User login
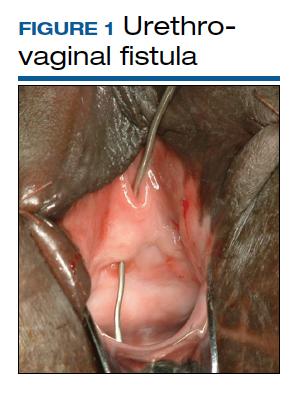
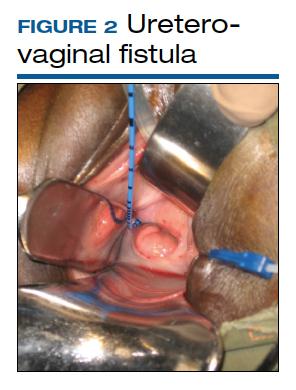
Although rare in the United States and more common in low-resource countries, fistulas due to obstructed labor do occur. In developed countries, other obstetric causes for fistula are usually surgery, trauma, or infection related. An abnormal communication between organs—be it the urethra, bladder, ureter, uterus, cervix, or rectum—can develop1 and lead to vesicovaginal fistula (VVF), urethrovaginal fistula (FIGURE 1), vesicocervical fistula, vesicouterine fistula, ureterovaginal fistula (FIGURE 2), and rectovaginal fistula (RVF). Other nonobstetric causes include gynecologic surgery, radiation, malignancy, and congenital malformations.
During labor, hypoxia, subsequent ischemia, and pressure necrosis contribute to fistula formation. Injury sustained during a cesarean delivery (CD) or cesarean hysterectomy can lead to fistula formation; at times, however, complications are unavoidable given the nature of the pathologic condition that the patient presents with.
VVF and RVF have a devastating impact on a woman’s quality of life as they lead to significant morbidity and short- and long-term psychological distress. The fistula may not be recognized at the time of injury. The presenting signs and symptoms may be intermittent and confusing. Immediate surgical intervention may not be possible due to ongoing inflammation or infection. Recovery often is prolonged. As there is significant concomitant postpartum anxiety and depression, patients with fistula often require psychosocial support and counseling. After repair, there is still a risk for recurrence and voiding dysfunction.
Fistula signs and symptoms and evaluation
In cases of VVF, patients present with continuing large or small volume urinary incontinence. Depending on the time to diagnosis, patients may have calculi formation, prolapse, scarring, external perineal dermatitis, perineal nerve injury, and even motor weakness. Cyclic hematuria may be seen in vesicouterine fistulas.2
Multiple classification systems for diagnosis and staging of VVF have been suggested.3,4 A classification system for RVF was published by Tsang and colleagues.5 All these classification systems have attempted to characterize fistulas in terms of level of surgical complexity for repair, providing a guideline for preoperative assessment. These classification systems do not translate into prediction regarding outcomes.
Evaluation of pelvic fistula from the urinary tract starts with a thorough history that includes onset, duration, and description of leakage (continuous, intermittent, or positional) and whether there is concomitant stress and urge incontinence. A detailed obstetric history, including circumstances around the mode of delivery, underlying risk factors, and psychosocial history, should be obtained.
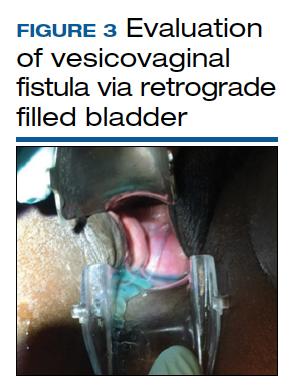
The pelvic examination with a plastic speculum and adequate lighting should assess the external perineum for dermatitis; bulbocavernosus and anal reflexes; and the vagina for length, caliber, level of scarring, and any prolapse. For VVFs, the location, size, and number of the fistula tracts can be visualized and confirmed with a retrograde fill of the bladder via a Foley catheter with saline or water mixed with methylene blue or any other blue dye (FIGURE 3). If a ureterovaginal fistula is suspected, the patient can simultaneously be given oral phenazopyridine and a tampon inserted within the vagina; the patient can then ambulate, and re-examination of the end of the tampon can reveal orange staining. The bladder meanwhile is retrograde filled with blue dye, with no blue staining of the tampon.
For RVF, history taking should include the onset, duration, and description of leakage, and the external anal sphincter should be assessed, with careful examination of the distal vagina at the vestibule as this is the most common location for RVF (fistula in ano). Patients may describe vaginal flatus and sometimes only brownish discharge, which can be intermittent, leading to an incorrect diagnosis of vaginitis that is treated repeatedly without success.
There is no consensus regarding optimal imaging for the assessment of VVF. Imaging used for diagnosis of VVF includes a voiding cystogram with opacification of the vagina after filling the bladder with contrast if there is a fistula. A cystoscopy can evaluate for calculi, retained suture, level of inflammation, and location of the ureters in relation to the fistula. Renal ultrasonography is of limited use. Intravenous pyelography can miss lesions by the trigone. In general, a computed tomography (CT) urogram and magnetic resonance imaging (MRI) with bladder contrast are more sensitive.
In the diagnosis of RVF, contrast vaginoscopy, double contrast barium enema, CT scan with contrast, and MRI can be used. MRI is more sensitive.6 A high index of suspicion is required based on the patient’s history as these imaging modalities do not always confirm RVF despite patient’s clear history of leakage. When the history is convincing, a thorough rectovaginal exam under anesthesia may be imperative. If rectal trauma is present, endoanal ultrasonography can delineate external and internal anal sphincter defects.
Prolonged Foley catheter placement after obstetric injury can lead to successful closure of a VVF. Prior to surgical intervention, assessing if there is possible ureteral involvement and use of intraoperative ureteral stents is a consideration. The route of surgery can be vaginal, abdominal, combined abdominal-vaginal, laparoscopic, or robotic.7 The robotic approach is increasingly utilized.8,9 However, the general consensus among fistula surgeons is that the vaginal approach should be considered first.
Continue to: Surgical repair...
Surgical repair
VVF repair. Factors that influence successful repair of VVF include the size and number of fistula, location, degree of scarring, bladder capacity, and urethral length.
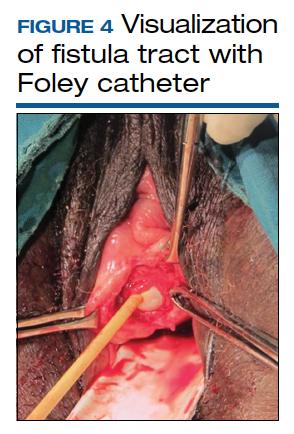
Surgical technique requires wide mobilization and adequate exposure. The fistula tract can be delineated and manipulated with a pediatric Foley catheter, ureteral stent, or even a ureteral guidewire to aid in dissection (FIGURE 4). Intraoperative visualization of the ureters, including stenting, often is needed. The fistulous track is excised depending on the level of scarring. Closure of the bladder uroepithelium for the first layer is with absorbable interrupted 3-0 or 2-0 sutures in a tension-free closure. The bladder is then evaluated with a retrograde fill with saline and methylene blue to ensure a watertight closure for the first layer. If the first layer is not watertight, the second layer closure will not compensate and the fistula will persist. Particular attention is paid to the angles of the fistula at the first layer closure to prevent recurrence of the fistula at the angles. A running second layer with absorbable 2-0 suture is done. At times, a Martius flap or an omental J flap can be used to provide an additional layer for support and to increase vascularity.10 The patient is sent home with a Foley catheter for drainage for 10 to 14 days.11 Antibiotics are not needed postoperatively for VVF surgery.12
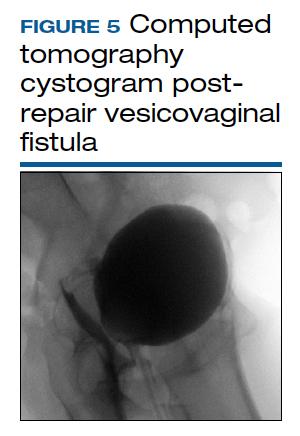
CT cystogram or retrograde cystogram is usually done to evaluate closure of the fistula prior to removal of the Foley catheter; retrograde fill with contrast directly into the bladder with 300 mL is sufficient (FIGURE 5). Patients are advised to refrain from sexual activity for a minimum of 6 weeks, but depending on the level of complexity and scarring, this can be up to 12 weeks.
The success rate in general is in the 95% range. Patients with successful closure of VVF are at risk for urge incontinence due to decreased bladder capacity, stress incontinence especially if the continence mechanism or urethra is involved, vaginal scarring, dyspareunia, and infertility.13 In general, sexual function improves after surgical repair.
RVF repair. Prior to surgical repair of RVF, the integrity of the external anal sphincter must be determined. If it is not involved, a vertical incision is made in the posterior vaginal wall, the vaginal epithelium is separated from the vaginal muscularis, and the fistula tract is identified. After complete wide mobilization of the tissue surrounding the tract, it is excised. The rectal wall is repaired with 3-0 or 4-0 absorbable interrupted sutures; a second layer and if possible even a third layer and finally the vaginal epithelium are all closed with 2-0 absorbable interrupted sutures.
If the sphincter complex is involved, the dissection involves an inverted U incision separating the vaginal wall from the rectum. The fistula tract is excised, the rectal wall is closed, and the internal anal sphincter is identified and reapproximated with interrupted absorbable 2-0 or 0 sutures. The disrupted external sphincter is then reapproximated with 2-0 or 0 sutures, and finally the transverse perineal and bulbocavernosus muscles are brought together with Lembert 0 sutures prior to closure of the external skin. Perioperative antibiotics have been shown to improve success rates in the correction of RVF.5 In patients with sphincter trauma and known RVF, outcomes with a sphincteroplasty are better, compared with endorectal advancement flaps. The patient is discharged with a bowel regimen and dietary precautions that aim for daily soft bowel movements.
After surgical treatment of fistulas, patients benefit from pelvic floor physical therapy that focuses on pelvic floor strengthening. Incorporating the habit of Kegel exercises after every void, timed (scheduled) bladder voiding, and avoidance of straining with urination or defecation should be emphasized.
Continue to: CASE 1 Pregnant woman with rectal bleeding...
CASE 1 Pregnant woman with rectal bleeding
A 37-year-old woman at 36 3/7 weeks’ gestation presented with acute rectal bleeding and pain. This was found to result from a catastrophic rupture of a pelvic arteriovenous malformation that caused an 11 x 7 x 9.5 cm size inferior pelvic hematoma and a full-thickness rectal tear at the dentate line. During examination under anesthesia, the baby was delivered by a stat CD due to breech presentation and a prolonged fetal heart rate deceleration. The patient underwent embolization of the right middle rectal artery and right internal iliac artery by a radiologic intervention. Further bleeding required surgical intervention for evacuation of about 1,000 mL of hematoma, repair of the rectal tear, and laparoscopic diverting loop ileostomy. In total, the patient received 8 U of packed red blood cells, 6 U of fresh frozen plasma, 5 L of crystalloid solution, and 2 g of tranexamic acid. The patient reported increased foul-smelling vaginal discharge, bedside exam suggested possible fistulous tract, and on postoperative day 16, an exam under anesthesia by Urogynecology confirmed a rectovaginal fistula in the right mid vagina. After 2 months of observation to allow resolution of inflammation, successful excision of the fistula tract and repair of RVF using the above-mentioned technique was accomplished.
CASE 2 Patient with VVF after cesarean hysterectomy
A 40-year-old (G6P2222) patient underwent cesarean hysterectomy for placenta percreta and uterine rupture at 24 weeks’ gestation. Intraoperatively, there were right ureteral ligation and posterior bladder wall cystotomies. The right ureter was reimplanted in the right upper posterior wall and the cystostomies were closed. As the patient had continuous urinary leakage postoperatively, a CT urogram was obtained, which showed left ureteral obstruction and VVF. Urinary incontinence persisted despite bilateral robotic ureteral reimplantation with omental flap by the urology team. Percutaneous nephrostomy tubes were placed bilaterally. The patient underwent additional imaging studies, including MRI, with findings of VVF and possible ureterovaginal fistula.
On referral to Urogynecology, the patient underwent cystoscopy with antegrade pyelogram, and the bilateral ureteroneocystostomy orifices had 5 French open-ended ureteral stents placed. A 10 French pediatric Foley catheter was inserted intravaginally into the bladder through the VVF. Via the vaginal approach, cervical remnant and skin bridges overlying the VVF were excised. The scarred fistula tract was excised with a circumferential incision. Horizontal interrupted Lembert sutures with 3-0 absorbable suture were used to reapproximate the first layer, which was confirmed to be watertight on testing with retrograde fill. Second-layer closure was completed with horizontal mattress 2-0 absorbable sutures, followed by a third-layer closure done in similar fashion. Fibrin glue was then placed. The vaginal epithelium was closed with 2-0 absorbable suture. Percutaneous nephrostomy tubes were removed. Postoperatively, the patient had a CT cystogram with no leak and no incontinence, but she developed urgency, which was controlled with timed voids and oxybutynin.
- Adler AJ, Ronsmans C, Calvert C, et al. Estimating the presence of obstetric fistula: a systematic review and meta-analysis BMC Pregnancy Childbirth. 2013;13:246.
- Battacharjee S, Kohli UA, Sood A, et al. Vesicouterine fistula: Youssef’s syndrome. Med J Armed Forces India. 2015;71(suppl 1):S175-S177. doi: 10.1016/j.mjafi.2013.11.006.
- Waaldijk K. Step-by-Step Surgery of Vesicovaginal Fistulas. Campion Press; 1994.
- Goh, JTW. A new classification for female genital tract fistula. Aust N Z J Ob Gynecol. 2004:44:502-504.
- Tsang CB, Rothenberger DA. Rectovaginal fistulas: therapeutic options. Surg Clin North Am. 1997;77:95-114.
- Champagne BJ, McGee MF. Rectovaginal fistula. Surg Clin North Am. 2010;90:69-82.
- Bodner-Adler B, Hanzal E, Pablik E, et al. Management of vesicovaginal fistulas in women following benign gynecologic surgery: a systematic review and meta-analysis. PLoS One. 2017;12:e0171554.
- Randazzo M, Lengauer L, Rochat CH, et al. Best practices in robotic-assisted repair of vesicovaginal fistula: a consensus report from the European Association of Urology Robotic Urology Section Scientific Working Group for Reconstructive Urology. Eur Urol. 2020;78: 432-442.
- Miklos JR, Moore RD, Chinthakanan O. Laparoscopic and robotic assisted vesicovaginal fistula repair: a systematic review of the literature. J Minim Invasive Gynecol. 2015:22:727-736.
- Hancock B. Practical Obstetric Fistula Surgery. Royal Society of Medicine Press; 2009.
- Nardos R, Menber B, Browning A. Outcome of obstetric fistula repair after 10-day versus 14-day Foley catheterization. Int J Gynaecol 0bstet. 2012;118:21-23.
- Tomlinson AJ, Thornton JG. A randomized controlled trial of antibiotic prophylaxis for vesico-vaginal fistula repair. Br J Obstet Gynaecol. 2005;105:397-399.
- Bengtson AM, Kopp D, Tang JH, et al. Identifying patients with vesicovaginal fistula at high risk of urinary incontinence after surgery. Obstet Gynecol. 2016;128:945-953.
Although rare in the United States and more common in low-resource countries, fistulas due to obstructed labor do occur. In developed countries, other obstetric causes for fistula are usually surgery, trauma, or infection related. An abnormal communication between organs—be it the urethra, bladder, ureter, uterus, cervix, or rectum—can develop1 and lead to vesicovaginal fistula (VVF), urethrovaginal fistula (FIGURE 1), vesicocervical fistula, vesicouterine fistula, ureterovaginal fistula (FIGURE 2), and rectovaginal fistula (RVF). Other nonobstetric causes include gynecologic surgery, radiation, malignancy, and congenital malformations.
During labor, hypoxia, subsequent ischemia, and pressure necrosis contribute to fistula formation. Injury sustained during a cesarean delivery (CD) or cesarean hysterectomy can lead to fistula formation; at times, however, complications are unavoidable given the nature of the pathologic condition that the patient presents with.
VVF and RVF have a devastating impact on a woman’s quality of life as they lead to significant morbidity and short- and long-term psychological distress. The fistula may not be recognized at the time of injury. The presenting signs and symptoms may be intermittent and confusing. Immediate surgical intervention may not be possible due to ongoing inflammation or infection. Recovery often is prolonged. As there is significant concomitant postpartum anxiety and depression, patients with fistula often require psychosocial support and counseling. After repair, there is still a risk for recurrence and voiding dysfunction.
Fistula signs and symptoms and evaluation
In cases of VVF, patients present with continuing large or small volume urinary incontinence. Depending on the time to diagnosis, patients may have calculi formation, prolapse, scarring, external perineal dermatitis, perineal nerve injury, and even motor weakness. Cyclic hematuria may be seen in vesicouterine fistulas.2
Multiple classification systems for diagnosis and staging of VVF have been suggested.3,4 A classification system for RVF was published by Tsang and colleagues.5 All these classification systems have attempted to characterize fistulas in terms of level of surgical complexity for repair, providing a guideline for preoperative assessment. These classification systems do not translate into prediction regarding outcomes.
Evaluation of pelvic fistula from the urinary tract starts with a thorough history that includes onset, duration, and description of leakage (continuous, intermittent, or positional) and whether there is concomitant stress and urge incontinence. A detailed obstetric history, including circumstances around the mode of delivery, underlying risk factors, and psychosocial history, should be obtained.
The pelvic examination with a plastic speculum and adequate lighting should assess the external perineum for dermatitis; bulbocavernosus and anal reflexes; and the vagina for length, caliber, level of scarring, and any prolapse. For VVFs, the location, size, and number of the fistula tracts can be visualized and confirmed with a retrograde fill of the bladder via a Foley catheter with saline or water mixed with methylene blue or any other blue dye (FIGURE 3). If a ureterovaginal fistula is suspected, the patient can simultaneously be given oral phenazopyridine and a tampon inserted within the vagina; the patient can then ambulate, and re-examination of the end of the tampon can reveal orange staining. The bladder meanwhile is retrograde filled with blue dye, with no blue staining of the tampon.
For RVF, history taking should include the onset, duration, and description of leakage, and the external anal sphincter should be assessed, with careful examination of the distal vagina at the vestibule as this is the most common location for RVF (fistula in ano). Patients may describe vaginal flatus and sometimes only brownish discharge, which can be intermittent, leading to an incorrect diagnosis of vaginitis that is treated repeatedly without success.
There is no consensus regarding optimal imaging for the assessment of VVF. Imaging used for diagnosis of VVF includes a voiding cystogram with opacification of the vagina after filling the bladder with contrast if there is a fistula. A cystoscopy can evaluate for calculi, retained suture, level of inflammation, and location of the ureters in relation to the fistula. Renal ultrasonography is of limited use. Intravenous pyelography can miss lesions by the trigone. In general, a computed tomography (CT) urogram and magnetic resonance imaging (MRI) with bladder contrast are more sensitive.
In the diagnosis of RVF, contrast vaginoscopy, double contrast barium enema, CT scan with contrast, and MRI can be used. MRI is more sensitive.6 A high index of suspicion is required based on the patient’s history as these imaging modalities do not always confirm RVF despite patient’s clear history of leakage. When the history is convincing, a thorough rectovaginal exam under anesthesia may be imperative. If rectal trauma is present, endoanal ultrasonography can delineate external and internal anal sphincter defects.
Prolonged Foley catheter placement after obstetric injury can lead to successful closure of a VVF. Prior to surgical intervention, assessing if there is possible ureteral involvement and use of intraoperative ureteral stents is a consideration. The route of surgery can be vaginal, abdominal, combined abdominal-vaginal, laparoscopic, or robotic.7 The robotic approach is increasingly utilized.8,9 However, the general consensus among fistula surgeons is that the vaginal approach should be considered first.
Continue to: Surgical repair...
Surgical repair
VVF repair. Factors that influence successful repair of VVF include the size and number of fistula, location, degree of scarring, bladder capacity, and urethral length.
Surgical technique requires wide mobilization and adequate exposure. The fistula tract can be delineated and manipulated with a pediatric Foley catheter, ureteral stent, or even a ureteral guidewire to aid in dissection (FIGURE 4). Intraoperative visualization of the ureters, including stenting, often is needed. The fistulous track is excised depending on the level of scarring. Closure of the bladder uroepithelium for the first layer is with absorbable interrupted 3-0 or 2-0 sutures in a tension-free closure. The bladder is then evaluated with a retrograde fill with saline and methylene blue to ensure a watertight closure for the first layer. If the first layer is not watertight, the second layer closure will not compensate and the fistula will persist. Particular attention is paid to the angles of the fistula at the first layer closure to prevent recurrence of the fistula at the angles. A running second layer with absorbable 2-0 suture is done. At times, a Martius flap or an omental J flap can be used to provide an additional layer for support and to increase vascularity.10 The patient is sent home with a Foley catheter for drainage for 10 to 14 days.11 Antibiotics are not needed postoperatively for VVF surgery.12
CT cystogram or retrograde cystogram is usually done to evaluate closure of the fistula prior to removal of the Foley catheter; retrograde fill with contrast directly into the bladder with 300 mL is sufficient (FIGURE 5). Patients are advised to refrain from sexual activity for a minimum of 6 weeks, but depending on the level of complexity and scarring, this can be up to 12 weeks.
The success rate in general is in the 95% range. Patients with successful closure of VVF are at risk for urge incontinence due to decreased bladder capacity, stress incontinence especially if the continence mechanism or urethra is involved, vaginal scarring, dyspareunia, and infertility.13 In general, sexual function improves after surgical repair.
RVF repair. Prior to surgical repair of RVF, the integrity of the external anal sphincter must be determined. If it is not involved, a vertical incision is made in the posterior vaginal wall, the vaginal epithelium is separated from the vaginal muscularis, and the fistula tract is identified. After complete wide mobilization of the tissue surrounding the tract, it is excised. The rectal wall is repaired with 3-0 or 4-0 absorbable interrupted sutures; a second layer and if possible even a third layer and finally the vaginal epithelium are all closed with 2-0 absorbable interrupted sutures.
If the sphincter complex is involved, the dissection involves an inverted U incision separating the vaginal wall from the rectum. The fistula tract is excised, the rectal wall is closed, and the internal anal sphincter is identified and reapproximated with interrupted absorbable 2-0 or 0 sutures. The disrupted external sphincter is then reapproximated with 2-0 or 0 sutures, and finally the transverse perineal and bulbocavernosus muscles are brought together with Lembert 0 sutures prior to closure of the external skin. Perioperative antibiotics have been shown to improve success rates in the correction of RVF.5 In patients with sphincter trauma and known RVF, outcomes with a sphincteroplasty are better, compared with endorectal advancement flaps. The patient is discharged with a bowel regimen and dietary precautions that aim for daily soft bowel movements.
After surgical treatment of fistulas, patients benefit from pelvic floor physical therapy that focuses on pelvic floor strengthening. Incorporating the habit of Kegel exercises after every void, timed (scheduled) bladder voiding, and avoidance of straining with urination or defecation should be emphasized.
Continue to: CASE 1 Pregnant woman with rectal bleeding...
CASE 1 Pregnant woman with rectal bleeding
A 37-year-old woman at 36 3/7 weeks’ gestation presented with acute rectal bleeding and pain. This was found to result from a catastrophic rupture of a pelvic arteriovenous malformation that caused an 11 x 7 x 9.5 cm size inferior pelvic hematoma and a full-thickness rectal tear at the dentate line. During examination under anesthesia, the baby was delivered by a stat CD due to breech presentation and a prolonged fetal heart rate deceleration. The patient underwent embolization of the right middle rectal artery and right internal iliac artery by a radiologic intervention. Further bleeding required surgical intervention for evacuation of about 1,000 mL of hematoma, repair of the rectal tear, and laparoscopic diverting loop ileostomy. In total, the patient received 8 U of packed red blood cells, 6 U of fresh frozen plasma, 5 L of crystalloid solution, and 2 g of tranexamic acid. The patient reported increased foul-smelling vaginal discharge, bedside exam suggested possible fistulous tract, and on postoperative day 16, an exam under anesthesia by Urogynecology confirmed a rectovaginal fistula in the right mid vagina. After 2 months of observation to allow resolution of inflammation, successful excision of the fistula tract and repair of RVF using the above-mentioned technique was accomplished.
CASE 2 Patient with VVF after cesarean hysterectomy
A 40-year-old (G6P2222) patient underwent cesarean hysterectomy for placenta percreta and uterine rupture at 24 weeks’ gestation. Intraoperatively, there were right ureteral ligation and posterior bladder wall cystotomies. The right ureter was reimplanted in the right upper posterior wall and the cystostomies were closed. As the patient had continuous urinary leakage postoperatively, a CT urogram was obtained, which showed left ureteral obstruction and VVF. Urinary incontinence persisted despite bilateral robotic ureteral reimplantation with omental flap by the urology team. Percutaneous nephrostomy tubes were placed bilaterally. The patient underwent additional imaging studies, including MRI, with findings of VVF and possible ureterovaginal fistula.
On referral to Urogynecology, the patient underwent cystoscopy with antegrade pyelogram, and the bilateral ureteroneocystostomy orifices had 5 French open-ended ureteral stents placed. A 10 French pediatric Foley catheter was inserted intravaginally into the bladder through the VVF. Via the vaginal approach, cervical remnant and skin bridges overlying the VVF were excised. The scarred fistula tract was excised with a circumferential incision. Horizontal interrupted Lembert sutures with 3-0 absorbable suture were used to reapproximate the first layer, which was confirmed to be watertight on testing with retrograde fill. Second-layer closure was completed with horizontal mattress 2-0 absorbable sutures, followed by a third-layer closure done in similar fashion. Fibrin glue was then placed. The vaginal epithelium was closed with 2-0 absorbable suture. Percutaneous nephrostomy tubes were removed. Postoperatively, the patient had a CT cystogram with no leak and no incontinence, but she developed urgency, which was controlled with timed voids and oxybutynin.
Although rare in the United States and more common in low-resource countries, fistulas due to obstructed labor do occur. In developed countries, other obstetric causes for fistula are usually surgery, trauma, or infection related. An abnormal communication between organs—be it the urethra, bladder, ureter, uterus, cervix, or rectum—can develop1 and lead to vesicovaginal fistula (VVF), urethrovaginal fistula (FIGURE 1), vesicocervical fistula, vesicouterine fistula, ureterovaginal fistula (FIGURE 2), and rectovaginal fistula (RVF). Other nonobstetric causes include gynecologic surgery, radiation, malignancy, and congenital malformations.
During labor, hypoxia, subsequent ischemia, and pressure necrosis contribute to fistula formation. Injury sustained during a cesarean delivery (CD) or cesarean hysterectomy can lead to fistula formation; at times, however, complications are unavoidable given the nature of the pathologic condition that the patient presents with.
VVF and RVF have a devastating impact on a woman’s quality of life as they lead to significant morbidity and short- and long-term psychological distress. The fistula may not be recognized at the time of injury. The presenting signs and symptoms may be intermittent and confusing. Immediate surgical intervention may not be possible due to ongoing inflammation or infection. Recovery often is prolonged. As there is significant concomitant postpartum anxiety and depression, patients with fistula often require psychosocial support and counseling. After repair, there is still a risk for recurrence and voiding dysfunction.
Fistula signs and symptoms and evaluation
In cases of VVF, patients present with continuing large or small volume urinary incontinence. Depending on the time to diagnosis, patients may have calculi formation, prolapse, scarring, external perineal dermatitis, perineal nerve injury, and even motor weakness. Cyclic hematuria may be seen in vesicouterine fistulas.2
Multiple classification systems for diagnosis and staging of VVF have been suggested.3,4 A classification system for RVF was published by Tsang and colleagues.5 All these classification systems have attempted to characterize fistulas in terms of level of surgical complexity for repair, providing a guideline for preoperative assessment. These classification systems do not translate into prediction regarding outcomes.
Evaluation of pelvic fistula from the urinary tract starts with a thorough history that includes onset, duration, and description of leakage (continuous, intermittent, or positional) and whether there is concomitant stress and urge incontinence. A detailed obstetric history, including circumstances around the mode of delivery, underlying risk factors, and psychosocial history, should be obtained.
The pelvic examination with a plastic speculum and adequate lighting should assess the external perineum for dermatitis; bulbocavernosus and anal reflexes; and the vagina for length, caliber, level of scarring, and any prolapse. For VVFs, the location, size, and number of the fistula tracts can be visualized and confirmed with a retrograde fill of the bladder via a Foley catheter with saline or water mixed with methylene blue or any other blue dye (FIGURE 3). If a ureterovaginal fistula is suspected, the patient can simultaneously be given oral phenazopyridine and a tampon inserted within the vagina; the patient can then ambulate, and re-examination of the end of the tampon can reveal orange staining. The bladder meanwhile is retrograde filled with blue dye, with no blue staining of the tampon.
For RVF, history taking should include the onset, duration, and description of leakage, and the external anal sphincter should be assessed, with careful examination of the distal vagina at the vestibule as this is the most common location for RVF (fistula in ano). Patients may describe vaginal flatus and sometimes only brownish discharge, which can be intermittent, leading to an incorrect diagnosis of vaginitis that is treated repeatedly without success.
There is no consensus regarding optimal imaging for the assessment of VVF. Imaging used for diagnosis of VVF includes a voiding cystogram with opacification of the vagina after filling the bladder with contrast if there is a fistula. A cystoscopy can evaluate for calculi, retained suture, level of inflammation, and location of the ureters in relation to the fistula. Renal ultrasonography is of limited use. Intravenous pyelography can miss lesions by the trigone. In general, a computed tomography (CT) urogram and magnetic resonance imaging (MRI) with bladder contrast are more sensitive.
In the diagnosis of RVF, contrast vaginoscopy, double contrast barium enema, CT scan with contrast, and MRI can be used. MRI is more sensitive.6 A high index of suspicion is required based on the patient’s history as these imaging modalities do not always confirm RVF despite patient’s clear history of leakage. When the history is convincing, a thorough rectovaginal exam under anesthesia may be imperative. If rectal trauma is present, endoanal ultrasonography can delineate external and internal anal sphincter defects.
Prolonged Foley catheter placement after obstetric injury can lead to successful closure of a VVF. Prior to surgical intervention, assessing if there is possible ureteral involvement and use of intraoperative ureteral stents is a consideration. The route of surgery can be vaginal, abdominal, combined abdominal-vaginal, laparoscopic, or robotic.7 The robotic approach is increasingly utilized.8,9 However, the general consensus among fistula surgeons is that the vaginal approach should be considered first.
Continue to: Surgical repair...
Surgical repair
VVF repair. Factors that influence successful repair of VVF include the size and number of fistula, location, degree of scarring, bladder capacity, and urethral length.
Surgical technique requires wide mobilization and adequate exposure. The fistula tract can be delineated and manipulated with a pediatric Foley catheter, ureteral stent, or even a ureteral guidewire to aid in dissection (FIGURE 4). Intraoperative visualization of the ureters, including stenting, often is needed. The fistulous track is excised depending on the level of scarring. Closure of the bladder uroepithelium for the first layer is with absorbable interrupted 3-0 or 2-0 sutures in a tension-free closure. The bladder is then evaluated with a retrograde fill with saline and methylene blue to ensure a watertight closure for the first layer. If the first layer is not watertight, the second layer closure will not compensate and the fistula will persist. Particular attention is paid to the angles of the fistula at the first layer closure to prevent recurrence of the fistula at the angles. A running second layer with absorbable 2-0 suture is done. At times, a Martius flap or an omental J flap can be used to provide an additional layer for support and to increase vascularity.10 The patient is sent home with a Foley catheter for drainage for 10 to 14 days.11 Antibiotics are not needed postoperatively for VVF surgery.12
CT cystogram or retrograde cystogram is usually done to evaluate closure of the fistula prior to removal of the Foley catheter; retrograde fill with contrast directly into the bladder with 300 mL is sufficient (FIGURE 5). Patients are advised to refrain from sexual activity for a minimum of 6 weeks, but depending on the level of complexity and scarring, this can be up to 12 weeks.
The success rate in general is in the 95% range. Patients with successful closure of VVF are at risk for urge incontinence due to decreased bladder capacity, stress incontinence especially if the continence mechanism or urethra is involved, vaginal scarring, dyspareunia, and infertility.13 In general, sexual function improves after surgical repair.
RVF repair. Prior to surgical repair of RVF, the integrity of the external anal sphincter must be determined. If it is not involved, a vertical incision is made in the posterior vaginal wall, the vaginal epithelium is separated from the vaginal muscularis, and the fistula tract is identified. After complete wide mobilization of the tissue surrounding the tract, it is excised. The rectal wall is repaired with 3-0 or 4-0 absorbable interrupted sutures; a second layer and if possible even a third layer and finally the vaginal epithelium are all closed with 2-0 absorbable interrupted sutures.
If the sphincter complex is involved, the dissection involves an inverted U incision separating the vaginal wall from the rectum. The fistula tract is excised, the rectal wall is closed, and the internal anal sphincter is identified and reapproximated with interrupted absorbable 2-0 or 0 sutures. The disrupted external sphincter is then reapproximated with 2-0 or 0 sutures, and finally the transverse perineal and bulbocavernosus muscles are brought together with Lembert 0 sutures prior to closure of the external skin. Perioperative antibiotics have been shown to improve success rates in the correction of RVF.5 In patients with sphincter trauma and known RVF, outcomes with a sphincteroplasty are better, compared with endorectal advancement flaps. The patient is discharged with a bowel regimen and dietary precautions that aim for daily soft bowel movements.
After surgical treatment of fistulas, patients benefit from pelvic floor physical therapy that focuses on pelvic floor strengthening. Incorporating the habit of Kegel exercises after every void, timed (scheduled) bladder voiding, and avoidance of straining with urination or defecation should be emphasized.
Continue to: CASE 1 Pregnant woman with rectal bleeding...
CASE 1 Pregnant woman with rectal bleeding
A 37-year-old woman at 36 3/7 weeks’ gestation presented with acute rectal bleeding and pain. This was found to result from a catastrophic rupture of a pelvic arteriovenous malformation that caused an 11 x 7 x 9.5 cm size inferior pelvic hematoma and a full-thickness rectal tear at the dentate line. During examination under anesthesia, the baby was delivered by a stat CD due to breech presentation and a prolonged fetal heart rate deceleration. The patient underwent embolization of the right middle rectal artery and right internal iliac artery by a radiologic intervention. Further bleeding required surgical intervention for evacuation of about 1,000 mL of hematoma, repair of the rectal tear, and laparoscopic diverting loop ileostomy. In total, the patient received 8 U of packed red blood cells, 6 U of fresh frozen plasma, 5 L of crystalloid solution, and 2 g of tranexamic acid. The patient reported increased foul-smelling vaginal discharge, bedside exam suggested possible fistulous tract, and on postoperative day 16, an exam under anesthesia by Urogynecology confirmed a rectovaginal fistula in the right mid vagina. After 2 months of observation to allow resolution of inflammation, successful excision of the fistula tract and repair of RVF using the above-mentioned technique was accomplished.
CASE 2 Patient with VVF after cesarean hysterectomy
A 40-year-old (G6P2222) patient underwent cesarean hysterectomy for placenta percreta and uterine rupture at 24 weeks’ gestation. Intraoperatively, there were right ureteral ligation and posterior bladder wall cystotomies. The right ureter was reimplanted in the right upper posterior wall and the cystostomies were closed. As the patient had continuous urinary leakage postoperatively, a CT urogram was obtained, which showed left ureteral obstruction and VVF. Urinary incontinence persisted despite bilateral robotic ureteral reimplantation with omental flap by the urology team. Percutaneous nephrostomy tubes were placed bilaterally. The patient underwent additional imaging studies, including MRI, with findings of VVF and possible ureterovaginal fistula.
On referral to Urogynecology, the patient underwent cystoscopy with antegrade pyelogram, and the bilateral ureteroneocystostomy orifices had 5 French open-ended ureteral stents placed. A 10 French pediatric Foley catheter was inserted intravaginally into the bladder through the VVF. Via the vaginal approach, cervical remnant and skin bridges overlying the VVF were excised. The scarred fistula tract was excised with a circumferential incision. Horizontal interrupted Lembert sutures with 3-0 absorbable suture were used to reapproximate the first layer, which was confirmed to be watertight on testing with retrograde fill. Second-layer closure was completed with horizontal mattress 2-0 absorbable sutures, followed by a third-layer closure done in similar fashion. Fibrin glue was then placed. The vaginal epithelium was closed with 2-0 absorbable suture. Percutaneous nephrostomy tubes were removed. Postoperatively, the patient had a CT cystogram with no leak and no incontinence, but she developed urgency, which was controlled with timed voids and oxybutynin.
- Adler AJ, Ronsmans C, Calvert C, et al. Estimating the presence of obstetric fistula: a systematic review and meta-analysis BMC Pregnancy Childbirth. 2013;13:246.
- Battacharjee S, Kohli UA, Sood A, et al. Vesicouterine fistula: Youssef’s syndrome. Med J Armed Forces India. 2015;71(suppl 1):S175-S177. doi: 10.1016/j.mjafi.2013.11.006.
- Waaldijk K. Step-by-Step Surgery of Vesicovaginal Fistulas. Campion Press; 1994.
- Goh, JTW. A new classification for female genital tract fistula. Aust N Z J Ob Gynecol. 2004:44:502-504.
- Tsang CB, Rothenberger DA. Rectovaginal fistulas: therapeutic options. Surg Clin North Am. 1997;77:95-114.
- Champagne BJ, McGee MF. Rectovaginal fistula. Surg Clin North Am. 2010;90:69-82.
- Bodner-Adler B, Hanzal E, Pablik E, et al. Management of vesicovaginal fistulas in women following benign gynecologic surgery: a systematic review and meta-analysis. PLoS One. 2017;12:e0171554.
- Randazzo M, Lengauer L, Rochat CH, et al. Best practices in robotic-assisted repair of vesicovaginal fistula: a consensus report from the European Association of Urology Robotic Urology Section Scientific Working Group for Reconstructive Urology. Eur Urol. 2020;78: 432-442.
- Miklos JR, Moore RD, Chinthakanan O. Laparoscopic and robotic assisted vesicovaginal fistula repair: a systematic review of the literature. J Minim Invasive Gynecol. 2015:22:727-736.
- Hancock B. Practical Obstetric Fistula Surgery. Royal Society of Medicine Press; 2009.
- Nardos R, Menber B, Browning A. Outcome of obstetric fistula repair after 10-day versus 14-day Foley catheterization. Int J Gynaecol 0bstet. 2012;118:21-23.
- Tomlinson AJ, Thornton JG. A randomized controlled trial of antibiotic prophylaxis for vesico-vaginal fistula repair. Br J Obstet Gynaecol. 2005;105:397-399.
- Bengtson AM, Kopp D, Tang JH, et al. Identifying patients with vesicovaginal fistula at high risk of urinary incontinence after surgery. Obstet Gynecol. 2016;128:945-953.
- Adler AJ, Ronsmans C, Calvert C, et al. Estimating the presence of obstetric fistula: a systematic review and meta-analysis BMC Pregnancy Childbirth. 2013;13:246.
- Battacharjee S, Kohli UA, Sood A, et al. Vesicouterine fistula: Youssef’s syndrome. Med J Armed Forces India. 2015;71(suppl 1):S175-S177. doi: 10.1016/j.mjafi.2013.11.006.
- Waaldijk K. Step-by-Step Surgery of Vesicovaginal Fistulas. Campion Press; 1994.
- Goh, JTW. A new classification for female genital tract fistula. Aust N Z J Ob Gynecol. 2004:44:502-504.
- Tsang CB, Rothenberger DA. Rectovaginal fistulas: therapeutic options. Surg Clin North Am. 1997;77:95-114.
- Champagne BJ, McGee MF. Rectovaginal fistula. Surg Clin North Am. 2010;90:69-82.
- Bodner-Adler B, Hanzal E, Pablik E, et al. Management of vesicovaginal fistulas in women following benign gynecologic surgery: a systematic review and meta-analysis. PLoS One. 2017;12:e0171554.
- Randazzo M, Lengauer L, Rochat CH, et al. Best practices in robotic-assisted repair of vesicovaginal fistula: a consensus report from the European Association of Urology Robotic Urology Section Scientific Working Group for Reconstructive Urology. Eur Urol. 2020;78: 432-442.
- Miklos JR, Moore RD, Chinthakanan O. Laparoscopic and robotic assisted vesicovaginal fistula repair: a systematic review of the literature. J Minim Invasive Gynecol. 2015:22:727-736.
- Hancock B. Practical Obstetric Fistula Surgery. Royal Society of Medicine Press; 2009.
- Nardos R, Menber B, Browning A. Outcome of obstetric fistula repair after 10-day versus 14-day Foley catheterization. Int J Gynaecol 0bstet. 2012;118:21-23.
- Tomlinson AJ, Thornton JG. A randomized controlled trial of antibiotic prophylaxis for vesico-vaginal fistula repair. Br J Obstet Gynaecol. 2005;105:397-399.
- Bengtson AM, Kopp D, Tang JH, et al. Identifying patients with vesicovaginal fistula at high risk of urinary incontinence after surgery. Obstet Gynecol. 2016;128:945-953.