User login
A patient-centered approach to treating Graves’ disease is among new recommendations for diagnosing and treating thyrotoxicosis.
The guidelines, commissioned by the American Thyroid Association (ATA) and the American Association of Clinical Endocrinologists (AACE), include 100 recommendations and took a 13-member task force 2 years to produce (Thyroid 2011;21:593-646 [doi:10.1089/thy.2010.0417] and Endocr. Pract. 2011;17:e1-e65).
The guidelines update the 1995 ATA recommendations and the 2002 AACE recommendations on treating thyrotoxicosis and, for the first time, the recommendations are given evidence-based ratings, Dr. Rebecca S. Bahn, chair of the task force, said in an interview.
"Physicians who don’t treat hyperthyroidism often may not be aware of new developments in the field and recent changes in practice," said Dr. Bahn.
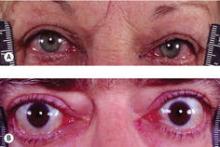
The guidelines address in specific detail initial evaluation and management of thyrotoxicosis; management of Graves’ hyperthyroidism using radioactive iodine, antithyroid drugs, or surgery; management of toxic multinodular goiter or toxic adenoma using radioactive iodine or surgery; Graves’ disease in children, adolescents, or pregnant patients; subclinical hyperthyroidism; hyperthyroidism in patients with Graves’ ophthalmopathy; and management of other miscellaneous causes of thyrotoxicosis.
Among the recommendations that could lead to a change in practice is the concept that antithyroid drugs could be used more often in place of radioactive iodine in Graves’ disease, and a recommendation that patients with Graves’ hyperthyroidism and active moderate to severe or sight-threatening ophthalmopathy should be treated with methimazole or surgery, said Dr. Bahn.
Patients with Graves’ disease have traditionally been prescribed radioactive iodine, but the new guidelines acknowledge the three treatment options: surgery, antithyroid medications, and radioactive iodine.
"It’s important to talk to patients about the pros and cons of each treatment and make appropriate choices. It’s about making patient-centric choices," said Dr. Bahn, past president of the American Thyroid Association and professor of medicine at the Mayo Clinic in Rochester, Minn.
Also recommended is a more cautious approach to the use of propylthiouracil (PTU). PTU can cause hepatic necrosis, "so our guideline is that methimazole should be the first-line treatment," she said.
PTU is still recommended as the first-line medication for pregnant women in their first trimester. "So, [pregnant women] should start with PTU, but then switch to methimazole during the second trimester should they need it."
Another recommendation advises that if surgery is the main therapy for the Graves’ disease, the patient should be sent to a surgeon who does a high volume of thyroid procedures.
"This means that if a high-volume surgeon is not available, then surgery should not be the choice," said Dr. Bahn.
In an editorial, Dr. Elizabeth N. Pearce of Boston University and her colleagues wrote that the guidelines "are much needed." (Thyroid 2011;21:573-6 [doi:10.1089/thy.2011.0104]).
But an important shortcoming of the new guidelines "is that they are based on relatively limited available evidence," she added.
"This indicates that clinical management of hyperthyroidism is still largely rooted in expert opinion and personal experience. Given this, it is perhaps remarkable that 98 of the recommendations were unanimous, whereas two of the recommendations had a single dissenting member," wrote Dr. Pearce and colleagues, who disclosed that they had no conflicts of interest.
The fact that there are not a lot of high-quality randomized trials "means that there does need to be more research in this area. We would hope that the NIH [National Institutes of Health] agrees with that" assessment, said Dr. Bahn.
Dr. Hossein Gharib, who was not involved in the task force, said that the new recommendations are useful. But he added that guidelines are only recommendations and do not necessarily apply to every practice and every patient.
"Doctors should read the guidelines and then apply their own experience, expertise, and resources," said Dr. Gharib, a clinical endocrinologist and professor of medicine at the Mayo Clinic in Rochester, Minn.
Dr. Bahn, Dr. Pearce, and Dr. Gharib reported no relevant financial disclosures.
Selected recommendations:
Recommendation 13: Methimazole should be used in virtually every patient who chooses antithyroid drug therapy for GD [Graves’ disease], except during the first trimester of pregnancy when propylthiouracil is preferred, in the treatment of thyroid storm, and in patients with minor reactions to methimazole who refuse radioactive iodine therapy or surgery.
Recommendation 25: If surgery is chosen as the primary therapy for GD, the patient should be referred to a high-volume thyroid surgeon.
Recommendation 31: We suggest that patients with overtly TMNG [toxic multinodar goiter] or TA [thyroid adenoma] be treated with either 131I therapy or thyroidectomy. On occasion, long-term, low-dose treatment with methimazole may be appropriate.
Recommendation 50: Children with GD should be treated with methimazole, 131I therapy, or thyroidectomy. 131I therapy should be avoided in very young children [younger than 5 years]. 131I therapy in patients between 5 and 10 years of age is acceptable if the calculated 131I administered activity is [less than] 10 mCi. 131I therapy in patients older than 10 years of age is acceptable if the activity is [greater than] 150 mcCi/g of thyroid tissue. Thyroidectomy should be chosen when definitive therapy is required, the child is too young for 131I, and surgery can be performed by a high-volume thyroid surgeon.
Recommendation 71: We suggest that patients taking methimazole who decide to become pregnant obtain pregnancy testing at the earliest suggestion of pregnancy and be switched to propylthiouracil as soon as possible in the first trimester and changed back to methimazole at the beginning of the second trimester. Similarly, we suggest that patients started on propylthiouracil during the first trimester be switched to methimazole at the beginning of the second trimester
Recommendation 86: Patients with Graves’ hyperthyroidism and active moderate to severe or sight-threatening ophthalmopathy should be treated with either methimazole or surgery.
A patient-centered approach to treating Graves’ disease is among new recommendations for diagnosing and treating thyrotoxicosis.
The guidelines, commissioned by the American Thyroid Association (ATA) and the American Association of Clinical Endocrinologists (AACE), include 100 recommendations and took a 13-member task force 2 years to produce (Thyroid 2011;21:593-646 [doi:10.1089/thy.2010.0417] and Endocr. Pract. 2011;17:e1-e65).
The guidelines update the 1995 ATA recommendations and the 2002 AACE recommendations on treating thyrotoxicosis and, for the first time, the recommendations are given evidence-based ratings, Dr. Rebecca S. Bahn, chair of the task force, said in an interview.
"Physicians who don’t treat hyperthyroidism often may not be aware of new developments in the field and recent changes in practice," said Dr. Bahn.
The guidelines address in specific detail initial evaluation and management of thyrotoxicosis; management of Graves’ hyperthyroidism using radioactive iodine, antithyroid drugs, or surgery; management of toxic multinodular goiter or toxic adenoma using radioactive iodine or surgery; Graves’ disease in children, adolescents, or pregnant patients; subclinical hyperthyroidism; hyperthyroidism in patients with Graves’ ophthalmopathy; and management of other miscellaneous causes of thyrotoxicosis.
Among the recommendations that could lead to a change in practice is the concept that antithyroid drugs could be used more often in place of radioactive iodine in Graves’ disease, and a recommendation that patients with Graves’ hyperthyroidism and active moderate to severe or sight-threatening ophthalmopathy should be treated with methimazole or surgery, said Dr. Bahn.
Patients with Graves’ disease have traditionally been prescribed radioactive iodine, but the new guidelines acknowledge the three treatment options: surgery, antithyroid medications, and radioactive iodine.
"It’s important to talk to patients about the pros and cons of each treatment and make appropriate choices. It’s about making patient-centric choices," said Dr. Bahn, past president of the American Thyroid Association and professor of medicine at the Mayo Clinic in Rochester, Minn.
Also recommended is a more cautious approach to the use of propylthiouracil (PTU). PTU can cause hepatic necrosis, "so our guideline is that methimazole should be the first-line treatment," she said.
PTU is still recommended as the first-line medication for pregnant women in their first trimester. "So, [pregnant women] should start with PTU, but then switch to methimazole during the second trimester should they need it."
Another recommendation advises that if surgery is the main therapy for the Graves’ disease, the patient should be sent to a surgeon who does a high volume of thyroid procedures.
"This means that if a high-volume surgeon is not available, then surgery should not be the choice," said Dr. Bahn.
In an editorial, Dr. Elizabeth N. Pearce of Boston University and her colleagues wrote that the guidelines "are much needed." (Thyroid 2011;21:573-6 [doi:10.1089/thy.2011.0104]).
But an important shortcoming of the new guidelines "is that they are based on relatively limited available evidence," she added.
"This indicates that clinical management of hyperthyroidism is still largely rooted in expert opinion and personal experience. Given this, it is perhaps remarkable that 98 of the recommendations were unanimous, whereas two of the recommendations had a single dissenting member," wrote Dr. Pearce and colleagues, who disclosed that they had no conflicts of interest.
The fact that there are not a lot of high-quality randomized trials "means that there does need to be more research in this area. We would hope that the NIH [National Institutes of Health] agrees with that" assessment, said Dr. Bahn.
Dr. Hossein Gharib, who was not involved in the task force, said that the new recommendations are useful. But he added that guidelines are only recommendations and do not necessarily apply to every practice and every patient.
"Doctors should read the guidelines and then apply their own experience, expertise, and resources," said Dr. Gharib, a clinical endocrinologist and professor of medicine at the Mayo Clinic in Rochester, Minn.
Dr. Bahn, Dr. Pearce, and Dr. Gharib reported no relevant financial disclosures.
Selected recommendations:
Recommendation 13: Methimazole should be used in virtually every patient who chooses antithyroid drug therapy for GD [Graves’ disease], except during the first trimester of pregnancy when propylthiouracil is preferred, in the treatment of thyroid storm, and in patients with minor reactions to methimazole who refuse radioactive iodine therapy or surgery.
Recommendation 25: If surgery is chosen as the primary therapy for GD, the patient should be referred to a high-volume thyroid surgeon.
Recommendation 31: We suggest that patients with overtly TMNG [toxic multinodar goiter] or TA [thyroid adenoma] be treated with either 131I therapy or thyroidectomy. On occasion, long-term, low-dose treatment with methimazole may be appropriate.
Recommendation 50: Children with GD should be treated with methimazole, 131I therapy, or thyroidectomy. 131I therapy should be avoided in very young children [younger than 5 years]. 131I therapy in patients between 5 and 10 years of age is acceptable if the calculated 131I administered activity is [less than] 10 mCi. 131I therapy in patients older than 10 years of age is acceptable if the activity is [greater than] 150 mcCi/g of thyroid tissue. Thyroidectomy should be chosen when definitive therapy is required, the child is too young for 131I, and surgery can be performed by a high-volume thyroid surgeon.
Recommendation 71: We suggest that patients taking methimazole who decide to become pregnant obtain pregnancy testing at the earliest suggestion of pregnancy and be switched to propylthiouracil as soon as possible in the first trimester and changed back to methimazole at the beginning of the second trimester. Similarly, we suggest that patients started on propylthiouracil during the first trimester be switched to methimazole at the beginning of the second trimester
Recommendation 86: Patients with Graves’ hyperthyroidism and active moderate to severe or sight-threatening ophthalmopathy should be treated with either methimazole or surgery.
A patient-centered approach to treating Graves’ disease is among new recommendations for diagnosing and treating thyrotoxicosis.
The guidelines, commissioned by the American Thyroid Association (ATA) and the American Association of Clinical Endocrinologists (AACE), include 100 recommendations and took a 13-member task force 2 years to produce (Thyroid 2011;21:593-646 [doi:10.1089/thy.2010.0417] and Endocr. Pract. 2011;17:e1-e65).
The guidelines update the 1995 ATA recommendations and the 2002 AACE recommendations on treating thyrotoxicosis and, for the first time, the recommendations are given evidence-based ratings, Dr. Rebecca S. Bahn, chair of the task force, said in an interview.
"Physicians who don’t treat hyperthyroidism often may not be aware of new developments in the field and recent changes in practice," said Dr. Bahn.
The guidelines address in specific detail initial evaluation and management of thyrotoxicosis; management of Graves’ hyperthyroidism using radioactive iodine, antithyroid drugs, or surgery; management of toxic multinodular goiter or toxic adenoma using radioactive iodine or surgery; Graves’ disease in children, adolescents, or pregnant patients; subclinical hyperthyroidism; hyperthyroidism in patients with Graves’ ophthalmopathy; and management of other miscellaneous causes of thyrotoxicosis.
Among the recommendations that could lead to a change in practice is the concept that antithyroid drugs could be used more often in place of radioactive iodine in Graves’ disease, and a recommendation that patients with Graves’ hyperthyroidism and active moderate to severe or sight-threatening ophthalmopathy should be treated with methimazole or surgery, said Dr. Bahn.
Patients with Graves’ disease have traditionally been prescribed radioactive iodine, but the new guidelines acknowledge the three treatment options: surgery, antithyroid medications, and radioactive iodine.
"It’s important to talk to patients about the pros and cons of each treatment and make appropriate choices. It’s about making patient-centric choices," said Dr. Bahn, past president of the American Thyroid Association and professor of medicine at the Mayo Clinic in Rochester, Minn.
Also recommended is a more cautious approach to the use of propylthiouracil (PTU). PTU can cause hepatic necrosis, "so our guideline is that methimazole should be the first-line treatment," she said.
PTU is still recommended as the first-line medication for pregnant women in their first trimester. "So, [pregnant women] should start with PTU, but then switch to methimazole during the second trimester should they need it."
Another recommendation advises that if surgery is the main therapy for the Graves’ disease, the patient should be sent to a surgeon who does a high volume of thyroid procedures.
"This means that if a high-volume surgeon is not available, then surgery should not be the choice," said Dr. Bahn.
In an editorial, Dr. Elizabeth N. Pearce of Boston University and her colleagues wrote that the guidelines "are much needed." (Thyroid 2011;21:573-6 [doi:10.1089/thy.2011.0104]).
But an important shortcoming of the new guidelines "is that they are based on relatively limited available evidence," she added.
"This indicates that clinical management of hyperthyroidism is still largely rooted in expert opinion and personal experience. Given this, it is perhaps remarkable that 98 of the recommendations were unanimous, whereas two of the recommendations had a single dissenting member," wrote Dr. Pearce and colleagues, who disclosed that they had no conflicts of interest.
The fact that there are not a lot of high-quality randomized trials "means that there does need to be more research in this area. We would hope that the NIH [National Institutes of Health] agrees with that" assessment, said Dr. Bahn.
Dr. Hossein Gharib, who was not involved in the task force, said that the new recommendations are useful. But he added that guidelines are only recommendations and do not necessarily apply to every practice and every patient.
"Doctors should read the guidelines and then apply their own experience, expertise, and resources," said Dr. Gharib, a clinical endocrinologist and professor of medicine at the Mayo Clinic in Rochester, Minn.
Dr. Bahn, Dr. Pearce, and Dr. Gharib reported no relevant financial disclosures.
Selected recommendations:
Recommendation 13: Methimazole should be used in virtually every patient who chooses antithyroid drug therapy for GD [Graves’ disease], except during the first trimester of pregnancy when propylthiouracil is preferred, in the treatment of thyroid storm, and in patients with minor reactions to methimazole who refuse radioactive iodine therapy or surgery.
Recommendation 25: If surgery is chosen as the primary therapy for GD, the patient should be referred to a high-volume thyroid surgeon.
Recommendation 31: We suggest that patients with overtly TMNG [toxic multinodar goiter] or TA [thyroid adenoma] be treated with either 131I therapy or thyroidectomy. On occasion, long-term, low-dose treatment with methimazole may be appropriate.
Recommendation 50: Children with GD should be treated with methimazole, 131I therapy, or thyroidectomy. 131I therapy should be avoided in very young children [younger than 5 years]. 131I therapy in patients between 5 and 10 years of age is acceptable if the calculated 131I administered activity is [less than] 10 mCi. 131I therapy in patients older than 10 years of age is acceptable if the activity is [greater than] 150 mcCi/g of thyroid tissue. Thyroidectomy should be chosen when definitive therapy is required, the child is too young for 131I, and surgery can be performed by a high-volume thyroid surgeon.
Recommendation 71: We suggest that patients taking methimazole who decide to become pregnant obtain pregnancy testing at the earliest suggestion of pregnancy and be switched to propylthiouracil as soon as possible in the first trimester and changed back to methimazole at the beginning of the second trimester. Similarly, we suggest that patients started on propylthiouracil during the first trimester be switched to methimazole at the beginning of the second trimester
Recommendation 86: Patients with Graves’ hyperthyroidism and active moderate to severe or sight-threatening ophthalmopathy should be treated with either methimazole or surgery.
FROM ENDOCRINE PRACTICE