User login
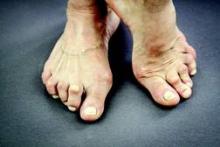
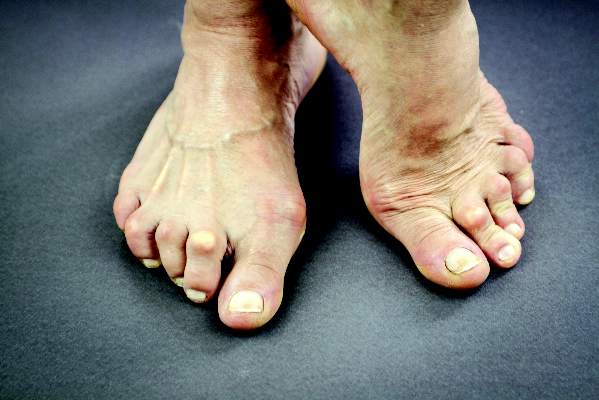
Three months marks a critical time point that determines whether a rheumatoid arthritis patient will reach a treatment target at 6 months, according to results of a study published in Annals of the Rheumatic Diseases.
Dr. Daniel Aletaha of the Medical University of Vienna and his associates performed a pooled analysis of clinical data from RA trials in the last decade. They found that, regardless of the starting point, 6-month success rates were clearly related to disease activity state reached at 3 months, and not so much to the number of disease activity categories improved.
The results show that a response at 3 months can be used as a decision criterion in two clinical situations. Failure to achieve minor responses (for example, ACR 20, Simplified Disease Activity Index [SDAI] 50) at the 3-month mark had the potential to almost rule out successfully reaching the target at 6 months, the investigators wrote (Ann Rheum Dis. 2015. doi: 10.1136/annrheumdis-2015-208324).
Conversely, achievement of major response definitions at 3 months (for example, ACR 70, SDAI 85%) can reliably predict achievement of a good state at 6 months.
“It is also obvious therefore that for many patients in the gray zone between these response levels, the prediction may be less solid, and may – to a greater extent – rest on the physician’s decision,” they wrote.
The findings were in line with updated treat-to-target (T2T) recommendations that suggest a goal of significant improvement at 3 months and attainment of the treatment target at 6 months, they added.
The study results showed that in order to be at least 80% sensitive for achieving the low disease activity target at 6 months, a change of 58% in SDAI/Clinical Disease Activity Index needed to be observed at 3 months.
Patients who did not achieve the (minor) SDAI 50% response level had very low negative likelihood ratios of 0.28 for low disease activity and 0.07 for remission at 6 months. Patients who achieved the (major) SDAI 85% response had substantial positive likelihood ratios of 9.2 for reaching low disease activity and 6.2 for reaching remission at 6 months.
In logistic regression, the change at 3 months was significantly associated with reaching the target at 6 months.
Dr. Aletaha and one of his associates received consulting and/or speaking honoraria from AbbVie; Merck, Sharp & Dohme; Pfizer; and Roche.
Three months marks a critical time point that determines whether a rheumatoid arthritis patient will reach a treatment target at 6 months, according to results of a study published in Annals of the Rheumatic Diseases.
Dr. Daniel Aletaha of the Medical University of Vienna and his associates performed a pooled analysis of clinical data from RA trials in the last decade. They found that, regardless of the starting point, 6-month success rates were clearly related to disease activity state reached at 3 months, and not so much to the number of disease activity categories improved.
The results show that a response at 3 months can be used as a decision criterion in two clinical situations. Failure to achieve minor responses (for example, ACR 20, Simplified Disease Activity Index [SDAI] 50) at the 3-month mark had the potential to almost rule out successfully reaching the target at 6 months, the investigators wrote (Ann Rheum Dis. 2015. doi: 10.1136/annrheumdis-2015-208324).
Conversely, achievement of major response definitions at 3 months (for example, ACR 70, SDAI 85%) can reliably predict achievement of a good state at 6 months.
“It is also obvious therefore that for many patients in the gray zone between these response levels, the prediction may be less solid, and may – to a greater extent – rest on the physician’s decision,” they wrote.
The findings were in line with updated treat-to-target (T2T) recommendations that suggest a goal of significant improvement at 3 months and attainment of the treatment target at 6 months, they added.
The study results showed that in order to be at least 80% sensitive for achieving the low disease activity target at 6 months, a change of 58% in SDAI/Clinical Disease Activity Index needed to be observed at 3 months.
Patients who did not achieve the (minor) SDAI 50% response level had very low negative likelihood ratios of 0.28 for low disease activity and 0.07 for remission at 6 months. Patients who achieved the (major) SDAI 85% response had substantial positive likelihood ratios of 9.2 for reaching low disease activity and 6.2 for reaching remission at 6 months.
In logistic regression, the change at 3 months was significantly associated with reaching the target at 6 months.
Dr. Aletaha and one of his associates received consulting and/or speaking honoraria from AbbVie; Merck, Sharp & Dohme; Pfizer; and Roche.
Three months marks a critical time point that determines whether a rheumatoid arthritis patient will reach a treatment target at 6 months, according to results of a study published in Annals of the Rheumatic Diseases.
Dr. Daniel Aletaha of the Medical University of Vienna and his associates performed a pooled analysis of clinical data from RA trials in the last decade. They found that, regardless of the starting point, 6-month success rates were clearly related to disease activity state reached at 3 months, and not so much to the number of disease activity categories improved.
The results show that a response at 3 months can be used as a decision criterion in two clinical situations. Failure to achieve minor responses (for example, ACR 20, Simplified Disease Activity Index [SDAI] 50) at the 3-month mark had the potential to almost rule out successfully reaching the target at 6 months, the investigators wrote (Ann Rheum Dis. 2015. doi: 10.1136/annrheumdis-2015-208324).
Conversely, achievement of major response definitions at 3 months (for example, ACR 70, SDAI 85%) can reliably predict achievement of a good state at 6 months.
“It is also obvious therefore that for many patients in the gray zone between these response levels, the prediction may be less solid, and may – to a greater extent – rest on the physician’s decision,” they wrote.
The findings were in line with updated treat-to-target (T2T) recommendations that suggest a goal of significant improvement at 3 months and attainment of the treatment target at 6 months, they added.
The study results showed that in order to be at least 80% sensitive for achieving the low disease activity target at 6 months, a change of 58% in SDAI/Clinical Disease Activity Index needed to be observed at 3 months.
Patients who did not achieve the (minor) SDAI 50% response level had very low negative likelihood ratios of 0.28 for low disease activity and 0.07 for remission at 6 months. Patients who achieved the (major) SDAI 85% response had substantial positive likelihood ratios of 9.2 for reaching low disease activity and 6.2 for reaching remission at 6 months.
In logistic regression, the change at 3 months was significantly associated with reaching the target at 6 months.
Dr. Aletaha and one of his associates received consulting and/or speaking honoraria from AbbVie; Merck, Sharp & Dohme; Pfizer; and Roche.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point:Failure to achieve minor responses at 3 months almost ruled out successfully reaching the target at 6 months.
Major finding: To be at least 80% sensitive for achieving the low disease activity target at 6 months, a change of 58% in SDAI or Clinical Disease Activity Index needed to be observed at 3 months.
Data source: Pooled analysis of clinical data from RA trials in the last decade.
Disclosures: Dr. Aletaha and one of his associates received consulting and/or speaking honoraria from AbbVie; Merck, Sharp & Dohme; Pfizer; and Roche.