User login
CHICAGO – Driving LDL cholesterol below 25 mg/dL for a year or more in high-cardiovascular-risk patients using the investigational human monoclonal antibody alirocumab proved well tolerated and displayed a treatment-emergent adverse event profile reassuringly comparable to that of placebo in an interim analysis of the largest double-blind phase III clinical trial to date involving any PCSK9 inhibitor.
While this is encouraging preliminary evidence that it may prove safe to pharmacologically maintain unnaturally low LDL concentrations – levels below those seen in newborns – in high-risk patients, Dr. Jennifer G. Robinson nonetheless urged her cardiology colleagues to curb their enthusiasm as she presented an interim analysis of the 2,341-patient ODYSSEY Long Term trial at the American Heart Association scientific sessions.
“We’re all concerned about the long-term safety of very low LDL levels. The fact that there are no signals so far is good, but it’s only been 52 weeks. In my own mind, we’re treating people with very-high-risk cardiovascular disease who are older and have comorbidities, and I think we just have to establish that there really aren’t any adverse effects of prolonged low levels of LDL,” said Dr. Robinson, professor of medicine at the University of Iowa in Iowa City.
Session cochair Dr. David C. Goff applauded her cautionary stance.
“The 1-year data on safety are promising, although these are still relatively small numbers of patients who have been exposed to the medication for at least a year. So rare adverse events will still be difficult to detect in just a couple thousand people treated for that length of time,” observed Dr. Goff, a cardiovascular epidemiologist and dean of the University of Colorado School of Public Health, Denver.
He was particularly intrigued by a different part of her presentation, a new post hoc pooled analysis of hard cardiovascular outcomes in ODYSSEY Long Term and two other ongoing, phase III, placebo-controlled alirocumab trials totaling 3,459 high-risk patients with follow-up of 52 weeks or more. In this Cox regression analysis, the relative risk of the composite endpoint of adjudicated death from coronary heart disease, nonfatal myocardial infarction, fatal or nonfatal ischemic stroke, or unstable angina requiring hospitalization was 35% lower in alirocumab- than placebo-treated patients. While this hefty-looking difference in risk didn’t achieve statistical significance a mere year into follow-up, the fact that the two event curves diverged from the very beginning of follow-up and the separation continues to expand bodes well indeed.
“Dr. Robinson has shown us a first glimpse of a promise of cardiovascular disease prevention from the pooled data. I think we’re all now looking forward to the results of the ongoing ODYSSEY Outcomes trial, with 18,000 randomized ACS [acute coronary syndrome] patients being followed for 5-6 years, which will provide us with much better evidence with regard to safety and cardiovascular event prevention. But so far so good, from what I’ve seen here today,” Dr. Goff commented.
ODYSSEY Long Term included 2,341 patients with high cardiovascular risk, either because of known coronary heart disease or, in the case of 415 subjects, heterozygous familial hypercholesterolemia (FH). At entry, participants were on maximum tolerated statin therapy, often accompanied by additional lipid-lowering agents, yet their mean baseline LDL was 122 mg/dL. While remaining on their maximum tolerated statin and other LDL-lowering drugs throughout the trial, they were randomized 2:1 to 150 mg of alirocumab by self-administered subcutaneous injection every 2 weeks or placebo. Alirocumab is a human monoclonal antibody designed to inhibit PCSK9 (proprotein convertase subtilisin/kexin type 9), an enzyme that helps govern cholesterol homeostasis.
Seventy-nine percent of alirocumab-treated patients achieved an LDL below 70 mg/dL at week 24. At week 52, mean LDL in the alirocumab group was 53 mg/dL, compared with 123 mg/dL in the control group.
The potential for adverse neurocognitive effects with very low achieved LDL levels has been of particular concern with regard to the PCSK9 inhibitors. The issue is quite relevant because patients on alirocumab commonly develop LDL levels below 25 mg/dL. In ODYSSEY Long Term, for example, 36% of alirocumab-treated patients did so. Lipidologists have been eager to learn more about the impact of maintaining patients in this state of subphysiologic LDL, and Dr. Robinson’s data provided the first meaningful opportunity to do so.
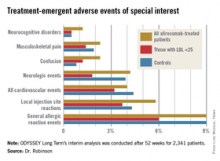
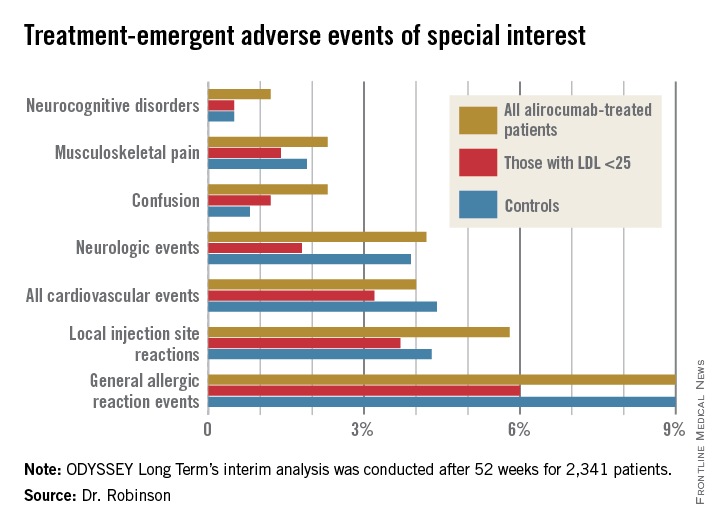
Reassuringly, the rate of treatment-emergent neurocognitive disorders was 1.2% in the total alirocumab-treated population, 0.5% in those with an on-treatment LDL below 25 mg/dL, and 0.5% in placebo-treated controls who, it must be remembered, were on maximum-tolerated therapy with statins and other conventional lipid-lowering agents. Other treatment-emergent adverse events – and more than 30 categories of such events were tracked – followed a similar pattern (see graphic).
There was one event of possible concern that has been reported to the Food and Drug Administration. An alirocumab-treated patient was diagnosed with Miller Fisher syndrome, a rare variant of Guillain-Barre syndrome, at week 27 after a prodromal severe gastroenteritis. At week 24, just 3 weeks earlier, the patient had an LDL of 1.5 mg/dL. The double vision and other symptoms of Miller Fisher syndrome resolved completely after treatment discontinuation.
During a panel discussion of the ODYSSEY clinical trial series, which includes 14 phase III studies, Dr. Henry N. Ginsburg predicted that further experience will eventually show that the dramatic LDL-lowering achieved with alirocumab not only doesn’t promote neurocognitive dysfunction, but actually protects against it.
“I think an important point to make is that, despite our neurology colleagues having tried for about a decade to completely separate the clinical phenotype of Alzheimer’s disease from vascular dementia, we all know that if you have both you’ll do much worse. So if we reduce cerebrovascular atherosclerosis and you have any predisposition to Alzheimer’s disease, you’ll do better. I think there will be studies to show that,” said Dr. Ginsburg, professor of medicine and director of the Irving Institute for Clinical and Translational Research at Columbia University in New York, who earlier in the session presented the findings from the phase III ODYSSEY High FH trial.
Another potential safety concern emerged during Dr. Dean J. Kereiakes’ presentation of the results of the phase III ODYSSEY Combo I study. Thirteen of 199 alirocumab-treated patients (6.6%) developed treatment-emergent, low-titer antialirocumab antibodies. The median time to their detection was 12 weeks. Four of the 13 patients had neutralizing antibodies, and all 4 became antibody negative by 24 weeks.
The presence of these antidrug antibodies had no discernible effect on treatment safety or efficacy. However, Combo I was a 52-week study. Whether the prevalence of these antibodies will increase over time and interfere with what is expected to be lifelong treatment is an unanswered question, noted Dr. Kereiakes, medical director for the Christ Hospital Heart and Vascular Center, Cincinnati.
Discussant Dr. Joshua W. Knowles, medical director of the FH Foundation, commented that “it’s really incredible to see the reductions in LDL that we’re getting with PCSK9 inhibitors in FH patients, the vast majority of whom require more than two standard medications to get to optimal LDL cholesterol levels.”
“The PCSK9 inhibitors are really the poster child for drug discovery in the modern genetic era. It has been a beautiful 10-year journey for them,” declared Dr. Knowles, a cardiologist at Stanford (Calif.) University.
In light of the powerful LDL-lowering effect of these agents, it’s not too soon to start thinking about how to carry out studies of atherosclerotic plaque stabilization and/or regression in treated patients, he added.
Other PCSK9 inhibitors in phase III clinical trials, besides alirocumab, are Amgen’s evolocumab and Pfizer/Renat’s bococizumab. In addition, Eli Lilly’s LY3015014 is in phase II studies, as is Roche/Genentech’s RG7652.
The ODYSSEY studies are funded by Sanofi and Regeneron Pharmaceuticals. Dr. Robinson, Dr. Ginsburg, and Dr. Kereiakes reported serving as consultants to those and other pharmaceutical companies.
CHICAGO – Driving LDL cholesterol below 25 mg/dL for a year or more in high-cardiovascular-risk patients using the investigational human monoclonal antibody alirocumab proved well tolerated and displayed a treatment-emergent adverse event profile reassuringly comparable to that of placebo in an interim analysis of the largest double-blind phase III clinical trial to date involving any PCSK9 inhibitor.
While this is encouraging preliminary evidence that it may prove safe to pharmacologically maintain unnaturally low LDL concentrations – levels below those seen in newborns – in high-risk patients, Dr. Jennifer G. Robinson nonetheless urged her cardiology colleagues to curb their enthusiasm as she presented an interim analysis of the 2,341-patient ODYSSEY Long Term trial at the American Heart Association scientific sessions.
“We’re all concerned about the long-term safety of very low LDL levels. The fact that there are no signals so far is good, but it’s only been 52 weeks. In my own mind, we’re treating people with very-high-risk cardiovascular disease who are older and have comorbidities, and I think we just have to establish that there really aren’t any adverse effects of prolonged low levels of LDL,” said Dr. Robinson, professor of medicine at the University of Iowa in Iowa City.
Session cochair Dr. David C. Goff applauded her cautionary stance.
“The 1-year data on safety are promising, although these are still relatively small numbers of patients who have been exposed to the medication for at least a year. So rare adverse events will still be difficult to detect in just a couple thousand people treated for that length of time,” observed Dr. Goff, a cardiovascular epidemiologist and dean of the University of Colorado School of Public Health, Denver.
He was particularly intrigued by a different part of her presentation, a new post hoc pooled analysis of hard cardiovascular outcomes in ODYSSEY Long Term and two other ongoing, phase III, placebo-controlled alirocumab trials totaling 3,459 high-risk patients with follow-up of 52 weeks or more. In this Cox regression analysis, the relative risk of the composite endpoint of adjudicated death from coronary heart disease, nonfatal myocardial infarction, fatal or nonfatal ischemic stroke, or unstable angina requiring hospitalization was 35% lower in alirocumab- than placebo-treated patients. While this hefty-looking difference in risk didn’t achieve statistical significance a mere year into follow-up, the fact that the two event curves diverged from the very beginning of follow-up and the separation continues to expand bodes well indeed.
“Dr. Robinson has shown us a first glimpse of a promise of cardiovascular disease prevention from the pooled data. I think we’re all now looking forward to the results of the ongoing ODYSSEY Outcomes trial, with 18,000 randomized ACS [acute coronary syndrome] patients being followed for 5-6 years, which will provide us with much better evidence with regard to safety and cardiovascular event prevention. But so far so good, from what I’ve seen here today,” Dr. Goff commented.
ODYSSEY Long Term included 2,341 patients with high cardiovascular risk, either because of known coronary heart disease or, in the case of 415 subjects, heterozygous familial hypercholesterolemia (FH). At entry, participants were on maximum tolerated statin therapy, often accompanied by additional lipid-lowering agents, yet their mean baseline LDL was 122 mg/dL. While remaining on their maximum tolerated statin and other LDL-lowering drugs throughout the trial, they were randomized 2:1 to 150 mg of alirocumab by self-administered subcutaneous injection every 2 weeks or placebo. Alirocumab is a human monoclonal antibody designed to inhibit PCSK9 (proprotein convertase subtilisin/kexin type 9), an enzyme that helps govern cholesterol homeostasis.
Seventy-nine percent of alirocumab-treated patients achieved an LDL below 70 mg/dL at week 24. At week 52, mean LDL in the alirocumab group was 53 mg/dL, compared with 123 mg/dL in the control group.
The potential for adverse neurocognitive effects with very low achieved LDL levels has been of particular concern with regard to the PCSK9 inhibitors. The issue is quite relevant because patients on alirocumab commonly develop LDL levels below 25 mg/dL. In ODYSSEY Long Term, for example, 36% of alirocumab-treated patients did so. Lipidologists have been eager to learn more about the impact of maintaining patients in this state of subphysiologic LDL, and Dr. Robinson’s data provided the first meaningful opportunity to do so.
Reassuringly, the rate of treatment-emergent neurocognitive disorders was 1.2% in the total alirocumab-treated population, 0.5% in those with an on-treatment LDL below 25 mg/dL, and 0.5% in placebo-treated controls who, it must be remembered, were on maximum-tolerated therapy with statins and other conventional lipid-lowering agents. Other treatment-emergent adverse events – and more than 30 categories of such events were tracked – followed a similar pattern (see graphic).
There was one event of possible concern that has been reported to the Food and Drug Administration. An alirocumab-treated patient was diagnosed with Miller Fisher syndrome, a rare variant of Guillain-Barre syndrome, at week 27 after a prodromal severe gastroenteritis. At week 24, just 3 weeks earlier, the patient had an LDL of 1.5 mg/dL. The double vision and other symptoms of Miller Fisher syndrome resolved completely after treatment discontinuation.
During a panel discussion of the ODYSSEY clinical trial series, which includes 14 phase III studies, Dr. Henry N. Ginsburg predicted that further experience will eventually show that the dramatic LDL-lowering achieved with alirocumab not only doesn’t promote neurocognitive dysfunction, but actually protects against it.
“I think an important point to make is that, despite our neurology colleagues having tried for about a decade to completely separate the clinical phenotype of Alzheimer’s disease from vascular dementia, we all know that if you have both you’ll do much worse. So if we reduce cerebrovascular atherosclerosis and you have any predisposition to Alzheimer’s disease, you’ll do better. I think there will be studies to show that,” said Dr. Ginsburg, professor of medicine and director of the Irving Institute for Clinical and Translational Research at Columbia University in New York, who earlier in the session presented the findings from the phase III ODYSSEY High FH trial.
Another potential safety concern emerged during Dr. Dean J. Kereiakes’ presentation of the results of the phase III ODYSSEY Combo I study. Thirteen of 199 alirocumab-treated patients (6.6%) developed treatment-emergent, low-titer antialirocumab antibodies. The median time to their detection was 12 weeks. Four of the 13 patients had neutralizing antibodies, and all 4 became antibody negative by 24 weeks.
The presence of these antidrug antibodies had no discernible effect on treatment safety or efficacy. However, Combo I was a 52-week study. Whether the prevalence of these antibodies will increase over time and interfere with what is expected to be lifelong treatment is an unanswered question, noted Dr. Kereiakes, medical director for the Christ Hospital Heart and Vascular Center, Cincinnati.
Discussant Dr. Joshua W. Knowles, medical director of the FH Foundation, commented that “it’s really incredible to see the reductions in LDL that we’re getting with PCSK9 inhibitors in FH patients, the vast majority of whom require more than two standard medications to get to optimal LDL cholesterol levels.”
“The PCSK9 inhibitors are really the poster child for drug discovery in the modern genetic era. It has been a beautiful 10-year journey for them,” declared Dr. Knowles, a cardiologist at Stanford (Calif.) University.
In light of the powerful LDL-lowering effect of these agents, it’s not too soon to start thinking about how to carry out studies of atherosclerotic plaque stabilization and/or regression in treated patients, he added.
Other PCSK9 inhibitors in phase III clinical trials, besides alirocumab, are Amgen’s evolocumab and Pfizer/Renat’s bococizumab. In addition, Eli Lilly’s LY3015014 is in phase II studies, as is Roche/Genentech’s RG7652.
The ODYSSEY studies are funded by Sanofi and Regeneron Pharmaceuticals. Dr. Robinson, Dr. Ginsburg, and Dr. Kereiakes reported serving as consultants to those and other pharmaceutical companies.
CHICAGO – Driving LDL cholesterol below 25 mg/dL for a year or more in high-cardiovascular-risk patients using the investigational human monoclonal antibody alirocumab proved well tolerated and displayed a treatment-emergent adverse event profile reassuringly comparable to that of placebo in an interim analysis of the largest double-blind phase III clinical trial to date involving any PCSK9 inhibitor.
While this is encouraging preliminary evidence that it may prove safe to pharmacologically maintain unnaturally low LDL concentrations – levels below those seen in newborns – in high-risk patients, Dr. Jennifer G. Robinson nonetheless urged her cardiology colleagues to curb their enthusiasm as she presented an interim analysis of the 2,341-patient ODYSSEY Long Term trial at the American Heart Association scientific sessions.
“We’re all concerned about the long-term safety of very low LDL levels. The fact that there are no signals so far is good, but it’s only been 52 weeks. In my own mind, we’re treating people with very-high-risk cardiovascular disease who are older and have comorbidities, and I think we just have to establish that there really aren’t any adverse effects of prolonged low levels of LDL,” said Dr. Robinson, professor of medicine at the University of Iowa in Iowa City.
Session cochair Dr. David C. Goff applauded her cautionary stance.
“The 1-year data on safety are promising, although these are still relatively small numbers of patients who have been exposed to the medication for at least a year. So rare adverse events will still be difficult to detect in just a couple thousand people treated for that length of time,” observed Dr. Goff, a cardiovascular epidemiologist and dean of the University of Colorado School of Public Health, Denver.
He was particularly intrigued by a different part of her presentation, a new post hoc pooled analysis of hard cardiovascular outcomes in ODYSSEY Long Term and two other ongoing, phase III, placebo-controlled alirocumab trials totaling 3,459 high-risk patients with follow-up of 52 weeks or more. In this Cox regression analysis, the relative risk of the composite endpoint of adjudicated death from coronary heart disease, nonfatal myocardial infarction, fatal or nonfatal ischemic stroke, or unstable angina requiring hospitalization was 35% lower in alirocumab- than placebo-treated patients. While this hefty-looking difference in risk didn’t achieve statistical significance a mere year into follow-up, the fact that the two event curves diverged from the very beginning of follow-up and the separation continues to expand bodes well indeed.
“Dr. Robinson has shown us a first glimpse of a promise of cardiovascular disease prevention from the pooled data. I think we’re all now looking forward to the results of the ongoing ODYSSEY Outcomes trial, with 18,000 randomized ACS [acute coronary syndrome] patients being followed for 5-6 years, which will provide us with much better evidence with regard to safety and cardiovascular event prevention. But so far so good, from what I’ve seen here today,” Dr. Goff commented.
ODYSSEY Long Term included 2,341 patients with high cardiovascular risk, either because of known coronary heart disease or, in the case of 415 subjects, heterozygous familial hypercholesterolemia (FH). At entry, participants were on maximum tolerated statin therapy, often accompanied by additional lipid-lowering agents, yet their mean baseline LDL was 122 mg/dL. While remaining on their maximum tolerated statin and other LDL-lowering drugs throughout the trial, they were randomized 2:1 to 150 mg of alirocumab by self-administered subcutaneous injection every 2 weeks or placebo. Alirocumab is a human monoclonal antibody designed to inhibit PCSK9 (proprotein convertase subtilisin/kexin type 9), an enzyme that helps govern cholesterol homeostasis.
Seventy-nine percent of alirocumab-treated patients achieved an LDL below 70 mg/dL at week 24. At week 52, mean LDL in the alirocumab group was 53 mg/dL, compared with 123 mg/dL in the control group.
The potential for adverse neurocognitive effects with very low achieved LDL levels has been of particular concern with regard to the PCSK9 inhibitors. The issue is quite relevant because patients on alirocumab commonly develop LDL levels below 25 mg/dL. In ODYSSEY Long Term, for example, 36% of alirocumab-treated patients did so. Lipidologists have been eager to learn more about the impact of maintaining patients in this state of subphysiologic LDL, and Dr. Robinson’s data provided the first meaningful opportunity to do so.
Reassuringly, the rate of treatment-emergent neurocognitive disorders was 1.2% in the total alirocumab-treated population, 0.5% in those with an on-treatment LDL below 25 mg/dL, and 0.5% in placebo-treated controls who, it must be remembered, were on maximum-tolerated therapy with statins and other conventional lipid-lowering agents. Other treatment-emergent adverse events – and more than 30 categories of such events were tracked – followed a similar pattern (see graphic).
There was one event of possible concern that has been reported to the Food and Drug Administration. An alirocumab-treated patient was diagnosed with Miller Fisher syndrome, a rare variant of Guillain-Barre syndrome, at week 27 after a prodromal severe gastroenteritis. At week 24, just 3 weeks earlier, the patient had an LDL of 1.5 mg/dL. The double vision and other symptoms of Miller Fisher syndrome resolved completely after treatment discontinuation.
During a panel discussion of the ODYSSEY clinical trial series, which includes 14 phase III studies, Dr. Henry N. Ginsburg predicted that further experience will eventually show that the dramatic LDL-lowering achieved with alirocumab not only doesn’t promote neurocognitive dysfunction, but actually protects against it.
“I think an important point to make is that, despite our neurology colleagues having tried for about a decade to completely separate the clinical phenotype of Alzheimer’s disease from vascular dementia, we all know that if you have both you’ll do much worse. So if we reduce cerebrovascular atherosclerosis and you have any predisposition to Alzheimer’s disease, you’ll do better. I think there will be studies to show that,” said Dr. Ginsburg, professor of medicine and director of the Irving Institute for Clinical and Translational Research at Columbia University in New York, who earlier in the session presented the findings from the phase III ODYSSEY High FH trial.
Another potential safety concern emerged during Dr. Dean J. Kereiakes’ presentation of the results of the phase III ODYSSEY Combo I study. Thirteen of 199 alirocumab-treated patients (6.6%) developed treatment-emergent, low-titer antialirocumab antibodies. The median time to their detection was 12 weeks. Four of the 13 patients had neutralizing antibodies, and all 4 became antibody negative by 24 weeks.
The presence of these antidrug antibodies had no discernible effect on treatment safety or efficacy. However, Combo I was a 52-week study. Whether the prevalence of these antibodies will increase over time and interfere with what is expected to be lifelong treatment is an unanswered question, noted Dr. Kereiakes, medical director for the Christ Hospital Heart and Vascular Center, Cincinnati.
Discussant Dr. Joshua W. Knowles, medical director of the FH Foundation, commented that “it’s really incredible to see the reductions in LDL that we’re getting with PCSK9 inhibitors in FH patients, the vast majority of whom require more than two standard medications to get to optimal LDL cholesterol levels.”
“The PCSK9 inhibitors are really the poster child for drug discovery in the modern genetic era. It has been a beautiful 10-year journey for them,” declared Dr. Knowles, a cardiologist at Stanford (Calif.) University.
In light of the powerful LDL-lowering effect of these agents, it’s not too soon to start thinking about how to carry out studies of atherosclerotic plaque stabilization and/or regression in treated patients, he added.
Other PCSK9 inhibitors in phase III clinical trials, besides alirocumab, are Amgen’s evolocumab and Pfizer/Renat’s bococizumab. In addition, Eli Lilly’s LY3015014 is in phase II studies, as is Roche/Genentech’s RG7652.
The ODYSSEY studies are funded by Sanofi and Regeneron Pharmaceuticals. Dr. Robinson, Dr. Ginsburg, and Dr. Kereiakes reported serving as consultants to those and other pharmaceutical companies.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: LDL cholesterol levels below 25 mg/dL induced by a superpotent investigational lipid-lowering agent appear to be safe through year 1 of follow-up.
Major finding: Thirty-six percent of high-cardiovascular-risk patients with a mean baseline LDL of 122 mg/dL despite maximum tolerated statin therapy responded to alirocumab with an LDL below 25 mg/dL, with treatment-emergent adverse events similar to those of placebo.
Data source: A prespecified interim analysis at week 52 of the ongoing double-blind, phase III ODYSSEY Long Term study, involving 2,341 high-cardiovascular-risk patients randomized 2:1 to self-administered subcutaneous injections of alirocumab at 150 mg or placebo every 2 weeks.
Disclosures: The study is sponsored by Sanofi and Regeneron Pharmaceuticals. Dr. Robinson reported serving as a consultant to those and other pharmaceutical companies.