User login
A 68-year-old woman with a history of well-controlled hypertension and diabetes presents to the office for routine follow-up. She says she has adhered to her current medications, and her blood pressure and hemoglobin A1c remain at goal. At the close of the visit, she mentions that she is worried she may be developing dementia. She says she has been having difficulty finding the right word in conversation and needs to write things down more than she used to.
What might be causing this patient’s changes in cognition?
In primary care settings, when patients complain of memory loss, there is a 20% to 30% chance they will be found to have mild cognitive impairment (MCI) or some level of dementia.1 Given the significant consequences of dementia, it’s important to maximize opportunities to distinguish those with age-related changes in cognition or reversible causes of memory loss from those who have irreversible pathologic changes.
Age-related changes in cognition
Changes in cognition associated with aging vary considerably among individuals and across domains of cognition. By their 7th decade, most people experience a decline in processing speed and working memory.2 However, some individuals retain excellent function into their 80s and perform as well as younger adults.3
Changes long thought to be due to brain senescence may, in fact, be related to the effects of age-related medical conditions on the brain’s function.4 Consistent with this theory is the observation that cognitive changes tend to occur earlier in individuals with cardiovascular disease, diabetes, and cancer.2 What constitutes a normal change depends on an individual’s baseline cognitive function, educational background, medical comorbidities, and the potential impact of sensory impairment on performance.
General cognitive trends with aging. Awareness of normal changes in an aging population is useful when assessing patients concerned about their memory. In general, an individual’s ability to maintain attention to a single task is preserved into late life. Ability to perform tasks requiring divided attention or attention-switching tends to decline.3 This has implications for driving, given the need to constantly switch one’s attention in response to the environment and the ability to sort relevant from irrelevant information.
Remote memory, semantic memory (factual information), and procedural memory (knowledge of skills and procedures) tend to remain intact with aging.4 Short-term memory (simple maintenance of information over a short period of time) shows little change with aging. However, working memory, which requires the manipulation of information in short-term memory, declines.
A simple demonstration of this is that performance on digit span testing tends to remain preserved (7±2), but digit span backwards declines. Holding digits in mind in the order they are received can be achieved through rehearsal. But to reverse the order requires reorganization of the information, and this ability declines with aging.3
Prospective memory (remembering to do things in the future) often requires increased dependence on external aids, such as a to-do list.3 The capacity to learn and recall new information declines. Even when given repeated opportunity to practice, older adults demonstrate a slower learning curve and lower total amount learned.4 Therefore, it becomes easier relying on well-learned cognitive processes such as cooking a familiar meal or relying on previously used principles for decision making.2
Language comprehension and vocabulary remain stable over time. However, difficulty with spontaneous word finding—the “tip-of-the-tongue” phenomenon—tends to increase. In contrast to the dysnomia related to dementia, the word-finding difficulties with normal aging typically improve with cues, indicating that the problem is in retrieval of information rather than storage. Verbal fluency, the rate at which words from a single category can be produced, shows decline. This is particularly true in tests of semantic verbal fluency (name all the animals you can think of); phonemic fluency (words that start with a certain letter) tends to be preserved.4
Some studies using neurocognitive testing have suggested a decline in executive functioning. But, in general, aging has little impact on “real world” executive functions that are required for planning and executing tasks.4 On the whole, cognitive changes related to aging typically do not interfere with an individual’s ability to function independently.
Mild cognitive impairment/mild neurocognitive disorder
Originally conceived as a precursor to Alzheimer’s dementia,5 mild cognitive impairment (MCI) is a diagnosis that has evolved to describe a heterogeneous syndrome of abnormal cognition characterized by:6-8
- a suspected change in cognition expressed by the patient, an acquaintance who knows the patient well, or a clinician;
- objectively measured impairment in one or more cognitive domains beyond what would be expected based on an individual’s age and educational background;
- preservation of functional abilities; and
- a lack of findings that would fulfill criteria for dementia.
In the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM V), this concept is identified as mild neurocognitive disorder, with the additional caveats that an individual’s cognitive deficits do not occur exclusively in the context of delirium and are not better explained by another mental disorder such as depression or schizophrenia.9
An accurate assessment of cognitive change is best measured against the individual’s baseline, which may necessitate the report of a reliable acquaintance. An assessment of functional abilities is also critical. Mild problems in performing complex functions (bill paying, shopping, etc) could be present and still allow a patient to meet the criteria for MCI. An individual may take more time, be less efficient, or make more errors than before; however, independence with minimal aid or assistance is preserved. It
MCI can be divided into 4 subtypes depending upon the cognitive domains affected (complex attention, executive function, learning and memory, language, visuospatial, social cognition):
- Amnestic MCI single domain, if only memory is affected.
- Amnestic MCI multiple domain, if memory and any other cognitive domains are affected.
- Non-amnestic MCI single domain, if any other cognitive domain aside from memory is the only one affected.
- Non-amnestic MCI multiple domain, if multiple domains other than memory are affected.
These distinctions may provide clues to the underlying cause of dysfunction and provide prognostic information regarding the risk of progression to dementia.6,7
Prevalence estimates for MCI vary widely due to differences in definitions used and populations studied. The best estimate is 5% to 10% prevalence among those ages 65 to 69 years old, and 12% to 25% among those ages 80 to 84.10 Similarly, estimates of the rate of progression to dementia vary. Among MCI populations identified through referral sources such as memory centers, the rate of progression to dementia has been 10% to 15% per year.11 In epidemiologic studies of general populations, the rate has been 6% to 10% per year.11 The rate of development of dementia among normal subjects is 1% to 2% per year.5
Dementia/major neurocognitive disorder
The primary feature distinguishing MCI/mild neurocognitive disorder from dementia or major neurocognitive disorder is a patient’s functional status. The core clinical criteria for all-cause dementia are cognitive or neurobehavioral symptoms that: 12
- interfere with work or usual daily function,
- represent a change from the prior baseline function,
- are not explained by delirium or a psychiatric illness, and
- include detectable impairment in 2 cognitive domains.
Criteria outlined in the DSM-V for major neurocognitive disorder are essentially the same but describe the functional change criteria as cognitive changes that “interfere with independence in everyday activities.”9 The DSM-V elaborates: “at a minimum, requiring assistance with complex instrumental activities of daily living such as paying bills or managing medications.”
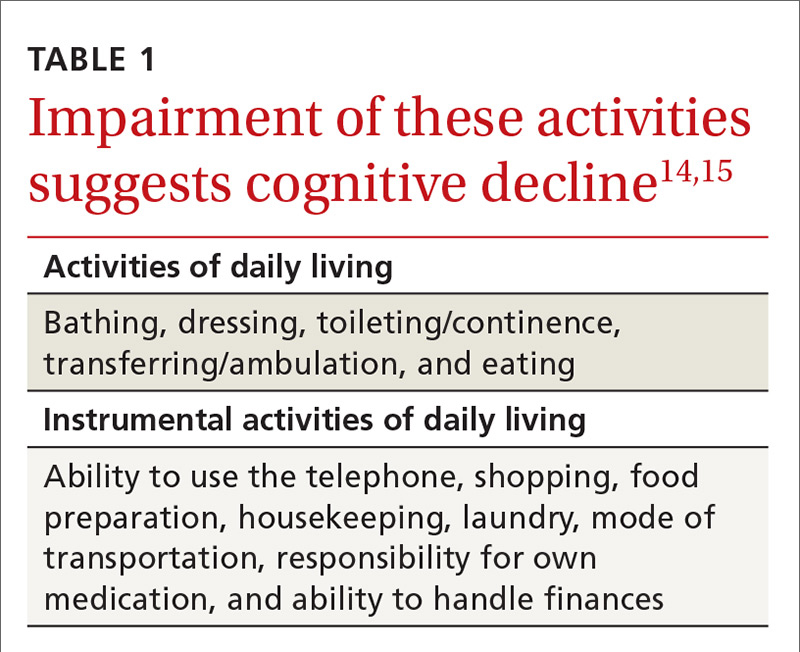
Assessing functional status accurately in clinical practice typically requires the assistance of a collateral informant who knows the patient well. The Informant Questionnaire on Cognitive Decline in the Elderly (https://www.alz.org/documents_custom/shortiqcode_english.pdf) is one validated assessment tool that can be used for this purpose.13 With this self-administered form, the informant answers 16 questions regarding changes in the patient’s performance of different activities over the 10 years prior. Alternatively, a structured interview based on indices of activities of daily living (ADLs) and instrumental activities of daily living (IADLs) as listed in TABLE 1 can be employed.14,15
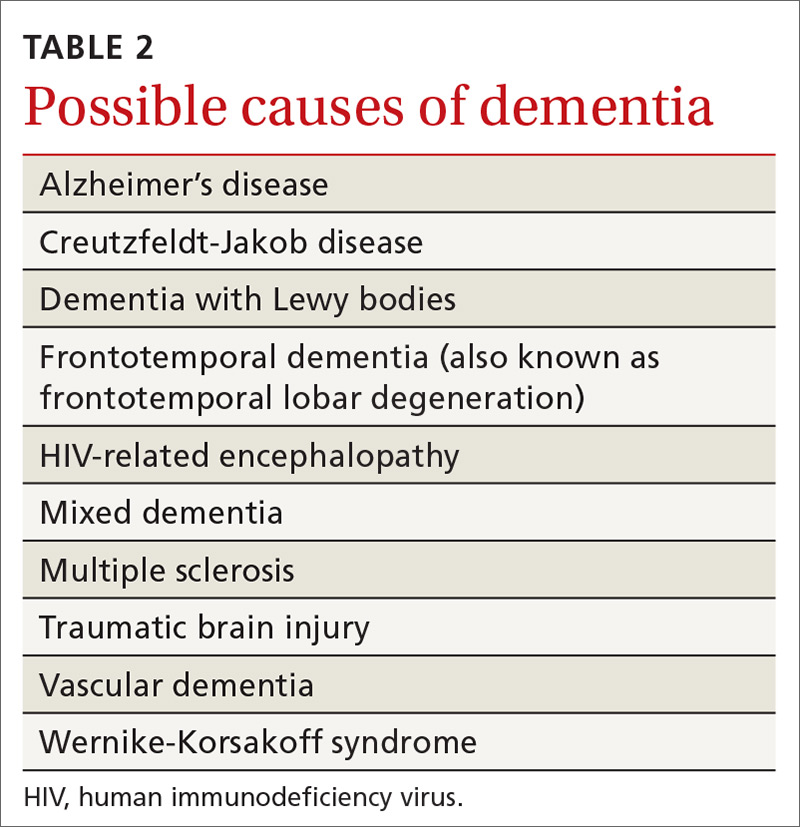
Review of the various causes of dementia is beyond the scope of this article, but a list of common diagnoses is presented in TABLE 2.
Dementia syndrome of depression (pseudodementia)
Elderly patients with depression commonly complain of memory impairment, and this interaction between depression and dementia has been investigated for decades. The term “pseudodementia” has been used since 1961 to describe signs of dementia in a patient with any psychiatric illness,16 but it has since been refined to apply solely to depression. The prevalence of depression among older adults varies depending on the population studied and how depression is defined. Approximately 2% to 3% of community-dwelling elders meet criteria for major depression, with 10% to 30% showing some symptoms of depression.17,18
Twenty percent to 40% of elderly patients diagnosed with depression will have evidence of cognitive impairment.19-21 Most improve with antidepressive treatment, though evidence of cognitive impairment may continue for some.19
A broad range of cognitive deficits have been associated with depression. Most consistently described are deficits in processing speed,22-25 attention,26-28 and executive function.22,25-29 Memory deficits can be apparent with tests of delayed recall, but recognition (the ability to identify items from a list) generally is preserved.26,28-30
Distinguishing between pseudodementia and true dementia can be challenging. An increased severity of deficits, particularly with delayed recall, is more indicative of dementia.31 Additionally, on clock drawing tasks, individuals with depression perform more comparably to controls than do those with true dementia.32
A 2013 meta-analysis reported a significant association of late-life depression with subsequent development of dementia, with an odds ratio (OR) of 1.85. The risk of subsequently developing vascular dementia (OR=2.52) was significantly higher than that for Alzheimer’s disease (OR=1.65). Individuals with evidence of reversible cognitive impairment at the time of diagnosis of depression seem to be particularly vulnerable, with dementia developing in 43% to 71%, compared with rates of 12% to 18% among elders diagnosed with depression but lacking signs of cognitive impairment.20,21
Other causes of reversible dementia
A meta-analysis performed in 1988 found that 11% of cases of dementia were reversible.33 However, an update using the same methodology in 2003 revealed the number had dropped to less than 1%.34 In the latest meta-analysis, one of the authors’ leading hypotheses for the dramatic decline in apparent prevalence was a significant shift in the study population from the inpatient to outpatient setting. In studies of community-based populations used in the re-analysis, the reported prevalence of reversibility was near zero.34
Metabolic abnormalities—most often B12 deficiency and hypothyroidism—are commonly cited as potential causes of dementia. Four systematic reviews, including one conducted by the Cochrane Collaborative, concluded there is a lack of evidence that treating low vitamin B12 in individuals with dementia improves cognition.35,36 There is some evidence, though, of a time-limited window for successful treatment within 12 months of the onset of symptoms.37,38 A study reviewing causes of dementia in nearly 3000 individuals found one case of reversible dementia attributable to hypothyroidism.39 A subsequent review reached similar conclusions about the lack of data to support the notion that treatment of hypothyroidism reverses dementia.40
Similarly, imaging for cerebral tumors, subdural hematomas, or normal-pressure hydrocephalus rarely identifies these as a cause of dementia.41 This is particularly true of unselected community-based populations, as there are typically signs or symptoms suggesting an intracranial pathology.
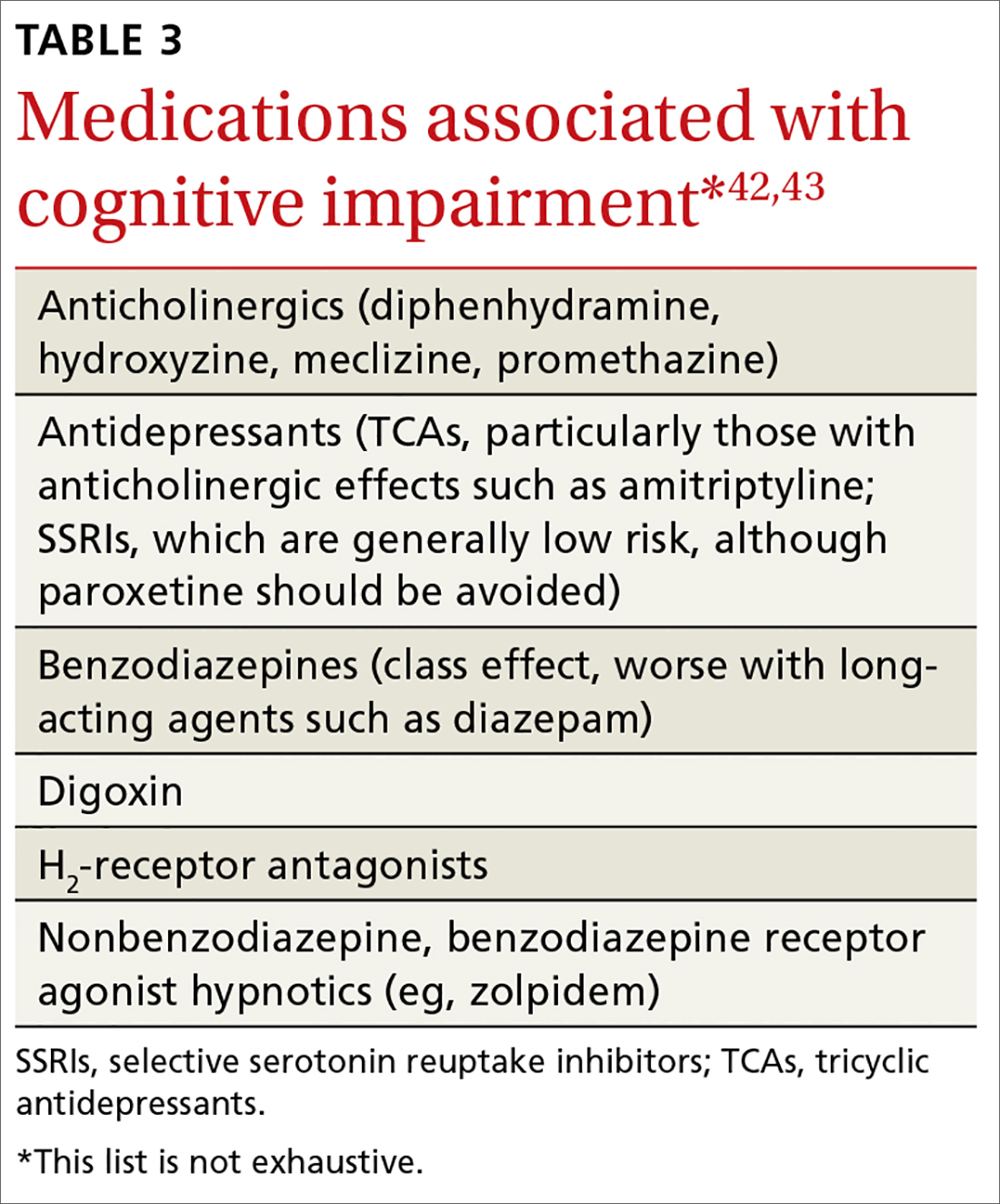
Numerous medications have been implicated in causing acute confusional states, and there is some evidence for their role in chronic confusion (TABLE 3).42,43 In my experience, many who experience adverse effects on cognition with medications will also have an underlying neurodegenerative process, and symptoms do not completely resolve with withdrawal of the offending agent.
Further assessment of the patient yielded a score of 29/30 on the Montreal Cognitive Assessment* and a zero on the Patient Health Questionnaire-2. Careful review of her daily function revealed no significant deficits in ADL or IADL performance, and her daughter confirmed that she had not observed any significant decline in her mother’s function. There was no significant family history of dementia. The patient was reassured that her cognitive changes were normal and age related.
Unfortunately, few data support specific interventions to reduce this patient’s risk of developing dementia. She was commended for keeping her blood pressure and blood sugar levels under control, thereby reducing her risk of vascular disease.
She and her daughter were directed to the Alzheimer’s Association Web site (alz.org) as a resource for information about signs and symptoms to watch for and for caregiving resources, should they be needed. She was briefly counseled to eliminate distractions to improve her ability to complete tasks and improve recall along with rehearsing or writing down information that she wished to retain.
Finally, she was counseled to remain physically, cognitively, and socially active as these are factors generally associated with healthy aging, have some evidence to support efficacy in reducing the risk of cognitive decline,44,45 and are unlikely to be of harm.
*The Montreal Cognitive Assessment is a validated office-based tool for the evaluation of cognitive impairment that is highly sensitive for the detection of mild cognitive impairment.
CORRESPONDENCE
Ian M. Deutchki, MD, Professor of Family Medicine and Geriatrics, University of Rochester Medical Center, 777 S. Clinton Avenue, Rochester, NY 14620; Ian_Deutchki@URMC.Rochester.edu.
1. Mitchell AJ. The clinical significance of subjective memory complaints in the diagnosis of mild cognitive impairment and dementia: a meta-analysis. Int J Geriatr Psychiatry. 2008;23:1191-1202.
2. Burnette V, Howell T. Cognitive changes in aging. In: Capezuti EA, Malone ML, Katz PR, et al, eds. The Encyclopedia of Elder Care. New York, NY, USA: Springer Publishing Company; 2013.
3. Glisky EL. Changes in cognitive function in human aging. In: Riddle DR, ed. Brain Aging: Models, Methods, and Mechanisms. Boca Raton, FL: Taylor & Francis Group, LLC; 2007:4-20.
4. Craft S, Cholerton B, Reger M. Cognitive changes associated with normal and pathological aging. In: Halter JB, Ouslander JG, Tinetti ME, Studenski S, et al, eds. Hazzard’s Geriatric Medicine and Gerontology. 6th ed. New York, NY: McGraw-Hill; 2009:751-766.
5. Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303-308.
6. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183-194.
7. Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240-246.
8. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270-279.
9. Neurocognitive disorders. In: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
10. Ward A. Arrighi HM, Michels S, et al. Mild cognitive impairment: disparity of incidence and prevalence estimates. Alzheimers Dement. 2012;8:14-21.
11. Petersen RC, Roberts RO, Knopman DS, et al. Mild cognitive impairment: ten years later. Arch Neurol. 2009;66:1447-1455.
12. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263-269.
13. Jorm AF. A short form of the informant questionnaire on cognitive decline in the elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24:145-153.
14. Katz S, Downs TD, Cash HR, et al. Progress in development of the index of ADL. Gerontologist. 1970;10:20-30.
15. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179-186.
16. Kiloh LG. Pseudo-dementia. Acta Psychiatr Scand. 1961;37:336-351.
17. Beekman AT, Copeland JR, Prince MJ. Review of community prevalence of depression in later life. Br J Psychiatry. 1999;174:307-311.
18. Birrer RB, Vemuri SP. Depression in later life: a diagnostic and therapeutic challenge. Am Fam Physician. 2004;69:2375-2382.
19. Butters MA, Becker JT, Nebes RD, et al. Changes in cognitive functioning following treatment of late-life depression. Am J Psychiatry. 2000;157:1949-1954.
20. Alexopoulos GS, Meyers BS, Young RC, et al. The course of geriatric depression with “reversible dementia”: a controlled study. Am J Psychiatry. 1993;150:1693-1699.
21. Saez-Fonseca JA, Lee L, Walker Z. Long-term outcome of depressive pseudodementia in the elderly. J Affect Disord. 2007;101:123-129.
22. Dillon C, Allegri RF, Serrano CM, et al. Late- versus early-onset geriatric depression in a memory research center. Neuropsychiatr Dis Treat. 2009;5:517-526.
23. Lockwood KA, Alexopoulos GS, van Gorp WG. Executive dysfunction in geriatric depression. Am J Psychiatry. 2002;159:1119-1126.
24. Shimada H, Park H, Makizako H, et al. Depressive symptoms and cognitive performance in older adults. J Psychiatr Res. 2014;57:149-156.
25. Butters MA, Whyte EM, Nebes RD, et al. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry. 2004;61:587-595.
26. Dillon C, Machnicki G, Serrano CM, et al. Clinical manifestations of geriatric depression in a memory clinic: toward a proposed subtyping of geriatric depression. J Affect Disord. 2011;134:177-187.
27. Rapp MA, Dahlman K, Sano M, et al. Neuropsychological differences between late-onset and recurrent geriatric major depression. Am J Psychiatry. 2005;162:691-698.
28. Zihl J, Reppermund S, Thum S, et al. Neuropsychological profiles in MCI and in depression: differential cognitive dysfunction patterns or similar final common pathway disorder? J Psychiatr Res. 2010;44:647-654.
29. Dillon C, Tartaglini MF, Stefani D, et al. Geriatric depression and its relation with cognitive impairment and dementia. Arch Gerontol Geriatr. 2014;59:450-456.
30. Wright SL, Persad C. Distinguishing between depression and dementia in older persons: neuropsychological and neuropathological correlates. J Geriatr Psychiatry Neurol. 2007;20:189-198.
31. Visser PJ, Verhey FR, Ponds RW, et al. Distinction between preclinical Alzheimer’s disease and depression. J Am Geriatr Soc. 2000;48:479-484.
32. Bodner T, Delazer M, Kemmler G, et al. Clock drawing, clock reading, clock setting, and judgment of clock faces in elderly people with dementia and depression. J Am Geriatr Soc. 2004;52:1146-1150.
33. Clarfield AM. The reversible dementias: do they reverse? Ann Intern Med. 1988;109:476-486.
34. Clarfield AM. The decreasing prevalence of reversible dementias: an updated meta-analysis. Arch Intern Med. 2003;163:2219-2229.
35. Malouf R, Areosa Sastre A. Vitamin B12 for cognition. Cochrane Database Syst Rev. 2003;(3):CD004326.
36. Health Quality Ontario. Vitamin B12 and cognitive function: an evidence-based analysis. Ont Health Technol Assess Ser. 2013;13:1-45.
37. Abyad A. Prevalence of vitamin B12 deficiency among demented patients and cognitive recovery with cobalamin replacement. J Nutr Health Aging. 2002;6:254-260.
38. Martin DC, Francis J, Protetch J, et al. Time dependency of cognitive recovery with cobalamin replacement: Report of a pilot study. J Am Geriatr Soc. 1992;40:168-172.
39. Clarnette RM, Patterson CJ. Hypothyroidism: does treatment cure dementia? J Geriatr Psychiatry Neurol. 1994;7:23-27.
40. Dugbartey AT. Neurocognitive aspects of hypothyroidism. Arch Intern Med. 1998;158:1413-1418.
41. Alexander EM, Wagner EH, Buchner DM, et al. Do surgical brain lesions present as isolated dementia? A population-based study. J Am Geriatr Soc. 1995;43:138-143.
42. Moore AR, O’Keeffe ST. Drug-induced cognitive impairment in the elderly. Drugs Aging. 1999;15:15-28.
43. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American geriatrics society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
44. Middleton LE, Yaffe K. Promising strategies for the prevention of dementia. Arch Neurol. 2009;66:1210-1215.
45. Shatenstein B, Barberger-Gateau P, Mecocci P. Prevention of age-related cognitive decline: which strategies, when, and for whom? J Alzheimers Dis. 2015;48:35-53.
A 68-year-old woman with a history of well-controlled hypertension and diabetes presents to the office for routine follow-up. She says she has adhered to her current medications, and her blood pressure and hemoglobin A1c remain at goal. At the close of the visit, she mentions that she is worried she may be developing dementia. She says she has been having difficulty finding the right word in conversation and needs to write things down more than she used to.
What might be causing this patient’s changes in cognition?
In primary care settings, when patients complain of memory loss, there is a 20% to 30% chance they will be found to have mild cognitive impairment (MCI) or some level of dementia.1 Given the significant consequences of dementia, it’s important to maximize opportunities to distinguish those with age-related changes in cognition or reversible causes of memory loss from those who have irreversible pathologic changes.
Age-related changes in cognition
Changes in cognition associated with aging vary considerably among individuals and across domains of cognition. By their 7th decade, most people experience a decline in processing speed and working memory.2 However, some individuals retain excellent function into their 80s and perform as well as younger adults.3
Changes long thought to be due to brain senescence may, in fact, be related to the effects of age-related medical conditions on the brain’s function.4 Consistent with this theory is the observation that cognitive changes tend to occur earlier in individuals with cardiovascular disease, diabetes, and cancer.2 What constitutes a normal change depends on an individual’s baseline cognitive function, educational background, medical comorbidities, and the potential impact of sensory impairment on performance.
General cognitive trends with aging. Awareness of normal changes in an aging population is useful when assessing patients concerned about their memory. In general, an individual’s ability to maintain attention to a single task is preserved into late life. Ability to perform tasks requiring divided attention or attention-switching tends to decline.3 This has implications for driving, given the need to constantly switch one’s attention in response to the environment and the ability to sort relevant from irrelevant information.
Remote memory, semantic memory (factual information), and procedural memory (knowledge of skills and procedures) tend to remain intact with aging.4 Short-term memory (simple maintenance of information over a short period of time) shows little change with aging. However, working memory, which requires the manipulation of information in short-term memory, declines.
A simple demonstration of this is that performance on digit span testing tends to remain preserved (7±2), but digit span backwards declines. Holding digits in mind in the order they are received can be achieved through rehearsal. But to reverse the order requires reorganization of the information, and this ability declines with aging.3
Prospective memory (remembering to do things in the future) often requires increased dependence on external aids, such as a to-do list.3 The capacity to learn and recall new information declines. Even when given repeated opportunity to practice, older adults demonstrate a slower learning curve and lower total amount learned.4 Therefore, it becomes easier relying on well-learned cognitive processes such as cooking a familiar meal or relying on previously used principles for decision making.2
Language comprehension and vocabulary remain stable over time. However, difficulty with spontaneous word finding—the “tip-of-the-tongue” phenomenon—tends to increase. In contrast to the dysnomia related to dementia, the word-finding difficulties with normal aging typically improve with cues, indicating that the problem is in retrieval of information rather than storage. Verbal fluency, the rate at which words from a single category can be produced, shows decline. This is particularly true in tests of semantic verbal fluency (name all the animals you can think of); phonemic fluency (words that start with a certain letter) tends to be preserved.4
Some studies using neurocognitive testing have suggested a decline in executive functioning. But, in general, aging has little impact on “real world” executive functions that are required for planning and executing tasks.4 On the whole, cognitive changes related to aging typically do not interfere with an individual’s ability to function independently.
Mild cognitive impairment/mild neurocognitive disorder
Originally conceived as a precursor to Alzheimer’s dementia,5 mild cognitive impairment (MCI) is a diagnosis that has evolved to describe a heterogeneous syndrome of abnormal cognition characterized by:6-8
- a suspected change in cognition expressed by the patient, an acquaintance who knows the patient well, or a clinician;
- objectively measured impairment in one or more cognitive domains beyond what would be expected based on an individual’s age and educational background;
- preservation of functional abilities; and
- a lack of findings that would fulfill criteria for dementia.
In the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM V), this concept is identified as mild neurocognitive disorder, with the additional caveats that an individual’s cognitive deficits do not occur exclusively in the context of delirium and are not better explained by another mental disorder such as depression or schizophrenia.9
An accurate assessment of cognitive change is best measured against the individual’s baseline, which may necessitate the report of a reliable acquaintance. An assessment of functional abilities is also critical. Mild problems in performing complex functions (bill paying, shopping, etc) could be present and still allow a patient to meet the criteria for MCI. An individual may take more time, be less efficient, or make more errors than before; however, independence with minimal aid or assistance is preserved. It
MCI can be divided into 4 subtypes depending upon the cognitive domains affected (complex attention, executive function, learning and memory, language, visuospatial, social cognition):
- Amnestic MCI single domain, if only memory is affected.
- Amnestic MCI multiple domain, if memory and any other cognitive domains are affected.
- Non-amnestic MCI single domain, if any other cognitive domain aside from memory is the only one affected.
- Non-amnestic MCI multiple domain, if multiple domains other than memory are affected.
These distinctions may provide clues to the underlying cause of dysfunction and provide prognostic information regarding the risk of progression to dementia.6,7
Prevalence estimates for MCI vary widely due to differences in definitions used and populations studied. The best estimate is 5% to 10% prevalence among those ages 65 to 69 years old, and 12% to 25% among those ages 80 to 84.10 Similarly, estimates of the rate of progression to dementia vary. Among MCI populations identified through referral sources such as memory centers, the rate of progression to dementia has been 10% to 15% per year.11 In epidemiologic studies of general populations, the rate has been 6% to 10% per year.11 The rate of development of dementia among normal subjects is 1% to 2% per year.5
Dementia/major neurocognitive disorder
The primary feature distinguishing MCI/mild neurocognitive disorder from dementia or major neurocognitive disorder is a patient’s functional status. The core clinical criteria for all-cause dementia are cognitive or neurobehavioral symptoms that: 12
- interfere with work or usual daily function,
- represent a change from the prior baseline function,
- are not explained by delirium or a psychiatric illness, and
- include detectable impairment in 2 cognitive domains.
Criteria outlined in the DSM-V for major neurocognitive disorder are essentially the same but describe the functional change criteria as cognitive changes that “interfere with independence in everyday activities.”9 The DSM-V elaborates: “at a minimum, requiring assistance with complex instrumental activities of daily living such as paying bills or managing medications.”
Assessing functional status accurately in clinical practice typically requires the assistance of a collateral informant who knows the patient well. The Informant Questionnaire on Cognitive Decline in the Elderly (https://www.alz.org/documents_custom/shortiqcode_english.pdf) is one validated assessment tool that can be used for this purpose.13 With this self-administered form, the informant answers 16 questions regarding changes in the patient’s performance of different activities over the 10 years prior. Alternatively, a structured interview based on indices of activities of daily living (ADLs) and instrumental activities of daily living (IADLs) as listed in TABLE 1 can be employed.14,15
Review of the various causes of dementia is beyond the scope of this article, but a list of common diagnoses is presented in TABLE 2.
Dementia syndrome of depression (pseudodementia)
Elderly patients with depression commonly complain of memory impairment, and this interaction between depression and dementia has been investigated for decades. The term “pseudodementia” has been used since 1961 to describe signs of dementia in a patient with any psychiatric illness,16 but it has since been refined to apply solely to depression. The prevalence of depression among older adults varies depending on the population studied and how depression is defined. Approximately 2% to 3% of community-dwelling elders meet criteria for major depression, with 10% to 30% showing some symptoms of depression.17,18
Twenty percent to 40% of elderly patients diagnosed with depression will have evidence of cognitive impairment.19-21 Most improve with antidepressive treatment, though evidence of cognitive impairment may continue for some.19
A broad range of cognitive deficits have been associated with depression. Most consistently described are deficits in processing speed,22-25 attention,26-28 and executive function.22,25-29 Memory deficits can be apparent with tests of delayed recall, but recognition (the ability to identify items from a list) generally is preserved.26,28-30
Distinguishing between pseudodementia and true dementia can be challenging. An increased severity of deficits, particularly with delayed recall, is more indicative of dementia.31 Additionally, on clock drawing tasks, individuals with depression perform more comparably to controls than do those with true dementia.32
A 2013 meta-analysis reported a significant association of late-life depression with subsequent development of dementia, with an odds ratio (OR) of 1.85. The risk of subsequently developing vascular dementia (OR=2.52) was significantly higher than that for Alzheimer’s disease (OR=1.65). Individuals with evidence of reversible cognitive impairment at the time of diagnosis of depression seem to be particularly vulnerable, with dementia developing in 43% to 71%, compared with rates of 12% to 18% among elders diagnosed with depression but lacking signs of cognitive impairment.20,21
Other causes of reversible dementia
A meta-analysis performed in 1988 found that 11% of cases of dementia were reversible.33 However, an update using the same methodology in 2003 revealed the number had dropped to less than 1%.34 In the latest meta-analysis, one of the authors’ leading hypotheses for the dramatic decline in apparent prevalence was a significant shift in the study population from the inpatient to outpatient setting. In studies of community-based populations used in the re-analysis, the reported prevalence of reversibility was near zero.34
Metabolic abnormalities—most often B12 deficiency and hypothyroidism—are commonly cited as potential causes of dementia. Four systematic reviews, including one conducted by the Cochrane Collaborative, concluded there is a lack of evidence that treating low vitamin B12 in individuals with dementia improves cognition.35,36 There is some evidence, though, of a time-limited window for successful treatment within 12 months of the onset of symptoms.37,38 A study reviewing causes of dementia in nearly 3000 individuals found one case of reversible dementia attributable to hypothyroidism.39 A subsequent review reached similar conclusions about the lack of data to support the notion that treatment of hypothyroidism reverses dementia.40
Similarly, imaging for cerebral tumors, subdural hematomas, or normal-pressure hydrocephalus rarely identifies these as a cause of dementia.41 This is particularly true of unselected community-based populations, as there are typically signs or symptoms suggesting an intracranial pathology.
Numerous medications have been implicated in causing acute confusional states, and there is some evidence for their role in chronic confusion (TABLE 3).42,43 In my experience, many who experience adverse effects on cognition with medications will also have an underlying neurodegenerative process, and symptoms do not completely resolve with withdrawal of the offending agent.
Further assessment of the patient yielded a score of 29/30 on the Montreal Cognitive Assessment* and a zero on the Patient Health Questionnaire-2. Careful review of her daily function revealed no significant deficits in ADL or IADL performance, and her daughter confirmed that she had not observed any significant decline in her mother’s function. There was no significant family history of dementia. The patient was reassured that her cognitive changes were normal and age related.
Unfortunately, few data support specific interventions to reduce this patient’s risk of developing dementia. She was commended for keeping her blood pressure and blood sugar levels under control, thereby reducing her risk of vascular disease.
She and her daughter were directed to the Alzheimer’s Association Web site (alz.org) as a resource for information about signs and symptoms to watch for and for caregiving resources, should they be needed. She was briefly counseled to eliminate distractions to improve her ability to complete tasks and improve recall along with rehearsing or writing down information that she wished to retain.
Finally, she was counseled to remain physically, cognitively, and socially active as these are factors generally associated with healthy aging, have some evidence to support efficacy in reducing the risk of cognitive decline,44,45 and are unlikely to be of harm.
*The Montreal Cognitive Assessment is a validated office-based tool for the evaluation of cognitive impairment that is highly sensitive for the detection of mild cognitive impairment.
CORRESPONDENCE
Ian M. Deutchki, MD, Professor of Family Medicine and Geriatrics, University of Rochester Medical Center, 777 S. Clinton Avenue, Rochester, NY 14620; Ian_Deutchki@URMC.Rochester.edu.
A 68-year-old woman with a history of well-controlled hypertension and diabetes presents to the office for routine follow-up. She says she has adhered to her current medications, and her blood pressure and hemoglobin A1c remain at goal. At the close of the visit, she mentions that she is worried she may be developing dementia. She says she has been having difficulty finding the right word in conversation and needs to write things down more than she used to.
What might be causing this patient’s changes in cognition?
In primary care settings, when patients complain of memory loss, there is a 20% to 30% chance they will be found to have mild cognitive impairment (MCI) or some level of dementia.1 Given the significant consequences of dementia, it’s important to maximize opportunities to distinguish those with age-related changes in cognition or reversible causes of memory loss from those who have irreversible pathologic changes.
Age-related changes in cognition
Changes in cognition associated with aging vary considerably among individuals and across domains of cognition. By their 7th decade, most people experience a decline in processing speed and working memory.2 However, some individuals retain excellent function into their 80s and perform as well as younger adults.3
Changes long thought to be due to brain senescence may, in fact, be related to the effects of age-related medical conditions on the brain’s function.4 Consistent with this theory is the observation that cognitive changes tend to occur earlier in individuals with cardiovascular disease, diabetes, and cancer.2 What constitutes a normal change depends on an individual’s baseline cognitive function, educational background, medical comorbidities, and the potential impact of sensory impairment on performance.
General cognitive trends with aging. Awareness of normal changes in an aging population is useful when assessing patients concerned about their memory. In general, an individual’s ability to maintain attention to a single task is preserved into late life. Ability to perform tasks requiring divided attention or attention-switching tends to decline.3 This has implications for driving, given the need to constantly switch one’s attention in response to the environment and the ability to sort relevant from irrelevant information.
Remote memory, semantic memory (factual information), and procedural memory (knowledge of skills and procedures) tend to remain intact with aging.4 Short-term memory (simple maintenance of information over a short period of time) shows little change with aging. However, working memory, which requires the manipulation of information in short-term memory, declines.
A simple demonstration of this is that performance on digit span testing tends to remain preserved (7±2), but digit span backwards declines. Holding digits in mind in the order they are received can be achieved through rehearsal. But to reverse the order requires reorganization of the information, and this ability declines with aging.3
Prospective memory (remembering to do things in the future) often requires increased dependence on external aids, such as a to-do list.3 The capacity to learn and recall new information declines. Even when given repeated opportunity to practice, older adults demonstrate a slower learning curve and lower total amount learned.4 Therefore, it becomes easier relying on well-learned cognitive processes such as cooking a familiar meal or relying on previously used principles for decision making.2
Language comprehension and vocabulary remain stable over time. However, difficulty with spontaneous word finding—the “tip-of-the-tongue” phenomenon—tends to increase. In contrast to the dysnomia related to dementia, the word-finding difficulties with normal aging typically improve with cues, indicating that the problem is in retrieval of information rather than storage. Verbal fluency, the rate at which words from a single category can be produced, shows decline. This is particularly true in tests of semantic verbal fluency (name all the animals you can think of); phonemic fluency (words that start with a certain letter) tends to be preserved.4
Some studies using neurocognitive testing have suggested a decline in executive functioning. But, in general, aging has little impact on “real world” executive functions that are required for planning and executing tasks.4 On the whole, cognitive changes related to aging typically do not interfere with an individual’s ability to function independently.
Mild cognitive impairment/mild neurocognitive disorder
Originally conceived as a precursor to Alzheimer’s dementia,5 mild cognitive impairment (MCI) is a diagnosis that has evolved to describe a heterogeneous syndrome of abnormal cognition characterized by:6-8
- a suspected change in cognition expressed by the patient, an acquaintance who knows the patient well, or a clinician;
- objectively measured impairment in one or more cognitive domains beyond what would be expected based on an individual’s age and educational background;
- preservation of functional abilities; and
- a lack of findings that would fulfill criteria for dementia.
In the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM V), this concept is identified as mild neurocognitive disorder, with the additional caveats that an individual’s cognitive deficits do not occur exclusively in the context of delirium and are not better explained by another mental disorder such as depression or schizophrenia.9
An accurate assessment of cognitive change is best measured against the individual’s baseline, which may necessitate the report of a reliable acquaintance. An assessment of functional abilities is also critical. Mild problems in performing complex functions (bill paying, shopping, etc) could be present and still allow a patient to meet the criteria for MCI. An individual may take more time, be less efficient, or make more errors than before; however, independence with minimal aid or assistance is preserved. It
MCI can be divided into 4 subtypes depending upon the cognitive domains affected (complex attention, executive function, learning and memory, language, visuospatial, social cognition):
- Amnestic MCI single domain, if only memory is affected.
- Amnestic MCI multiple domain, if memory and any other cognitive domains are affected.
- Non-amnestic MCI single domain, if any other cognitive domain aside from memory is the only one affected.
- Non-amnestic MCI multiple domain, if multiple domains other than memory are affected.
These distinctions may provide clues to the underlying cause of dysfunction and provide prognostic information regarding the risk of progression to dementia.6,7
Prevalence estimates for MCI vary widely due to differences in definitions used and populations studied. The best estimate is 5% to 10% prevalence among those ages 65 to 69 years old, and 12% to 25% among those ages 80 to 84.10 Similarly, estimates of the rate of progression to dementia vary. Among MCI populations identified through referral sources such as memory centers, the rate of progression to dementia has been 10% to 15% per year.11 In epidemiologic studies of general populations, the rate has been 6% to 10% per year.11 The rate of development of dementia among normal subjects is 1% to 2% per year.5
Dementia/major neurocognitive disorder
The primary feature distinguishing MCI/mild neurocognitive disorder from dementia or major neurocognitive disorder is a patient’s functional status. The core clinical criteria for all-cause dementia are cognitive or neurobehavioral symptoms that: 12
- interfere with work or usual daily function,
- represent a change from the prior baseline function,
- are not explained by delirium or a psychiatric illness, and
- include detectable impairment in 2 cognitive domains.
Criteria outlined in the DSM-V for major neurocognitive disorder are essentially the same but describe the functional change criteria as cognitive changes that “interfere with independence in everyday activities.”9 The DSM-V elaborates: “at a minimum, requiring assistance with complex instrumental activities of daily living such as paying bills or managing medications.”
Assessing functional status accurately in clinical practice typically requires the assistance of a collateral informant who knows the patient well. The Informant Questionnaire on Cognitive Decline in the Elderly (https://www.alz.org/documents_custom/shortiqcode_english.pdf) is one validated assessment tool that can be used for this purpose.13 With this self-administered form, the informant answers 16 questions regarding changes in the patient’s performance of different activities over the 10 years prior. Alternatively, a structured interview based on indices of activities of daily living (ADLs) and instrumental activities of daily living (IADLs) as listed in TABLE 1 can be employed.14,15
Review of the various causes of dementia is beyond the scope of this article, but a list of common diagnoses is presented in TABLE 2.
Dementia syndrome of depression (pseudodementia)
Elderly patients with depression commonly complain of memory impairment, and this interaction between depression and dementia has been investigated for decades. The term “pseudodementia” has been used since 1961 to describe signs of dementia in a patient with any psychiatric illness,16 but it has since been refined to apply solely to depression. The prevalence of depression among older adults varies depending on the population studied and how depression is defined. Approximately 2% to 3% of community-dwelling elders meet criteria for major depression, with 10% to 30% showing some symptoms of depression.17,18
Twenty percent to 40% of elderly patients diagnosed with depression will have evidence of cognitive impairment.19-21 Most improve with antidepressive treatment, though evidence of cognitive impairment may continue for some.19
A broad range of cognitive deficits have been associated with depression. Most consistently described are deficits in processing speed,22-25 attention,26-28 and executive function.22,25-29 Memory deficits can be apparent with tests of delayed recall, but recognition (the ability to identify items from a list) generally is preserved.26,28-30
Distinguishing between pseudodementia and true dementia can be challenging. An increased severity of deficits, particularly with delayed recall, is more indicative of dementia.31 Additionally, on clock drawing tasks, individuals with depression perform more comparably to controls than do those with true dementia.32
A 2013 meta-analysis reported a significant association of late-life depression with subsequent development of dementia, with an odds ratio (OR) of 1.85. The risk of subsequently developing vascular dementia (OR=2.52) was significantly higher than that for Alzheimer’s disease (OR=1.65). Individuals with evidence of reversible cognitive impairment at the time of diagnosis of depression seem to be particularly vulnerable, with dementia developing in 43% to 71%, compared with rates of 12% to 18% among elders diagnosed with depression but lacking signs of cognitive impairment.20,21
Other causes of reversible dementia
A meta-analysis performed in 1988 found that 11% of cases of dementia were reversible.33 However, an update using the same methodology in 2003 revealed the number had dropped to less than 1%.34 In the latest meta-analysis, one of the authors’ leading hypotheses for the dramatic decline in apparent prevalence was a significant shift in the study population from the inpatient to outpatient setting. In studies of community-based populations used in the re-analysis, the reported prevalence of reversibility was near zero.34
Metabolic abnormalities—most often B12 deficiency and hypothyroidism—are commonly cited as potential causes of dementia. Four systematic reviews, including one conducted by the Cochrane Collaborative, concluded there is a lack of evidence that treating low vitamin B12 in individuals with dementia improves cognition.35,36 There is some evidence, though, of a time-limited window for successful treatment within 12 months of the onset of symptoms.37,38 A study reviewing causes of dementia in nearly 3000 individuals found one case of reversible dementia attributable to hypothyroidism.39 A subsequent review reached similar conclusions about the lack of data to support the notion that treatment of hypothyroidism reverses dementia.40
Similarly, imaging for cerebral tumors, subdural hematomas, or normal-pressure hydrocephalus rarely identifies these as a cause of dementia.41 This is particularly true of unselected community-based populations, as there are typically signs or symptoms suggesting an intracranial pathology.
Numerous medications have been implicated in causing acute confusional states, and there is some evidence for their role in chronic confusion (TABLE 3).42,43 In my experience, many who experience adverse effects on cognition with medications will also have an underlying neurodegenerative process, and symptoms do not completely resolve with withdrawal of the offending agent.
Further assessment of the patient yielded a score of 29/30 on the Montreal Cognitive Assessment* and a zero on the Patient Health Questionnaire-2. Careful review of her daily function revealed no significant deficits in ADL or IADL performance, and her daughter confirmed that she had not observed any significant decline in her mother’s function. There was no significant family history of dementia. The patient was reassured that her cognitive changes were normal and age related.
Unfortunately, few data support specific interventions to reduce this patient’s risk of developing dementia. She was commended for keeping her blood pressure and blood sugar levels under control, thereby reducing her risk of vascular disease.
She and her daughter were directed to the Alzheimer’s Association Web site (alz.org) as a resource for information about signs and symptoms to watch for and for caregiving resources, should they be needed. She was briefly counseled to eliminate distractions to improve her ability to complete tasks and improve recall along with rehearsing or writing down information that she wished to retain.
Finally, she was counseled to remain physically, cognitively, and socially active as these are factors generally associated with healthy aging, have some evidence to support efficacy in reducing the risk of cognitive decline,44,45 and are unlikely to be of harm.
*The Montreal Cognitive Assessment is a validated office-based tool for the evaluation of cognitive impairment that is highly sensitive for the detection of mild cognitive impairment.
CORRESPONDENCE
Ian M. Deutchki, MD, Professor of Family Medicine and Geriatrics, University of Rochester Medical Center, 777 S. Clinton Avenue, Rochester, NY 14620; Ian_Deutchki@URMC.Rochester.edu.
1. Mitchell AJ. The clinical significance of subjective memory complaints in the diagnosis of mild cognitive impairment and dementia: a meta-analysis. Int J Geriatr Psychiatry. 2008;23:1191-1202.
2. Burnette V, Howell T. Cognitive changes in aging. In: Capezuti EA, Malone ML, Katz PR, et al, eds. The Encyclopedia of Elder Care. New York, NY, USA: Springer Publishing Company; 2013.
3. Glisky EL. Changes in cognitive function in human aging. In: Riddle DR, ed. Brain Aging: Models, Methods, and Mechanisms. Boca Raton, FL: Taylor & Francis Group, LLC; 2007:4-20.
4. Craft S, Cholerton B, Reger M. Cognitive changes associated with normal and pathological aging. In: Halter JB, Ouslander JG, Tinetti ME, Studenski S, et al, eds. Hazzard’s Geriatric Medicine and Gerontology. 6th ed. New York, NY: McGraw-Hill; 2009:751-766.
5. Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303-308.
6. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183-194.
7. Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240-246.
8. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270-279.
9. Neurocognitive disorders. In: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
10. Ward A. Arrighi HM, Michels S, et al. Mild cognitive impairment: disparity of incidence and prevalence estimates. Alzheimers Dement. 2012;8:14-21.
11. Petersen RC, Roberts RO, Knopman DS, et al. Mild cognitive impairment: ten years later. Arch Neurol. 2009;66:1447-1455.
12. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263-269.
13. Jorm AF. A short form of the informant questionnaire on cognitive decline in the elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24:145-153.
14. Katz S, Downs TD, Cash HR, et al. Progress in development of the index of ADL. Gerontologist. 1970;10:20-30.
15. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179-186.
16. Kiloh LG. Pseudo-dementia. Acta Psychiatr Scand. 1961;37:336-351.
17. Beekman AT, Copeland JR, Prince MJ. Review of community prevalence of depression in later life. Br J Psychiatry. 1999;174:307-311.
18. Birrer RB, Vemuri SP. Depression in later life: a diagnostic and therapeutic challenge. Am Fam Physician. 2004;69:2375-2382.
19. Butters MA, Becker JT, Nebes RD, et al. Changes in cognitive functioning following treatment of late-life depression. Am J Psychiatry. 2000;157:1949-1954.
20. Alexopoulos GS, Meyers BS, Young RC, et al. The course of geriatric depression with “reversible dementia”: a controlled study. Am J Psychiatry. 1993;150:1693-1699.
21. Saez-Fonseca JA, Lee L, Walker Z. Long-term outcome of depressive pseudodementia in the elderly. J Affect Disord. 2007;101:123-129.
22. Dillon C, Allegri RF, Serrano CM, et al. Late- versus early-onset geriatric depression in a memory research center. Neuropsychiatr Dis Treat. 2009;5:517-526.
23. Lockwood KA, Alexopoulos GS, van Gorp WG. Executive dysfunction in geriatric depression. Am J Psychiatry. 2002;159:1119-1126.
24. Shimada H, Park H, Makizako H, et al. Depressive symptoms and cognitive performance in older adults. J Psychiatr Res. 2014;57:149-156.
25. Butters MA, Whyte EM, Nebes RD, et al. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry. 2004;61:587-595.
26. Dillon C, Machnicki G, Serrano CM, et al. Clinical manifestations of geriatric depression in a memory clinic: toward a proposed subtyping of geriatric depression. J Affect Disord. 2011;134:177-187.
27. Rapp MA, Dahlman K, Sano M, et al. Neuropsychological differences between late-onset and recurrent geriatric major depression. Am J Psychiatry. 2005;162:691-698.
28. Zihl J, Reppermund S, Thum S, et al. Neuropsychological profiles in MCI and in depression: differential cognitive dysfunction patterns or similar final common pathway disorder? J Psychiatr Res. 2010;44:647-654.
29. Dillon C, Tartaglini MF, Stefani D, et al. Geriatric depression and its relation with cognitive impairment and dementia. Arch Gerontol Geriatr. 2014;59:450-456.
30. Wright SL, Persad C. Distinguishing between depression and dementia in older persons: neuropsychological and neuropathological correlates. J Geriatr Psychiatry Neurol. 2007;20:189-198.
31. Visser PJ, Verhey FR, Ponds RW, et al. Distinction between preclinical Alzheimer’s disease and depression. J Am Geriatr Soc. 2000;48:479-484.
32. Bodner T, Delazer M, Kemmler G, et al. Clock drawing, clock reading, clock setting, and judgment of clock faces in elderly people with dementia and depression. J Am Geriatr Soc. 2004;52:1146-1150.
33. Clarfield AM. The reversible dementias: do they reverse? Ann Intern Med. 1988;109:476-486.
34. Clarfield AM. The decreasing prevalence of reversible dementias: an updated meta-analysis. Arch Intern Med. 2003;163:2219-2229.
35. Malouf R, Areosa Sastre A. Vitamin B12 for cognition. Cochrane Database Syst Rev. 2003;(3):CD004326.
36. Health Quality Ontario. Vitamin B12 and cognitive function: an evidence-based analysis. Ont Health Technol Assess Ser. 2013;13:1-45.
37. Abyad A. Prevalence of vitamin B12 deficiency among demented patients and cognitive recovery with cobalamin replacement. J Nutr Health Aging. 2002;6:254-260.
38. Martin DC, Francis J, Protetch J, et al. Time dependency of cognitive recovery with cobalamin replacement: Report of a pilot study. J Am Geriatr Soc. 1992;40:168-172.
39. Clarnette RM, Patterson CJ. Hypothyroidism: does treatment cure dementia? J Geriatr Psychiatry Neurol. 1994;7:23-27.
40. Dugbartey AT. Neurocognitive aspects of hypothyroidism. Arch Intern Med. 1998;158:1413-1418.
41. Alexander EM, Wagner EH, Buchner DM, et al. Do surgical brain lesions present as isolated dementia? A population-based study. J Am Geriatr Soc. 1995;43:138-143.
42. Moore AR, O’Keeffe ST. Drug-induced cognitive impairment in the elderly. Drugs Aging. 1999;15:15-28.
43. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American geriatrics society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
44. Middleton LE, Yaffe K. Promising strategies for the prevention of dementia. Arch Neurol. 2009;66:1210-1215.
45. Shatenstein B, Barberger-Gateau P, Mecocci P. Prevention of age-related cognitive decline: which strategies, when, and for whom? J Alzheimers Dis. 2015;48:35-53.
1. Mitchell AJ. The clinical significance of subjective memory complaints in the diagnosis of mild cognitive impairment and dementia: a meta-analysis. Int J Geriatr Psychiatry. 2008;23:1191-1202.
2. Burnette V, Howell T. Cognitive changes in aging. In: Capezuti EA, Malone ML, Katz PR, et al, eds. The Encyclopedia of Elder Care. New York, NY, USA: Springer Publishing Company; 2013.
3. Glisky EL. Changes in cognitive function in human aging. In: Riddle DR, ed. Brain Aging: Models, Methods, and Mechanisms. Boca Raton, FL: Taylor & Francis Group, LLC; 2007:4-20.
4. Craft S, Cholerton B, Reger M. Cognitive changes associated with normal and pathological aging. In: Halter JB, Ouslander JG, Tinetti ME, Studenski S, et al, eds. Hazzard’s Geriatric Medicine and Gerontology. 6th ed. New York, NY: McGraw-Hill; 2009:751-766.
5. Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303-308.
6. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183-194.
7. Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240-246.
8. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270-279.
9. Neurocognitive disorders. In: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
10. Ward A. Arrighi HM, Michels S, et al. Mild cognitive impairment: disparity of incidence and prevalence estimates. Alzheimers Dement. 2012;8:14-21.
11. Petersen RC, Roberts RO, Knopman DS, et al. Mild cognitive impairment: ten years later. Arch Neurol. 2009;66:1447-1455.
12. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263-269.
13. Jorm AF. A short form of the informant questionnaire on cognitive decline in the elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24:145-153.
14. Katz S, Downs TD, Cash HR, et al. Progress in development of the index of ADL. Gerontologist. 1970;10:20-30.
15. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179-186.
16. Kiloh LG. Pseudo-dementia. Acta Psychiatr Scand. 1961;37:336-351.
17. Beekman AT, Copeland JR, Prince MJ. Review of community prevalence of depression in later life. Br J Psychiatry. 1999;174:307-311.
18. Birrer RB, Vemuri SP. Depression in later life: a diagnostic and therapeutic challenge. Am Fam Physician. 2004;69:2375-2382.
19. Butters MA, Becker JT, Nebes RD, et al. Changes in cognitive functioning following treatment of late-life depression. Am J Psychiatry. 2000;157:1949-1954.
20. Alexopoulos GS, Meyers BS, Young RC, et al. The course of geriatric depression with “reversible dementia”: a controlled study. Am J Psychiatry. 1993;150:1693-1699.
21. Saez-Fonseca JA, Lee L, Walker Z. Long-term outcome of depressive pseudodementia in the elderly. J Affect Disord. 2007;101:123-129.
22. Dillon C, Allegri RF, Serrano CM, et al. Late- versus early-onset geriatric depression in a memory research center. Neuropsychiatr Dis Treat. 2009;5:517-526.
23. Lockwood KA, Alexopoulos GS, van Gorp WG. Executive dysfunction in geriatric depression. Am J Psychiatry. 2002;159:1119-1126.
24. Shimada H, Park H, Makizako H, et al. Depressive symptoms and cognitive performance in older adults. J Psychiatr Res. 2014;57:149-156.
25. Butters MA, Whyte EM, Nebes RD, et al. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry. 2004;61:587-595.
26. Dillon C, Machnicki G, Serrano CM, et al. Clinical manifestations of geriatric depression in a memory clinic: toward a proposed subtyping of geriatric depression. J Affect Disord. 2011;134:177-187.
27. Rapp MA, Dahlman K, Sano M, et al. Neuropsychological differences between late-onset and recurrent geriatric major depression. Am J Psychiatry. 2005;162:691-698.
28. Zihl J, Reppermund S, Thum S, et al. Neuropsychological profiles in MCI and in depression: differential cognitive dysfunction patterns or similar final common pathway disorder? J Psychiatr Res. 2010;44:647-654.
29. Dillon C, Tartaglini MF, Stefani D, et al. Geriatric depression and its relation with cognitive impairment and dementia. Arch Gerontol Geriatr. 2014;59:450-456.
30. Wright SL, Persad C. Distinguishing between depression and dementia in older persons: neuropsychological and neuropathological correlates. J Geriatr Psychiatry Neurol. 2007;20:189-198.
31. Visser PJ, Verhey FR, Ponds RW, et al. Distinction between preclinical Alzheimer’s disease and depression. J Am Geriatr Soc. 2000;48:479-484.
32. Bodner T, Delazer M, Kemmler G, et al. Clock drawing, clock reading, clock setting, and judgment of clock faces in elderly people with dementia and depression. J Am Geriatr Soc. 2004;52:1146-1150.
33. Clarfield AM. The reversible dementias: do they reverse? Ann Intern Med. 1988;109:476-486.
34. Clarfield AM. The decreasing prevalence of reversible dementias: an updated meta-analysis. Arch Intern Med. 2003;163:2219-2229.
35. Malouf R, Areosa Sastre A. Vitamin B12 for cognition. Cochrane Database Syst Rev. 2003;(3):CD004326.
36. Health Quality Ontario. Vitamin B12 and cognitive function: an evidence-based analysis. Ont Health Technol Assess Ser. 2013;13:1-45.
37. Abyad A. Prevalence of vitamin B12 deficiency among demented patients and cognitive recovery with cobalamin replacement. J Nutr Health Aging. 2002;6:254-260.
38. Martin DC, Francis J, Protetch J, et al. Time dependency of cognitive recovery with cobalamin replacement: Report of a pilot study. J Am Geriatr Soc. 1992;40:168-172.
39. Clarnette RM, Patterson CJ. Hypothyroidism: does treatment cure dementia? J Geriatr Psychiatry Neurol. 1994;7:23-27.
40. Dugbartey AT. Neurocognitive aspects of hypothyroidism. Arch Intern Med. 1998;158:1413-1418.
41. Alexander EM, Wagner EH, Buchner DM, et al. Do surgical brain lesions present as isolated dementia? A population-based study. J Am Geriatr Soc. 1995;43:138-143.
42. Moore AR, O’Keeffe ST. Drug-induced cognitive impairment in the elderly. Drugs Aging. 1999;15:15-28.
43. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American geriatrics society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
44. Middleton LE, Yaffe K. Promising strategies for the prevention of dementia. Arch Neurol. 2009;66:1210-1215.
45. Shatenstein B, Barberger-Gateau P, Mecocci P. Prevention of age-related cognitive decline: which strategies, when, and for whom? J Alzheimers Dis. 2015;48:35-53.
From The Journal of Family Practice | 2017;66(11):670-676.
PRACTICE RECOMMENDATIONS
› Evaluate cognitive domain involvement in cases of suspected mild cognitive impairment; findings could suggest an underlying cause and indicate risk of progression to dementia. C
› Consider the severity of a cognitive deficit (eg, delayed recall) when depression is diagnosed; a marked deficit is usually more indicative of true dementia than pseudodementia. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series