User login
EVIDENCE-BASED ANSWER:
We don’t know which selective serotonin reuptake inhibitors (SSRIs) are the most effective and safe because no studies have compared these antidepressants with each other.
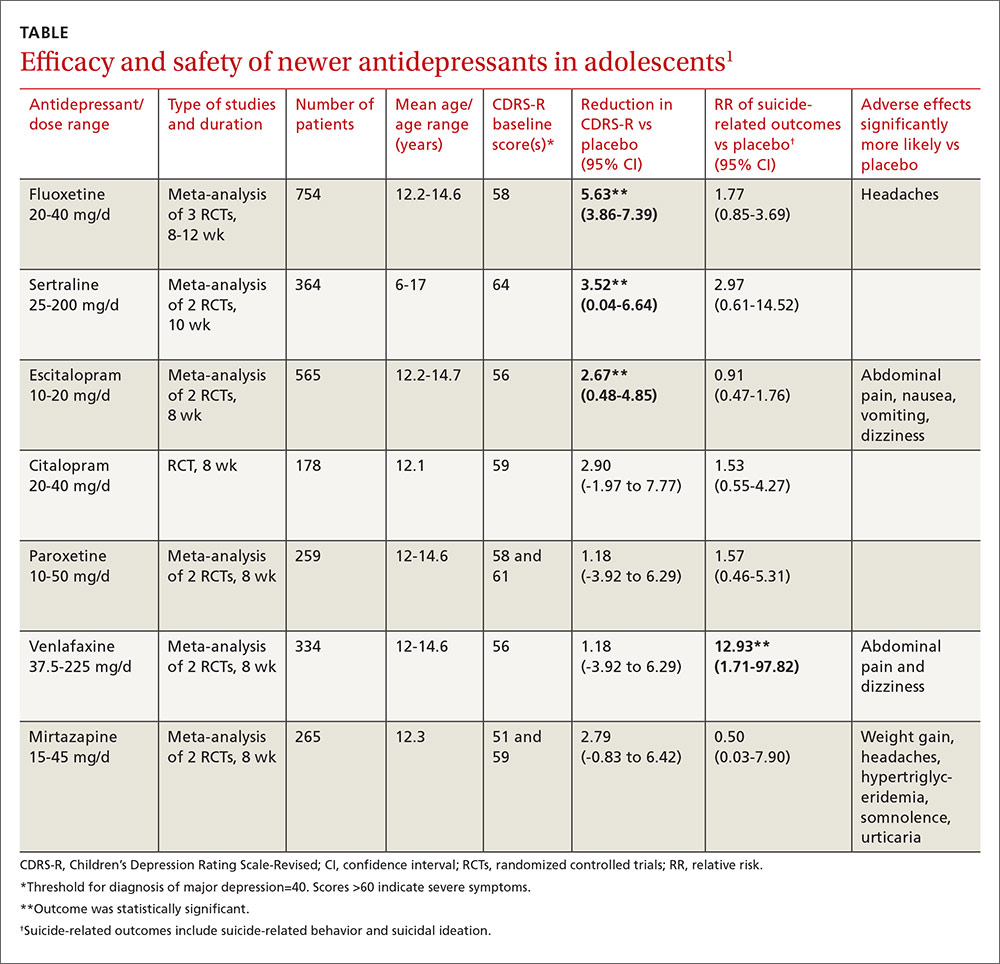
Three SSRI antidepressant medications—fluoxetine, sertraline, and escitalopram—produce modest improvements (about 5% to 10%) in standardized depression scores without a significant increase in the risk of suicide-related outcomes (suicidal behavior or ideation) in adolescent patients with major depression of moderate severity. As a group, however, the newer-generation antidepressants, including SSRIs, increase suicide-related outcomes by 50%. Citalopram, paroxetine, venlafaxine, and mirtazapine don’t improve depression scores (strength of recommendation [SOR]: A, meta-analyses of randomized controlled trials [RCTs]).
An updated national guideline recommends specific psychological therapy for adolescents with mild depression and combined psychotherapy and fluoxetine for moderate or severe depression, with sertraline or citalopram as second-line agents (SOR: A, RCTs).
EVIDENCE SUMMARY
A Cochrane systematic review (19 RCTs; 3335 patients, total) of newer-generation antidepressants for treating depression in adolescents found that, overall, they produced both a small decrease in symptom severity scores and an increased risk of suicide-related outcomes.1
Three SSRIs slightly lower one symptom severity score
Investigators performed a meta-analysis of all trials (14 RCTs; 2490 patients, total) that used the same standardized symptom severity score (the Children’s Depression Rating Scale—Revised [CDRS-R], range 17 to 113 points) to evaluate the following medications: fluoxetine, sertraline, escitalopram, citalopram, paroxetine, venlafaxine, and mirtazapine.1
All participants were outpatients who met criteria for a primary diagnosis of major depression, excluding comorbid conditions. The CDRS-R scores were evaluated by clinicians; the mean baseline score was 57 (40 is considered a threshold score for diagnosis, and above 60 indicates severe symptoms). Only 5 trials reported patients’ self-rated depression symptom severity (in patients taking fluoxetine and paroxetine) and none reported improvement. Treatment courses ranged from 8 to 12 weeks.
As a group, the newer antidepressants slightly reduced CDRS-R scores in adolescents (by 4.21 points, 95% confidence interval [CI], 0.41-5.95) but increased suicide-related outcomes (relative risk [RR]=1.47; 95% CI, 0.99-2.19). The individual antidepressants fluoxetine, sertraline, and escitalopram each produced statistically significant but clinically small reductions in CDRS-R scores of 5% to 10% without significantly increasing suicide-related outcomes (TABLE1). The other medications evaluated individually didn’t improve CDRS-R scores, and only venlafaxine increased suicide-related outcomes.
Other symptom severity scores show no improvement with SSRIs
Five additional RCTs not included in the meta-analysis that used standardized symptom severity scores other than the CDRS-R (Schedule for Affective Disorders and Schizophrenia for School-Aged Children [K-SADS], Montgomery-Asberg Depression Rating Scale [MADR], and Hamilton Depression Rating Scale [HAM-D]) found no improvement with fluoxetine (2 RCTs; 63 patients, total), citalopram (one RCT, 233 patients), or paroxetine (2 RCTs; 466 patients, total).
Certain drugs cause significantly more adverse events than placebo
Ten RCTs evaluated adverse events in adolescents treated with fluoxetine, escitalopram, citalopram, and paroxetine and reported a small increase over placebo when all medications were combined as a group (RR=1.11; 95% CI, 1.05-1.17). Investigators reported that the individual antidepressants fluoxetine, escitalopram, venlafaxine, and mirtazapine produced significantly more adverse events than placebo (P values not given). No studies compared antidepressant medications against each other for either efficacy or potential harms.
RECOMMENDATIONS
A newly revised expert guideline recommends treating mildly depressed adolescents with a specific psychological therapy—individual cognitive behavioral therapy, interpersonal therapy, family therapy, or psychodynamic psychotherapy—for at least 3 months.2
For adolescents with moderate to severe depression, the guideline advocates psychotherapy with the option of adding fluoxetine, although using antidepressants in adolescents who haven’t at least tried psychotherapy is outside of the drug’s indications.
The guideline also recommends careful monitoring for adverse effects and close review of mental state—weekly for the first 4 weeks of treatment, for example. If fluoxetine doesn’t help, sertraline and citalopram are recommended as alternatives.
1. Hetrick SE, McKenzie JE, Cox GR, et al. Newer generation antidepressants for depressive disorders in children and adolescents. Cochrane Database Syst Rev. 2012;11:CD004851.
2. Hopkins K, Crosland P, Elliott N, et al. Diagnosis and management of depression in children and young people: summary of updated NICE guidance. BMJ. 2015;350:h824.
EVIDENCE-BASED ANSWER:
We don’t know which selective serotonin reuptake inhibitors (SSRIs) are the most effective and safe because no studies have compared these antidepressants with each other.
Three SSRI antidepressant medications—fluoxetine, sertraline, and escitalopram—produce modest improvements (about 5% to 10%) in standardized depression scores without a significant increase in the risk of suicide-related outcomes (suicidal behavior or ideation) in adolescent patients with major depression of moderate severity. As a group, however, the newer-generation antidepressants, including SSRIs, increase suicide-related outcomes by 50%. Citalopram, paroxetine, venlafaxine, and mirtazapine don’t improve depression scores (strength of recommendation [SOR]: A, meta-analyses of randomized controlled trials [RCTs]).
An updated national guideline recommends specific psychological therapy for adolescents with mild depression and combined psychotherapy and fluoxetine for moderate or severe depression, with sertraline or citalopram as second-line agents (SOR: A, RCTs).
EVIDENCE SUMMARY
A Cochrane systematic review (19 RCTs; 3335 patients, total) of newer-generation antidepressants for treating depression in adolescents found that, overall, they produced both a small decrease in symptom severity scores and an increased risk of suicide-related outcomes.1
Three SSRIs slightly lower one symptom severity score
Investigators performed a meta-analysis of all trials (14 RCTs; 2490 patients, total) that used the same standardized symptom severity score (the Children’s Depression Rating Scale—Revised [CDRS-R], range 17 to 113 points) to evaluate the following medications: fluoxetine, sertraline, escitalopram, citalopram, paroxetine, venlafaxine, and mirtazapine.1
All participants were outpatients who met criteria for a primary diagnosis of major depression, excluding comorbid conditions. The CDRS-R scores were evaluated by clinicians; the mean baseline score was 57 (40 is considered a threshold score for diagnosis, and above 60 indicates severe symptoms). Only 5 trials reported patients’ self-rated depression symptom severity (in patients taking fluoxetine and paroxetine) and none reported improvement. Treatment courses ranged from 8 to 12 weeks.
As a group, the newer antidepressants slightly reduced CDRS-R scores in adolescents (by 4.21 points, 95% confidence interval [CI], 0.41-5.95) but increased suicide-related outcomes (relative risk [RR]=1.47; 95% CI, 0.99-2.19). The individual antidepressants fluoxetine, sertraline, and escitalopram each produced statistically significant but clinically small reductions in CDRS-R scores of 5% to 10% without significantly increasing suicide-related outcomes (TABLE1). The other medications evaluated individually didn’t improve CDRS-R scores, and only venlafaxine increased suicide-related outcomes.

Other symptom severity scores show no improvement with SSRIs
Five additional RCTs not included in the meta-analysis that used standardized symptom severity scores other than the CDRS-R (Schedule for Affective Disorders and Schizophrenia for School-Aged Children [K-SADS], Montgomery-Asberg Depression Rating Scale [MADR], and Hamilton Depression Rating Scale [HAM-D]) found no improvement with fluoxetine (2 RCTs; 63 patients, total), citalopram (one RCT, 233 patients), or paroxetine (2 RCTs; 466 patients, total).
Certain drugs cause significantly more adverse events than placebo
Ten RCTs evaluated adverse events in adolescents treated with fluoxetine, escitalopram, citalopram, and paroxetine and reported a small increase over placebo when all medications were combined as a group (RR=1.11; 95% CI, 1.05-1.17). Investigators reported that the individual antidepressants fluoxetine, escitalopram, venlafaxine, and mirtazapine produced significantly more adverse events than placebo (P values not given). No studies compared antidepressant medications against each other for either efficacy or potential harms.
RECOMMENDATIONS
A newly revised expert guideline recommends treating mildly depressed adolescents with a specific psychological therapy—individual cognitive behavioral therapy, interpersonal therapy, family therapy, or psychodynamic psychotherapy—for at least 3 months.2
For adolescents with moderate to severe depression, the guideline advocates psychotherapy with the option of adding fluoxetine, although using antidepressants in adolescents who haven’t at least tried psychotherapy is outside of the drug’s indications.
The guideline also recommends careful monitoring for adverse effects and close review of mental state—weekly for the first 4 weeks of treatment, for example. If fluoxetine doesn’t help, sertraline and citalopram are recommended as alternatives.
EVIDENCE-BASED ANSWER:
We don’t know which selective serotonin reuptake inhibitors (SSRIs) are the most effective and safe because no studies have compared these antidepressants with each other.
Three SSRI antidepressant medications—fluoxetine, sertraline, and escitalopram—produce modest improvements (about 5% to 10%) in standardized depression scores without a significant increase in the risk of suicide-related outcomes (suicidal behavior or ideation) in adolescent patients with major depression of moderate severity. As a group, however, the newer-generation antidepressants, including SSRIs, increase suicide-related outcomes by 50%. Citalopram, paroxetine, venlafaxine, and mirtazapine don’t improve depression scores (strength of recommendation [SOR]: A, meta-analyses of randomized controlled trials [RCTs]).
An updated national guideline recommends specific psychological therapy for adolescents with mild depression and combined psychotherapy and fluoxetine for moderate or severe depression, with sertraline or citalopram as second-line agents (SOR: A, RCTs).
EVIDENCE SUMMARY
A Cochrane systematic review (19 RCTs; 3335 patients, total) of newer-generation antidepressants for treating depression in adolescents found that, overall, they produced both a small decrease in symptom severity scores and an increased risk of suicide-related outcomes.1
Three SSRIs slightly lower one symptom severity score
Investigators performed a meta-analysis of all trials (14 RCTs; 2490 patients, total) that used the same standardized symptom severity score (the Children’s Depression Rating Scale—Revised [CDRS-R], range 17 to 113 points) to evaluate the following medications: fluoxetine, sertraline, escitalopram, citalopram, paroxetine, venlafaxine, and mirtazapine.1
All participants were outpatients who met criteria for a primary diagnosis of major depression, excluding comorbid conditions. The CDRS-R scores were evaluated by clinicians; the mean baseline score was 57 (40 is considered a threshold score for diagnosis, and above 60 indicates severe symptoms). Only 5 trials reported patients’ self-rated depression symptom severity (in patients taking fluoxetine and paroxetine) and none reported improvement. Treatment courses ranged from 8 to 12 weeks.
As a group, the newer antidepressants slightly reduced CDRS-R scores in adolescents (by 4.21 points, 95% confidence interval [CI], 0.41-5.95) but increased suicide-related outcomes (relative risk [RR]=1.47; 95% CI, 0.99-2.19). The individual antidepressants fluoxetine, sertraline, and escitalopram each produced statistically significant but clinically small reductions in CDRS-R scores of 5% to 10% without significantly increasing suicide-related outcomes (TABLE1). The other medications evaluated individually didn’t improve CDRS-R scores, and only venlafaxine increased suicide-related outcomes.

Other symptom severity scores show no improvement with SSRIs
Five additional RCTs not included in the meta-analysis that used standardized symptom severity scores other than the CDRS-R (Schedule for Affective Disorders and Schizophrenia for School-Aged Children [K-SADS], Montgomery-Asberg Depression Rating Scale [MADR], and Hamilton Depression Rating Scale [HAM-D]) found no improvement with fluoxetine (2 RCTs; 63 patients, total), citalopram (one RCT, 233 patients), or paroxetine (2 RCTs; 466 patients, total).
Certain drugs cause significantly more adverse events than placebo
Ten RCTs evaluated adverse events in adolescents treated with fluoxetine, escitalopram, citalopram, and paroxetine and reported a small increase over placebo when all medications were combined as a group (RR=1.11; 95% CI, 1.05-1.17). Investigators reported that the individual antidepressants fluoxetine, escitalopram, venlafaxine, and mirtazapine produced significantly more adverse events than placebo (P values not given). No studies compared antidepressant medications against each other for either efficacy or potential harms.
RECOMMENDATIONS
A newly revised expert guideline recommends treating mildly depressed adolescents with a specific psychological therapy—individual cognitive behavioral therapy, interpersonal therapy, family therapy, or psychodynamic psychotherapy—for at least 3 months.2
For adolescents with moderate to severe depression, the guideline advocates psychotherapy with the option of adding fluoxetine, although using antidepressants in adolescents who haven’t at least tried psychotherapy is outside of the drug’s indications.
The guideline also recommends careful monitoring for adverse effects and close review of mental state—weekly for the first 4 weeks of treatment, for example. If fluoxetine doesn’t help, sertraline and citalopram are recommended as alternatives.
1. Hetrick SE, McKenzie JE, Cox GR, et al. Newer generation antidepressants for depressive disorders in children and adolescents. Cochrane Database Syst Rev. 2012;11:CD004851.
2. Hopkins K, Crosland P, Elliott N, et al. Diagnosis and management of depression in children and young people: summary of updated NICE guidance. BMJ. 2015;350:h824.
1. Hetrick SE, McKenzie JE, Cox GR, et al. Newer generation antidepressants for depressive disorders in children and adolescents. Cochrane Database Syst Rev. 2012;11:CD004851.
2. Hopkins K, Crosland P, Elliott N, et al. Diagnosis and management of depression in children and young people: summary of updated NICE guidance. BMJ. 2015;350:h824.
Evidence-based answers from the Family Physicians Inquiries Network
