User login
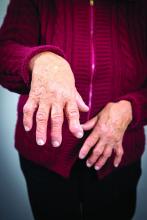
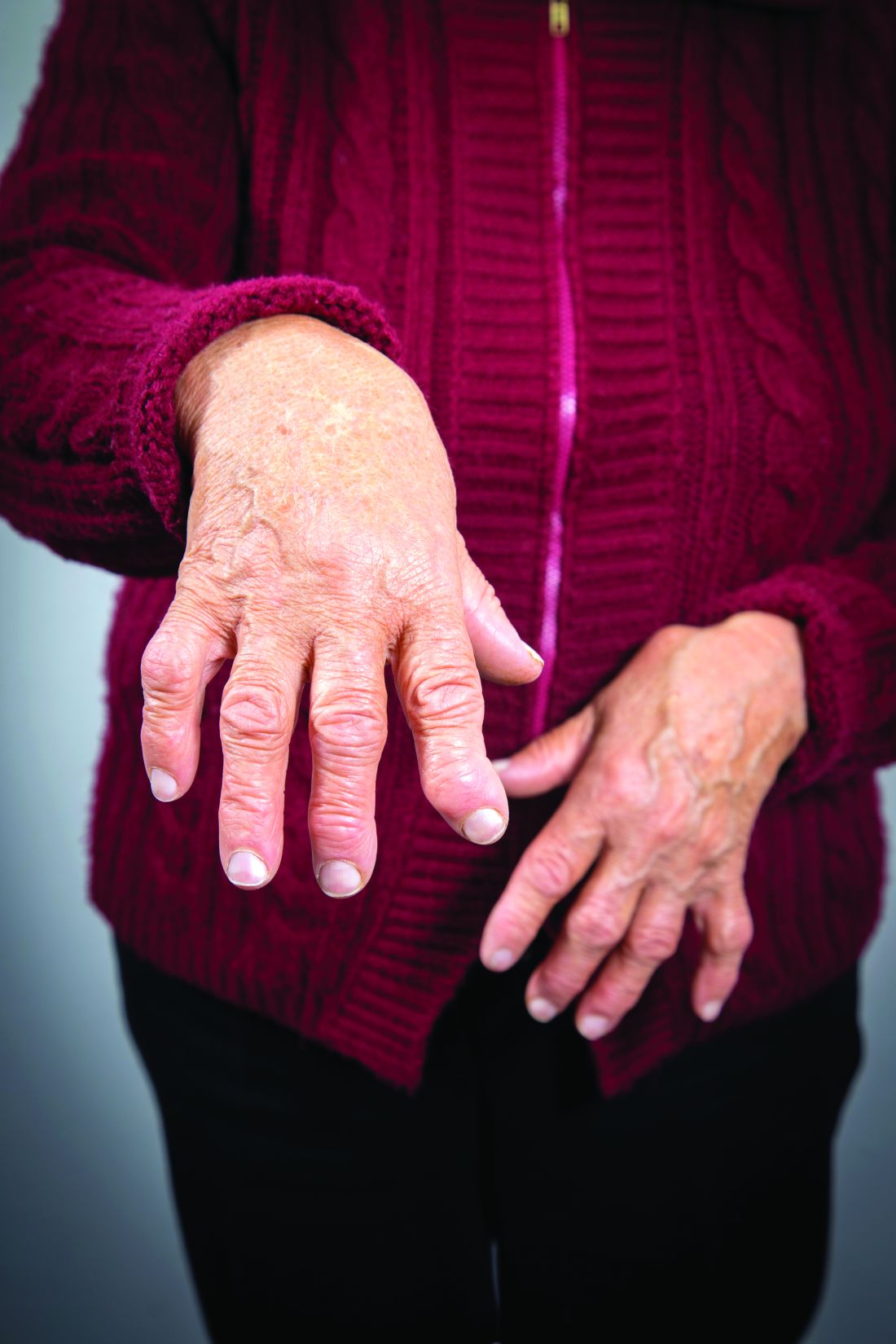
Researchers have identified three different synovial subtypes of rheumatoid arthritis that exhibit different mechanisms of pain and correlate with specific clinical phenotypes.
The findings could be clinically meaningful and may help guide optimal treatment strategies for patients, as well as provide a better understanding of the cause of pain in patients with high tender and swollen joint counts but little tissue inflammation, according to the research team led by Dana E. Orange, MD, of the Hospital for Special Surgery and Rockefeller University in New York.
The report was published in Arthritis & Rheumatology.
The assessment of the synovium in rheumatoid arthritis (RA) has the potential to provide guidance on optimal treatment strategies, they noted, but its classification has not yet factored into current diagnosis or treatment guidelines of RA.
In total, the research team analyzed 20 histologic features on 129 synovial tissue samples.
The researchers used machine learning integration to identify three distinct molecular subtypes of RA from a consensus clustering of the 500 most variable genes expressed in a subset of 45 synovial samples, including 39 from RA patients. The subtypes were high inflammatory, low inflammatory, and a mixed phenotype.
The researchers then took the histologic features that best corresponded to each subtype to develop a histology scoring algorithm that predicted the three gene expression subtypes (using only histology features), each of which were each associated with levels of erythrocyte sedimentation rate, C-reactive protein, and autoantibodies.
The histologic features that most strongly defined the high inflammatory subtype included three plasma cell features: binucleate plasma cells, plasma cell percentage, and Russell bodies. Patients with a high inflammatory synovial subtype also exhibited higher levels of markers of systemic inflammation and autoantibodies. For example, C-reactive protein was significantly correlated with pain in the high inflammatory group.
“This suggests that pain is associated with inflammation in patients with high inflammatory subtype and that pain may be driven by distinct mechanisms in the other patients,” the study authors wrote.
The low inflammatory subgroup was characterized by high neuronal and glycoprotein gene expression. But in this group, pain scores were not associated with elevated inflammatory markers.
“It is interesting that this subtype is characterized by a paucity of inflammatory infiltrates, yet maintains high pain scores and multiple tender/swollen joints – this too is consistent with other findings of patients with established RA,” the research team noted.
The mixed subtype shared features with both the high and low subtypes, the researchers said.
“Our work suggests that RA patients with longstanding disease and poor response to anti-inflammatory treatment may warrant synovial biopsy to determine their inflammatory subtype,” the researchers concluded.
Several research institutions and the Accelerating Medicines Partnership in Rheumatoid Arthritis and Lupus Network, a public-private partnership involving several pharmaceutical companies, patient advocacy groups, and the National Institutes of Health, funded the study.
SOURCE: Orange D et al. Arthritis Rheumatol. 2018 Feb 22. doi: 10.1002/art.40428.
Researchers have identified three different synovial subtypes of rheumatoid arthritis that exhibit different mechanisms of pain and correlate with specific clinical phenotypes.
The findings could be clinically meaningful and may help guide optimal treatment strategies for patients, as well as provide a better understanding of the cause of pain in patients with high tender and swollen joint counts but little tissue inflammation, according to the research team led by Dana E. Orange, MD, of the Hospital for Special Surgery and Rockefeller University in New York.
The report was published in Arthritis & Rheumatology.
The assessment of the synovium in rheumatoid arthritis (RA) has the potential to provide guidance on optimal treatment strategies, they noted, but its classification has not yet factored into current diagnosis or treatment guidelines of RA.
In total, the research team analyzed 20 histologic features on 129 synovial tissue samples.
The researchers used machine learning integration to identify three distinct molecular subtypes of RA from a consensus clustering of the 500 most variable genes expressed in a subset of 45 synovial samples, including 39 from RA patients. The subtypes were high inflammatory, low inflammatory, and a mixed phenotype.
The researchers then took the histologic features that best corresponded to each subtype to develop a histology scoring algorithm that predicted the three gene expression subtypes (using only histology features), each of which were each associated with levels of erythrocyte sedimentation rate, C-reactive protein, and autoantibodies.
The histologic features that most strongly defined the high inflammatory subtype included three plasma cell features: binucleate plasma cells, plasma cell percentage, and Russell bodies. Patients with a high inflammatory synovial subtype also exhibited higher levels of markers of systemic inflammation and autoantibodies. For example, C-reactive protein was significantly correlated with pain in the high inflammatory group.
“This suggests that pain is associated with inflammation in patients with high inflammatory subtype and that pain may be driven by distinct mechanisms in the other patients,” the study authors wrote.
The low inflammatory subgroup was characterized by high neuronal and glycoprotein gene expression. But in this group, pain scores were not associated with elevated inflammatory markers.
“It is interesting that this subtype is characterized by a paucity of inflammatory infiltrates, yet maintains high pain scores and multiple tender/swollen joints – this too is consistent with other findings of patients with established RA,” the research team noted.
The mixed subtype shared features with both the high and low subtypes, the researchers said.
“Our work suggests that RA patients with longstanding disease and poor response to anti-inflammatory treatment may warrant synovial biopsy to determine their inflammatory subtype,” the researchers concluded.
Several research institutions and the Accelerating Medicines Partnership in Rheumatoid Arthritis and Lupus Network, a public-private partnership involving several pharmaceutical companies, patient advocacy groups, and the National Institutes of Health, funded the study.
SOURCE: Orange D et al. Arthritis Rheumatol. 2018 Feb 22. doi: 10.1002/art.40428.
Researchers have identified three different synovial subtypes of rheumatoid arthritis that exhibit different mechanisms of pain and correlate with specific clinical phenotypes.
The findings could be clinically meaningful and may help guide optimal treatment strategies for patients, as well as provide a better understanding of the cause of pain in patients with high tender and swollen joint counts but little tissue inflammation, according to the research team led by Dana E. Orange, MD, of the Hospital for Special Surgery and Rockefeller University in New York.
The report was published in Arthritis & Rheumatology.
The assessment of the synovium in rheumatoid arthritis (RA) has the potential to provide guidance on optimal treatment strategies, they noted, but its classification has not yet factored into current diagnosis or treatment guidelines of RA.
In total, the research team analyzed 20 histologic features on 129 synovial tissue samples.
The researchers used machine learning integration to identify three distinct molecular subtypes of RA from a consensus clustering of the 500 most variable genes expressed in a subset of 45 synovial samples, including 39 from RA patients. The subtypes were high inflammatory, low inflammatory, and a mixed phenotype.
The researchers then took the histologic features that best corresponded to each subtype to develop a histology scoring algorithm that predicted the three gene expression subtypes (using only histology features), each of which were each associated with levels of erythrocyte sedimentation rate, C-reactive protein, and autoantibodies.
The histologic features that most strongly defined the high inflammatory subtype included three plasma cell features: binucleate plasma cells, plasma cell percentage, and Russell bodies. Patients with a high inflammatory synovial subtype also exhibited higher levels of markers of systemic inflammation and autoantibodies. For example, C-reactive protein was significantly correlated with pain in the high inflammatory group.
“This suggests that pain is associated with inflammation in patients with high inflammatory subtype and that pain may be driven by distinct mechanisms in the other patients,” the study authors wrote.
The low inflammatory subgroup was characterized by high neuronal and glycoprotein gene expression. But in this group, pain scores were not associated with elevated inflammatory markers.
“It is interesting that this subtype is characterized by a paucity of inflammatory infiltrates, yet maintains high pain scores and multiple tender/swollen joints – this too is consistent with other findings of patients with established RA,” the research team noted.
The mixed subtype shared features with both the high and low subtypes, the researchers said.
“Our work suggests that RA patients with longstanding disease and poor response to anti-inflammatory treatment may warrant synovial biopsy to determine their inflammatory subtype,” the researchers concluded.
Several research institutions and the Accelerating Medicines Partnership in Rheumatoid Arthritis and Lupus Network, a public-private partnership involving several pharmaceutical companies, patient advocacy groups, and the National Institutes of Health, funded the study.
SOURCE: Orange D et al. Arthritis Rheumatol. 2018 Feb 22. doi: 10.1002/art.40428.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: Mechanisms of pain may differ in RA patients with different synovial subtypes.
Major findings:
Study details: Twenty histologic features were assessed on 129 synovial tissue samples from 123 RA patients and 6 OA patients.
Disclosures: Several research institutions and the Accelerating Medicines Partnership in Rheumatoid Arthritis and Lupus Network, a public-private partnership involving several pharmaceutical companies, patient advocacy groups, and the National Institutes of Health, funded the study.
Source: Orange D et al. Arthritis Rheumatol. 2018 Feb 22. doi: 10.1002/art.40428.