User login
CASE: A 26-year-old woman came to our emergency department with shortness of breath—a symptom that had begun 2 weeks earlier and was getting steadily worse. She had a history of severe, persistent asthma, and had been admitted to the ICU—but not intubated—9 months earlier. Now, she reported dyspnea with even mild exertion, which inhalers failed to relieve.
The patient also had a cough and had recently begun producing thick, green sputum, but said she’d had no chest pain, fever, or lower extremity swelling. The young woman lived with her parents, both of whom smoked heavily, but denied tobacco or alcohol use herself. She had not taken antibiotics in the past 6 months.
On physical examination, the patient was afebrile, mildly tachypneic, and had a heart rate of 125 bpm. She was saturating 80% on ambient air and 95% on 2 liters per minute via nasal cannula; her lung exam was significant for diffuse wheezes and rhonchi, but the remainder of the physical exam was normal.
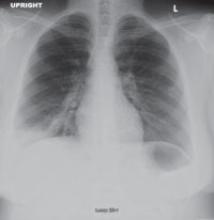
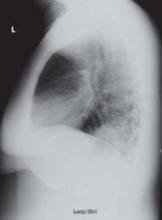
Lab tests revealed a white blood cell count of 25,200/mcL, with 81% neutrophils. Blood and urine cultures were preliminarily negative. We sent a sputum sample to be cultured and took chest x-rays. Posterior-anterior (PA) (FIGURE 1) and lateral (FIGURE 2) radiographs showed a dense right lower lobe inflltrate.
We diagnosed community-acquired pneumonia (CAP), coupled with an acute exacerbation of asthma, and admitted the patient to the hospital. We started her on intravenous (IV) moxifloxacin, parenteral steroids, and nebulized albuterol and ipratropium. But by the patient’s third day in the hospital, her white blood cell count had climbed to 34,500/mcL, and she still required oxygen by nasal cannula.
FIGURE 1 PA x-ray shows right lower lobe infiltrate
FIGURE 2 Dense infiltrate on lateral view
WHY DIDN’T THIS PATIENT RESPOND TO DRUG THERAPY?
CAP complicated by CA-MRSA
Initially, this appeared to be a rather straightforward case of CAP and asthma. The return of the sputum cultures on day 4, showing positive results for methicillin-resistant Staphylococcus aureus (MRSA), indicated that this was not the case. Sensitivity analysis revealed intermediate susceptibility to moxifloxacin and sensitivity to trimethoprim-sulfamethoxazole (TMP-SMX), vancomycin, and linezolid.
We switched the patient’s antibiotic from moxifloxacin to parenteral linezolid. She responded rapidly. On day 5, the patient was successfully weaned from O2 and converted to oral linezolid; the following day, she was discharged, with orders to complete an 8-day course of linezolid and an appointment with her family physician for the following week. At follow-up, she was doing well.
Cultures are crucial if results would alter Tx
In 2007, the Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS) published guidelines on the management of CAP1—a clinical diagnosis based on the presence of select features, including cough, fever, sputum production, and pleuritic chest pain. (Imaging of the chest may be used to support the diagnosis, but is not required.)
Following a site-of-care decision, ideally made with the help of a severity-of-illness scoring system such as the CURB-65 (Confusion, Urea nitrogen, Respiratory rate, Blood pressure, 65 years of age and older) or Pneumonia Severity Index, the guidelines recommend cultures whenever the result is likely to significantly alter standard empiric treatment.1
Pretreatment blood samples for culture should be obtained (strength of recommendation: A, well-conducted randomized controlled trials). The IDSA/ATS CAP guidelines also recommend an expectorated sputum sample for stain and culture for patients admitted to the ICU, as well as for those with a presentation suggestive of CAP who:
- have failed outpatient treatment;
- abuse alcohol;
- have severe structural or obstructive lung disease;
- or have pleural effusion.1
In our patient’s case, we obtained a sputum culture on admission because of her severe asthma. She was persistently hypoxic during her initial days in the hospital, and when the sputum culture returned positive for MRSA, her regimen of antibiotics was adjusted.
Suspect CA-MRSA when CAP is severe
Although MRSA has traditionally been thought of as a nosocomial pathogen, infections caused by distinct CA-MRSA strains are on the rise. An estimated 1% to 10% of CAP cases are caused by S aureus, but the percentage of those strains that are resistant to methicillin is not known.2
Clinical risk factors for S aureus CAP include end-stage renal disease, injection drug use, prior influenza, and prior antibiotic therapy, especially with fluoroquinolones.1 How-ever, CA-MRSA CAP often affects children and healthy young adults without any risk factors.3,4
Additionally, CAP that is caused by CA-MRSA is typically severe, often involving empyema, acute respiratory distress syndrome (ARDS), cavitary pneumonia, or severe sepsis.5 Consider a diagnosis of CA-MRSA CAP in patients who present with cavitary infiltrates without risk factors for anaerobic aspiration, such as syncope, seizures, alcohol abuse, or esophageal motility disorders.1
Start treatment without delay in patients at risk
Standard empiric treatment options for hospitalized patients with CAP (with the exception of those in the ICU) include either a beta-lactam and azithromycin or a respiratory fluoroquinolone (neither of which is active against MRSA).1 All patients who are hospitalized for CAP should be started on this regimen without delay. If gram-positive cocci in clusters suggestive of S aureus are isolated from sputum, this standard empiric regimen should be adequate until further microbiologic identification is available.1 While most cases of S aureus CAP are not MRSA, the increased mortality rate associated with inappropriate antibiotic selection led the IDSA/ATS to recommend broader empiric coverage for MRSA for patients with risk factors for S aureus.1
CA-MRSA strains are distinct from their nosocomial counterpart, and the optimal therapy for confirmed CA-MRSA CAP has not been determined. Frequently, CA-MRSA isolates are sensitive to TMP-SMX, clindamycin, and fluoroquinolones. But neither fluoroquinolones nor TMP-SMX have a clear effect on toxin production, and bacterial resistance frequently emerges during therapy with clindamycin.1
The IDSA/ATS guidelines recommend either vancomycin or linezolid for CAP due to CA-MRSA, and these are the most widely used drugs for invasive CA-MRSA infections. Linezolid has been recognized as having a theoretical advantage in treating pneumonia, as it achieves higher lung epithelial concentrations than vancomycin.6 Particularly in cases of necrotizing CA-MRSA pneumonia, vancomycin has not been found to decrease toxin production, and linezolid is therefore the preferred treatment.1 Because of the limitations of vancomycin, we chose linezolid for our patient.
Treatment duration is not evidence-based
Although most patients with CAP are treated for 7 to 10 days, there are few well-designed studies evaluating the optimal time frame. Guidelines for the duration of antibiotics for CAP recommend a minimum of 5 days of therapy, presuming the patient is clinically stable.1,7 Short-duration therapy for patients with bacteremic S aureus CAP infection has been associated with an increased risk of endocarditis, and the presence of pulmonary cavities may warrant prolonged therapy.1 Our patient had an isolate of S aureus with intermediate susceptibility to moxifloxacin, which likely explains her rapid response to linezolid. She remained on linezolid for 8 days.
• Consider CA-MRSA as a cause of CAP, especially in cases in which the presentation is severe, as the guidelines for empiric treatment of CAP feature antibiotics that are not active against the CA-MRSA pathogen.
• Order sputum cultures for all patients admitted to the ICU, as well as for patients with a clinical presentation suggestive of CAP who abuse alcohol, have severe asthma or other lung disease, or have a pleural effusion.
• Start patients with CA-MRSA CAP on linezolid without delay.
The author reported no potential conflict of interest relevant to this article.
1. Mandell LA, Wunderink RG, Anzueto A, et al. IDSA/ATS Guidelines for CAP in adults. Clin Infect Dis. 2007;44(suppl 2):S27-S72.
2. Hidron AI, Low CE, Honig EG, et al. Emergence of community-acquired methicillin-resistant Staphylococcus aureus strain USA300 as a cause of necrotizing community-onset pneumonia. Lancet Infect Dis. 2009;9:384-392.
3. Torell E, Molin D, Tano E, et al. CAP and bacteraemia in a healthy young woman caused by methicillin-resistant Staphylococcus aureus (MRSA) carrying the genes encoding Panton-Valentine leukocidin (PVL). Scandinavian J Inf Dis. 2005;37:902-904.
4. Soderquist B, Berglund C, Stralin K. CAP and bacteremia caused by an unusual methicillin-resistant Staphylococcus aureus (MRSA) strain with sequence type 36, staphylococcal cassette chromosome mec type IV and Panton-Valentine leukocidin genes. Eur J Clin Microbiol Infec Dis. 2006;25:604-606.
5. Mizell KN, Patterson KV, Carter JE. Empyema necessitatis due to methicillin-resistant Staphylococcus aureus: case report and review of the literature. J Clin Microbiol. 2008;46:3534-3536.
6. Dunbar LM, Wunderink RG, Habib MP, et al. High-dose, short-course levofloxacin for CAP: a new treatment paradigm. Clin Infect Dis. 2003;37:752-760.
7. Martino JL, McMillian WD, Polish LB, et al. Community-acquired methicillin-resistant Staphylococcus aureus pneumonia. Resp Med. 2008;102:932-943.
CORRESPONDENCE Jeffrey D. Schlaudecker, MD, The Christ Hospital/University of Cincinnati, Family Medicine Residency, 2123 Auburn Avenue, Suite 340, Cincinnati, OH 45219; schlaj@fammed.uc.edu
CASE: A 26-year-old woman came to our emergency department with shortness of breath—a symptom that had begun 2 weeks earlier and was getting steadily worse. She had a history of severe, persistent asthma, and had been admitted to the ICU—but not intubated—9 months earlier. Now, she reported dyspnea with even mild exertion, which inhalers failed to relieve.
The patient also had a cough and had recently begun producing thick, green sputum, but said she’d had no chest pain, fever, or lower extremity swelling. The young woman lived with her parents, both of whom smoked heavily, but denied tobacco or alcohol use herself. She had not taken antibiotics in the past 6 months.
On physical examination, the patient was afebrile, mildly tachypneic, and had a heart rate of 125 bpm. She was saturating 80% on ambient air and 95% on 2 liters per minute via nasal cannula; her lung exam was significant for diffuse wheezes and rhonchi, but the remainder of the physical exam was normal.
Lab tests revealed a white blood cell count of 25,200/mcL, with 81% neutrophils. Blood and urine cultures were preliminarily negative. We sent a sputum sample to be cultured and took chest x-rays. Posterior-anterior (PA) (FIGURE 1) and lateral (FIGURE 2) radiographs showed a dense right lower lobe inflltrate.
We diagnosed community-acquired pneumonia (CAP), coupled with an acute exacerbation of asthma, and admitted the patient to the hospital. We started her on intravenous (IV) moxifloxacin, parenteral steroids, and nebulized albuterol and ipratropium. But by the patient’s third day in the hospital, her white blood cell count had climbed to 34,500/mcL, and she still required oxygen by nasal cannula.
FIGURE 1 PA x-ray shows right lower lobe infiltrate
FIGURE 2 Dense infiltrate on lateral view
WHY DIDN’T THIS PATIENT RESPOND TO DRUG THERAPY?
CAP complicated by CA-MRSA
Initially, this appeared to be a rather straightforward case of CAP and asthma. The return of the sputum cultures on day 4, showing positive results for methicillin-resistant Staphylococcus aureus (MRSA), indicated that this was not the case. Sensitivity analysis revealed intermediate susceptibility to moxifloxacin and sensitivity to trimethoprim-sulfamethoxazole (TMP-SMX), vancomycin, and linezolid.
We switched the patient’s antibiotic from moxifloxacin to parenteral linezolid. She responded rapidly. On day 5, the patient was successfully weaned from O2 and converted to oral linezolid; the following day, she was discharged, with orders to complete an 8-day course of linezolid and an appointment with her family physician for the following week. At follow-up, she was doing well.
Cultures are crucial if results would alter Tx
In 2007, the Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS) published guidelines on the management of CAP1—a clinical diagnosis based on the presence of select features, including cough, fever, sputum production, and pleuritic chest pain. (Imaging of the chest may be used to support the diagnosis, but is not required.)
Following a site-of-care decision, ideally made with the help of a severity-of-illness scoring system such as the CURB-65 (Confusion, Urea nitrogen, Respiratory rate, Blood pressure, 65 years of age and older) or Pneumonia Severity Index, the guidelines recommend cultures whenever the result is likely to significantly alter standard empiric treatment.1
Pretreatment blood samples for culture should be obtained (strength of recommendation: A, well-conducted randomized controlled trials). The IDSA/ATS CAP guidelines also recommend an expectorated sputum sample for stain and culture for patients admitted to the ICU, as well as for those with a presentation suggestive of CAP who:
- have failed outpatient treatment;
- abuse alcohol;
- have severe structural or obstructive lung disease;
- or have pleural effusion.1
In our patient’s case, we obtained a sputum culture on admission because of her severe asthma. She was persistently hypoxic during her initial days in the hospital, and when the sputum culture returned positive for MRSA, her regimen of antibiotics was adjusted.
Suspect CA-MRSA when CAP is severe
Although MRSA has traditionally been thought of as a nosocomial pathogen, infections caused by distinct CA-MRSA strains are on the rise. An estimated 1% to 10% of CAP cases are caused by S aureus, but the percentage of those strains that are resistant to methicillin is not known.2
Clinical risk factors for S aureus CAP include end-stage renal disease, injection drug use, prior influenza, and prior antibiotic therapy, especially with fluoroquinolones.1 How-ever, CA-MRSA CAP often affects children and healthy young adults without any risk factors.3,4
Additionally, CAP that is caused by CA-MRSA is typically severe, often involving empyema, acute respiratory distress syndrome (ARDS), cavitary pneumonia, or severe sepsis.5 Consider a diagnosis of CA-MRSA CAP in patients who present with cavitary infiltrates without risk factors for anaerobic aspiration, such as syncope, seizures, alcohol abuse, or esophageal motility disorders.1
Start treatment without delay in patients at risk
Standard empiric treatment options for hospitalized patients with CAP (with the exception of those in the ICU) include either a beta-lactam and azithromycin or a respiratory fluoroquinolone (neither of which is active against MRSA).1 All patients who are hospitalized for CAP should be started on this regimen without delay. If gram-positive cocci in clusters suggestive of S aureus are isolated from sputum, this standard empiric regimen should be adequate until further microbiologic identification is available.1 While most cases of S aureus CAP are not MRSA, the increased mortality rate associated with inappropriate antibiotic selection led the IDSA/ATS to recommend broader empiric coverage for MRSA for patients with risk factors for S aureus.1
CA-MRSA strains are distinct from their nosocomial counterpart, and the optimal therapy for confirmed CA-MRSA CAP has not been determined. Frequently, CA-MRSA isolates are sensitive to TMP-SMX, clindamycin, and fluoroquinolones. But neither fluoroquinolones nor TMP-SMX have a clear effect on toxin production, and bacterial resistance frequently emerges during therapy with clindamycin.1
The IDSA/ATS guidelines recommend either vancomycin or linezolid for CAP due to CA-MRSA, and these are the most widely used drugs for invasive CA-MRSA infections. Linezolid has been recognized as having a theoretical advantage in treating pneumonia, as it achieves higher lung epithelial concentrations than vancomycin.6 Particularly in cases of necrotizing CA-MRSA pneumonia, vancomycin has not been found to decrease toxin production, and linezolid is therefore the preferred treatment.1 Because of the limitations of vancomycin, we chose linezolid for our patient.
Treatment duration is not evidence-based
Although most patients with CAP are treated for 7 to 10 days, there are few well-designed studies evaluating the optimal time frame. Guidelines for the duration of antibiotics for CAP recommend a minimum of 5 days of therapy, presuming the patient is clinically stable.1,7 Short-duration therapy for patients with bacteremic S aureus CAP infection has been associated with an increased risk of endocarditis, and the presence of pulmonary cavities may warrant prolonged therapy.1 Our patient had an isolate of S aureus with intermediate susceptibility to moxifloxacin, which likely explains her rapid response to linezolid. She remained on linezolid for 8 days.
• Consider CA-MRSA as a cause of CAP, especially in cases in which the presentation is severe, as the guidelines for empiric treatment of CAP feature antibiotics that are not active against the CA-MRSA pathogen.
• Order sputum cultures for all patients admitted to the ICU, as well as for patients with a clinical presentation suggestive of CAP who abuse alcohol, have severe asthma or other lung disease, or have a pleural effusion.
• Start patients with CA-MRSA CAP on linezolid without delay.
The author reported no potential conflict of interest relevant to this article.
CASE: A 26-year-old woman came to our emergency department with shortness of breath—a symptom that had begun 2 weeks earlier and was getting steadily worse. She had a history of severe, persistent asthma, and had been admitted to the ICU—but not intubated—9 months earlier. Now, she reported dyspnea with even mild exertion, which inhalers failed to relieve.
The patient also had a cough and had recently begun producing thick, green sputum, but said she’d had no chest pain, fever, or lower extremity swelling. The young woman lived with her parents, both of whom smoked heavily, but denied tobacco or alcohol use herself. She had not taken antibiotics in the past 6 months.
On physical examination, the patient was afebrile, mildly tachypneic, and had a heart rate of 125 bpm. She was saturating 80% on ambient air and 95% on 2 liters per minute via nasal cannula; her lung exam was significant for diffuse wheezes and rhonchi, but the remainder of the physical exam was normal.
Lab tests revealed a white blood cell count of 25,200/mcL, with 81% neutrophils. Blood and urine cultures were preliminarily negative. We sent a sputum sample to be cultured and took chest x-rays. Posterior-anterior (PA) (FIGURE 1) and lateral (FIGURE 2) radiographs showed a dense right lower lobe inflltrate.
We diagnosed community-acquired pneumonia (CAP), coupled with an acute exacerbation of asthma, and admitted the patient to the hospital. We started her on intravenous (IV) moxifloxacin, parenteral steroids, and nebulized albuterol and ipratropium. But by the patient’s third day in the hospital, her white blood cell count had climbed to 34,500/mcL, and she still required oxygen by nasal cannula.
FIGURE 1 PA x-ray shows right lower lobe infiltrate
FIGURE 2 Dense infiltrate on lateral view
WHY DIDN’T THIS PATIENT RESPOND TO DRUG THERAPY?
CAP complicated by CA-MRSA
Initially, this appeared to be a rather straightforward case of CAP and asthma. The return of the sputum cultures on day 4, showing positive results for methicillin-resistant Staphylococcus aureus (MRSA), indicated that this was not the case. Sensitivity analysis revealed intermediate susceptibility to moxifloxacin and sensitivity to trimethoprim-sulfamethoxazole (TMP-SMX), vancomycin, and linezolid.
We switched the patient’s antibiotic from moxifloxacin to parenteral linezolid. She responded rapidly. On day 5, the patient was successfully weaned from O2 and converted to oral linezolid; the following day, she was discharged, with orders to complete an 8-day course of linezolid and an appointment with her family physician for the following week. At follow-up, she was doing well.
Cultures are crucial if results would alter Tx
In 2007, the Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS) published guidelines on the management of CAP1—a clinical diagnosis based on the presence of select features, including cough, fever, sputum production, and pleuritic chest pain. (Imaging of the chest may be used to support the diagnosis, but is not required.)
Following a site-of-care decision, ideally made with the help of a severity-of-illness scoring system such as the CURB-65 (Confusion, Urea nitrogen, Respiratory rate, Blood pressure, 65 years of age and older) or Pneumonia Severity Index, the guidelines recommend cultures whenever the result is likely to significantly alter standard empiric treatment.1
Pretreatment blood samples for culture should be obtained (strength of recommendation: A, well-conducted randomized controlled trials). The IDSA/ATS CAP guidelines also recommend an expectorated sputum sample for stain and culture for patients admitted to the ICU, as well as for those with a presentation suggestive of CAP who:
- have failed outpatient treatment;
- abuse alcohol;
- have severe structural or obstructive lung disease;
- or have pleural effusion.1
In our patient’s case, we obtained a sputum culture on admission because of her severe asthma. She was persistently hypoxic during her initial days in the hospital, and when the sputum culture returned positive for MRSA, her regimen of antibiotics was adjusted.
Suspect CA-MRSA when CAP is severe
Although MRSA has traditionally been thought of as a nosocomial pathogen, infections caused by distinct CA-MRSA strains are on the rise. An estimated 1% to 10% of CAP cases are caused by S aureus, but the percentage of those strains that are resistant to methicillin is not known.2
Clinical risk factors for S aureus CAP include end-stage renal disease, injection drug use, prior influenza, and prior antibiotic therapy, especially with fluoroquinolones.1 How-ever, CA-MRSA CAP often affects children and healthy young adults without any risk factors.3,4
Additionally, CAP that is caused by CA-MRSA is typically severe, often involving empyema, acute respiratory distress syndrome (ARDS), cavitary pneumonia, or severe sepsis.5 Consider a diagnosis of CA-MRSA CAP in patients who present with cavitary infiltrates without risk factors for anaerobic aspiration, such as syncope, seizures, alcohol abuse, or esophageal motility disorders.1
Start treatment without delay in patients at risk
Standard empiric treatment options for hospitalized patients with CAP (with the exception of those in the ICU) include either a beta-lactam and azithromycin or a respiratory fluoroquinolone (neither of which is active against MRSA).1 All patients who are hospitalized for CAP should be started on this regimen without delay. If gram-positive cocci in clusters suggestive of S aureus are isolated from sputum, this standard empiric regimen should be adequate until further microbiologic identification is available.1 While most cases of S aureus CAP are not MRSA, the increased mortality rate associated with inappropriate antibiotic selection led the IDSA/ATS to recommend broader empiric coverage for MRSA for patients with risk factors for S aureus.1
CA-MRSA strains are distinct from their nosocomial counterpart, and the optimal therapy for confirmed CA-MRSA CAP has not been determined. Frequently, CA-MRSA isolates are sensitive to TMP-SMX, clindamycin, and fluoroquinolones. But neither fluoroquinolones nor TMP-SMX have a clear effect on toxin production, and bacterial resistance frequently emerges during therapy with clindamycin.1
The IDSA/ATS guidelines recommend either vancomycin or linezolid for CAP due to CA-MRSA, and these are the most widely used drugs for invasive CA-MRSA infections. Linezolid has been recognized as having a theoretical advantage in treating pneumonia, as it achieves higher lung epithelial concentrations than vancomycin.6 Particularly in cases of necrotizing CA-MRSA pneumonia, vancomycin has not been found to decrease toxin production, and linezolid is therefore the preferred treatment.1 Because of the limitations of vancomycin, we chose linezolid for our patient.
Treatment duration is not evidence-based
Although most patients with CAP are treated for 7 to 10 days, there are few well-designed studies evaluating the optimal time frame. Guidelines for the duration of antibiotics for CAP recommend a minimum of 5 days of therapy, presuming the patient is clinically stable.1,7 Short-duration therapy for patients with bacteremic S aureus CAP infection has been associated with an increased risk of endocarditis, and the presence of pulmonary cavities may warrant prolonged therapy.1 Our patient had an isolate of S aureus with intermediate susceptibility to moxifloxacin, which likely explains her rapid response to linezolid. She remained on linezolid for 8 days.
• Consider CA-MRSA as a cause of CAP, especially in cases in which the presentation is severe, as the guidelines for empiric treatment of CAP feature antibiotics that are not active against the CA-MRSA pathogen.
• Order sputum cultures for all patients admitted to the ICU, as well as for patients with a clinical presentation suggestive of CAP who abuse alcohol, have severe asthma or other lung disease, or have a pleural effusion.
• Start patients with CA-MRSA CAP on linezolid without delay.
The author reported no potential conflict of interest relevant to this article.
1. Mandell LA, Wunderink RG, Anzueto A, et al. IDSA/ATS Guidelines for CAP in adults. Clin Infect Dis. 2007;44(suppl 2):S27-S72.
2. Hidron AI, Low CE, Honig EG, et al. Emergence of community-acquired methicillin-resistant Staphylococcus aureus strain USA300 as a cause of necrotizing community-onset pneumonia. Lancet Infect Dis. 2009;9:384-392.
3. Torell E, Molin D, Tano E, et al. CAP and bacteraemia in a healthy young woman caused by methicillin-resistant Staphylococcus aureus (MRSA) carrying the genes encoding Panton-Valentine leukocidin (PVL). Scandinavian J Inf Dis. 2005;37:902-904.
4. Soderquist B, Berglund C, Stralin K. CAP and bacteremia caused by an unusual methicillin-resistant Staphylococcus aureus (MRSA) strain with sequence type 36, staphylococcal cassette chromosome mec type IV and Panton-Valentine leukocidin genes. Eur J Clin Microbiol Infec Dis. 2006;25:604-606.
5. Mizell KN, Patterson KV, Carter JE. Empyema necessitatis due to methicillin-resistant Staphylococcus aureus: case report and review of the literature. J Clin Microbiol. 2008;46:3534-3536.
6. Dunbar LM, Wunderink RG, Habib MP, et al. High-dose, short-course levofloxacin for CAP: a new treatment paradigm. Clin Infect Dis. 2003;37:752-760.
7. Martino JL, McMillian WD, Polish LB, et al. Community-acquired methicillin-resistant Staphylococcus aureus pneumonia. Resp Med. 2008;102:932-943.
CORRESPONDENCE Jeffrey D. Schlaudecker, MD, The Christ Hospital/University of Cincinnati, Family Medicine Residency, 2123 Auburn Avenue, Suite 340, Cincinnati, OH 45219; schlaj@fammed.uc.edu
1. Mandell LA, Wunderink RG, Anzueto A, et al. IDSA/ATS Guidelines for CAP in adults. Clin Infect Dis. 2007;44(suppl 2):S27-S72.
2. Hidron AI, Low CE, Honig EG, et al. Emergence of community-acquired methicillin-resistant Staphylococcus aureus strain USA300 as a cause of necrotizing community-onset pneumonia. Lancet Infect Dis. 2009;9:384-392.
3. Torell E, Molin D, Tano E, et al. CAP and bacteraemia in a healthy young woman caused by methicillin-resistant Staphylococcus aureus (MRSA) carrying the genes encoding Panton-Valentine leukocidin (PVL). Scandinavian J Inf Dis. 2005;37:902-904.
4. Soderquist B, Berglund C, Stralin K. CAP and bacteremia caused by an unusual methicillin-resistant Staphylococcus aureus (MRSA) strain with sequence type 36, staphylococcal cassette chromosome mec type IV and Panton-Valentine leukocidin genes. Eur J Clin Microbiol Infec Dis. 2006;25:604-606.
5. Mizell KN, Patterson KV, Carter JE. Empyema necessitatis due to methicillin-resistant Staphylococcus aureus: case report and review of the literature. J Clin Microbiol. 2008;46:3534-3536.
6. Dunbar LM, Wunderink RG, Habib MP, et al. High-dose, short-course levofloxacin for CAP: a new treatment paradigm. Clin Infect Dis. 2003;37:752-760.
7. Martino JL, McMillian WD, Polish LB, et al. Community-acquired methicillin-resistant Staphylococcus aureus pneumonia. Resp Med. 2008;102:932-943.
CORRESPONDENCE Jeffrey D. Schlaudecker, MD, The Christ Hospital/University of Cincinnati, Family Medicine Residency, 2123 Auburn Avenue, Suite 340, Cincinnati, OH 45219; schlaj@fammed.uc.edu