User login
One of the most important medical interventions to improve maternal-child health is providing effective contraception to men and women of reproductive age. The 52-mg levonorgestrel-intrauterine device (LNG-IUD; Mirena) is one of the most effective forms of reversible contraception available to women, with a failure rate of 1.1% over 5 years of use.1 The TCu380A copper-IUD (ParaGard), another highly effective reversible contraceptive, is reported to have failure rates of approximately 1.4% and 2.2%, over 5 and 10 years of use.2
An interesting question is whether—in certain clinical situations—a single IUD can be used for longer than the currently recommended 5 and 10 years for a Mirena IUD and a ParaGard IUD, respectively.
The LNG-IUD containing 52 mg LNG may be effective up to 7 years
The US Food and Drug Administration (FDA) package insert for the Mirena 52-mg LNG-IUD states that the device is “indicated for contraception for up to 5 years. Thereafter if continued contraception is desired, the system should be replaced.”1 The FDA package insert for the levonorgestrel-releasing intrauterine system, Liletta 52-mg LNG-IUD, states that it is “indicated for prevention of pregnancy up to 3 years.”3 The FDA guidance is based on data submitted to the agency by the manufacturers to support the approval process. Completing large-scale clinical trials that extend past 5 years or more is challenging, because of the cost and the loss of study participants to follow-up. Hence, few clinical trials of contraceptive IUDs continue for more than 5 to 10 years.
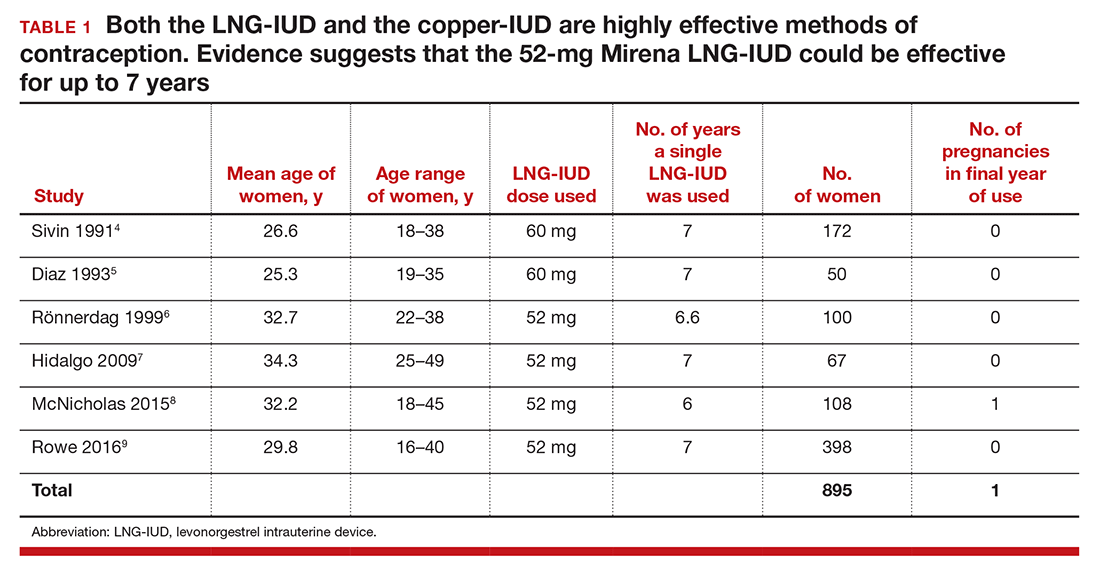
Although the FDA-approved indication for Mirena and Liletta is 5 and 3 years, respectively, evidence suggests that the 52-mg LNG-IUD is an effective contraceptive beyond 5 years. In fact, multiple studies report that this IUD is an effective contraceptive for at least 6 or 7 years (TABLE 1).4–9 Among 895 women using the 52-mg LNG-IUD for 6 to 7 years, only 1 pregnancy was reported in the last year of use. In that case, the IUD was in the cervix and partially expelled from the uterus.8 These data indicate that the 52-mg LNG-IUD is likely an effective contraceptive for up to 7 years, with pregnancy rates below 1% in the last year of use.
The TCu380A copper-IUD is effective up to 12 years
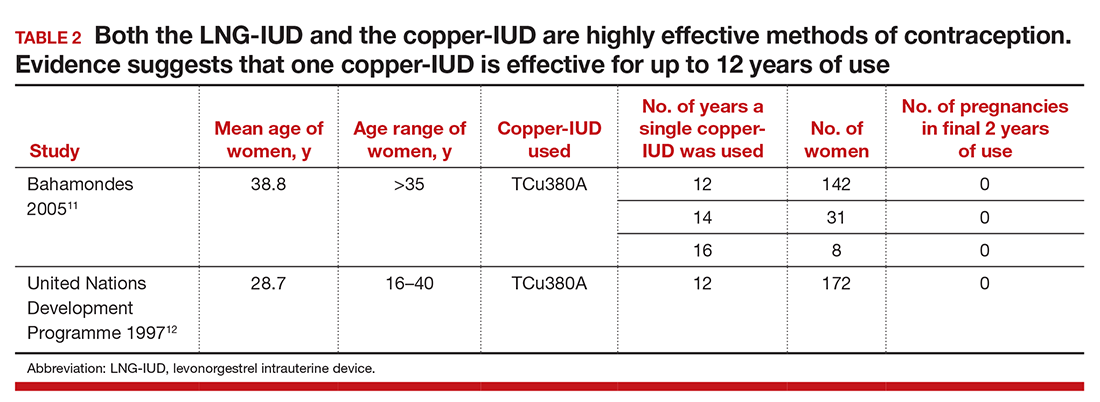
The currently available TCu380A copper-IUD (ParaGard) is FDA approved for 10 years.2 Studies evaluating the efficacy of this copper-IUD are limited, but those that have been published reported that it is effective for at least 12 years and possibly up to 20 years (TABLE 2).10−13
Recently I saw a patient who had a copper-IUD (ParaGard, TCu380A) inserted as a teen after a birth, and had successfully used the same device for 17 years. She presented for removal of the IUD so that she could attempt conception. After removal of the IUD, copper wire was visible on the device. Long-term studies of the TCu220 copper-IUD, which contains less copper than the ParaGard, report pregnancies with the use of the device beyond 10 years.12 These devices, which are not available in the United States, should not be used past their recommended interval.
- Mirena [package insert]. Wayne, NJ: Bayer HealthCare Pharmaceuticals; July 2008. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021225s019lbl.pdf. Accessed July 28, 2016.
- ParaGard [package insert]. N. Tonawanda, NY: FEI Women’s Health LLC; revised September 2005. http://www.accessdata.fda.gov/drugsatfda_docs/label/2005/018680s060lbl.pdf. Accessed July 28, 2016.
- Liletta [package insert]. Parsippany, NJ: Actavis Pharma, Inc; February 2015. http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206229s000lbl.pdf. Accessed July 28, 2016.
- Sivin I, Stern J, Coutinho E, et al. Prolonged intrauterine contraception: a seven-year randomized study of the levonorgestrel 20 mcg/day (LNg 20) and the copper T380 Ag IUDS. Contraception. 1991;44(5):473–480.
- Díaz J, Faúndes A, Díaz M, Marchi N. Evaluation of the clinical performance of a levonorgestrel-releasing IUD, up to seven years of use, in Campinas, Brazil. Contraception. 1993;47(2):169–175.
- Rönnerdag M, Odlind V. Health effects of long-term use of the intrauterine levonorgestrel-releasing system. A follow-up study over 12 years of continuous use. Acta Obstet Gynecol Scand. 1999;78(8):716–721.
- Hidalgo MM, Hidalgo-Regina C, Bahamondes MV, Monteiro I, Petta CA, Bahamondes L. Serum levonorgestrel levels and endometrial thickness during extended use of the levonorgestrel-releasing intrauterine system. Contraception. 2009;80(1):84–89.
- McNicholas C, Maddipati R, Zhao Q, Swor E, Peipert JF. Use of the etonogestrel implant and levonorgestrel intrauterine device beyond the U.S. Food and Drug Administration-approved duration. Obstet Gynecol. 2015;125(3):599–604.
- Rowe P, Farley T, Peregoudov A, et al. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93(6):498–506.
- Wu JP, Pickle S. Extended use of the intrauterine device: a literature review and recommendations for clinical practice. Contraception. 2014;89(6):495–503.
- Bahamondes L, Faundes A, Sobreira-Lima B, Liu-Filho JF, Pecci P, Matera S. TCu 380A IUD: a reversible permanent contraceptive method in women over 35 years of age. Contraception. 2005;72(5):337–341.
- United Nations Development Programme. Long-term reversible contraception. Twelve years of experience with the TCu380A and TCu220C. Contraception. 1997;56(6):341–352.
- Sivin I. Utility and drawbacks of continuous use of a copper T IUD for 20 years. Contraception. 2007;75(6 suppl):S70–S75.
One of the most important medical interventions to improve maternal-child health is providing effective contraception to men and women of reproductive age. The 52-mg levonorgestrel-intrauterine device (LNG-IUD; Mirena) is one of the most effective forms of reversible contraception available to women, with a failure rate of 1.1% over 5 years of use.1 The TCu380A copper-IUD (ParaGard), another highly effective reversible contraceptive, is reported to have failure rates of approximately 1.4% and 2.2%, over 5 and 10 years of use.2
An interesting question is whether—in certain clinical situations—a single IUD can be used for longer than the currently recommended 5 and 10 years for a Mirena IUD and a ParaGard IUD, respectively.
The LNG-IUD containing 52 mg LNG may be effective up to 7 years
The US Food and Drug Administration (FDA) package insert for the Mirena 52-mg LNG-IUD states that the device is “indicated for contraception for up to 5 years. Thereafter if continued contraception is desired, the system should be replaced.”1 The FDA package insert for the levonorgestrel-releasing intrauterine system, Liletta 52-mg LNG-IUD, states that it is “indicated for prevention of pregnancy up to 3 years.”3 The FDA guidance is based on data submitted to the agency by the manufacturers to support the approval process. Completing large-scale clinical trials that extend past 5 years or more is challenging, because of the cost and the loss of study participants to follow-up. Hence, few clinical trials of contraceptive IUDs continue for more than 5 to 10 years.
Although the FDA-approved indication for Mirena and Liletta is 5 and 3 years, respectively, evidence suggests that the 52-mg LNG-IUD is an effective contraceptive beyond 5 years. In fact, multiple studies report that this IUD is an effective contraceptive for at least 6 or 7 years (TABLE 1).4–9 Among 895 women using the 52-mg LNG-IUD for 6 to 7 years, only 1 pregnancy was reported in the last year of use. In that case, the IUD was in the cervix and partially expelled from the uterus.8 These data indicate that the 52-mg LNG-IUD is likely an effective contraceptive for up to 7 years, with pregnancy rates below 1% in the last year of use.

The TCu380A copper-IUD is effective up to 12 years
The currently available TCu380A copper-IUD (ParaGard) is FDA approved for 10 years.2 Studies evaluating the efficacy of this copper-IUD are limited, but those that have been published reported that it is effective for at least 12 years and possibly up to 20 years (TABLE 2).10−13

Recently I saw a patient who had a copper-IUD (ParaGard, TCu380A) inserted as a teen after a birth, and had successfully used the same device for 17 years. She presented for removal of the IUD so that she could attempt conception. After removal of the IUD, copper wire was visible on the device. Long-term studies of the TCu220 copper-IUD, which contains less copper than the ParaGard, report pregnancies with the use of the device beyond 10 years.12 These devices, which are not available in the United States, should not be used past their recommended interval.
One of the most important medical interventions to improve maternal-child health is providing effective contraception to men and women of reproductive age. The 52-mg levonorgestrel-intrauterine device (LNG-IUD; Mirena) is one of the most effective forms of reversible contraception available to women, with a failure rate of 1.1% over 5 years of use.1 The TCu380A copper-IUD (ParaGard), another highly effective reversible contraceptive, is reported to have failure rates of approximately 1.4% and 2.2%, over 5 and 10 years of use.2
An interesting question is whether—in certain clinical situations—a single IUD can be used for longer than the currently recommended 5 and 10 years for a Mirena IUD and a ParaGard IUD, respectively.
The LNG-IUD containing 52 mg LNG may be effective up to 7 years
The US Food and Drug Administration (FDA) package insert for the Mirena 52-mg LNG-IUD states that the device is “indicated for contraception for up to 5 years. Thereafter if continued contraception is desired, the system should be replaced.”1 The FDA package insert for the levonorgestrel-releasing intrauterine system, Liletta 52-mg LNG-IUD, states that it is “indicated for prevention of pregnancy up to 3 years.”3 The FDA guidance is based on data submitted to the agency by the manufacturers to support the approval process. Completing large-scale clinical trials that extend past 5 years or more is challenging, because of the cost and the loss of study participants to follow-up. Hence, few clinical trials of contraceptive IUDs continue for more than 5 to 10 years.
Although the FDA-approved indication for Mirena and Liletta is 5 and 3 years, respectively, evidence suggests that the 52-mg LNG-IUD is an effective contraceptive beyond 5 years. In fact, multiple studies report that this IUD is an effective contraceptive for at least 6 or 7 years (TABLE 1).4–9 Among 895 women using the 52-mg LNG-IUD for 6 to 7 years, only 1 pregnancy was reported in the last year of use. In that case, the IUD was in the cervix and partially expelled from the uterus.8 These data indicate that the 52-mg LNG-IUD is likely an effective contraceptive for up to 7 years, with pregnancy rates below 1% in the last year of use.

The TCu380A copper-IUD is effective up to 12 years
The currently available TCu380A copper-IUD (ParaGard) is FDA approved for 10 years.2 Studies evaluating the efficacy of this copper-IUD are limited, but those that have been published reported that it is effective for at least 12 years and possibly up to 20 years (TABLE 2).10−13

Recently I saw a patient who had a copper-IUD (ParaGard, TCu380A) inserted as a teen after a birth, and had successfully used the same device for 17 years. She presented for removal of the IUD so that she could attempt conception. After removal of the IUD, copper wire was visible on the device. Long-term studies of the TCu220 copper-IUD, which contains less copper than the ParaGard, report pregnancies with the use of the device beyond 10 years.12 These devices, which are not available in the United States, should not be used past their recommended interval.
- Mirena [package insert]. Wayne, NJ: Bayer HealthCare Pharmaceuticals; July 2008. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021225s019lbl.pdf. Accessed July 28, 2016.
- ParaGard [package insert]. N. Tonawanda, NY: FEI Women’s Health LLC; revised September 2005. http://www.accessdata.fda.gov/drugsatfda_docs/label/2005/018680s060lbl.pdf. Accessed July 28, 2016.
- Liletta [package insert]. Parsippany, NJ: Actavis Pharma, Inc; February 2015. http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206229s000lbl.pdf. Accessed July 28, 2016.
- Sivin I, Stern J, Coutinho E, et al. Prolonged intrauterine contraception: a seven-year randomized study of the levonorgestrel 20 mcg/day (LNg 20) and the copper T380 Ag IUDS. Contraception. 1991;44(5):473–480.
- Díaz J, Faúndes A, Díaz M, Marchi N. Evaluation of the clinical performance of a levonorgestrel-releasing IUD, up to seven years of use, in Campinas, Brazil. Contraception. 1993;47(2):169–175.
- Rönnerdag M, Odlind V. Health effects of long-term use of the intrauterine levonorgestrel-releasing system. A follow-up study over 12 years of continuous use. Acta Obstet Gynecol Scand. 1999;78(8):716–721.
- Hidalgo MM, Hidalgo-Regina C, Bahamondes MV, Monteiro I, Petta CA, Bahamondes L. Serum levonorgestrel levels and endometrial thickness during extended use of the levonorgestrel-releasing intrauterine system. Contraception. 2009;80(1):84–89.
- McNicholas C, Maddipati R, Zhao Q, Swor E, Peipert JF. Use of the etonogestrel implant and levonorgestrel intrauterine device beyond the U.S. Food and Drug Administration-approved duration. Obstet Gynecol. 2015;125(3):599–604.
- Rowe P, Farley T, Peregoudov A, et al. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93(6):498–506.
- Wu JP, Pickle S. Extended use of the intrauterine device: a literature review and recommendations for clinical practice. Contraception. 2014;89(6):495–503.
- Bahamondes L, Faundes A, Sobreira-Lima B, Liu-Filho JF, Pecci P, Matera S. TCu 380A IUD: a reversible permanent contraceptive method in women over 35 years of age. Contraception. 2005;72(5):337–341.
- United Nations Development Programme. Long-term reversible contraception. Twelve years of experience with the TCu380A and TCu220C. Contraception. 1997;56(6):341–352.
- Sivin I. Utility and drawbacks of continuous use of a copper T IUD for 20 years. Contraception. 2007;75(6 suppl):S70–S75.
- Mirena [package insert]. Wayne, NJ: Bayer HealthCare Pharmaceuticals; July 2008. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021225s019lbl.pdf. Accessed July 28, 2016.
- ParaGard [package insert]. N. Tonawanda, NY: FEI Women’s Health LLC; revised September 2005. http://www.accessdata.fda.gov/drugsatfda_docs/label/2005/018680s060lbl.pdf. Accessed July 28, 2016.
- Liletta [package insert]. Parsippany, NJ: Actavis Pharma, Inc; February 2015. http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206229s000lbl.pdf. Accessed July 28, 2016.
- Sivin I, Stern J, Coutinho E, et al. Prolonged intrauterine contraception: a seven-year randomized study of the levonorgestrel 20 mcg/day (LNg 20) and the copper T380 Ag IUDS. Contraception. 1991;44(5):473–480.
- Díaz J, Faúndes A, Díaz M, Marchi N. Evaluation of the clinical performance of a levonorgestrel-releasing IUD, up to seven years of use, in Campinas, Brazil. Contraception. 1993;47(2):169–175.
- Rönnerdag M, Odlind V. Health effects of long-term use of the intrauterine levonorgestrel-releasing system. A follow-up study over 12 years of continuous use. Acta Obstet Gynecol Scand. 1999;78(8):716–721.
- Hidalgo MM, Hidalgo-Regina C, Bahamondes MV, Monteiro I, Petta CA, Bahamondes L. Serum levonorgestrel levels and endometrial thickness during extended use of the levonorgestrel-releasing intrauterine system. Contraception. 2009;80(1):84–89.
- McNicholas C, Maddipati R, Zhao Q, Swor E, Peipert JF. Use of the etonogestrel implant and levonorgestrel intrauterine device beyond the U.S. Food and Drug Administration-approved duration. Obstet Gynecol. 2015;125(3):599–604.
- Rowe P, Farley T, Peregoudov A, et al. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93(6):498–506.
- Wu JP, Pickle S. Extended use of the intrauterine device: a literature review and recommendations for clinical practice. Contraception. 2014;89(6):495–503.
- Bahamondes L, Faundes A, Sobreira-Lima B, Liu-Filho JF, Pecci P, Matera S. TCu 380A IUD: a reversible permanent contraceptive method in women over 35 years of age. Contraception. 2005;72(5):337–341.
- United Nations Development Programme. Long-term reversible contraception. Twelve years of experience with the TCu380A and TCu220C. Contraception. 1997;56(6):341–352.
- Sivin I. Utility and drawbacks of continuous use of a copper T IUD for 20 years. Contraception. 2007;75(6 suppl):S70–S75.