User login
Solfenacin succinate, an antimuscarinic agent, is approved for the treatment of overactive bladder and described as well tolerated in the elderly.1 We present the case of solifenacin‐induced small bowel pseudo‐obstruction in an 89‐year‐old woman.
FINDINGS
An 89‐year‐old woman with untreated stage 0 chronic lymphocytic leukemia and a history of stage III colorectal cancer treated with hemicolectomy and adjuvant capecitabine in 2003 was admitted to Johns Hopkins Hospital in 2006. She reported feeling dehydrated, nauseated, and constipated, with decreased output from her colostomy. She also noted no urine output for 4 days and felt that she had to urinate, but I can't. This coincided with a decrease in fluid intake. She denied fevers, chills, abdominal pain, or loss of appetite. While waiting to be seen in the emergency department, the patient was finally able to urinate.
She had no evidence of colon cancer recurrence, with a normal postoperative positron‐emission tomography (PET) scan in 2003, colonoscopy in 2005, and screening computerized tomography (CT) scan in 2005. She also had a history of well‐controlled hypertension and hypothyroidism, hyperlipidemia, chemotherapy‐induced neuropathy, and anxiety.
Her home medication regimen included solifenacin 5 mg once daily (started 10 days prior to her admission) for bladder overactivity, buspirone 5 mg 3 times a day, metoprolol 25 mg twice a day, pantoprazole 40 mg once daily, levothyroxine 100 g once daily, lisinopril/hydrochlorathiazide 20 mg/25 mg twice daily, gabapentin 300 mg twice a day, and fenofibrate 145 mg nightly.
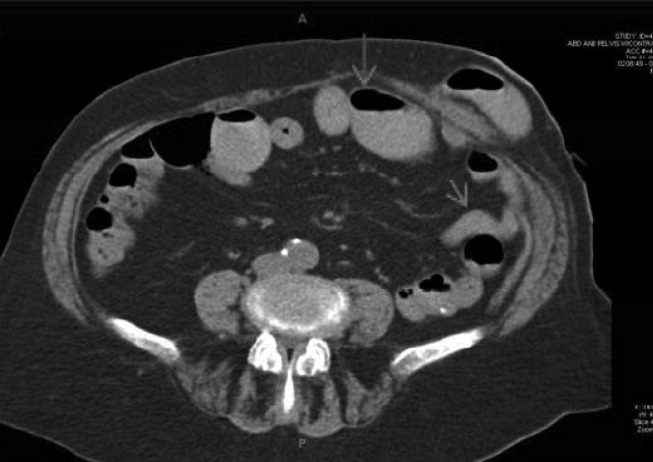
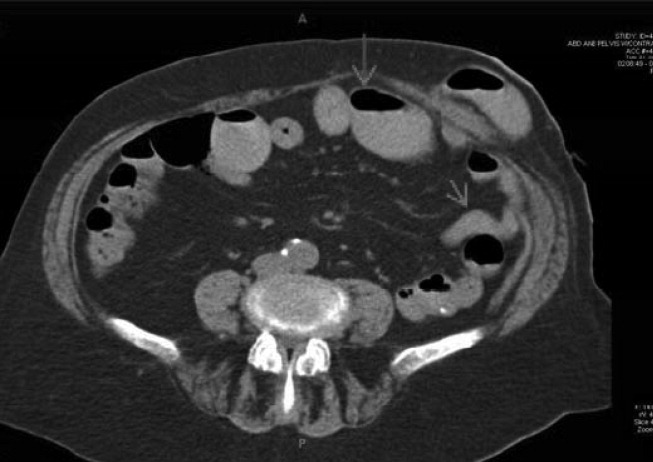
The patient appeared nontoxic. Her exam was remarkable only for hypoactive bowel sounds and mild diffuse abdominal tenderness without distension or peritoneal signs. A Foley catheter was placed, and her postvoid residual was only 50 cc of urine. Her admission serum blood urea nitrogen and creatinine were 90 and 3.4 mg/dL, respectively, as compared with 18 and 0.8 mg/dL 2 months prior to presentation. A CT scan of the abdomen (Figure 1) revealed multiple dilated loops of small bowel with a transition point at the left lower quadrant ostomy site, consistent with a small bowel obstruction. A PET scan revealed no evidence of malignancy. A renal ultrasound showed no evidence of obstruction.
With cessation of solifenacin and lisinopril/hydrochlorothiazide and hydration with normal saline, her constipation resolved, as did her acute renal failure and perception of urinary retention. She began to tolerate a regular diet after 4 days of hospitalization, and her colostomy output normalized. At follow‐up 8 months after admission, her creatinine was 0.8 mg/dL, and a screening abdominal CT showed complete resolution of the small bowel obstruction.
DISCUSSION
We believe that this patient developed small bowel pseudo‐obstruction as well the feeling of urinary retention because of treatment with solifenacin, an antimuscarinic agent approved for the treatment of bladder overactivity. Her acute renal failure was a result of prerenal azotemia. This particular patient was at increased risk for developing antimuscarinic‐induced bowel obstruction because of her previous surgery and exposure to chemotherapy.
In the 4 randomized trials cited in the prescribing information for solifenacin,2 only 189 patients of the 1811 who received the active drug were greater than 75 years old. Healthy elderly patients ranging from 64 to 78 years of age (mean 68.0 years) who received 2 weeks of treatment with solifenacin 5 and 10 mg had a mean AUC024 that was approximately 20% higher than that of younger subjects.3 In the 4 12‐week double‐blind clinical trials in which 1158 patients were treated with solifenacin 10 mg, there were 3 serious intestinal adverse events: 1 patient had a fecal impaction, 1 patient had a colonic obstruction, and 1 patient had an intestinal obstruction.2 Patients receiving solifenacin 5 and 10 mg were more likely to experience constipation than those receiving placebo (5.4%, 13.4%, and 2.9%, respectively).2 Given the dearth of clinical data on patients greater than 75 years old, the effects of age on the pharmacokinetics, the higher likelihood of bowel pathology in the elderly, the increased risk of solifenacin‐induced side effects in the elderly as reported in the pooled analysis of patients at least 65 years old,4 and the small clinical benefit of solifenacin,46 physicians should seriously consider whether the benefits of solifenacin outweigh both the known and the possible risks. 0
| Patients in safety analysis (n) | Constipation, n (%) | Micturition/24 hours | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | 5 mg | 10 mg | Placebo | 5 mg | 10 mg | Baseline | Mean decrease from baseline | |||
| Placebo | 5 mg | 10 mg | ||||||||
| ||||||||||
| Chapple et al.6* | 267 | 279 | 268 | 5 (1.9) | 20 (7.2) | 21 (7.8) | 12.0812.32 | 1.2 | 2.19 | 2.61 |
| Cardozo et al.4* | 301 | 299 | 307 | 6 (2.0) | 11 (3.7) | 28 (9.1) | 12.0512.31 | 1.59 | 2.37 | 2.81 |
| Wagg3 | 422 | 192 | 431 | 18 (4.3) | 18 (9.4) | 78 (18.1) | 11.611.7 | 1.1 | 2.0 | 2.5 |
- .Solifenacin provides effective antimuscarinic therapy for the complete management of overactive bladder.Expert Opin Pharmacother.2006;7:2421–2434.
- Yamanouchi Pharma America, Inc.United States prescribing information for solifenacin succinate (Vesicare®), November2004.
- ,,,,.Effect of age on the pharmacokinetics of solifenacin in men and women.Int J Clin Pharmacol Ther.2005;43:227–238.
- ,,.Efficacy and tolerability of solifenacin in elderly subjects with overactive bladder syndrome: a pooled analysis.Am J Geriatr Pharmacother.2006;4(1):14–24.
- ,,, et al.Randomized, double‐blind placebo controlled trial of the once daily antimuscarinic agent solifenacin succinate in patients with overactive bladder.J Urol.2004;172(5 Pt 1):1919–1924.
- ,,, et al.Randomized, double‐blind placebo‐ and tolterodine‐controlled trial of the once‐daily antimuscarinic agent solifenacin in patients with symptomatic overactive bladder.BJU Int.2004;93:303–310.
Solfenacin succinate, an antimuscarinic agent, is approved for the treatment of overactive bladder and described as well tolerated in the elderly.1 We present the case of solifenacin‐induced small bowel pseudo‐obstruction in an 89‐year‐old woman.
FINDINGS
An 89‐year‐old woman with untreated stage 0 chronic lymphocytic leukemia and a history of stage III colorectal cancer treated with hemicolectomy and adjuvant capecitabine in 2003 was admitted to Johns Hopkins Hospital in 2006. She reported feeling dehydrated, nauseated, and constipated, with decreased output from her colostomy. She also noted no urine output for 4 days and felt that she had to urinate, but I can't. This coincided with a decrease in fluid intake. She denied fevers, chills, abdominal pain, or loss of appetite. While waiting to be seen in the emergency department, the patient was finally able to urinate.
She had no evidence of colon cancer recurrence, with a normal postoperative positron‐emission tomography (PET) scan in 2003, colonoscopy in 2005, and screening computerized tomography (CT) scan in 2005. She also had a history of well‐controlled hypertension and hypothyroidism, hyperlipidemia, chemotherapy‐induced neuropathy, and anxiety.
Her home medication regimen included solifenacin 5 mg once daily (started 10 days prior to her admission) for bladder overactivity, buspirone 5 mg 3 times a day, metoprolol 25 mg twice a day, pantoprazole 40 mg once daily, levothyroxine 100 g once daily, lisinopril/hydrochlorathiazide 20 mg/25 mg twice daily, gabapentin 300 mg twice a day, and fenofibrate 145 mg nightly.
The patient appeared nontoxic. Her exam was remarkable only for hypoactive bowel sounds and mild diffuse abdominal tenderness without distension or peritoneal signs. A Foley catheter was placed, and her postvoid residual was only 50 cc of urine. Her admission serum blood urea nitrogen and creatinine were 90 and 3.4 mg/dL, respectively, as compared with 18 and 0.8 mg/dL 2 months prior to presentation. A CT scan of the abdomen (Figure 1) revealed multiple dilated loops of small bowel with a transition point at the left lower quadrant ostomy site, consistent with a small bowel obstruction. A PET scan revealed no evidence of malignancy. A renal ultrasound showed no evidence of obstruction.

With cessation of solifenacin and lisinopril/hydrochlorothiazide and hydration with normal saline, her constipation resolved, as did her acute renal failure and perception of urinary retention. She began to tolerate a regular diet after 4 days of hospitalization, and her colostomy output normalized. At follow‐up 8 months after admission, her creatinine was 0.8 mg/dL, and a screening abdominal CT showed complete resolution of the small bowel obstruction.
DISCUSSION
We believe that this patient developed small bowel pseudo‐obstruction as well the feeling of urinary retention because of treatment with solifenacin, an antimuscarinic agent approved for the treatment of bladder overactivity. Her acute renal failure was a result of prerenal azotemia. This particular patient was at increased risk for developing antimuscarinic‐induced bowel obstruction because of her previous surgery and exposure to chemotherapy.
In the 4 randomized trials cited in the prescribing information for solifenacin,2 only 189 patients of the 1811 who received the active drug were greater than 75 years old. Healthy elderly patients ranging from 64 to 78 years of age (mean 68.0 years) who received 2 weeks of treatment with solifenacin 5 and 10 mg had a mean AUC024 that was approximately 20% higher than that of younger subjects.3 In the 4 12‐week double‐blind clinical trials in which 1158 patients were treated with solifenacin 10 mg, there were 3 serious intestinal adverse events: 1 patient had a fecal impaction, 1 patient had a colonic obstruction, and 1 patient had an intestinal obstruction.2 Patients receiving solifenacin 5 and 10 mg were more likely to experience constipation than those receiving placebo (5.4%, 13.4%, and 2.9%, respectively).2 Given the dearth of clinical data on patients greater than 75 years old, the effects of age on the pharmacokinetics, the higher likelihood of bowel pathology in the elderly, the increased risk of solifenacin‐induced side effects in the elderly as reported in the pooled analysis of patients at least 65 years old,4 and the small clinical benefit of solifenacin,46 physicians should seriously consider whether the benefits of solifenacin outweigh both the known and the possible risks. 0
| Patients in safety analysis (n) | Constipation, n (%) | Micturition/24 hours | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | 5 mg | 10 mg | Placebo | 5 mg | 10 mg | Baseline | Mean decrease from baseline | |||
| Placebo | 5 mg | 10 mg | ||||||||
| ||||||||||
| Chapple et al.6* | 267 | 279 | 268 | 5 (1.9) | 20 (7.2) | 21 (7.8) | 12.0812.32 | 1.2 | 2.19 | 2.61 |
| Cardozo et al.4* | 301 | 299 | 307 | 6 (2.0) | 11 (3.7) | 28 (9.1) | 12.0512.31 | 1.59 | 2.37 | 2.81 |
| Wagg3 | 422 | 192 | 431 | 18 (4.3) | 18 (9.4) | 78 (18.1) | 11.611.7 | 1.1 | 2.0 | 2.5 |
Solfenacin succinate, an antimuscarinic agent, is approved for the treatment of overactive bladder and described as well tolerated in the elderly.1 We present the case of solifenacin‐induced small bowel pseudo‐obstruction in an 89‐year‐old woman.
FINDINGS
An 89‐year‐old woman with untreated stage 0 chronic lymphocytic leukemia and a history of stage III colorectal cancer treated with hemicolectomy and adjuvant capecitabine in 2003 was admitted to Johns Hopkins Hospital in 2006. She reported feeling dehydrated, nauseated, and constipated, with decreased output from her colostomy. She also noted no urine output for 4 days and felt that she had to urinate, but I can't. This coincided with a decrease in fluid intake. She denied fevers, chills, abdominal pain, or loss of appetite. While waiting to be seen in the emergency department, the patient was finally able to urinate.
She had no evidence of colon cancer recurrence, with a normal postoperative positron‐emission tomography (PET) scan in 2003, colonoscopy in 2005, and screening computerized tomography (CT) scan in 2005. She also had a history of well‐controlled hypertension and hypothyroidism, hyperlipidemia, chemotherapy‐induced neuropathy, and anxiety.
Her home medication regimen included solifenacin 5 mg once daily (started 10 days prior to her admission) for bladder overactivity, buspirone 5 mg 3 times a day, metoprolol 25 mg twice a day, pantoprazole 40 mg once daily, levothyroxine 100 g once daily, lisinopril/hydrochlorathiazide 20 mg/25 mg twice daily, gabapentin 300 mg twice a day, and fenofibrate 145 mg nightly.
The patient appeared nontoxic. Her exam was remarkable only for hypoactive bowel sounds and mild diffuse abdominal tenderness without distension or peritoneal signs. A Foley catheter was placed, and her postvoid residual was only 50 cc of urine. Her admission serum blood urea nitrogen and creatinine were 90 and 3.4 mg/dL, respectively, as compared with 18 and 0.8 mg/dL 2 months prior to presentation. A CT scan of the abdomen (Figure 1) revealed multiple dilated loops of small bowel with a transition point at the left lower quadrant ostomy site, consistent with a small bowel obstruction. A PET scan revealed no evidence of malignancy. A renal ultrasound showed no evidence of obstruction.

With cessation of solifenacin and lisinopril/hydrochlorothiazide and hydration with normal saline, her constipation resolved, as did her acute renal failure and perception of urinary retention. She began to tolerate a regular diet after 4 days of hospitalization, and her colostomy output normalized. At follow‐up 8 months after admission, her creatinine was 0.8 mg/dL, and a screening abdominal CT showed complete resolution of the small bowel obstruction.
DISCUSSION
We believe that this patient developed small bowel pseudo‐obstruction as well the feeling of urinary retention because of treatment with solifenacin, an antimuscarinic agent approved for the treatment of bladder overactivity. Her acute renal failure was a result of prerenal azotemia. This particular patient was at increased risk for developing antimuscarinic‐induced bowel obstruction because of her previous surgery and exposure to chemotherapy.
In the 4 randomized trials cited in the prescribing information for solifenacin,2 only 189 patients of the 1811 who received the active drug were greater than 75 years old. Healthy elderly patients ranging from 64 to 78 years of age (mean 68.0 years) who received 2 weeks of treatment with solifenacin 5 and 10 mg had a mean AUC024 that was approximately 20% higher than that of younger subjects.3 In the 4 12‐week double‐blind clinical trials in which 1158 patients were treated with solifenacin 10 mg, there were 3 serious intestinal adverse events: 1 patient had a fecal impaction, 1 patient had a colonic obstruction, and 1 patient had an intestinal obstruction.2 Patients receiving solifenacin 5 and 10 mg were more likely to experience constipation than those receiving placebo (5.4%, 13.4%, and 2.9%, respectively).2 Given the dearth of clinical data on patients greater than 75 years old, the effects of age on the pharmacokinetics, the higher likelihood of bowel pathology in the elderly, the increased risk of solifenacin‐induced side effects in the elderly as reported in the pooled analysis of patients at least 65 years old,4 and the small clinical benefit of solifenacin,46 physicians should seriously consider whether the benefits of solifenacin outweigh both the known and the possible risks. 0
| Patients in safety analysis (n) | Constipation, n (%) | Micturition/24 hours | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | 5 mg | 10 mg | Placebo | 5 mg | 10 mg | Baseline | Mean decrease from baseline | |||
| Placebo | 5 mg | 10 mg | ||||||||
| ||||||||||
| Chapple et al.6* | 267 | 279 | 268 | 5 (1.9) | 20 (7.2) | 21 (7.8) | 12.0812.32 | 1.2 | 2.19 | 2.61 |
| Cardozo et al.4* | 301 | 299 | 307 | 6 (2.0) | 11 (3.7) | 28 (9.1) | 12.0512.31 | 1.59 | 2.37 | 2.81 |
| Wagg3 | 422 | 192 | 431 | 18 (4.3) | 18 (9.4) | 78 (18.1) | 11.611.7 | 1.1 | 2.0 | 2.5 |
- .Solifenacin provides effective antimuscarinic therapy for the complete management of overactive bladder.Expert Opin Pharmacother.2006;7:2421–2434.
- Yamanouchi Pharma America, Inc.United States prescribing information for solifenacin succinate (Vesicare®), November2004.
- ,,,,.Effect of age on the pharmacokinetics of solifenacin in men and women.Int J Clin Pharmacol Ther.2005;43:227–238.
- ,,.Efficacy and tolerability of solifenacin in elderly subjects with overactive bladder syndrome: a pooled analysis.Am J Geriatr Pharmacother.2006;4(1):14–24.
- ,,, et al.Randomized, double‐blind placebo controlled trial of the once daily antimuscarinic agent solifenacin succinate in patients with overactive bladder.J Urol.2004;172(5 Pt 1):1919–1924.
- ,,, et al.Randomized, double‐blind placebo‐ and tolterodine‐controlled trial of the once‐daily antimuscarinic agent solifenacin in patients with symptomatic overactive bladder.BJU Int.2004;93:303–310.
- .Solifenacin provides effective antimuscarinic therapy for the complete management of overactive bladder.Expert Opin Pharmacother.2006;7:2421–2434.
- Yamanouchi Pharma America, Inc.United States prescribing information for solifenacin succinate (Vesicare®), November2004.
- ,,,,.Effect of age on the pharmacokinetics of solifenacin in men and women.Int J Clin Pharmacol Ther.2005;43:227–238.
- ,,.Efficacy and tolerability of solifenacin in elderly subjects with overactive bladder syndrome: a pooled analysis.Am J Geriatr Pharmacother.2006;4(1):14–24.
- ,,, et al.Randomized, double‐blind placebo controlled trial of the once daily antimuscarinic agent solifenacin succinate in patients with overactive bladder.J Urol.2004;172(5 Pt 1):1919–1924.
- ,,, et al.Randomized, double‐blind placebo‐ and tolterodine‐controlled trial of the once‐daily antimuscarinic agent solifenacin in patients with symptomatic overactive bladder.BJU Int.2004;93:303–310.