User login
BOSTON — There is “no standard of care and no clear pattern” for discontinuing treatment in multiple myeloma, according to a speaker at the International Myeloma Workshop.
Data from a large, observational study revealed that a wide range of treatment regimens are used for first-, second-, and third-line therapy in multiple myeloma. The duration of therapy and time to next treatment were shorter in this real-world study than in prior clinical trials, and reasons for treatment discontinuation varied by regimen and line of therapy.
Katja Weisel, MD, of University Medical Center Hamburg-Eppendorf in Germany, presented these findings at the workshop, held by the International Myeloma Society.
The study, INSIGHT MM, is the largest global, prospective, observational study of multiple myeloma to date, according to Dr. Weisel. The study, which began July 1, 2016, has enrolled patients in the United States (n = 1,004), Europe (n = 1,612), Latin America (n = 367), and Asia (n = 218).
Dr. Weisel and her colleagues evaluated duration of therapy, reasons for treatment discontinuation, and subsequent therapies in a subset of patients on INSIGHT MM. The researchers’ analysis revealed “broad heterogeneity” across lines of therapy, Dr. Weisel said, adding that patients are receiving multiple regimens in addition to the most commonly prescribed regimens in myeloma.
First-line therapy
“In first-line treatment, we see predominantly bortezomib-based triplets ... regardless of transplant-eligible or transplant-ineligible patients,” Dr. Weisel said. “This is followed by doublets and other more rarely [applied] regimens.”
First-line therapies in 1,175 patients included:
- Bortezomib, cyclophosphamide, and dexamethasone (VCd) – 323 patients.
- Bortezomib, lenalidomide, and dexamethasone (VRd) – 321 patients.
- Bortezomib, thalidomide, and dexamethasone (VTd) – 200 patients.
- Bortezomib and dexamethasone (Vd) – 102 patients.
- Lenalidomide and dexamethasone (Rd) – 90 patients.
- Bortezomib, melphalan, and prednisone (VMP) – 53 patients.
- Carfilzomib, lenalidomide, and dexamethasone (KRd) – 47 patients.
- Daratumumab-based regimens (Dara) – 32 patients.
- Carfilzomib and dexamethasone (Kd) – 5 patients.
- Ixazomib, lenalidomide, and dexamethasone (IRd) – 2 patients.
Of the 1,175 newly diagnosed patients, 894 did not proceed to transplant after first-line therapy, but 281 did. Most of the patients who went on to transplant had received VRd (n = 82), VTd (n = 76), or VCd (n = 75).
Second- and third-line therapies
“In second-line treatment, we have still a dominance of the len-dex regimen all over the world,” Dr. Weisel said. “There is an emerging use of daratumumab in various combinations, and then you see the whole spectrum of approved triplet and doublet regimens.”
In the third line, the most commonly used regimens are daratumumab-based combinations and Rd.
There were 548 patients who received second-line treatment and 332 who received third-line therapy. The regimens used were:
- Rd – 130 patients second line, 71 third line.
- Dara – 121 patients second line, 105 third line.
- KRd – 61 patients second line, 17 third line.
- VCd – 57 patients second line, 19 third line.
- Vd – 48 patients second line, 29 third line.
- VRd – 36 patients second line, 8 third line.
- Kd – 33 patients for both second and third line.
- IRd – 29 patients second line, 43 third line.
- VTd – 25 patients second line, 4 third line.
- VMP – 8 patients second line, 3 third line.
Duration of therapy
Most transplant-eligible patients received any first-line therapy (VRd, VTd, or VCd) for longer than 12 months. Among transplant-ineligible patients, Rd was the first-line therapy most likely to be given for 12 months or more.
None of the second-line regimens lasted longer than 12 months in a majority of patients, but daratumumab-based regimens and IRd were the therapies most likely to exceed 12 months’ duration in both second- and third-line treatment.
Time to next treatment
“The vast majority of [transplant-eligible] patients, close to 90% ... do not need a second-line treatment during the first year of treatment,” Dr. Weisel said. “However, for transplant-ineligible patients, this accounts only for the most effective regimens, VMP and Rd.”
For second- and third-line therapies, a 12-month or longer time to next treatment was most likely among patients who received IRd or daratumumab-based regimens.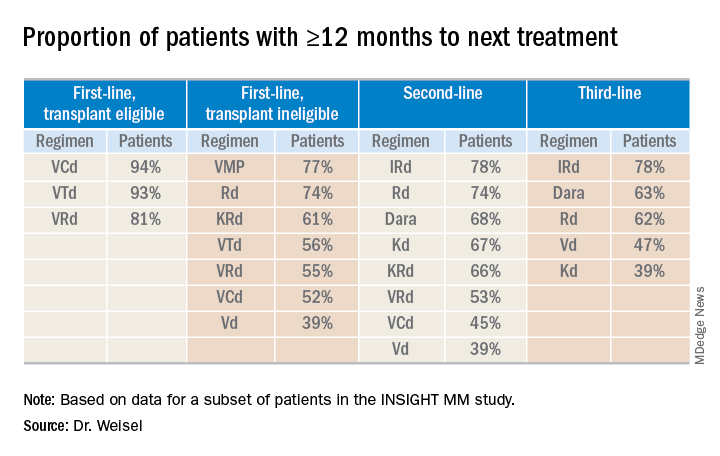
Reasons for discontinuation
“Planned end of therapy only accounts for a small proportion of treatment discontinuations, especially in the relapsed setting,” Dr. Weisel said. “Patients are discontinuing treatment due to reasons other than relapse, ultimately receiving fixed-duration therapy.”
The most common reasons for discontinuation of first-line therapy were:
- Relapse for VCd.
- Planned end of therapy for VRd.
- Adverse events (AEs) for VD and VTd.
- AEs and “other reasons” for Rd.
The most common reasons for discontinuation of second-line therapy were:
- Planned end of therapy for VCd.
- AEs, relapse, and other reasons for VRd.
- Relapse for VD, KRd, and Dara.
- AEs for Rd and IRd.
- AEs and other reasons for Kd.
The most common reasons for discontinuation of third-line therapy were:
- AEs for VCd, Vd, and KRd.
- Relapse for Kd, IRd, and Dara.
- Relapse and other reasons for VRd.
- AEs and other reasons for Rd.
The most common AE leading to discontinuation, across all treatment regimens, was peripheral neuropathy. This suggests peripheral neuropathy is still the “biggest impediment for continuous treatment,” Dr. Weisel said.
INSIGHT MM is sponsored by Takeda. Dr. Weisel reported relationships with Takeda and several other companies.
SOURCE: Weisel K et al. IMW 2019, Abstract OAB-005.
BOSTON — There is “no standard of care and no clear pattern” for discontinuing treatment in multiple myeloma, according to a speaker at the International Myeloma Workshop.
Data from a large, observational study revealed that a wide range of treatment regimens are used for first-, second-, and third-line therapy in multiple myeloma. The duration of therapy and time to next treatment were shorter in this real-world study than in prior clinical trials, and reasons for treatment discontinuation varied by regimen and line of therapy.
Katja Weisel, MD, of University Medical Center Hamburg-Eppendorf in Germany, presented these findings at the workshop, held by the International Myeloma Society.
The study, INSIGHT MM, is the largest global, prospective, observational study of multiple myeloma to date, according to Dr. Weisel. The study, which began July 1, 2016, has enrolled patients in the United States (n = 1,004), Europe (n = 1,612), Latin America (n = 367), and Asia (n = 218).
Dr. Weisel and her colleagues evaluated duration of therapy, reasons for treatment discontinuation, and subsequent therapies in a subset of patients on INSIGHT MM. The researchers’ analysis revealed “broad heterogeneity” across lines of therapy, Dr. Weisel said, adding that patients are receiving multiple regimens in addition to the most commonly prescribed regimens in myeloma.
First-line therapy
“In first-line treatment, we see predominantly bortezomib-based triplets ... regardless of transplant-eligible or transplant-ineligible patients,” Dr. Weisel said. “This is followed by doublets and other more rarely [applied] regimens.”
First-line therapies in 1,175 patients included:
- Bortezomib, cyclophosphamide, and dexamethasone (VCd) – 323 patients.
- Bortezomib, lenalidomide, and dexamethasone (VRd) – 321 patients.
- Bortezomib, thalidomide, and dexamethasone (VTd) – 200 patients.
- Bortezomib and dexamethasone (Vd) – 102 patients.
- Lenalidomide and dexamethasone (Rd) – 90 patients.
- Bortezomib, melphalan, and prednisone (VMP) – 53 patients.
- Carfilzomib, lenalidomide, and dexamethasone (KRd) – 47 patients.
- Daratumumab-based regimens (Dara) – 32 patients.
- Carfilzomib and dexamethasone (Kd) – 5 patients.
- Ixazomib, lenalidomide, and dexamethasone (IRd) – 2 patients.
Of the 1,175 newly diagnosed patients, 894 did not proceed to transplant after first-line therapy, but 281 did. Most of the patients who went on to transplant had received VRd (n = 82), VTd (n = 76), or VCd (n = 75).
Second- and third-line therapies
“In second-line treatment, we have still a dominance of the len-dex regimen all over the world,” Dr. Weisel said. “There is an emerging use of daratumumab in various combinations, and then you see the whole spectrum of approved triplet and doublet regimens.”
In the third line, the most commonly used regimens are daratumumab-based combinations and Rd.
There were 548 patients who received second-line treatment and 332 who received third-line therapy. The regimens used were:
- Rd – 130 patients second line, 71 third line.
- Dara – 121 patients second line, 105 third line.
- KRd – 61 patients second line, 17 third line.
- VCd – 57 patients second line, 19 third line.
- Vd – 48 patients second line, 29 third line.
- VRd – 36 patients second line, 8 third line.
- Kd – 33 patients for both second and third line.
- IRd – 29 patients second line, 43 third line.
- VTd – 25 patients second line, 4 third line.
- VMP – 8 patients second line, 3 third line.
Duration of therapy
Most transplant-eligible patients received any first-line therapy (VRd, VTd, or VCd) for longer than 12 months. Among transplant-ineligible patients, Rd was the first-line therapy most likely to be given for 12 months or more.
None of the second-line regimens lasted longer than 12 months in a majority of patients, but daratumumab-based regimens and IRd were the therapies most likely to exceed 12 months’ duration in both second- and third-line treatment.
Time to next treatment
“The vast majority of [transplant-eligible] patients, close to 90% ... do not need a second-line treatment during the first year of treatment,” Dr. Weisel said. “However, for transplant-ineligible patients, this accounts only for the most effective regimens, VMP and Rd.”
For second- and third-line therapies, a 12-month or longer time to next treatment was most likely among patients who received IRd or daratumumab-based regimens.
Reasons for discontinuation
“Planned end of therapy only accounts for a small proportion of treatment discontinuations, especially in the relapsed setting,” Dr. Weisel said. “Patients are discontinuing treatment due to reasons other than relapse, ultimately receiving fixed-duration therapy.”
The most common reasons for discontinuation of first-line therapy were:
- Relapse for VCd.
- Planned end of therapy for VRd.
- Adverse events (AEs) for VD and VTd.
- AEs and “other reasons” for Rd.
The most common reasons for discontinuation of second-line therapy were:
- Planned end of therapy for VCd.
- AEs, relapse, and other reasons for VRd.
- Relapse for VD, KRd, and Dara.
- AEs for Rd and IRd.
- AEs and other reasons for Kd.
The most common reasons for discontinuation of third-line therapy were:
- AEs for VCd, Vd, and KRd.
- Relapse for Kd, IRd, and Dara.
- Relapse and other reasons for VRd.
- AEs and other reasons for Rd.
The most common AE leading to discontinuation, across all treatment regimens, was peripheral neuropathy. This suggests peripheral neuropathy is still the “biggest impediment for continuous treatment,” Dr. Weisel said.
INSIGHT MM is sponsored by Takeda. Dr. Weisel reported relationships with Takeda and several other companies.
SOURCE: Weisel K et al. IMW 2019, Abstract OAB-005.
BOSTON — There is “no standard of care and no clear pattern” for discontinuing treatment in multiple myeloma, according to a speaker at the International Myeloma Workshop.
Data from a large, observational study revealed that a wide range of treatment regimens are used for first-, second-, and third-line therapy in multiple myeloma. The duration of therapy and time to next treatment were shorter in this real-world study than in prior clinical trials, and reasons for treatment discontinuation varied by regimen and line of therapy.
Katja Weisel, MD, of University Medical Center Hamburg-Eppendorf in Germany, presented these findings at the workshop, held by the International Myeloma Society.
The study, INSIGHT MM, is the largest global, prospective, observational study of multiple myeloma to date, according to Dr. Weisel. The study, which began July 1, 2016, has enrolled patients in the United States (n = 1,004), Europe (n = 1,612), Latin America (n = 367), and Asia (n = 218).
Dr. Weisel and her colleagues evaluated duration of therapy, reasons for treatment discontinuation, and subsequent therapies in a subset of patients on INSIGHT MM. The researchers’ analysis revealed “broad heterogeneity” across lines of therapy, Dr. Weisel said, adding that patients are receiving multiple regimens in addition to the most commonly prescribed regimens in myeloma.
First-line therapy
“In first-line treatment, we see predominantly bortezomib-based triplets ... regardless of transplant-eligible or transplant-ineligible patients,” Dr. Weisel said. “This is followed by doublets and other more rarely [applied] regimens.”
First-line therapies in 1,175 patients included:
- Bortezomib, cyclophosphamide, and dexamethasone (VCd) – 323 patients.
- Bortezomib, lenalidomide, and dexamethasone (VRd) – 321 patients.
- Bortezomib, thalidomide, and dexamethasone (VTd) – 200 patients.
- Bortezomib and dexamethasone (Vd) – 102 patients.
- Lenalidomide and dexamethasone (Rd) – 90 patients.
- Bortezomib, melphalan, and prednisone (VMP) – 53 patients.
- Carfilzomib, lenalidomide, and dexamethasone (KRd) – 47 patients.
- Daratumumab-based regimens (Dara) – 32 patients.
- Carfilzomib and dexamethasone (Kd) – 5 patients.
- Ixazomib, lenalidomide, and dexamethasone (IRd) – 2 patients.
Of the 1,175 newly diagnosed patients, 894 did not proceed to transplant after first-line therapy, but 281 did. Most of the patients who went on to transplant had received VRd (n = 82), VTd (n = 76), or VCd (n = 75).
Second- and third-line therapies
“In second-line treatment, we have still a dominance of the len-dex regimen all over the world,” Dr. Weisel said. “There is an emerging use of daratumumab in various combinations, and then you see the whole spectrum of approved triplet and doublet regimens.”
In the third line, the most commonly used regimens are daratumumab-based combinations and Rd.
There were 548 patients who received second-line treatment and 332 who received third-line therapy. The regimens used were:
- Rd – 130 patients second line, 71 third line.
- Dara – 121 patients second line, 105 third line.
- KRd – 61 patients second line, 17 third line.
- VCd – 57 patients second line, 19 third line.
- Vd – 48 patients second line, 29 third line.
- VRd – 36 patients second line, 8 third line.
- Kd – 33 patients for both second and third line.
- IRd – 29 patients second line, 43 third line.
- VTd – 25 patients second line, 4 third line.
- VMP – 8 patients second line, 3 third line.
Duration of therapy
Most transplant-eligible patients received any first-line therapy (VRd, VTd, or VCd) for longer than 12 months. Among transplant-ineligible patients, Rd was the first-line therapy most likely to be given for 12 months or more.
None of the second-line regimens lasted longer than 12 months in a majority of patients, but daratumumab-based regimens and IRd were the therapies most likely to exceed 12 months’ duration in both second- and third-line treatment.
Time to next treatment
“The vast majority of [transplant-eligible] patients, close to 90% ... do not need a second-line treatment during the first year of treatment,” Dr. Weisel said. “However, for transplant-ineligible patients, this accounts only for the most effective regimens, VMP and Rd.”
For second- and third-line therapies, a 12-month or longer time to next treatment was most likely among patients who received IRd or daratumumab-based regimens.
Reasons for discontinuation
“Planned end of therapy only accounts for a small proportion of treatment discontinuations, especially in the relapsed setting,” Dr. Weisel said. “Patients are discontinuing treatment due to reasons other than relapse, ultimately receiving fixed-duration therapy.”
The most common reasons for discontinuation of first-line therapy were:
- Relapse for VCd.
- Planned end of therapy for VRd.
- Adverse events (AEs) for VD and VTd.
- AEs and “other reasons” for Rd.
The most common reasons for discontinuation of second-line therapy were:
- Planned end of therapy for VCd.
- AEs, relapse, and other reasons for VRd.
- Relapse for VD, KRd, and Dara.
- AEs for Rd and IRd.
- AEs and other reasons for Kd.
The most common reasons for discontinuation of third-line therapy were:
- AEs for VCd, Vd, and KRd.
- Relapse for Kd, IRd, and Dara.
- Relapse and other reasons for VRd.
- AEs and other reasons for Rd.
The most common AE leading to discontinuation, across all treatment regimens, was peripheral neuropathy. This suggests peripheral neuropathy is still the “biggest impediment for continuous treatment,” Dr. Weisel said.
INSIGHT MM is sponsored by Takeda. Dr. Weisel reported relationships with Takeda and several other companies.
SOURCE: Weisel K et al. IMW 2019, Abstract OAB-005.
REPORTING FROM IMW 2019

