User login
Stimulants are first-line psychopharmacologic interventions for attention-deficit/hyperactivity disorder (ADHD), and their efficacy is supported by clinical trials and meta-analyses in children and adolescents1 as well as adults.2 Despite decades of tolerability and efficacy data supporting their use, a major drawback of stimulants is that their salutary therapeutic effects wane once the medication is cleared or metabolized. Both mixed amphetamine- and methylphenidate-based preparations have short half-lives, necessitating multiple doses per day (eg, 3 or 4 times a day) when short-acting preparations are used. Over the past 15 years, nearly a dozen formulations were developed that extend the duration of action through delayed release, delayed absorption, or utilizing prodrugs.
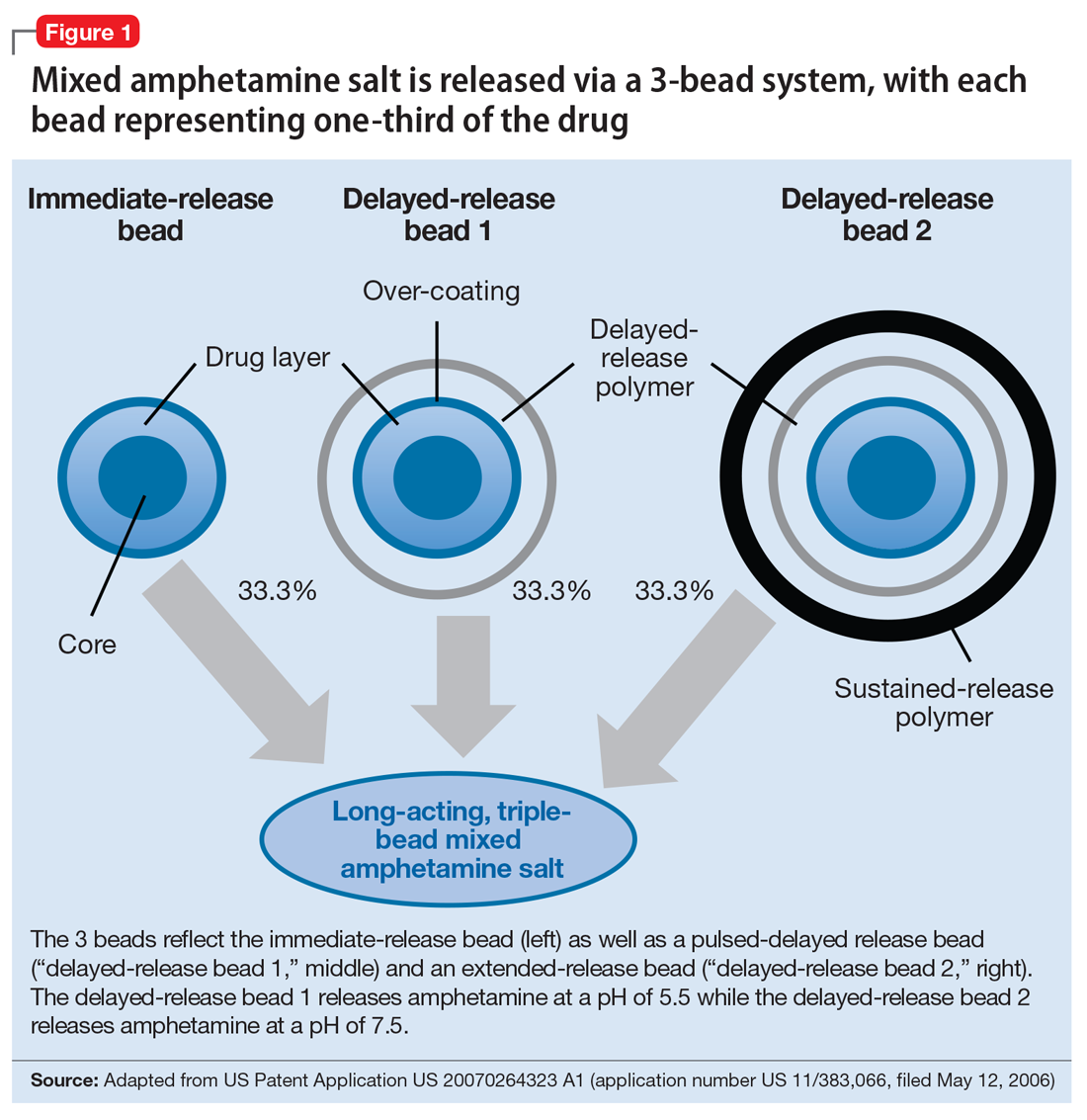
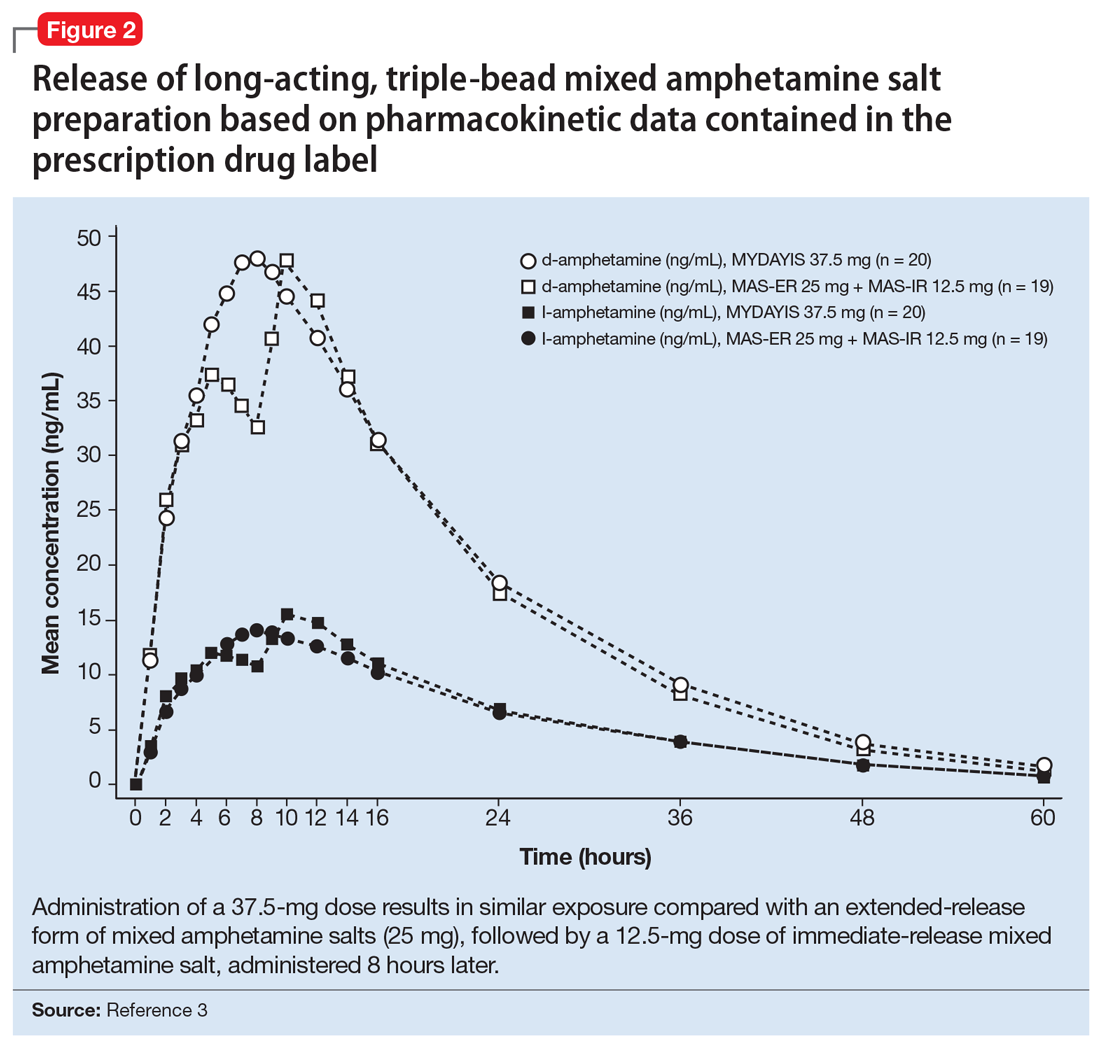
The encapsulated preparation contains 3 MAS beads: an immediate-release amphetamine salt bead, a pulsed-delayed release bead, and an extended-release bead (Figure 1), which give rise to a unique pharmacokinetic profile (Figure 2).3
Mechanism of action
Like all MAS, this formulation blocks the reuptake of norepinephrine and dopamine, increasing synaptic concentrations of these monoamine neurotransmitters. Additionally, amphetamine salts may inhibit the activity of monoamine oxidase (MAO), further increasing synaptic levels of monoamines.4 Enhancing noradrenergic, dopaminergic neurotransmission, particularly within the prefrontal cortex, increases attention, working memory, and processing speed in patients with ADHD.4
Pharmacokinetics
Cmax occurs approximately 7 to 10 hours and 8 hours following administration in adolescent and adult patients, respectively (Figure 2).3 In adolescents who were administered a single dose of long-acting, triple-bead MAS, Cmax and area under the curve (AUC) for d- and l-amphetamine were both 21% to 31% higher compared with adults3 and did not appear to be affected by sex or race.3
Half-life is 10 to 11 hours for d-amphetamine and 10 to 13 hours for l-amphetamine and does not statistically differ between pediatric and adult studies.3
Metabolism and elimination. Amphetamines are partially metabolized through cytochrome 450 (CYP) 2D6-dependent mechanisms, and thus in CYP2D6 poor metabolizers medication exposure may be increased, while decreased exposure may occur in ultra-rapid metabolizers; however, there are no guidelines from the Clinical Pharmacogenetics Implementation Consortium regarding alternate dosing strategies for patients based on CYP2D6 genotype or activity phenotype.5 Because amphetamines are renally excreted, dosages should be adjusted in patients with renal impairment.
Drug interactions. Medications that affect gastrointestinal and urinary pH may affect serum concentrations of amphetamine. Specifically, agents that increase gastric pH (eg, proton pump inhibitors) as well as urinary alkalinizing agents (eg, acetazolamide, some thiazide diuretics) increase serum amphetamine concentrations.3 Because amphetamine is a weak MAOI, there is a theoretical risk of serotonin syndrome when amphetamine-based preparations are used concurrently with SSRIs, TCAs, and MAOIs. However, the concurrent use of MAS and SSRIs generally is considered safe and common practice in patients with ADHD and co-occurring anxiety6,7 or depressive disorders.
Dosing
Long-acting, triple-bead MAS is available in 12.5-, 25-, 37.5-, and 50-mg capsules. The capsule may be opened and sprinkled in food for patients who cannot swallow capsules. Opening of the capsule results in similar absorption relative to oral administration of the intact capsule.3
In adults with ADHD, long-acting, triple-bead MAS should be initiated at 12.5 mg in the morning (Table 2). However, in some individuals, long-acting, triple-bead MAS may be initiated at 25 mg each morning. Titration should occur in 12.5-mg weekly increments to a maximum dosage of 50 mg/d.3
In adults with severe renal impairment (glomerular filtrate rate, 15 to 30 mL/min/1.73 m2), the recommended starting dose is 12.5 mg/d, with a maximum dosage of 25 mg/d.3
The efficacy of long-acting, triple-bead MAS in adults with ADHD was demonstrated in 3 studies involving adults ages 18 to 55, and the effectiveness of the medication, with regard to duration of action, was assessed using the Time-Sensitive ADHD Symptom Scale—a self-report scale that consists of items indexed by the ADHD Rating Scale-IV (ADHD-RS-IV) which assesses ADHD symptom severity. Doses up to 75 mg/d were studied; however, there were no significant effects. It should be noted that this maximum daily dose was not determined by any safety parameter.
Study 1 (dose-optimization, triple-bead MAS, n = 137; placebo, n = 135, dosing: 12.5 to 75 mg) and Study 2 (forced dose-titration study, triple-bead MAS, n = 308; placebo, n = 104, dosing: 25 mg, 50 mg, 75 mg) demonstrated efficacy of triple-bead MAS for treating ADHD in adults. Despite differences in study designs, statistically significant and similar clinically relevant improvement was observed with triple-bead MAS (vs placebo) on ADHD-RS-IV total scores in both Study 1 and Study 2.8 An additional study in adults ages 18 to 55 (N = 275) with ADHD (DSM-5 criteria) involved randomization to either 12.5 mg (fixed dose) or forced titration (12.5 to 37.5 mg) or placebo and, as with the first 2 studies, improvement in ADHD symptoms was observed in triple-bead MAS-treated patients relative to those who had received placebo. (See Reference 3 for a summary of the clinical trials of triple-bead MAS in adults with ADHD.)
The tolerability of this medication was evaluated in a 12-month open-label study of adults with ADHD (DSM-IV-TR criteria) in which discontinuation was higher at doses >25 mg/d.7 Treatment-related increases in blood pressure and heart rate were consistent with the known hemodynamic adverse effect profile of stimulants.9
In adolescents with ADHD ages 13 to 17, long-acting, triple-bead MAS should be initiated at 12.5 mg/d and may be increased to 25 mg/d (Table 2). Importantly, in younger patients, including those younger than age 12, triple-bead MAS was associated with an increased risk of adverse events including insomnia and anorexia, and this was thought to be related to increased drug exposure (ie, AUC).
The efficacy of long-acting, triple-bead MAS was evaluated in 2 studies of adolescents ages 13 to 17, including 1 fixed-dose trial (25 mg/d) and 1 flexibly-dosed trial (12.5 to 25 mg/d). These unpublished studies utilized the ADHD-RS-IV score and the Average Permanent Product Measure of Performance, an age-adjusted math test and measure of sustained attention, and revealed statistically significant differences between medication and placebo in the primary outcomes.3
Adverse effects
Long-acting, triple-bead MAS was developed to treat ADHD symptoms throughout the day, and serum concentrations of the medication may be higher with this formulation compared with other long-acting preparations. Therefore, adverse effects that are directly related to plasma exposure (eg, insomnia and appetite suppression) may occur at higher rates with this preparation compared with alternatives. For example, in some of the registration trials, insomnia occurred in more than one-third of patients receiving the active medication (38%).9 Although insomnia was the most frequently reported adverse event in adults with ADHD, most reports of insomnia occurred early in the course of treatment. Of these insomnia-related adverse events, 94% were mild to moderate and resulted in discontinuation of the medication in approximately 2% of patients. Further, 73.9% of treatment-emergent, insomnia–related adverse events resolved during the course of the study. It is also important to note that the Pittsburgh Sleep Quality Index did not differ from placebo in studies of triple-bead MAS in adults with ADHD.10 Similarly, rates of stimulant-induced appetite suppression may be higher with this preparation compared with other long-acting preparations.9
Adverse effects observed in adults with ADHD that occurred in ≥2% of patients receiving triple-bead MAS and at least twice the incidence in patients randomized to placebo included:
- anxiety (7% vs 3%)
- feeling jittery (2% vs 1%)
- agitation (2% vs 0%)
- insomnia (31% vs 8%)
- depression (3% vs 0%)
- decreased appetite (30% vs 4%)
- weight loss (9% vs 0%)
- xerostomia (23% vs 4%)
- diarrhea (3% vs 0%)
- increased heart rate (9% vs 0%)
- palpitations (4% vs 2%)
- dysmenorrhea (4% vs 2%)
- erectile dysfunction (2% vs 1%).
In adolescents receiving triple-bea
1. Punja S, Shamseer L, Hartling L, et al. Amphetamines for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev. 2016;2016(2):CD009996.
2. Castells X, Ramos-Quiroga J, Bosch R, et al. Amphetamines for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst Rev. 2011;(6):CD007813.
3. Mydayis [package insert]. Lexington, MA: Shire; 2017.
4. Heal DJ, Smith SL, Gosden J, et al. Amphetamine, past and present—a pharmacological and clinical perspective. J Psychopharmacol. 2013;27(6):479-496.
5. Hoffman JM, Dunnenberger HM, Kevin Hicks J, et al. Developing knowledge resources to support precision medicine: principles from the Clinical Pharmacogenetics Implementation Consortium (CPIC). J Am Med Inform Assoc. 2016;23(4):766-801.
6. Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753-2766.
7. Connolly SD, Bernstein GA; Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(2):267-283.
8. Goodman DW, Spencer TJ, Adler LA, et al. Clinical evaluation of triple-bead mixed amphetamine salts in adult ADHD. Presented at: 54th Annual Meeting of the American Academy of Child and Adolescent Psychiatry; October 25, 2007; Boston, MA.
9. Adler LA, Frick G, Yan B. A Long-term, open-label, safety study of triple-bead mixed amphetamine salts (SHP465) in adults with ADHD [published online April 1, 2017]. J Atten Disord. doi: 10.1177/1087054717696770.
10. Backhaus J, Junghanns K, Broocks A, et al. test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53(3):737-740.
Stimulants are first-line psychopharmacologic interventions for attention-deficit/hyperactivity disorder (ADHD), and their efficacy is supported by clinical trials and meta-analyses in children and adolescents1 as well as adults.2 Despite decades of tolerability and efficacy data supporting their use, a major drawback of stimulants is that their salutary therapeutic effects wane once the medication is cleared or metabolized. Both mixed amphetamine- and methylphenidate-based preparations have short half-lives, necessitating multiple doses per day (eg, 3 or 4 times a day) when short-acting preparations are used. Over the past 15 years, nearly a dozen formulations were developed that extend the duration of action through delayed release, delayed absorption, or utilizing prodrugs.
The encapsulated preparation contains 3 MAS beads: an immediate-release amphetamine salt bead, a pulsed-delayed release bead, and an extended-release bead (Figure 1), which give rise to a unique pharmacokinetic profile (Figure 2).3
Mechanism of action
Like all MAS, this formulation blocks the reuptake of norepinephrine and dopamine, increasing synaptic concentrations of these monoamine neurotransmitters. Additionally, amphetamine salts may inhibit the activity of monoamine oxidase (MAO), further increasing synaptic levels of monoamines.4 Enhancing noradrenergic, dopaminergic neurotransmission, particularly within the prefrontal cortex, increases attention, working memory, and processing speed in patients with ADHD.4
Pharmacokinetics
Cmax occurs approximately 7 to 10 hours and 8 hours following administration in adolescent and adult patients, respectively (Figure 2).3 In adolescents who were administered a single dose of long-acting, triple-bead MAS, Cmax and area under the curve (AUC) for d- and l-amphetamine were both 21% to 31% higher compared with adults3 and did not appear to be affected by sex or race.3
Half-life is 10 to 11 hours for d-amphetamine and 10 to 13 hours for l-amphetamine and does not statistically differ between pediatric and adult studies.3
Metabolism and elimination. Amphetamines are partially metabolized through cytochrome 450 (CYP) 2D6-dependent mechanisms, and thus in CYP2D6 poor metabolizers medication exposure may be increased, while decreased exposure may occur in ultra-rapid metabolizers; however, there are no guidelines from the Clinical Pharmacogenetics Implementation Consortium regarding alternate dosing strategies for patients based on CYP2D6 genotype or activity phenotype.5 Because amphetamines are renally excreted, dosages should be adjusted in patients with renal impairment.
Drug interactions. Medications that affect gastrointestinal and urinary pH may affect serum concentrations of amphetamine. Specifically, agents that increase gastric pH (eg, proton pump inhibitors) as well as urinary alkalinizing agents (eg, acetazolamide, some thiazide diuretics) increase serum amphetamine concentrations.3 Because amphetamine is a weak MAOI, there is a theoretical risk of serotonin syndrome when amphetamine-based preparations are used concurrently with SSRIs, TCAs, and MAOIs. However, the concurrent use of MAS and SSRIs generally is considered safe and common practice in patients with ADHD and co-occurring anxiety6,7 or depressive disorders.
Dosing
Long-acting, triple-bead MAS is available in 12.5-, 25-, 37.5-, and 50-mg capsules. The capsule may be opened and sprinkled in food for patients who cannot swallow capsules. Opening of the capsule results in similar absorption relative to oral administration of the intact capsule.3
In adults with ADHD, long-acting, triple-bead MAS should be initiated at 12.5 mg in the morning (Table 2). However, in some individuals, long-acting, triple-bead MAS may be initiated at 25 mg each morning. Titration should occur in 12.5-mg weekly increments to a maximum dosage of 50 mg/d.3
In adults with severe renal impairment (glomerular filtrate rate, 15 to 30 mL/min/1.73 m2), the recommended starting dose is 12.5 mg/d, with a maximum dosage of 25 mg/d.3
The efficacy of long-acting, triple-bead MAS in adults with ADHD was demonstrated in 3 studies involving adults ages 18 to 55, and the effectiveness of the medication, with regard to duration of action, was assessed using the Time-Sensitive ADHD Symptom Scale—a self-report scale that consists of items indexed by the ADHD Rating Scale-IV (ADHD-RS-IV) which assesses ADHD symptom severity. Doses up to 75 mg/d were studied; however, there were no significant effects. It should be noted that this maximum daily dose was not determined by any safety parameter.
Study 1 (dose-optimization, triple-bead MAS, n = 137; placebo, n = 135, dosing: 12.5 to 75 mg) and Study 2 (forced dose-titration study, triple-bead MAS, n = 308; placebo, n = 104, dosing: 25 mg, 50 mg, 75 mg) demonstrated efficacy of triple-bead MAS for treating ADHD in adults. Despite differences in study designs, statistically significant and similar clinically relevant improvement was observed with triple-bead MAS (vs placebo) on ADHD-RS-IV total scores in both Study 1 and Study 2.8 An additional study in adults ages 18 to 55 (N = 275) with ADHD (DSM-5 criteria) involved randomization to either 12.5 mg (fixed dose) or forced titration (12.5 to 37.5 mg) or placebo and, as with the first 2 studies, improvement in ADHD symptoms was observed in triple-bead MAS-treated patients relative to those who had received placebo. (See Reference 3 for a summary of the clinical trials of triple-bead MAS in adults with ADHD.)
The tolerability of this medication was evaluated in a 12-month open-label study of adults with ADHD (DSM-IV-TR criteria) in which discontinuation was higher at doses >25 mg/d.7 Treatment-related increases in blood pressure and heart rate were consistent with the known hemodynamic adverse effect profile of stimulants.9
In adolescents with ADHD ages 13 to 17, long-acting, triple-bead MAS should be initiated at 12.5 mg/d and may be increased to 25 mg/d (Table 2). Importantly, in younger patients, including those younger than age 12, triple-bead MAS was associated with an increased risk of adverse events including insomnia and anorexia, and this was thought to be related to increased drug exposure (ie, AUC).
The efficacy of long-acting, triple-bead MAS was evaluated in 2 studies of adolescents ages 13 to 17, including 1 fixed-dose trial (25 mg/d) and 1 flexibly-dosed trial (12.5 to 25 mg/d). These unpublished studies utilized the ADHD-RS-IV score and the Average Permanent Product Measure of Performance, an age-adjusted math test and measure of sustained attention, and revealed statistically significant differences between medication and placebo in the primary outcomes.3
Adverse effects
Long-acting, triple-bead MAS was developed to treat ADHD symptoms throughout the day, and serum concentrations of the medication may be higher with this formulation compared with other long-acting preparations. Therefore, adverse effects that are directly related to plasma exposure (eg, insomnia and appetite suppression) may occur at higher rates with this preparation compared with alternatives. For example, in some of the registration trials, insomnia occurred in more than one-third of patients receiving the active medication (38%).9 Although insomnia was the most frequently reported adverse event in adults with ADHD, most reports of insomnia occurred early in the course of treatment. Of these insomnia-related adverse events, 94% were mild to moderate and resulted in discontinuation of the medication in approximately 2% of patients. Further, 73.9% of treatment-emergent, insomnia–related adverse events resolved during the course of the study. It is also important to note that the Pittsburgh Sleep Quality Index did not differ from placebo in studies of triple-bead MAS in adults with ADHD.10 Similarly, rates of stimulant-induced appetite suppression may be higher with this preparation compared with other long-acting preparations.9
Adverse effects observed in adults with ADHD that occurred in ≥2% of patients receiving triple-bead MAS and at least twice the incidence in patients randomized to placebo included:
- anxiety (7% vs 3%)
- feeling jittery (2% vs 1%)
- agitation (2% vs 0%)
- insomnia (31% vs 8%)
- depression (3% vs 0%)
- decreased appetite (30% vs 4%)
- weight loss (9% vs 0%)
- xerostomia (23% vs 4%)
- diarrhea (3% vs 0%)
- increased heart rate (9% vs 0%)
- palpitations (4% vs 2%)
- dysmenorrhea (4% vs 2%)
- erectile dysfunction (2% vs 1%).
In adolescents receiving triple-bea
Stimulants are first-line psychopharmacologic interventions for attention-deficit/hyperactivity disorder (ADHD), and their efficacy is supported by clinical trials and meta-analyses in children and adolescents1 as well as adults.2 Despite decades of tolerability and efficacy data supporting their use, a major drawback of stimulants is that their salutary therapeutic effects wane once the medication is cleared or metabolized. Both mixed amphetamine- and methylphenidate-based preparations have short half-lives, necessitating multiple doses per day (eg, 3 or 4 times a day) when short-acting preparations are used. Over the past 15 years, nearly a dozen formulations were developed that extend the duration of action through delayed release, delayed absorption, or utilizing prodrugs.
The encapsulated preparation contains 3 MAS beads: an immediate-release amphetamine salt bead, a pulsed-delayed release bead, and an extended-release bead (Figure 1), which give rise to a unique pharmacokinetic profile (Figure 2).3
Mechanism of action
Like all MAS, this formulation blocks the reuptake of norepinephrine and dopamine, increasing synaptic concentrations of these monoamine neurotransmitters. Additionally, amphetamine salts may inhibit the activity of monoamine oxidase (MAO), further increasing synaptic levels of monoamines.4 Enhancing noradrenergic, dopaminergic neurotransmission, particularly within the prefrontal cortex, increases attention, working memory, and processing speed in patients with ADHD.4
Pharmacokinetics
Cmax occurs approximately 7 to 10 hours and 8 hours following administration in adolescent and adult patients, respectively (Figure 2).3 In adolescents who were administered a single dose of long-acting, triple-bead MAS, Cmax and area under the curve (AUC) for d- and l-amphetamine were both 21% to 31% higher compared with adults3 and did not appear to be affected by sex or race.3
Half-life is 10 to 11 hours for d-amphetamine and 10 to 13 hours for l-amphetamine and does not statistically differ between pediatric and adult studies.3
Metabolism and elimination. Amphetamines are partially metabolized through cytochrome 450 (CYP) 2D6-dependent mechanisms, and thus in CYP2D6 poor metabolizers medication exposure may be increased, while decreased exposure may occur in ultra-rapid metabolizers; however, there are no guidelines from the Clinical Pharmacogenetics Implementation Consortium regarding alternate dosing strategies for patients based on CYP2D6 genotype or activity phenotype.5 Because amphetamines are renally excreted, dosages should be adjusted in patients with renal impairment.
Drug interactions. Medications that affect gastrointestinal and urinary pH may affect serum concentrations of amphetamine. Specifically, agents that increase gastric pH (eg, proton pump inhibitors) as well as urinary alkalinizing agents (eg, acetazolamide, some thiazide diuretics) increase serum amphetamine concentrations.3 Because amphetamine is a weak MAOI, there is a theoretical risk of serotonin syndrome when amphetamine-based preparations are used concurrently with SSRIs, TCAs, and MAOIs. However, the concurrent use of MAS and SSRIs generally is considered safe and common practice in patients with ADHD and co-occurring anxiety6,7 or depressive disorders.
Dosing
Long-acting, triple-bead MAS is available in 12.5-, 25-, 37.5-, and 50-mg capsules. The capsule may be opened and sprinkled in food for patients who cannot swallow capsules. Opening of the capsule results in similar absorption relative to oral administration of the intact capsule.3
In adults with ADHD, long-acting, triple-bead MAS should be initiated at 12.5 mg in the morning (Table 2). However, in some individuals, long-acting, triple-bead MAS may be initiated at 25 mg each morning. Titration should occur in 12.5-mg weekly increments to a maximum dosage of 50 mg/d.3
In adults with severe renal impairment (glomerular filtrate rate, 15 to 30 mL/min/1.73 m2), the recommended starting dose is 12.5 mg/d, with a maximum dosage of 25 mg/d.3
The efficacy of long-acting, triple-bead MAS in adults with ADHD was demonstrated in 3 studies involving adults ages 18 to 55, and the effectiveness of the medication, with regard to duration of action, was assessed using the Time-Sensitive ADHD Symptom Scale—a self-report scale that consists of items indexed by the ADHD Rating Scale-IV (ADHD-RS-IV) which assesses ADHD symptom severity. Doses up to 75 mg/d were studied; however, there were no significant effects. It should be noted that this maximum daily dose was not determined by any safety parameter.
Study 1 (dose-optimization, triple-bead MAS, n = 137; placebo, n = 135, dosing: 12.5 to 75 mg) and Study 2 (forced dose-titration study, triple-bead MAS, n = 308; placebo, n = 104, dosing: 25 mg, 50 mg, 75 mg) demonstrated efficacy of triple-bead MAS for treating ADHD in adults. Despite differences in study designs, statistically significant and similar clinically relevant improvement was observed with triple-bead MAS (vs placebo) on ADHD-RS-IV total scores in both Study 1 and Study 2.8 An additional study in adults ages 18 to 55 (N = 275) with ADHD (DSM-5 criteria) involved randomization to either 12.5 mg (fixed dose) or forced titration (12.5 to 37.5 mg) or placebo and, as with the first 2 studies, improvement in ADHD symptoms was observed in triple-bead MAS-treated patients relative to those who had received placebo. (See Reference 3 for a summary of the clinical trials of triple-bead MAS in adults with ADHD.)
The tolerability of this medication was evaluated in a 12-month open-label study of adults with ADHD (DSM-IV-TR criteria) in which discontinuation was higher at doses >25 mg/d.7 Treatment-related increases in blood pressure and heart rate were consistent with the known hemodynamic adverse effect profile of stimulants.9
In adolescents with ADHD ages 13 to 17, long-acting, triple-bead MAS should be initiated at 12.5 mg/d and may be increased to 25 mg/d (Table 2). Importantly, in younger patients, including those younger than age 12, triple-bead MAS was associated with an increased risk of adverse events including insomnia and anorexia, and this was thought to be related to increased drug exposure (ie, AUC).
The efficacy of long-acting, triple-bead MAS was evaluated in 2 studies of adolescents ages 13 to 17, including 1 fixed-dose trial (25 mg/d) and 1 flexibly-dosed trial (12.5 to 25 mg/d). These unpublished studies utilized the ADHD-RS-IV score and the Average Permanent Product Measure of Performance, an age-adjusted math test and measure of sustained attention, and revealed statistically significant differences between medication and placebo in the primary outcomes.3
Adverse effects
Long-acting, triple-bead MAS was developed to treat ADHD symptoms throughout the day, and serum concentrations of the medication may be higher with this formulation compared with other long-acting preparations. Therefore, adverse effects that are directly related to plasma exposure (eg, insomnia and appetite suppression) may occur at higher rates with this preparation compared with alternatives. For example, in some of the registration trials, insomnia occurred in more than one-third of patients receiving the active medication (38%).9 Although insomnia was the most frequently reported adverse event in adults with ADHD, most reports of insomnia occurred early in the course of treatment. Of these insomnia-related adverse events, 94% were mild to moderate and resulted in discontinuation of the medication in approximately 2% of patients. Further, 73.9% of treatment-emergent, insomnia–related adverse events resolved during the course of the study. It is also important to note that the Pittsburgh Sleep Quality Index did not differ from placebo in studies of triple-bead MAS in adults with ADHD.10 Similarly, rates of stimulant-induced appetite suppression may be higher with this preparation compared with other long-acting preparations.9
Adverse effects observed in adults with ADHD that occurred in ≥2% of patients receiving triple-bead MAS and at least twice the incidence in patients randomized to placebo included:
- anxiety (7% vs 3%)
- feeling jittery (2% vs 1%)
- agitation (2% vs 0%)
- insomnia (31% vs 8%)
- depression (3% vs 0%)
- decreased appetite (30% vs 4%)
- weight loss (9% vs 0%)
- xerostomia (23% vs 4%)
- diarrhea (3% vs 0%)
- increased heart rate (9% vs 0%)
- palpitations (4% vs 2%)
- dysmenorrhea (4% vs 2%)
- erectile dysfunction (2% vs 1%).
In adolescents receiving triple-bea
1. Punja S, Shamseer L, Hartling L, et al. Amphetamines for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev. 2016;2016(2):CD009996.
2. Castells X, Ramos-Quiroga J, Bosch R, et al. Amphetamines for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst Rev. 2011;(6):CD007813.
3. Mydayis [package insert]. Lexington, MA: Shire; 2017.
4. Heal DJ, Smith SL, Gosden J, et al. Amphetamine, past and present—a pharmacological and clinical perspective. J Psychopharmacol. 2013;27(6):479-496.
5. Hoffman JM, Dunnenberger HM, Kevin Hicks J, et al. Developing knowledge resources to support precision medicine: principles from the Clinical Pharmacogenetics Implementation Consortium (CPIC). J Am Med Inform Assoc. 2016;23(4):766-801.
6. Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753-2766.
7. Connolly SD, Bernstein GA; Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(2):267-283.
8. Goodman DW, Spencer TJ, Adler LA, et al. Clinical evaluation of triple-bead mixed amphetamine salts in adult ADHD. Presented at: 54th Annual Meeting of the American Academy of Child and Adolescent Psychiatry; October 25, 2007; Boston, MA.
9. Adler LA, Frick G, Yan B. A Long-term, open-label, safety study of triple-bead mixed amphetamine salts (SHP465) in adults with ADHD [published online April 1, 2017]. J Atten Disord. doi: 10.1177/1087054717696770.
10. Backhaus J, Junghanns K, Broocks A, et al. test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53(3):737-740.
1. Punja S, Shamseer L, Hartling L, et al. Amphetamines for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev. 2016;2016(2):CD009996.
2. Castells X, Ramos-Quiroga J, Bosch R, et al. Amphetamines for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst Rev. 2011;(6):CD007813.
3. Mydayis [package insert]. Lexington, MA: Shire; 2017.
4. Heal DJ, Smith SL, Gosden J, et al. Amphetamine, past and present—a pharmacological and clinical perspective. J Psychopharmacol. 2013;27(6):479-496.
5. Hoffman JM, Dunnenberger HM, Kevin Hicks J, et al. Developing knowledge resources to support precision medicine: principles from the Clinical Pharmacogenetics Implementation Consortium (CPIC). J Am Med Inform Assoc. 2016;23(4):766-801.
6. Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753-2766.
7. Connolly SD, Bernstein GA; Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(2):267-283.
8. Goodman DW, Spencer TJ, Adler LA, et al. Clinical evaluation of triple-bead mixed amphetamine salts in adult ADHD. Presented at: 54th Annual Meeting of the American Academy of Child and Adolescent Psychiatry; October 25, 2007; Boston, MA.
9. Adler LA, Frick G, Yan B. A Long-term, open-label, safety study of triple-bead mixed amphetamine salts (SHP465) in adults with ADHD [published online April 1, 2017]. J Atten Disord. doi: 10.1177/1087054717696770.
10. Backhaus J, Junghanns K, Broocks A, et al. test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53(3):737-740.