supported at all levels, both nationally and locally, because it makes implementing very significant changes to research and treatment possible.”
Only about 5% of hereditary prostate cancer (HPC) cases can be explained by known genetic variants, but a groundbreaking US Department of Veterans Affairs (VA) study could revolutionize the diagnosis, prevention, and treatment of HPC in a similar fashion that the discovery of the BRAC2 gene did in breast cancer.
The study, conducted at the VA Tennessee Valley Healthcare System in accordance with Vanderbilt University Medical Center and the VA Million Veteran Program (MVP), linked variants of the WNT9B gene with a greater risk of prostate cancer.
About 15,000 veterans are diagnosed with prostate cancer and treated at the VA annually, and > 200,000 veterans are prostate cancer survivors. According to Bruce Montgomery, MD, an oncologist with VA Puget Sound Health Care System, “Veterans are unique in that those men exposed to Agent Orange during the Vietnam War are at elevated risk for prostate cancer.” Montgomery added that germline pathogenic variants in genes such as BRCA2 and HOXB13 are other risk factors.
This genome-wide study searched for recurrently observed variants that carried the most risk. The study gathered data from a familial case-control population in the Nashville Familial Prostate Cancer Study (NFPCS) and International Consortium for Prostate Cancer Genetics (ICPCG). For evidence of replication, the study turned to 4 biobanks: the MVP, All of Us, the UK Biobank, and FinnGen.
The NFPCS is a case-control study based on family history. Patients included those undergoing treatment for prostate cancer and controls undergoing routine screening at Vanderbilt University Medical Center and the Nashville VA Medical Center between 2003 and 2009. Patients were included in the analysis if they had also had a first- or second-degree relative with prostate cancer.
The ICPCG dataset encompasses unrelated HPC cases aggregated from 12 study sites across Finland, France, Germany, the UK, and the US. The MVP is the nation’s largest biorepository of veteran data and has one of the world’s most diverse cohorts of any genetic research program. More than 1 million veterans are enrolled, and 800-plus researchers are working on > 100 projects.
Pathogenic variants of only 2 genes met the replication requirement with genome-wide significance: HOXB13 and WNT9B. HOXB13 has been reported on in the literature, but this is the first study to investigate WNT9B.
Researchers identified 2 variants of the WNT9B gene: WNT9B E152K carried 2.5-fold risk and reached genome-wide significance under meta-analysis, collectively encompassing one-half million patients. The association of WNT9B E152K with prostate cancer was supported by the familial study populations and each biobank, with genome-wide significance. Variant WNT9B Q47R reached genome-wide significance in the Finnish study. The Q47R founder haplotype was also carried by familial prostate cancer cases in the US and UK.
Autosomal dominant WNT9B pathogenic variants are already known to cause embryonic developmental sequence defects, leading later to prostatic cysts, enlarged prostate, and seminal vesicle cysts. Seminal vesicle adenocarcinoma (or squamous cell carcinoma) and clear cell carcinoma of the prostate have also been reported.
The study found that HOXB13 and WNT9B “share an unexpected commonality.” Both genes function in embryonic genitourinary development. WNT9B pathogenic variants cause the autosomal dominant Mayer-Rokitansky-Küster-Hauser syndrome, featuring genitourinary developmental defects. The study concluded: “Collectively, our observations implicate inherited variation in pathways guiding embryonic genitourinary development in the development of prostate cancer.”
“Significant investments” in VA-specific clinical trials recently have been pursued through a joint agreement between the VA and the Prostate Cancer Foundation, Montgomery said: “The Prostate Cancer Foundation is supporting tumor and germline sequencing of prostate cancer for veterans with advanced disease and providing resources to set up research infrastructure at 10 centers nationwide.”
The VA has also published a prostate cancer clinical pathway and is in the process of creating a national prostate cancer registry. Such a database, as well as the MVP are both unique to the VA and key to research such as the Predicting Metastatic Progression of High Risk Localized Prostate Cancer study, which began in 2023. Five VA medical centers are collaborating on an artificial intelligence algorithm that will detect patterns indicative of aggressive prostate cancer.
“A digital repository for data will allow for development, testing, and validation of prognostic classifiers that could positively impact clinical management of veterans with high-risk prostate cancer,” said Matthew Rettig, MD, chief of oncology and hematology at the Greater Los Angeles VA Medical Center who was coprincipal investigator for the study. “The infrastructure developed by this research will serve as a valuable hub for future discovery.”
About 12% of men with metastatic prostate cancer carry a pathogenic germline alteration that could warrant the use of PARP (poly [ADP-ribose] polymerase) inhibitors or platinum chemotherapy, neither of which is part of standard care. National Comprehensive Cancer Network guidelines recommend germline testing in men with metastatic prostate cancer. In addition, “the family members of veterans who carry these alterations could benefit from undergoing testing and taking advantage of potentially life-saving interventions and surveillance strategies if they are also carriers,” Montgomery wrote.
The VA is committed to improving access to germline testing for men with metastatic prostate cancer in several ways. Montgomery pointed to the system-wide VA genetic counseling and testing resource, the Genomic Medicine Service, and said somatic testing is available across the VA through the National Precision Oncology Program. Both programs can be extremely important to veterans because they provide access to precision oncology studies, along with off-label use of effective treatments.
Precision oncology is the most rapidly moving area in prostate cancer, according to Montgomery. “In the VA, this has been embraced as a very specific need to find these therapeutic options for all veterans as quickly as possible. I am most excited by how the enthusiasm for these approaches is supported at all levels, both nationally and locally, because it makes implementing very significant changes to research and treatment possible.”