User login
A 63-YEAR-OLD SOUTHEAST ASIAN WOMAN presented with early satiety, mild swelling of her lower extremities, and several months of progressive shortness of breath that had become severe (provoked by activities of daily living). She had a history of longstanding, rate-controlled atrial fibrillation on oral anticoagulation. She also had a history of mitral valve stenosis that was treated 30 years earlier with mechanical valve replacement. The patient had previously been treated out of state and prior records were not available.
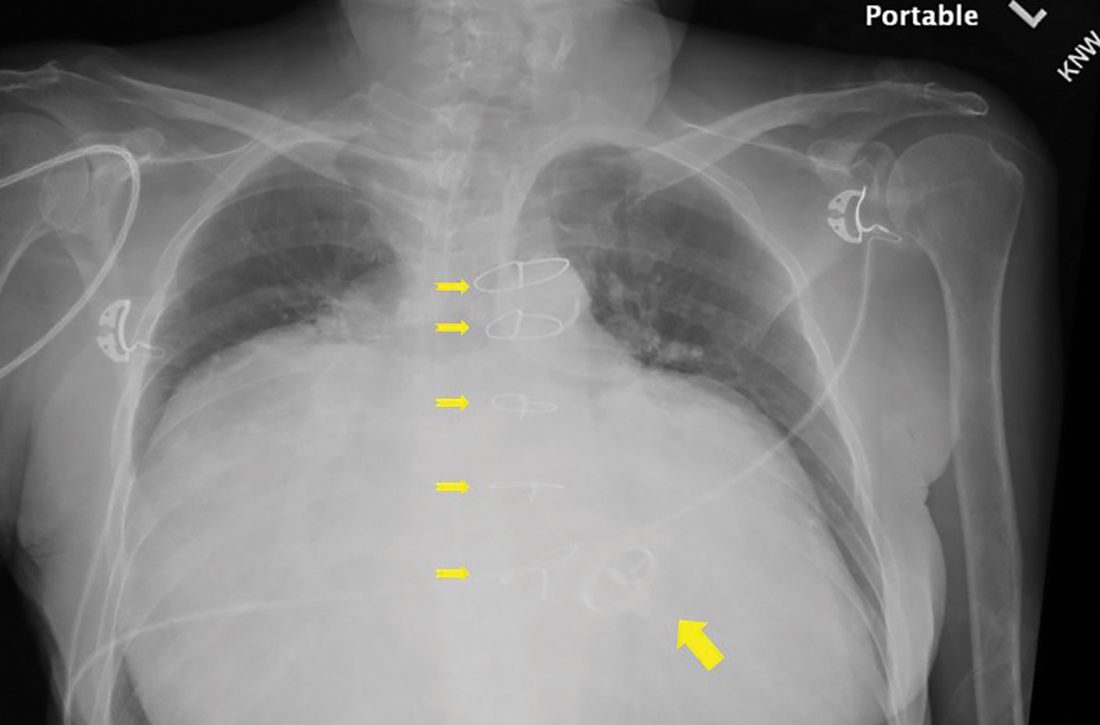
Chest radiography (CXR) was performed as part of the initial work-up (FIGURE 1) and demonstrated a substantially enlarged cardiac silhouette spanning the entire width of the chest without significant pleural effusion or evidence of airspace disease. Suspecting a primary cardiac pathology in this patient, we explored clinical findings of heart failure with transthoracic echocardiography.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Severe tricuspid valve regurgitation secondary to rheumatic heart disease
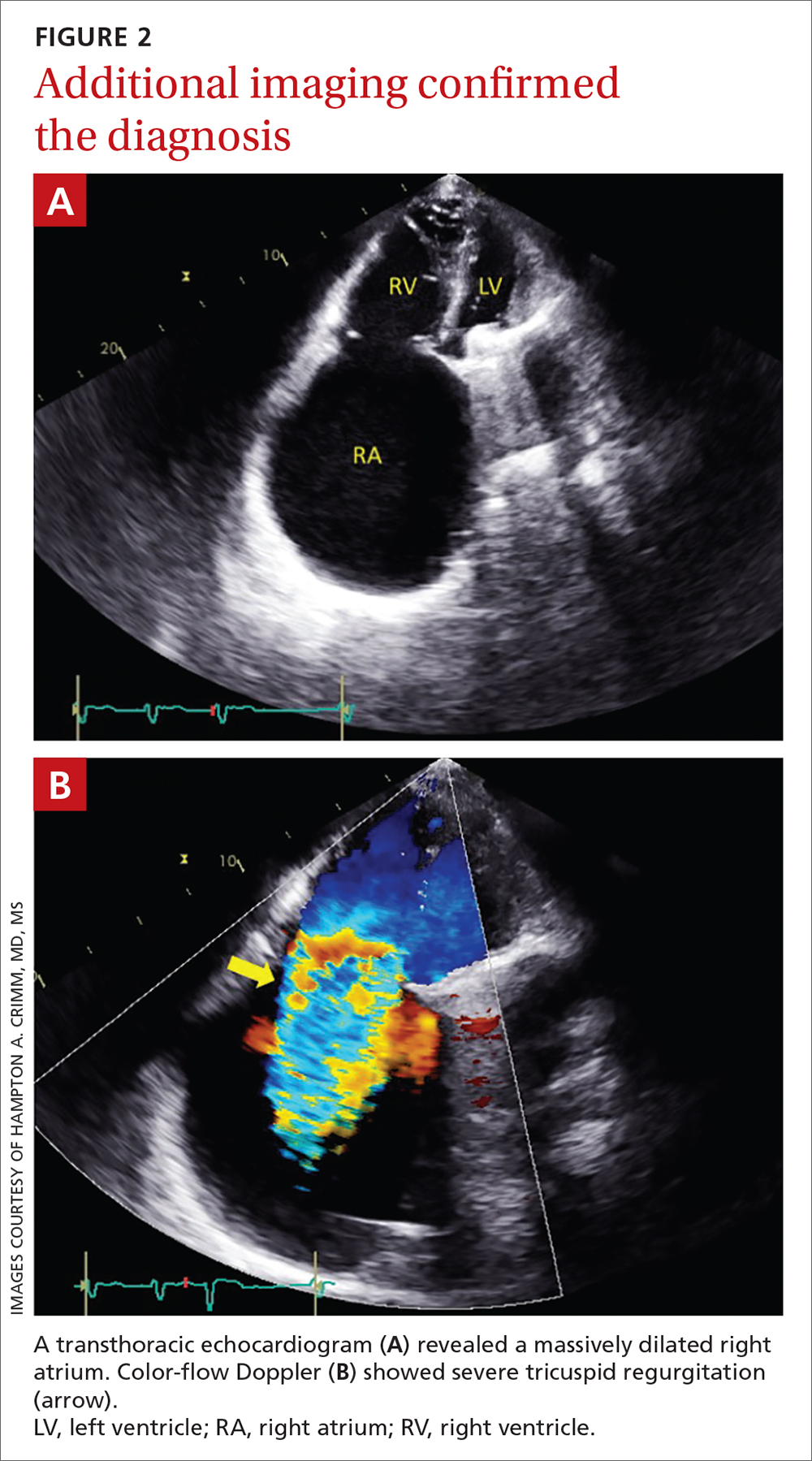
A transthoracic echocardiogram (FIGURE 2A) revealed cardiomegaly with massive right atrial enlargement; a color-flow Doppler (FIGURE 2B) revealed severe tricuspid regurgitation, reduced right ventricular systolic function, and preserved left ventricular systolic function. All of these findings pointed to the diagnosis of rheumatic heart disease (RHD), especially in the context of prior mitral valve stenosis.
RHD affects more than 33 million people annually and remains a significant problem globally.1 It’s associated with a relatively poor prognosis, especially if heart failure is present (as it was in this case).2,3 Although the mitral and aortic valves are most commonly affected, approximately 34% of patients will develop tricuspid regurgitation.4 Right-side cardiac manifestations of RHD may lead to clinical heart failure with chronic venous congestion and, ultimately, cirrhosis.
Suspect RHD when encountering a new murmur in a patient with prior history of acute rheumatic fever, especially if they are living in or are from a country where rheumatic disease is endemic (most of the developing world).
The diagnosis is confirmed when echocardiographic findings demonstrate characteristic pathologic valve changes (eg, thickening of the anterior mitral valve leaflet, especially the leaflet tips and subvalvular apparatus).
The differential for an enlarged cardiac silhouette
The differential diagnosis for an enlarged cardiac silhouette on CXR includes cardiomegaly (as in this case), pericardial effusion, or a thoracic mass (either mediastinal or pericardial). Imaging artifact from patient orientation may also yield the appearance of an enlarged cardiac silhouette. Distinguishing between these entities may be accomplished by incorporating the history with selection of more definitive imaging (eg, echocardiogram or computed tomography).
Continue to: Management depends on the severity and symptoms
Management depends on the severity and symptoms
Percutaneous or surgical intervention may be required with RHD, depending on the clinical scenario. If the patient also has atrial fibrillation, medical management includes oral anticoagulation (with a vitamin K antagonist). Additionally, secondary prophylaxis with long-term antibiotics (directed against recurrent group A Streptococcus infection) is recommended for RHD patients with mitral stenosis.5 If the patient in this case had engaged in more regular cardiology follow-up, the progression of her tricuspid regurgitation may have been mitigated by surgical intervention and aggressive medical management (although the progression of RHD can eclipse standard treatments).5
In this case, a liver biopsy was pursued for prognostication. Unfortunately, the biopsy demonstrated cirrhosis with perisinusoidal fibrosis suggesting an advanced, end-stage clinical state. This diagnosis precluded the patient’s eligibility for advanced therapies such as right ventricular assist device implantation or cardiac transplantation. Surgical intervention (repair or replacement) was also deemed likely to be futile due to right ventricular dilatation and systolic dysfunction in the context of antecedent left-side valve intervention.
The patient elected to pursue palliative care and died at home several months later. In the years since this case occurred, less invasive tricuspid valve interventions have been explored, offering promise of amelioration of such cases in the future.6
1. Watkins DA, Johnson CO, Colquhoun SM, et. al. Global, regional, and national burden of rheumatic heart disease, 1990-2015. N Engl J Med. 2017; 377:713-722. doi: 10.1056/NEJMoa1603693
2. Zühlke L, Karthikeyan G, Engel ME, et al. Clinical outcomes in 3343 children and adults with rheumatic heart disease from 14 low- and middle-income countries: 2-year follow-up of the global rheumatic heart disease registry (the REMEDY study). Circulation. 2016;134:1456-1466. doi: 10.1161/CIRCULATIONAHA
3. Reményi B, Wilson N, Steer A, et al. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease—an evidence-based guideline. Nat Rev Cardiol. 2012;9:297-309. doi: 10.1038/nrcardio.2012.7
4. Sriharibabu M, Himabindu Y, Kabir, et al. Rheumatic heart disease in rural south India: a clinico-observational study. J Cardiovasc Dis Res. 2013;4:25-29. doi: 10.1016/j.jcdr.2013.02.011
5. Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77:4:e25-e197. doi: 10.1016/j.jacc.2020.11.018
6. Fam NP, von Bardeleben RS, Hensey M, et al. Transfemoral transcatheter tricuspid valve replacement with the EVOQUE System: a multicenter, observational, first-in-human experience. JACC Cardiovasc Interv. 2021;14:501-511. doi: 10.1016/j.jcin.2020.11.045
A 63-YEAR-OLD SOUTHEAST ASIAN WOMAN presented with early satiety, mild swelling of her lower extremities, and several months of progressive shortness of breath that had become severe (provoked by activities of daily living). She had a history of longstanding, rate-controlled atrial fibrillation on oral anticoagulation. She also had a history of mitral valve stenosis that was treated 30 years earlier with mechanical valve replacement. The patient had previously been treated out of state and prior records were not available.
Chest radiography (CXR) was performed as part of the initial work-up (FIGURE 1) and demonstrated a substantially enlarged cardiac silhouette spanning the entire width of the chest without significant pleural effusion or evidence of airspace disease. Suspecting a primary cardiac pathology in this patient, we explored clinical findings of heart failure with transthoracic echocardiography.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Severe tricuspid valve regurgitation secondary to rheumatic heart disease
A transthoracic echocardiogram (FIGURE 2A) revealed cardiomegaly with massive right atrial enlargement; a color-flow Doppler (FIGURE 2B) revealed severe tricuspid regurgitation, reduced right ventricular systolic function, and preserved left ventricular systolic function. All of these findings pointed to the diagnosis of rheumatic heart disease (RHD), especially in the context of prior mitral valve stenosis.

RHD affects more than 33 million people annually and remains a significant problem globally.1 It’s associated with a relatively poor prognosis, especially if heart failure is present (as it was in this case).2,3 Although the mitral and aortic valves are most commonly affected, approximately 34% of patients will develop tricuspid regurgitation.4 Right-side cardiac manifestations of RHD may lead to clinical heart failure with chronic venous congestion and, ultimately, cirrhosis.
Suspect RHD when encountering a new murmur in a patient with prior history of acute rheumatic fever, especially if they are living in or are from a country where rheumatic disease is endemic (most of the developing world).
The diagnosis is confirmed when echocardiographic findings demonstrate characteristic pathologic valve changes (eg, thickening of the anterior mitral valve leaflet, especially the leaflet tips and subvalvular apparatus).
The differential for an enlarged cardiac silhouette
The differential diagnosis for an enlarged cardiac silhouette on CXR includes cardiomegaly (as in this case), pericardial effusion, or a thoracic mass (either mediastinal or pericardial). Imaging artifact from patient orientation may also yield the appearance of an enlarged cardiac silhouette. Distinguishing between these entities may be accomplished by incorporating the history with selection of more definitive imaging (eg, echocardiogram or computed tomography).
Continue to: Management depends on the severity and symptoms
Management depends on the severity and symptoms
Percutaneous or surgical intervention may be required with RHD, depending on the clinical scenario. If the patient also has atrial fibrillation, medical management includes oral anticoagulation (with a vitamin K antagonist). Additionally, secondary prophylaxis with long-term antibiotics (directed against recurrent group A Streptococcus infection) is recommended for RHD patients with mitral stenosis.5 If the patient in this case had engaged in more regular cardiology follow-up, the progression of her tricuspid regurgitation may have been mitigated by surgical intervention and aggressive medical management (although the progression of RHD can eclipse standard treatments).5
In this case, a liver biopsy was pursued for prognostication. Unfortunately, the biopsy demonstrated cirrhosis with perisinusoidal fibrosis suggesting an advanced, end-stage clinical state. This diagnosis precluded the patient’s eligibility for advanced therapies such as right ventricular assist device implantation or cardiac transplantation. Surgical intervention (repair or replacement) was also deemed likely to be futile due to right ventricular dilatation and systolic dysfunction in the context of antecedent left-side valve intervention.
The patient elected to pursue palliative care and died at home several months later. In the years since this case occurred, less invasive tricuspid valve interventions have been explored, offering promise of amelioration of such cases in the future.6
A 63-YEAR-OLD SOUTHEAST ASIAN WOMAN presented with early satiety, mild swelling of her lower extremities, and several months of progressive shortness of breath that had become severe (provoked by activities of daily living). She had a history of longstanding, rate-controlled atrial fibrillation on oral anticoagulation. She also had a history of mitral valve stenosis that was treated 30 years earlier with mechanical valve replacement. The patient had previously been treated out of state and prior records were not available.
Chest radiography (CXR) was performed as part of the initial work-up (FIGURE 1) and demonstrated a substantially enlarged cardiac silhouette spanning the entire width of the chest without significant pleural effusion or evidence of airspace disease. Suspecting a primary cardiac pathology in this patient, we explored clinical findings of heart failure with transthoracic echocardiography.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Severe tricuspid valve regurgitation secondary to rheumatic heart disease
A transthoracic echocardiogram (FIGURE 2A) revealed cardiomegaly with massive right atrial enlargement; a color-flow Doppler (FIGURE 2B) revealed severe tricuspid regurgitation, reduced right ventricular systolic function, and preserved left ventricular systolic function. All of these findings pointed to the diagnosis of rheumatic heart disease (RHD), especially in the context of prior mitral valve stenosis.

RHD affects more than 33 million people annually and remains a significant problem globally.1 It’s associated with a relatively poor prognosis, especially if heart failure is present (as it was in this case).2,3 Although the mitral and aortic valves are most commonly affected, approximately 34% of patients will develop tricuspid regurgitation.4 Right-side cardiac manifestations of RHD may lead to clinical heart failure with chronic venous congestion and, ultimately, cirrhosis.
Suspect RHD when encountering a new murmur in a patient with prior history of acute rheumatic fever, especially if they are living in or are from a country where rheumatic disease is endemic (most of the developing world).
The diagnosis is confirmed when echocardiographic findings demonstrate characteristic pathologic valve changes (eg, thickening of the anterior mitral valve leaflet, especially the leaflet tips and subvalvular apparatus).
The differential for an enlarged cardiac silhouette
The differential diagnosis for an enlarged cardiac silhouette on CXR includes cardiomegaly (as in this case), pericardial effusion, or a thoracic mass (either mediastinal or pericardial). Imaging artifact from patient orientation may also yield the appearance of an enlarged cardiac silhouette. Distinguishing between these entities may be accomplished by incorporating the history with selection of more definitive imaging (eg, echocardiogram or computed tomography).
Continue to: Management depends on the severity and symptoms
Management depends on the severity and symptoms
Percutaneous or surgical intervention may be required with RHD, depending on the clinical scenario. If the patient also has atrial fibrillation, medical management includes oral anticoagulation (with a vitamin K antagonist). Additionally, secondary prophylaxis with long-term antibiotics (directed against recurrent group A Streptococcus infection) is recommended for RHD patients with mitral stenosis.5 If the patient in this case had engaged in more regular cardiology follow-up, the progression of her tricuspid regurgitation may have been mitigated by surgical intervention and aggressive medical management (although the progression of RHD can eclipse standard treatments).5
In this case, a liver biopsy was pursued for prognostication. Unfortunately, the biopsy demonstrated cirrhosis with perisinusoidal fibrosis suggesting an advanced, end-stage clinical state. This diagnosis precluded the patient’s eligibility for advanced therapies such as right ventricular assist device implantation or cardiac transplantation. Surgical intervention (repair or replacement) was also deemed likely to be futile due to right ventricular dilatation and systolic dysfunction in the context of antecedent left-side valve intervention.
The patient elected to pursue palliative care and died at home several months later. In the years since this case occurred, less invasive tricuspid valve interventions have been explored, offering promise of amelioration of such cases in the future.6
1. Watkins DA, Johnson CO, Colquhoun SM, et. al. Global, regional, and national burden of rheumatic heart disease, 1990-2015. N Engl J Med. 2017; 377:713-722. doi: 10.1056/NEJMoa1603693
2. Zühlke L, Karthikeyan G, Engel ME, et al. Clinical outcomes in 3343 children and adults with rheumatic heart disease from 14 low- and middle-income countries: 2-year follow-up of the global rheumatic heart disease registry (the REMEDY study). Circulation. 2016;134:1456-1466. doi: 10.1161/CIRCULATIONAHA
3. Reményi B, Wilson N, Steer A, et al. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease—an evidence-based guideline. Nat Rev Cardiol. 2012;9:297-309. doi: 10.1038/nrcardio.2012.7
4. Sriharibabu M, Himabindu Y, Kabir, et al. Rheumatic heart disease in rural south India: a clinico-observational study. J Cardiovasc Dis Res. 2013;4:25-29. doi: 10.1016/j.jcdr.2013.02.011
5. Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77:4:e25-e197. doi: 10.1016/j.jacc.2020.11.018
6. Fam NP, von Bardeleben RS, Hensey M, et al. Transfemoral transcatheter tricuspid valve replacement with the EVOQUE System: a multicenter, observational, first-in-human experience. JACC Cardiovasc Interv. 2021;14:501-511. doi: 10.1016/j.jcin.2020.11.045
1. Watkins DA, Johnson CO, Colquhoun SM, et. al. Global, regional, and national burden of rheumatic heart disease, 1990-2015. N Engl J Med. 2017; 377:713-722. doi: 10.1056/NEJMoa1603693
2. Zühlke L, Karthikeyan G, Engel ME, et al. Clinical outcomes in 3343 children and adults with rheumatic heart disease from 14 low- and middle-income countries: 2-year follow-up of the global rheumatic heart disease registry (the REMEDY study). Circulation. 2016;134:1456-1466. doi: 10.1161/CIRCULATIONAHA
3. Reményi B, Wilson N, Steer A, et al. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease—an evidence-based guideline. Nat Rev Cardiol. 2012;9:297-309. doi: 10.1038/nrcardio.2012.7
4. Sriharibabu M, Himabindu Y, Kabir, et al. Rheumatic heart disease in rural south India: a clinico-observational study. J Cardiovasc Dis Res. 2013;4:25-29. doi: 10.1016/j.jcdr.2013.02.011
5. Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77:4:e25-e197. doi: 10.1016/j.jacc.2020.11.018
6. Fam NP, von Bardeleben RS, Hensey M, et al. Transfemoral transcatheter tricuspid valve replacement with the EVOQUE System: a multicenter, observational, first-in-human experience. JACC Cardiovasc Interv. 2021;14:501-511. doi: 10.1016/j.jcin.2020.11.045