User login
. I see numerous social media posts, blogs, and magazine articles about toxic skin care ingredients, while more patients are asking their dermatologists about clean beauty products. So, I decided it was time to dissect the issues and figure out what “clean” really means to me.
The problem is that no one agrees on a clean ingredient standard for beauty products. Many companies, like Target, Walgreens/Boots, Sephora, Neiman Marcus, Whole Foods, and Ulta, have their own varying clean standards. Even Allure magazine has a “Clean Best of Beauty” seal. California has Proposition 65, otherwise known as the Safe Drinking Water and Toxic Enforcement Act of 1986, which contains a list of banned chemicals “known to the state to cause cancer or reproductive toxicity.” In January 2021, Hawai‘i law prohibited the sale of oxybenzone and octinoxate in sunscreens in response to scientific studies showing that these ingredients “are toxic to corals and other marine life.” The Environmental Working Group (EWG) rates the safety of ingredients based on carcinogenicity, developmental and reproductive toxicity, allergenicity, and immunotoxicity. The Cosmetic Ingredient Review (CIR), funded by the Personal Care Products Council, consists of a seven-member steering committee that has at least one dermatologist representing the American Academy of Dermatology and a toxicologist representing the Society of Toxicology. The CIR publishes detailed reviews of ingredients that can be easily found on PubMed and Google Scholar and closely reviews animal and human data and reports on safety and contact dermatitis risk.
Which clean beauty standard is best?
I reviewed most of the various standards, clean seals, laws, and safety reports and found significant discrepancies resulting from misunderstandings of the science, lack of depth in the scientific evaluations, lumping of ingredients into a larger category, or lack of data. The most salient cause of misinformation and confusion seems to be hyperbolic claims by the media and clean beauty advocates who do not understand the basic science.
When I conducted a survey of cosmetic chemists on my LinkedIn account, most of the chemists stated that “ ‘Clean Beauty’ is a marketing term, more than a scientific term.” None of the chemists could give an exact definition of clean beauty. However, I thought I needed a good answer for my patients and for doctors who want to use and recommend “clean skin care brands.”
A dermatologist’s approach to develop a clean beauty standard
Many of the standards combine all of the following into the “clean” designation: nontoxic to the environment (both the production process and the resulting ingredient), nontoxic to marine life and coral, cruelty-free (not tested on animals), hypoallergenic, lacking in known health risks (carcinogenicity, reproductive toxicity), vegan, and gluten free. As a dermatologist, I am a splitter more than a lumper, so I prefer that “clean” be split into categories to make it easier to understand. With that in mind, I will focus on clean beauty ingredients only as they pertain to health: carcinogenicity, endocrine effects, nephrotoxicity, hepatotoxicity, immunotoxicity, etc. This discussion will not consider environmental effects, reproductive toxicity (some ingredients may decrease fertility, which is beyond the scope of this article), ingredient sources, and sustainability, animal testing, or human rights violations during production. Those issues are important, of course, but for clarity and simplicity, we will focus on the health risks of skin care ingredients.
In this month’s column, I will focus on a few ingredients and will continue the discussion in subsequent columns. Please note that commercial standards such as Target standards are based on the product type (e.g., cleansers, sunscreens, or moisturizers). So, when I mention an ingredient not allowed by certain company standards, note that it can vary by product type. My comments pertain mostly to facial moisturizers and facial serums to try and simplify the information. The Good Face Project has a complete list of standards by product type, which I recommend as a resource if you want more detailed information.
Are ethanolamines safe or toxic in cosmetics?
Ethanolamines are common ingredients in surfactants, fragrances, and emulsifying agents and include cocamide diethanolamine (DEA), cocamide monoethanolamine (MEA), and triethanolamine (TEA). Cocamide DEA, lauramide DEA, linoleamide DEA, and oleamide DEA are fatty acid diethanolamides that may contain 4% to 33% diethanolamine.1 A Google search of toxic ingredients in beauty products consistently identifies ethanolamines among such offending product constituents. Table 1 reveals that ethanolamines are excluded from some standards and included in others (N = not allowed or restricted by amount used and Y = allowed with no restrictions). As you can see, the standards don’t correspond to the EWG rating of the ingredients, which ranges from 1 (low hazard) to 10 (high hazard).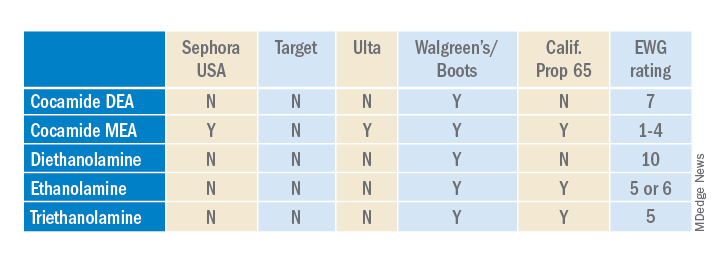
Why are ethanolamines sometimes considered safe and sometimes not?
Ethanolamines are reputed to be allergenic, but as we know as dermatologists, that does not mean that everyone will react to them. (In my opinion, allergenicity is a separate issue than the clean issue.) One study showed that TEA in 2.5% petrolatum had a 0.4% positive patch test rate in humans, which was thought to be related more to irritation than allergenicity.2 Cocamide DEA allergy is seen in those with hand dermatitis resulting from hand cleansers but is more commonly seen in metal workers.3 For this reason, these ethanolamines are usually found in rinse-off products to decrease exposure time. But there are many irritating ingredients not banned by Target, Sephora, and Ulta, so why does ethanolamine end up on toxic ingredient lists?
First, there is the issue of oral studies in animals. Oral forms of some ethanolamines have shown mild toxicity in rats, but topical forms have not been demonstrated to cause mutagenicity.1
For this reason, ethanolamines in their native form are considered safe.
The main issue with ethanolamines is that, when they are formulated with ingredients that break down into nitrogen, such as certain preservatives, the combination forms nitrosamines, such as N-nitrosodiethylamine (NDEA), which are carcinogenic.4 The European Commission prohibits DEA in cosmetics based on concerns about formation of these carcinogenic nitrosamines. Some standards limit ethanolamines to rinse-off products.5 The CIR panel concluded that diethanolamine and its 16 salts are safe if they are not used in cosmetic products in which N-nitroso compounds can be formed and that TEA and TEA-related compounds are safe if they are not used in cosmetic products in which N-nitroso compounds can be formed.6,7 The FDA states that there is no reason for consumers to be alarmed based on the use of DEA in cosmetics.8
The safety issues surrounding the use of ethanolamines in a skin care routine illustrate an important point: Every single product in the skin care routine should be compatible with the other products in the regimen. Using ethanolamines in a rinse-off product is one solution, as is ensuring that no other products in the skin care routine contain N-nitroso compounds that can combine with ethanolamines to form nitrosamines.
Are natural products safer?
Natural products are not necessarily any safer than synthetic products. Considering ethanolamines as the example here, note that cocamide DEA is an ethanolamine derived from coconut. It is often found in “green” or “natural” skin care products.9 It can still combine with N-nitroso compounds to form carcinogenic nitrosamines.
What is the bottom line? Are ethanolamines safe in cosmetics?
For now, if a patient asks if ethanolamine is safe in skin care, my answer would be yes, so long as the following is true:
- It is in a rinse-off product.
- The patient is not allergic to it.
- They do not have hand dermatitis.
- Their skin care routine does not include nitrogen-containing compounds like N-nitrosodiethanolamine (NDELA) or NDEA.
Conclusion
This column uses ethanolamines as an example to show the disparity in clean standards in the cosmetic industry. As you can see, there are multiple factors to consider. I will begin including clean information in my cosmeceutical critique columns to address some of these issues.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Cocamide DE. J Am Coll Toxicol. 1986;5(5).
2. Lessmann H et al. Contact Dermatitis. 2009 May;60(5):243-55.
3. Aalto-Korte K et al. 2014 Mar;70(3):169-74.
4. Kraeling ME et al. Food Chem Toxicol. 2004 Oct;42(10):1553-61.
5. Fiume MM et al. Int J Toxicol. 2015 Sep;34(2 Suppl):84S-98S.
6. Fiume MM.. Int J Toxicol. 2017 Sep/Oct;36(5_suppl2):89S-110S.
7. Fiume MM et al. Int J Toxicol. 2013 May-Jun;32(3 Suppl):59S-83S.
8. U.S. Food & Drug Administration. Diethanolamine. https://www.fda.gov/cosmetics/cosmetic-ingredients/diethanolamine. Accessed Feb. 12, 2022.
9. Aryanti N et al. IOP Conference Series: Materials Science and Engineering 2021 Feb 1 (Vol. 1053, No. 1, p. 012066). IOP Publishing.
. I see numerous social media posts, blogs, and magazine articles about toxic skin care ingredients, while more patients are asking their dermatologists about clean beauty products. So, I decided it was time to dissect the issues and figure out what “clean” really means to me.
The problem is that no one agrees on a clean ingredient standard for beauty products. Many companies, like Target, Walgreens/Boots, Sephora, Neiman Marcus, Whole Foods, and Ulta, have their own varying clean standards. Even Allure magazine has a “Clean Best of Beauty” seal. California has Proposition 65, otherwise known as the Safe Drinking Water and Toxic Enforcement Act of 1986, which contains a list of banned chemicals “known to the state to cause cancer or reproductive toxicity.” In January 2021, Hawai‘i law prohibited the sale of oxybenzone and octinoxate in sunscreens in response to scientific studies showing that these ingredients “are toxic to corals and other marine life.” The Environmental Working Group (EWG) rates the safety of ingredients based on carcinogenicity, developmental and reproductive toxicity, allergenicity, and immunotoxicity. The Cosmetic Ingredient Review (CIR), funded by the Personal Care Products Council, consists of a seven-member steering committee that has at least one dermatologist representing the American Academy of Dermatology and a toxicologist representing the Society of Toxicology. The CIR publishes detailed reviews of ingredients that can be easily found on PubMed and Google Scholar and closely reviews animal and human data and reports on safety and contact dermatitis risk.
Which clean beauty standard is best?
I reviewed most of the various standards, clean seals, laws, and safety reports and found significant discrepancies resulting from misunderstandings of the science, lack of depth in the scientific evaluations, lumping of ingredients into a larger category, or lack of data. The most salient cause of misinformation and confusion seems to be hyperbolic claims by the media and clean beauty advocates who do not understand the basic science.
When I conducted a survey of cosmetic chemists on my LinkedIn account, most of the chemists stated that “ ‘Clean Beauty’ is a marketing term, more than a scientific term.” None of the chemists could give an exact definition of clean beauty. However, I thought I needed a good answer for my patients and for doctors who want to use and recommend “clean skin care brands.”
A dermatologist’s approach to develop a clean beauty standard
Many of the standards combine all of the following into the “clean” designation: nontoxic to the environment (both the production process and the resulting ingredient), nontoxic to marine life and coral, cruelty-free (not tested on animals), hypoallergenic, lacking in known health risks (carcinogenicity, reproductive toxicity), vegan, and gluten free. As a dermatologist, I am a splitter more than a lumper, so I prefer that “clean” be split into categories to make it easier to understand. With that in mind, I will focus on clean beauty ingredients only as they pertain to health: carcinogenicity, endocrine effects, nephrotoxicity, hepatotoxicity, immunotoxicity, etc. This discussion will not consider environmental effects, reproductive toxicity (some ingredients may decrease fertility, which is beyond the scope of this article), ingredient sources, and sustainability, animal testing, or human rights violations during production. Those issues are important, of course, but for clarity and simplicity, we will focus on the health risks of skin care ingredients.
In this month’s column, I will focus on a few ingredients and will continue the discussion in subsequent columns. Please note that commercial standards such as Target standards are based on the product type (e.g., cleansers, sunscreens, or moisturizers). So, when I mention an ingredient not allowed by certain company standards, note that it can vary by product type. My comments pertain mostly to facial moisturizers and facial serums to try and simplify the information. The Good Face Project has a complete list of standards by product type, which I recommend as a resource if you want more detailed information.
Are ethanolamines safe or toxic in cosmetics?
Ethanolamines are common ingredients in surfactants, fragrances, and emulsifying agents and include cocamide diethanolamine (DEA), cocamide monoethanolamine (MEA), and triethanolamine (TEA). Cocamide DEA, lauramide DEA, linoleamide DEA, and oleamide DEA are fatty acid diethanolamides that may contain 4% to 33% diethanolamine.1 A Google search of toxic ingredients in beauty products consistently identifies ethanolamines among such offending product constituents. Table 1 reveals that ethanolamines are excluded from some standards and included in others (N = not allowed or restricted by amount used and Y = allowed with no restrictions). As you can see, the standards don’t correspond to the EWG rating of the ingredients, which ranges from 1 (low hazard) to 10 (high hazard).
Why are ethanolamines sometimes considered safe and sometimes not?
Ethanolamines are reputed to be allergenic, but as we know as dermatologists, that does not mean that everyone will react to them. (In my opinion, allergenicity is a separate issue than the clean issue.) One study showed that TEA in 2.5% petrolatum had a 0.4% positive patch test rate in humans, which was thought to be related more to irritation than allergenicity.2 Cocamide DEA allergy is seen in those with hand dermatitis resulting from hand cleansers but is more commonly seen in metal workers.3 For this reason, these ethanolamines are usually found in rinse-off products to decrease exposure time. But there are many irritating ingredients not banned by Target, Sephora, and Ulta, so why does ethanolamine end up on toxic ingredient lists?
First, there is the issue of oral studies in animals. Oral forms of some ethanolamines have shown mild toxicity in rats, but topical forms have not been demonstrated to cause mutagenicity.1
For this reason, ethanolamines in their native form are considered safe.
The main issue with ethanolamines is that, when they are formulated with ingredients that break down into nitrogen, such as certain preservatives, the combination forms nitrosamines, such as N-nitrosodiethylamine (NDEA), which are carcinogenic.4 The European Commission prohibits DEA in cosmetics based on concerns about formation of these carcinogenic nitrosamines. Some standards limit ethanolamines to rinse-off products.5 The CIR panel concluded that diethanolamine and its 16 salts are safe if they are not used in cosmetic products in which N-nitroso compounds can be formed and that TEA and TEA-related compounds are safe if they are not used in cosmetic products in which N-nitroso compounds can be formed.6,7 The FDA states that there is no reason for consumers to be alarmed based on the use of DEA in cosmetics.8
The safety issues surrounding the use of ethanolamines in a skin care routine illustrate an important point: Every single product in the skin care routine should be compatible with the other products in the regimen. Using ethanolamines in a rinse-off product is one solution, as is ensuring that no other products in the skin care routine contain N-nitroso compounds that can combine with ethanolamines to form nitrosamines.
Are natural products safer?
Natural products are not necessarily any safer than synthetic products. Considering ethanolamines as the example here, note that cocamide DEA is an ethanolamine derived from coconut. It is often found in “green” or “natural” skin care products.9 It can still combine with N-nitroso compounds to form carcinogenic nitrosamines.
What is the bottom line? Are ethanolamines safe in cosmetics?
For now, if a patient asks if ethanolamine is safe in skin care, my answer would be yes, so long as the following is true:
- It is in a rinse-off product.
- The patient is not allergic to it.
- They do not have hand dermatitis.
- Their skin care routine does not include nitrogen-containing compounds like N-nitrosodiethanolamine (NDELA) or NDEA.
Conclusion
This column uses ethanolamines as an example to show the disparity in clean standards in the cosmetic industry. As you can see, there are multiple factors to consider. I will begin including clean information in my cosmeceutical critique columns to address some of these issues.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Cocamide DE. J Am Coll Toxicol. 1986;5(5).
2. Lessmann H et al. Contact Dermatitis. 2009 May;60(5):243-55.
3. Aalto-Korte K et al. 2014 Mar;70(3):169-74.
4. Kraeling ME et al. Food Chem Toxicol. 2004 Oct;42(10):1553-61.
5. Fiume MM et al. Int J Toxicol. 2015 Sep;34(2 Suppl):84S-98S.
6. Fiume MM.. Int J Toxicol. 2017 Sep/Oct;36(5_suppl2):89S-110S.
7. Fiume MM et al. Int J Toxicol. 2013 May-Jun;32(3 Suppl):59S-83S.
8. U.S. Food & Drug Administration. Diethanolamine. https://www.fda.gov/cosmetics/cosmetic-ingredients/diethanolamine. Accessed Feb. 12, 2022.
9. Aryanti N et al. IOP Conference Series: Materials Science and Engineering 2021 Feb 1 (Vol. 1053, No. 1, p. 012066). IOP Publishing.
. I see numerous social media posts, blogs, and magazine articles about toxic skin care ingredients, while more patients are asking their dermatologists about clean beauty products. So, I decided it was time to dissect the issues and figure out what “clean” really means to me.
The problem is that no one agrees on a clean ingredient standard for beauty products. Many companies, like Target, Walgreens/Boots, Sephora, Neiman Marcus, Whole Foods, and Ulta, have their own varying clean standards. Even Allure magazine has a “Clean Best of Beauty” seal. California has Proposition 65, otherwise known as the Safe Drinking Water and Toxic Enforcement Act of 1986, which contains a list of banned chemicals “known to the state to cause cancer or reproductive toxicity.” In January 2021, Hawai‘i law prohibited the sale of oxybenzone and octinoxate in sunscreens in response to scientific studies showing that these ingredients “are toxic to corals and other marine life.” The Environmental Working Group (EWG) rates the safety of ingredients based on carcinogenicity, developmental and reproductive toxicity, allergenicity, and immunotoxicity. The Cosmetic Ingredient Review (CIR), funded by the Personal Care Products Council, consists of a seven-member steering committee that has at least one dermatologist representing the American Academy of Dermatology and a toxicologist representing the Society of Toxicology. The CIR publishes detailed reviews of ingredients that can be easily found on PubMed and Google Scholar and closely reviews animal and human data and reports on safety and contact dermatitis risk.
Which clean beauty standard is best?
I reviewed most of the various standards, clean seals, laws, and safety reports and found significant discrepancies resulting from misunderstandings of the science, lack of depth in the scientific evaluations, lumping of ingredients into a larger category, or lack of data. The most salient cause of misinformation and confusion seems to be hyperbolic claims by the media and clean beauty advocates who do not understand the basic science.
When I conducted a survey of cosmetic chemists on my LinkedIn account, most of the chemists stated that “ ‘Clean Beauty’ is a marketing term, more than a scientific term.” None of the chemists could give an exact definition of clean beauty. However, I thought I needed a good answer for my patients and for doctors who want to use and recommend “clean skin care brands.”
A dermatologist’s approach to develop a clean beauty standard
Many of the standards combine all of the following into the “clean” designation: nontoxic to the environment (both the production process and the resulting ingredient), nontoxic to marine life and coral, cruelty-free (not tested on animals), hypoallergenic, lacking in known health risks (carcinogenicity, reproductive toxicity), vegan, and gluten free. As a dermatologist, I am a splitter more than a lumper, so I prefer that “clean” be split into categories to make it easier to understand. With that in mind, I will focus on clean beauty ingredients only as they pertain to health: carcinogenicity, endocrine effects, nephrotoxicity, hepatotoxicity, immunotoxicity, etc. This discussion will not consider environmental effects, reproductive toxicity (some ingredients may decrease fertility, which is beyond the scope of this article), ingredient sources, and sustainability, animal testing, or human rights violations during production. Those issues are important, of course, but for clarity and simplicity, we will focus on the health risks of skin care ingredients.
In this month’s column, I will focus on a few ingredients and will continue the discussion in subsequent columns. Please note that commercial standards such as Target standards are based on the product type (e.g., cleansers, sunscreens, or moisturizers). So, when I mention an ingredient not allowed by certain company standards, note that it can vary by product type. My comments pertain mostly to facial moisturizers and facial serums to try and simplify the information. The Good Face Project has a complete list of standards by product type, which I recommend as a resource if you want more detailed information.
Are ethanolamines safe or toxic in cosmetics?
Ethanolamines are common ingredients in surfactants, fragrances, and emulsifying agents and include cocamide diethanolamine (DEA), cocamide monoethanolamine (MEA), and triethanolamine (TEA). Cocamide DEA, lauramide DEA, linoleamide DEA, and oleamide DEA are fatty acid diethanolamides that may contain 4% to 33% diethanolamine.1 A Google search of toxic ingredients in beauty products consistently identifies ethanolamines among such offending product constituents. Table 1 reveals that ethanolamines are excluded from some standards and included in others (N = not allowed or restricted by amount used and Y = allowed with no restrictions). As you can see, the standards don’t correspond to the EWG rating of the ingredients, which ranges from 1 (low hazard) to 10 (high hazard).
Why are ethanolamines sometimes considered safe and sometimes not?
Ethanolamines are reputed to be allergenic, but as we know as dermatologists, that does not mean that everyone will react to them. (In my opinion, allergenicity is a separate issue than the clean issue.) One study showed that TEA in 2.5% petrolatum had a 0.4% positive patch test rate in humans, which was thought to be related more to irritation than allergenicity.2 Cocamide DEA allergy is seen in those with hand dermatitis resulting from hand cleansers but is more commonly seen in metal workers.3 For this reason, these ethanolamines are usually found in rinse-off products to decrease exposure time. But there are many irritating ingredients not banned by Target, Sephora, and Ulta, so why does ethanolamine end up on toxic ingredient lists?
First, there is the issue of oral studies in animals. Oral forms of some ethanolamines have shown mild toxicity in rats, but topical forms have not been demonstrated to cause mutagenicity.1
For this reason, ethanolamines in their native form are considered safe.
The main issue with ethanolamines is that, when they are formulated with ingredients that break down into nitrogen, such as certain preservatives, the combination forms nitrosamines, such as N-nitrosodiethylamine (NDEA), which are carcinogenic.4 The European Commission prohibits DEA in cosmetics based on concerns about formation of these carcinogenic nitrosamines. Some standards limit ethanolamines to rinse-off products.5 The CIR panel concluded that diethanolamine and its 16 salts are safe if they are not used in cosmetic products in which N-nitroso compounds can be formed and that TEA and TEA-related compounds are safe if they are not used in cosmetic products in which N-nitroso compounds can be formed.6,7 The FDA states that there is no reason for consumers to be alarmed based on the use of DEA in cosmetics.8
The safety issues surrounding the use of ethanolamines in a skin care routine illustrate an important point: Every single product in the skin care routine should be compatible with the other products in the regimen. Using ethanolamines in a rinse-off product is one solution, as is ensuring that no other products in the skin care routine contain N-nitroso compounds that can combine with ethanolamines to form nitrosamines.
Are natural products safer?
Natural products are not necessarily any safer than synthetic products. Considering ethanolamines as the example here, note that cocamide DEA is an ethanolamine derived from coconut. It is often found in “green” or “natural” skin care products.9 It can still combine with N-nitroso compounds to form carcinogenic nitrosamines.
What is the bottom line? Are ethanolamines safe in cosmetics?
For now, if a patient asks if ethanolamine is safe in skin care, my answer would be yes, so long as the following is true:
- It is in a rinse-off product.
- The patient is not allergic to it.
- They do not have hand dermatitis.
- Their skin care routine does not include nitrogen-containing compounds like N-nitrosodiethanolamine (NDELA) or NDEA.
Conclusion
This column uses ethanolamines as an example to show the disparity in clean standards in the cosmetic industry. As you can see, there are multiple factors to consider. I will begin including clean information in my cosmeceutical critique columns to address some of these issues.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Cocamide DE. J Am Coll Toxicol. 1986;5(5).
2. Lessmann H et al. Contact Dermatitis. 2009 May;60(5):243-55.
3. Aalto-Korte K et al. 2014 Mar;70(3):169-74.
4. Kraeling ME et al. Food Chem Toxicol. 2004 Oct;42(10):1553-61.
5. Fiume MM et al. Int J Toxicol. 2015 Sep;34(2 Suppl):84S-98S.
6. Fiume MM.. Int J Toxicol. 2017 Sep/Oct;36(5_suppl2):89S-110S.
7. Fiume MM et al. Int J Toxicol. 2013 May-Jun;32(3 Suppl):59S-83S.
8. U.S. Food & Drug Administration. Diethanolamine. https://www.fda.gov/cosmetics/cosmetic-ingredients/diethanolamine. Accessed Feb. 12, 2022.
9. Aryanti N et al. IOP Conference Series: Materials Science and Engineering 2021 Feb 1 (Vol. 1053, No. 1, p. 012066). IOP Publishing.

