User login
The circulation of cerebrospinal fluid (CSF) is crucial for maintaining homeostasis for the optimal functioning of the multiple complex activities of the brain and spinal cord, including the disposal of metabolic waste products of brain and spinal cord activity into the cerebral venous drainage. Throughout the brain, the arachnoid mater forms small outpouchings or diverticula that penetrate the dura mater and communicate with the dural venous sinuses. These outpuchings are called arachnoid granulations or arachnoid villi, and most are found within the dural sinuses, primarily in the transverse sinuses and superior sagittal sinus, but can occasionally be seen extending into the inner table of the calvarium.1,2
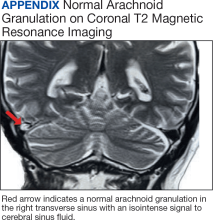
The amount of arachnoid granulations seen in bone, particularly around the superior sagittal sinus, may increase with age.2 Arachnoid granulations are generally small but the largest ones can be seen on gross examination during intracranial procedures or autopsy.3 Magnetic resonance imaging (MRI) can detect arachnoid granulations, which are characterized as T1 hypointense and T2 hyperintense (CSF isointense), well-circumscribed, small, nonenhancing masses within the dural sinuses or in the diploic space (Figure 1). Even small arachnoid granulations < 1 mm in length can be detected.2
Smaller arachnoid granulations have been described histologically as entirely covered by a dural membrane, thus creating a subdural space that separates the body of the arachnoid granulation from the lumen of the accompanying venous sinus.4 However, larger arachnoid granulations may not be completely covered by a dural membrane, thus creating a point of contact between the arachnoid granulation and the venous sinus.4 Larger arachnoid granulations are normally filled with CSF, and their signal characteristics are similar to CSF on imaging.5,6 Arachnoid granulations also often contain vessels draining into the adjacent venous sinus.5,6
When larger arachnoid granulations are present, they may permit the protrusion of herniated brain tissue. There has been an increasing number of reports of these brain herniations into arachnoid granulations (BHAGs) in the literature.7-10 While these herniations have been associated with nonspecific neurologic symptoms like tinnitus and idiopathic intracranial hypertension, their true clinical significance remains undetermined.10,11 This article presents 5 cases of BHAG, discusses their clinical presentations and image findings, and reviews the current literature.
Case 1
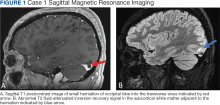
A 30-year-old male with a history of multiple traumatic brain injuries presented for evaluation of seizures. The patient described the semiology of the seizures as a bright, colorful light in his right visual field, followed by loss of vision, then loss of awareness and full body convulsion. The semiology of this patient’s seizures was consistent with left temporo-occipital lobe seizure. The only abnormality seen in the brain MRI was the herniation of brain parenchyma originating from the occipital lobe into the transverse sinus, presumably through an arachnoid granulation (Figure 1). An electroencephalogram (EEG) was unremarkable, though the semiology of the seizure historically described by the patient was localized to the area of BHAG. The patient is currently taking antiseizure medications and has experienced no additional seizures.
Case 2
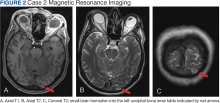
A male aged 53 years with a history of peripheral artery disease presented with a 6-month history of headaches and dizziness. The patient reported the onset of visual aura to his right visual field, starting as a fingernail-sized scintillating kaleidoscope light that would gradually increase in size to a round shape with fading kaleidoscope colors. This episode would last for a few minutes and was immediately followed by a headache. There was no alteration of consciousness during visual aura, although sometimes the patient would have right-sided scalp tingling. These episodes were often unprovoked, but occasionally triggered by bright lights. A single routine EEG was unremarkable. The patient reported headaches without aura, but not aura without headaches, which made occipital lobe seizure less likely. MRI demonstrated a small herniation of brain parenchyma into the inner table of the left occipital bone (Figure 2). The patient was diagnosed with migraine with aura, and the semiology of the visual aura corresponded to the location of the herniation in the left occipital region.
Case 3
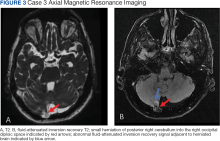
A 77-year-old male with a history of left ear diving injury presented with left-sided asymmetric hearing loss and word recognition difficulty for several years. MRI obtained as part of his work-up to evaluate for possible schwannoma of the eighth left cranial nerve instead demonstrated an incidental right cerebellar herniation within an arachnoid granulation into the diploic space of the occipital bone (Figure 3). The BHAG for this patient appeared to be an incidental finding unrelated to his asymmetric hearing loss.
Case 4
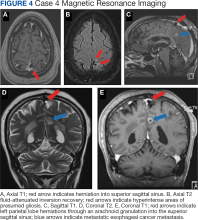
A male aged 62 years with a history of metastatic esophageal cancer, substance abuse, and a prior presumed alcohol withdrawal seizure underwent an MRI for evaluation of brain metastasis after presenting to the hospital with confusion 1 day after starting chemotherapy (Figure 4). Nine years prior, the patient had an isolated generalized tonic-clonic seizure approximately 72 hours following a period of alcohol cessation. The MRI demonstrated an incidental left parasagittal herniation of left parietal lobe tissue through an arachnoid granulation into the superior sagittal sinus, in addition to metastatic brain lesions. An EEG showed mild encephalopathy without evidence of seizures. It was determined that the patient's confusion was most likely due to toxic-metabolic encephalopathy from chemotherapy.
Case 5
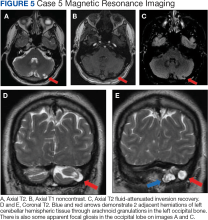
A 51-year-old male presented with worsening headache severity and frequency. He had a history of chronic headaches for about 20 years that occurred annually, but were now occurring twice weekly. The headaches often started with a left eye visual aura followed by pressure in the left eye, left frontal region, and left ear, with at times a cervicogenic component. No cervical spine imaging was available. An MRI revealed 2 small adjacent areas of cerebellar herniation into arachnoid granulations in the left occipital bone (Figure 5).
Discussion
Arachnoid granulations appear very early in life, although they are uncommon before age 2 years.2 Classically, they have been understood to act as 1-way valves permitting the outflow of CSF from the subarachnoid space to the dural venous sinuses. However, increasing evidence shows they may only play a minor role in that process.12 The structure of arachnoid granulations is being reexamined. A recent microscopy study demonstrated structural heterogeneity with a fine, porous lining that permits flow.13 Additionally, associated immune components in the microenvironment suggests that arachnoid granulations may function similarly to lymph nodes as part of a central nervous system lymphatic network.13 Evidence is lacking for arachnoid granulations being the primary route of CSF outflow, and newer models include CSF exit pathways along the cranial nerves and drainage through lymphatics within the dura mater.12
New MRI systems have demonstrated that the prevalence of arachnoid granulations increases with age. One study found that all subjects in the aged 40 years cohort had detectable arachnoid granulations on images obtained with a 3T MRI system, with the main site being the superior sagittal sinus.2 The prevalence increased until age 40 years and then noticeably decreased. Not only did the prevalence increase in this pattern, but the total number of detectable arachnoid granulations followed a similar pattern.2 In addition, the detectable arachnoid granulations tend to be larger in older patients. Arachnoid granulations are very common in adults, but little is known about when and why brain tissue herniates through these structures.
This case series illustrates how a small amount of adult cerebral or cerebellar matter in large arachnoid granulations can herniate into the dural sinuses and diploic space. Although arachnoid granulations extending into the dural sinuses and diploic space are a relatively common finding on MRI,BHAGs are rare in these locations.1,2,8 Improved spatial resolution afforded by newer high-field scanners with thinner sections, such as very thin (1 mm) T1- and heavily T2-weighted 3 dimensional sequences may lead to increased detection of BHAG. Some of these herniations are small and may be easily missed or confused for normal arachnoid granulations on 3 to 5 mm thickness MRIs.
Despite increased recognition, it is still uncertain to what degree these herniations contribute to the clinical presentations. Associated neurologic symptoms may include seizures, headaches, tinnitus, syncope, and increased intracranial pressure.7-10
Three cases presented in this article demonstrated abnormal signals adjacent to the herniated brain, presumably due to dysplasia of gliotic tissue. In 1 study, parenchymal signal and structural changes occurred in about one-half of the reported BHAG, all of which were cerebellar herniations.7 In Case 1, the herniation and adjacent abnormal MRI signal corresponded to localization of the seizure semiology as obtained from patient history, strongly suggesting the BHAG played a role in the presentation. Signal abnormality accompanying an adjacent BHAG may suggest a higher likelihood that the BHAG has clinical relevance. However, the patient in Case 2 had a visual aura that corresponded to the BHAG location, so a signal abnormality may not be necessary for a patient to develop symptoms. Case 1 also included a history of documented traumatic brain injuries, suggesting that perhaps head trauma may facilitate BHAG development. Regardless, there is likely also a congenital component to their formation, as BHAG has been observed in the pediatric population.14
The patient's asymmetric left-sided hearing loss in Case 3 appeared unrelated to the BHAG as its location was in the contralateral cerebellar region and did not correspond to the patient’s clinical findings. The patient in Case 4 had a limited history regarding localization details of their prior presumed alcohol withdrawal seizure, such as head movements, eye deviation, or lateralized onset of convulsions. Given this limited data, it is unclear whether their prior seizure could have been related to BHAG or not. The patient in case 5 reported worsening headaches on the left side of his head, which corresponded to BHAG occurring on the left side. However, given that the increased T2 signal occurred in the left cerebellar hemisphere with BHAG in the left occipital bone, the occipital cortex was not involved. In this case, the BHAG would not explain the patient’s visual aura as such a lesion would have been expected in the right occipital cortex rather than its actual location in this patient’s left cerebellar hemisphere.
CONCLUSIONS
Understanding the clinical impact of brain herniations is important because they are probably more common than previously thought. Improved MRI capabilities suggest that more BHAG will be detected moving forward as radiologists interpret images with higher resolution and thinner slices. Until its significance is fully understood, BHAG will continue to complicate the diagnosis of patients with neurologic complaints whose brain MRIs and EEGs are otherwise unremarkable.
There have been no cases of surgical BHAG intervention and pathology analysis that would help determine their clinical significance. A related entity, temporal lobe encephalocele, has been linked to focal temporal lobe epilepsy, which has demonstrated significant symptom improvement following surgical correction.15 However, encephaloceles have been distinguished from BHAG in part because they do not necessarily herniate through an arachnoid granulation.8 BHAG has only begun to be characterized in detail over the last decade, so more research is needed to understand how it develops and what clinical significance it truly holds.
1. Ikushima I, Korogi Y, Makita O, et al. MRI of arachnoid granulations within the dural sinuses using a FLAIR pulse sequence. Br J Radiol. 1999;72(863):1046-1051. doi:10.1259/bjr.72.863.10700819
2. Rados M, Zivko M, Perisa A, Oreskovic D, Klarica M. No arachnoid granulations-no problems: number, size, and distribution of arachnoid granulations from birth to 80 years of age. Front Aging Neurosci. 2021;13:698865. doi:10.3389/fnagi.2021.698865
3. Grossman CB, Potts DG. Arachnoid granulations: radiology and anatomy. Radiology. 1974;113(1):95-100. doi:10.1148/113.1.95
4. Wolpow ER, Schaumburg HH. Structure of the human arachnoid granulation. J Neurosurg. 1972;37(6):724-727. doi:10.3171/jns.1972.37.6.0724
5. Leach JL, Jones BV, Tomsick TA, Stewart CA, Balko MG. Normal appearance of arachnoid granulations on contrast-enhanced CT and MR of the brain: differentiation from dural sinus disease. AJNR Am J Neuroradiol. 1996;17(8):1523-1532.
6. Roche J, Warner D. Arachnoid granulations in the transverse and sigmoid sinuses: CT, MR, and MR angiographic appearance of a normal anatomic variation. AJNR Am J Neuroradiol. 1996;17(4):677-683.
7. Malekzadehlashkariani S, Wanke I, Rufenacht DA, San Millan D. Brain herniations into arachnoid granulations: about 68 cases in 38 patients and review of the literature. Neuroradiology. 2016;58(5):443-457. doi:10.1007/s00234-016-1662-5
8. Battal B, Castillo M. Brain herniations into the dural venous sinuses or calvarium: MRI of a recently recognized entity. Neuroradiol J. 2014;27(1):55-62. doi:10.15274/NRJ-2014-10006
9. Liebo GB, Lane JJ, Van Gompel JJ, Eckel LJ, Schwartz KM, Lehman VT. Brain herniation into arachnoid granulations: clinical and neuroimaging features. J Neuroimaging. 2016;26(6):592-598. doi:10.1111/jon.12366
10. Smith ER, Caton MT, Villanueva-Meyer JE, et al. Brain herniation (encephalocele) into arachnoid granulations: Prevalence and association with pulsatile tinnitus and idiopathic intracranial hypertension. Neuroradiology. 2022;64(9):1747-1754.
11. Battal B, Hamcan S, Akgun V, et al. Brain herniations into the dural venous sinus or calvarium: MRI findings, possible causes and clinical significance. Eur Radiol. 2016;26(6):1723-1731.
12. Proulx ST. Cerebrospinal fluid outflow: A review of the historical and contemporary evidence for arachnoid villi, perineural routes, and dural lymphatics. Cell Mol Life Sci. 2021;78(6):2429-2457.
13. Shah T, Leurgans SE, Mehta RI, et al. Arachnoid granulations are lymphatic conduits that communicate with bone marrow and dura-arachnoid stroma. J Exp Med. 2023;220(2).
14. Sade R, Ogul H, Polat G, Pirimoglu B, Kantarci M. Brain herniation into the transverse sinuses’ arachnoid granulations in the pediatric population investigated with 3 T MRI. Acta Neurol Belg. 2019;119(2):225-231.
15. Saavalainen T, Jutila L, Mervaala E, Kalviainen R, Vanninen R, Immonen A. Temporal anteroinferior encephalocele: An underrecognized etiology of temporal lobe epilepsy? Neurology. 2015;85(17):1467-1474.
The circulation of cerebrospinal fluid (CSF) is crucial for maintaining homeostasis for the optimal functioning of the multiple complex activities of the brain and spinal cord, including the disposal of metabolic waste products of brain and spinal cord activity into the cerebral venous drainage. Throughout the brain, the arachnoid mater forms small outpouchings or diverticula that penetrate the dura mater and communicate with the dural venous sinuses. These outpuchings are called arachnoid granulations or arachnoid villi, and most are found within the dural sinuses, primarily in the transverse sinuses and superior sagittal sinus, but can occasionally be seen extending into the inner table of the calvarium.1,2
The amount of arachnoid granulations seen in bone, particularly around the superior sagittal sinus, may increase with age.2 Arachnoid granulations are generally small but the largest ones can be seen on gross examination during intracranial procedures or autopsy.3 Magnetic resonance imaging (MRI) can detect arachnoid granulations, which are characterized as T1 hypointense and T2 hyperintense (CSF isointense), well-circumscribed, small, nonenhancing masses within the dural sinuses or in the diploic space (Figure 1). Even small arachnoid granulations < 1 mm in length can be detected.2
Smaller arachnoid granulations have been described histologically as entirely covered by a dural membrane, thus creating a subdural space that separates the body of the arachnoid granulation from the lumen of the accompanying venous sinus.4 However, larger arachnoid granulations may not be completely covered by a dural membrane, thus creating a point of contact between the arachnoid granulation and the venous sinus.4 Larger arachnoid granulations are normally filled with CSF, and their signal characteristics are similar to CSF on imaging.5,6 Arachnoid granulations also often contain vessels draining into the adjacent venous sinus.5,6
When larger arachnoid granulations are present, they may permit the protrusion of herniated brain tissue. There has been an increasing number of reports of these brain herniations into arachnoid granulations (BHAGs) in the literature.7-10 While these herniations have been associated with nonspecific neurologic symptoms like tinnitus and idiopathic intracranial hypertension, their true clinical significance remains undetermined.10,11 This article presents 5 cases of BHAG, discusses their clinical presentations and image findings, and reviews the current literature.
Case 1
A 30-year-old male with a history of multiple traumatic brain injuries presented for evaluation of seizures. The patient described the semiology of the seizures as a bright, colorful light in his right visual field, followed by loss of vision, then loss of awareness and full body convulsion. The semiology of this patient’s seizures was consistent with left temporo-occipital lobe seizure. The only abnormality seen in the brain MRI was the herniation of brain parenchyma originating from the occipital lobe into the transverse sinus, presumably through an arachnoid granulation (Figure 1). An electroencephalogram (EEG) was unremarkable, though the semiology of the seizure historically described by the patient was localized to the area of BHAG. The patient is currently taking antiseizure medications and has experienced no additional seizures.
Case 2
A male aged 53 years with a history of peripheral artery disease presented with a 6-month history of headaches and dizziness. The patient reported the onset of visual aura to his right visual field, starting as a fingernail-sized scintillating kaleidoscope light that would gradually increase in size to a round shape with fading kaleidoscope colors. This episode would last for a few minutes and was immediately followed by a headache. There was no alteration of consciousness during visual aura, although sometimes the patient would have right-sided scalp tingling. These episodes were often unprovoked, but occasionally triggered by bright lights. A single routine EEG was unremarkable. The patient reported headaches without aura, but not aura without headaches, which made occipital lobe seizure less likely. MRI demonstrated a small herniation of brain parenchyma into the inner table of the left occipital bone (Figure 2). The patient was diagnosed with migraine with aura, and the semiology of the visual aura corresponded to the location of the herniation in the left occipital region.
Case 3
A 77-year-old male with a history of left ear diving injury presented with left-sided asymmetric hearing loss and word recognition difficulty for several years. MRI obtained as part of his work-up to evaluate for possible schwannoma of the eighth left cranial nerve instead demonstrated an incidental right cerebellar herniation within an arachnoid granulation into the diploic space of the occipital bone (Figure 3). The BHAG for this patient appeared to be an incidental finding unrelated to his asymmetric hearing loss.
Case 4
A male aged 62 years with a history of metastatic esophageal cancer, substance abuse, and a prior presumed alcohol withdrawal seizure underwent an MRI for evaluation of brain metastasis after presenting to the hospital with confusion 1 day after starting chemotherapy (Figure 4). Nine years prior, the patient had an isolated generalized tonic-clonic seizure approximately 72 hours following a period of alcohol cessation. The MRI demonstrated an incidental left parasagittal herniation of left parietal lobe tissue through an arachnoid granulation into the superior sagittal sinus, in addition to metastatic brain lesions. An EEG showed mild encephalopathy without evidence of seizures. It was determined that the patient's confusion was most likely due to toxic-metabolic encephalopathy from chemotherapy.
Case 5
A 51-year-old male presented with worsening headache severity and frequency. He had a history of chronic headaches for about 20 years that occurred annually, but were now occurring twice weekly. The headaches often started with a left eye visual aura followed by pressure in the left eye, left frontal region, and left ear, with at times a cervicogenic component. No cervical spine imaging was available. An MRI revealed 2 small adjacent areas of cerebellar herniation into arachnoid granulations in the left occipital bone (Figure 5).
Discussion
Arachnoid granulations appear very early in life, although they are uncommon before age 2 years.2 Classically, they have been understood to act as 1-way valves permitting the outflow of CSF from the subarachnoid space to the dural venous sinuses. However, increasing evidence shows they may only play a minor role in that process.12 The structure of arachnoid granulations is being reexamined. A recent microscopy study demonstrated structural heterogeneity with a fine, porous lining that permits flow.13 Additionally, associated immune components in the microenvironment suggests that arachnoid granulations may function similarly to lymph nodes as part of a central nervous system lymphatic network.13 Evidence is lacking for arachnoid granulations being the primary route of CSF outflow, and newer models include CSF exit pathways along the cranial nerves and drainage through lymphatics within the dura mater.12
New MRI systems have demonstrated that the prevalence of arachnoid granulations increases with age. One study found that all subjects in the aged 40 years cohort had detectable arachnoid granulations on images obtained with a 3T MRI system, with the main site being the superior sagittal sinus.2 The prevalence increased until age 40 years and then noticeably decreased. Not only did the prevalence increase in this pattern, but the total number of detectable arachnoid granulations followed a similar pattern.2 In addition, the detectable arachnoid granulations tend to be larger in older patients. Arachnoid granulations are very common in adults, but little is known about when and why brain tissue herniates through these structures.
This case series illustrates how a small amount of adult cerebral or cerebellar matter in large arachnoid granulations can herniate into the dural sinuses and diploic space. Although arachnoid granulations extending into the dural sinuses and diploic space are a relatively common finding on MRI,BHAGs are rare in these locations.1,2,8 Improved spatial resolution afforded by newer high-field scanners with thinner sections, such as very thin (1 mm) T1- and heavily T2-weighted 3 dimensional sequences may lead to increased detection of BHAG. Some of these herniations are small and may be easily missed or confused for normal arachnoid granulations on 3 to 5 mm thickness MRIs.
Despite increased recognition, it is still uncertain to what degree these herniations contribute to the clinical presentations. Associated neurologic symptoms may include seizures, headaches, tinnitus, syncope, and increased intracranial pressure.7-10
Three cases presented in this article demonstrated abnormal signals adjacent to the herniated brain, presumably due to dysplasia of gliotic tissue. In 1 study, parenchymal signal and structural changes occurred in about one-half of the reported BHAG, all of which were cerebellar herniations.7 In Case 1, the herniation and adjacent abnormal MRI signal corresponded to localization of the seizure semiology as obtained from patient history, strongly suggesting the BHAG played a role in the presentation. Signal abnormality accompanying an adjacent BHAG may suggest a higher likelihood that the BHAG has clinical relevance. However, the patient in Case 2 had a visual aura that corresponded to the BHAG location, so a signal abnormality may not be necessary for a patient to develop symptoms. Case 1 also included a history of documented traumatic brain injuries, suggesting that perhaps head trauma may facilitate BHAG development. Regardless, there is likely also a congenital component to their formation, as BHAG has been observed in the pediatric population.14
The patient's asymmetric left-sided hearing loss in Case 3 appeared unrelated to the BHAG as its location was in the contralateral cerebellar region and did not correspond to the patient’s clinical findings. The patient in Case 4 had a limited history regarding localization details of their prior presumed alcohol withdrawal seizure, such as head movements, eye deviation, or lateralized onset of convulsions. Given this limited data, it is unclear whether their prior seizure could have been related to BHAG or not. The patient in case 5 reported worsening headaches on the left side of his head, which corresponded to BHAG occurring on the left side. However, given that the increased T2 signal occurred in the left cerebellar hemisphere with BHAG in the left occipital bone, the occipital cortex was not involved. In this case, the BHAG would not explain the patient’s visual aura as such a lesion would have been expected in the right occipital cortex rather than its actual location in this patient’s left cerebellar hemisphere.
CONCLUSIONS
Understanding the clinical impact of brain herniations is important because they are probably more common than previously thought. Improved MRI capabilities suggest that more BHAG will be detected moving forward as radiologists interpret images with higher resolution and thinner slices. Until its significance is fully understood, BHAG will continue to complicate the diagnosis of patients with neurologic complaints whose brain MRIs and EEGs are otherwise unremarkable.
There have been no cases of surgical BHAG intervention and pathology analysis that would help determine their clinical significance. A related entity, temporal lobe encephalocele, has been linked to focal temporal lobe epilepsy, which has demonstrated significant symptom improvement following surgical correction.15 However, encephaloceles have been distinguished from BHAG in part because they do not necessarily herniate through an arachnoid granulation.8 BHAG has only begun to be characterized in detail over the last decade, so more research is needed to understand how it develops and what clinical significance it truly holds.
The circulation of cerebrospinal fluid (CSF) is crucial for maintaining homeostasis for the optimal functioning of the multiple complex activities of the brain and spinal cord, including the disposal of metabolic waste products of brain and spinal cord activity into the cerebral venous drainage. Throughout the brain, the arachnoid mater forms small outpouchings or diverticula that penetrate the dura mater and communicate with the dural venous sinuses. These outpuchings are called arachnoid granulations or arachnoid villi, and most are found within the dural sinuses, primarily in the transverse sinuses and superior sagittal sinus, but can occasionally be seen extending into the inner table of the calvarium.1,2
The amount of arachnoid granulations seen in bone, particularly around the superior sagittal sinus, may increase with age.2 Arachnoid granulations are generally small but the largest ones can be seen on gross examination during intracranial procedures or autopsy.3 Magnetic resonance imaging (MRI) can detect arachnoid granulations, which are characterized as T1 hypointense and T2 hyperintense (CSF isointense), well-circumscribed, small, nonenhancing masses within the dural sinuses or in the diploic space (Figure 1). Even small arachnoid granulations < 1 mm in length can be detected.2
Smaller arachnoid granulations have been described histologically as entirely covered by a dural membrane, thus creating a subdural space that separates the body of the arachnoid granulation from the lumen of the accompanying venous sinus.4 However, larger arachnoid granulations may not be completely covered by a dural membrane, thus creating a point of contact between the arachnoid granulation and the venous sinus.4 Larger arachnoid granulations are normally filled with CSF, and their signal characteristics are similar to CSF on imaging.5,6 Arachnoid granulations also often contain vessels draining into the adjacent venous sinus.5,6
When larger arachnoid granulations are present, they may permit the protrusion of herniated brain tissue. There has been an increasing number of reports of these brain herniations into arachnoid granulations (BHAGs) in the literature.7-10 While these herniations have been associated with nonspecific neurologic symptoms like tinnitus and idiopathic intracranial hypertension, their true clinical significance remains undetermined.10,11 This article presents 5 cases of BHAG, discusses their clinical presentations and image findings, and reviews the current literature.
Case 1
A 30-year-old male with a history of multiple traumatic brain injuries presented for evaluation of seizures. The patient described the semiology of the seizures as a bright, colorful light in his right visual field, followed by loss of vision, then loss of awareness and full body convulsion. The semiology of this patient’s seizures was consistent with left temporo-occipital lobe seizure. The only abnormality seen in the brain MRI was the herniation of brain parenchyma originating from the occipital lobe into the transverse sinus, presumably through an arachnoid granulation (Figure 1). An electroencephalogram (EEG) was unremarkable, though the semiology of the seizure historically described by the patient was localized to the area of BHAG. The patient is currently taking antiseizure medications and has experienced no additional seizures.
Case 2
A male aged 53 years with a history of peripheral artery disease presented with a 6-month history of headaches and dizziness. The patient reported the onset of visual aura to his right visual field, starting as a fingernail-sized scintillating kaleidoscope light that would gradually increase in size to a round shape with fading kaleidoscope colors. This episode would last for a few minutes and was immediately followed by a headache. There was no alteration of consciousness during visual aura, although sometimes the patient would have right-sided scalp tingling. These episodes were often unprovoked, but occasionally triggered by bright lights. A single routine EEG was unremarkable. The patient reported headaches without aura, but not aura without headaches, which made occipital lobe seizure less likely. MRI demonstrated a small herniation of brain parenchyma into the inner table of the left occipital bone (Figure 2). The patient was diagnosed with migraine with aura, and the semiology of the visual aura corresponded to the location of the herniation in the left occipital region.
Case 3
A 77-year-old male with a history of left ear diving injury presented with left-sided asymmetric hearing loss and word recognition difficulty for several years. MRI obtained as part of his work-up to evaluate for possible schwannoma of the eighth left cranial nerve instead demonstrated an incidental right cerebellar herniation within an arachnoid granulation into the diploic space of the occipital bone (Figure 3). The BHAG for this patient appeared to be an incidental finding unrelated to his asymmetric hearing loss.
Case 4
A male aged 62 years with a history of metastatic esophageal cancer, substance abuse, and a prior presumed alcohol withdrawal seizure underwent an MRI for evaluation of brain metastasis after presenting to the hospital with confusion 1 day after starting chemotherapy (Figure 4). Nine years prior, the patient had an isolated generalized tonic-clonic seizure approximately 72 hours following a period of alcohol cessation. The MRI demonstrated an incidental left parasagittal herniation of left parietal lobe tissue through an arachnoid granulation into the superior sagittal sinus, in addition to metastatic brain lesions. An EEG showed mild encephalopathy without evidence of seizures. It was determined that the patient's confusion was most likely due to toxic-metabolic encephalopathy from chemotherapy.
Case 5
A 51-year-old male presented with worsening headache severity and frequency. He had a history of chronic headaches for about 20 years that occurred annually, but were now occurring twice weekly. The headaches often started with a left eye visual aura followed by pressure in the left eye, left frontal region, and left ear, with at times a cervicogenic component. No cervical spine imaging was available. An MRI revealed 2 small adjacent areas of cerebellar herniation into arachnoid granulations in the left occipital bone (Figure 5).
Discussion
Arachnoid granulations appear very early in life, although they are uncommon before age 2 years.2 Classically, they have been understood to act as 1-way valves permitting the outflow of CSF from the subarachnoid space to the dural venous sinuses. However, increasing evidence shows they may only play a minor role in that process.12 The structure of arachnoid granulations is being reexamined. A recent microscopy study demonstrated structural heterogeneity with a fine, porous lining that permits flow.13 Additionally, associated immune components in the microenvironment suggests that arachnoid granulations may function similarly to lymph nodes as part of a central nervous system lymphatic network.13 Evidence is lacking for arachnoid granulations being the primary route of CSF outflow, and newer models include CSF exit pathways along the cranial nerves and drainage through lymphatics within the dura mater.12
New MRI systems have demonstrated that the prevalence of arachnoid granulations increases with age. One study found that all subjects in the aged 40 years cohort had detectable arachnoid granulations on images obtained with a 3T MRI system, with the main site being the superior sagittal sinus.2 The prevalence increased until age 40 years and then noticeably decreased. Not only did the prevalence increase in this pattern, but the total number of detectable arachnoid granulations followed a similar pattern.2 In addition, the detectable arachnoid granulations tend to be larger in older patients. Arachnoid granulations are very common in adults, but little is known about when and why brain tissue herniates through these structures.
This case series illustrates how a small amount of adult cerebral or cerebellar matter in large arachnoid granulations can herniate into the dural sinuses and diploic space. Although arachnoid granulations extending into the dural sinuses and diploic space are a relatively common finding on MRI,BHAGs are rare in these locations.1,2,8 Improved spatial resolution afforded by newer high-field scanners with thinner sections, such as very thin (1 mm) T1- and heavily T2-weighted 3 dimensional sequences may lead to increased detection of BHAG. Some of these herniations are small and may be easily missed or confused for normal arachnoid granulations on 3 to 5 mm thickness MRIs.
Despite increased recognition, it is still uncertain to what degree these herniations contribute to the clinical presentations. Associated neurologic symptoms may include seizures, headaches, tinnitus, syncope, and increased intracranial pressure.7-10
Three cases presented in this article demonstrated abnormal signals adjacent to the herniated brain, presumably due to dysplasia of gliotic tissue. In 1 study, parenchymal signal and structural changes occurred in about one-half of the reported BHAG, all of which were cerebellar herniations.7 In Case 1, the herniation and adjacent abnormal MRI signal corresponded to localization of the seizure semiology as obtained from patient history, strongly suggesting the BHAG played a role in the presentation. Signal abnormality accompanying an adjacent BHAG may suggest a higher likelihood that the BHAG has clinical relevance. However, the patient in Case 2 had a visual aura that corresponded to the BHAG location, so a signal abnormality may not be necessary for a patient to develop symptoms. Case 1 also included a history of documented traumatic brain injuries, suggesting that perhaps head trauma may facilitate BHAG development. Regardless, there is likely also a congenital component to their formation, as BHAG has been observed in the pediatric population.14
The patient's asymmetric left-sided hearing loss in Case 3 appeared unrelated to the BHAG as its location was in the contralateral cerebellar region and did not correspond to the patient’s clinical findings. The patient in Case 4 had a limited history regarding localization details of their prior presumed alcohol withdrawal seizure, such as head movements, eye deviation, or lateralized onset of convulsions. Given this limited data, it is unclear whether their prior seizure could have been related to BHAG or not. The patient in case 5 reported worsening headaches on the left side of his head, which corresponded to BHAG occurring on the left side. However, given that the increased T2 signal occurred in the left cerebellar hemisphere with BHAG in the left occipital bone, the occipital cortex was not involved. In this case, the BHAG would not explain the patient’s visual aura as such a lesion would have been expected in the right occipital cortex rather than its actual location in this patient’s left cerebellar hemisphere.
CONCLUSIONS
Understanding the clinical impact of brain herniations is important because they are probably more common than previously thought. Improved MRI capabilities suggest that more BHAG will be detected moving forward as radiologists interpret images with higher resolution and thinner slices. Until its significance is fully understood, BHAG will continue to complicate the diagnosis of patients with neurologic complaints whose brain MRIs and EEGs are otherwise unremarkable.
There have been no cases of surgical BHAG intervention and pathology analysis that would help determine their clinical significance. A related entity, temporal lobe encephalocele, has been linked to focal temporal lobe epilepsy, which has demonstrated significant symptom improvement following surgical correction.15 However, encephaloceles have been distinguished from BHAG in part because they do not necessarily herniate through an arachnoid granulation.8 BHAG has only begun to be characterized in detail over the last decade, so more research is needed to understand how it develops and what clinical significance it truly holds.
1. Ikushima I, Korogi Y, Makita O, et al. MRI of arachnoid granulations within the dural sinuses using a FLAIR pulse sequence. Br J Radiol. 1999;72(863):1046-1051. doi:10.1259/bjr.72.863.10700819
2. Rados M, Zivko M, Perisa A, Oreskovic D, Klarica M. No arachnoid granulations-no problems: number, size, and distribution of arachnoid granulations from birth to 80 years of age. Front Aging Neurosci. 2021;13:698865. doi:10.3389/fnagi.2021.698865
3. Grossman CB, Potts DG. Arachnoid granulations: radiology and anatomy. Radiology. 1974;113(1):95-100. doi:10.1148/113.1.95
4. Wolpow ER, Schaumburg HH. Structure of the human arachnoid granulation. J Neurosurg. 1972;37(6):724-727. doi:10.3171/jns.1972.37.6.0724
5. Leach JL, Jones BV, Tomsick TA, Stewart CA, Balko MG. Normal appearance of arachnoid granulations on contrast-enhanced CT and MR of the brain: differentiation from dural sinus disease. AJNR Am J Neuroradiol. 1996;17(8):1523-1532.
6. Roche J, Warner D. Arachnoid granulations in the transverse and sigmoid sinuses: CT, MR, and MR angiographic appearance of a normal anatomic variation. AJNR Am J Neuroradiol. 1996;17(4):677-683.
7. Malekzadehlashkariani S, Wanke I, Rufenacht DA, San Millan D. Brain herniations into arachnoid granulations: about 68 cases in 38 patients and review of the literature. Neuroradiology. 2016;58(5):443-457. doi:10.1007/s00234-016-1662-5
8. Battal B, Castillo M. Brain herniations into the dural venous sinuses or calvarium: MRI of a recently recognized entity. Neuroradiol J. 2014;27(1):55-62. doi:10.15274/NRJ-2014-10006
9. Liebo GB, Lane JJ, Van Gompel JJ, Eckel LJ, Schwartz KM, Lehman VT. Brain herniation into arachnoid granulations: clinical and neuroimaging features. J Neuroimaging. 2016;26(6):592-598. doi:10.1111/jon.12366
10. Smith ER, Caton MT, Villanueva-Meyer JE, et al. Brain herniation (encephalocele) into arachnoid granulations: Prevalence and association with pulsatile tinnitus and idiopathic intracranial hypertension. Neuroradiology. 2022;64(9):1747-1754.
11. Battal B, Hamcan S, Akgun V, et al. Brain herniations into the dural venous sinus or calvarium: MRI findings, possible causes and clinical significance. Eur Radiol. 2016;26(6):1723-1731.
12. Proulx ST. Cerebrospinal fluid outflow: A review of the historical and contemporary evidence for arachnoid villi, perineural routes, and dural lymphatics. Cell Mol Life Sci. 2021;78(6):2429-2457.
13. Shah T, Leurgans SE, Mehta RI, et al. Arachnoid granulations are lymphatic conduits that communicate with bone marrow and dura-arachnoid stroma. J Exp Med. 2023;220(2).
14. Sade R, Ogul H, Polat G, Pirimoglu B, Kantarci M. Brain herniation into the transverse sinuses’ arachnoid granulations in the pediatric population investigated with 3 T MRI. Acta Neurol Belg. 2019;119(2):225-231.
15. Saavalainen T, Jutila L, Mervaala E, Kalviainen R, Vanninen R, Immonen A. Temporal anteroinferior encephalocele: An underrecognized etiology of temporal lobe epilepsy? Neurology. 2015;85(17):1467-1474.
1. Ikushima I, Korogi Y, Makita O, et al. MRI of arachnoid granulations within the dural sinuses using a FLAIR pulse sequence. Br J Radiol. 1999;72(863):1046-1051. doi:10.1259/bjr.72.863.10700819
2. Rados M, Zivko M, Perisa A, Oreskovic D, Klarica M. No arachnoid granulations-no problems: number, size, and distribution of arachnoid granulations from birth to 80 years of age. Front Aging Neurosci. 2021;13:698865. doi:10.3389/fnagi.2021.698865
3. Grossman CB, Potts DG. Arachnoid granulations: radiology and anatomy. Radiology. 1974;113(1):95-100. doi:10.1148/113.1.95
4. Wolpow ER, Schaumburg HH. Structure of the human arachnoid granulation. J Neurosurg. 1972;37(6):724-727. doi:10.3171/jns.1972.37.6.0724
5. Leach JL, Jones BV, Tomsick TA, Stewart CA, Balko MG. Normal appearance of arachnoid granulations on contrast-enhanced CT and MR of the brain: differentiation from dural sinus disease. AJNR Am J Neuroradiol. 1996;17(8):1523-1532.
6. Roche J, Warner D. Arachnoid granulations in the transverse and sigmoid sinuses: CT, MR, and MR angiographic appearance of a normal anatomic variation. AJNR Am J Neuroradiol. 1996;17(4):677-683.
7. Malekzadehlashkariani S, Wanke I, Rufenacht DA, San Millan D. Brain herniations into arachnoid granulations: about 68 cases in 38 patients and review of the literature. Neuroradiology. 2016;58(5):443-457. doi:10.1007/s00234-016-1662-5
8. Battal B, Castillo M. Brain herniations into the dural venous sinuses or calvarium: MRI of a recently recognized entity. Neuroradiol J. 2014;27(1):55-62. doi:10.15274/NRJ-2014-10006
9. Liebo GB, Lane JJ, Van Gompel JJ, Eckel LJ, Schwartz KM, Lehman VT. Brain herniation into arachnoid granulations: clinical and neuroimaging features. J Neuroimaging. 2016;26(6):592-598. doi:10.1111/jon.12366
10. Smith ER, Caton MT, Villanueva-Meyer JE, et al. Brain herniation (encephalocele) into arachnoid granulations: Prevalence and association with pulsatile tinnitus and idiopathic intracranial hypertension. Neuroradiology. 2022;64(9):1747-1754.
11. Battal B, Hamcan S, Akgun V, et al. Brain herniations into the dural venous sinus or calvarium: MRI findings, possible causes and clinical significance. Eur Radiol. 2016;26(6):1723-1731.
12. Proulx ST. Cerebrospinal fluid outflow: A review of the historical and contemporary evidence for arachnoid villi, perineural routes, and dural lymphatics. Cell Mol Life Sci. 2021;78(6):2429-2457.
13. Shah T, Leurgans SE, Mehta RI, et al. Arachnoid granulations are lymphatic conduits that communicate with bone marrow and dura-arachnoid stroma. J Exp Med. 2023;220(2).
14. Sade R, Ogul H, Polat G, Pirimoglu B, Kantarci M. Brain herniation into the transverse sinuses’ arachnoid granulations in the pediatric population investigated with 3 T MRI. Acta Neurol Belg. 2019;119(2):225-231.
15. Saavalainen T, Jutila L, Mervaala E, Kalviainen R, Vanninen R, Immonen A. Temporal anteroinferior encephalocele: An underrecognized etiology of temporal lobe epilepsy? Neurology. 2015;85(17):1467-1474.