User login
Case
A 63-year-old man with hypertension, diabetes, and recently diagnosed squamous-cell lung cancer presents with diffuse abdominal pain and confusion of two-day duration. He weighs 105 Kg, his blood pressure is 105/65 mm/Hg, heart rate is 105 beats per minute, and temperature is 99.0 degrees Fahrenheit. His respirations are 18 breaths per minute, oxygen saturation is 95% on room air, and his orthostatics are positive. Dry mucus membranes with decreased skin turgor are noted on physical examination. Laboratory evaluation reveals a calcium level of 15.5 mg/dL, creatinine level of 1.2 mg/dL, albumin level of 4.3 g/dL, and a phosphorous level of 2.9 mg/dL.
What is the best treatment of this condition?
Overview
Calcium homeostasis involves complex interactions between the kidney, gastrointestinal (GI) tract, and the skeletal system via hormonal influences. Although 99% of the body’s calcium is stored in the bones, 50% of serum calcium is in the active ionized form, 40% is bound to albumin, and 10% is complexed with anions.1 It’s important to remember these percentages when evaluating a patient’s serum calcium; elevated serum calcium can be validated by using either a correction formula (corrected calcium=measured total calcium + [0.8 x (4.5-albumin)]) or by direct measurement of the ionized calcium, which is the physiologically active form.
Hypercalcemia of malignancy is the most common cause of hypercalcemia in the hospitalized patient. Twenty to 30% of patients with cancer will develop hypercalcemia at some point in their disease course.2 Overall, this portends a poor prognosis with a median survival of three to four months.3
Four general mechanisms are involved in the pathogenesis of malignant hypercalcemia; these mechanisms form the basis for available treatment strategies available:
- Osteolytic tumors, such as multiple myeloma, can directly act on bone, leading to osteoclast activation and release of calcium;
- Humoral mediators elaborated by malignant cells, such as parathyroid hormone-related peptide (PTH-RP), can effect activation of osteoclasts and decrease renal elimination of calcium, causing humoral hypercalcemia of malignancy;
- Some malignancies (most commonly lymphomas) can directly synthesize 1,25 (OH)2 vitamin D, leading to increased luminal absorption of both calcium and phosphorus from the GI tract; and
- Direct production of parathyroid hormone (PTH) by the malignant cells is rare, but has been reported.2
Other factors, including impaired mobility, might lead to further bone resorption and a worsening of the hypercalcemic state.
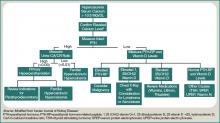
A patient with hypercalcemia must have a systematic workup, with knowledge of other causes of hypercalcemia that could be present, irrespective of malignancy. Examples include primary hyperparathyroidism, medications effect, and genetic etiologies. Although further discussion is beyond the scope of this article, a broad diagnostic approach is represented in Figure 1 (at right).
Effective management of hypercalcemia demands consideration of both the patient’s immediate, as well as longer-term, clinical situation in light of the patient’s prognosis. The primary aim in the acute management of hypercalcemia is to normalize serum values and decrease symptoms. However, this must be done with appreciation that the metabolic derangement was generated by an underlying malignancy. The main focus of clinical therapeutics should be aimed at this.
Review of the Data
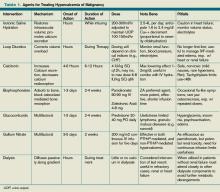
Intravenous (IV) fluids. IV hydration with isotonic saline represents the most immediate and critical intervention in the acute management of malignant hypercalcemia. This condition has multiple, potentially deleterious effects on the kidney, including vasoconstriction, inhibition of salt absorption distally, and antagonism of anti-diuretic hormone (ADH), leading to both salt and water loss. The decrease in intravascular volume then potentiates increased sodium re-absorption proximally in the kidney.
Isotonic saline restores the volume depletion that invariably occurs in the setting of hypercalcemia-provoked urinary salt wasting. The restoration of intravascular volume results in an increase in the glomerular filtration rate and, thus, an increase in calcium filtration. Furthermore, proximal tubular sodium and calcium re-absorption decrease as the glomerular filtration rate increases. Additionally, an increase in sodium and water presentation to the distal renal tubular sites provokes a further calciuresis.
It is estimated that with saline hydration, the calcium concentration should decline, at least by the degree to which dehydration raised it, typically in the range of 1.6 mg to 2.4 mg per deciliter.4 Hydration alone, however, rarely leads to normalization of the serum calcium concentration in patients with severe hypercalcemia.
The rate of infusion is based on the severity of hypercalcemia, and the patient’s age and comorbidities, with particular attention to cardiac or renal disease. A standard approach for most patients without edema and without heart or renal failure is to begin a saline infusion at an initial rate between 200 mL/h to 300 mL/h. The goal is to maintain urine output at 100 mL/h to 150 mL/h.
Furosemide. Following the administration of intravenous fluids to re-establish a euvolemic state, furosemide historically has been used because it has a calcinuric effect with forced diuresis. It also is useful for managing and preventing the fluid overload that occurs with saline hydration. However, data does not support its routine use to lower calcium levels in hypercalcemic patients.
The majority of articles studying the use of furosemide were published in the 1970s and ’80s, and they involve a variety of doses and administration schedules ranging from 40 mg orally daily to 100 mg IV hourly with variable improvement in serum calcium levels and effects that were short-lived. Although some studies have shown that these high doses (2,400 mg/24 hours) of furosemide can decrease calcium levels, resultant severe metabolic derangements in other electrolytes were encountered. This approach required frequent and invasive monitoring to prevent such derangements.5 The clinical application of these studies have led to published recommendations that are as variable as the doses used in the initial studies more than 30 years ago.
This includes the consideration that, in light of the availability and efficacy of bisphosphonates, furosemide might no longer be clinically helpful in this endeavor.6 The current role of furosemide in the management in hypercalcemic patients remains on an as-needed basis for management of fluid overload states brought about after aggressive IV fluid resuscitation.
Bisphosphonates. Bisphosph-onates first became available for the management of hypercalcemia in the early 1990s and have dramatically changed the acute intervention and improved the long-term clinical course of patients with malignant hypercalcemia. Though first developed in the 19th century with industrial applications, it wasn’t until the 1960s that their role in bone metabolism was appreciated.
While their complex mechanism of action remains an issue of ongoing investigations, it is known that bisphosphonates are directed to the bones, where they inhibit an enzyme in the HMG-CoA reductase pathway and promote apoptotic cell death of osteoclasts.7 By blocking osteoclast-mediated bone resorption, the bisphosphonates are effective in treating the hypercalcemia that occurs with a variety of bone-resorbing disease processes, malignant hypercalcemia included. As relatively nontoxic compounds capable of conferring a profound and sustained diminution in serum calcium, these agents have become preferred in the management of acute and chronic hypercalcemia of malignancy.
There are five parenteral bisphosphonates available for the treatment of malignant hypercalcemia: pamidronate, zoledronic acid, ibandronate, etidronate, and clodronate. Etidronate and clodronate are first-generation agents, which are less potent and have more side effects than other agents and are not as commonly used. Ibandronate is a useful agent with a long half-life shown to be as effective as pamidronate, though it has not been as extensively studied as the other agents.
Pamidronate has been studied thoroughly in multiple observational and randomized trials, and has been shown to be highly efficacious and minimally toxic in the treatment of hypercalcemia due to multiple causes, including malignant hypercalcemia.8,9 A maximum calcium-lowering effect occurs at a dose of 90 mg, and the dose is often titrated based on the measured serum calcium. It is infused over two to four hours, effects a lowering of serum calcium within one to two days, and has a sustained effect lasting for up to two weeks or more.
As the most potent and most easily administered bisphosphonate, zoledronic acid is considered by many the agent of choice in the treatment of malignant hypercalcemia. It can be administered as a 4 mg-8 mg dose intravenously over 15 minutes (compared with two hours for pamidronate). Two Phase III trials comprising 275 patients have demonstrated zoledronic acid’s superior efficacy compared with pamidronate, with 88% of patients accomplishing a normalized serum calcium (compared with 70% of patients receiving a 90-mg dose of pamidronate).10
Even though these agents are relatively nontoxic, each can provoke a mild, transient flulike illness in recipients. Renal dysfunction has been noted rarely. These agents should be renally dosed and used with caution in patients with advanced renal insufficiency (serum creatinine >2.5). Osteonecrosis of the jaw has been observed in less than 2% of patients receiving IV bisphosphonates. Accordingly, it is recommended that patients undergo dental evaluation prior to receiving the agent (if feasible) and avoid invasive dental procedures around the time that they receive the agent.11
Other therapeutic interventions. The bisphosphonates represent the best studied and most efficacious pharmaceutical agents available to treat hypercalcemia. Straying from these agents should be considered only when they are contraindicated, in severe circumstances, or after the patient has failed to respond.
Calcitonin has long had FDA approval for treatment of hypercalcemia in adults. It has been shown in small, nonrandomized studies from the 1970s and ’80s to rapidly (within two hours) decrease calcium levels in hypercalcemic patients.12,13,14 However, these reductions are small (<10%) and transient (usually persisting up to 72 to 96 hours) due to the tachyphylaxsis noted with this medication. Nonetheless, calcitonin can be used as an adjuvant bridge to lower calcium levels in severely hypercalcemic patients for the first few days before other agents start taking effect.
Glucocorticoids have been used to treat hypercalcemia since the 1950s. Prednisone, dexamethasone, and methylprednisolone all carry FDA indications for hypercalcemia, but data are lacking and contradictory. A small (n=28) randomized controlled trial (RCT) conducted in 1984 showed no additional efficacy of glucocorticoids with IV fluids when compared with IV fluids alone.15 Another small (n=30) RCT done in 1992 on women with metastatic breast cancer showed a significant improvement in patients treated with prednisolone, IV fluids, and furosemide when compared with IV fluids and furosemide.16 Other nonrandomized trials have shown response to be unpredictable at best.17 Despite this, glucocorticoids likely retain a limited role for treatment in specific cases, including hypercalcemia induced by lymphomas elevating levels of 1,25(OH)2 vitamin D (as this interacts with a steroid-regulated receptor), or multiple myelomas where they potentially impact disease progression.
Gallium nitrate, an anhydrous salt of a heavy metal, has been shown in several randomized trials to be an effective therapeutic agent in lowering calcium levels in hypercalcemic patients.18,19 Furthermore, a double-blinded trial of 64 patients with hypercalcemia of malignancy showed gallium nitrate to be at least as effective as pamidronate for acute control of cancer-related hypercalcemia.20 However, the need for continuous infusion over a five-day period has limited the application of this agent.
Hemodialysis with a calcium-lacking dialysate has been shown in small, nonrandomized studies to be a temporarily effective method of reducing serum calcium levels.21,22 However, this treatment modality would best be reserved for patients with severe hypercalcemia, in whom aggressive intravascular volume repletion and bisphosphonates are not advisable (e.g. those with significant heart or kidney failure) and have an underlying etiology that is likely to be responsive to other treatment. Furthermore, consideration as to the appropriateness of such invasive temporizing procedures in patients with metastatic cancer should be undertaken.
Back to the Case
This patient had an ionized calcium level of 1.9 mmol/L (normal 1.1-1.4 mmol/L). He was started on aggressive IV hydration with normal saline and zoledronic acid. His home medications were reviewed, and it was confirmed that he was not taking such contraindicated medications as thiazides or calcium/vitamin D supplementation.
Further workup for the etiology of his hypercalcemia revealed an appropriately suppressed, intact PTH and normal 25 (OH) Vitamin D and 1,25 (OH)2 Vitamin D levels. His intact PTH-RP was elevated at 10pmol/L, and consistent with hypercalcemia of malignancy.
Oncology and palliative-care consults were requested to assist with coordination of the treatment of the patient’s underlying lung cancer; plans were made for systemic chemotherapy. His symptoms slowly improved, and 72 hours after admission, his serum calcium had normalized. He was discharged with a plan to initiate chemotherapy and continued follow-up with oncology.
Bottom Line
Acute management of hypercalcemia of malignancy focuses on lowering the serum calcium through a variety of pharmacologic agents. However, such long-term issues as treatment of the underlying malignancy and discussions about goals of care in this high-mortality patient population is paramount. TH
Dr. Hartley and Dr. Repaskey are clinical instructors in internal medicine at the University of Michigan Health System. Dr. Rohde is a clinical assistant professor of internal medicine at UMHS.
References
- Assadi F. Hypercalcemia: an evidence-based approach to clinical cases. Iran J Kidney Dis. 2009;3:(2):71-79.
- Stewart A. Hypercalcemia associated with cancer. N Engl J Med. 2005;542(4):373-379.
- Seccareccia D. Cancer-related hypercalcemia. Can Fam Physician. 2010;56:(3):244-246.
- Bilezikian JP. Management of acute hypercalcemia. N Engl J Med. 1992; 326(18):1196-1203.
- Suki WN, Yium JJ, VonMinden M, et al. Acute treatment of hypercalcemia with furosemide. N Engl J Med. 1970;283:836-840.
- LeGrand SB, Leskuski D, Zama I. Narrative review: furosemide for hypercalcemia: an unproven yet common practice. Ann Intern Med. 2008;149:259-263.
- Drake MT, Bart LC, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clinic Proc. 2008;83(9):1032-1045.
- Nussbaum SR, Younger J, Vandepol CJ, et al. Single-dose intravenous therapy with pamidronate for the treatment of hypercalcemia of malignancy: comparison of 30-, 60-, and 90-mg dosages. Am J Med. 1993; 95(3):297-304.
- Gucalp R, Ritch P, Riernik PH, et al. Comparative study of pamidronate disodium and etidronate disodium in the treatment of cancer-related hypercalcemia. J Clin Oncol. 1992;10(1):134-142.
- Major P, Lortholary A, Hon J, et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: a pooled analysis of two randomized, controlled clinical trials. J Clin Oncol. 2001;19(2): 558-567.
- Tanvetyanon T. Management of the adverse effects associated with intravenous bisphosphonates. Ann Oncol. 2006;17(6):897-907.
- Wisneski LA, Croom WP, Silva OL, et al. Salmon calcitonin in hypercalcemia. Clin Pharmacol Ther. 1978; 24:219-222.
- Binstock ML, Mundy GR. Effect of calcitonin and glucocorticoids in combination on the hypercalcemia of malignancy. Ann Intern Med. 1980;93(2):269-272.
- Nilsson O, Almqvist S, Karlberg BE. Salmon calcitonin in the acute treatment of moderate and severe hypercalcemia in man. Acta Med Scand. 1978;204(4): 249-252.
- Percival RC, Yates AJ, Gray RE, et al. Role of glucocorticoids in management of malignant hypercalcemia. Br Med J. 1984;289(6440):287.
- Kristensen B, Ejlertsen B, Holmegaard SN, et al. Prednisolone in the treatment of severe malignant hypercalcemia in metastatic breast cancer: a randomized study. J Intern Med. 1992;232(3):237-245.
- Thalassinos NC, Joplin GF. Failure of corticosteroid therapy to correct the hypercalcemia of malignant disease. Lancet. 1970;2(7672):537-538.
- Warrell RP Jr, Murphy WK, Schulman P, et al. A randomized double-blind study of gallium nitrate compared with etidronate for acute control of cancer-related hypercalcemia. J Clin Oncol. 1991;9(8):1467-1475.
- Warrell RP Jr, Israel R, Frisone M, et al. Gallium nitrate for acute treatment of cancer-related hypercalcemia: a randomized, double-blinded comparison to calcitonin. Ann Intern Med. 1988;108:669-674.
- Cvitkovic F, Armand JP, Tubiana-Hulin M, et al. Randomized, double-blind, phase II trial of gallium nitrate compared with pamidronate for acute control of cancer-related hypercalcemia. Cancer J. 2006;12 (1):47-53.
- Cardella CJ, Birkin BL, Rapoport A. Role of dialysis in the treatment of severe hypercalcemia: report of two cases successfully treated with hemodialysis and review of the literature. Clin Nephrol. 1979; 12(6):285-290.
- Koo WS, Jeon DS, Ahn SJ, et al. Calcium-free hemodialysis for the management of hypercalcemia. Nephron. 1996;72(3):424-428.
Case
A 63-year-old man with hypertension, diabetes, and recently diagnosed squamous-cell lung cancer presents with diffuse abdominal pain and confusion of two-day duration. He weighs 105 Kg, his blood pressure is 105/65 mm/Hg, heart rate is 105 beats per minute, and temperature is 99.0 degrees Fahrenheit. His respirations are 18 breaths per minute, oxygen saturation is 95% on room air, and his orthostatics are positive. Dry mucus membranes with decreased skin turgor are noted on physical examination. Laboratory evaluation reveals a calcium level of 15.5 mg/dL, creatinine level of 1.2 mg/dL, albumin level of 4.3 g/dL, and a phosphorous level of 2.9 mg/dL.
What is the best treatment of this condition?
Overview
Calcium homeostasis involves complex interactions between the kidney, gastrointestinal (GI) tract, and the skeletal system via hormonal influences. Although 99% of the body’s calcium is stored in the bones, 50% of serum calcium is in the active ionized form, 40% is bound to albumin, and 10% is complexed with anions.1 It’s important to remember these percentages when evaluating a patient’s serum calcium; elevated serum calcium can be validated by using either a correction formula (corrected calcium=measured total calcium + [0.8 x (4.5-albumin)]) or by direct measurement of the ionized calcium, which is the physiologically active form.
Hypercalcemia of malignancy is the most common cause of hypercalcemia in the hospitalized patient. Twenty to 30% of patients with cancer will develop hypercalcemia at some point in their disease course.2 Overall, this portends a poor prognosis with a median survival of three to four months.3
Four general mechanisms are involved in the pathogenesis of malignant hypercalcemia; these mechanisms form the basis for available treatment strategies available:
- Osteolytic tumors, such as multiple myeloma, can directly act on bone, leading to osteoclast activation and release of calcium;
- Humoral mediators elaborated by malignant cells, such as parathyroid hormone-related peptide (PTH-RP), can effect activation of osteoclasts and decrease renal elimination of calcium, causing humoral hypercalcemia of malignancy;
- Some malignancies (most commonly lymphomas) can directly synthesize 1,25 (OH)2 vitamin D, leading to increased luminal absorption of both calcium and phosphorus from the GI tract; and
- Direct production of parathyroid hormone (PTH) by the malignant cells is rare, but has been reported.2
Other factors, including impaired mobility, might lead to further bone resorption and a worsening of the hypercalcemic state.
A patient with hypercalcemia must have a systematic workup, with knowledge of other causes of hypercalcemia that could be present, irrespective of malignancy. Examples include primary hyperparathyroidism, medications effect, and genetic etiologies. Although further discussion is beyond the scope of this article, a broad diagnostic approach is represented in Figure 1 (at right).
Effective management of hypercalcemia demands consideration of both the patient’s immediate, as well as longer-term, clinical situation in light of the patient’s prognosis. The primary aim in the acute management of hypercalcemia is to normalize serum values and decrease symptoms. However, this must be done with appreciation that the metabolic derangement was generated by an underlying malignancy. The main focus of clinical therapeutics should be aimed at this.
Review of the Data
Intravenous (IV) fluids. IV hydration with isotonic saline represents the most immediate and critical intervention in the acute management of malignant hypercalcemia. This condition has multiple, potentially deleterious effects on the kidney, including vasoconstriction, inhibition of salt absorption distally, and antagonism of anti-diuretic hormone (ADH), leading to both salt and water loss. The decrease in intravascular volume then potentiates increased sodium re-absorption proximally in the kidney.
Isotonic saline restores the volume depletion that invariably occurs in the setting of hypercalcemia-provoked urinary salt wasting. The restoration of intravascular volume results in an increase in the glomerular filtration rate and, thus, an increase in calcium filtration. Furthermore, proximal tubular sodium and calcium re-absorption decrease as the glomerular filtration rate increases. Additionally, an increase in sodium and water presentation to the distal renal tubular sites provokes a further calciuresis.
It is estimated that with saline hydration, the calcium concentration should decline, at least by the degree to which dehydration raised it, typically in the range of 1.6 mg to 2.4 mg per deciliter.4 Hydration alone, however, rarely leads to normalization of the serum calcium concentration in patients with severe hypercalcemia.
The rate of infusion is based on the severity of hypercalcemia, and the patient’s age and comorbidities, with particular attention to cardiac or renal disease. A standard approach for most patients without edema and without heart or renal failure is to begin a saline infusion at an initial rate between 200 mL/h to 300 mL/h. The goal is to maintain urine output at 100 mL/h to 150 mL/h.
Furosemide. Following the administration of intravenous fluids to re-establish a euvolemic state, furosemide historically has been used because it has a calcinuric effect with forced diuresis. It also is useful for managing and preventing the fluid overload that occurs with saline hydration. However, data does not support its routine use to lower calcium levels in hypercalcemic patients.
The majority of articles studying the use of furosemide were published in the 1970s and ’80s, and they involve a variety of doses and administration schedules ranging from 40 mg orally daily to 100 mg IV hourly with variable improvement in serum calcium levels and effects that were short-lived. Although some studies have shown that these high doses (2,400 mg/24 hours) of furosemide can decrease calcium levels, resultant severe metabolic derangements in other electrolytes were encountered. This approach required frequent and invasive monitoring to prevent such derangements.5 The clinical application of these studies have led to published recommendations that are as variable as the doses used in the initial studies more than 30 years ago.
This includes the consideration that, in light of the availability and efficacy of bisphosphonates, furosemide might no longer be clinically helpful in this endeavor.6 The current role of furosemide in the management in hypercalcemic patients remains on an as-needed basis for management of fluid overload states brought about after aggressive IV fluid resuscitation.
Bisphosphonates. Bisphosph-onates first became available for the management of hypercalcemia in the early 1990s and have dramatically changed the acute intervention and improved the long-term clinical course of patients with malignant hypercalcemia. Though first developed in the 19th century with industrial applications, it wasn’t until the 1960s that their role in bone metabolism was appreciated.
While their complex mechanism of action remains an issue of ongoing investigations, it is known that bisphosphonates are directed to the bones, where they inhibit an enzyme in the HMG-CoA reductase pathway and promote apoptotic cell death of osteoclasts.7 By blocking osteoclast-mediated bone resorption, the bisphosphonates are effective in treating the hypercalcemia that occurs with a variety of bone-resorbing disease processes, malignant hypercalcemia included. As relatively nontoxic compounds capable of conferring a profound and sustained diminution in serum calcium, these agents have become preferred in the management of acute and chronic hypercalcemia of malignancy.
There are five parenteral bisphosphonates available for the treatment of malignant hypercalcemia: pamidronate, zoledronic acid, ibandronate, etidronate, and clodronate. Etidronate and clodronate are first-generation agents, which are less potent and have more side effects than other agents and are not as commonly used. Ibandronate is a useful agent with a long half-life shown to be as effective as pamidronate, though it has not been as extensively studied as the other agents.
Pamidronate has been studied thoroughly in multiple observational and randomized trials, and has been shown to be highly efficacious and minimally toxic in the treatment of hypercalcemia due to multiple causes, including malignant hypercalcemia.8,9 A maximum calcium-lowering effect occurs at a dose of 90 mg, and the dose is often titrated based on the measured serum calcium. It is infused over two to four hours, effects a lowering of serum calcium within one to two days, and has a sustained effect lasting for up to two weeks or more.
As the most potent and most easily administered bisphosphonate, zoledronic acid is considered by many the agent of choice in the treatment of malignant hypercalcemia. It can be administered as a 4 mg-8 mg dose intravenously over 15 minutes (compared with two hours for pamidronate). Two Phase III trials comprising 275 patients have demonstrated zoledronic acid’s superior efficacy compared with pamidronate, with 88% of patients accomplishing a normalized serum calcium (compared with 70% of patients receiving a 90-mg dose of pamidronate).10
Even though these agents are relatively nontoxic, each can provoke a mild, transient flulike illness in recipients. Renal dysfunction has been noted rarely. These agents should be renally dosed and used with caution in patients with advanced renal insufficiency (serum creatinine >2.5). Osteonecrosis of the jaw has been observed in less than 2% of patients receiving IV bisphosphonates. Accordingly, it is recommended that patients undergo dental evaluation prior to receiving the agent (if feasible) and avoid invasive dental procedures around the time that they receive the agent.11
Other therapeutic interventions. The bisphosphonates represent the best studied and most efficacious pharmaceutical agents available to treat hypercalcemia. Straying from these agents should be considered only when they are contraindicated, in severe circumstances, or after the patient has failed to respond.
Calcitonin has long had FDA approval for treatment of hypercalcemia in adults. It has been shown in small, nonrandomized studies from the 1970s and ’80s to rapidly (within two hours) decrease calcium levels in hypercalcemic patients.12,13,14 However, these reductions are small (<10%) and transient (usually persisting up to 72 to 96 hours) due to the tachyphylaxsis noted with this medication. Nonetheless, calcitonin can be used as an adjuvant bridge to lower calcium levels in severely hypercalcemic patients for the first few days before other agents start taking effect.
Glucocorticoids have been used to treat hypercalcemia since the 1950s. Prednisone, dexamethasone, and methylprednisolone all carry FDA indications for hypercalcemia, but data are lacking and contradictory. A small (n=28) randomized controlled trial (RCT) conducted in 1984 showed no additional efficacy of glucocorticoids with IV fluids when compared with IV fluids alone.15 Another small (n=30) RCT done in 1992 on women with metastatic breast cancer showed a significant improvement in patients treated with prednisolone, IV fluids, and furosemide when compared with IV fluids and furosemide.16 Other nonrandomized trials have shown response to be unpredictable at best.17 Despite this, glucocorticoids likely retain a limited role for treatment in specific cases, including hypercalcemia induced by lymphomas elevating levels of 1,25(OH)2 vitamin D (as this interacts with a steroid-regulated receptor), or multiple myelomas where they potentially impact disease progression.
Gallium nitrate, an anhydrous salt of a heavy metal, has been shown in several randomized trials to be an effective therapeutic agent in lowering calcium levels in hypercalcemic patients.18,19 Furthermore, a double-blinded trial of 64 patients with hypercalcemia of malignancy showed gallium nitrate to be at least as effective as pamidronate for acute control of cancer-related hypercalcemia.20 However, the need for continuous infusion over a five-day period has limited the application of this agent.
Hemodialysis with a calcium-lacking dialysate has been shown in small, nonrandomized studies to be a temporarily effective method of reducing serum calcium levels.21,22 However, this treatment modality would best be reserved for patients with severe hypercalcemia, in whom aggressive intravascular volume repletion and bisphosphonates are not advisable (e.g. those with significant heart or kidney failure) and have an underlying etiology that is likely to be responsive to other treatment. Furthermore, consideration as to the appropriateness of such invasive temporizing procedures in patients with metastatic cancer should be undertaken.
Back to the Case
This patient had an ionized calcium level of 1.9 mmol/L (normal 1.1-1.4 mmol/L). He was started on aggressive IV hydration with normal saline and zoledronic acid. His home medications were reviewed, and it was confirmed that he was not taking such contraindicated medications as thiazides or calcium/vitamin D supplementation.
Further workup for the etiology of his hypercalcemia revealed an appropriately suppressed, intact PTH and normal 25 (OH) Vitamin D and 1,25 (OH)2 Vitamin D levels. His intact PTH-RP was elevated at 10pmol/L, and consistent with hypercalcemia of malignancy.
Oncology and palliative-care consults were requested to assist with coordination of the treatment of the patient’s underlying lung cancer; plans were made for systemic chemotherapy. His symptoms slowly improved, and 72 hours after admission, his serum calcium had normalized. He was discharged with a plan to initiate chemotherapy and continued follow-up with oncology.
Bottom Line
Acute management of hypercalcemia of malignancy focuses on lowering the serum calcium through a variety of pharmacologic agents. However, such long-term issues as treatment of the underlying malignancy and discussions about goals of care in this high-mortality patient population is paramount. TH
Dr. Hartley and Dr. Repaskey are clinical instructors in internal medicine at the University of Michigan Health System. Dr. Rohde is a clinical assistant professor of internal medicine at UMHS.
References
- Assadi F. Hypercalcemia: an evidence-based approach to clinical cases. Iran J Kidney Dis. 2009;3:(2):71-79.
- Stewart A. Hypercalcemia associated with cancer. N Engl J Med. 2005;542(4):373-379.
- Seccareccia D. Cancer-related hypercalcemia. Can Fam Physician. 2010;56:(3):244-246.
- Bilezikian JP. Management of acute hypercalcemia. N Engl J Med. 1992; 326(18):1196-1203.
- Suki WN, Yium JJ, VonMinden M, et al. Acute treatment of hypercalcemia with furosemide. N Engl J Med. 1970;283:836-840.
- LeGrand SB, Leskuski D, Zama I. Narrative review: furosemide for hypercalcemia: an unproven yet common practice. Ann Intern Med. 2008;149:259-263.
- Drake MT, Bart LC, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clinic Proc. 2008;83(9):1032-1045.
- Nussbaum SR, Younger J, Vandepol CJ, et al. Single-dose intravenous therapy with pamidronate for the treatment of hypercalcemia of malignancy: comparison of 30-, 60-, and 90-mg dosages. Am J Med. 1993; 95(3):297-304.
- Gucalp R, Ritch P, Riernik PH, et al. Comparative study of pamidronate disodium and etidronate disodium in the treatment of cancer-related hypercalcemia. J Clin Oncol. 1992;10(1):134-142.
- Major P, Lortholary A, Hon J, et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: a pooled analysis of two randomized, controlled clinical trials. J Clin Oncol. 2001;19(2): 558-567.
- Tanvetyanon T. Management of the adverse effects associated with intravenous bisphosphonates. Ann Oncol. 2006;17(6):897-907.
- Wisneski LA, Croom WP, Silva OL, et al. Salmon calcitonin in hypercalcemia. Clin Pharmacol Ther. 1978; 24:219-222.
- Binstock ML, Mundy GR. Effect of calcitonin and glucocorticoids in combination on the hypercalcemia of malignancy. Ann Intern Med. 1980;93(2):269-272.
- Nilsson O, Almqvist S, Karlberg BE. Salmon calcitonin in the acute treatment of moderate and severe hypercalcemia in man. Acta Med Scand. 1978;204(4): 249-252.
- Percival RC, Yates AJ, Gray RE, et al. Role of glucocorticoids in management of malignant hypercalcemia. Br Med J. 1984;289(6440):287.
- Kristensen B, Ejlertsen B, Holmegaard SN, et al. Prednisolone in the treatment of severe malignant hypercalcemia in metastatic breast cancer: a randomized study. J Intern Med. 1992;232(3):237-245.
- Thalassinos NC, Joplin GF. Failure of corticosteroid therapy to correct the hypercalcemia of malignant disease. Lancet. 1970;2(7672):537-538.
- Warrell RP Jr, Murphy WK, Schulman P, et al. A randomized double-blind study of gallium nitrate compared with etidronate for acute control of cancer-related hypercalcemia. J Clin Oncol. 1991;9(8):1467-1475.
- Warrell RP Jr, Israel R, Frisone M, et al. Gallium nitrate for acute treatment of cancer-related hypercalcemia: a randomized, double-blinded comparison to calcitonin. Ann Intern Med. 1988;108:669-674.
- Cvitkovic F, Armand JP, Tubiana-Hulin M, et al. Randomized, double-blind, phase II trial of gallium nitrate compared with pamidronate for acute control of cancer-related hypercalcemia. Cancer J. 2006;12 (1):47-53.
- Cardella CJ, Birkin BL, Rapoport A. Role of dialysis in the treatment of severe hypercalcemia: report of two cases successfully treated with hemodialysis and review of the literature. Clin Nephrol. 1979; 12(6):285-290.
- Koo WS, Jeon DS, Ahn SJ, et al. Calcium-free hemodialysis for the management of hypercalcemia. Nephron. 1996;72(3):424-428.
Case
A 63-year-old man with hypertension, diabetes, and recently diagnosed squamous-cell lung cancer presents with diffuse abdominal pain and confusion of two-day duration. He weighs 105 Kg, his blood pressure is 105/65 mm/Hg, heart rate is 105 beats per minute, and temperature is 99.0 degrees Fahrenheit. His respirations are 18 breaths per minute, oxygen saturation is 95% on room air, and his orthostatics are positive. Dry mucus membranes with decreased skin turgor are noted on physical examination. Laboratory evaluation reveals a calcium level of 15.5 mg/dL, creatinine level of 1.2 mg/dL, albumin level of 4.3 g/dL, and a phosphorous level of 2.9 mg/dL.
What is the best treatment of this condition?
Overview
Calcium homeostasis involves complex interactions between the kidney, gastrointestinal (GI) tract, and the skeletal system via hormonal influences. Although 99% of the body’s calcium is stored in the bones, 50% of serum calcium is in the active ionized form, 40% is bound to albumin, and 10% is complexed with anions.1 It’s important to remember these percentages when evaluating a patient’s serum calcium; elevated serum calcium can be validated by using either a correction formula (corrected calcium=measured total calcium + [0.8 x (4.5-albumin)]) or by direct measurement of the ionized calcium, which is the physiologically active form.
Hypercalcemia of malignancy is the most common cause of hypercalcemia in the hospitalized patient. Twenty to 30% of patients with cancer will develop hypercalcemia at some point in their disease course.2 Overall, this portends a poor prognosis with a median survival of three to four months.3
Four general mechanisms are involved in the pathogenesis of malignant hypercalcemia; these mechanisms form the basis for available treatment strategies available:
- Osteolytic tumors, such as multiple myeloma, can directly act on bone, leading to osteoclast activation and release of calcium;
- Humoral mediators elaborated by malignant cells, such as parathyroid hormone-related peptide (PTH-RP), can effect activation of osteoclasts and decrease renal elimination of calcium, causing humoral hypercalcemia of malignancy;
- Some malignancies (most commonly lymphomas) can directly synthesize 1,25 (OH)2 vitamin D, leading to increased luminal absorption of both calcium and phosphorus from the GI tract; and
- Direct production of parathyroid hormone (PTH) by the malignant cells is rare, but has been reported.2
Other factors, including impaired mobility, might lead to further bone resorption and a worsening of the hypercalcemic state.
A patient with hypercalcemia must have a systematic workup, with knowledge of other causes of hypercalcemia that could be present, irrespective of malignancy. Examples include primary hyperparathyroidism, medications effect, and genetic etiologies. Although further discussion is beyond the scope of this article, a broad diagnostic approach is represented in Figure 1 (at right).
Effective management of hypercalcemia demands consideration of both the patient’s immediate, as well as longer-term, clinical situation in light of the patient’s prognosis. The primary aim in the acute management of hypercalcemia is to normalize serum values and decrease symptoms. However, this must be done with appreciation that the metabolic derangement was generated by an underlying malignancy. The main focus of clinical therapeutics should be aimed at this.
Review of the Data
Intravenous (IV) fluids. IV hydration with isotonic saline represents the most immediate and critical intervention in the acute management of malignant hypercalcemia. This condition has multiple, potentially deleterious effects on the kidney, including vasoconstriction, inhibition of salt absorption distally, and antagonism of anti-diuretic hormone (ADH), leading to both salt and water loss. The decrease in intravascular volume then potentiates increased sodium re-absorption proximally in the kidney.
Isotonic saline restores the volume depletion that invariably occurs in the setting of hypercalcemia-provoked urinary salt wasting. The restoration of intravascular volume results in an increase in the glomerular filtration rate and, thus, an increase in calcium filtration. Furthermore, proximal tubular sodium and calcium re-absorption decrease as the glomerular filtration rate increases. Additionally, an increase in sodium and water presentation to the distal renal tubular sites provokes a further calciuresis.
It is estimated that with saline hydration, the calcium concentration should decline, at least by the degree to which dehydration raised it, typically in the range of 1.6 mg to 2.4 mg per deciliter.4 Hydration alone, however, rarely leads to normalization of the serum calcium concentration in patients with severe hypercalcemia.
The rate of infusion is based on the severity of hypercalcemia, and the patient’s age and comorbidities, with particular attention to cardiac or renal disease. A standard approach for most patients without edema and without heart or renal failure is to begin a saline infusion at an initial rate between 200 mL/h to 300 mL/h. The goal is to maintain urine output at 100 mL/h to 150 mL/h.
Furosemide. Following the administration of intravenous fluids to re-establish a euvolemic state, furosemide historically has been used because it has a calcinuric effect with forced diuresis. It also is useful for managing and preventing the fluid overload that occurs with saline hydration. However, data does not support its routine use to lower calcium levels in hypercalcemic patients.
The majority of articles studying the use of furosemide were published in the 1970s and ’80s, and they involve a variety of doses and administration schedules ranging from 40 mg orally daily to 100 mg IV hourly with variable improvement in serum calcium levels and effects that were short-lived. Although some studies have shown that these high doses (2,400 mg/24 hours) of furosemide can decrease calcium levels, resultant severe metabolic derangements in other electrolytes were encountered. This approach required frequent and invasive monitoring to prevent such derangements.5 The clinical application of these studies have led to published recommendations that are as variable as the doses used in the initial studies more than 30 years ago.
This includes the consideration that, in light of the availability and efficacy of bisphosphonates, furosemide might no longer be clinically helpful in this endeavor.6 The current role of furosemide in the management in hypercalcemic patients remains on an as-needed basis for management of fluid overload states brought about after aggressive IV fluid resuscitation.
Bisphosphonates. Bisphosph-onates first became available for the management of hypercalcemia in the early 1990s and have dramatically changed the acute intervention and improved the long-term clinical course of patients with malignant hypercalcemia. Though first developed in the 19th century with industrial applications, it wasn’t until the 1960s that their role in bone metabolism was appreciated.
While their complex mechanism of action remains an issue of ongoing investigations, it is known that bisphosphonates are directed to the bones, where they inhibit an enzyme in the HMG-CoA reductase pathway and promote apoptotic cell death of osteoclasts.7 By blocking osteoclast-mediated bone resorption, the bisphosphonates are effective in treating the hypercalcemia that occurs with a variety of bone-resorbing disease processes, malignant hypercalcemia included. As relatively nontoxic compounds capable of conferring a profound and sustained diminution in serum calcium, these agents have become preferred in the management of acute and chronic hypercalcemia of malignancy.
There are five parenteral bisphosphonates available for the treatment of malignant hypercalcemia: pamidronate, zoledronic acid, ibandronate, etidronate, and clodronate. Etidronate and clodronate are first-generation agents, which are less potent and have more side effects than other agents and are not as commonly used. Ibandronate is a useful agent with a long half-life shown to be as effective as pamidronate, though it has not been as extensively studied as the other agents.
Pamidronate has been studied thoroughly in multiple observational and randomized trials, and has been shown to be highly efficacious and minimally toxic in the treatment of hypercalcemia due to multiple causes, including malignant hypercalcemia.8,9 A maximum calcium-lowering effect occurs at a dose of 90 mg, and the dose is often titrated based on the measured serum calcium. It is infused over two to four hours, effects a lowering of serum calcium within one to two days, and has a sustained effect lasting for up to two weeks or more.
As the most potent and most easily administered bisphosphonate, zoledronic acid is considered by many the agent of choice in the treatment of malignant hypercalcemia. It can be administered as a 4 mg-8 mg dose intravenously over 15 minutes (compared with two hours for pamidronate). Two Phase III trials comprising 275 patients have demonstrated zoledronic acid’s superior efficacy compared with pamidronate, with 88% of patients accomplishing a normalized serum calcium (compared with 70% of patients receiving a 90-mg dose of pamidronate).10
Even though these agents are relatively nontoxic, each can provoke a mild, transient flulike illness in recipients. Renal dysfunction has been noted rarely. These agents should be renally dosed and used with caution in patients with advanced renal insufficiency (serum creatinine >2.5). Osteonecrosis of the jaw has been observed in less than 2% of patients receiving IV bisphosphonates. Accordingly, it is recommended that patients undergo dental evaluation prior to receiving the agent (if feasible) and avoid invasive dental procedures around the time that they receive the agent.11
Other therapeutic interventions. The bisphosphonates represent the best studied and most efficacious pharmaceutical agents available to treat hypercalcemia. Straying from these agents should be considered only when they are contraindicated, in severe circumstances, or after the patient has failed to respond.
Calcitonin has long had FDA approval for treatment of hypercalcemia in adults. It has been shown in small, nonrandomized studies from the 1970s and ’80s to rapidly (within two hours) decrease calcium levels in hypercalcemic patients.12,13,14 However, these reductions are small (<10%) and transient (usually persisting up to 72 to 96 hours) due to the tachyphylaxsis noted with this medication. Nonetheless, calcitonin can be used as an adjuvant bridge to lower calcium levels in severely hypercalcemic patients for the first few days before other agents start taking effect.
Glucocorticoids have been used to treat hypercalcemia since the 1950s. Prednisone, dexamethasone, and methylprednisolone all carry FDA indications for hypercalcemia, but data are lacking and contradictory. A small (n=28) randomized controlled trial (RCT) conducted in 1984 showed no additional efficacy of glucocorticoids with IV fluids when compared with IV fluids alone.15 Another small (n=30) RCT done in 1992 on women with metastatic breast cancer showed a significant improvement in patients treated with prednisolone, IV fluids, and furosemide when compared with IV fluids and furosemide.16 Other nonrandomized trials have shown response to be unpredictable at best.17 Despite this, glucocorticoids likely retain a limited role for treatment in specific cases, including hypercalcemia induced by lymphomas elevating levels of 1,25(OH)2 vitamin D (as this interacts with a steroid-regulated receptor), or multiple myelomas where they potentially impact disease progression.
Gallium nitrate, an anhydrous salt of a heavy metal, has been shown in several randomized trials to be an effective therapeutic agent in lowering calcium levels in hypercalcemic patients.18,19 Furthermore, a double-blinded trial of 64 patients with hypercalcemia of malignancy showed gallium nitrate to be at least as effective as pamidronate for acute control of cancer-related hypercalcemia.20 However, the need for continuous infusion over a five-day period has limited the application of this agent.
Hemodialysis with a calcium-lacking dialysate has been shown in small, nonrandomized studies to be a temporarily effective method of reducing serum calcium levels.21,22 However, this treatment modality would best be reserved for patients with severe hypercalcemia, in whom aggressive intravascular volume repletion and bisphosphonates are not advisable (e.g. those with significant heart or kidney failure) and have an underlying etiology that is likely to be responsive to other treatment. Furthermore, consideration as to the appropriateness of such invasive temporizing procedures in patients with metastatic cancer should be undertaken.
Back to the Case
This patient had an ionized calcium level of 1.9 mmol/L (normal 1.1-1.4 mmol/L). He was started on aggressive IV hydration with normal saline and zoledronic acid. His home medications were reviewed, and it was confirmed that he was not taking such contraindicated medications as thiazides or calcium/vitamin D supplementation.
Further workup for the etiology of his hypercalcemia revealed an appropriately suppressed, intact PTH and normal 25 (OH) Vitamin D and 1,25 (OH)2 Vitamin D levels. His intact PTH-RP was elevated at 10pmol/L, and consistent with hypercalcemia of malignancy.
Oncology and palliative-care consults were requested to assist with coordination of the treatment of the patient’s underlying lung cancer; plans were made for systemic chemotherapy. His symptoms slowly improved, and 72 hours after admission, his serum calcium had normalized. He was discharged with a plan to initiate chemotherapy and continued follow-up with oncology.
Bottom Line
Acute management of hypercalcemia of malignancy focuses on lowering the serum calcium through a variety of pharmacologic agents. However, such long-term issues as treatment of the underlying malignancy and discussions about goals of care in this high-mortality patient population is paramount. TH
Dr. Hartley and Dr. Repaskey are clinical instructors in internal medicine at the University of Michigan Health System. Dr. Rohde is a clinical assistant professor of internal medicine at UMHS.
References
- Assadi F. Hypercalcemia: an evidence-based approach to clinical cases. Iran J Kidney Dis. 2009;3:(2):71-79.
- Stewart A. Hypercalcemia associated with cancer. N Engl J Med. 2005;542(4):373-379.
- Seccareccia D. Cancer-related hypercalcemia. Can Fam Physician. 2010;56:(3):244-246.
- Bilezikian JP. Management of acute hypercalcemia. N Engl J Med. 1992; 326(18):1196-1203.
- Suki WN, Yium JJ, VonMinden M, et al. Acute treatment of hypercalcemia with furosemide. N Engl J Med. 1970;283:836-840.
- LeGrand SB, Leskuski D, Zama I. Narrative review: furosemide for hypercalcemia: an unproven yet common practice. Ann Intern Med. 2008;149:259-263.
- Drake MT, Bart LC, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clinic Proc. 2008;83(9):1032-1045.
- Nussbaum SR, Younger J, Vandepol CJ, et al. Single-dose intravenous therapy with pamidronate for the treatment of hypercalcemia of malignancy: comparison of 30-, 60-, and 90-mg dosages. Am J Med. 1993; 95(3):297-304.
- Gucalp R, Ritch P, Riernik PH, et al. Comparative study of pamidronate disodium and etidronate disodium in the treatment of cancer-related hypercalcemia. J Clin Oncol. 1992;10(1):134-142.
- Major P, Lortholary A, Hon J, et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: a pooled analysis of two randomized, controlled clinical trials. J Clin Oncol. 2001;19(2): 558-567.
- Tanvetyanon T. Management of the adverse effects associated with intravenous bisphosphonates. Ann Oncol. 2006;17(6):897-907.
- Wisneski LA, Croom WP, Silva OL, et al. Salmon calcitonin in hypercalcemia. Clin Pharmacol Ther. 1978; 24:219-222.
- Binstock ML, Mundy GR. Effect of calcitonin and glucocorticoids in combination on the hypercalcemia of malignancy. Ann Intern Med. 1980;93(2):269-272.
- Nilsson O, Almqvist S, Karlberg BE. Salmon calcitonin in the acute treatment of moderate and severe hypercalcemia in man. Acta Med Scand. 1978;204(4): 249-252.
- Percival RC, Yates AJ, Gray RE, et al. Role of glucocorticoids in management of malignant hypercalcemia. Br Med J. 1984;289(6440):287.
- Kristensen B, Ejlertsen B, Holmegaard SN, et al. Prednisolone in the treatment of severe malignant hypercalcemia in metastatic breast cancer: a randomized study. J Intern Med. 1992;232(3):237-245.
- Thalassinos NC, Joplin GF. Failure of corticosteroid therapy to correct the hypercalcemia of malignant disease. Lancet. 1970;2(7672):537-538.
- Warrell RP Jr, Murphy WK, Schulman P, et al. A randomized double-blind study of gallium nitrate compared with etidronate for acute control of cancer-related hypercalcemia. J Clin Oncol. 1991;9(8):1467-1475.
- Warrell RP Jr, Israel R, Frisone M, et al. Gallium nitrate for acute treatment of cancer-related hypercalcemia: a randomized, double-blinded comparison to calcitonin. Ann Intern Med. 1988;108:669-674.
- Cvitkovic F, Armand JP, Tubiana-Hulin M, et al. Randomized, double-blind, phase II trial of gallium nitrate compared with pamidronate for acute control of cancer-related hypercalcemia. Cancer J. 2006;12 (1):47-53.
- Cardella CJ, Birkin BL, Rapoport A. Role of dialysis in the treatment of severe hypercalcemia: report of two cases successfully treated with hemodialysis and review of the literature. Clin Nephrol. 1979; 12(6):285-290.
- Koo WS, Jeon DS, Ahn SJ, et al. Calcium-free hemodialysis for the management of hypercalcemia. Nephron. 1996;72(3):424-428.