User login
Case Report
A 49-year-old woman presented to the dermatology department with a concern of itching distributed along the V1 branch of the trigeminal nerve on the left frontoparietal scalp following a herpes zoster (HZ) outbreak in the same dermatome 2 months prior. She initially presented to the emergency department 2 months earlier with vesicular lesions distributed along the V1 branch of the trigeminal nerve, along with facial swelling, periorbital edema, inability to open the left eye, and “excruciating” pain. Her left eye was “itchy” but no ophthalmologic pathology was noted on examination. She was diagnosed with HZ and was treated with valacyclovir and prednisone. Oxycodone-acetaminophen followed by hydromorphone was prescribed for the severe pain with limited benefit. After completing treatment with valacyclovir, oral gabapentin was added for additional pain management, with an initial dose of 100 mg 3 times daily.
At the current presentation, the patient reported profound pruritus in the left frontoparietal scalp region that was intractable and debilitating. Some improvement of the itching was achieved with scratching that resulted in deep ulcerations of the scalp with moderate associated pain. In addition to the prior HZ outbreak, her medical history was remarkable for recurrent lymphoma, uterine cancer, chronic bronchitis, depression, hypothyroidism, osteoarthritis, and primary varicella-zoster virus infection in childhood. Her current medications included oral gabapentin (600 mg 3 times daily), diphenhydramine, levothyroxine, simvastatin, and topical ointments for itching.
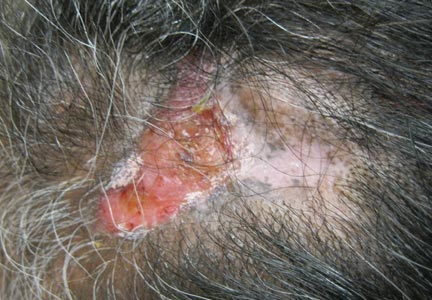
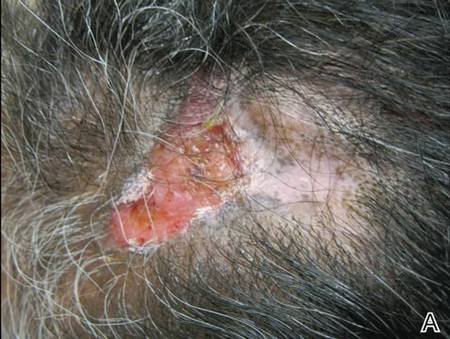
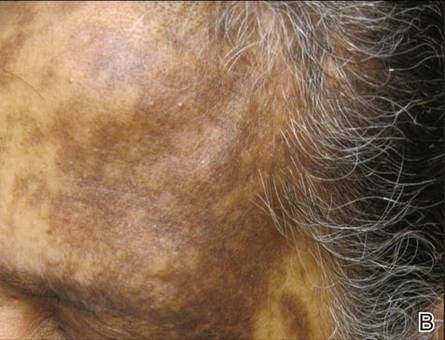
On dermatologic evaluation, the patient rated her pain as a 5 on a 10-point scale of intensity. Alopecia involving the left frontoparietal scalp with a 2×3-cm ulceration in a geometric pattern with surrounding erythema was noted (Figure 1A). There also was hyperpigmentation on the forehead distributed along the V1 branch of the trigeminal nerve (Figure 1B). The patient also had been seen in the pain clinic where examination revealed sensory loss to both light touch and sharp stimulus along the left V1 branch of the trigeminal nerve. Visual fields were full, ocular movements were intact, and the face was symmetric with lower cranial nerves intact.
  |
A diagnosis of trigeminal trophic syndrome (TTS) with chronic pain and pruritus due to a complex sensory neural disorder associated with HZ reactivation was made. Treatment included an increase in the dosage of oral gabapentin (1200 mg 3 times daily), oral oxycodone (5 mg every 4 to 6 hours as needed), and sphenopalatine ganglion block on the left side in an attempt to decrease pain and pruritus. At 6-week follow-up, the patient had no improvement in symptoms.
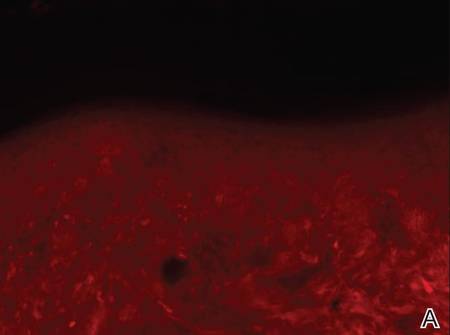
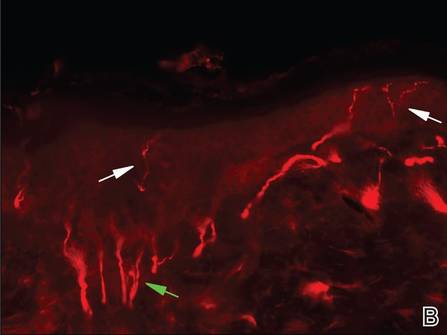
Three scalp punch biopsies were performed on presentation to the dermatology clinic including 2 from the affected area on the left frontoparietal scalp, and one from normal skin on the right side to assess the small nerve fibers affected. Protein gene product 9.5 (PGP 9.5) immunostaining was performed to assess epidermal nerve fiber density. The left scalp biopsies were consistent with a complete focal sensory neuropathy affecting sensory and autonomic axons (Figure 2A). The right scalp biopsy revealed well-innervated skin (Figure 2B).
  |
One year after the original HZ outbreak, the patient continued to have debilitating pruritus and pain in the affected dermatome. On physical examination at 1-year follow-up, the hyperpigmentation on the left side of the forehead showed minimal improvement. The ulcerations were healed, but excoriations were noted in the area. Having experienced some relief from titration of the dose of gabapentin 800 mg 3 times daily and doxepin 25 mg nightly at 1-year follow-up, the patient returned to work but remained highly distressed by her symptoms. Neurosurgery was consulted for possible balloon rhizotomy of the left trigeminal nerve, which she ultimately refused due to concerns about side effects.
Comment
Trophic trigeminal syndrome is characterized by unilateral ulceration of the face with anesthesia, paresthesia, and a crescent-shaped erosion or ulcer.1,2 It is one of 2 causes of self-induced facial ulcerations, the other being factitial dermatitis.1,3,4 A 2008 retrospective medical chart review and report of 14 cases helped elucidate the epidemiology of TTS.2 In this case series, the female to male ratio was 6 to 1, and the mean age of TTS onset was 45 years (age range, 6–82 years). The cause of disease in most patients was iatrogenic and the latent period to onset ranged from days to almost one decade. Most patients self-manipulated the face (n=9), and most ulcers affected the second trigeminal division. Pain intensity was severe in most (n=6), and gabapentin offered relief in only 2 cases.2
The etiologies of TTS are wide ranging, and the differential diagnosis should be contemplated when patients present with facial ulcers. Most cases are iatrogenic secondary to trigeminal rhizotomy,5 alcohol injections into the gasserian ganglion, or electrocoagulation. Also common are cases caused by ischemic damage to the trigeminal ganglion6 or Wallenberg syndrome.7 More rare etiologies include trauma,7 craniotomy,7 astrocytoma, acoustic neuroma, meningioma,8 idiopathic causes, basal cell carcinoma, infectious diseases (eg, tertiary syphilis, recurrent herpes simplex virus, leishmaniasis, cutaneous tuberculosis, leprosy, HZ),9-11 or systemic disease (eg, Wegener granulomatosis, Horton arteritis).
Trigeminal trophic syndrome is rare and there is little agreement on a treatment algorithm. As in our case, a methodical trial-and-error approach is suggested while encouraging the patient not to abandon treatment when efforts are not fruitful. The most important treatment strategy is behavioral modification; patients must become aware of the role of self-manipulation and assiduously avoid it. Using occlusive dressings at the affected site also may be helpful3,12 Transcutaneous electrical nerve stimulation may lead to improvement, but relapse is common with treatment discontinuation. Therapies directed at reducing paresthesia (eg, carbamazepine, diazepam, amitriptyline, chlorpromazine, pimozide) are sometimes successful, but relapse is common.1,3 Transplantation of in vitro–cultured epidermal cells is a new experimental treatment that offers hope for future success.13 Facial reconstruction of the affected area may help patients who can restrain themselves from self-manipulation.4
Skin biopsy findings in our case revealed an interesting aspect of the disease process of TTS. Skin biopsies are helpful in ruling out malignancy and specific stains can be used to further elucidate disease or pathologic processes occurring in the skin. In TTS, no specific changes are seen on hematoxylin and eosin staining, revealing only nonspecific inflammatory changes.1,5 Strikingly, the pathology of affected skin in patients with postherpetic neuralgia often reveals distal nociceptive axon loss,9 as was seen in the skin biopsies from our patient’s left scalp. It has been proven by many researchers in many neuropathic pain conditions that the pathological signature of chronic neuropathic pain is reduction in the density of cutaneous nociceptive innervation.9 The most common method for visualizing cutaneous neuritis is using an immunohistochemical labeling method in which antibodies are directed against PGP 9.5. A pan-axonal neurofilament marker, PGP 9.5 allows for visualization of small sensory nerve endings in the skin. As nociceptive axons degenerate in neuropathic pain conditions, it is believed that initiation of proalgesic changes within remaining peripheral nerves and the central nervous system (CNS) occur. Another interesting aspect of our case was the patient’s persistent intractable itching and chronic pain 2 months following the initial HZ outbreak. Although pain and itching can be evoked by similar stimuli and injuries, it has been shown that both have separate neuronal pathways because they produce different conscious and reflex motor actions.14 For instance, pain causes a withdrawal reflex, while itching causes mechanical stimulation of the affected area. The act of itching is thought to have evolved to protect against threats by the act of dislodging the stimulus rather than withdrawing as seen in pain.14 It has been hypothesized that postherpetic itching (chronic pruritus following an HZ outbreak) is due to spontaneous firing of denervated CNS itch neurons.9
Postherpetic neuralgia–related pain seems to be most closely correlated with degeneration of varicella-zoster virus–infected primary afferent neurons. With deceased afferent neurons sending signals to the CNS and death or dysfunction of inhibitory interneurons in the dorsal horn of the spinal cord due to peripheral nerve injury, there is increased paradoxical electrical activity in specific CNS neurons. This CNS plasticity results in neuropathic pain and other altered sensory abnormalities in patients with TTS.9
Conclusion
We present a case of TTS distributed along the V1 branch of the trigeminal nerve on the left frontoparietal scalp following an HZ outbreak in a 49-year-old woman. Skin biopsies were consistent with this diagnosis, which revealed no neuronal innervation of the affected scalp despite intractable itching and chronic pain. Further research of TTS and postherpetic neuralgia is necessary to find appropriate treatment for patients with these conditions.
1. Kautz O, Bruckner-Tuderman L, Müller ML, et al. Trigeminal trophic syndrome with extensive ulceration following herpes zoster. Eur J Dermatol. 2009;19:61-63.
2. Garza I. The trigeminal trophic syndrome: an unusual cause of face pain, dysaesthesias, anaesthesia and skin/soft tissue lesions. Cephalalgia. 2008;28:980-985.
3. Farahani RM, Marsee DK, Baden LR, et al. Trigeminal trophic syndrome with features of oral CMV disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:15-18.
4. Tollefson TT, Kriet JD, Wang TD, et al. Self-induced nasal ulceration. Arch Facial Plast Surg. 2004;6:162-166.
5. Monrad SU, Terrell JE, Aronoff DM. The trigeminal trophic syndrome: an unusual cause of nasal ulceration. J Am Acad Dermatol. 2004;50:949-952.
6. Elloumi-Jellouli A, Ben Ammar S, Fenniche S, et al. Trigeminal trophic syndrome: a report of two cases with review of literature. Dermatol Online J. 2003;9:26.
7. Sadeghi P, Papay FA, Vidimos AT. Trigeminal trophic syndrome—report of four cases and review of the literature. Dermatol Surg. 2004;30:807-812.
8. Luksi´c I, Luksi´c I, Sestan-Crnek S, et al. Trigeminal trophic syndrome of all three nerve branches: an underrecognized complication after brain surgery. J Neurosurg. 2008;108:170-173.
9. Oaklander AL. Mechanisms of pain and itch caused by herpes zoster (shingles). J Pain. 2008;9(1 suppl 1):S10-S18.
10. Gawande A. The itch. The New Yorker. June 2008:58-67.
11. Oaklander AL, Cohen SP, Raju SV. Intractable postherpetic itch and cutaneous deafferentation after facial shingles. Pain. 2002;96:9-12.
12. Preston PW, Orpin SD, Tucker WF, et al. Successful use of a thermoplastic dressing in two cases of the trigeminal trophic syndrome. Clin Exp Dermatol. 2006;31:525-527.
13. Schwerdtner O, Damaskos T, Kage A, et al. Autologous epidermal cells can induce wound closure of neurotrophic ulceration caused by trigeminal trophic syndrome. Int J Oral Maxillofac Surg. 2005;34:443-445.
14. Oaklander AL, Siegel SM. Cutaneous innervation: form and function. J Am Acad Dermatol. 2005;53:1027-1037.
Case Report
A 49-year-old woman presented to the dermatology department with a concern of itching distributed along the V1 branch of the trigeminal nerve on the left frontoparietal scalp following a herpes zoster (HZ) outbreak in the same dermatome 2 months prior. She initially presented to the emergency department 2 months earlier with vesicular lesions distributed along the V1 branch of the trigeminal nerve, along with facial swelling, periorbital edema, inability to open the left eye, and “excruciating” pain. Her left eye was “itchy” but no ophthalmologic pathology was noted on examination. She was diagnosed with HZ and was treated with valacyclovir and prednisone. Oxycodone-acetaminophen followed by hydromorphone was prescribed for the severe pain with limited benefit. After completing treatment with valacyclovir, oral gabapentin was added for additional pain management, with an initial dose of 100 mg 3 times daily.
At the current presentation, the patient reported profound pruritus in the left frontoparietal scalp region that was intractable and debilitating. Some improvement of the itching was achieved with scratching that resulted in deep ulcerations of the scalp with moderate associated pain. In addition to the prior HZ outbreak, her medical history was remarkable for recurrent lymphoma, uterine cancer, chronic bronchitis, depression, hypothyroidism, osteoarthritis, and primary varicella-zoster virus infection in childhood. Her current medications included oral gabapentin (600 mg 3 times daily), diphenhydramine, levothyroxine, simvastatin, and topical ointments for itching.
On dermatologic evaluation, the patient rated her pain as a 5 on a 10-point scale of intensity. Alopecia involving the left frontoparietal scalp with a 2×3-cm ulceration in a geometric pattern with surrounding erythema was noted (Figure 1A). There also was hyperpigmentation on the forehead distributed along the V1 branch of the trigeminal nerve (Figure 1B). The patient also had been seen in the pain clinic where examination revealed sensory loss to both light touch and sharp stimulus along the left V1 branch of the trigeminal nerve. Visual fields were full, ocular movements were intact, and the face was symmetric with lower cranial nerves intact.
  |
A diagnosis of trigeminal trophic syndrome (TTS) with chronic pain and pruritus due to a complex sensory neural disorder associated with HZ reactivation was made. Treatment included an increase in the dosage of oral gabapentin (1200 mg 3 times daily), oral oxycodone (5 mg every 4 to 6 hours as needed), and sphenopalatine ganglion block on the left side in an attempt to decrease pain and pruritus. At 6-week follow-up, the patient had no improvement in symptoms.
Three scalp punch biopsies were performed on presentation to the dermatology clinic including 2 from the affected area on the left frontoparietal scalp, and one from normal skin on the right side to assess the small nerve fibers affected. Protein gene product 9.5 (PGP 9.5) immunostaining was performed to assess epidermal nerve fiber density. The left scalp biopsies were consistent with a complete focal sensory neuropathy affecting sensory and autonomic axons (Figure 2A). The right scalp biopsy revealed well-innervated skin (Figure 2B).
  |
One year after the original HZ outbreak, the patient continued to have debilitating pruritus and pain in the affected dermatome. On physical examination at 1-year follow-up, the hyperpigmentation on the left side of the forehead showed minimal improvement. The ulcerations were healed, but excoriations were noted in the area. Having experienced some relief from titration of the dose of gabapentin 800 mg 3 times daily and doxepin 25 mg nightly at 1-year follow-up, the patient returned to work but remained highly distressed by her symptoms. Neurosurgery was consulted for possible balloon rhizotomy of the left trigeminal nerve, which she ultimately refused due to concerns about side effects.
Comment
Trophic trigeminal syndrome is characterized by unilateral ulceration of the face with anesthesia, paresthesia, and a crescent-shaped erosion or ulcer.1,2 It is one of 2 causes of self-induced facial ulcerations, the other being factitial dermatitis.1,3,4 A 2008 retrospective medical chart review and report of 14 cases helped elucidate the epidemiology of TTS.2 In this case series, the female to male ratio was 6 to 1, and the mean age of TTS onset was 45 years (age range, 6–82 years). The cause of disease in most patients was iatrogenic and the latent period to onset ranged from days to almost one decade. Most patients self-manipulated the face (n=9), and most ulcers affected the second trigeminal division. Pain intensity was severe in most (n=6), and gabapentin offered relief in only 2 cases.2
The etiologies of TTS are wide ranging, and the differential diagnosis should be contemplated when patients present with facial ulcers. Most cases are iatrogenic secondary to trigeminal rhizotomy,5 alcohol injections into the gasserian ganglion, or electrocoagulation. Also common are cases caused by ischemic damage to the trigeminal ganglion6 or Wallenberg syndrome.7 More rare etiologies include trauma,7 craniotomy,7 astrocytoma, acoustic neuroma, meningioma,8 idiopathic causes, basal cell carcinoma, infectious diseases (eg, tertiary syphilis, recurrent herpes simplex virus, leishmaniasis, cutaneous tuberculosis, leprosy, HZ),9-11 or systemic disease (eg, Wegener granulomatosis, Horton arteritis).
Trigeminal trophic syndrome is rare and there is little agreement on a treatment algorithm. As in our case, a methodical trial-and-error approach is suggested while encouraging the patient not to abandon treatment when efforts are not fruitful. The most important treatment strategy is behavioral modification; patients must become aware of the role of self-manipulation and assiduously avoid it. Using occlusive dressings at the affected site also may be helpful3,12 Transcutaneous electrical nerve stimulation may lead to improvement, but relapse is common with treatment discontinuation. Therapies directed at reducing paresthesia (eg, carbamazepine, diazepam, amitriptyline, chlorpromazine, pimozide) are sometimes successful, but relapse is common.1,3 Transplantation of in vitro–cultured epidermal cells is a new experimental treatment that offers hope for future success.13 Facial reconstruction of the affected area may help patients who can restrain themselves from self-manipulation.4
Skin biopsy findings in our case revealed an interesting aspect of the disease process of TTS. Skin biopsies are helpful in ruling out malignancy and specific stains can be used to further elucidate disease or pathologic processes occurring in the skin. In TTS, no specific changes are seen on hematoxylin and eosin staining, revealing only nonspecific inflammatory changes.1,5 Strikingly, the pathology of affected skin in patients with postherpetic neuralgia often reveals distal nociceptive axon loss,9 as was seen in the skin biopsies from our patient’s left scalp. It has been proven by many researchers in many neuropathic pain conditions that the pathological signature of chronic neuropathic pain is reduction in the density of cutaneous nociceptive innervation.9 The most common method for visualizing cutaneous neuritis is using an immunohistochemical labeling method in which antibodies are directed against PGP 9.5. A pan-axonal neurofilament marker, PGP 9.5 allows for visualization of small sensory nerve endings in the skin. As nociceptive axons degenerate in neuropathic pain conditions, it is believed that initiation of proalgesic changes within remaining peripheral nerves and the central nervous system (CNS) occur. Another interesting aspect of our case was the patient’s persistent intractable itching and chronic pain 2 months following the initial HZ outbreak. Although pain and itching can be evoked by similar stimuli and injuries, it has been shown that both have separate neuronal pathways because they produce different conscious and reflex motor actions.14 For instance, pain causes a withdrawal reflex, while itching causes mechanical stimulation of the affected area. The act of itching is thought to have evolved to protect against threats by the act of dislodging the stimulus rather than withdrawing as seen in pain.14 It has been hypothesized that postherpetic itching (chronic pruritus following an HZ outbreak) is due to spontaneous firing of denervated CNS itch neurons.9
Postherpetic neuralgia–related pain seems to be most closely correlated with degeneration of varicella-zoster virus–infected primary afferent neurons. With deceased afferent neurons sending signals to the CNS and death or dysfunction of inhibitory interneurons in the dorsal horn of the spinal cord due to peripheral nerve injury, there is increased paradoxical electrical activity in specific CNS neurons. This CNS plasticity results in neuropathic pain and other altered sensory abnormalities in patients with TTS.9
Conclusion
We present a case of TTS distributed along the V1 branch of the trigeminal nerve on the left frontoparietal scalp following an HZ outbreak in a 49-year-old woman. Skin biopsies were consistent with this diagnosis, which revealed no neuronal innervation of the affected scalp despite intractable itching and chronic pain. Further research of TTS and postherpetic neuralgia is necessary to find appropriate treatment for patients with these conditions.
Case Report
A 49-year-old woman presented to the dermatology department with a concern of itching distributed along the V1 branch of the trigeminal nerve on the left frontoparietal scalp following a herpes zoster (HZ) outbreak in the same dermatome 2 months prior. She initially presented to the emergency department 2 months earlier with vesicular lesions distributed along the V1 branch of the trigeminal nerve, along with facial swelling, periorbital edema, inability to open the left eye, and “excruciating” pain. Her left eye was “itchy” but no ophthalmologic pathology was noted on examination. She was diagnosed with HZ and was treated with valacyclovir and prednisone. Oxycodone-acetaminophen followed by hydromorphone was prescribed for the severe pain with limited benefit. After completing treatment with valacyclovir, oral gabapentin was added for additional pain management, with an initial dose of 100 mg 3 times daily.
At the current presentation, the patient reported profound pruritus in the left frontoparietal scalp region that was intractable and debilitating. Some improvement of the itching was achieved with scratching that resulted in deep ulcerations of the scalp with moderate associated pain. In addition to the prior HZ outbreak, her medical history was remarkable for recurrent lymphoma, uterine cancer, chronic bronchitis, depression, hypothyroidism, osteoarthritis, and primary varicella-zoster virus infection in childhood. Her current medications included oral gabapentin (600 mg 3 times daily), diphenhydramine, levothyroxine, simvastatin, and topical ointments for itching.
On dermatologic evaluation, the patient rated her pain as a 5 on a 10-point scale of intensity. Alopecia involving the left frontoparietal scalp with a 2×3-cm ulceration in a geometric pattern with surrounding erythema was noted (Figure 1A). There also was hyperpigmentation on the forehead distributed along the V1 branch of the trigeminal nerve (Figure 1B). The patient also had been seen in the pain clinic where examination revealed sensory loss to both light touch and sharp stimulus along the left V1 branch of the trigeminal nerve. Visual fields were full, ocular movements were intact, and the face was symmetric with lower cranial nerves intact.
  |
A diagnosis of trigeminal trophic syndrome (TTS) with chronic pain and pruritus due to a complex sensory neural disorder associated with HZ reactivation was made. Treatment included an increase in the dosage of oral gabapentin (1200 mg 3 times daily), oral oxycodone (5 mg every 4 to 6 hours as needed), and sphenopalatine ganglion block on the left side in an attempt to decrease pain and pruritus. At 6-week follow-up, the patient had no improvement in symptoms.
Three scalp punch biopsies were performed on presentation to the dermatology clinic including 2 from the affected area on the left frontoparietal scalp, and one from normal skin on the right side to assess the small nerve fibers affected. Protein gene product 9.5 (PGP 9.5) immunostaining was performed to assess epidermal nerve fiber density. The left scalp biopsies were consistent with a complete focal sensory neuropathy affecting sensory and autonomic axons (Figure 2A). The right scalp biopsy revealed well-innervated skin (Figure 2B).
  |
One year after the original HZ outbreak, the patient continued to have debilitating pruritus and pain in the affected dermatome. On physical examination at 1-year follow-up, the hyperpigmentation on the left side of the forehead showed minimal improvement. The ulcerations were healed, but excoriations were noted in the area. Having experienced some relief from titration of the dose of gabapentin 800 mg 3 times daily and doxepin 25 mg nightly at 1-year follow-up, the patient returned to work but remained highly distressed by her symptoms. Neurosurgery was consulted for possible balloon rhizotomy of the left trigeminal nerve, which she ultimately refused due to concerns about side effects.
Comment
Trophic trigeminal syndrome is characterized by unilateral ulceration of the face with anesthesia, paresthesia, and a crescent-shaped erosion or ulcer.1,2 It is one of 2 causes of self-induced facial ulcerations, the other being factitial dermatitis.1,3,4 A 2008 retrospective medical chart review and report of 14 cases helped elucidate the epidemiology of TTS.2 In this case series, the female to male ratio was 6 to 1, and the mean age of TTS onset was 45 years (age range, 6–82 years). The cause of disease in most patients was iatrogenic and the latent period to onset ranged from days to almost one decade. Most patients self-manipulated the face (n=9), and most ulcers affected the second trigeminal division. Pain intensity was severe in most (n=6), and gabapentin offered relief in only 2 cases.2
The etiologies of TTS are wide ranging, and the differential diagnosis should be contemplated when patients present with facial ulcers. Most cases are iatrogenic secondary to trigeminal rhizotomy,5 alcohol injections into the gasserian ganglion, or electrocoagulation. Also common are cases caused by ischemic damage to the trigeminal ganglion6 or Wallenberg syndrome.7 More rare etiologies include trauma,7 craniotomy,7 astrocytoma, acoustic neuroma, meningioma,8 idiopathic causes, basal cell carcinoma, infectious diseases (eg, tertiary syphilis, recurrent herpes simplex virus, leishmaniasis, cutaneous tuberculosis, leprosy, HZ),9-11 or systemic disease (eg, Wegener granulomatosis, Horton arteritis).
Trigeminal trophic syndrome is rare and there is little agreement on a treatment algorithm. As in our case, a methodical trial-and-error approach is suggested while encouraging the patient not to abandon treatment when efforts are not fruitful. The most important treatment strategy is behavioral modification; patients must become aware of the role of self-manipulation and assiduously avoid it. Using occlusive dressings at the affected site also may be helpful3,12 Transcutaneous electrical nerve stimulation may lead to improvement, but relapse is common with treatment discontinuation. Therapies directed at reducing paresthesia (eg, carbamazepine, diazepam, amitriptyline, chlorpromazine, pimozide) are sometimes successful, but relapse is common.1,3 Transplantation of in vitro–cultured epidermal cells is a new experimental treatment that offers hope for future success.13 Facial reconstruction of the affected area may help patients who can restrain themselves from self-manipulation.4
Skin biopsy findings in our case revealed an interesting aspect of the disease process of TTS. Skin biopsies are helpful in ruling out malignancy and specific stains can be used to further elucidate disease or pathologic processes occurring in the skin. In TTS, no specific changes are seen on hematoxylin and eosin staining, revealing only nonspecific inflammatory changes.1,5 Strikingly, the pathology of affected skin in patients with postherpetic neuralgia often reveals distal nociceptive axon loss,9 as was seen in the skin biopsies from our patient’s left scalp. It has been proven by many researchers in many neuropathic pain conditions that the pathological signature of chronic neuropathic pain is reduction in the density of cutaneous nociceptive innervation.9 The most common method for visualizing cutaneous neuritis is using an immunohistochemical labeling method in which antibodies are directed against PGP 9.5. A pan-axonal neurofilament marker, PGP 9.5 allows for visualization of small sensory nerve endings in the skin. As nociceptive axons degenerate in neuropathic pain conditions, it is believed that initiation of proalgesic changes within remaining peripheral nerves and the central nervous system (CNS) occur. Another interesting aspect of our case was the patient’s persistent intractable itching and chronic pain 2 months following the initial HZ outbreak. Although pain and itching can be evoked by similar stimuli and injuries, it has been shown that both have separate neuronal pathways because they produce different conscious and reflex motor actions.14 For instance, pain causes a withdrawal reflex, while itching causes mechanical stimulation of the affected area. The act of itching is thought to have evolved to protect against threats by the act of dislodging the stimulus rather than withdrawing as seen in pain.14 It has been hypothesized that postherpetic itching (chronic pruritus following an HZ outbreak) is due to spontaneous firing of denervated CNS itch neurons.9
Postherpetic neuralgia–related pain seems to be most closely correlated with degeneration of varicella-zoster virus–infected primary afferent neurons. With deceased afferent neurons sending signals to the CNS and death or dysfunction of inhibitory interneurons in the dorsal horn of the spinal cord due to peripheral nerve injury, there is increased paradoxical electrical activity in specific CNS neurons. This CNS plasticity results in neuropathic pain and other altered sensory abnormalities in patients with TTS.9
Conclusion
We present a case of TTS distributed along the V1 branch of the trigeminal nerve on the left frontoparietal scalp following an HZ outbreak in a 49-year-old woman. Skin biopsies were consistent with this diagnosis, which revealed no neuronal innervation of the affected scalp despite intractable itching and chronic pain. Further research of TTS and postherpetic neuralgia is necessary to find appropriate treatment for patients with these conditions.
1. Kautz O, Bruckner-Tuderman L, Müller ML, et al. Trigeminal trophic syndrome with extensive ulceration following herpes zoster. Eur J Dermatol. 2009;19:61-63.
2. Garza I. The trigeminal trophic syndrome: an unusual cause of face pain, dysaesthesias, anaesthesia and skin/soft tissue lesions. Cephalalgia. 2008;28:980-985.
3. Farahani RM, Marsee DK, Baden LR, et al. Trigeminal trophic syndrome with features of oral CMV disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:15-18.
4. Tollefson TT, Kriet JD, Wang TD, et al. Self-induced nasal ulceration. Arch Facial Plast Surg. 2004;6:162-166.
5. Monrad SU, Terrell JE, Aronoff DM. The trigeminal trophic syndrome: an unusual cause of nasal ulceration. J Am Acad Dermatol. 2004;50:949-952.
6. Elloumi-Jellouli A, Ben Ammar S, Fenniche S, et al. Trigeminal trophic syndrome: a report of two cases with review of literature. Dermatol Online J. 2003;9:26.
7. Sadeghi P, Papay FA, Vidimos AT. Trigeminal trophic syndrome—report of four cases and review of the literature. Dermatol Surg. 2004;30:807-812.
8. Luksi´c I, Luksi´c I, Sestan-Crnek S, et al. Trigeminal trophic syndrome of all three nerve branches: an underrecognized complication after brain surgery. J Neurosurg. 2008;108:170-173.
9. Oaklander AL. Mechanisms of pain and itch caused by herpes zoster (shingles). J Pain. 2008;9(1 suppl 1):S10-S18.
10. Gawande A. The itch. The New Yorker. June 2008:58-67.
11. Oaklander AL, Cohen SP, Raju SV. Intractable postherpetic itch and cutaneous deafferentation after facial shingles. Pain. 2002;96:9-12.
12. Preston PW, Orpin SD, Tucker WF, et al. Successful use of a thermoplastic dressing in two cases of the trigeminal trophic syndrome. Clin Exp Dermatol. 2006;31:525-527.
13. Schwerdtner O, Damaskos T, Kage A, et al. Autologous epidermal cells can induce wound closure of neurotrophic ulceration caused by trigeminal trophic syndrome. Int J Oral Maxillofac Surg. 2005;34:443-445.
14. Oaklander AL, Siegel SM. Cutaneous innervation: form and function. J Am Acad Dermatol. 2005;53:1027-1037.
1. Kautz O, Bruckner-Tuderman L, Müller ML, et al. Trigeminal trophic syndrome with extensive ulceration following herpes zoster. Eur J Dermatol. 2009;19:61-63.
2. Garza I. The trigeminal trophic syndrome: an unusual cause of face pain, dysaesthesias, anaesthesia and skin/soft tissue lesions. Cephalalgia. 2008;28:980-985.
3. Farahani RM, Marsee DK, Baden LR, et al. Trigeminal trophic syndrome with features of oral CMV disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:15-18.
4. Tollefson TT, Kriet JD, Wang TD, et al. Self-induced nasal ulceration. Arch Facial Plast Surg. 2004;6:162-166.
5. Monrad SU, Terrell JE, Aronoff DM. The trigeminal trophic syndrome: an unusual cause of nasal ulceration. J Am Acad Dermatol. 2004;50:949-952.
6. Elloumi-Jellouli A, Ben Ammar S, Fenniche S, et al. Trigeminal trophic syndrome: a report of two cases with review of literature. Dermatol Online J. 2003;9:26.
7. Sadeghi P, Papay FA, Vidimos AT. Trigeminal trophic syndrome—report of four cases and review of the literature. Dermatol Surg. 2004;30:807-812.
8. Luksi´c I, Luksi´c I, Sestan-Crnek S, et al. Trigeminal trophic syndrome of all three nerve branches: an underrecognized complication after brain surgery. J Neurosurg. 2008;108:170-173.
9. Oaklander AL. Mechanisms of pain and itch caused by herpes zoster (shingles). J Pain. 2008;9(1 suppl 1):S10-S18.
10. Gawande A. The itch. The New Yorker. June 2008:58-67.
11. Oaklander AL, Cohen SP, Raju SV. Intractable postherpetic itch and cutaneous deafferentation after facial shingles. Pain. 2002;96:9-12.
12. Preston PW, Orpin SD, Tucker WF, et al. Successful use of a thermoplastic dressing in two cases of the trigeminal trophic syndrome. Clin Exp Dermatol. 2006;31:525-527.
13. Schwerdtner O, Damaskos T, Kage A, et al. Autologous epidermal cells can induce wound closure of neurotrophic ulceration caused by trigeminal trophic syndrome. Int J Oral Maxillofac Surg. 2005;34:443-445.
14. Oaklander AL, Siegel SM. Cutaneous innervation: form and function. J Am Acad Dermatol. 2005;53:1027-1037.
Practice Points
- Clinicians should remember to include trigeminal trophic syndrome in the differential diagnosis of patients with facial ulcers.
- Trigeminal trophic syndrome is a rare syndrome with a variety of treatment options, though no gold standard for treatment exists.