User login
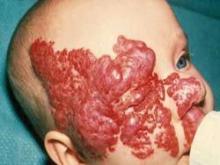
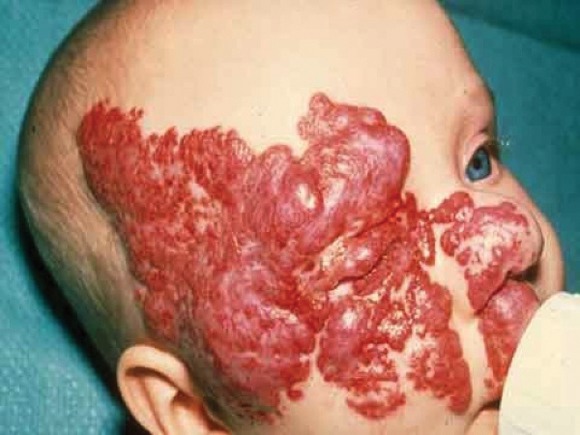
MIAMI BEACH – Approximately 60% of infants with proliferating hemangiomas showed significant improvement after treatment with oral propranolol, compared with a placebo, in a randomized, double-blind adaptive phase 2/3 study.
After 24 weeks, 61 of 101 patients (60.4%) treated daily with 3 mg/kg of propranolol had complete or nearly complete resolution of the hemangiomas, compared with only 2 of 55 patients (3.6%) who received a placebo, Dr. Christine Léauté-Labrèze of University Hospital of Bordeaux (France) reported at the annual meeting of the American Academy of Dermatology.
The complete or nearly complete resolution of hemangiomas was assessed by blinded independent investigators, based on a comparison of baseline photographs of the target lesions and photographs taken at week 24.
Patients in the international, multicenter study included infants aged 1-5 months with proliferating infantile hemangiomas requiring systemic therapy. Initially, 456 infants from 60 sites in 15 countries were randomized to receive one of four treatment regimens with oral propranolol: 1 or 3 mg/kg per day for either 3 or 6 months. After an interim efficacy and safety analysis based on the first 188 patients, only the treatment group receiving 3 mg/kg per day for 6 months was retained for trial completion.
The outcomes in both 3-month treatment arms were similar to those in the placebo group. However, the outcomes in the patients who received 3 mg/kg per day for 6 months were better – with no evidence of an increased risk of adverse events – than those in the patients who received 1 mg/kg per day for 6 months, reported Dr. Léauté-Labrèze, principal investigator in the study.
Of note, about half of the placebo patients dropped out of the study after 1 month because of nonefficacy; at the end of the study only about a third of the babies remained in the placebo arm. Similarly, in both of the 3-month treatment arms, dropout rates were high when patients switched from active treatment to placebo. However, approximately 80% of the patients in the group receiving 3 mg/kg per day for 6 months completed the study.
Treatment was well tolerated, and no unexpected safety signals emerged during the course of the study.
The findings of "a highly significant efficacy level" in this randomized, controlled study are important, because 5% of all babies born in the United States are affected by infantile hemangiomas, Dr. Léauté-Labrèze said. Approximately 12% of those babies will likely have complications such as ulceration, impairment of vision, or risk of disfigurement; therefore, effective treatment is needed, she said.
Although several smaller studies and open-label case series involving propranolol have demonstrated efficacy, no treatment has yet received Food and Drug Administration approval for the treatment of infantile hemangiomas, she added.
The study was sponsored by Pierre Fabre Dermatologie.
MIAMI BEACH – Approximately 60% of infants with proliferating hemangiomas showed significant improvement after treatment with oral propranolol, compared with a placebo, in a randomized, double-blind adaptive phase 2/3 study.
After 24 weeks, 61 of 101 patients (60.4%) treated daily with 3 mg/kg of propranolol had complete or nearly complete resolution of the hemangiomas, compared with only 2 of 55 patients (3.6%) who received a placebo, Dr. Christine Léauté-Labrèze of University Hospital of Bordeaux (France) reported at the annual meeting of the American Academy of Dermatology.
The complete or nearly complete resolution of hemangiomas was assessed by blinded independent investigators, based on a comparison of baseline photographs of the target lesions and photographs taken at week 24.
Patients in the international, multicenter study included infants aged 1-5 months with proliferating infantile hemangiomas requiring systemic therapy. Initially, 456 infants from 60 sites in 15 countries were randomized to receive one of four treatment regimens with oral propranolol: 1 or 3 mg/kg per day for either 3 or 6 months. After an interim efficacy and safety analysis based on the first 188 patients, only the treatment group receiving 3 mg/kg per day for 6 months was retained for trial completion.
The outcomes in both 3-month treatment arms were similar to those in the placebo group. However, the outcomes in the patients who received 3 mg/kg per day for 6 months were better – with no evidence of an increased risk of adverse events – than those in the patients who received 1 mg/kg per day for 6 months, reported Dr. Léauté-Labrèze, principal investigator in the study.
Of note, about half of the placebo patients dropped out of the study after 1 month because of nonefficacy; at the end of the study only about a third of the babies remained in the placebo arm. Similarly, in both of the 3-month treatment arms, dropout rates were high when patients switched from active treatment to placebo. However, approximately 80% of the patients in the group receiving 3 mg/kg per day for 6 months completed the study.
Treatment was well tolerated, and no unexpected safety signals emerged during the course of the study.
The findings of "a highly significant efficacy level" in this randomized, controlled study are important, because 5% of all babies born in the United States are affected by infantile hemangiomas, Dr. Léauté-Labrèze said. Approximately 12% of those babies will likely have complications such as ulceration, impairment of vision, or risk of disfigurement; therefore, effective treatment is needed, she said.
Although several smaller studies and open-label case series involving propranolol have demonstrated efficacy, no treatment has yet received Food and Drug Administration approval for the treatment of infantile hemangiomas, she added.
The study was sponsored by Pierre Fabre Dermatologie.
MIAMI BEACH – Approximately 60% of infants with proliferating hemangiomas showed significant improvement after treatment with oral propranolol, compared with a placebo, in a randomized, double-blind adaptive phase 2/3 study.
After 24 weeks, 61 of 101 patients (60.4%) treated daily with 3 mg/kg of propranolol had complete or nearly complete resolution of the hemangiomas, compared with only 2 of 55 patients (3.6%) who received a placebo, Dr. Christine Léauté-Labrèze of University Hospital of Bordeaux (France) reported at the annual meeting of the American Academy of Dermatology.
The complete or nearly complete resolution of hemangiomas was assessed by blinded independent investigators, based on a comparison of baseline photographs of the target lesions and photographs taken at week 24.
Patients in the international, multicenter study included infants aged 1-5 months with proliferating infantile hemangiomas requiring systemic therapy. Initially, 456 infants from 60 sites in 15 countries were randomized to receive one of four treatment regimens with oral propranolol: 1 or 3 mg/kg per day for either 3 or 6 months. After an interim efficacy and safety analysis based on the first 188 patients, only the treatment group receiving 3 mg/kg per day for 6 months was retained for trial completion.
The outcomes in both 3-month treatment arms were similar to those in the placebo group. However, the outcomes in the patients who received 3 mg/kg per day for 6 months were better – with no evidence of an increased risk of adverse events – than those in the patients who received 1 mg/kg per day for 6 months, reported Dr. Léauté-Labrèze, principal investigator in the study.
Of note, about half of the placebo patients dropped out of the study after 1 month because of nonefficacy; at the end of the study only about a third of the babies remained in the placebo arm. Similarly, in both of the 3-month treatment arms, dropout rates were high when patients switched from active treatment to placebo. However, approximately 80% of the patients in the group receiving 3 mg/kg per day for 6 months completed the study.
Treatment was well tolerated, and no unexpected safety signals emerged during the course of the study.
The findings of "a highly significant efficacy level" in this randomized, controlled study are important, because 5% of all babies born in the United States are affected by infantile hemangiomas, Dr. Léauté-Labrèze said. Approximately 12% of those babies will likely have complications such as ulceration, impairment of vision, or risk of disfigurement; therefore, effective treatment is needed, she said.
Although several smaller studies and open-label case series involving propranolol have demonstrated efficacy, no treatment has yet received Food and Drug Administration approval for the treatment of infantile hemangiomas, she added.
The study was sponsored by Pierre Fabre Dermatologie.
AT THE AAD ANNUAL MEETING
Major finding: Among children treated with propranolol, 60% had complete or nearly complete resolution of hemangiomas after 24 weeks, compared with 4% of children treated with placebo.
Data source: A randomized, double-blind, placebo-controlled adaptive phase 2/3 study.
Disclosures: This study was sponsored by Pierre Fabre Dermatologie.