User login
Q) My hematuria patient had more significant proteinuria recently, so the nephrologist sent him for kidney biopsy. It was read as IgA nephropathy: “classic mesangial staining on IF with moderate-advanced chronic injury (15/32 gloms globally sclerosed, 40% IFTA, mild arteriosclerosis).” What exactly does this mean, and what is IgA nephropathy?
Immunoglobulin A (IgA) nephropathy is the most common type of glomerulonephritis; up to 40% of patients with IgA nephropathy develop end-stage renal disease within 20 years of diagnosis. More common in men, IgA nephropathy is usually diagnosed in people in their second or third decades of life.2,3
Considered an autoimmune disease, IgA nephropathy typically presents with macroscopic or gross hematuria that occurs within 24 hours of the onset of an upper respiratory infection (URI). The hematuria typically resolves quickly, in one to three days. An individual bacterial or viral element has not yet been identified.
IgA nephropathy is an immune response to the URI. IgA is secreted from mucosal surfaces at the back of the mouth and then deposited in the glomerular mesangium, a “stalk of cells” associated with the capillaries of the renal glomerulus.1 It is speculated that genetics, environment, and/or hypersensitivity to food antigens may play a part in IgA nephropathy. Results from biopsies of blood relatives of patients with confirmed IgA nephropathy suggest a familial role.1
IgA nephropathy is prevalent in persons who live in the Pacific Rim and Southern Europe. However, this association may be the result of a sampling error due to investigation of all microscopic hematuria in these areas. In all, 90% of IgA is sporadic.4 It is often asymptomatic, aside from occasional back and flank pain secondary to inflammation of the renal capsule. Unfortunately, many patients develop renal impairment and hypertension by the time they are diagnosed.
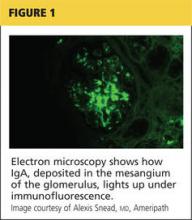
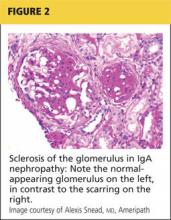
Renal biopsy is used to confirm/diagnose IgA nephropathy. IgA, deposited in the mesangium of the glomerulus, lights up under immunofluorescence (IF; see Figure 1). In some patients, this mesangial deposition results in sclerosis, scarring, and/or inflammation of the glomerulus (see Figure 2).
An international panel of experts created guidelines (the Oxford classification system) for reporting IgA kidney biopsies. Six adverse pathologic features have been identified:
• Mesangial cellularity score
• Percentage of segmental sclerosis
• Endocapillary hypercellularity
• Cellular and/or fibrocellular crescents
• Percentage of interstitial fibrosis/tubular atrophy (IFTA)
• Arteriosclerosis score5,6
Interstitial fibrosis, crescents, and as little as 25% glomerular sclerosis found on biopsy increases the likelihood of disease progression.5 Clinically, hypertension, a reduced glomerular filtration rate, increasing age, and proteinuria of > 1g/24h have been identified as risk factors for progression of IgA nephropathy. Up to 30% of patients diagnosed will require renal replacement therapy within 20 years.1
The case patient’s findings include the typical IF staining of IgA in the glomerulus. The biopsy report also indicates that 40% of the glomeruli (less than half) have interstitial fibrosis and that the structural integrity of the tubules has been affected secondary to IgA accumulation in the mesangium. These findings are suggestive of progressive disease.
There is no known way to stop IgA deposition in the mesangium. Tonsillectomy to reduce mucosal IgA release has been suggested but is controversial.
Treatment of IgA nephropathy focuses on preserving renal function by reducing proteinuria through the use of ACE inhibitors and/or angiotensin receptor blockers. Aggressive blood pressure management is achieved by blocking the renin-angiotensin-aldosterone system (RAAS).
Other methods for decreasing progression of renal disease are directed at reducing the immune and inflammatory response via immunosuppressant medications.3 The use of immunosuppressive agents, though controversial, is recommended for those who have progressive disease and/or proteinuria despite achieving target blood pressure with full RAAS blockade.1
Amy L. Hazel, RN, MSN, CNP
Kidney & Hypertension Consultants, Canton, Ohio
REFERENCES
1. Greenberg A. Primer on Kidney Diseases. 5th ed. Philadelphia, PA: Elsevier Saunders; 2005.
2. Barratt J, Feehally J. IgA nephropathy [disease of the month]. J Am Soc Nephrol. 2005;16(7): 2088-2097.
3. Barratt J, Feehally J. Treatment of IgA nephropathy. Kidney Int. 2006;69(11):1934-1938.
4. Johnson R, Feehally J. Comprehensive Clinical Nephrology. 2nd ed. London: Mosby; 2000.
5. Walsh M, Sar A, Lee D, et al. Histopathologic features aid in predicting risk for progression of IgA nephropathy. Clin J Am Soc Nephrol. 2010; 5(3):425-430.
6. Roberts I. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009; 76(5):546-556.
Q) My hematuria patient had more significant proteinuria recently, so the nephrologist sent him for kidney biopsy. It was read as IgA nephropathy: “classic mesangial staining on IF with moderate-advanced chronic injury (15/32 gloms globally sclerosed, 40% IFTA, mild arteriosclerosis).” What exactly does this mean, and what is IgA nephropathy?
Immunoglobulin A (IgA) nephropathy is the most common type of glomerulonephritis; up to 40% of patients with IgA nephropathy develop end-stage renal disease within 20 years of diagnosis. More common in men, IgA nephropathy is usually diagnosed in people in their second or third decades of life.2,3
Considered an autoimmune disease, IgA nephropathy typically presents with macroscopic or gross hematuria that occurs within 24 hours of the onset of an upper respiratory infection (URI). The hematuria typically resolves quickly, in one to three days. An individual bacterial or viral element has not yet been identified.
IgA nephropathy is an immune response to the URI. IgA is secreted from mucosal surfaces at the back of the mouth and then deposited in the glomerular mesangium, a “stalk of cells” associated with the capillaries of the renal glomerulus.1 It is speculated that genetics, environment, and/or hypersensitivity to food antigens may play a part in IgA nephropathy. Results from biopsies of blood relatives of patients with confirmed IgA nephropathy suggest a familial role.1
IgA nephropathy is prevalent in persons who live in the Pacific Rim and Southern Europe. However, this association may be the result of a sampling error due to investigation of all microscopic hematuria in these areas. In all, 90% of IgA is sporadic.4 It is often asymptomatic, aside from occasional back and flank pain secondary to inflammation of the renal capsule. Unfortunately, many patients develop renal impairment and hypertension by the time they are diagnosed.
Renal biopsy is used to confirm/diagnose IgA nephropathy. IgA, deposited in the mesangium of the glomerulus, lights up under immunofluorescence (IF; see Figure 1). In some patients, this mesangial deposition results in sclerosis, scarring, and/or inflammation of the glomerulus (see Figure 2).
An international panel of experts created guidelines (the Oxford classification system) for reporting IgA kidney biopsies. Six adverse pathologic features have been identified:
• Mesangial cellularity score
• Percentage of segmental sclerosis
• Endocapillary hypercellularity
• Cellular and/or fibrocellular crescents
• Percentage of interstitial fibrosis/tubular atrophy (IFTA)
• Arteriosclerosis score5,6
Interstitial fibrosis, crescents, and as little as 25% glomerular sclerosis found on biopsy increases the likelihood of disease progression.5 Clinically, hypertension, a reduced glomerular filtration rate, increasing age, and proteinuria of > 1g/24h have been identified as risk factors for progression of IgA nephropathy. Up to 30% of patients diagnosed will require renal replacement therapy within 20 years.1
The case patient’s findings include the typical IF staining of IgA in the glomerulus. The biopsy report also indicates that 40% of the glomeruli (less than half) have interstitial fibrosis and that the structural integrity of the tubules has been affected secondary to IgA accumulation in the mesangium. These findings are suggestive of progressive disease.
There is no known way to stop IgA deposition in the mesangium. Tonsillectomy to reduce mucosal IgA release has been suggested but is controversial.
Treatment of IgA nephropathy focuses on preserving renal function by reducing proteinuria through the use of ACE inhibitors and/or angiotensin receptor blockers. Aggressive blood pressure management is achieved by blocking the renin-angiotensin-aldosterone system (RAAS).
Other methods for decreasing progression of renal disease are directed at reducing the immune and inflammatory response via immunosuppressant medications.3 The use of immunosuppressive agents, though controversial, is recommended for those who have progressive disease and/or proteinuria despite achieving target blood pressure with full RAAS blockade.1
Amy L. Hazel, RN, MSN, CNP
Kidney & Hypertension Consultants, Canton, Ohio
REFERENCES
1. Greenberg A. Primer on Kidney Diseases. 5th ed. Philadelphia, PA: Elsevier Saunders; 2005.
2. Barratt J, Feehally J. IgA nephropathy [disease of the month]. J Am Soc Nephrol. 2005;16(7): 2088-2097.
3. Barratt J, Feehally J. Treatment of IgA nephropathy. Kidney Int. 2006;69(11):1934-1938.
4. Johnson R, Feehally J. Comprehensive Clinical Nephrology. 2nd ed. London: Mosby; 2000.
5. Walsh M, Sar A, Lee D, et al. Histopathologic features aid in predicting risk for progression of IgA nephropathy. Clin J Am Soc Nephrol. 2010; 5(3):425-430.
6. Roberts I. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009; 76(5):546-556.
Q) My hematuria patient had more significant proteinuria recently, so the nephrologist sent him for kidney biopsy. It was read as IgA nephropathy: “classic mesangial staining on IF with moderate-advanced chronic injury (15/32 gloms globally sclerosed, 40% IFTA, mild arteriosclerosis).” What exactly does this mean, and what is IgA nephropathy?
Immunoglobulin A (IgA) nephropathy is the most common type of glomerulonephritis; up to 40% of patients with IgA nephropathy develop end-stage renal disease within 20 years of diagnosis. More common in men, IgA nephropathy is usually diagnosed in people in their second or third decades of life.2,3
Considered an autoimmune disease, IgA nephropathy typically presents with macroscopic or gross hematuria that occurs within 24 hours of the onset of an upper respiratory infection (URI). The hematuria typically resolves quickly, in one to three days. An individual bacterial or viral element has not yet been identified.
IgA nephropathy is an immune response to the URI. IgA is secreted from mucosal surfaces at the back of the mouth and then deposited in the glomerular mesangium, a “stalk of cells” associated with the capillaries of the renal glomerulus.1 It is speculated that genetics, environment, and/or hypersensitivity to food antigens may play a part in IgA nephropathy. Results from biopsies of blood relatives of patients with confirmed IgA nephropathy suggest a familial role.1
IgA nephropathy is prevalent in persons who live in the Pacific Rim and Southern Europe. However, this association may be the result of a sampling error due to investigation of all microscopic hematuria in these areas. In all, 90% of IgA is sporadic.4 It is often asymptomatic, aside from occasional back and flank pain secondary to inflammation of the renal capsule. Unfortunately, many patients develop renal impairment and hypertension by the time they are diagnosed.
Renal biopsy is used to confirm/diagnose IgA nephropathy. IgA, deposited in the mesangium of the glomerulus, lights up under immunofluorescence (IF; see Figure 1). In some patients, this mesangial deposition results in sclerosis, scarring, and/or inflammation of the glomerulus (see Figure 2).
An international panel of experts created guidelines (the Oxford classification system) for reporting IgA kidney biopsies. Six adverse pathologic features have been identified:
• Mesangial cellularity score
• Percentage of segmental sclerosis
• Endocapillary hypercellularity
• Cellular and/or fibrocellular crescents
• Percentage of interstitial fibrosis/tubular atrophy (IFTA)
• Arteriosclerosis score5,6
Interstitial fibrosis, crescents, and as little as 25% glomerular sclerosis found on biopsy increases the likelihood of disease progression.5 Clinically, hypertension, a reduced glomerular filtration rate, increasing age, and proteinuria of > 1g/24h have been identified as risk factors for progression of IgA nephropathy. Up to 30% of patients diagnosed will require renal replacement therapy within 20 years.1
The case patient’s findings include the typical IF staining of IgA in the glomerulus. The biopsy report also indicates that 40% of the glomeruli (less than half) have interstitial fibrosis and that the structural integrity of the tubules has been affected secondary to IgA accumulation in the mesangium. These findings are suggestive of progressive disease.
There is no known way to stop IgA deposition in the mesangium. Tonsillectomy to reduce mucosal IgA release has been suggested but is controversial.
Treatment of IgA nephropathy focuses on preserving renal function by reducing proteinuria through the use of ACE inhibitors and/or angiotensin receptor blockers. Aggressive blood pressure management is achieved by blocking the renin-angiotensin-aldosterone system (RAAS).
Other methods for decreasing progression of renal disease are directed at reducing the immune and inflammatory response via immunosuppressant medications.3 The use of immunosuppressive agents, though controversial, is recommended for those who have progressive disease and/or proteinuria despite achieving target blood pressure with full RAAS blockade.1
Amy L. Hazel, RN, MSN, CNP
Kidney & Hypertension Consultants, Canton, Ohio
REFERENCES
1. Greenberg A. Primer on Kidney Diseases. 5th ed. Philadelphia, PA: Elsevier Saunders; 2005.
2. Barratt J, Feehally J. IgA nephropathy [disease of the month]. J Am Soc Nephrol. 2005;16(7): 2088-2097.
3. Barratt J, Feehally J. Treatment of IgA nephropathy. Kidney Int. 2006;69(11):1934-1938.
4. Johnson R, Feehally J. Comprehensive Clinical Nephrology. 2nd ed. London: Mosby; 2000.
5. Walsh M, Sar A, Lee D, et al. Histopathologic features aid in predicting risk for progression of IgA nephropathy. Clin J Am Soc Nephrol. 2010; 5(3):425-430.
6. Roberts I. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009; 76(5):546-556.