User login
AUSTIN, TEX. – A program of frequent monitoring in Crohn’s disease and ulcerative colitis that includes fecal calprotectin (FC) and C-reactive protein (CRP) testing may be cost effective to significantly reduce disease recurrence and hospitalization rates, according to a review of published studies presented at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
“Some data show that calprotectin levels rise months before the onset of symptoms, so it’s my practice that every 3-4 months patients should undergo CRP and calprotectin testing, if they’re willing to do so, while they’re on biologic therapy,” Frank I. Scott, MD, MSCE, of the University of Colorado in Aurora, Denver, said in an interview after the presentation.
Regular monitoring of the two levels makes sense as the practice of tight control of IBD symptoms and treating to target has emerged over the past decade, Dr. Scott said. He noted the 2015 Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) guidelines called for using CRP and FC as adjunctive targets only in symptom assessment (Am J Gastroenterol. 2015:110[9]:1324-58). “I argue that we’ve had a growing body of literature that we should be using these tests regularly as well,” he said.
STRIDE calls for endoscopic assessment 6-9 months after therapy change and consideration of cross-sectional imaging if the small bowel is involved, with assessment every 3 months until symptoms improve and then every 6-12 months thereafter.
However, Dr. Scott noted potential drawbacks to these follow-up steps. “They currently focus on clinical symptoms in the short-term follow-up, and we know from looking at our disease activity indices, such as the CDAI [Crohn’s disease activity index] or Harvey-Bradshaw index, that they don’t always perfectly correlate with actual mucosal healing or resolution of inflammation in Crohn’s or [ulcerative colitis],” he said, pointing to a 2014 study that found CDAI had an area under the curve of 0.57, “which is pretty poor correlation” (Gut. 2014;63[1]:88-95).
Whereas a study of 2,499 patients that showed CRP had an area under the curve of 0.72 and FC of 0.89 (Am J Gastroentrol. 2015;110[6]:802-19). “CRP is a really attractive potential noninvasive marker of inflammation,” he said. “It’s relatively inexpensive, it’s widely available, and the cutoff ranges are well defined.”
He noted four potential drawbacks of CRP: the false-positive rate is relatively high; as a marker of systemic inflammation it’s not specific to the GI tract; false negatives have been well described, with up to 15% of patients not registering a response; and levels can depend on disease location. “Those with isolated ileal disease, for instance, may have relatively low CRP elevations when their disease is active,” Dr. Scott said.
Stool-based FC “represents a potentially more attractive option,” Dr. Scott said. Along with an area under the curve superior to CRP, FC has a documented sensitivity and specificity of 88% and 73%, respectively, versus 49% and 92% for CRP. Drawbacks of fecal calprotectin are that it’s specific to the GI tract but not inflammatory bowel disease, it costs more, and insurance coverage is not as universal as it is for CRP, although more carriers are covering the test, he said.
“However, we do know that through clinical trial data that the use of CRP and FC, in addition to clinical symptom monitoring, does appear to improve care,” Dr. Scott said, noting that the CALM trial of tight disease control through the frequent use of biochemical markers of inflammation with anti–tumor necrosis therapy bore this out (Lancet. 2018;390[10114]:2779-89). “This trial was able to demonstrate at 48 weeks that mucosal healing rates were improved in those receiving CRP and FC monitoring, compared to symptom monitoring alone, with higher rates of steroid-free remission at each visit, which persisted over the follow-up time.”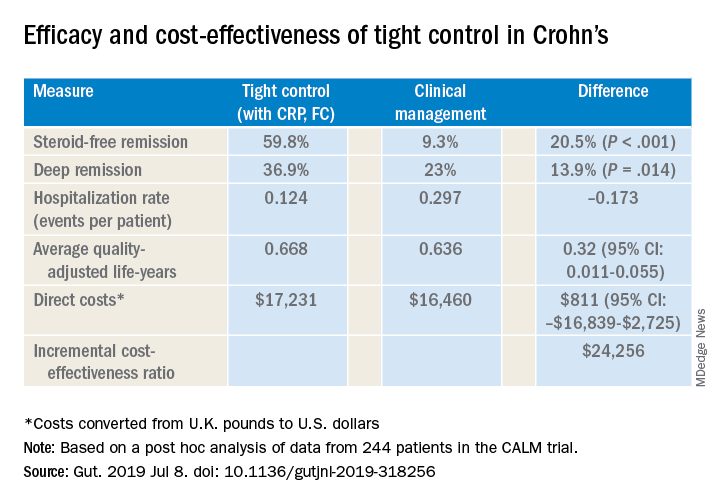
Dr. Scott also cited a post hoc analysis of CALM trial data that validated CRP and FC monitoring to improve steroid-free remission rates and other outcomes (Gut. 2019 Jul 8. doi: 10.1136/gutjnl-2019-318256). That trial reported steroid-free remission rates of 39.3% with clinical management and 59.8% with tight control, a 34% overall difference (P less than .001). “And it was cost effective to incorporate this monitoring at a cost of about $24,300 per quality-adjusted life-year, well below the typically used $50,000 willingness-to-pay threshold when considering new tests,” Dr. Scott said.
Dr. Scott acknowledged that FC testing may pose some inconvenience to patients when collecting their stool samples, but accuracy has improved. “Laboratories are becoming more reliable in terms of what the values are, and the cutoffs are becoming more defined as far as what’s positive and what’s negative, so it’s good way to monitor whether or not patients are at increased risk of a future flare,” he said.
Dr. Scott reported financial relationships with Takeda, Janssen, Merck and PRIME.
SOURCE: Scott FI et al. Crohn’s & Colitis Congress 2020, Session Sp125.
AUSTIN, TEX. – A program of frequent monitoring in Crohn’s disease and ulcerative colitis that includes fecal calprotectin (FC) and C-reactive protein (CRP) testing may be cost effective to significantly reduce disease recurrence and hospitalization rates, according to a review of published studies presented at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
“Some data show that calprotectin levels rise months before the onset of symptoms, so it’s my practice that every 3-4 months patients should undergo CRP and calprotectin testing, if they’re willing to do so, while they’re on biologic therapy,” Frank I. Scott, MD, MSCE, of the University of Colorado in Aurora, Denver, said in an interview after the presentation.
Regular monitoring of the two levels makes sense as the practice of tight control of IBD symptoms and treating to target has emerged over the past decade, Dr. Scott said. He noted the 2015 Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) guidelines called for using CRP and FC as adjunctive targets only in symptom assessment (Am J Gastroenterol. 2015:110[9]:1324-58). “I argue that we’ve had a growing body of literature that we should be using these tests regularly as well,” he said.
STRIDE calls for endoscopic assessment 6-9 months after therapy change and consideration of cross-sectional imaging if the small bowel is involved, with assessment every 3 months until symptoms improve and then every 6-12 months thereafter.
However, Dr. Scott noted potential drawbacks to these follow-up steps. “They currently focus on clinical symptoms in the short-term follow-up, and we know from looking at our disease activity indices, such as the CDAI [Crohn’s disease activity index] or Harvey-Bradshaw index, that they don’t always perfectly correlate with actual mucosal healing or resolution of inflammation in Crohn’s or [ulcerative colitis],” he said, pointing to a 2014 study that found CDAI had an area under the curve of 0.57, “which is pretty poor correlation” (Gut. 2014;63[1]:88-95).
Whereas a study of 2,499 patients that showed CRP had an area under the curve of 0.72 and FC of 0.89 (Am J Gastroentrol. 2015;110[6]:802-19). “CRP is a really attractive potential noninvasive marker of inflammation,” he said. “It’s relatively inexpensive, it’s widely available, and the cutoff ranges are well defined.”
He noted four potential drawbacks of CRP: the false-positive rate is relatively high; as a marker of systemic inflammation it’s not specific to the GI tract; false negatives have been well described, with up to 15% of patients not registering a response; and levels can depend on disease location. “Those with isolated ileal disease, for instance, may have relatively low CRP elevations when their disease is active,” Dr. Scott said.
Stool-based FC “represents a potentially more attractive option,” Dr. Scott said. Along with an area under the curve superior to CRP, FC has a documented sensitivity and specificity of 88% and 73%, respectively, versus 49% and 92% for CRP. Drawbacks of fecal calprotectin are that it’s specific to the GI tract but not inflammatory bowel disease, it costs more, and insurance coverage is not as universal as it is for CRP, although more carriers are covering the test, he said.
“However, we do know that through clinical trial data that the use of CRP and FC, in addition to clinical symptom monitoring, does appear to improve care,” Dr. Scott said, noting that the CALM trial of tight disease control through the frequent use of biochemical markers of inflammation with anti–tumor necrosis therapy bore this out (Lancet. 2018;390[10114]:2779-89). “This trial was able to demonstrate at 48 weeks that mucosal healing rates were improved in those receiving CRP and FC monitoring, compared to symptom monitoring alone, with higher rates of steroid-free remission at each visit, which persisted over the follow-up time.”
Dr. Scott also cited a post hoc analysis of CALM trial data that validated CRP and FC monitoring to improve steroid-free remission rates and other outcomes (Gut. 2019 Jul 8. doi: 10.1136/gutjnl-2019-318256). That trial reported steroid-free remission rates of 39.3% with clinical management and 59.8% with tight control, a 34% overall difference (P less than .001). “And it was cost effective to incorporate this monitoring at a cost of about $24,300 per quality-adjusted life-year, well below the typically used $50,000 willingness-to-pay threshold when considering new tests,” Dr. Scott said.
Dr. Scott acknowledged that FC testing may pose some inconvenience to patients when collecting their stool samples, but accuracy has improved. “Laboratories are becoming more reliable in terms of what the values are, and the cutoffs are becoming more defined as far as what’s positive and what’s negative, so it’s good way to monitor whether or not patients are at increased risk of a future flare,” he said.
Dr. Scott reported financial relationships with Takeda, Janssen, Merck and PRIME.
SOURCE: Scott FI et al. Crohn’s & Colitis Congress 2020, Session Sp125.
AUSTIN, TEX. – A program of frequent monitoring in Crohn’s disease and ulcerative colitis that includes fecal calprotectin (FC) and C-reactive protein (CRP) testing may be cost effective to significantly reduce disease recurrence and hospitalization rates, according to a review of published studies presented at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
“Some data show that calprotectin levels rise months before the onset of symptoms, so it’s my practice that every 3-4 months patients should undergo CRP and calprotectin testing, if they’re willing to do so, while they’re on biologic therapy,” Frank I. Scott, MD, MSCE, of the University of Colorado in Aurora, Denver, said in an interview after the presentation.
Regular monitoring of the two levels makes sense as the practice of tight control of IBD symptoms and treating to target has emerged over the past decade, Dr. Scott said. He noted the 2015 Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) guidelines called for using CRP and FC as adjunctive targets only in symptom assessment (Am J Gastroenterol. 2015:110[9]:1324-58). “I argue that we’ve had a growing body of literature that we should be using these tests regularly as well,” he said.
STRIDE calls for endoscopic assessment 6-9 months after therapy change and consideration of cross-sectional imaging if the small bowel is involved, with assessment every 3 months until symptoms improve and then every 6-12 months thereafter.
However, Dr. Scott noted potential drawbacks to these follow-up steps. “They currently focus on clinical symptoms in the short-term follow-up, and we know from looking at our disease activity indices, such as the CDAI [Crohn’s disease activity index] or Harvey-Bradshaw index, that they don’t always perfectly correlate with actual mucosal healing or resolution of inflammation in Crohn’s or [ulcerative colitis],” he said, pointing to a 2014 study that found CDAI had an area under the curve of 0.57, “which is pretty poor correlation” (Gut. 2014;63[1]:88-95).
Whereas a study of 2,499 patients that showed CRP had an area under the curve of 0.72 and FC of 0.89 (Am J Gastroentrol. 2015;110[6]:802-19). “CRP is a really attractive potential noninvasive marker of inflammation,” he said. “It’s relatively inexpensive, it’s widely available, and the cutoff ranges are well defined.”
He noted four potential drawbacks of CRP: the false-positive rate is relatively high; as a marker of systemic inflammation it’s not specific to the GI tract; false negatives have been well described, with up to 15% of patients not registering a response; and levels can depend on disease location. “Those with isolated ileal disease, for instance, may have relatively low CRP elevations when their disease is active,” Dr. Scott said.
Stool-based FC “represents a potentially more attractive option,” Dr. Scott said. Along with an area under the curve superior to CRP, FC has a documented sensitivity and specificity of 88% and 73%, respectively, versus 49% and 92% for CRP. Drawbacks of fecal calprotectin are that it’s specific to the GI tract but not inflammatory bowel disease, it costs more, and insurance coverage is not as universal as it is for CRP, although more carriers are covering the test, he said.
“However, we do know that through clinical trial data that the use of CRP and FC, in addition to clinical symptom monitoring, does appear to improve care,” Dr. Scott said, noting that the CALM trial of tight disease control through the frequent use of biochemical markers of inflammation with anti–tumor necrosis therapy bore this out (Lancet. 2018;390[10114]:2779-89). “This trial was able to demonstrate at 48 weeks that mucosal healing rates were improved in those receiving CRP and FC monitoring, compared to symptom monitoring alone, with higher rates of steroid-free remission at each visit, which persisted over the follow-up time.”
Dr. Scott also cited a post hoc analysis of CALM trial data that validated CRP and FC monitoring to improve steroid-free remission rates and other outcomes (Gut. 2019 Jul 8. doi: 10.1136/gutjnl-2019-318256). That trial reported steroid-free remission rates of 39.3% with clinical management and 59.8% with tight control, a 34% overall difference (P less than .001). “And it was cost effective to incorporate this monitoring at a cost of about $24,300 per quality-adjusted life-year, well below the typically used $50,000 willingness-to-pay threshold when considering new tests,” Dr. Scott said.
Dr. Scott acknowledged that FC testing may pose some inconvenience to patients when collecting their stool samples, but accuracy has improved. “Laboratories are becoming more reliable in terms of what the values are, and the cutoffs are becoming more defined as far as what’s positive and what’s negative, so it’s good way to monitor whether or not patients are at increased risk of a future flare,” he said.
Dr. Scott reported financial relationships with Takeda, Janssen, Merck and PRIME.
SOURCE: Scott FI et al. Crohn’s & Colitis Congress 2020, Session Sp125.
REPORTING FROM CROHN’S & COLITIS CONGRESS

