User login
To the Editor:
Most cutaneous squamous cell carcinomas (cSCCs) are successfully treated with standard modalities such as surgical excision; however, a subset of tumors is not amenable to surgical resection.1,2 Patients who are not able to undergo surgical treatment may instead receive radiation therapy, topical 5-fluorouracil (5-FU), imiquimod, cryosurgery, photodynamic therapy, or systemic treatment (eg, immunotherapy) in addition to intralesional approaches for localized disease.1-4 However, the adverse effects associated with these treatments and their modest effect in preventing the recurrence of cutaneous lesions limit their efficacy against unresectable cSCC.4-6 We present a case that demonstrates the efficacy of electrodesiccation and curettage (ED&C) followed by topical 5-FU for an invasive cSCC not amenable to surgical therapy.
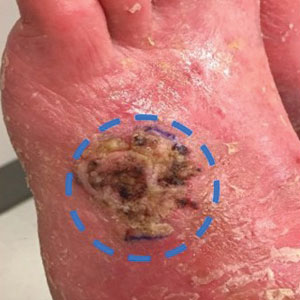
A 58-year-old woman presented for evaluation of a 3.5×3.4-cm, incisional biopsy–proven, invasive stage T2a cSCC (Brigham and Women’s Hospital tumor staging system [Boston, Massachusetts]) on the dorsal aspect of the left foot, which had developed over several months (Figure 1A). She had a history of treatment with psoralen plus UV light therapy for erythroderma of unknown cause and peripheral neuropathy. She was not a surgical candidate because of suspected underlying cutaneous sclerosis and a history of poor wound healing on the lower legs.
Prior to presentation to dermatology, the patient had been treated with intralesional methotrexate, intralesional 5-FU, and the antiangiogenic and antiproliferative combination agent OLCAT-0053—consisting of equal parts [by volume] of diclofenac gel 3%, imiquimod cream 5%, hydrocortisone valerate cream 0.2%, calcipotriene cream 0.005%, and tretinoin cream 0.05—which failed, and the patient reported that OLCAT-005 made the pain from the cSCC worse.
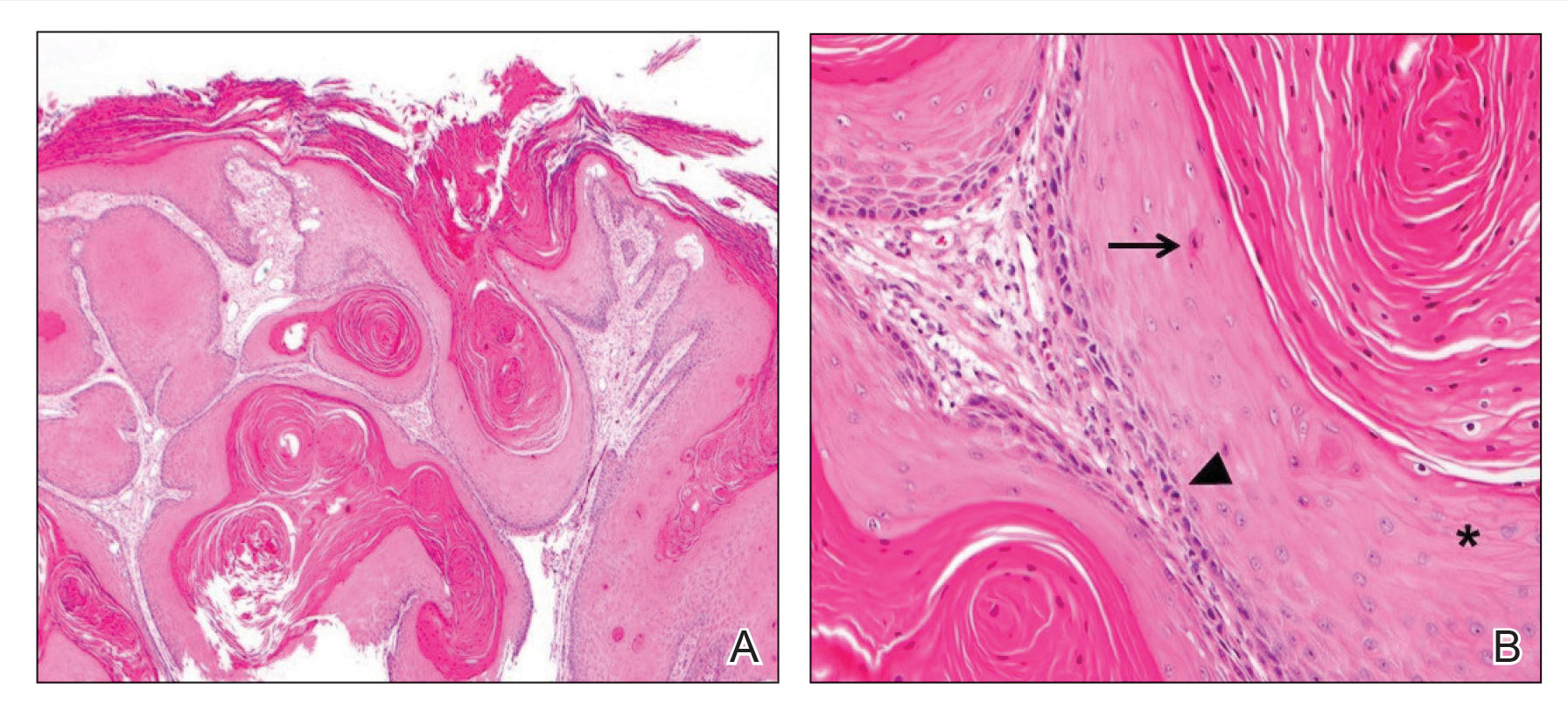
Upon growth of the lesion over several months, the patient was referred to the High-Risk Skin Cancer Clinic at Massachusetts General Hospital (Boston, Massachusetts). A repeat biopsy demonstrated an invasive well-differentiated cSCC (Figure 2). The size and invasive features of the lesion on clinical examination prompted a referral to surgical oncology for a wide local excision. However, surgical oncology concluded she was not a surgical candidate.
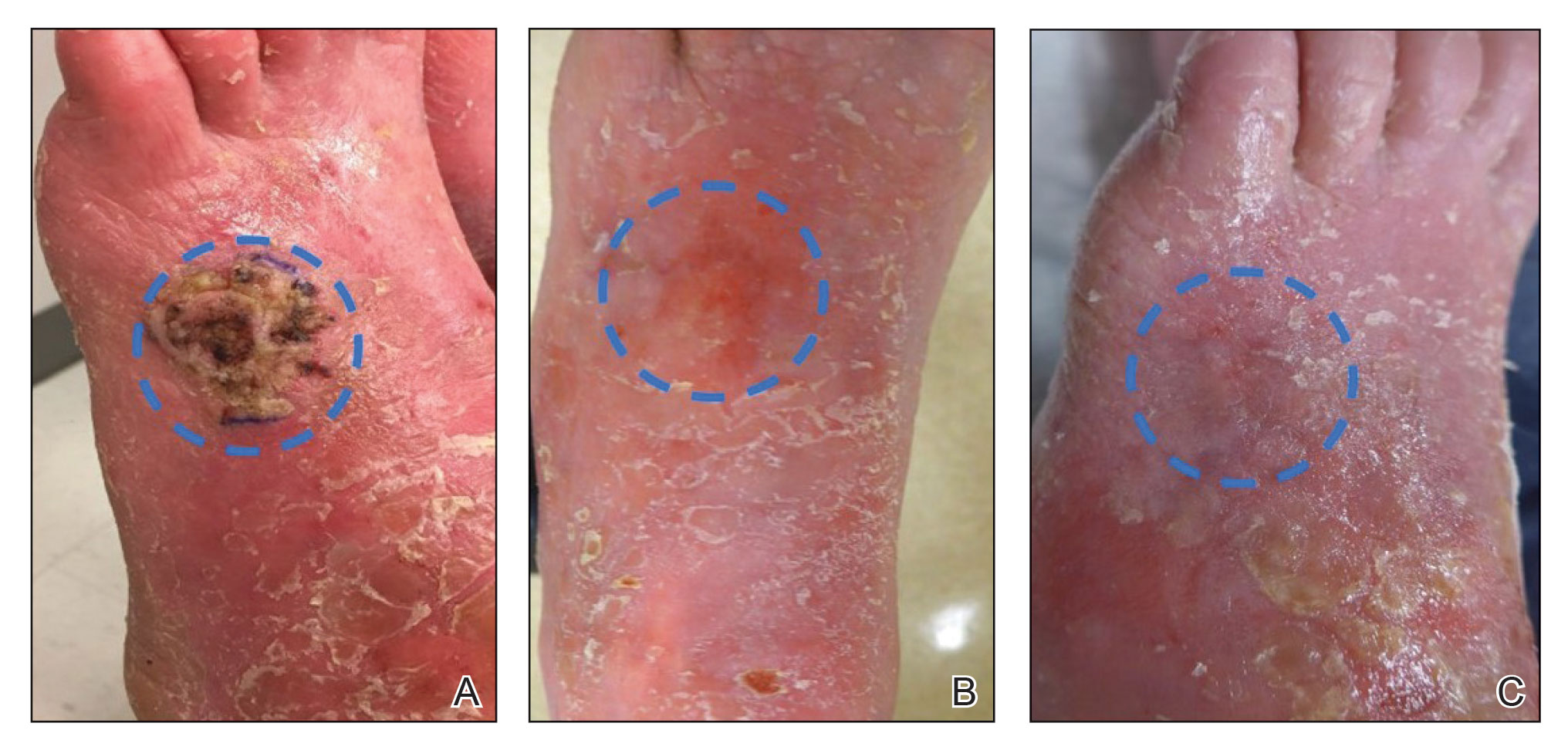
Magnetic resonance imaging showed no deep invasion of the cSCC to the tendons or bones. Electrodesiccation and curettage was performed to debulk the tumor, followed by twice-daily application of topical 5-FU for 4 weeks to improve the odds of tumor clearance (Figure 1B). Fourteen weeks after completion of 5-FU treatment, the cSCC showed complete clinical regression (Figure 1C). No recurrence has been detected clinically more than 3 years following treatment.
Prior to the advent of Mohs micrographic surgery, ED&C commonly was used to treat skin cancer, with a lower cost and a cure rate close to 95%.7,8 We postulate that the mechanism of tumor regression in our patient was ED&C-mediated removal and necrosis of neoplastic tissue combined with 5-FU–induced cancer-cell DNA damage and apoptosis. An antitumor immune response also may have contributed to the complete regression of the cSCC.
Although antiangiogenic and antiproliferative agents are suitable for primary cSCC treatment, it is possible that this patient’s prior therapies alone—in the absence of debulking by ED&C to sufficiently reduce disease burden—did not allow for tumor clearance and were ineffective. Many clinicians are reluctant to apply 5-FU to a wound bed because it can impede wound healing.9 In this case, re-epithelialization likely occurred primarily after completion of 5-FU treatment.
We recommend consideration of ED&C with 5-FU for similar malignant lesions that are not amenable to surgical excision. Nevertheless, Mohs micrographic surgery and wide local excision remain the gold standards for definitive treatment of invasive skin cancer in a patient who is a candidate for surgical treatment.
- Nehal KS, Bichakjian CK. Update on keratinocyte carcinomas. N Engl J Med. 2018;379:363-374. doi:10.1056/NEJMra1708701
- de Jong E, Lammerts MUPA, Genders RE, et al. Update of advanced cutaneous squamous cell carcinoma. J Eur Acad Dermatol Venereol. 2022;36(suppl 1):6-10. doi:10.1111/jdv.17728
- Li VW, Ball RA, Vasan N, et al. Antiangiogenic therapy for squamous cell carcinoma using combinatorial agents [abstract]. J Clin Oncol. 2005;23(16 suppl):3032. doi:10.1200/jco.2005.23.16_suppl.3032
- Lansbury L, Bath-Hextall F, Perkins W, et al. Interventions for non-metastatic squamous cell carcinoma of the skin: systematic review and pooled analysis of observational studies. BMJ. 2013;347:f6153. doi:10.1136/bmj.f6153
- Behshad R, Garcia‐Zuazaga J, Bordeaux J. Systemic treatment of locally advanced nonmetastatic cutaneous squamous cell carcinoma: a review of the literature. Br J Dermatol. 2011;165:1169-1177. doi:10.1111/j.1365-2133.2011.10524.x
- Rowe DE, Carroll RJ, Day CL Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. implications for treatment modality selection. J Am Acad Dermatol. 1992;26:976-990. doi:10.1016/0190-9622(92)70144-5
- Knox JM, Lyles TW, Shapiro EM, et al. Curettage and electrodesiccation in the treatment of skin cancer. Arch Dermatol. 1960;82:197-204.
- Chren M-M, Linos E, Torres JS, et al. Tumor recurrence 5 years after treatment of cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2013;133:1188-1196. doi:10.1038/jid.2012.403
- Berman B, Maderal A, Raphael B. Keloids and hypertrophic scars: pathophysiology, classification, and treatment. Dermatologic Surgery. 2017;43:S3-S18.
To the Editor:
Most cutaneous squamous cell carcinomas (cSCCs) are successfully treated with standard modalities such as surgical excision; however, a subset of tumors is not amenable to surgical resection.1,2 Patients who are not able to undergo surgical treatment may instead receive radiation therapy, topical 5-fluorouracil (5-FU), imiquimod, cryosurgery, photodynamic therapy, or systemic treatment (eg, immunotherapy) in addition to intralesional approaches for localized disease.1-4 However, the adverse effects associated with these treatments and their modest effect in preventing the recurrence of cutaneous lesions limit their efficacy against unresectable cSCC.4-6 We present a case that demonstrates the efficacy of electrodesiccation and curettage (ED&C) followed by topical 5-FU for an invasive cSCC not amenable to surgical therapy.
A 58-year-old woman presented for evaluation of a 3.5×3.4-cm, incisional biopsy–proven, invasive stage T2a cSCC (Brigham and Women’s Hospital tumor staging system [Boston, Massachusetts]) on the dorsal aspect of the left foot, which had developed over several months (Figure 1A). She had a history of treatment with psoralen plus UV light therapy for erythroderma of unknown cause and peripheral neuropathy. She was not a surgical candidate because of suspected underlying cutaneous sclerosis and a history of poor wound healing on the lower legs.

Prior to presentation to dermatology, the patient had been treated with intralesional methotrexate, intralesional 5-FU, and the antiangiogenic and antiproliferative combination agent OLCAT-0053—consisting of equal parts [by volume] of diclofenac gel 3%, imiquimod cream 5%, hydrocortisone valerate cream 0.2%, calcipotriene cream 0.005%, and tretinoin cream 0.05—which failed, and the patient reported that OLCAT-005 made the pain from the cSCC worse.
Upon growth of the lesion over several months, the patient was referred to the High-Risk Skin Cancer Clinic at Massachusetts General Hospital (Boston, Massachusetts). A repeat biopsy demonstrated an invasive well-differentiated cSCC (Figure 2). The size and invasive features of the lesion on clinical examination prompted a referral to surgical oncology for a wide local excision. However, surgical oncology concluded she was not a surgical candidate.

Magnetic resonance imaging showed no deep invasion of the cSCC to the tendons or bones. Electrodesiccation and curettage was performed to debulk the tumor, followed by twice-daily application of topical 5-FU for 4 weeks to improve the odds of tumor clearance (Figure 1B). Fourteen weeks after completion of 5-FU treatment, the cSCC showed complete clinical regression (Figure 1C). No recurrence has been detected clinically more than 3 years following treatment.
Prior to the advent of Mohs micrographic surgery, ED&C commonly was used to treat skin cancer, with a lower cost and a cure rate close to 95%.7,8 We postulate that the mechanism of tumor regression in our patient was ED&C-mediated removal and necrosis of neoplastic tissue combined with 5-FU–induced cancer-cell DNA damage and apoptosis. An antitumor immune response also may have contributed to the complete regression of the cSCC.
Although antiangiogenic and antiproliferative agents are suitable for primary cSCC treatment, it is possible that this patient’s prior therapies alone—in the absence of debulking by ED&C to sufficiently reduce disease burden—did not allow for tumor clearance and were ineffective. Many clinicians are reluctant to apply 5-FU to a wound bed because it can impede wound healing.9 In this case, re-epithelialization likely occurred primarily after completion of 5-FU treatment.
We recommend consideration of ED&C with 5-FU for similar malignant lesions that are not amenable to surgical excision. Nevertheless, Mohs micrographic surgery and wide local excision remain the gold standards for definitive treatment of invasive skin cancer in a patient who is a candidate for surgical treatment.
To the Editor:
Most cutaneous squamous cell carcinomas (cSCCs) are successfully treated with standard modalities such as surgical excision; however, a subset of tumors is not amenable to surgical resection.1,2 Patients who are not able to undergo surgical treatment may instead receive radiation therapy, topical 5-fluorouracil (5-FU), imiquimod, cryosurgery, photodynamic therapy, or systemic treatment (eg, immunotherapy) in addition to intralesional approaches for localized disease.1-4 However, the adverse effects associated with these treatments and their modest effect in preventing the recurrence of cutaneous lesions limit their efficacy against unresectable cSCC.4-6 We present a case that demonstrates the efficacy of electrodesiccation and curettage (ED&C) followed by topical 5-FU for an invasive cSCC not amenable to surgical therapy.
A 58-year-old woman presented for evaluation of a 3.5×3.4-cm, incisional biopsy–proven, invasive stage T2a cSCC (Brigham and Women’s Hospital tumor staging system [Boston, Massachusetts]) on the dorsal aspect of the left foot, which had developed over several months (Figure 1A). She had a history of treatment with psoralen plus UV light therapy for erythroderma of unknown cause and peripheral neuropathy. She was not a surgical candidate because of suspected underlying cutaneous sclerosis and a history of poor wound healing on the lower legs.

Prior to presentation to dermatology, the patient had been treated with intralesional methotrexate, intralesional 5-FU, and the antiangiogenic and antiproliferative combination agent OLCAT-0053—consisting of equal parts [by volume] of diclofenac gel 3%, imiquimod cream 5%, hydrocortisone valerate cream 0.2%, calcipotriene cream 0.005%, and tretinoin cream 0.05—which failed, and the patient reported that OLCAT-005 made the pain from the cSCC worse.
Upon growth of the lesion over several months, the patient was referred to the High-Risk Skin Cancer Clinic at Massachusetts General Hospital (Boston, Massachusetts). A repeat biopsy demonstrated an invasive well-differentiated cSCC (Figure 2). The size and invasive features of the lesion on clinical examination prompted a referral to surgical oncology for a wide local excision. However, surgical oncology concluded she was not a surgical candidate.

Magnetic resonance imaging showed no deep invasion of the cSCC to the tendons or bones. Electrodesiccation and curettage was performed to debulk the tumor, followed by twice-daily application of topical 5-FU for 4 weeks to improve the odds of tumor clearance (Figure 1B). Fourteen weeks after completion of 5-FU treatment, the cSCC showed complete clinical regression (Figure 1C). No recurrence has been detected clinically more than 3 years following treatment.
Prior to the advent of Mohs micrographic surgery, ED&C commonly was used to treat skin cancer, with a lower cost and a cure rate close to 95%.7,8 We postulate that the mechanism of tumor regression in our patient was ED&C-mediated removal and necrosis of neoplastic tissue combined with 5-FU–induced cancer-cell DNA damage and apoptosis. An antitumor immune response also may have contributed to the complete regression of the cSCC.
Although antiangiogenic and antiproliferative agents are suitable for primary cSCC treatment, it is possible that this patient’s prior therapies alone—in the absence of debulking by ED&C to sufficiently reduce disease burden—did not allow for tumor clearance and were ineffective. Many clinicians are reluctant to apply 5-FU to a wound bed because it can impede wound healing.9 In this case, re-epithelialization likely occurred primarily after completion of 5-FU treatment.
We recommend consideration of ED&C with 5-FU for similar malignant lesions that are not amenable to surgical excision. Nevertheless, Mohs micrographic surgery and wide local excision remain the gold standards for definitive treatment of invasive skin cancer in a patient who is a candidate for surgical treatment.
- Nehal KS, Bichakjian CK. Update on keratinocyte carcinomas. N Engl J Med. 2018;379:363-374. doi:10.1056/NEJMra1708701
- de Jong E, Lammerts MUPA, Genders RE, et al. Update of advanced cutaneous squamous cell carcinoma. J Eur Acad Dermatol Venereol. 2022;36(suppl 1):6-10. doi:10.1111/jdv.17728
- Li VW, Ball RA, Vasan N, et al. Antiangiogenic therapy for squamous cell carcinoma using combinatorial agents [abstract]. J Clin Oncol. 2005;23(16 suppl):3032. doi:10.1200/jco.2005.23.16_suppl.3032
- Lansbury L, Bath-Hextall F, Perkins W, et al. Interventions for non-metastatic squamous cell carcinoma of the skin: systematic review and pooled analysis of observational studies. BMJ. 2013;347:f6153. doi:10.1136/bmj.f6153
- Behshad R, Garcia‐Zuazaga J, Bordeaux J. Systemic treatment of locally advanced nonmetastatic cutaneous squamous cell carcinoma: a review of the literature. Br J Dermatol. 2011;165:1169-1177. doi:10.1111/j.1365-2133.2011.10524.x
- Rowe DE, Carroll RJ, Day CL Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. implications for treatment modality selection. J Am Acad Dermatol. 1992;26:976-990. doi:10.1016/0190-9622(92)70144-5
- Knox JM, Lyles TW, Shapiro EM, et al. Curettage and electrodesiccation in the treatment of skin cancer. Arch Dermatol. 1960;82:197-204.
- Chren M-M, Linos E, Torres JS, et al. Tumor recurrence 5 years after treatment of cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2013;133:1188-1196. doi:10.1038/jid.2012.403
- Berman B, Maderal A, Raphael B. Keloids and hypertrophic scars: pathophysiology, classification, and treatment. Dermatologic Surgery. 2017;43:S3-S18.
- Nehal KS, Bichakjian CK. Update on keratinocyte carcinomas. N Engl J Med. 2018;379:363-374. doi:10.1056/NEJMra1708701
- de Jong E, Lammerts MUPA, Genders RE, et al. Update of advanced cutaneous squamous cell carcinoma. J Eur Acad Dermatol Venereol. 2022;36(suppl 1):6-10. doi:10.1111/jdv.17728
- Li VW, Ball RA, Vasan N, et al. Antiangiogenic therapy for squamous cell carcinoma using combinatorial agents [abstract]. J Clin Oncol. 2005;23(16 suppl):3032. doi:10.1200/jco.2005.23.16_suppl.3032
- Lansbury L, Bath-Hextall F, Perkins W, et al. Interventions for non-metastatic squamous cell carcinoma of the skin: systematic review and pooled analysis of observational studies. BMJ. 2013;347:f6153. doi:10.1136/bmj.f6153
- Behshad R, Garcia‐Zuazaga J, Bordeaux J. Systemic treatment of locally advanced nonmetastatic cutaneous squamous cell carcinoma: a review of the literature. Br J Dermatol. 2011;165:1169-1177. doi:10.1111/j.1365-2133.2011.10524.x
- Rowe DE, Carroll RJ, Day CL Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. implications for treatment modality selection. J Am Acad Dermatol. 1992;26:976-990. doi:10.1016/0190-9622(92)70144-5
- Knox JM, Lyles TW, Shapiro EM, et al. Curettage and electrodesiccation in the treatment of skin cancer. Arch Dermatol. 1960;82:197-204.
- Chren M-M, Linos E, Torres JS, et al. Tumor recurrence 5 years after treatment of cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2013;133:1188-1196. doi:10.1038/jid.2012.403
- Berman B, Maderal A, Raphael B. Keloids and hypertrophic scars: pathophysiology, classification, and treatment. Dermatologic Surgery. 2017;43:S3-S18.
Practice Points
- In a subset of cases of cutaneous squamous cell carcinoma (cSCC), the tumor is not amenable to surgical resection or other standard treatment modalities.
- Electrodesiccation and curettage followed by topical 5-fluorouracil may be an effective option in eliminating unresectable primary cSCCs that do not respond to intralesional treatment.