User login
Improving Colorectal Cancer Screening via Mailed Fecal Immunochemical Testing in a Veterans Affairs Health System
Colorectal cancer (CRC) is among the most common cancers and causes of cancer-related deaths in the United States.1 Reflective of a nationwide trend, CRC screening rates at the Veterans Affairs Connecticut Healthcare System (VACHS) decreased during the COVID-19 pandemic.2-5 Contributing factors to this decrease included cancellations of elective colonoscopies during the initial phase of the pandemic and concurrent turnover of endoscopists. In 2021, the US Preventive Services Task Force lowered the recommended initial CRC screening age from 50 years to 45 years, further increasing the backlog of unscreened patients.6
Fecal immunochemical testing (FIT) is a noninvasive screening method in which antibodies are used to detect hemoglobin in the stool. The sensitivity and specificity of 1-time FIT are 79% to 80% and 94%, respectively, for the detection of CRC, with sensitivity improving with successive testing.7,8 Annual FIT is recognized as a tier 1 preferred screening method by the US Multi-Society Task Force on Colorectal Cancer.7,9 Programs that mail FIT kits to eligible patients outside of physician visits have been successfully implemented in health care systems.10,11
The VACHS designed and implemented a mailed FIT program using existing infrastructure and staffing.
Program Description
A team of local stakeholders comprised of VACHS leadership, primary care, nursing, and gastroenterology staff, as well as representatives from laboratory, informatics, mail services, and group practice management, was established to execute the project. The team met monthly to plan the project.
The team developed a dataset consisting of patients aged 45 to 75 years who were at average risk for CRC and due for CRC screening. Patients were defined as due for CRC screening if they had not had a colonoscopy in the previous 9 years or a FIT or fecal occult blood test in the previous 11 months. Average risk for CRC was defined by excluding patients with associated diagnosis codes for CRC, colectomy, inflammatory bowel disease, and anemia. The program also excluded patients with diagnosis codes associated with dementia, deferring discussions about cancer screening to their primary care practitioners (PCPs). Patients with invalid mailing addresses were also excluded, as well as those whose PCPs had indicated in the electronic health record that the patient received CRC screening outside the US Department of Veterans Affairs (VA) system.
Letter Templates
Two patient letter electronic health record templates were developed. The first was a primer letter, which was mailed to patients 2 to 3 weeks before the mailed FIT kit as an introduction to the program.12 The purpose of the primer letter was to give advance notice to patients that they could expect a FIT kit to arrive in the mail. The goal was to prepare patients to complete FIT when the kit arrived and prompt them to call the VA to opt out of the mailed FIT program if they were up to date with CRC screening or if they had a condition which made them at high risk for CRC.
The second FIT letter arrived with the FIT kit, introduced FIT and described the importance of CRC screening. The letter detailed instructions for completing FIT and automatically created a FIT order. It also included a list of common conditions that may exclude patients, with a recommendation for patients to contact their medical team if they felt they were not candidates for FIT.
Staff Education
A previous VACHS pilot project demonstrated the success of a mailed FIT program to increase FIT use. Implemented as part of the pilot program, staff education consisted of a session for clinicians about the role of FIT in CRC screening and an all-staff education session. An additional education session about CRC and FIT for all staff was repeated with the program launch.
Program Launch
The mailed FIT program was introduced during a VACHS primary care all-staff meeting. After the meeting, each patient aligned care team (PACT) received an encrypted email that included a list of the patients on their team who were candidates for the program, a patient-facing FIT instruction sheet, detailed instructions on how to send the FIT primer letter, and a FIT package consisting of the labeled FIT kit, FIT letter, and patient instruction sheet. A reminder letter was sent to each patient 3 weeks after the FIT package was mailed. The patient lists were populated into a shared, encrypted Microsoft Teams folder that was edited in real time by PACT teams and viewed by VACHS leadership to track progress.
Program Metrics
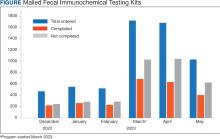
At program launch, the VACHS had 4642 patients due for CRC screening who were eligible for the mailed FIT program. On March 7, 2023, the data consisting of FIT tests ordered between December 2022 and May 2023—3 months before and after the launch of the program—were reviewed and categorized. In the 3 months before program launch, 1528 FIT were ordered and 714 were returned (46.7%). In the 3 months after the launch of the program, 4383 FIT were ordered and 1712 were returned (39.1%) (Figure). Test orders increased 287% from the preintervention to the postintervention period. The mean (SD) number of monthly FIT tests prelaunch was 509 (32.7), which increased to 1461 (331.6) postlaunch.
At the VACHS, 61.4% of patients aged 45 to 75 years were up to date with CRC screening before the program launch. In the 3 months after program launch, the rate increased to 63.8% among patients aged 45 to 75 years, the highest rate in our Veterans Integrated Services Network and exceeding the VA national average CRC screening rate, according to unpublished VA Monthly Management Report data.
In the 3 months following the program launch, 139 FIT kits tested positive for potential CRC. Of these, 79 (56.8%) patients had completed a diagnostic colonoscopy. PACT PCPs and nurses received reports on patients with positive FIT tests and those with no colonoscopy scheduled or completed and were asked to follow up.
Discussion
Through a proactive, population-based CRC screening program centered on mailed FIT kits outside of the traditional patient visit, the VACHS increased the use of FIT and rates of CRC screening. The numbers of FIT kits ordered and completed substantially increased in the 3 months after program launch.
Compared to mailed FIT programs described in the literature that rely on centralized processes in that a separate team operates the mailed FIT program for the entire organization, this program used existing PACT infrastructure and staff.10,11 This strategy allowed VACHS to design and implement the program in several months. Not needing to hire new staff or create a central team for the sole purpose of implementing the program allowed us to save on any organizational funding and efforts that would have accompanied the additional staff. The program described in this article may be more attainable for primary care practices or smaller health systems that do not have the capacity for the creation of a centralized process.
Limitations
Although the total number of FIT completions substantially increased during the program, the rate of FIT completion during the mailed FIT program was lower than the rate of completion prior to program launch. This decreased rate of FIT kit completion may be related to separation from a patient visit and potential loss of real-time education with a clinician. The program’s decentralized design increased the existing workload for primary care staff, and as a result, consideration must be given to local staffing levels. Additionally, the report of eligible patients depended on diagnosis codes and may have captured patients with higher-than-average risk of CRC, such as patients with prior history of adenomatous polyps, family history of CRC, or other medical or genetic conditions. We attempted to mitigate this by including a list of conditions that would exclude patients from FIT eligibility in the FIT letter and giving them the option to opt out.
Conclusions
CRC screening rates improved following implementation of a primary care team-centered quality improvement process to proactively identify patients appropriate for FIT and mail them FIT kits. This project highlights that population-health interventions around CRC screening via use of FIT can be successful within a primary care patient-centered medical home model, considering the increases in both CRC screening rates and increase in FIT tests ordered.
1. American Cancer Society. Key statistics for colorectal cancer. Revised January 29, 2024. Accessed June 11, 2024. https://www.cancer.org/cancer/types/colon-rectal-cancer/about/key-statistics.html
2. Chen RC, Haynes K, Du S, Barron J, Katz AJ. Association of cancer screening deficit in the United States with the COVID-19 pandemic. JAMA Oncol. 2021;7(6):878-884. doi:10.1001/jamaoncol.2021.0884
3. Mazidimoradi A, Tiznobaik A, Salehiniya H. Impact of the COVID-19 pandemic on colorectal cancer screening: a systematic review. J Gastrointest Cancer. 2022;53(3):730-744. doi:10.1007/s12029-021-00679-x
4. Adams MA, Kurlander JE, Gao Y, Yankey N, Saini SD. Impact of coronavirus disease 2019 on screening colonoscopy utilization in a large integrated health system. Gastroenterology. 2022;162(7):2098-2100.e2. doi:10.1053/j.gastro.2022.02.034
5. Sundaram S, Olson S, Sharma P, Rajendra S. A review of the impact of the COVID-19 pandemic on colorectal cancer screening: implications and solutions. Pathogens. 2021;10(11):558. doi:10.3390/pathogens10111508
6. US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977. doi:10.1001/jama.2021.6238
7. Robertson DJ, Lee JK, Boland CR, et al. Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: a consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Gastrointest Endosc. 2017;85(1):2-21.e3. doi:10.1016/j.gie.2016.09.025
8. Lee JK, Liles EG, Bent S, Levin TR, Corley DA. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med. 2014;160(3):171. doi:10.7326/M13-1484
9. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323. doi:10.1053/j.gastro.2017.05.013
10. Deeds SA, Moore CB, Gunnink EJ, et al. Implementation of a mailed faecal immunochemical test programme for colorectal cancer screening among veterans. BMJ Open Qual. 2022;11(4):e001927. doi:10.1136/bmjoq-2022-001927
11. Selby K, Jensen CD, Levin TR, et al. Program components and results from an organized colorectal cancer screening program using annual fecal immunochemical testing. Clin Gastroenterol Hepatol. 2022;20(1):145-152. doi:10.1016/j.cgh.2020.09.042
12. Deeds S, Liu T, Schuttner L, et al. A postcard primer prior to mailed fecal immunochemical test among veterans: a randomized controlled trial. J Gen Intern Med. 2023:38(14):3235-3241. doi:10.1007/s11606-023-08248-7
Colorectal cancer (CRC) is among the most common cancers and causes of cancer-related deaths in the United States.1 Reflective of a nationwide trend, CRC screening rates at the Veterans Affairs Connecticut Healthcare System (VACHS) decreased during the COVID-19 pandemic.2-5 Contributing factors to this decrease included cancellations of elective colonoscopies during the initial phase of the pandemic and concurrent turnover of endoscopists. In 2021, the US Preventive Services Task Force lowered the recommended initial CRC screening age from 50 years to 45 years, further increasing the backlog of unscreened patients.6
Fecal immunochemical testing (FIT) is a noninvasive screening method in which antibodies are used to detect hemoglobin in the stool. The sensitivity and specificity of 1-time FIT are 79% to 80% and 94%, respectively, for the detection of CRC, with sensitivity improving with successive testing.7,8 Annual FIT is recognized as a tier 1 preferred screening method by the US Multi-Society Task Force on Colorectal Cancer.7,9 Programs that mail FIT kits to eligible patients outside of physician visits have been successfully implemented in health care systems.10,11
The VACHS designed and implemented a mailed FIT program using existing infrastructure and staffing.
Program Description
A team of local stakeholders comprised of VACHS leadership, primary care, nursing, and gastroenterology staff, as well as representatives from laboratory, informatics, mail services, and group practice management, was established to execute the project. The team met monthly to plan the project.
The team developed a dataset consisting of patients aged 45 to 75 years who were at average risk for CRC and due for CRC screening. Patients were defined as due for CRC screening if they had not had a colonoscopy in the previous 9 years or a FIT or fecal occult blood test in the previous 11 months. Average risk for CRC was defined by excluding patients with associated diagnosis codes for CRC, colectomy, inflammatory bowel disease, and anemia. The program also excluded patients with diagnosis codes associated with dementia, deferring discussions about cancer screening to their primary care practitioners (PCPs). Patients with invalid mailing addresses were also excluded, as well as those whose PCPs had indicated in the electronic health record that the patient received CRC screening outside the US Department of Veterans Affairs (VA) system.
Letter Templates
Two patient letter electronic health record templates were developed. The first was a primer letter, which was mailed to patients 2 to 3 weeks before the mailed FIT kit as an introduction to the program.12 The purpose of the primer letter was to give advance notice to patients that they could expect a FIT kit to arrive in the mail. The goal was to prepare patients to complete FIT when the kit arrived and prompt them to call the VA to opt out of the mailed FIT program if they were up to date with CRC screening or if they had a condition which made them at high risk for CRC.
The second FIT letter arrived with the FIT kit, introduced FIT and described the importance of CRC screening. The letter detailed instructions for completing FIT and automatically created a FIT order. It also included a list of common conditions that may exclude patients, with a recommendation for patients to contact their medical team if they felt they were not candidates for FIT.
Staff Education
A previous VACHS pilot project demonstrated the success of a mailed FIT program to increase FIT use. Implemented as part of the pilot program, staff education consisted of a session for clinicians about the role of FIT in CRC screening and an all-staff education session. An additional education session about CRC and FIT for all staff was repeated with the program launch.
Program Launch
The mailed FIT program was introduced during a VACHS primary care all-staff meeting. After the meeting, each patient aligned care team (PACT) received an encrypted email that included a list of the patients on their team who were candidates for the program, a patient-facing FIT instruction sheet, detailed instructions on how to send the FIT primer letter, and a FIT package consisting of the labeled FIT kit, FIT letter, and patient instruction sheet. A reminder letter was sent to each patient 3 weeks after the FIT package was mailed. The patient lists were populated into a shared, encrypted Microsoft Teams folder that was edited in real time by PACT teams and viewed by VACHS leadership to track progress.
Program Metrics
At program launch, the VACHS had 4642 patients due for CRC screening who were eligible for the mailed FIT program. On March 7, 2023, the data consisting of FIT tests ordered between December 2022 and May 2023—3 months before and after the launch of the program—were reviewed and categorized. In the 3 months before program launch, 1528 FIT were ordered and 714 were returned (46.7%). In the 3 months after the launch of the program, 4383 FIT were ordered and 1712 were returned (39.1%) (Figure). Test orders increased 287% from the preintervention to the postintervention period. The mean (SD) number of monthly FIT tests prelaunch was 509 (32.7), which increased to 1461 (331.6) postlaunch.
At the VACHS, 61.4% of patients aged 45 to 75 years were up to date with CRC screening before the program launch. In the 3 months after program launch, the rate increased to 63.8% among patients aged 45 to 75 years, the highest rate in our Veterans Integrated Services Network and exceeding the VA national average CRC screening rate, according to unpublished VA Monthly Management Report data.
In the 3 months following the program launch, 139 FIT kits tested positive for potential CRC. Of these, 79 (56.8%) patients had completed a diagnostic colonoscopy. PACT PCPs and nurses received reports on patients with positive FIT tests and those with no colonoscopy scheduled or completed and were asked to follow up.
Discussion
Through a proactive, population-based CRC screening program centered on mailed FIT kits outside of the traditional patient visit, the VACHS increased the use of FIT and rates of CRC screening. The numbers of FIT kits ordered and completed substantially increased in the 3 months after program launch.
Compared to mailed FIT programs described in the literature that rely on centralized processes in that a separate team operates the mailed FIT program for the entire organization, this program used existing PACT infrastructure and staff.10,11 This strategy allowed VACHS to design and implement the program in several months. Not needing to hire new staff or create a central team for the sole purpose of implementing the program allowed us to save on any organizational funding and efforts that would have accompanied the additional staff. The program described in this article may be more attainable for primary care practices or smaller health systems that do not have the capacity for the creation of a centralized process.
Limitations
Although the total number of FIT completions substantially increased during the program, the rate of FIT completion during the mailed FIT program was lower than the rate of completion prior to program launch. This decreased rate of FIT kit completion may be related to separation from a patient visit and potential loss of real-time education with a clinician. The program’s decentralized design increased the existing workload for primary care staff, and as a result, consideration must be given to local staffing levels. Additionally, the report of eligible patients depended on diagnosis codes and may have captured patients with higher-than-average risk of CRC, such as patients with prior history of adenomatous polyps, family history of CRC, or other medical or genetic conditions. We attempted to mitigate this by including a list of conditions that would exclude patients from FIT eligibility in the FIT letter and giving them the option to opt out.
Conclusions
CRC screening rates improved following implementation of a primary care team-centered quality improvement process to proactively identify patients appropriate for FIT and mail them FIT kits. This project highlights that population-health interventions around CRC screening via use of FIT can be successful within a primary care patient-centered medical home model, considering the increases in both CRC screening rates and increase in FIT tests ordered.
Colorectal cancer (CRC) is among the most common cancers and causes of cancer-related deaths in the United States.1 Reflective of a nationwide trend, CRC screening rates at the Veterans Affairs Connecticut Healthcare System (VACHS) decreased during the COVID-19 pandemic.2-5 Contributing factors to this decrease included cancellations of elective colonoscopies during the initial phase of the pandemic and concurrent turnover of endoscopists. In 2021, the US Preventive Services Task Force lowered the recommended initial CRC screening age from 50 years to 45 years, further increasing the backlog of unscreened patients.6
Fecal immunochemical testing (FIT) is a noninvasive screening method in which antibodies are used to detect hemoglobin in the stool. The sensitivity and specificity of 1-time FIT are 79% to 80% and 94%, respectively, for the detection of CRC, with sensitivity improving with successive testing.7,8 Annual FIT is recognized as a tier 1 preferred screening method by the US Multi-Society Task Force on Colorectal Cancer.7,9 Programs that mail FIT kits to eligible patients outside of physician visits have been successfully implemented in health care systems.10,11
The VACHS designed and implemented a mailed FIT program using existing infrastructure and staffing.
Program Description
A team of local stakeholders comprised of VACHS leadership, primary care, nursing, and gastroenterology staff, as well as representatives from laboratory, informatics, mail services, and group practice management, was established to execute the project. The team met monthly to plan the project.
The team developed a dataset consisting of patients aged 45 to 75 years who were at average risk for CRC and due for CRC screening. Patients were defined as due for CRC screening if they had not had a colonoscopy in the previous 9 years or a FIT or fecal occult blood test in the previous 11 months. Average risk for CRC was defined by excluding patients with associated diagnosis codes for CRC, colectomy, inflammatory bowel disease, and anemia. The program also excluded patients with diagnosis codes associated with dementia, deferring discussions about cancer screening to their primary care practitioners (PCPs). Patients with invalid mailing addresses were also excluded, as well as those whose PCPs had indicated in the electronic health record that the patient received CRC screening outside the US Department of Veterans Affairs (VA) system.
Letter Templates
Two patient letter electronic health record templates were developed. The first was a primer letter, which was mailed to patients 2 to 3 weeks before the mailed FIT kit as an introduction to the program.12 The purpose of the primer letter was to give advance notice to patients that they could expect a FIT kit to arrive in the mail. The goal was to prepare patients to complete FIT when the kit arrived and prompt them to call the VA to opt out of the mailed FIT program if they were up to date with CRC screening or if they had a condition which made them at high risk for CRC.
The second FIT letter arrived with the FIT kit, introduced FIT and described the importance of CRC screening. The letter detailed instructions for completing FIT and automatically created a FIT order. It also included a list of common conditions that may exclude patients, with a recommendation for patients to contact their medical team if they felt they were not candidates for FIT.
Staff Education
A previous VACHS pilot project demonstrated the success of a mailed FIT program to increase FIT use. Implemented as part of the pilot program, staff education consisted of a session for clinicians about the role of FIT in CRC screening and an all-staff education session. An additional education session about CRC and FIT for all staff was repeated with the program launch.
Program Launch
The mailed FIT program was introduced during a VACHS primary care all-staff meeting. After the meeting, each patient aligned care team (PACT) received an encrypted email that included a list of the patients on their team who were candidates for the program, a patient-facing FIT instruction sheet, detailed instructions on how to send the FIT primer letter, and a FIT package consisting of the labeled FIT kit, FIT letter, and patient instruction sheet. A reminder letter was sent to each patient 3 weeks after the FIT package was mailed. The patient lists were populated into a shared, encrypted Microsoft Teams folder that was edited in real time by PACT teams and viewed by VACHS leadership to track progress.
Program Metrics
At program launch, the VACHS had 4642 patients due for CRC screening who were eligible for the mailed FIT program. On March 7, 2023, the data consisting of FIT tests ordered between December 2022 and May 2023—3 months before and after the launch of the program—were reviewed and categorized. In the 3 months before program launch, 1528 FIT were ordered and 714 were returned (46.7%). In the 3 months after the launch of the program, 4383 FIT were ordered and 1712 were returned (39.1%) (Figure). Test orders increased 287% from the preintervention to the postintervention period. The mean (SD) number of monthly FIT tests prelaunch was 509 (32.7), which increased to 1461 (331.6) postlaunch.
At the VACHS, 61.4% of patients aged 45 to 75 years were up to date with CRC screening before the program launch. In the 3 months after program launch, the rate increased to 63.8% among patients aged 45 to 75 years, the highest rate in our Veterans Integrated Services Network and exceeding the VA national average CRC screening rate, according to unpublished VA Monthly Management Report data.
In the 3 months following the program launch, 139 FIT kits tested positive for potential CRC. Of these, 79 (56.8%) patients had completed a diagnostic colonoscopy. PACT PCPs and nurses received reports on patients with positive FIT tests and those with no colonoscopy scheduled or completed and were asked to follow up.
Discussion
Through a proactive, population-based CRC screening program centered on mailed FIT kits outside of the traditional patient visit, the VACHS increased the use of FIT and rates of CRC screening. The numbers of FIT kits ordered and completed substantially increased in the 3 months after program launch.
Compared to mailed FIT programs described in the literature that rely on centralized processes in that a separate team operates the mailed FIT program for the entire organization, this program used existing PACT infrastructure and staff.10,11 This strategy allowed VACHS to design and implement the program in several months. Not needing to hire new staff or create a central team for the sole purpose of implementing the program allowed us to save on any organizational funding and efforts that would have accompanied the additional staff. The program described in this article may be more attainable for primary care practices or smaller health systems that do not have the capacity for the creation of a centralized process.
Limitations
Although the total number of FIT completions substantially increased during the program, the rate of FIT completion during the mailed FIT program was lower than the rate of completion prior to program launch. This decreased rate of FIT kit completion may be related to separation from a patient visit and potential loss of real-time education with a clinician. The program’s decentralized design increased the existing workload for primary care staff, and as a result, consideration must be given to local staffing levels. Additionally, the report of eligible patients depended on diagnosis codes and may have captured patients with higher-than-average risk of CRC, such as patients with prior history of adenomatous polyps, family history of CRC, or other medical or genetic conditions. We attempted to mitigate this by including a list of conditions that would exclude patients from FIT eligibility in the FIT letter and giving them the option to opt out.
Conclusions
CRC screening rates improved following implementation of a primary care team-centered quality improvement process to proactively identify patients appropriate for FIT and mail them FIT kits. This project highlights that population-health interventions around CRC screening via use of FIT can be successful within a primary care patient-centered medical home model, considering the increases in both CRC screening rates and increase in FIT tests ordered.
1. American Cancer Society. Key statistics for colorectal cancer. Revised January 29, 2024. Accessed June 11, 2024. https://www.cancer.org/cancer/types/colon-rectal-cancer/about/key-statistics.html
2. Chen RC, Haynes K, Du S, Barron J, Katz AJ. Association of cancer screening deficit in the United States with the COVID-19 pandemic. JAMA Oncol. 2021;7(6):878-884. doi:10.1001/jamaoncol.2021.0884
3. Mazidimoradi A, Tiznobaik A, Salehiniya H. Impact of the COVID-19 pandemic on colorectal cancer screening: a systematic review. J Gastrointest Cancer. 2022;53(3):730-744. doi:10.1007/s12029-021-00679-x
4. Adams MA, Kurlander JE, Gao Y, Yankey N, Saini SD. Impact of coronavirus disease 2019 on screening colonoscopy utilization in a large integrated health system. Gastroenterology. 2022;162(7):2098-2100.e2. doi:10.1053/j.gastro.2022.02.034
5. Sundaram S, Olson S, Sharma P, Rajendra S. A review of the impact of the COVID-19 pandemic on colorectal cancer screening: implications and solutions. Pathogens. 2021;10(11):558. doi:10.3390/pathogens10111508
6. US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977. doi:10.1001/jama.2021.6238
7. Robertson DJ, Lee JK, Boland CR, et al. Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: a consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Gastrointest Endosc. 2017;85(1):2-21.e3. doi:10.1016/j.gie.2016.09.025
8. Lee JK, Liles EG, Bent S, Levin TR, Corley DA. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med. 2014;160(3):171. doi:10.7326/M13-1484
9. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323. doi:10.1053/j.gastro.2017.05.013
10. Deeds SA, Moore CB, Gunnink EJ, et al. Implementation of a mailed faecal immunochemical test programme for colorectal cancer screening among veterans. BMJ Open Qual. 2022;11(4):e001927. doi:10.1136/bmjoq-2022-001927
11. Selby K, Jensen CD, Levin TR, et al. Program components and results from an organized colorectal cancer screening program using annual fecal immunochemical testing. Clin Gastroenterol Hepatol. 2022;20(1):145-152. doi:10.1016/j.cgh.2020.09.042
12. Deeds S, Liu T, Schuttner L, et al. A postcard primer prior to mailed fecal immunochemical test among veterans: a randomized controlled trial. J Gen Intern Med. 2023:38(14):3235-3241. doi:10.1007/s11606-023-08248-7
1. American Cancer Society. Key statistics for colorectal cancer. Revised January 29, 2024. Accessed June 11, 2024. https://www.cancer.org/cancer/types/colon-rectal-cancer/about/key-statistics.html
2. Chen RC, Haynes K, Du S, Barron J, Katz AJ. Association of cancer screening deficit in the United States with the COVID-19 pandemic. JAMA Oncol. 2021;7(6):878-884. doi:10.1001/jamaoncol.2021.0884
3. Mazidimoradi A, Tiznobaik A, Salehiniya H. Impact of the COVID-19 pandemic on colorectal cancer screening: a systematic review. J Gastrointest Cancer. 2022;53(3):730-744. doi:10.1007/s12029-021-00679-x
4. Adams MA, Kurlander JE, Gao Y, Yankey N, Saini SD. Impact of coronavirus disease 2019 on screening colonoscopy utilization in a large integrated health system. Gastroenterology. 2022;162(7):2098-2100.e2. doi:10.1053/j.gastro.2022.02.034
5. Sundaram S, Olson S, Sharma P, Rajendra S. A review of the impact of the COVID-19 pandemic on colorectal cancer screening: implications and solutions. Pathogens. 2021;10(11):558. doi:10.3390/pathogens10111508
6. US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977. doi:10.1001/jama.2021.6238
7. Robertson DJ, Lee JK, Boland CR, et al. Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: a consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Gastrointest Endosc. 2017;85(1):2-21.e3. doi:10.1016/j.gie.2016.09.025
8. Lee JK, Liles EG, Bent S, Levin TR, Corley DA. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med. 2014;160(3):171. doi:10.7326/M13-1484
9. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323. doi:10.1053/j.gastro.2017.05.013
10. Deeds SA, Moore CB, Gunnink EJ, et al. Implementation of a mailed faecal immunochemical test programme for colorectal cancer screening among veterans. BMJ Open Qual. 2022;11(4):e001927. doi:10.1136/bmjoq-2022-001927
11. Selby K, Jensen CD, Levin TR, et al. Program components and results from an organized colorectal cancer screening program using annual fecal immunochemical testing. Clin Gastroenterol Hepatol. 2022;20(1):145-152. doi:10.1016/j.cgh.2020.09.042
12. Deeds S, Liu T, Schuttner L, et al. A postcard primer prior to mailed fecal immunochemical test among veterans: a randomized controlled trial. J Gen Intern Med. 2023:38(14):3235-3241. doi:10.1007/s11606-023-08248-7
Alcohol and CRC: These Drinking Patterns May Influence Risk
Alcohol and CRC: These Drinking Patterns May Influence Risk
New research sheds light on how chronic heavy alcohol use may contribute to colorectal cancer (CRC) development and how quitting may lower the risk for precancerous colorectal adenomas.
In a large US cancer screening trial, current heavy drinkers — with an average lifetime alcohol intake of 14 or more drinks per week — had a 25% higher risk for CRC and an almost twofold higher risk for rectal cancer than light drinkers averaging less than one drink per week.
When the research team further considered drinking consistency, steady heavy drinking throughout adulthood was associated with a 91% higher risk for CRC than consistent light drinking.
Additionally, no increased risk for CRC was found among former drinkers, and former drinkers were less likely than light drinkers to develop nonadvanced colorectal adenomas.
This analysis “adds to the growing amount of concerning literature showing that chronic heavy alcohol use can potentially contribute to colorectal cancer development,” Benjamin H. Levy III, MD, gastroenterologist and clinical associate of medicine at UChicago Medicine in Chicago, who wasn’t involved in the study, told Medscape Medical News.
The study’s co-senior author, Erikka Loftfield, PhD, MPH, also noted that the study “provides new evidence indicating that drinking cessation, compared to consistent light drinking, may lower adenoma risk.”
Current cancer prevention guidelines recommend limiting alcohol intake or ideally not drinking at all, and “our findings do not change this advice,” said Loftfield, with the National Cancer Institute (NCI) in Bethesda, Maryland.
The study was published online on January 26 in the journal Cancer.
Addressing a Data Gap
Alcoholic beverages are classified as carcinogenic to humans and are causally associated with CRC, Loftfield told Medscape Medical News. However, much of the evidence for this comes from cohort studies that only measure recent drinking patterns, generally among older adults, at study baseline. Fewer studies have looked at how drinking over a person’s lifetime and alcohol consumption patterns relate to colorectal adenoma and CRC risk, she explained.
To address these gaps, Loftfield and colleagues leveraged data on alcohol intake gathered as part of the NCI’s Prostate, Long, Colorectal, and Ovarian Cancer Screening Trial.
Average lifetime alcohol intake was calculated as drinks per week from age 18 through study baseline, and drinking patterns were further classified based on consistency and intensity over time.
During 20 years of follow-up, 1679 incident CRC cases occurred among 88,092 study participants. In multivariable-adjusted analyses, current heavy drinkers had a higher risk for CRC than those averaging less than one drink per week (hazard ratio [HR], 1.25), with the strongest association observed for rectal cancer (HR, 1.95).
“The increase in rectal cancer risk for heavy drinkers seen in this 20-year observational study was especially concerning,” Levy told Medscape Medical News.
What About Moderate Drinking?
Perhaps counterintuitively, moderate current drinkers (those consuming an average of 7 to less than 14 drinks per week) had a lower risk for CRC (HR, 0.79), especially distal colon cancer (HR, 0.64), than light drinkers.
Loftfield said that research in rodents suggests moderate alcohol intake may reduce inflammation and lower DNA damage, but it’s possible that the observed inverse association is due to residual confounding by unmeasured or poorly measured confounders, such as socioeconomic status.
She said it’s also important to note that the inverse association of moderate alcohol intake was strongest for distal colon cancer and in the screening arm of the trial. Those in the screening arm who screened positive with flexible sigmoidoscopy had polyps removed and were referred for colonoscopy during the trial period, making screening a potential intervention as well.
“Screening with flexible sigmoidoscopy has previously been found to decrease CRC incidence, specifically distal colon cancer, in this population. Thus, it is possible that better adherence to screening among moderate drinkers over the course of follow-up contributed to this finding,” Loftfield explained.
When looking at consistency of drinking, her team found that current drinkers who were consistent heavy drinkers throughout adulthood had a higher risk for CRC than consistent light drinkers (HR, 1.91).
Separate analyses of incident colorectal adenomas were directionally consistent with the CRC findings. These analyses included 12,327 participants with a negative baseline sigmoidoscopy, among whom 812 adenomas were detected on repeat screening.
Compared with current light drinkers, former drinkers had significantly lower odds of nonadvanced adenomas (odds ratio [OR], 0.58), but no significant association was observed for advanced adenomas (OR, 1.08; 95% CI, 0.62-1.90). The authors cautioned, however, that overall adenoma case numbers were limited, and estimates for advanced lesions were imprecise.
Educating Patients
Reached for comment, William Dahut, chief scientific officer for the American Cancer Society, told Medscape Medical News that this “very well done, large perspective study clearly demonstrates the significant increased risk of colorectal cancer for those that are heavy drinkers.”
He noted that the nearly twofold increased risk for rectal cancer among heavy drinkers “makes biological sense because the rectum is the area of the body where the toxins produced by alcohol potentially spend the most period of time.”
Heavy drinkers are at the highest risk, Dahut said, and “for them, screenings are particularly important.”
Even with this growing body of evidence, Levy noted that many patients in America and worldwide “have not been educated yet about the potential carcinogenic dangers of chronic alcohol use.”
Levy recommended that physicians get “accurate social histories about alcohol use” and “spend several minutes educating patients about their increased risk of cancer and liver problems from heavy alcohol use.”
Dahut encouraged health providers to tell patients that the risk for CRC from alcohol is also based on one’s lifetime alcohol consumption, “not simply what they had last weekend.”
Overall, this important research study, along with the Surgeon General’s recent publication about Alcohol and Cancer Risk, will hopefully “encourage physicians to have important conversations about alcohol reduction with their patients,” Levy said.
The study had no commercial funding. Loftfield, Dahult, and Levy reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
New research sheds light on how chronic heavy alcohol use may contribute to colorectal cancer (CRC) development and how quitting may lower the risk for precancerous colorectal adenomas.
In a large US cancer screening trial, current heavy drinkers — with an average lifetime alcohol intake of 14 or more drinks per week — had a 25% higher risk for CRC and an almost twofold higher risk for rectal cancer than light drinkers averaging less than one drink per week.
When the research team further considered drinking consistency, steady heavy drinking throughout adulthood was associated with a 91% higher risk for CRC than consistent light drinking.
Additionally, no increased risk for CRC was found among former drinkers, and former drinkers were less likely than light drinkers to develop nonadvanced colorectal adenomas.
This analysis “adds to the growing amount of concerning literature showing that chronic heavy alcohol use can potentially contribute to colorectal cancer development,” Benjamin H. Levy III, MD, gastroenterologist and clinical associate of medicine at UChicago Medicine in Chicago, who wasn’t involved in the study, told Medscape Medical News.
The study’s co-senior author, Erikka Loftfield, PhD, MPH, also noted that the study “provides new evidence indicating that drinking cessation, compared to consistent light drinking, may lower adenoma risk.”
Current cancer prevention guidelines recommend limiting alcohol intake or ideally not drinking at all, and “our findings do not change this advice,” said Loftfield, with the National Cancer Institute (NCI) in Bethesda, Maryland.
The study was published online on January 26 in the journal Cancer.
Addressing a Data Gap
Alcoholic beverages are classified as carcinogenic to humans and are causally associated with CRC, Loftfield told Medscape Medical News. However, much of the evidence for this comes from cohort studies that only measure recent drinking patterns, generally among older adults, at study baseline. Fewer studies have looked at how drinking over a person’s lifetime and alcohol consumption patterns relate to colorectal adenoma and CRC risk, she explained.
To address these gaps, Loftfield and colleagues leveraged data on alcohol intake gathered as part of the NCI’s Prostate, Long, Colorectal, and Ovarian Cancer Screening Trial.
Average lifetime alcohol intake was calculated as drinks per week from age 18 through study baseline, and drinking patterns were further classified based on consistency and intensity over time.
During 20 years of follow-up, 1679 incident CRC cases occurred among 88,092 study participants. In multivariable-adjusted analyses, current heavy drinkers had a higher risk for CRC than those averaging less than one drink per week (hazard ratio [HR], 1.25), with the strongest association observed for rectal cancer (HR, 1.95).
“The increase in rectal cancer risk for heavy drinkers seen in this 20-year observational study was especially concerning,” Levy told Medscape Medical News.
What About Moderate Drinking?
Perhaps counterintuitively, moderate current drinkers (those consuming an average of 7 to less than 14 drinks per week) had a lower risk for CRC (HR, 0.79), especially distal colon cancer (HR, 0.64), than light drinkers.
Loftfield said that research in rodents suggests moderate alcohol intake may reduce inflammation and lower DNA damage, but it’s possible that the observed inverse association is due to residual confounding by unmeasured or poorly measured confounders, such as socioeconomic status.
She said it’s also important to note that the inverse association of moderate alcohol intake was strongest for distal colon cancer and in the screening arm of the trial. Those in the screening arm who screened positive with flexible sigmoidoscopy had polyps removed and were referred for colonoscopy during the trial period, making screening a potential intervention as well.
“Screening with flexible sigmoidoscopy has previously been found to decrease CRC incidence, specifically distal colon cancer, in this population. Thus, it is possible that better adherence to screening among moderate drinkers over the course of follow-up contributed to this finding,” Loftfield explained.
When looking at consistency of drinking, her team found that current drinkers who were consistent heavy drinkers throughout adulthood had a higher risk for CRC than consistent light drinkers (HR, 1.91).
Separate analyses of incident colorectal adenomas were directionally consistent with the CRC findings. These analyses included 12,327 participants with a negative baseline sigmoidoscopy, among whom 812 adenomas were detected on repeat screening.
Compared with current light drinkers, former drinkers had significantly lower odds of nonadvanced adenomas (odds ratio [OR], 0.58), but no significant association was observed for advanced adenomas (OR, 1.08; 95% CI, 0.62-1.90). The authors cautioned, however, that overall adenoma case numbers were limited, and estimates for advanced lesions were imprecise.
Educating Patients
Reached for comment, William Dahut, chief scientific officer for the American Cancer Society, told Medscape Medical News that this “very well done, large perspective study clearly demonstrates the significant increased risk of colorectal cancer for those that are heavy drinkers.”
He noted that the nearly twofold increased risk for rectal cancer among heavy drinkers “makes biological sense because the rectum is the area of the body where the toxins produced by alcohol potentially spend the most period of time.”
Heavy drinkers are at the highest risk, Dahut said, and “for them, screenings are particularly important.”
Even with this growing body of evidence, Levy noted that many patients in America and worldwide “have not been educated yet about the potential carcinogenic dangers of chronic alcohol use.”
Levy recommended that physicians get “accurate social histories about alcohol use” and “spend several minutes educating patients about their increased risk of cancer and liver problems from heavy alcohol use.”
Dahut encouraged health providers to tell patients that the risk for CRC from alcohol is also based on one’s lifetime alcohol consumption, “not simply what they had last weekend.”
Overall, this important research study, along with the Surgeon General’s recent publication about Alcohol and Cancer Risk, will hopefully “encourage physicians to have important conversations about alcohol reduction with their patients,” Levy said.
The study had no commercial funding. Loftfield, Dahult, and Levy reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
New research sheds light on how chronic heavy alcohol use may contribute to colorectal cancer (CRC) development and how quitting may lower the risk for precancerous colorectal adenomas.
In a large US cancer screening trial, current heavy drinkers — with an average lifetime alcohol intake of 14 or more drinks per week — had a 25% higher risk for CRC and an almost twofold higher risk for rectal cancer than light drinkers averaging less than one drink per week.
When the research team further considered drinking consistency, steady heavy drinking throughout adulthood was associated with a 91% higher risk for CRC than consistent light drinking.
Additionally, no increased risk for CRC was found among former drinkers, and former drinkers were less likely than light drinkers to develop nonadvanced colorectal adenomas.
This analysis “adds to the growing amount of concerning literature showing that chronic heavy alcohol use can potentially contribute to colorectal cancer development,” Benjamin H. Levy III, MD, gastroenterologist and clinical associate of medicine at UChicago Medicine in Chicago, who wasn’t involved in the study, told Medscape Medical News.
The study’s co-senior author, Erikka Loftfield, PhD, MPH, also noted that the study “provides new evidence indicating that drinking cessation, compared to consistent light drinking, may lower adenoma risk.”
Current cancer prevention guidelines recommend limiting alcohol intake or ideally not drinking at all, and “our findings do not change this advice,” said Loftfield, with the National Cancer Institute (NCI) in Bethesda, Maryland.
The study was published online on January 26 in the journal Cancer.
Addressing a Data Gap
Alcoholic beverages are classified as carcinogenic to humans and are causally associated with CRC, Loftfield told Medscape Medical News. However, much of the evidence for this comes from cohort studies that only measure recent drinking patterns, generally among older adults, at study baseline. Fewer studies have looked at how drinking over a person’s lifetime and alcohol consumption patterns relate to colorectal adenoma and CRC risk, she explained.
To address these gaps, Loftfield and colleagues leveraged data on alcohol intake gathered as part of the NCI’s Prostate, Long, Colorectal, and Ovarian Cancer Screening Trial.
Average lifetime alcohol intake was calculated as drinks per week from age 18 through study baseline, and drinking patterns were further classified based on consistency and intensity over time.
During 20 years of follow-up, 1679 incident CRC cases occurred among 88,092 study participants. In multivariable-adjusted analyses, current heavy drinkers had a higher risk for CRC than those averaging less than one drink per week (hazard ratio [HR], 1.25), with the strongest association observed for rectal cancer (HR, 1.95).
“The increase in rectal cancer risk for heavy drinkers seen in this 20-year observational study was especially concerning,” Levy told Medscape Medical News.
What About Moderate Drinking?
Perhaps counterintuitively, moderate current drinkers (those consuming an average of 7 to less than 14 drinks per week) had a lower risk for CRC (HR, 0.79), especially distal colon cancer (HR, 0.64), than light drinkers.
Loftfield said that research in rodents suggests moderate alcohol intake may reduce inflammation and lower DNA damage, but it’s possible that the observed inverse association is due to residual confounding by unmeasured or poorly measured confounders, such as socioeconomic status.
She said it’s also important to note that the inverse association of moderate alcohol intake was strongest for distal colon cancer and in the screening arm of the trial. Those in the screening arm who screened positive with flexible sigmoidoscopy had polyps removed and were referred for colonoscopy during the trial period, making screening a potential intervention as well.
“Screening with flexible sigmoidoscopy has previously been found to decrease CRC incidence, specifically distal colon cancer, in this population. Thus, it is possible that better adherence to screening among moderate drinkers over the course of follow-up contributed to this finding,” Loftfield explained.
When looking at consistency of drinking, her team found that current drinkers who were consistent heavy drinkers throughout adulthood had a higher risk for CRC than consistent light drinkers (HR, 1.91).
Separate analyses of incident colorectal adenomas were directionally consistent with the CRC findings. These analyses included 12,327 participants with a negative baseline sigmoidoscopy, among whom 812 adenomas were detected on repeat screening.
Compared with current light drinkers, former drinkers had significantly lower odds of nonadvanced adenomas (odds ratio [OR], 0.58), but no significant association was observed for advanced adenomas (OR, 1.08; 95% CI, 0.62-1.90). The authors cautioned, however, that overall adenoma case numbers were limited, and estimates for advanced lesions were imprecise.
Educating Patients
Reached for comment, William Dahut, chief scientific officer for the American Cancer Society, told Medscape Medical News that this “very well done, large perspective study clearly demonstrates the significant increased risk of colorectal cancer for those that are heavy drinkers.”
He noted that the nearly twofold increased risk for rectal cancer among heavy drinkers “makes biological sense because the rectum is the area of the body where the toxins produced by alcohol potentially spend the most period of time.”
Heavy drinkers are at the highest risk, Dahut said, and “for them, screenings are particularly important.”
Even with this growing body of evidence, Levy noted that many patients in America and worldwide “have not been educated yet about the potential carcinogenic dangers of chronic alcohol use.”
Levy recommended that physicians get “accurate social histories about alcohol use” and “spend several minutes educating patients about their increased risk of cancer and liver problems from heavy alcohol use.”
Dahut encouraged health providers to tell patients that the risk for CRC from alcohol is also based on one’s lifetime alcohol consumption, “not simply what they had last weekend.”
Overall, this important research study, along with the Surgeon General’s recent publication about Alcohol and Cancer Risk, will hopefully “encourage physicians to have important conversations about alcohol reduction with their patients,” Levy said.
The study had no commercial funding. Loftfield, Dahult, and Levy reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Alcohol and CRC: These Drinking Patterns May Influence Risk
Alcohol and CRC: These Drinking Patterns May Influence Risk
New Insights on Treatment of Veterans With CLL From ASH 2025
New Insights on Treatment of Veterans With CLL From ASH 2025

Insights from phase 3 trials presented at the 2025 American Society of Hematology Annual Meeting may expand treatment options for veterans with chronic lymphocytic leukemia (CLL), as discussed by Dr Nicholas Burwick from University of Washington, Seattle.
Dr Burwick begins with the CLL17 trial examining continuous treatment vs fixed-duration therapy in previously untreated patients. The fixed-duration therapy showed noninferior results. Research pertaining to the veterans population in the phase 2 Benefit VA study may offer further insight on these results.
He next discusses the first study comparing the noncovalent BTKi pirtobrutinib to covalent ibrutinib in both treatment-naive patients and those with relapsed/refractory CLL. Pirtobrutinib demonstrated noninferiority in each subgroup.
Pirtobrutinib was compared to bendamustine plus rituximab in the treatment-naive setting in the next study, showing favorable progression-free survival and a notable trend in overall survival. These two trials could lead to use of a noncovalent BTKi as frontline therapy.
Dr Burwick then turns to 6-year follow-up in the SEQUOIA trial, in which zanubrutinib showed sustained superiority over bendamustine and rituximab. He notes that acalabrutinib is currently the preferred BTKi therapy for veterans with CLL.
Finally, he discusses a study examining combination acalabrutinib and venetoclax, to which obinutuzumab was added either early or late. The rate of infections was significantly higher in the early group, an issue of particular concern in the veterans population.
--
Nicholas R. Burwick, MD, VA Puget Sound Health Care System; Associate Professor, Department of Medicine, Division of Hematology, University of Washington, Seattle; President, AVAHO - Association of VA Hematology/Oncology
Nicholas R. Burwick, MD, has disclosed no relevant financial relationships.

Insights from phase 3 trials presented at the 2025 American Society of Hematology Annual Meeting may expand treatment options for veterans with chronic lymphocytic leukemia (CLL), as discussed by Dr Nicholas Burwick from University of Washington, Seattle.
Dr Burwick begins with the CLL17 trial examining continuous treatment vs fixed-duration therapy in previously untreated patients. The fixed-duration therapy showed noninferior results. Research pertaining to the veterans population in the phase 2 Benefit VA study may offer further insight on these results.
He next discusses the first study comparing the noncovalent BTKi pirtobrutinib to covalent ibrutinib in both treatment-naive patients and those with relapsed/refractory CLL. Pirtobrutinib demonstrated noninferiority in each subgroup.
Pirtobrutinib was compared to bendamustine plus rituximab in the treatment-naive setting in the next study, showing favorable progression-free survival and a notable trend in overall survival. These two trials could lead to use of a noncovalent BTKi as frontline therapy.
Dr Burwick then turns to 6-year follow-up in the SEQUOIA trial, in which zanubrutinib showed sustained superiority over bendamustine and rituximab. He notes that acalabrutinib is currently the preferred BTKi therapy for veterans with CLL.
Finally, he discusses a study examining combination acalabrutinib and venetoclax, to which obinutuzumab was added either early or late. The rate of infections was significantly higher in the early group, an issue of particular concern in the veterans population.
--
Nicholas R. Burwick, MD, VA Puget Sound Health Care System; Associate Professor, Department of Medicine, Division of Hematology, University of Washington, Seattle; President, AVAHO - Association of VA Hematology/Oncology
Nicholas R. Burwick, MD, has disclosed no relevant financial relationships.

Insights from phase 3 trials presented at the 2025 American Society of Hematology Annual Meeting may expand treatment options for veterans with chronic lymphocytic leukemia (CLL), as discussed by Dr Nicholas Burwick from University of Washington, Seattle.
Dr Burwick begins with the CLL17 trial examining continuous treatment vs fixed-duration therapy in previously untreated patients. The fixed-duration therapy showed noninferior results. Research pertaining to the veterans population in the phase 2 Benefit VA study may offer further insight on these results.
He next discusses the first study comparing the noncovalent BTKi pirtobrutinib to covalent ibrutinib in both treatment-naive patients and those with relapsed/refractory CLL. Pirtobrutinib demonstrated noninferiority in each subgroup.
Pirtobrutinib was compared to bendamustine plus rituximab in the treatment-naive setting in the next study, showing favorable progression-free survival and a notable trend in overall survival. These two trials could lead to use of a noncovalent BTKi as frontline therapy.
Dr Burwick then turns to 6-year follow-up in the SEQUOIA trial, in which zanubrutinib showed sustained superiority over bendamustine and rituximab. He notes that acalabrutinib is currently the preferred BTKi therapy for veterans with CLL.
Finally, he discusses a study examining combination acalabrutinib and venetoclax, to which obinutuzumab was added either early or late. The rate of infections was significantly higher in the early group, an issue of particular concern in the veterans population.
--
Nicholas R. Burwick, MD, VA Puget Sound Health Care System; Associate Professor, Department of Medicine, Division of Hematology, University of Washington, Seattle; President, AVAHO - Association of VA Hematology/Oncology
Nicholas R. Burwick, MD, has disclosed no relevant financial relationships.
New Insights on Treatment of Veterans With CLL From ASH 2025
New Insights on Treatment of Veterans With CLL From ASH 2025

When Does Spleen Size Signal Cancer Risk?
When Does Spleen Size Signal Cancer Risk?
TOPLINE:
Spleen volume larger than the 99th percentile was associated with an 11-fold increased risk for hematologic cancer compared with normal volumes, with 5-year risks as high as 46% among men aged 70 years or older. Significant risks for cirrhosis and liver cancer were also seen.
METHODOLOGY:
- Splenomegaly is often detected incidentally during imaging, but guidelines vary as to the threshold that should prompt evaluation — ranging from a spleen length of 120 mm to 150 mm. However, up to 21% of healthy individuals have spleen lengths > 120 mm, which could lead to unnecessary follow-up of low-risk patients.
- Researchers used data from two general population cohorts to evaluate the relative and absolute risks for hematologic cancer and liver disease (two common causes of spleen enlargement) among individuals with incidentally detected splenomegaly. They included 8459 Danish adults (57% female; median age, 61 years) and 38,607 UK adults (51.9% female; median age, 65 years) who underwent CT or MRI scans as part of study procedures.
- Spleen length and volume measurements were available from the Danish cohort, while only spleen volume was available from the UK group.
- Participants were followed for a median of 5 years after imaging to assess the incidence of hematologic cancers (both cohorts) and cirrhosis and liver cancer (UK cohort only). Hazard ratios were adjusted for age, sex, smoking status, alcohol consumption, comorbidities, and C-reactive protein levels.
TAKEAWAY:
- In the Danish cohort, the relative risk for any hematologic cancer was significantly increased among individuals with spleen lengths above the 99th percentile (≥ 135 mm) compared with those with spleen lengths in the 26th-74th percentile (hazard ratio [HR], 5.11; P < .001). Among individuals with a spleen length ≥ 140 mm, absolute 5-year risks reached 23% for men aged 70 years or older and 12% for women in that age group.
- Risks were even more pronounced for Danish adults with a spleen volume above the 99th percentile — > 433 mL. Relative to the 26th-74th percentile, their risk for any hematologic cancer was 11-fold higher (HR, 11.08; P < .001). Among people with a spleen volume ≥ 500 mL, 5-year risks reached 46% for men aged 70 years or older and 27% for women in that age group.
- Findings were similar in the UK cohort. Among individuals with a spleen volume above the 99th percentile (> 386 mL), the risk for hematologic cancer increased nearly 12-fold (HR, 11.82; P < .001). With a spleen volume ≥ 500 mL, 5-year risks reached 21% for men aged 70 years or older and 18% for women in that age group. Relative risks were also elevated — by 1.55-2.94 — among individuals in the 75th-99th percentile (199 mL-386 mL).
- The risks for liver disease began to rise substantially at a spleen volume ≥ 400 mL. Absolute 5-year risks for cirrhosis reached 10.8% for men and 9.3% for women aged 70 years or older with a spleen volume ≥ 500 mL. For liver cancer, 5-year risks reached 3.2% and 1.2% for men and women in that age group with a spleen volume ≥ 400 mL.
IN PRACTICE:
“To our knowledge, no previous studies have examined risk of hematologic cancers by spleen length or volume in incidentally detected splenomegaly,” the authors of the study wrote. “Risk was moderately increased at spleen length of 130-139 mm or spleen volume of 400-499 mL, where diagnostic workup may be considered, and more pronounced at spleen length of 140 mm or greater or spleen volume of 500 mL or greater, supporting that diagnostic workup may likely be relevant.”
They stressed, however, that the study participants were asymptomatic, and the underlying reason for imaging should always be considered.
SOURCE:
The study, led by Jens Helby, MD, PhD, Copenhagen University Hospital – Rigshospitalet, Copenhagen, Denmark, was published online in JAMA Oncology.
DISCLOSURES:
The study was funded by the Danish Cancer Society, the Boserup Foundation, Copenhagen University Hospital – Rigshospitalet, and Sanofi A/S. Helby reported having financial relationships with Sanofi and Disc Medicine. Additional disclosures are available in the full article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Spleen volume larger than the 99th percentile was associated with an 11-fold increased risk for hematologic cancer compared with normal volumes, with 5-year risks as high as 46% among men aged 70 years or older. Significant risks for cirrhosis and liver cancer were also seen.
METHODOLOGY:
- Splenomegaly is often detected incidentally during imaging, but guidelines vary as to the threshold that should prompt evaluation — ranging from a spleen length of 120 mm to 150 mm. However, up to 21% of healthy individuals have spleen lengths > 120 mm, which could lead to unnecessary follow-up of low-risk patients.
- Researchers used data from two general population cohorts to evaluate the relative and absolute risks for hematologic cancer and liver disease (two common causes of spleen enlargement) among individuals with incidentally detected splenomegaly. They included 8459 Danish adults (57% female; median age, 61 years) and 38,607 UK adults (51.9% female; median age, 65 years) who underwent CT or MRI scans as part of study procedures.
- Spleen length and volume measurements were available from the Danish cohort, while only spleen volume was available from the UK group.
- Participants were followed for a median of 5 years after imaging to assess the incidence of hematologic cancers (both cohorts) and cirrhosis and liver cancer (UK cohort only). Hazard ratios were adjusted for age, sex, smoking status, alcohol consumption, comorbidities, and C-reactive protein levels.
TAKEAWAY:
- In the Danish cohort, the relative risk for any hematologic cancer was significantly increased among individuals with spleen lengths above the 99th percentile (≥ 135 mm) compared with those with spleen lengths in the 26th-74th percentile (hazard ratio [HR], 5.11; P < .001). Among individuals with a spleen length ≥ 140 mm, absolute 5-year risks reached 23% for men aged 70 years or older and 12% for women in that age group.
- Risks were even more pronounced for Danish adults with a spleen volume above the 99th percentile — > 433 mL. Relative to the 26th-74th percentile, their risk for any hematologic cancer was 11-fold higher (HR, 11.08; P < .001). Among people with a spleen volume ≥ 500 mL, 5-year risks reached 46% for men aged 70 years or older and 27% for women in that age group.
- Findings were similar in the UK cohort. Among individuals with a spleen volume above the 99th percentile (> 386 mL), the risk for hematologic cancer increased nearly 12-fold (HR, 11.82; P < .001). With a spleen volume ≥ 500 mL, 5-year risks reached 21% for men aged 70 years or older and 18% for women in that age group. Relative risks were also elevated — by 1.55-2.94 — among individuals in the 75th-99th percentile (199 mL-386 mL).
- The risks for liver disease began to rise substantially at a spleen volume ≥ 400 mL. Absolute 5-year risks for cirrhosis reached 10.8% for men and 9.3% for women aged 70 years or older with a spleen volume ≥ 500 mL. For liver cancer, 5-year risks reached 3.2% and 1.2% for men and women in that age group with a spleen volume ≥ 400 mL.
IN PRACTICE:
“To our knowledge, no previous studies have examined risk of hematologic cancers by spleen length or volume in incidentally detected splenomegaly,” the authors of the study wrote. “Risk was moderately increased at spleen length of 130-139 mm or spleen volume of 400-499 mL, where diagnostic workup may be considered, and more pronounced at spleen length of 140 mm or greater or spleen volume of 500 mL or greater, supporting that diagnostic workup may likely be relevant.”
They stressed, however, that the study participants were asymptomatic, and the underlying reason for imaging should always be considered.
SOURCE:
The study, led by Jens Helby, MD, PhD, Copenhagen University Hospital – Rigshospitalet, Copenhagen, Denmark, was published online in JAMA Oncology.
DISCLOSURES:
The study was funded by the Danish Cancer Society, the Boserup Foundation, Copenhagen University Hospital – Rigshospitalet, and Sanofi A/S. Helby reported having financial relationships with Sanofi and Disc Medicine. Additional disclosures are available in the full article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Spleen volume larger than the 99th percentile was associated with an 11-fold increased risk for hematologic cancer compared with normal volumes, with 5-year risks as high as 46% among men aged 70 years or older. Significant risks for cirrhosis and liver cancer were also seen.
METHODOLOGY:
- Splenomegaly is often detected incidentally during imaging, but guidelines vary as to the threshold that should prompt evaluation — ranging from a spleen length of 120 mm to 150 mm. However, up to 21% of healthy individuals have spleen lengths > 120 mm, which could lead to unnecessary follow-up of low-risk patients.
- Researchers used data from two general population cohorts to evaluate the relative and absolute risks for hematologic cancer and liver disease (two common causes of spleen enlargement) among individuals with incidentally detected splenomegaly. They included 8459 Danish adults (57% female; median age, 61 years) and 38,607 UK adults (51.9% female; median age, 65 years) who underwent CT or MRI scans as part of study procedures.
- Spleen length and volume measurements were available from the Danish cohort, while only spleen volume was available from the UK group.
- Participants were followed for a median of 5 years after imaging to assess the incidence of hematologic cancers (both cohorts) and cirrhosis and liver cancer (UK cohort only). Hazard ratios were adjusted for age, sex, smoking status, alcohol consumption, comorbidities, and C-reactive protein levels.
TAKEAWAY:
- In the Danish cohort, the relative risk for any hematologic cancer was significantly increased among individuals with spleen lengths above the 99th percentile (≥ 135 mm) compared with those with spleen lengths in the 26th-74th percentile (hazard ratio [HR], 5.11; P < .001). Among individuals with a spleen length ≥ 140 mm, absolute 5-year risks reached 23% for men aged 70 years or older and 12% for women in that age group.
- Risks were even more pronounced for Danish adults with a spleen volume above the 99th percentile — > 433 mL. Relative to the 26th-74th percentile, their risk for any hematologic cancer was 11-fold higher (HR, 11.08; P < .001). Among people with a spleen volume ≥ 500 mL, 5-year risks reached 46% for men aged 70 years or older and 27% for women in that age group.
- Findings were similar in the UK cohort. Among individuals with a spleen volume above the 99th percentile (> 386 mL), the risk for hematologic cancer increased nearly 12-fold (HR, 11.82; P < .001). With a spleen volume ≥ 500 mL, 5-year risks reached 21% for men aged 70 years or older and 18% for women in that age group. Relative risks were also elevated — by 1.55-2.94 — among individuals in the 75th-99th percentile (199 mL-386 mL).
- The risks for liver disease began to rise substantially at a spleen volume ≥ 400 mL. Absolute 5-year risks for cirrhosis reached 10.8% for men and 9.3% for women aged 70 years or older with a spleen volume ≥ 500 mL. For liver cancer, 5-year risks reached 3.2% and 1.2% for men and women in that age group with a spleen volume ≥ 400 mL.
IN PRACTICE:
“To our knowledge, no previous studies have examined risk of hematologic cancers by spleen length or volume in incidentally detected splenomegaly,” the authors of the study wrote. “Risk was moderately increased at spleen length of 130-139 mm or spleen volume of 400-499 mL, where diagnostic workup may be considered, and more pronounced at spleen length of 140 mm or greater or spleen volume of 500 mL or greater, supporting that diagnostic workup may likely be relevant.”
They stressed, however, that the study participants were asymptomatic, and the underlying reason for imaging should always be considered.
SOURCE:
The study, led by Jens Helby, MD, PhD, Copenhagen University Hospital – Rigshospitalet, Copenhagen, Denmark, was published online in JAMA Oncology.
DISCLOSURES:
The study was funded by the Danish Cancer Society, the Boserup Foundation, Copenhagen University Hospital – Rigshospitalet, and Sanofi A/S. Helby reported having financial relationships with Sanofi and Disc Medicine. Additional disclosures are available in the full article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
When Does Spleen Size Signal Cancer Risk?
When Does Spleen Size Signal Cancer Risk?
A New First-Line Option in BRAF-Mutant Metastatic CRC?
A New First-Line Option in BRAF-Mutant Metastatic CRC?
The targeted therapy combination of encorafenib and cetuximab with FOLFIRI (leucovorin/5-fluorouracil [FU]/irinotecan) chemotherapy may be a new first-line option for patients with BRAF V600E-mutant metastatic colorectal cancer (CRC), according to new results from the BREAKWATER trial.
After a median follow-up of about 10 months, response rates were significantly better with encorafenib and cetuximab plus FOLFIRI than with FOLFIRI alone — without increasing side effects.
The findings, presented at the ASCO Gastrointestinal Cancers Symposium 2026, point to a potential new option for the 20%-25% of patients with BRAF V600E-mutant metastatic CRC who receive FOLFIRI as their chemotherapy.
Based on previous results from BREAKWATER, the FDA granted accelerated approval to first-line encorafenib (Braftovi) and cetuximab (Erbitux) plus mFOLFOX6 for this patient population. That regimen doubled median overall survival compared with standard chemotherapy with or without bevacizumab.
Cohort 3 of BREAKWATER was designed to address a specific question: Are the benefits with the targeted therapy duo a “FOLFOX-specific phenomenon?” lead investigator Scott Kopetz, MD, PhD, of MD Anderson Cancer Center, Houston, said during a press briefing.
Based on these early results, the answer is no. Instead, Kopetz said, there appears to be a “broader synergy” between the targeted therapies and cytotoxic chemotherapy.
Joel Saltzman, MD, ASCO expert in gastrointestinal cancers based at Taussig Cancer Center, Cleveland Clinic in Cleveland, agreed.
“The additional data from the BREAKWATER trial reveals that it is the targeted therapy backbone that provides the better disease control and response rate in BRAF V600E-mutant colorectal cancers,” he said.
BRAF V600E mutations occur in up to 12% of patients with metastatic CRC and are associated with poor outcomes. While many newly diagnosed patients receive FOLFOX (leucovorin/5-FU/oxaliplatin) in the first line, FOLFIRI is a common alternative — often due to concerns about oxaliplatin-associated peripheral neuropathy, Kopetz noted.
The safety lead-in portion of BREAKWATER showed that encorafenib and cetuximab plus FOLFIRI were tolerable and had promising antitumor activity.
Cohort 3 of the trial included 147 patients (mean age, 62 years; 46% male) with BRAF V600E-mutant metastatic CRC, no prior systemic treatment, and good performance status (Eastern Cooperative Oncology Group PS 0 or 1); 73 patients were randomly allocated to encorafenib and cetuximab plus FOLFIRI and 74 to FOLFIRI with or without bevacizumab. The primary endpoint was objective response rate assessed by blinded independent central review.
After a median follow-up of 10 months, patients in the targeted therapy group had an objective response rate of 64.4% vs 39.2% among patients who received FOLFIRI alone or with bevacizumab (odds ratio, 2.76; P = .001).
Responses to the targeted therapies were “rapid and durable,” Kopetz said. More than half (57.4%) of patients treated with encorafenib and cetuximab and FOLFIRI had a duration of response of 6 months or longer than 34.5% in the control group.
Data on overall survival, a secondary endpoint, were not yet mature, but there was a trend toward improved survival with targeted therapy.
Importantly, Kopetz reported, there were no new safety signals, and serious treatment-emergent adverse events occurred at a similar rate in both treatment groups: 39.4% in the targeted therapy group and 36.8% in the control group.
The most common adverse events in both groups included nausea, diarrhea, vomiting, fatigue, appetite loss, and alopecia. About 10% of patients in the targeted therapy group and 9% of those in the control group discontinued their treatment early, suggesting the severity of side effects was similar between the groups.
Kopetz cautioned that the data are still early and follow-up is ongoing. However, he said, the findings support the targeted drugs plus FOLFIRI as a “potential new standard of care” for this patient population.
“The addition of FOLFIRI chemotherapy in the frontline setting will give oncologists and patients more options when selecting a first-line regimen,” Saltzman said. “To have as many options as possible is certainly something we all hope for.”
The trial was funded by Pfizer. Kopetz reported consulting for Pfizer and several other pharmaceutical companies. Saltzman reported having no disclosures.
A version of this article first appeared on Medscape.com.
The targeted therapy combination of encorafenib and cetuximab with FOLFIRI (leucovorin/5-fluorouracil [FU]/irinotecan) chemotherapy may be a new first-line option for patients with BRAF V600E-mutant metastatic colorectal cancer (CRC), according to new results from the BREAKWATER trial.
After a median follow-up of about 10 months, response rates were significantly better with encorafenib and cetuximab plus FOLFIRI than with FOLFIRI alone — without increasing side effects.
The findings, presented at the ASCO Gastrointestinal Cancers Symposium 2026, point to a potential new option for the 20%-25% of patients with BRAF V600E-mutant metastatic CRC who receive FOLFIRI as their chemotherapy.
Based on previous results from BREAKWATER, the FDA granted accelerated approval to first-line encorafenib (Braftovi) and cetuximab (Erbitux) plus mFOLFOX6 for this patient population. That regimen doubled median overall survival compared with standard chemotherapy with or without bevacizumab.
Cohort 3 of BREAKWATER was designed to address a specific question: Are the benefits with the targeted therapy duo a “FOLFOX-specific phenomenon?” lead investigator Scott Kopetz, MD, PhD, of MD Anderson Cancer Center, Houston, said during a press briefing.
Based on these early results, the answer is no. Instead, Kopetz said, there appears to be a “broader synergy” between the targeted therapies and cytotoxic chemotherapy.
Joel Saltzman, MD, ASCO expert in gastrointestinal cancers based at Taussig Cancer Center, Cleveland Clinic in Cleveland, agreed.
“The additional data from the BREAKWATER trial reveals that it is the targeted therapy backbone that provides the better disease control and response rate in BRAF V600E-mutant colorectal cancers,” he said.
BRAF V600E mutations occur in up to 12% of patients with metastatic CRC and are associated with poor outcomes. While many newly diagnosed patients receive FOLFOX (leucovorin/5-FU/oxaliplatin) in the first line, FOLFIRI is a common alternative — often due to concerns about oxaliplatin-associated peripheral neuropathy, Kopetz noted.
The safety lead-in portion of BREAKWATER showed that encorafenib and cetuximab plus FOLFIRI were tolerable and had promising antitumor activity.
Cohort 3 of the trial included 147 patients (mean age, 62 years; 46% male) with BRAF V600E-mutant metastatic CRC, no prior systemic treatment, and good performance status (Eastern Cooperative Oncology Group PS 0 or 1); 73 patients were randomly allocated to encorafenib and cetuximab plus FOLFIRI and 74 to FOLFIRI with or without bevacizumab. The primary endpoint was objective response rate assessed by blinded independent central review.
After a median follow-up of 10 months, patients in the targeted therapy group had an objective response rate of 64.4% vs 39.2% among patients who received FOLFIRI alone or with bevacizumab (odds ratio, 2.76; P = .001).
Responses to the targeted therapies were “rapid and durable,” Kopetz said. More than half (57.4%) of patients treated with encorafenib and cetuximab and FOLFIRI had a duration of response of 6 months or longer than 34.5% in the control group.
Data on overall survival, a secondary endpoint, were not yet mature, but there was a trend toward improved survival with targeted therapy.
Importantly, Kopetz reported, there were no new safety signals, and serious treatment-emergent adverse events occurred at a similar rate in both treatment groups: 39.4% in the targeted therapy group and 36.8% in the control group.
The most common adverse events in both groups included nausea, diarrhea, vomiting, fatigue, appetite loss, and alopecia. About 10% of patients in the targeted therapy group and 9% of those in the control group discontinued their treatment early, suggesting the severity of side effects was similar between the groups.
Kopetz cautioned that the data are still early and follow-up is ongoing. However, he said, the findings support the targeted drugs plus FOLFIRI as a “potential new standard of care” for this patient population.
“The addition of FOLFIRI chemotherapy in the frontline setting will give oncologists and patients more options when selecting a first-line regimen,” Saltzman said. “To have as many options as possible is certainly something we all hope for.”
The trial was funded by Pfizer. Kopetz reported consulting for Pfizer and several other pharmaceutical companies. Saltzman reported having no disclosures.
A version of this article first appeared on Medscape.com.
The targeted therapy combination of encorafenib and cetuximab with FOLFIRI (leucovorin/5-fluorouracil [FU]/irinotecan) chemotherapy may be a new first-line option for patients with BRAF V600E-mutant metastatic colorectal cancer (CRC), according to new results from the BREAKWATER trial.
After a median follow-up of about 10 months, response rates were significantly better with encorafenib and cetuximab plus FOLFIRI than with FOLFIRI alone — without increasing side effects.
The findings, presented at the ASCO Gastrointestinal Cancers Symposium 2026, point to a potential new option for the 20%-25% of patients with BRAF V600E-mutant metastatic CRC who receive FOLFIRI as their chemotherapy.
Based on previous results from BREAKWATER, the FDA granted accelerated approval to first-line encorafenib (Braftovi) and cetuximab (Erbitux) plus mFOLFOX6 for this patient population. That regimen doubled median overall survival compared with standard chemotherapy with or without bevacizumab.
Cohort 3 of BREAKWATER was designed to address a specific question: Are the benefits with the targeted therapy duo a “FOLFOX-specific phenomenon?” lead investigator Scott Kopetz, MD, PhD, of MD Anderson Cancer Center, Houston, said during a press briefing.
Based on these early results, the answer is no. Instead, Kopetz said, there appears to be a “broader synergy” between the targeted therapies and cytotoxic chemotherapy.
Joel Saltzman, MD, ASCO expert in gastrointestinal cancers based at Taussig Cancer Center, Cleveland Clinic in Cleveland, agreed.
“The additional data from the BREAKWATER trial reveals that it is the targeted therapy backbone that provides the better disease control and response rate in BRAF V600E-mutant colorectal cancers,” he said.
BRAF V600E mutations occur in up to 12% of patients with metastatic CRC and are associated with poor outcomes. While many newly diagnosed patients receive FOLFOX (leucovorin/5-FU/oxaliplatin) in the first line, FOLFIRI is a common alternative — often due to concerns about oxaliplatin-associated peripheral neuropathy, Kopetz noted.
The safety lead-in portion of BREAKWATER showed that encorafenib and cetuximab plus FOLFIRI were tolerable and had promising antitumor activity.
Cohort 3 of the trial included 147 patients (mean age, 62 years; 46% male) with BRAF V600E-mutant metastatic CRC, no prior systemic treatment, and good performance status (Eastern Cooperative Oncology Group PS 0 or 1); 73 patients were randomly allocated to encorafenib and cetuximab plus FOLFIRI and 74 to FOLFIRI with or without bevacizumab. The primary endpoint was objective response rate assessed by blinded independent central review.
After a median follow-up of 10 months, patients in the targeted therapy group had an objective response rate of 64.4% vs 39.2% among patients who received FOLFIRI alone or with bevacizumab (odds ratio, 2.76; P = .001).
Responses to the targeted therapies were “rapid and durable,” Kopetz said. More than half (57.4%) of patients treated with encorafenib and cetuximab and FOLFIRI had a duration of response of 6 months or longer than 34.5% in the control group.
Data on overall survival, a secondary endpoint, were not yet mature, but there was a trend toward improved survival with targeted therapy.
Importantly, Kopetz reported, there were no new safety signals, and serious treatment-emergent adverse events occurred at a similar rate in both treatment groups: 39.4% in the targeted therapy group and 36.8% in the control group.
The most common adverse events in both groups included nausea, diarrhea, vomiting, fatigue, appetite loss, and alopecia. About 10% of patients in the targeted therapy group and 9% of those in the control group discontinued their treatment early, suggesting the severity of side effects was similar between the groups.
Kopetz cautioned that the data are still early and follow-up is ongoing. However, he said, the findings support the targeted drugs plus FOLFIRI as a “potential new standard of care” for this patient population.
“The addition of FOLFIRI chemotherapy in the frontline setting will give oncologists and patients more options when selecting a first-line regimen,” Saltzman said. “To have as many options as possible is certainly something we all hope for.”
The trial was funded by Pfizer. Kopetz reported consulting for Pfizer and several other pharmaceutical companies. Saltzman reported having no disclosures.
A version of this article first appeared on Medscape.com.
A New First-Line Option in BRAF-Mutant Metastatic CRC?
A New First-Line Option in BRAF-Mutant Metastatic CRC?
Cannabis May Ease Symptoms in Advanced Pancreatic Cancer
Cannabis May Ease Symptoms in Advanced Pancreatic Cancer
TOPLINE:
A randomized trial of 32 patients with advanced pancreatic cancer found that early access to medical cannabis patients' symptom burden, with minimal side effects.
METHODOLOGY:
- Patients with pancreatic cancer commonly experience moderate-to-severe pain, nausea, insomnia, and other symptoms that significantly affect their quality of life. Current management approaches are insufficient. Preliminary evidence suggests that medical cannabis has efficacy against multiple cancer-related symptoms, but high-quality data remain limited due to regulatory barriers.
- Researchers conducted a pilot randomized, waitlist-controlled trial involving 32 patients (median age, 71 years) with newly diagnosed locally advanced or metastatic pancreatic adenocarcinoma and at least one burdensome symptom.
- Patients were randomly assigned in a 1:1 ratio to early (0-8 weeks) or delayed (9-16 weeks) cannabis intervention through the Minnesota Medical Cannabis Program, which provided cannabis products and education in how to use them.
- Primary outcomes focused on feasibility, while secondary outcomes examined acceptability, changes in symptom burden, and quality of life in exploratory efficacy analyses.
TAKEAWAY:
- At baseline, patients reported a substantial moderate-to-severe symptom burden — most commonly insomnia (85%), pain (77%), and appetite loss (69%); 10 patients (31%) were using opioids.
- The study met all of its feasibility metrics, with 74% of the patients meeting enrollment eligibility and 81% complying with their random assignment. Patients in the arm with early cannabis access typically picked up their products 3 days after starting chemotherapy. Most used tablets or other oral cannabis formulations.
- At 8 weeks, patients in the early-access arm experienced numerically higher rates of improvement in pain (44% vs 20%; P = .35), appetite (56% vs 30%; P = .37), and insomnia (67% vs 30%; P = .18), as well as a reduction in opioid use. Their rates of potential cannabis side effects, including dry mouth, dizziness, and concentration problems, were lower compared with the waitlist group — possibly, the authors noted, due to their education to “start low, go slow.”
- Patients made a median of two trips to a cannabis dispensary during the study period, and most said that using cannabis was “easy” and “practical.”
IN PRACTICE:
“Early access to medical cannabis was associated with improvement in certain symptoms, such as insomnia, with minimal harms,” the authors wrote, adding that the research design offers a model collaboration between investigators and state cannabis programs.
“The encouraging preliminary efficacy and safety of cannabis in managing symptoms supports further exploration," they concluded.
SOURCE:
The study was led by Dylan Zylla, MD, MS, of HealthPartners Institute, Cancer Research Center, Minneapolis, Minnesota. It was presented on January 9 at the ASCO Gastrointestinal Cancers Symposium 2026 and simultaneously published in JCO Oncology Practice.
LIMITATIONS:
The trial was small and the 8-week primary study period precluded conclusions about longer-term benefits and safety. Generalizability may be limited as the trial was conducted in a single state with a predominantly urban and White patient population. Additionally, heterogeneity in state cannabis programs and laws may limit national applicability.
DISCLOSURES:
The study was supported by philanthropic support to the HealthPartners Cancer Research Center. Cannabis products were provided by Vireo Health (GreenGoods, Minnesota). Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
A randomized trial of 32 patients with advanced pancreatic cancer found that early access to medical cannabis patients' symptom burden, with minimal side effects.
METHODOLOGY:
- Patients with pancreatic cancer commonly experience moderate-to-severe pain, nausea, insomnia, and other symptoms that significantly affect their quality of life. Current management approaches are insufficient. Preliminary evidence suggests that medical cannabis has efficacy against multiple cancer-related symptoms, but high-quality data remain limited due to regulatory barriers.
- Researchers conducted a pilot randomized, waitlist-controlled trial involving 32 patients (median age, 71 years) with newly diagnosed locally advanced or metastatic pancreatic adenocarcinoma and at least one burdensome symptom.
- Patients were randomly assigned in a 1:1 ratio to early (0-8 weeks) or delayed (9-16 weeks) cannabis intervention through the Minnesota Medical Cannabis Program, which provided cannabis products and education in how to use them.
- Primary outcomes focused on feasibility, while secondary outcomes examined acceptability, changes in symptom burden, and quality of life in exploratory efficacy analyses.
TAKEAWAY:
- At baseline, patients reported a substantial moderate-to-severe symptom burden — most commonly insomnia (85%), pain (77%), and appetite loss (69%); 10 patients (31%) were using opioids.
- The study met all of its feasibility metrics, with 74% of the patients meeting enrollment eligibility and 81% complying with their random assignment. Patients in the arm with early cannabis access typically picked up their products 3 days after starting chemotherapy. Most used tablets or other oral cannabis formulations.
- At 8 weeks, patients in the early-access arm experienced numerically higher rates of improvement in pain (44% vs 20%; P = .35), appetite (56% vs 30%; P = .37), and insomnia (67% vs 30%; P = .18), as well as a reduction in opioid use. Their rates of potential cannabis side effects, including dry mouth, dizziness, and concentration problems, were lower compared with the waitlist group — possibly, the authors noted, due to their education to “start low, go slow.”
- Patients made a median of two trips to a cannabis dispensary during the study period, and most said that using cannabis was “easy” and “practical.”
IN PRACTICE:
“Early access to medical cannabis was associated with improvement in certain symptoms, such as insomnia, with minimal harms,” the authors wrote, adding that the research design offers a model collaboration between investigators and state cannabis programs.
“The encouraging preliminary efficacy and safety of cannabis in managing symptoms supports further exploration," they concluded.
SOURCE:
The study was led by Dylan Zylla, MD, MS, of HealthPartners Institute, Cancer Research Center, Minneapolis, Minnesota. It was presented on January 9 at the ASCO Gastrointestinal Cancers Symposium 2026 and simultaneously published in JCO Oncology Practice.
LIMITATIONS:
The trial was small and the 8-week primary study period precluded conclusions about longer-term benefits and safety. Generalizability may be limited as the trial was conducted in a single state with a predominantly urban and White patient population. Additionally, heterogeneity in state cannabis programs and laws may limit national applicability.
DISCLOSURES:
The study was supported by philanthropic support to the HealthPartners Cancer Research Center. Cannabis products were provided by Vireo Health (GreenGoods, Minnesota). Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
A randomized trial of 32 patients with advanced pancreatic cancer found that early access to medical cannabis patients' symptom burden, with minimal side effects.
METHODOLOGY:
- Patients with pancreatic cancer commonly experience moderate-to-severe pain, nausea, insomnia, and other symptoms that significantly affect their quality of life. Current management approaches are insufficient. Preliminary evidence suggests that medical cannabis has efficacy against multiple cancer-related symptoms, but high-quality data remain limited due to regulatory barriers.
- Researchers conducted a pilot randomized, waitlist-controlled trial involving 32 patients (median age, 71 years) with newly diagnosed locally advanced or metastatic pancreatic adenocarcinoma and at least one burdensome symptom.
- Patients were randomly assigned in a 1:1 ratio to early (0-8 weeks) or delayed (9-16 weeks) cannabis intervention through the Minnesota Medical Cannabis Program, which provided cannabis products and education in how to use them.
- Primary outcomes focused on feasibility, while secondary outcomes examined acceptability, changes in symptom burden, and quality of life in exploratory efficacy analyses.
TAKEAWAY:
- At baseline, patients reported a substantial moderate-to-severe symptom burden — most commonly insomnia (85%), pain (77%), and appetite loss (69%); 10 patients (31%) were using opioids.
- The study met all of its feasibility metrics, with 74% of the patients meeting enrollment eligibility and 81% complying with their random assignment. Patients in the arm with early cannabis access typically picked up their products 3 days after starting chemotherapy. Most used tablets or other oral cannabis formulations.
- At 8 weeks, patients in the early-access arm experienced numerically higher rates of improvement in pain (44% vs 20%; P = .35), appetite (56% vs 30%; P = .37), and insomnia (67% vs 30%; P = .18), as well as a reduction in opioid use. Their rates of potential cannabis side effects, including dry mouth, dizziness, and concentration problems, were lower compared with the waitlist group — possibly, the authors noted, due to their education to “start low, go slow.”
- Patients made a median of two trips to a cannabis dispensary during the study period, and most said that using cannabis was “easy” and “practical.”
IN PRACTICE:
“Early access to medical cannabis was associated with improvement in certain symptoms, such as insomnia, with minimal harms,” the authors wrote, adding that the research design offers a model collaboration between investigators and state cannabis programs.
“The encouraging preliminary efficacy and safety of cannabis in managing symptoms supports further exploration," they concluded.
SOURCE:
The study was led by Dylan Zylla, MD, MS, of HealthPartners Institute, Cancer Research Center, Minneapolis, Minnesota. It was presented on January 9 at the ASCO Gastrointestinal Cancers Symposium 2026 and simultaneously published in JCO Oncology Practice.
LIMITATIONS:
The trial was small and the 8-week primary study period precluded conclusions about longer-term benefits and safety. Generalizability may be limited as the trial was conducted in a single state with a predominantly urban and White patient population. Additionally, heterogeneity in state cannabis programs and laws may limit national applicability.
DISCLOSURES:
The study was supported by philanthropic support to the HealthPartners Cancer Research Center. Cannabis products were provided by Vireo Health (GreenGoods, Minnesota). Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Cannabis May Ease Symptoms in Advanced Pancreatic Cancer
Cannabis May Ease Symptoms in Advanced Pancreatic Cancer
Home Screening Cost-Effective for Anal Cancer
Home Screening Cost-Effective for Anal Cancer
TOPLINE:
A recent analysis suggested that home-based screening for anal cancer is a cost-effective way to increase screening compared to clinic-based screening. The study found that a home-based approach led to higher participation rates (89.2% vs 74.2% for a clinic-based approach) among sexual and gender minority individuals and was cost-effective, costing $25.19 per additional individual screened when accounting for both direct and indirect costs and $132.36 per additional individual screened when only accounting for direct medical costs.
METHODOLOGY:
- Anal cancer screening is recommended for high-risk populations, such as sexual and gender minority individuals. However, it's unclear how cost-effective home-based self-sampling is compared to clinic-based screening.
- Researchers conducted an economic evaluation using data from a randomized clinical trial that included 240 sexual and gender minority individuals in Milwaukee from January 2020 to August 2022.
- Participants, aged ≥ 25 years, were randomized to either home-based self-sampling or clinic-based screening.
- Researchers evaluated direct home-based screening costs from the trial, and sourced clinic-based costs from the Medicare reimbursement schedule. Travel and time costs were determined from participant self-reports.
- The primary outcome was the incremental cost-effectiveness ratio (ICER), which was the additional cost needed to increase screening participation by one person. The researchers calculated ICERs from both a healthcare payer and societal perspective. The healthcare perspective included only direct medical costs and the societal perspective accounted for direct medical costs as well as indirect time and travel costs.
TAKEAWAY:
- Home-based screening led to higher participation rates than clinic-based screening—89.2% vs 74.2%—with 107 participants completing home-based screening compared with 89 participants doing clinic-based screening.
- The cost per participant was $64.18 for home-based screening and $60.40 for clinic-based screening from the societal perspective, and $61.91 for home-based screening and $42.06 for clinic-based screening from the healthcare payer perspective.
- With home-based screening, the ICER per additional screened participant was $25.19 from a societal perspective and $132.36 from a healthcare payer perspective.
- From the societal perspective, the probability that home-based screening was cost-effective compared with clinic-based screening was nearly 50% at a willingness-to-pay threshold of $25 and 99.99% at a threshold of $100. From the healthcare perspective, the probability was 3.8% at a threshold of $100 and 90.9% at a threshold of $200.
IN PRACTICE:
"These findings suggest that home-based screening promises to be a cost-effective option to enhance anal cancer screening participation," the study authors concluded.
SOURCE:
The study, led by Haluk Damgacioglu, PhD, Department of Public Health Sciences, Medical University of South Carolina in Charleston, South Carolina, was published online in JAMA Network Open.
LIMITATIONS:
The study was conducted in an urban setting where proximity to clinics may reduce structural barriers, potentially limiting generalizability to rural areas where longer travel distances and limited clinician availability could affect participation rates. The analysis did not include downstream steps such as follow-up clinic visits, confirmatory testing, treatment of precancerous lesions, or cancer prevention outcomes. While home-based screening participants were required to visit clinics for digital anal rectal examination to exclude prevalent anal cancer, these follow-up visit costs were not included in the cost-effectiveness analysis.
DISCLOSURES:
Elizabeth Chiao, PhD, reported receiving grants from the National Institutes of Health during the study. Jennifer S. Smith, PhD, MPH, disclosed receiving personal fees from Hologic, Inc., and materials for research purposes from Rovers Medical Devices. Ashish A. Deshmukh, PhD, MPH, reported receiving personal fees from Value Analytics Lab. Alan G. Nyitray, PhD, reported receiving grants from the National Cancer Institute and test kits from Copan Diagnostics.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
A recent analysis suggested that home-based screening for anal cancer is a cost-effective way to increase screening compared to clinic-based screening. The study found that a home-based approach led to higher participation rates (89.2% vs 74.2% for a clinic-based approach) among sexual and gender minority individuals and was cost-effective, costing $25.19 per additional individual screened when accounting for both direct and indirect costs and $132.36 per additional individual screened when only accounting for direct medical costs.
METHODOLOGY:
- Anal cancer screening is recommended for high-risk populations, such as sexual and gender minority individuals. However, it's unclear how cost-effective home-based self-sampling is compared to clinic-based screening.
- Researchers conducted an economic evaluation using data from a randomized clinical trial that included 240 sexual and gender minority individuals in Milwaukee from January 2020 to August 2022.
- Participants, aged ≥ 25 years, were randomized to either home-based self-sampling or clinic-based screening.
- Researchers evaluated direct home-based screening costs from the trial, and sourced clinic-based costs from the Medicare reimbursement schedule. Travel and time costs were determined from participant self-reports.
- The primary outcome was the incremental cost-effectiveness ratio (ICER), which was the additional cost needed to increase screening participation by one person. The researchers calculated ICERs from both a healthcare payer and societal perspective. The healthcare perspective included only direct medical costs and the societal perspective accounted for direct medical costs as well as indirect time and travel costs.
TAKEAWAY:
- Home-based screening led to higher participation rates than clinic-based screening—89.2% vs 74.2%—with 107 participants completing home-based screening compared with 89 participants doing clinic-based screening.
- The cost per participant was $64.18 for home-based screening and $60.40 for clinic-based screening from the societal perspective, and $61.91 for home-based screening and $42.06 for clinic-based screening from the healthcare payer perspective.
- With home-based screening, the ICER per additional screened participant was $25.19 from a societal perspective and $132.36 from a healthcare payer perspective.
- From the societal perspective, the probability that home-based screening was cost-effective compared with clinic-based screening was nearly 50% at a willingness-to-pay threshold of $25 and 99.99% at a threshold of $100. From the healthcare perspective, the probability was 3.8% at a threshold of $100 and 90.9% at a threshold of $200.
IN PRACTICE:
"These findings suggest that home-based screening promises to be a cost-effective option to enhance anal cancer screening participation," the study authors concluded.
SOURCE:
The study, led by Haluk Damgacioglu, PhD, Department of Public Health Sciences, Medical University of South Carolina in Charleston, South Carolina, was published online in JAMA Network Open.
LIMITATIONS:
The study was conducted in an urban setting where proximity to clinics may reduce structural barriers, potentially limiting generalizability to rural areas where longer travel distances and limited clinician availability could affect participation rates. The analysis did not include downstream steps such as follow-up clinic visits, confirmatory testing, treatment of precancerous lesions, or cancer prevention outcomes. While home-based screening participants were required to visit clinics for digital anal rectal examination to exclude prevalent anal cancer, these follow-up visit costs were not included in the cost-effectiveness analysis.
DISCLOSURES:
Elizabeth Chiao, PhD, reported receiving grants from the National Institutes of Health during the study. Jennifer S. Smith, PhD, MPH, disclosed receiving personal fees from Hologic, Inc., and materials for research purposes from Rovers Medical Devices. Ashish A. Deshmukh, PhD, MPH, reported receiving personal fees from Value Analytics Lab. Alan G. Nyitray, PhD, reported receiving grants from the National Cancer Institute and test kits from Copan Diagnostics.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
A recent analysis suggested that home-based screening for anal cancer is a cost-effective way to increase screening compared to clinic-based screening. The study found that a home-based approach led to higher participation rates (89.2% vs 74.2% for a clinic-based approach) among sexual and gender minority individuals and was cost-effective, costing $25.19 per additional individual screened when accounting for both direct and indirect costs and $132.36 per additional individual screened when only accounting for direct medical costs.
METHODOLOGY:
- Anal cancer screening is recommended for high-risk populations, such as sexual and gender minority individuals. However, it's unclear how cost-effective home-based self-sampling is compared to clinic-based screening.
- Researchers conducted an economic evaluation using data from a randomized clinical trial that included 240 sexual and gender minority individuals in Milwaukee from January 2020 to August 2022.
- Participants, aged ≥ 25 years, were randomized to either home-based self-sampling or clinic-based screening.
- Researchers evaluated direct home-based screening costs from the trial, and sourced clinic-based costs from the Medicare reimbursement schedule. Travel and time costs were determined from participant self-reports.
- The primary outcome was the incremental cost-effectiveness ratio (ICER), which was the additional cost needed to increase screening participation by one person. The researchers calculated ICERs from both a healthcare payer and societal perspective. The healthcare perspective included only direct medical costs and the societal perspective accounted for direct medical costs as well as indirect time and travel costs.
TAKEAWAY:
- Home-based screening led to higher participation rates than clinic-based screening—89.2% vs 74.2%—with 107 participants completing home-based screening compared with 89 participants doing clinic-based screening.
- The cost per participant was $64.18 for home-based screening and $60.40 for clinic-based screening from the societal perspective, and $61.91 for home-based screening and $42.06 for clinic-based screening from the healthcare payer perspective.
- With home-based screening, the ICER per additional screened participant was $25.19 from a societal perspective and $132.36 from a healthcare payer perspective.
- From the societal perspective, the probability that home-based screening was cost-effective compared with clinic-based screening was nearly 50% at a willingness-to-pay threshold of $25 and 99.99% at a threshold of $100. From the healthcare perspective, the probability was 3.8% at a threshold of $100 and 90.9% at a threshold of $200.
IN PRACTICE:
"These findings suggest that home-based screening promises to be a cost-effective option to enhance anal cancer screening participation," the study authors concluded.
SOURCE:
The study, led by Haluk Damgacioglu, PhD, Department of Public Health Sciences, Medical University of South Carolina in Charleston, South Carolina, was published online in JAMA Network Open.
LIMITATIONS:
The study was conducted in an urban setting where proximity to clinics may reduce structural barriers, potentially limiting generalizability to rural areas where longer travel distances and limited clinician availability could affect participation rates. The analysis did not include downstream steps such as follow-up clinic visits, confirmatory testing, treatment of precancerous lesions, or cancer prevention outcomes. While home-based screening participants were required to visit clinics for digital anal rectal examination to exclude prevalent anal cancer, these follow-up visit costs were not included in the cost-effectiveness analysis.
DISCLOSURES:
Elizabeth Chiao, PhD, reported receiving grants from the National Institutes of Health during the study. Jennifer S. Smith, PhD, MPH, disclosed receiving personal fees from Hologic, Inc., and materials for research purposes from Rovers Medical Devices. Ashish A. Deshmukh, PhD, MPH, reported receiving personal fees from Value Analytics Lab. Alan G. Nyitray, PhD, reported receiving grants from the National Cancer Institute and test kits from Copan Diagnostics.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Home Screening Cost-Effective for Anal Cancer
Home Screening Cost-Effective for Anal Cancer
Rural Cancer Survivors Are More Likely to Have Chronic Pain
TOPLINE:
Rural cancer survivors experience significantly higher rates of chronic pain at 43.0% than those among urban survivors at 33.5%. Even after controlling for demographics and health conditions, rural residents showed 21% higher odds of experiencing chronic pain.
METHODOLOGY:
- Chronic pain prevalence among cancer survivors is twice that of the general US population and is associated with numerous negative outcomes. Rural residence is frequently linked to debilitating long-term survivorship effects, and current data lack information on whether chronic pain disparity exists specifically for rural cancer survivors.
- Researchers pooled data from the 2019–2021 and 2023 National Health Interview Survey, a cross–sectional survey conducted by the National Center for Health Statistics.
- Analysis included 5542 adult cancer survivors diagnosed within the previous 5 years, with 51.6% female participants and 48.4% male participants.
- Chronic pain was defined as pain experienced on most or all days over the past 3 months, following National Center for Health Statistics conventions.
- Rural residence classification was based on noncore or nonmetropolitan counties using the modified National Center for Health Statistics Urban–Rural Classification Scheme for Counties.
TAKEAWAY:
- Rural cancer survivors showed significantly higher odds of experiencing chronic pain compared with urban survivors (odds ratio [OR], 1.21; 95% CI, 1.01-1.45).
- Rural survivors were more likely to be non–Hispanic White, have less than a 4-year college degree, have an income below 200% of the federal poverty level, and have slightly more chronic health conditions.
- Having an income below 100% of the federal poverty level was associated with doubled odds of chronic pain (OR, 2.07; 95% CI, 1.54-2.77) compared with having an income at least four times the federal poverty level.
- Each additional health condition increased the odds of experiencing chronic pain by 32% (OR, 1.32; 95% CI, 1.26-1.39).
IN PRACTICE:
“Policymakers and health systems should work to close this gap by increasing the availability of pain management resources for rural cancer survivors. Approaches could include innovative payment models for integrative medicine in rural areas or supporting rural clinician access to pain specialists,” the authors of the study wrote.
SOURCE:
This study was led by Hyojin Choi, PhD, Department of Family Medicine, The Robert Larner MD College of Medicine, University of Vermont in Burlington, Vermont. It was published online in JAMA Network Open.
LIMITATIONS:
The authors note that the cross–sectional design of the study and limited information on individual respondents’ use of multimodal pain treatment options constrain the interpretation of findings.
DISCLOSURES:
The authors did not report any relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Rural cancer survivors experience significantly higher rates of chronic pain at 43.0% than those among urban survivors at 33.5%. Even after controlling for demographics and health conditions, rural residents showed 21% higher odds of experiencing chronic pain.
METHODOLOGY:
- Chronic pain prevalence among cancer survivors is twice that of the general US population and is associated with numerous negative outcomes. Rural residence is frequently linked to debilitating long-term survivorship effects, and current data lack information on whether chronic pain disparity exists specifically for rural cancer survivors.
- Researchers pooled data from the 2019–2021 and 2023 National Health Interview Survey, a cross–sectional survey conducted by the National Center for Health Statistics.
- Analysis included 5542 adult cancer survivors diagnosed within the previous 5 years, with 51.6% female participants and 48.4% male participants.
- Chronic pain was defined as pain experienced on most or all days over the past 3 months, following National Center for Health Statistics conventions.
- Rural residence classification was based on noncore or nonmetropolitan counties using the modified National Center for Health Statistics Urban–Rural Classification Scheme for Counties.
TAKEAWAY:
- Rural cancer survivors showed significantly higher odds of experiencing chronic pain compared with urban survivors (odds ratio [OR], 1.21; 95% CI, 1.01-1.45).
- Rural survivors were more likely to be non–Hispanic White, have less than a 4-year college degree, have an income below 200% of the federal poverty level, and have slightly more chronic health conditions.
- Having an income below 100% of the federal poverty level was associated with doubled odds of chronic pain (OR, 2.07; 95% CI, 1.54-2.77) compared with having an income at least four times the federal poverty level.
- Each additional health condition increased the odds of experiencing chronic pain by 32% (OR, 1.32; 95% CI, 1.26-1.39).
IN PRACTICE:
“Policymakers and health systems should work to close this gap by increasing the availability of pain management resources for rural cancer survivors. Approaches could include innovative payment models for integrative medicine in rural areas or supporting rural clinician access to pain specialists,” the authors of the study wrote.
SOURCE:
This study was led by Hyojin Choi, PhD, Department of Family Medicine, The Robert Larner MD College of Medicine, University of Vermont in Burlington, Vermont. It was published online in JAMA Network Open.
LIMITATIONS:
The authors note that the cross–sectional design of the study and limited information on individual respondents’ use of multimodal pain treatment options constrain the interpretation of findings.
DISCLOSURES:
The authors did not report any relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Rural cancer survivors experience significantly higher rates of chronic pain at 43.0% than those among urban survivors at 33.5%. Even after controlling for demographics and health conditions, rural residents showed 21% higher odds of experiencing chronic pain.
METHODOLOGY:
- Chronic pain prevalence among cancer survivors is twice that of the general US population and is associated with numerous negative outcomes. Rural residence is frequently linked to debilitating long-term survivorship effects, and current data lack information on whether chronic pain disparity exists specifically for rural cancer survivors.
- Researchers pooled data from the 2019–2021 and 2023 National Health Interview Survey, a cross–sectional survey conducted by the National Center for Health Statistics.
- Analysis included 5542 adult cancer survivors diagnosed within the previous 5 years, with 51.6% female participants and 48.4% male participants.
- Chronic pain was defined as pain experienced on most or all days over the past 3 months, following National Center for Health Statistics conventions.
- Rural residence classification was based on noncore or nonmetropolitan counties using the modified National Center for Health Statistics Urban–Rural Classification Scheme for Counties.
TAKEAWAY:
- Rural cancer survivors showed significantly higher odds of experiencing chronic pain compared with urban survivors (odds ratio [OR], 1.21; 95% CI, 1.01-1.45).
- Rural survivors were more likely to be non–Hispanic White, have less than a 4-year college degree, have an income below 200% of the federal poverty level, and have slightly more chronic health conditions.
- Having an income below 100% of the federal poverty level was associated with doubled odds of chronic pain (OR, 2.07; 95% CI, 1.54-2.77) compared with having an income at least four times the federal poverty level.
- Each additional health condition increased the odds of experiencing chronic pain by 32% (OR, 1.32; 95% CI, 1.26-1.39).
IN PRACTICE:
“Policymakers and health systems should work to close this gap by increasing the availability of pain management resources for rural cancer survivors. Approaches could include innovative payment models for integrative medicine in rural areas or supporting rural clinician access to pain specialists,” the authors of the study wrote.
SOURCE:
This study was led by Hyojin Choi, PhD, Department of Family Medicine, The Robert Larner MD College of Medicine, University of Vermont in Burlington, Vermont. It was published online in JAMA Network Open.
LIMITATIONS:
The authors note that the cross–sectional design of the study and limited information on individual respondents’ use of multimodal pain treatment options constrain the interpretation of findings.
DISCLOSURES:
The authors did not report any relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Novel Treatment Combo Ups Survival in Multiple Myeloma
Novel Treatment Combo Ups Survival in Multiple Myeloma
Adding teclistamab to daratumumab dramatically improved progression-free survival and overall survival vs standard-of-care (SOC) therapy in patients with relapsed or refractory multiple myeloma (RRMM) in the phase 3 MajesTEC-3 trial.
At median follow-up of 34.5 months, progression-free survival was not reached in study participants randomized to receive teclistamab plus daratumumab (Tec-Dara). Starkly contrasting that was the 18.1 months progression-free survival among those randomized to a control group who received the standard of care: investigator’s choice of daratumumab and dexamethasone plus either pomalidomide or bortezomib (DPd/DVd (hazard ratio [HR], 0.17). Overall survival also significantly favored Tec-Dara (HR, 0.46).
Lead investigator María-Victoria Mateos, MD, PhD, reported the findings in a late-breaking abstract session at American Society of Hematology (ASH) 2025 Annual Meeting. They were published simultaneously in The New England Journal of Medicine.
“[Tec-Dara in this setting] generated the greatest progression-free survival treatment effect to date [in RRMR] with a plateau phase after 6 months of therapy, suggesting potential for functional cure,” said Mateos, a consultant physician and associate professor at the University of Salamanca, Salamanca, Spain.
“We consider that this synergistic immunotherapy combination…is a new potential standard of care for relapsed or refractory multiple myeloma after at least on prior line of therapy, with broad potential across academic and community settings,” added Mateos, who also directs the Multiple Myeloma Program at the University.
Based on the MajesTEC-3 findings, the FDA proactively awarded a national priority voucher to Tec-Dara under the Commissioner’s National Priority Voucher pilot program designed to accelerate the review of certain promising products.
About Tec-Dara
Teclistamab (Tecvayli) is an off-the-shelf first-in-class bispecific monoclonal antibody shown in the MajesTEC-1 trial to provide deep, durable responses in RRMM, with improved efficacy and safety with earlier lines of therapy. The FDA approved the agent for use in 4th or greater lines of therapy in 2022 based on those findings.
Daratumumab (Darzalex) is a widely used anti-CD38 monoclonal antibody currently considered the SOC therapy for RRMM. Both agents are products of the Janssen Pharmaceutical Companies of Johnson & Johnson.
Although front-line therapy for multiple myeloma has dramatically improved, there is a need for new, more effective treatment strategies in patients with disease progression, Mateos noted.
Therefore, she and her colleagues conducted MajesTEC-3, a randomized trial exploring the fully immunotherapy-based regimen of Tec-Dara vs daratumumab-based SOC in patients who had received one to three prior lines of therapy, including a proteasome inhibitor and lenalidomide (Len).
The study was the first to test a bispecific monoclonal antibody as early as the first relapse after initial treatment, she noted.
About MajesTEC-3
Study participants were 587 adults aged 25-88 years. Those with one prior line of therapy were required to be Len-refractory with progressive disease on or after the last therapy. Prior anti-CD-38 Patients with prior B-cell maturation antigen-directed therapy or who were refractory to anti-CD38 treatment were excluded.
The 291 patients randomized to the Tec-Dara treatment group and 296 randomized to the control group were treated in 28-day cycles according to the standard daratumumab schedule: weekly treatment during cycles 1 and 2, biweekly treatment during cycles 3-6, and monthly treatments beginning with cycle 7.
Teclistamab was initiated with an approved step-up dose school followed by 1.5 mg/kg weekly in cycles 1 and 2, 3 mg/kg biweekly in cycles 3-6, and 3 mg/kg monthly beginning with cycle 7.
The 36-month progression-free survival rates with Tec-Dara vs DPd/DVd were 83.4% and 29.7%, with the 36-month overall survival rates having been 83.3% and 65.0%. More than 90% of patients in the Tec-Dara group who were alive at 6 months were also alive at 30 months, Mateos noted.
For both progression-free survival and overall survival, the “clinically remarkable and statistically significant” differences were apparent across all prespecified and clinically relevant subgroups, she added. These included patients who were 75 years or older, Len-refractory patients, and those with high-risk cytogenetics, ≥ 60% bone marrow plasma cells, soft-tissue plasmacytomas, and anti-CD38 exposure.
Patients receiving Tec-Dara also had significantly higher rates of complete or better responses (81.8% vs 32.1%; odds ratio [OR], 9.56), overall response (89.0% vs 75.3%; OR, 2.65), and minimal residual disease-negativity (58.4% vs 17.1%; OR, 6.78).
The median time to first response and first complete or better response was similar in the two groups, but 36-month duration of response was 88.5 vs 36.4 months. At data cutoff, 49.4% of patients remained on study treatment — 71.0% in the Tec-Dara group and 28.3% in the DPd/DVd group, and median treatment duration was twice as long with Tec-Dara (32.4 vs 16.1 months), she said.
Serious adverse events occurred at similar rates in the treatment and control groups (70.7% and 62.4%) and most (44.2%) were grade 1 cytokine release syndrome (CRS). No grade 3 CRS occurred, and all CRS cases resolved.
Immune effector-cell-associated neurotoxicity occurred in 1.1% of patients, and all cases resolved.
Treatment-related adverse events leading to discontinuation occurred in 4.6% and 5.5% of patients in the Tec-Dara and DPd/DVd groups. The rates of deaths due to treatment-emergent adverse events were also similar in the groups (7.1% vs 5.9%).
Infections of any grade occurred in 96.5% and 84.1% of Tec-Dara and DPd/DVd patients, and grade 3/4 infections occurred in 54.1% and 43.4%. New-onset grade 3 or greater infections decreased over time.
“It’s important to acknowledge that patients with infections needed to be supported with adequate prophylaxis and immunoglobulins,” Mateo stressed.
Implications for Patients With RRMM
Teclistamab is currently only approved after three prior lines of therapy, but under the FDA Commissioner’s National Priority Voucher program, the agency will aim to complete its review of Tec-Dara for earlier treatment within 1-2 months following submission of an application for approval by Johnson & Johnson.
If an approval for that indication were to occur, it would be transformative for patients with RRMM, said Michael Rosenzweig, MD, of City of Hope, Duarte, California, in an interview with Medscape Medical News.
The [MajesTEC-3] findings suggest that Tec-Dar “really gives patients a chance at long-term disease control,” added Rosenzweig, chief of the Division of Multiple Myeloma, and an associate professor in the Department of Hematology & Hematopoietic Cell Transplantation at City of Hope.
MajesTEC-3 was funded by Johnson & Johnson. Mateos disclosed relationships with numerous pharmaceutical companies, including Johnson & Johnson. Rosenzweig reported consulting for Johnson & Johnson and was previously on the company’s speakers bureau.
Sharon Worcester, MA, is an award-winning medical journalist based in Birmingham, Alabama, writing for Medscape, MDedge, and other affiliate sites. She currently covers oncology, but she has also written on a variety of other medical specialties and health care topics. She can be reached at sworcester@mdedge.com or on X: @SW_MedReporter.
Adding teclistamab to daratumumab dramatically improved progression-free survival and overall survival vs standard-of-care (SOC) therapy in patients with relapsed or refractory multiple myeloma (RRMM) in the phase 3 MajesTEC-3 trial.
At median follow-up of 34.5 months, progression-free survival was not reached in study participants randomized to receive teclistamab plus daratumumab (Tec-Dara). Starkly contrasting that was the 18.1 months progression-free survival among those randomized to a control group who received the standard of care: investigator’s choice of daratumumab and dexamethasone plus either pomalidomide or bortezomib (DPd/DVd (hazard ratio [HR], 0.17). Overall survival also significantly favored Tec-Dara (HR, 0.46).
Lead investigator María-Victoria Mateos, MD, PhD, reported the findings in a late-breaking abstract session at American Society of Hematology (ASH) 2025 Annual Meeting. They were published simultaneously in The New England Journal of Medicine.
“[Tec-Dara in this setting] generated the greatest progression-free survival treatment effect to date [in RRMR] with a plateau phase after 6 months of therapy, suggesting potential for functional cure,” said Mateos, a consultant physician and associate professor at the University of Salamanca, Salamanca, Spain.
“We consider that this synergistic immunotherapy combination…is a new potential standard of care for relapsed or refractory multiple myeloma after at least on prior line of therapy, with broad potential across academic and community settings,” added Mateos, who also directs the Multiple Myeloma Program at the University.
Based on the MajesTEC-3 findings, the FDA proactively awarded a national priority voucher to Tec-Dara under the Commissioner’s National Priority Voucher pilot program designed to accelerate the review of certain promising products.
About Tec-Dara
Teclistamab (Tecvayli) is an off-the-shelf first-in-class bispecific monoclonal antibody shown in the MajesTEC-1 trial to provide deep, durable responses in RRMM, with improved efficacy and safety with earlier lines of therapy. The FDA approved the agent for use in 4th or greater lines of therapy in 2022 based on those findings.
Daratumumab (Darzalex) is a widely used anti-CD38 monoclonal antibody currently considered the SOC therapy for RRMM. Both agents are products of the Janssen Pharmaceutical Companies of Johnson & Johnson.
Although front-line therapy for multiple myeloma has dramatically improved, there is a need for new, more effective treatment strategies in patients with disease progression, Mateos noted.
Therefore, she and her colleagues conducted MajesTEC-3, a randomized trial exploring the fully immunotherapy-based regimen of Tec-Dara vs daratumumab-based SOC in patients who had received one to three prior lines of therapy, including a proteasome inhibitor and lenalidomide (Len).
The study was the first to test a bispecific monoclonal antibody as early as the first relapse after initial treatment, she noted.
About MajesTEC-3
Study participants were 587 adults aged 25-88 years. Those with one prior line of therapy were required to be Len-refractory with progressive disease on or after the last therapy. Prior anti-CD-38 Patients with prior B-cell maturation antigen-directed therapy or who were refractory to anti-CD38 treatment were excluded.
The 291 patients randomized to the Tec-Dara treatment group and 296 randomized to the control group were treated in 28-day cycles according to the standard daratumumab schedule: weekly treatment during cycles 1 and 2, biweekly treatment during cycles 3-6, and monthly treatments beginning with cycle 7.
Teclistamab was initiated with an approved step-up dose school followed by 1.5 mg/kg weekly in cycles 1 and 2, 3 mg/kg biweekly in cycles 3-6, and 3 mg/kg monthly beginning with cycle 7.
The 36-month progression-free survival rates with Tec-Dara vs DPd/DVd were 83.4% and 29.7%, with the 36-month overall survival rates having been 83.3% and 65.0%. More than 90% of patients in the Tec-Dara group who were alive at 6 months were also alive at 30 months, Mateos noted.
For both progression-free survival and overall survival, the “clinically remarkable and statistically significant” differences were apparent across all prespecified and clinically relevant subgroups, she added. These included patients who were 75 years or older, Len-refractory patients, and those with high-risk cytogenetics, ≥ 60% bone marrow plasma cells, soft-tissue plasmacytomas, and anti-CD38 exposure.
Patients receiving Tec-Dara also had significantly higher rates of complete or better responses (81.8% vs 32.1%; odds ratio [OR], 9.56), overall response (89.0% vs 75.3%; OR, 2.65), and minimal residual disease-negativity (58.4% vs 17.1%; OR, 6.78).
The median time to first response and first complete or better response was similar in the two groups, but 36-month duration of response was 88.5 vs 36.4 months. At data cutoff, 49.4% of patients remained on study treatment — 71.0% in the Tec-Dara group and 28.3% in the DPd/DVd group, and median treatment duration was twice as long with Tec-Dara (32.4 vs 16.1 months), she said.
Serious adverse events occurred at similar rates in the treatment and control groups (70.7% and 62.4%) and most (44.2%) were grade 1 cytokine release syndrome (CRS). No grade 3 CRS occurred, and all CRS cases resolved.
Immune effector-cell-associated neurotoxicity occurred in 1.1% of patients, and all cases resolved.
Treatment-related adverse events leading to discontinuation occurred in 4.6% and 5.5% of patients in the Tec-Dara and DPd/DVd groups. The rates of deaths due to treatment-emergent adverse events were also similar in the groups (7.1% vs 5.9%).
Infections of any grade occurred in 96.5% and 84.1% of Tec-Dara and DPd/DVd patients, and grade 3/4 infections occurred in 54.1% and 43.4%. New-onset grade 3 or greater infections decreased over time.
“It’s important to acknowledge that patients with infections needed to be supported with adequate prophylaxis and immunoglobulins,” Mateo stressed.
Implications for Patients With RRMM
Teclistamab is currently only approved after three prior lines of therapy, but under the FDA Commissioner’s National Priority Voucher program, the agency will aim to complete its review of Tec-Dara for earlier treatment within 1-2 months following submission of an application for approval by Johnson & Johnson.
If an approval for that indication were to occur, it would be transformative for patients with RRMM, said Michael Rosenzweig, MD, of City of Hope, Duarte, California, in an interview with Medscape Medical News.
The [MajesTEC-3] findings suggest that Tec-Dar “really gives patients a chance at long-term disease control,” added Rosenzweig, chief of the Division of Multiple Myeloma, and an associate professor in the Department of Hematology & Hematopoietic Cell Transplantation at City of Hope.
MajesTEC-3 was funded by Johnson & Johnson. Mateos disclosed relationships with numerous pharmaceutical companies, including Johnson & Johnson. Rosenzweig reported consulting for Johnson & Johnson and was previously on the company’s speakers bureau.
Sharon Worcester, MA, is an award-winning medical journalist based in Birmingham, Alabama, writing for Medscape, MDedge, and other affiliate sites. She currently covers oncology, but she has also written on a variety of other medical specialties and health care topics. She can be reached at sworcester@mdedge.com or on X: @SW_MedReporter.
Adding teclistamab to daratumumab dramatically improved progression-free survival and overall survival vs standard-of-care (SOC) therapy in patients with relapsed or refractory multiple myeloma (RRMM) in the phase 3 MajesTEC-3 trial.
At median follow-up of 34.5 months, progression-free survival was not reached in study participants randomized to receive teclistamab plus daratumumab (Tec-Dara). Starkly contrasting that was the 18.1 months progression-free survival among those randomized to a control group who received the standard of care: investigator’s choice of daratumumab and dexamethasone plus either pomalidomide or bortezomib (DPd/DVd (hazard ratio [HR], 0.17). Overall survival also significantly favored Tec-Dara (HR, 0.46).
Lead investigator María-Victoria Mateos, MD, PhD, reported the findings in a late-breaking abstract session at American Society of Hematology (ASH) 2025 Annual Meeting. They were published simultaneously in The New England Journal of Medicine.
“[Tec-Dara in this setting] generated the greatest progression-free survival treatment effect to date [in RRMR] with a plateau phase after 6 months of therapy, suggesting potential for functional cure,” said Mateos, a consultant physician and associate professor at the University of Salamanca, Salamanca, Spain.
“We consider that this synergistic immunotherapy combination…is a new potential standard of care for relapsed or refractory multiple myeloma after at least on prior line of therapy, with broad potential across academic and community settings,” added Mateos, who also directs the Multiple Myeloma Program at the University.
Based on the MajesTEC-3 findings, the FDA proactively awarded a national priority voucher to Tec-Dara under the Commissioner’s National Priority Voucher pilot program designed to accelerate the review of certain promising products.
About Tec-Dara
Teclistamab (Tecvayli) is an off-the-shelf first-in-class bispecific monoclonal antibody shown in the MajesTEC-1 trial to provide deep, durable responses in RRMM, with improved efficacy and safety with earlier lines of therapy. The FDA approved the agent for use in 4th or greater lines of therapy in 2022 based on those findings.
Daratumumab (Darzalex) is a widely used anti-CD38 monoclonal antibody currently considered the SOC therapy for RRMM. Both agents are products of the Janssen Pharmaceutical Companies of Johnson & Johnson.
Although front-line therapy for multiple myeloma has dramatically improved, there is a need for new, more effective treatment strategies in patients with disease progression, Mateos noted.
Therefore, she and her colleagues conducted MajesTEC-3, a randomized trial exploring the fully immunotherapy-based regimen of Tec-Dara vs daratumumab-based SOC in patients who had received one to three prior lines of therapy, including a proteasome inhibitor and lenalidomide (Len).
The study was the first to test a bispecific monoclonal antibody as early as the first relapse after initial treatment, she noted.
About MajesTEC-3
Study participants were 587 adults aged 25-88 years. Those with one prior line of therapy were required to be Len-refractory with progressive disease on or after the last therapy. Prior anti-CD-38 Patients with prior B-cell maturation antigen-directed therapy or who were refractory to anti-CD38 treatment were excluded.
The 291 patients randomized to the Tec-Dara treatment group and 296 randomized to the control group were treated in 28-day cycles according to the standard daratumumab schedule: weekly treatment during cycles 1 and 2, biweekly treatment during cycles 3-6, and monthly treatments beginning with cycle 7.
Teclistamab was initiated with an approved step-up dose school followed by 1.5 mg/kg weekly in cycles 1 and 2, 3 mg/kg biweekly in cycles 3-6, and 3 mg/kg monthly beginning with cycle 7.
The 36-month progression-free survival rates with Tec-Dara vs DPd/DVd were 83.4% and 29.7%, with the 36-month overall survival rates having been 83.3% and 65.0%. More than 90% of patients in the Tec-Dara group who were alive at 6 months were also alive at 30 months, Mateos noted.
For both progression-free survival and overall survival, the “clinically remarkable and statistically significant” differences were apparent across all prespecified and clinically relevant subgroups, she added. These included patients who were 75 years or older, Len-refractory patients, and those with high-risk cytogenetics, ≥ 60% bone marrow plasma cells, soft-tissue plasmacytomas, and anti-CD38 exposure.
Patients receiving Tec-Dara also had significantly higher rates of complete or better responses (81.8% vs 32.1%; odds ratio [OR], 9.56), overall response (89.0% vs 75.3%; OR, 2.65), and minimal residual disease-negativity (58.4% vs 17.1%; OR, 6.78).
The median time to first response and first complete or better response was similar in the two groups, but 36-month duration of response was 88.5 vs 36.4 months. At data cutoff, 49.4% of patients remained on study treatment — 71.0% in the Tec-Dara group and 28.3% in the DPd/DVd group, and median treatment duration was twice as long with Tec-Dara (32.4 vs 16.1 months), she said.
Serious adverse events occurred at similar rates in the treatment and control groups (70.7% and 62.4%) and most (44.2%) were grade 1 cytokine release syndrome (CRS). No grade 3 CRS occurred, and all CRS cases resolved.
Immune effector-cell-associated neurotoxicity occurred in 1.1% of patients, and all cases resolved.
Treatment-related adverse events leading to discontinuation occurred in 4.6% and 5.5% of patients in the Tec-Dara and DPd/DVd groups. The rates of deaths due to treatment-emergent adverse events were also similar in the groups (7.1% vs 5.9%).
Infections of any grade occurred in 96.5% and 84.1% of Tec-Dara and DPd/DVd patients, and grade 3/4 infections occurred in 54.1% and 43.4%. New-onset grade 3 or greater infections decreased over time.
“It’s important to acknowledge that patients with infections needed to be supported with adequate prophylaxis and immunoglobulins,” Mateo stressed.
Implications for Patients With RRMM
Teclistamab is currently only approved after three prior lines of therapy, but under the FDA Commissioner’s National Priority Voucher program, the agency will aim to complete its review of Tec-Dara for earlier treatment within 1-2 months following submission of an application for approval by Johnson & Johnson.
If an approval for that indication were to occur, it would be transformative for patients with RRMM, said Michael Rosenzweig, MD, of City of Hope, Duarte, California, in an interview with Medscape Medical News.
The [MajesTEC-3] findings suggest that Tec-Dar “really gives patients a chance at long-term disease control,” added Rosenzweig, chief of the Division of Multiple Myeloma, and an associate professor in the Department of Hematology & Hematopoietic Cell Transplantation at City of Hope.
MajesTEC-3 was funded by Johnson & Johnson. Mateos disclosed relationships with numerous pharmaceutical companies, including Johnson & Johnson. Rosenzweig reported consulting for Johnson & Johnson and was previously on the company’s speakers bureau.
Sharon Worcester, MA, is an award-winning medical journalist based in Birmingham, Alabama, writing for Medscape, MDedge, and other affiliate sites. She currently covers oncology, but she has also written on a variety of other medical specialties and health care topics. She can be reached at sworcester@mdedge.com or on X: @SW_MedReporter.
Novel Treatment Combo Ups Survival in Multiple Myeloma
Novel Treatment Combo Ups Survival in Multiple Myeloma
NICE Endorses Chemo-Free First-Line Options for EGFR NSCLC
NICE Endorses Chemo-Free First-Line Options for EGFR NSCLC
The National Institute for Health and Care Excellence (NICE) has recommended amivantamab (Rybrevant) plus lazertinib (Lazcluze) as a first-line option for adults with previously untreated advanced non–small-cell lung cancer (NSCLC) harboring epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 L858R substitution mutations.
In final draft guidance, NICE said the combination therapy should be funded by the NHS in England for eligible patients when it is the most appropriate option. Around 1115 people are expected to benefit.
Lung cancer is the third most common cancer and the leading cause of cancer mortality in the UK. It accounted for 10% of all new cancer diagnoses and 20% of cancer deaths in 2020. Approximately 31,000 people received NSCLC diagnoses in England in 2021, comprising 91% of all lung cancer cases. EGFR mutation-positive NSCLC is more common in women, non-smokers, and individuals from Asian ethnic backgrounds.
Welcoming the decision, Virginia Harrison, research trustee, EGFR+ UK, said, “This is a meaningful advance for patients and their families facing this diagnosis. [It] provides something the EGFR community urgently needs: more choice in first-line treatment.”
How Practice May Shift
The recommendation adds an alternative to existing standards, including osimertinib monotherapy or osimertinib plus pemetrexed/platinum-based chemotherapy. Clinical specialists noted that no single standard care exists for this patient group.
Younger patients and those willing to accept greater side effects may choose between amivantamab plus lazertinib or osimertinib plus chemotherapy. Patients older than 80 years might prefer osimertinib monotherapy due to adverse event considerations.
Mechanism of Action and Clinical Evidence
Amivantamab is a bispecific antibody that simultaneously binds EGFR and mesenchymal-epithelial transition receptors, blocking downstream signaling pathways that drive tumor growth and promoting immune-mediated cancer cell killing. Lazertinib is an oral third-generation EGFR TKI that selectively inhibits mutant EGFR signaling. Together, the agents provides complementary suppression of EGFR-driven tumour growth and resistance mechanisms.
The NICE recommendation is supported by results from the phase 3 MARIPOSA trial, which met its primary endpoint of progression-free survival (PFS). Treatment with amivantamab plus lazertinib significantly prolonged median PFS to 23.7 months compared with 16.6 months with osimertinib. The combination also demonstrated a significant improvement in overall survival, reducing the risk for death by 25% vs osimertinib. Median OS was not reached in the combination arm and was 36.7 months with osimertinib.
The most common adverse reactions with the combination included rash, nail toxicity, hypoalbuminaemia, hepatotoxicity, and stomatitis.
A Medicines and Healthcare products Regulatory Agency-approved subcutaneous formulation of amivantamab, authorized after the committee’s initial meeting, may further improve tolerability and convenience. Administration-related reactions occurred in 63% of patients with the intravenous formulation vs 14% with the subcutaneous formulation. Clinicians expect subcutaneous dosing to replace intravenous use in practice.
Dosing, Access, and Implementation
Amivantamab is administered every 2 weeks, either intravenously or subcutaneously. Lazertinib is taken as a daily oral tablet.
Rybrevant costs £1079 for a 350-mg per 7-mL vial. Lazcluze is priced at £4128.50 for 56 x 80-mg tablets and £6192.75 for 28 x 240-mg tablets. Confidential NHS discounts are available through simple patient access schemes.
Integrated care boards, NHS England, and local authorities must implement the guidance within 90 days of publication. For drugs receiving positive draft recommendations for routine commissioning, interim funding becomes accessible from the Cancer Drugs Fund budget starting from the point of marketing authorisation or publication of draft guidance.
The National Institute for Health and Care Excellence (NICE) has recommended amivantamab (Rybrevant) plus lazertinib (Lazcluze) as a first-line option for adults with previously untreated advanced non–small-cell lung cancer (NSCLC) harboring epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 L858R substitution mutations.
In final draft guidance, NICE said the combination therapy should be funded by the NHS in England for eligible patients when it is the most appropriate option. Around 1115 people are expected to benefit.
Lung cancer is the third most common cancer and the leading cause of cancer mortality in the UK. It accounted for 10% of all new cancer diagnoses and 20% of cancer deaths in 2020. Approximately 31,000 people received NSCLC diagnoses in England in 2021, comprising 91% of all lung cancer cases. EGFR mutation-positive NSCLC is more common in women, non-smokers, and individuals from Asian ethnic backgrounds.
Welcoming the decision, Virginia Harrison, research trustee, EGFR+ UK, said, “This is a meaningful advance for patients and their families facing this diagnosis. [It] provides something the EGFR community urgently needs: more choice in first-line treatment.”
How Practice May Shift
The recommendation adds an alternative to existing standards, including osimertinib monotherapy or osimertinib plus pemetrexed/platinum-based chemotherapy. Clinical specialists noted that no single standard care exists for this patient group.
Younger patients and those willing to accept greater side effects may choose between amivantamab plus lazertinib or osimertinib plus chemotherapy. Patients older than 80 years might prefer osimertinib monotherapy due to adverse event considerations.
Mechanism of Action and Clinical Evidence
Amivantamab is a bispecific antibody that simultaneously binds EGFR and mesenchymal-epithelial transition receptors, blocking downstream signaling pathways that drive tumor growth and promoting immune-mediated cancer cell killing. Lazertinib is an oral third-generation EGFR TKI that selectively inhibits mutant EGFR signaling. Together, the agents provides complementary suppression of EGFR-driven tumour growth and resistance mechanisms.
The NICE recommendation is supported by results from the phase 3 MARIPOSA trial, which met its primary endpoint of progression-free survival (PFS). Treatment with amivantamab plus lazertinib significantly prolonged median PFS to 23.7 months compared with 16.6 months with osimertinib. The combination also demonstrated a significant improvement in overall survival, reducing the risk for death by 25% vs osimertinib. Median OS was not reached in the combination arm and was 36.7 months with osimertinib.
The most common adverse reactions with the combination included rash, nail toxicity, hypoalbuminaemia, hepatotoxicity, and stomatitis.
A Medicines and Healthcare products Regulatory Agency-approved subcutaneous formulation of amivantamab, authorized after the committee’s initial meeting, may further improve tolerability and convenience. Administration-related reactions occurred in 63% of patients with the intravenous formulation vs 14% with the subcutaneous formulation. Clinicians expect subcutaneous dosing to replace intravenous use in practice.
Dosing, Access, and Implementation
Amivantamab is administered every 2 weeks, either intravenously or subcutaneously. Lazertinib is taken as a daily oral tablet.
Rybrevant costs £1079 for a 350-mg per 7-mL vial. Lazcluze is priced at £4128.50 for 56 x 80-mg tablets and £6192.75 for 28 x 240-mg tablets. Confidential NHS discounts are available through simple patient access schemes.
Integrated care boards, NHS England, and local authorities must implement the guidance within 90 days of publication. For drugs receiving positive draft recommendations for routine commissioning, interim funding becomes accessible from the Cancer Drugs Fund budget starting from the point of marketing authorisation or publication of draft guidance.
The National Institute for Health and Care Excellence (NICE) has recommended amivantamab (Rybrevant) plus lazertinib (Lazcluze) as a first-line option for adults with previously untreated advanced non–small-cell lung cancer (NSCLC) harboring epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 L858R substitution mutations.
In final draft guidance, NICE said the combination therapy should be funded by the NHS in England for eligible patients when it is the most appropriate option. Around 1115 people are expected to benefit.
Lung cancer is the third most common cancer and the leading cause of cancer mortality in the UK. It accounted for 10% of all new cancer diagnoses and 20% of cancer deaths in 2020. Approximately 31,000 people received NSCLC diagnoses in England in 2021, comprising 91% of all lung cancer cases. EGFR mutation-positive NSCLC is more common in women, non-smokers, and individuals from Asian ethnic backgrounds.
Welcoming the decision, Virginia Harrison, research trustee, EGFR+ UK, said, “This is a meaningful advance for patients and their families facing this diagnosis. [It] provides something the EGFR community urgently needs: more choice in first-line treatment.”
How Practice May Shift
The recommendation adds an alternative to existing standards, including osimertinib monotherapy or osimertinib plus pemetrexed/platinum-based chemotherapy. Clinical specialists noted that no single standard care exists for this patient group.
Younger patients and those willing to accept greater side effects may choose between amivantamab plus lazertinib or osimertinib plus chemotherapy. Patients older than 80 years might prefer osimertinib monotherapy due to adverse event considerations.
Mechanism of Action and Clinical Evidence
Amivantamab is a bispecific antibody that simultaneously binds EGFR and mesenchymal-epithelial transition receptors, blocking downstream signaling pathways that drive tumor growth and promoting immune-mediated cancer cell killing. Lazertinib is an oral third-generation EGFR TKI that selectively inhibits mutant EGFR signaling. Together, the agents provides complementary suppression of EGFR-driven tumour growth and resistance mechanisms.
The NICE recommendation is supported by results from the phase 3 MARIPOSA trial, which met its primary endpoint of progression-free survival (PFS). Treatment with amivantamab plus lazertinib significantly prolonged median PFS to 23.7 months compared with 16.6 months with osimertinib. The combination also demonstrated a significant improvement in overall survival, reducing the risk for death by 25% vs osimertinib. Median OS was not reached in the combination arm and was 36.7 months with osimertinib.
The most common adverse reactions with the combination included rash, nail toxicity, hypoalbuminaemia, hepatotoxicity, and stomatitis.
A Medicines and Healthcare products Regulatory Agency-approved subcutaneous formulation of amivantamab, authorized after the committee’s initial meeting, may further improve tolerability and convenience. Administration-related reactions occurred in 63% of patients with the intravenous formulation vs 14% with the subcutaneous formulation. Clinicians expect subcutaneous dosing to replace intravenous use in practice.
Dosing, Access, and Implementation
Amivantamab is administered every 2 weeks, either intravenously or subcutaneously. Lazertinib is taken as a daily oral tablet.
Rybrevant costs £1079 for a 350-mg per 7-mL vial. Lazcluze is priced at £4128.50 for 56 x 80-mg tablets and £6192.75 for 28 x 240-mg tablets. Confidential NHS discounts are available through simple patient access schemes.
Integrated care boards, NHS England, and local authorities must implement the guidance within 90 days of publication. For drugs receiving positive draft recommendations for routine commissioning, interim funding becomes accessible from the Cancer Drugs Fund budget starting from the point of marketing authorisation or publication of draft guidance.
NICE Endorses Chemo-Free First-Line Options for EGFR NSCLC
NICE Endorses Chemo-Free First-Line Options for EGFR NSCLC