User login
Incidence and Mortality
By 2040, the number of women diagnosed with ovarian cancer annually worldwide is expected to increase by 100% in low Human Development Index (HDI) countries, and by 19-28% in high HDI countries.2 The causes of this increasing incidence are likely to be multifactorial, including both hereditary and modifiable risk factors.3 In addition to increasing population size, the growing prevalence of obesity, estrogen exposures, and nulliparity are particularly pertinent as potential causes of the rising incidence of ovarian cancer in younger women. The number of ovarian cancer-related deaths is also projected to rise from about 200,000 to nearly 314,000 annually, an increase of over 50% from 2020.2,4 Although outcomes in developed regions and nations continue to improve somewhat, 5-year survival rates range from 36% to 46%.5 These outcomes are nevertheless dismal when compared with 5-year survival rates from other cancer types, such as breast cancer, which are approaching 90%.6
Principal Histotypes
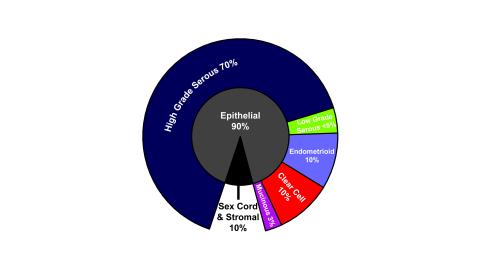
The principal histotypes in ovarian cancer are epithelial in origin and include high-grade serous carcinoma, clear-cell carcinoma, endometrioid carcinoma, low-grade serous carcinoma, and mucinous carcinoma. Other rarer types are nonepithelial, ie, arising from stromal or germ cell lines.7 Incidence rates appear to be affected over time by trends such as birth rates, use of combination oral contraceptives, and menopausal hormone therapy.8 Figure 1 shows that most ovarian cancers—approximately 70%—are high-grade serous carcinoma, although in Asian countries clear cell and endometrioid carcinomas comprise a higher proportion.9
Most ovarian cancers are epithelial carcinomas. High-grade serous carcinoma is the most common, whereas the other subtypes represent 10% or fewer cases each.9
Into the Fallopian Tube
One of the most salient and dramatic discoveries of the last 2 decades has been the finding that high-grade, clear-cell, and endometrioid tumors appear to arise from tissues not normally present in the ovary.1 As a result of risk-reducing efforts to prevent serous cancers in women with genetic predisposition to develop ovarian cancer (ie, those with BRCA1 or BRCA2 mutations), it became increasingly clear that many early cancers arose in the fallopian tube,10-12 with the distal portion—the fimbria—as the most common site of origin.13-16
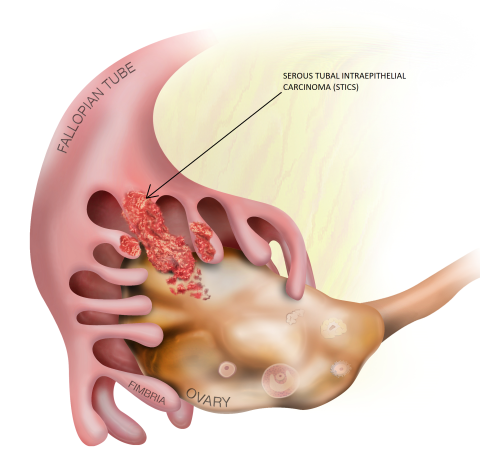
Figure 2 depicts the female reproductive tract, including the location of the fimbria compared with the ovaries. Moreover, lesions observed in the fallopian tube fimbria—serous tubal intraepithelial carcinomas (STICs)—were identified as precursors of ovarian cancer, with a window of 7 years between development of STIC and the beginning of an ovarian cancer.14,16
Lesions that develop in the fallopian tube fimbria, called serous tubal intraepithelial carcinomas (STICs), have been identified as precursors of ovarian cancer.
Early Detection
Early, localized ovarian cancer is asymptomatic; by the time a patient presents with symptoms, even with nonspecific abdominal complaints, the disease is almost invariably advanced. The concept of early detection has improved both the rate of cancer diagnoses and outcomes for some malignancies, such as cervical, colorectal, breast, and lung cancers,17 but this strategy is yet to be effectively applied in ovarian cancer. A large, population-based study, for instance, yielded negative results when multimodal screening (using both measurement of CA125 blood levels and transvaginal ultrasound imaging) failed to improve survival, even though such screening was able to detect lower stage disease.18 Emerging technologies, such as liquid biopsies and uterine lavage, which seek to detect potential biomarkers (new types of blood tests) of ovarian cancer at an early stage and closer to the site of tumor origin, are being investigated and refined but are not yet ready for clinical use, particularly at the population level for screening.19
Risk-Reducing Interventions
Use of oral contraceptives has been associated with a significant reduction in risk for ovarian cancer, but the potential risks (eg, increased risk for breast cancer, increased risk for venous thromboembolism) preclude its universal recommendation.20-22 Simple removal of the fallopian tube, salpingectomy, was proposed as a potential intervention to “intercept” the progression of a STIC to cancer. Researchers recently compared simple salpingectomy with salpingo-oophorectomy as a risk-reduction procedure in carriers of BRCA 1/2 pathogenic variants after they had completed childbearing.23
These investigators proposed that later removal of the ovaries would delay menopause and would contribute to fewer/less severe symptoms, such as hot flashes, disturbed sleep, and sexual issues, as well as maintain or improve overall quality of life. The hypothesis was supported by results, which showed that patients had better menopause-related quality of life after salpingectomy than after salpingo-oophorectomy, regardless of the use of hormone replacement therapy.23 The oncologic safety of this approach was subsequently demonstrated by other studies that showed a significantly lower incidence of ovarian cancers in women who had undergone opportunistic salpingectomy.22,24,25
An international prospective trial, TUBA-WISPII, is now underway to test the hypothesis that postponement of oophorectomy after salpingectomy is non-inferior to standard salpingo-oophorectomy in terms of ovarian cancer risk for patients at high risk.26
Treatment
First-Line Therapy
Currently, there are no durable curative therapies for ovarian cancer once advanced disease has been diagnosed.
Surgery plus platinum-based chemotherapy. Most patients, even those diagnosed with advanced disease, are treated initially with debulking surgery, ideally by a gynecologic oncologist, and adjuvant chemotherapy. Most ovarian carcinomas are initially platinum-sensitive, but resistance and disease recurrence are almost inevitable. According to the National Comprehensive Cancer Network (NCCN) guidelines for ovarian cancer,27 preferred chemotherapy regimens include paclitaxel and carboplatin with or without bevacizumab, docetaxel and carboplatin, or carboplatin and liposomal doxorubicin. Numerous other regimens, combinations, and agents are included in the guidelines to help providers customize treatment plans.
Neoadjuvant vs adjuvant regimens. Neoadjuvant chemotherapy has been used for other malignancies to gauge sensitivity to systemic treatments and to improve surgical margins.28 Thus far, though, outcomes in ovarian cancer have been similar whether patients were given neoadjuvant or adjuvant treatment in the perioperative period. Individualizing these decisions based on ability to surgically resect, patient age, tumor histology, disease stage, and performance status is recommended.29
Intraperitoneal chemotherapy. Other approaches have been explored to reduce risk for micrometastases after surgery. Hyperthermic intraperitoneal chemotherapy,32 for instance, administered immediately after cytoreductive surgery was studied as a technique that might prevent some of the risks and adverse effects associated with intraperitoneal chemotherapy.31 Results showed some improvement in progression-free survival and overall survival in a subgroup of patients who underwent interval cytoreductive surgery after neoadjuvant therapy, but no differences were observed for the larger population with advanced epithelial ovarian cancer. Adverse reactions to intraperitoneal chemotherapy were also observed.
Angiogenesis inhibition. Tumors need energy and oxygen to grow. Angiogenesis is the process of new blood vessel formation that provides the tumor with nutrients. Blocking angiogenesis can thwart tumor growth and improve patient outcomes. Bevacizumab is an antiangiogenic agent that has been extensively studied for 2 decades for many cancers including ovarian carcinoma. The NCCN guidelines note that bevacizumab may be considered as part of a first-line regimen with platinum agents, as maintenance in patients with wild-type or unknown BRCA mutation status and a good response to first-line therapy, or in combination with a poly (ADP-ribose) polymerase (PARP) inhibitor in eligible patients.27
PARP inhibitors. Approximately half of all high-grade serous ovarian carcinomas exhibit some defect in the ability to repair DNA damage using the homologous recombination (HR) pathway. These tumors include those with mutations in the BRCA1, BRCA2, and other HR genes. Defects in HR make tumors more dependent on back-up DNA repair systems, including the activity of PARP. PARP inhibitors were developed to specifically target HR-deficient tumors. To date, 3 PARP inhibitors have been approved for use in ovarian cancer—olaparib, rucaparib, and niraparib. Their use has expanded from later-line use in patients with BRCA1/2-mutated tumors to include frontline maintenance regimens for women with high-grade serous and high-grade endometrioid carcinomas, as well as women with recurrent disease.32 Numerous clinical trials are ongoing to develop next-generation PARP inhibitors and to explore their efficacy in combination with chemotherapy and other targeted agents.
Resistance and Disease Progression: Second-Line and Subsequent Treatment
A number of second-line and subsequent systemic treatment regimens may be considered when primary platinum-based chemotherapy and/or maintenance are no longer effective.33,34 As emphasized by the NCCN, a clinical trial is always an appropriate option, depending on eligibility, and sometimes a second cytoreductive surgery35,36 may be considered for patients who experience radiographic and/or clinical relapse after a long disease-free interval (6+ months). Each line of treatment is associated with progressively lower response rates and shorter durations of response. According to the NCCN guidelines, as patient performance status decreases and the toxicities of each line of therapy accumulate, assessment for palliative care should be considered and discussed.27
Investigational Approaches
With the high mortality rate associated with ovarian cancer, the challenges of detecting the disease at its early stages, and the lack of therapies that can significantly extend progression-free and overall survival in patients with advanced disease, many investigators are focused on novel treatment approaches. Preclinical observations, for instance, showing synergy between ataxia telangiectasia and RAD3-related (ATR) kinase inhibitors and PARP inhibitors led researchers to initiate a phase 2 study of olaparib plus ceralasertib (an ATR inhibitor) in patients with recurrent, platinum-resistant epithelial ovarian cancer.37 No objective responses were noted, but some signals of activity were seen among patients with BRCA1 mutations.
Due to success in other malignancies, immunotherapy is also being explored. Although some promising signals were reported at 6 months when nivolumab, a PD-1 (programmed cell death protein 1) inhibitor, and ipilimumab, a cytotoxic T-lymphocyte-associated antigen 4 antibody, were combined to treat patients with platinum-resistant epithelial ovarian cancer; final results are not yet available.38
Ovarian cancer is sometimes characterized as immunologically “cold.” This description means that immune cells, especially T cells, are not able to enter the tumor and destroy the cancer cells. It also means that these tumors are not as responsive to immune-based treatments. Therefore, some researchers are examining novel alternative immunotherapy strategies, such as chimeric antigen receptor T-cell (CAR-T) therapy.39 When a CAR T-cell encounters a tumor antigen, the CAR T-cell becomes activated. Activated CAR T-cells multiply, signal to other immune cells, and ultimately kill the tumor cells. Although CAR T-cell therapy has been tremendously successful in hematologic malignancies, to date, the benefits in solid tumors have been modest.39 However, there is significant enthusiasm for novel tumor antigens that can be targeted by CAR-T therapy, including mesothelin, folate receptor, Claudin-6, B7-H3, B7-H4, HER2, CD47, and L1-CAM, among others.40
Other investigational strategies include a p53 vaccine that would enhance the patient’s immunologic response to abnormal proteins produced by a mutated p53 gene, which is the most common finding in ovarian tumors.
Although researchers are investigating many approaches to treating advanced ovarian cancer, one strategy that has been pursued in other cancer settings—development of antibody-drug conjugates (ADCs)41—has seen promising results. In the late fall of 2022, the US Food and Drug Administration granted accelerated approval for mirvetuximab soravtansine-gynx for use in patients with a specific type of type of tumor (folate receptor alpha [FRα]-positive) when platinum resistance emerges.42 A companion diagnostic assay was also approved for selecting patients with FRα-positive disease. Several other clinical trials are investigating the efficacy of targeting other ovarian tumor antigens using the ADC approach. These targets include NaPi2b, mesothelin, B7-H4, Claudin-6, and Trop-2.43,44
Progress to Come
Progress in ovarian cancer will be made through a multipronged approach that includes interventions that may proactively “intercept” the development of cancer (eg, salpingectomy for women planning to have other simple gynecologic procedures after childbearing is complete). Although prophylactic surgeries are often undertaken by individuals at high risk for ovarian cancer because of genetic findings, such as BRCA1/2 abnormalities, even women with normal risk may consider when planning tubal ligation, removing their tubes, and other routine procedures. A substantial number of malignant tumors, and associated morbidity and mortality, may be thwarted as a result. The question of whether to treat when a STIC is detected remains to be answered.
The search for better methods of early detection continues, as local therapies for early-stage disease are invariably more effective than treatments in the advanced and/or metastatic setting.
Finally, as with certain other malignancies, even in the advanced setting, effective, often targeted, treatments can significantly prolong both progression-free and overall survival, transforming an often-lethal disease into a chronic one that allows patients to enjoy a better life expectancy with good quality of life.
- Karnezis AN, Cho KR, Gilks CB, Pearce CL, Huntsman DG. The disparate origins of ovarian cancers: pathogenesis and prevention strategies. Nat Rev Cancer. 2017;17(1):65-74. doi:10.1038/nrc.2016.113
- Cabasag CJ, Fagan PJ, Ferlay J, et al. Ovarian cancer today and tomorrow: A global assessment by world region and Human Development Index using GLOBOCAN 2020. Int J Cancer. 2022;151(9):1535-1541. doi:10.1002/ijc.34002
- Huang J, Chan WC, Ngai CH, et al. Worldwide burden, risk factors, and temporal trends of ovarian cancer: a global study. Cancers (Basel). 2022;14(9):2230. doi:10.3390/cancers14092230
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17-48. doi:10.3322/caac.21763
- World Ovarian Cancer Coalition. Ovarian cancer key stats. Accessed May 8, 2023. https://worldovariancancercoalition.org/about-ovarian-cancer/key-stats/
- American Cancer Society. Survival rates for breast cancer. Updated March 1, 2023. Accessed May 8, 2023. https://www.cancer.org/cancer/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-survival-rates.html
- Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: a review. Cancer Biol Med. 2017;14(1):9-32. doi:10.20892/j.issn.2095-3941.2016.0084
- Phung MT, Pearce CL, Meza R, Jeon J. Trends of ovarian cancer incidence by histotype and race/ethnicity in the United States 1992–2019. Cancer Res Commun. 2023;3(1):1-8. doi:10.1158/2767-9764.CRC-22-0410
- Coburn SB, Bray F, Sherman ME, Trabert B. International patterns and trends in ovarian cancer incidence, overall and by histologic subtype. Int J Cancer. 2017;140(11):2451-2460. doi:10.1002/ijc.30676
- Kroeger PT Jr, Drapkin R. Pathogenesis and heterogeneity of ovarian cancer. Curr Obstet Gynecol. 2017;29(1):26-34. doi:10.1097/GCO.0000000000000340
- Shih lM, Wang Y, Wang TL. The origin of ovarian cancer species and precancerous landscape. Am J Pathol. 2021;191(1):26-39. doi:10.1016/j.ajpath.2020.09.006
- Meserve EEK, Brouwer J, Crum CP. Serous tubal intraepithelial neoplasia: the concept and its application. Mod Pathol. 2017;30(5):710-721. doi:10.1038/modpathol.2016.23
- Crum CP, Drapkin R, Kindelberger D, Medeiros F, Miron A, Lee Y. Lessons from BRCA: the tubal fimbria emerges as an origin for pelvic serous cancer. Clin Med Res. 2007;5(1):35-44. doi:10.3121/cmr.2007.702
- Wu RC, Wang P, Lin SF, et al. Genomic landscape and evolutionary trajectories of ovarian cancer precursor lesions. J Pathol. 2019;248(1):41-50. doi:10.1002/path.5219
- Eckert MA, Pan S, Hernandez KM, et al. Genomics of ovarian cancer progression reveals diverse metastatic trajectories including intraepithelial metastasis to the fallopian tube. Cancer Discov. 2016;6(12):1342-1351. doi:10.1158/2159-8290.CD-16-0607
- Labidi-Galy SI, Papp E, Hallberg D, et al. High grade serous ovarian carcinomas originate in the fallopian tube. Nat Comm. 2017;8(1):1093. doi:10.1038/s41467-017-00962-1
- Centers for Disease Control and Prevention; National Comprehensive Cancer Control Program (NCCP). Promoting early detection and treatment of cancer. Reviewed July 30, 2021. Accessed May 8, 2023. https://www.cdc.gov/cancer/ncccp/priorities/early-detection-treatment.htm
- Menon U, Gentry-Maharaj A, Burnell M, et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2021;397(10290):2182-2193. doi:10.1016/S0140-6736(21)00731-5
- Žilovic D, Ciurliene R, Sabaliauskaite R, Jarmalaitė S. Future screening prospects for ovarian cancer. Cancers (Basel). 2021;13(15):3840. doi:10.3390/cancers1315384
- Michels KA, Pfeiffer RM, Brinton LA, Trabert B. Modification of the associations between duration of oral contraceptive use and ovarian, endometrial, breast, and colorectal cancers. JAMA Oncol. 2018;4(4):516-521. doi:10.1001/jamaoncol.2017.4942
- Havrilesky LJ, Moorman PG, Lowery WJ, et al. Oral contraceptive pills as primary prevention for ovarian cancer: a systematic review and meta-analysis. Obstet Gynecol. 2013;122(1):139-147. doi:10.1097/AOG.0b013e318291c235
- Kotsopoulos J, Narod SA. Prophylactic salpingectomy for the prevention of ovarian cancer: who should we target? Int J Cancer. 2020;147(5):1245-1251. doi:10.1002/ijc.32916
- Steenbeek MP, Harmsen MG, Hoogerbrugge N, et al. Association of salpingectomy with delayed oophorectomy versus salpingo-oophorectomy with quality of life in BRCA 1/2 pathogenic variant carriers. A nonrandomized controlled trial. JAMA Oncol. 2021;7(8):1203-1212. doi:10.1001/jamaoncol.2021.1590
- Hanley GE, Pearce CL, Talhouk A, et al. Outcomes from opportunistic salpingectomy for ovarian cancer prevention. JAMA Netw Open. 2022;5(2):e2147343. doi:10.1001/jamanetworkopen.2021.47343
- Society of Gynecologic Oncology (SGO). SGO clinical practice statement: salpingectomy for ovarian cancer prevention (SGO, November 2013). Published November 1, 2013. Accessed May 8, 2023. https://www.sgo.org/resources/sgo-clinical-practice-statement-salpingectomy-for-ovarian-cancer-prevention/
- Steenbeek MP, van Bommel MHD, intHout J, et al. TUBectomy with delayed oophorectomy as an alternative to risk-reducing salpingo-oophorectomy in high-risk women to assess the safety of prevention: the TUBA-WISP II study protocol [published online ahead of print, 2023 Apr 12]. Int J Gynecol Cancer. 2023;ijgc-2023-004377. doi:10.1136/ijgc-2023-004377
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: Ovarian cancer including fallopian tube cancer and primary peritoneal cancer. Version 1.2023. December 22, 2022. Accessed May 8, 2023. https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf
- Chawla A, Hunt KK, Mittendorf EA. Surgical considerations in patients receiving neoadjuvant systemic therapy. Future Oncol. 2012;8(3):239-250. doi:10.2217/fon.12.12
- Coleridge SL, Bryant A, Kehoe S, Morrison J. Neoadjuvant chemotherapy before surgery versus surgery followed by chemotherapy for initial treatment in advanced ovarian epithelial cancer. Cochrane Database Syst Rev. 2021;7(7):CD005343. doi:10.1002/14651858.CD005343.pub6
- Lim MC, Chang SJ, Park B, et al; HIPEC for Ovarian Cancer Collaborators. Survival after hyperthermic intraperitoneal chemotherapy and primary or interval cytoreductive surgery in ovarian cancer: a randomized clinical trial. JAMA Surg. 2022;157(5):374-383. doi:10.1001/jamasurg.2022.0143
- Walker JL, Brady MF, Wenzel L, et al. Randomized trial of intravenous versus intraperitoneal chemotherapy plus bevacizumab in advanced ovarian carcinoma: an NRG Oncology/Gynecologic Oncology Group study. J Clin Oncol. 2019;37(16):1380-1390. doi:10.1200/JCO.18.01568
- Konstantinopoulos PA, Lheureux S, Moore KN. PARP inhibitors for ovarian cancer: current indications, future combinations, and novel assets in development to target DNA damage repair. Am Soc Clin Oncol Educ Book. 2020;40:1-16. doi:10.1200/EDBK_288015
- Markman M, Bookman MA. Second-line treatment of ovarian cancer. Oncologist. 2000;5(1):26-35. doi:10.1634/theoncologist.5-1-26
- Markman M. Pharmaceutical management of ovarian cancer: current status. Drugs. 2019;79(11):1231-1239. doi:10.1007/s40265-019-01158-1
- Bristow RE, Puri I, Chi DS. Cytoreductive surgery for recurrent ovarian cancer: a meta-analysis. Gynecol Oncol. 2009;112(1):265-274. doi:10.1016/j.ygyno.2008.08.033
- de Bree E, Michelakis D, Anagnostopoulou E. The current role of secondary cytoreductive surgery for recurrent ovarian cancer. Front Oncol. 2022;12:1029976. doi:10.3389/fonc.2022.1029976
- Shah PD, Wethington SL, Pagan C, et al. Combination ATR and PARP inhibitor (CAPRI): a phase 2 study of ceralasertib plus olaparib in patients with recurrent, platinum-resistant epithelial ovarian cancer. Gynecol Oncol. 2021;163(2): 246-253. doi:10.1016/j.ygyno.2021.08.024
- Borella F, Ghisoni E, Giannone G, et al. Immune checkpoint inhibitors in epithelial ovarian cancer: an overview on efficacy and future perspectives. Diagnostics (Basel). 2020;10(3):146. doi:10.3390/diagnostics10030146
- Wu JWY, Dand S, Doig L, et al. T-cell receptor therapy in the treatment of ovarian cancer: a mini review. Front Immunol. 2021;12:672502. doi:10.3389/fimmu.2021.672502
- Benard E, Casey NP, Inderberg EM, Wälchli S. SJI 2020 special issue: a catalogue of ovarian cancer targets for CAR therapy. Scand J Immunol. 2020;92(4):e12917 doi:10.1111/sji.12917
- Martín-Sabroso C, Lozza I, Torres-Suárez AI, Fraguas-Sánchez AI. Antibodyantineoplastic conjugates in gynecological malignancies: current status and future perspectives. Pharmaceutics. 2021;13(10):1705. doi:10.3390/pharmaceutics13101705
- US Food and Drug Administration. FDA grants accelerated approval to mirvetuximab soravtansine-gynx for FRα positive, platinum-resistant epithelial ovarian, fallopian tube, or peritoneal cancer. Published November 14, 2022. Accessed May 8, 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-mirvetuximab-soravtansine-gynx-fra-positive-platinum-resistant
- Tolcher A, Hamilton E, Coleman RL. The evolving landscape of antibody-drug conjugates in gynecologic cancers. Cancer Treat Rev. 2023;116:102546. doi:10.1016/j.ctrv.2023.102546
- Banerjee S, Drapkin R, Richarson DL, Birrer M. Targeting NaPi2b in ovarian cancer. Cancer Treat Rev. 2023;112:102489. doi:10.1016/j.ctrv.2022.102489
Incidence and Mortality
By 2040, the number of women diagnosed with ovarian cancer annually worldwide is expected to increase by 100% in low Human Development Index (HDI) countries, and by 19-28% in high HDI countries.2 The causes of this increasing incidence are likely to be multifactorial, including both hereditary and modifiable risk factors.3 In addition to increasing population size, the growing prevalence of obesity, estrogen exposures, and nulliparity are particularly pertinent as potential causes of the rising incidence of ovarian cancer in younger women. The number of ovarian cancer-related deaths is also projected to rise from about 200,000 to nearly 314,000 annually, an increase of over 50% from 2020.2,4 Although outcomes in developed regions and nations continue to improve somewhat, 5-year survival rates range from 36% to 46%.5 These outcomes are nevertheless dismal when compared with 5-year survival rates from other cancer types, such as breast cancer, which are approaching 90%.6
Principal Histotypes
The principal histotypes in ovarian cancer are epithelial in origin and include high-grade serous carcinoma, clear-cell carcinoma, endometrioid carcinoma, low-grade serous carcinoma, and mucinous carcinoma. Other rarer types are nonepithelial, ie, arising from stromal or germ cell lines.7 Incidence rates appear to be affected over time by trends such as birth rates, use of combination oral contraceptives, and menopausal hormone therapy.8 Figure 1 shows that most ovarian cancers—approximately 70%—are high-grade serous carcinoma, although in Asian countries clear cell and endometrioid carcinomas comprise a higher proportion.9
Most ovarian cancers are epithelial carcinomas. High-grade serous carcinoma is the most common, whereas the other subtypes represent 10% or fewer cases each.9
Into the Fallopian Tube
One of the most salient and dramatic discoveries of the last 2 decades has been the finding that high-grade, clear-cell, and endometrioid tumors appear to arise from tissues not normally present in the ovary.1 As a result of risk-reducing efforts to prevent serous cancers in women with genetic predisposition to develop ovarian cancer (ie, those with BRCA1 or BRCA2 mutations), it became increasingly clear that many early cancers arose in the fallopian tube,10-12 with the distal portion—the fimbria—as the most common site of origin.13-16
Figure 2 depicts the female reproductive tract, including the location of the fimbria compared with the ovaries. Moreover, lesions observed in the fallopian tube fimbria—serous tubal intraepithelial carcinomas (STICs)—were identified as precursors of ovarian cancer, with a window of 7 years between development of STIC and the beginning of an ovarian cancer.14,16
Lesions that develop in the fallopian tube fimbria, called serous tubal intraepithelial carcinomas (STICs), have been identified as precursors of ovarian cancer.
Early Detection
Early, localized ovarian cancer is asymptomatic; by the time a patient presents with symptoms, even with nonspecific abdominal complaints, the disease is almost invariably advanced. The concept of early detection has improved both the rate of cancer diagnoses and outcomes for some malignancies, such as cervical, colorectal, breast, and lung cancers,17 but this strategy is yet to be effectively applied in ovarian cancer. A large, population-based study, for instance, yielded negative results when multimodal screening (using both measurement of CA125 blood levels and transvaginal ultrasound imaging) failed to improve survival, even though such screening was able to detect lower stage disease.18 Emerging technologies, such as liquid biopsies and uterine lavage, which seek to detect potential biomarkers (new types of blood tests) of ovarian cancer at an early stage and closer to the site of tumor origin, are being investigated and refined but are not yet ready for clinical use, particularly at the population level for screening.19
Risk-Reducing Interventions
Use of oral contraceptives has been associated with a significant reduction in risk for ovarian cancer, but the potential risks (eg, increased risk for breast cancer, increased risk for venous thromboembolism) preclude its universal recommendation.20-22 Simple removal of the fallopian tube, salpingectomy, was proposed as a potential intervention to “intercept” the progression of a STIC to cancer. Researchers recently compared simple salpingectomy with salpingo-oophorectomy as a risk-reduction procedure in carriers of BRCA 1/2 pathogenic variants after they had completed childbearing.23
These investigators proposed that later removal of the ovaries would delay menopause and would contribute to fewer/less severe symptoms, such as hot flashes, disturbed sleep, and sexual issues, as well as maintain or improve overall quality of life. The hypothesis was supported by results, which showed that patients had better menopause-related quality of life after salpingectomy than after salpingo-oophorectomy, regardless of the use of hormone replacement therapy.23 The oncologic safety of this approach was subsequently demonstrated by other studies that showed a significantly lower incidence of ovarian cancers in women who had undergone opportunistic salpingectomy.22,24,25
An international prospective trial, TUBA-WISPII, is now underway to test the hypothesis that postponement of oophorectomy after salpingectomy is non-inferior to standard salpingo-oophorectomy in terms of ovarian cancer risk for patients at high risk.26
Treatment
First-Line Therapy
Currently, there are no durable curative therapies for ovarian cancer once advanced disease has been diagnosed.
Surgery plus platinum-based chemotherapy. Most patients, even those diagnosed with advanced disease, are treated initially with debulking surgery, ideally by a gynecologic oncologist, and adjuvant chemotherapy. Most ovarian carcinomas are initially platinum-sensitive, but resistance and disease recurrence are almost inevitable. According to the National Comprehensive Cancer Network (NCCN) guidelines for ovarian cancer,27 preferred chemotherapy regimens include paclitaxel and carboplatin with or without bevacizumab, docetaxel and carboplatin, or carboplatin and liposomal doxorubicin. Numerous other regimens, combinations, and agents are included in the guidelines to help providers customize treatment plans.
Neoadjuvant vs adjuvant regimens. Neoadjuvant chemotherapy has been used for other malignancies to gauge sensitivity to systemic treatments and to improve surgical margins.28 Thus far, though, outcomes in ovarian cancer have been similar whether patients were given neoadjuvant or adjuvant treatment in the perioperative period. Individualizing these decisions based on ability to surgically resect, patient age, tumor histology, disease stage, and performance status is recommended.29
Intraperitoneal chemotherapy. Other approaches have been explored to reduce risk for micrometastases after surgery. Hyperthermic intraperitoneal chemotherapy,32 for instance, administered immediately after cytoreductive surgery was studied as a technique that might prevent some of the risks and adverse effects associated with intraperitoneal chemotherapy.31 Results showed some improvement in progression-free survival and overall survival in a subgroup of patients who underwent interval cytoreductive surgery after neoadjuvant therapy, but no differences were observed for the larger population with advanced epithelial ovarian cancer. Adverse reactions to intraperitoneal chemotherapy were also observed.
Angiogenesis inhibition. Tumors need energy and oxygen to grow. Angiogenesis is the process of new blood vessel formation that provides the tumor with nutrients. Blocking angiogenesis can thwart tumor growth and improve patient outcomes. Bevacizumab is an antiangiogenic agent that has been extensively studied for 2 decades for many cancers including ovarian carcinoma. The NCCN guidelines note that bevacizumab may be considered as part of a first-line regimen with platinum agents, as maintenance in patients with wild-type or unknown BRCA mutation status and a good response to first-line therapy, or in combination with a poly (ADP-ribose) polymerase (PARP) inhibitor in eligible patients.27
PARP inhibitors. Approximately half of all high-grade serous ovarian carcinomas exhibit some defect in the ability to repair DNA damage using the homologous recombination (HR) pathway. These tumors include those with mutations in the BRCA1, BRCA2, and other HR genes. Defects in HR make tumors more dependent on back-up DNA repair systems, including the activity of PARP. PARP inhibitors were developed to specifically target HR-deficient tumors. To date, 3 PARP inhibitors have been approved for use in ovarian cancer—olaparib, rucaparib, and niraparib. Their use has expanded from later-line use in patients with BRCA1/2-mutated tumors to include frontline maintenance regimens for women with high-grade serous and high-grade endometrioid carcinomas, as well as women with recurrent disease.32 Numerous clinical trials are ongoing to develop next-generation PARP inhibitors and to explore their efficacy in combination with chemotherapy and other targeted agents.
Resistance and Disease Progression: Second-Line and Subsequent Treatment
A number of second-line and subsequent systemic treatment regimens may be considered when primary platinum-based chemotherapy and/or maintenance are no longer effective.33,34 As emphasized by the NCCN, a clinical trial is always an appropriate option, depending on eligibility, and sometimes a second cytoreductive surgery35,36 may be considered for patients who experience radiographic and/or clinical relapse after a long disease-free interval (6+ months). Each line of treatment is associated with progressively lower response rates and shorter durations of response. According to the NCCN guidelines, as patient performance status decreases and the toxicities of each line of therapy accumulate, assessment for palliative care should be considered and discussed.27
Investigational Approaches
With the high mortality rate associated with ovarian cancer, the challenges of detecting the disease at its early stages, and the lack of therapies that can significantly extend progression-free and overall survival in patients with advanced disease, many investigators are focused on novel treatment approaches. Preclinical observations, for instance, showing synergy between ataxia telangiectasia and RAD3-related (ATR) kinase inhibitors and PARP inhibitors led researchers to initiate a phase 2 study of olaparib plus ceralasertib (an ATR inhibitor) in patients with recurrent, platinum-resistant epithelial ovarian cancer.37 No objective responses were noted, but some signals of activity were seen among patients with BRCA1 mutations.
Due to success in other malignancies, immunotherapy is also being explored. Although some promising signals were reported at 6 months when nivolumab, a PD-1 (programmed cell death protein 1) inhibitor, and ipilimumab, a cytotoxic T-lymphocyte-associated antigen 4 antibody, were combined to treat patients with platinum-resistant epithelial ovarian cancer; final results are not yet available.38
Ovarian cancer is sometimes characterized as immunologically “cold.” This description means that immune cells, especially T cells, are not able to enter the tumor and destroy the cancer cells. It also means that these tumors are not as responsive to immune-based treatments. Therefore, some researchers are examining novel alternative immunotherapy strategies, such as chimeric antigen receptor T-cell (CAR-T) therapy.39 When a CAR T-cell encounters a tumor antigen, the CAR T-cell becomes activated. Activated CAR T-cells multiply, signal to other immune cells, and ultimately kill the tumor cells. Although CAR T-cell therapy has been tremendously successful in hematologic malignancies, to date, the benefits in solid tumors have been modest.39 However, there is significant enthusiasm for novel tumor antigens that can be targeted by CAR-T therapy, including mesothelin, folate receptor, Claudin-6, B7-H3, B7-H4, HER2, CD47, and L1-CAM, among others.40
Other investigational strategies include a p53 vaccine that would enhance the patient’s immunologic response to abnormal proteins produced by a mutated p53 gene, which is the most common finding in ovarian tumors.
Although researchers are investigating many approaches to treating advanced ovarian cancer, one strategy that has been pursued in other cancer settings—development of antibody-drug conjugates (ADCs)41—has seen promising results. In the late fall of 2022, the US Food and Drug Administration granted accelerated approval for mirvetuximab soravtansine-gynx for use in patients with a specific type of type of tumor (folate receptor alpha [FRα]-positive) when platinum resistance emerges.42 A companion diagnostic assay was also approved for selecting patients with FRα-positive disease. Several other clinical trials are investigating the efficacy of targeting other ovarian tumor antigens using the ADC approach. These targets include NaPi2b, mesothelin, B7-H4, Claudin-6, and Trop-2.43,44
Progress to Come
Progress in ovarian cancer will be made through a multipronged approach that includes interventions that may proactively “intercept” the development of cancer (eg, salpingectomy for women planning to have other simple gynecologic procedures after childbearing is complete). Although prophylactic surgeries are often undertaken by individuals at high risk for ovarian cancer because of genetic findings, such as BRCA1/2 abnormalities, even women with normal risk may consider when planning tubal ligation, removing their tubes, and other routine procedures. A substantial number of malignant tumors, and associated morbidity and mortality, may be thwarted as a result. The question of whether to treat when a STIC is detected remains to be answered.
The search for better methods of early detection continues, as local therapies for early-stage disease are invariably more effective than treatments in the advanced and/or metastatic setting.
Finally, as with certain other malignancies, even in the advanced setting, effective, often targeted, treatments can significantly prolong both progression-free and overall survival, transforming an often-lethal disease into a chronic one that allows patients to enjoy a better life expectancy with good quality of life.
Incidence and Mortality
By 2040, the number of women diagnosed with ovarian cancer annually worldwide is expected to increase by 100% in low Human Development Index (HDI) countries, and by 19-28% in high HDI countries.2 The causes of this increasing incidence are likely to be multifactorial, including both hereditary and modifiable risk factors.3 In addition to increasing population size, the growing prevalence of obesity, estrogen exposures, and nulliparity are particularly pertinent as potential causes of the rising incidence of ovarian cancer in younger women. The number of ovarian cancer-related deaths is also projected to rise from about 200,000 to nearly 314,000 annually, an increase of over 50% from 2020.2,4 Although outcomes in developed regions and nations continue to improve somewhat, 5-year survival rates range from 36% to 46%.5 These outcomes are nevertheless dismal when compared with 5-year survival rates from other cancer types, such as breast cancer, which are approaching 90%.6
Principal Histotypes
The principal histotypes in ovarian cancer are epithelial in origin and include high-grade serous carcinoma, clear-cell carcinoma, endometrioid carcinoma, low-grade serous carcinoma, and mucinous carcinoma. Other rarer types are nonepithelial, ie, arising from stromal or germ cell lines.7 Incidence rates appear to be affected over time by trends such as birth rates, use of combination oral contraceptives, and menopausal hormone therapy.8 Figure 1 shows that most ovarian cancers—approximately 70%—are high-grade serous carcinoma, although in Asian countries clear cell and endometrioid carcinomas comprise a higher proportion.9
Most ovarian cancers are epithelial carcinomas. High-grade serous carcinoma is the most common, whereas the other subtypes represent 10% or fewer cases each.9
Into the Fallopian Tube
One of the most salient and dramatic discoveries of the last 2 decades has been the finding that high-grade, clear-cell, and endometrioid tumors appear to arise from tissues not normally present in the ovary.1 As a result of risk-reducing efforts to prevent serous cancers in women with genetic predisposition to develop ovarian cancer (ie, those with BRCA1 or BRCA2 mutations), it became increasingly clear that many early cancers arose in the fallopian tube,10-12 with the distal portion—the fimbria—as the most common site of origin.13-16
Figure 2 depicts the female reproductive tract, including the location of the fimbria compared with the ovaries. Moreover, lesions observed in the fallopian tube fimbria—serous tubal intraepithelial carcinomas (STICs)—were identified as precursors of ovarian cancer, with a window of 7 years between development of STIC and the beginning of an ovarian cancer.14,16
Lesions that develop in the fallopian tube fimbria, called serous tubal intraepithelial carcinomas (STICs), have been identified as precursors of ovarian cancer.
Early Detection
Early, localized ovarian cancer is asymptomatic; by the time a patient presents with symptoms, even with nonspecific abdominal complaints, the disease is almost invariably advanced. The concept of early detection has improved both the rate of cancer diagnoses and outcomes for some malignancies, such as cervical, colorectal, breast, and lung cancers,17 but this strategy is yet to be effectively applied in ovarian cancer. A large, population-based study, for instance, yielded negative results when multimodal screening (using both measurement of CA125 blood levels and transvaginal ultrasound imaging) failed to improve survival, even though such screening was able to detect lower stage disease.18 Emerging technologies, such as liquid biopsies and uterine lavage, which seek to detect potential biomarkers (new types of blood tests) of ovarian cancer at an early stage and closer to the site of tumor origin, are being investigated and refined but are not yet ready for clinical use, particularly at the population level for screening.19
Risk-Reducing Interventions
Use of oral contraceptives has been associated with a significant reduction in risk for ovarian cancer, but the potential risks (eg, increased risk for breast cancer, increased risk for venous thromboembolism) preclude its universal recommendation.20-22 Simple removal of the fallopian tube, salpingectomy, was proposed as a potential intervention to “intercept” the progression of a STIC to cancer. Researchers recently compared simple salpingectomy with salpingo-oophorectomy as a risk-reduction procedure in carriers of BRCA 1/2 pathogenic variants after they had completed childbearing.23
These investigators proposed that later removal of the ovaries would delay menopause and would contribute to fewer/less severe symptoms, such as hot flashes, disturbed sleep, and sexual issues, as well as maintain or improve overall quality of life. The hypothesis was supported by results, which showed that patients had better menopause-related quality of life after salpingectomy than after salpingo-oophorectomy, regardless of the use of hormone replacement therapy.23 The oncologic safety of this approach was subsequently demonstrated by other studies that showed a significantly lower incidence of ovarian cancers in women who had undergone opportunistic salpingectomy.22,24,25
An international prospective trial, TUBA-WISPII, is now underway to test the hypothesis that postponement of oophorectomy after salpingectomy is non-inferior to standard salpingo-oophorectomy in terms of ovarian cancer risk for patients at high risk.26
Treatment
First-Line Therapy
Currently, there are no durable curative therapies for ovarian cancer once advanced disease has been diagnosed.
Surgery plus platinum-based chemotherapy. Most patients, even those diagnosed with advanced disease, are treated initially with debulking surgery, ideally by a gynecologic oncologist, and adjuvant chemotherapy. Most ovarian carcinomas are initially platinum-sensitive, but resistance and disease recurrence are almost inevitable. According to the National Comprehensive Cancer Network (NCCN) guidelines for ovarian cancer,27 preferred chemotherapy regimens include paclitaxel and carboplatin with or without bevacizumab, docetaxel and carboplatin, or carboplatin and liposomal doxorubicin. Numerous other regimens, combinations, and agents are included in the guidelines to help providers customize treatment plans.
Neoadjuvant vs adjuvant regimens. Neoadjuvant chemotherapy has been used for other malignancies to gauge sensitivity to systemic treatments and to improve surgical margins.28 Thus far, though, outcomes in ovarian cancer have been similar whether patients were given neoadjuvant or adjuvant treatment in the perioperative period. Individualizing these decisions based on ability to surgically resect, patient age, tumor histology, disease stage, and performance status is recommended.29
Intraperitoneal chemotherapy. Other approaches have been explored to reduce risk for micrometastases after surgery. Hyperthermic intraperitoneal chemotherapy,32 for instance, administered immediately after cytoreductive surgery was studied as a technique that might prevent some of the risks and adverse effects associated with intraperitoneal chemotherapy.31 Results showed some improvement in progression-free survival and overall survival in a subgroup of patients who underwent interval cytoreductive surgery after neoadjuvant therapy, but no differences were observed for the larger population with advanced epithelial ovarian cancer. Adverse reactions to intraperitoneal chemotherapy were also observed.
Angiogenesis inhibition. Tumors need energy and oxygen to grow. Angiogenesis is the process of new blood vessel formation that provides the tumor with nutrients. Blocking angiogenesis can thwart tumor growth and improve patient outcomes. Bevacizumab is an antiangiogenic agent that has been extensively studied for 2 decades for many cancers including ovarian carcinoma. The NCCN guidelines note that bevacizumab may be considered as part of a first-line regimen with platinum agents, as maintenance in patients with wild-type or unknown BRCA mutation status and a good response to first-line therapy, or in combination with a poly (ADP-ribose) polymerase (PARP) inhibitor in eligible patients.27
PARP inhibitors. Approximately half of all high-grade serous ovarian carcinomas exhibit some defect in the ability to repair DNA damage using the homologous recombination (HR) pathway. These tumors include those with mutations in the BRCA1, BRCA2, and other HR genes. Defects in HR make tumors more dependent on back-up DNA repair systems, including the activity of PARP. PARP inhibitors were developed to specifically target HR-deficient tumors. To date, 3 PARP inhibitors have been approved for use in ovarian cancer—olaparib, rucaparib, and niraparib. Their use has expanded from later-line use in patients with BRCA1/2-mutated tumors to include frontline maintenance regimens for women with high-grade serous and high-grade endometrioid carcinomas, as well as women with recurrent disease.32 Numerous clinical trials are ongoing to develop next-generation PARP inhibitors and to explore their efficacy in combination with chemotherapy and other targeted agents.
Resistance and Disease Progression: Second-Line and Subsequent Treatment
A number of second-line and subsequent systemic treatment regimens may be considered when primary platinum-based chemotherapy and/or maintenance are no longer effective.33,34 As emphasized by the NCCN, a clinical trial is always an appropriate option, depending on eligibility, and sometimes a second cytoreductive surgery35,36 may be considered for patients who experience radiographic and/or clinical relapse after a long disease-free interval (6+ months). Each line of treatment is associated with progressively lower response rates and shorter durations of response. According to the NCCN guidelines, as patient performance status decreases and the toxicities of each line of therapy accumulate, assessment for palliative care should be considered and discussed.27
Investigational Approaches
With the high mortality rate associated with ovarian cancer, the challenges of detecting the disease at its early stages, and the lack of therapies that can significantly extend progression-free and overall survival in patients with advanced disease, many investigators are focused on novel treatment approaches. Preclinical observations, for instance, showing synergy between ataxia telangiectasia and RAD3-related (ATR) kinase inhibitors and PARP inhibitors led researchers to initiate a phase 2 study of olaparib plus ceralasertib (an ATR inhibitor) in patients with recurrent, platinum-resistant epithelial ovarian cancer.37 No objective responses were noted, but some signals of activity were seen among patients with BRCA1 mutations.
Due to success in other malignancies, immunotherapy is also being explored. Although some promising signals were reported at 6 months when nivolumab, a PD-1 (programmed cell death protein 1) inhibitor, and ipilimumab, a cytotoxic T-lymphocyte-associated antigen 4 antibody, were combined to treat patients with platinum-resistant epithelial ovarian cancer; final results are not yet available.38
Ovarian cancer is sometimes characterized as immunologically “cold.” This description means that immune cells, especially T cells, are not able to enter the tumor and destroy the cancer cells. It also means that these tumors are not as responsive to immune-based treatments. Therefore, some researchers are examining novel alternative immunotherapy strategies, such as chimeric antigen receptor T-cell (CAR-T) therapy.39 When a CAR T-cell encounters a tumor antigen, the CAR T-cell becomes activated. Activated CAR T-cells multiply, signal to other immune cells, and ultimately kill the tumor cells. Although CAR T-cell therapy has been tremendously successful in hematologic malignancies, to date, the benefits in solid tumors have been modest.39 However, there is significant enthusiasm for novel tumor antigens that can be targeted by CAR-T therapy, including mesothelin, folate receptor, Claudin-6, B7-H3, B7-H4, HER2, CD47, and L1-CAM, among others.40
Other investigational strategies include a p53 vaccine that would enhance the patient’s immunologic response to abnormal proteins produced by a mutated p53 gene, which is the most common finding in ovarian tumors.
Although researchers are investigating many approaches to treating advanced ovarian cancer, one strategy that has been pursued in other cancer settings—development of antibody-drug conjugates (ADCs)41—has seen promising results. In the late fall of 2022, the US Food and Drug Administration granted accelerated approval for mirvetuximab soravtansine-gynx for use in patients with a specific type of type of tumor (folate receptor alpha [FRα]-positive) when platinum resistance emerges.42 A companion diagnostic assay was also approved for selecting patients with FRα-positive disease. Several other clinical trials are investigating the efficacy of targeting other ovarian tumor antigens using the ADC approach. These targets include NaPi2b, mesothelin, B7-H4, Claudin-6, and Trop-2.43,44
Progress to Come
Progress in ovarian cancer will be made through a multipronged approach that includes interventions that may proactively “intercept” the development of cancer (eg, salpingectomy for women planning to have other simple gynecologic procedures after childbearing is complete). Although prophylactic surgeries are often undertaken by individuals at high risk for ovarian cancer because of genetic findings, such as BRCA1/2 abnormalities, even women with normal risk may consider when planning tubal ligation, removing their tubes, and other routine procedures. A substantial number of malignant tumors, and associated morbidity and mortality, may be thwarted as a result. The question of whether to treat when a STIC is detected remains to be answered.
The search for better methods of early detection continues, as local therapies for early-stage disease are invariably more effective than treatments in the advanced and/or metastatic setting.
Finally, as with certain other malignancies, even in the advanced setting, effective, often targeted, treatments can significantly prolong both progression-free and overall survival, transforming an often-lethal disease into a chronic one that allows patients to enjoy a better life expectancy with good quality of life.
- Karnezis AN, Cho KR, Gilks CB, Pearce CL, Huntsman DG. The disparate origins of ovarian cancers: pathogenesis and prevention strategies. Nat Rev Cancer. 2017;17(1):65-74. doi:10.1038/nrc.2016.113
- Cabasag CJ, Fagan PJ, Ferlay J, et al. Ovarian cancer today and tomorrow: A global assessment by world region and Human Development Index using GLOBOCAN 2020. Int J Cancer. 2022;151(9):1535-1541. doi:10.1002/ijc.34002
- Huang J, Chan WC, Ngai CH, et al. Worldwide burden, risk factors, and temporal trends of ovarian cancer: a global study. Cancers (Basel). 2022;14(9):2230. doi:10.3390/cancers14092230
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17-48. doi:10.3322/caac.21763
- World Ovarian Cancer Coalition. Ovarian cancer key stats. Accessed May 8, 2023. https://worldovariancancercoalition.org/about-ovarian-cancer/key-stats/
- American Cancer Society. Survival rates for breast cancer. Updated March 1, 2023. Accessed May 8, 2023. https://www.cancer.org/cancer/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-survival-rates.html
- Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: a review. Cancer Biol Med. 2017;14(1):9-32. doi:10.20892/j.issn.2095-3941.2016.0084
- Phung MT, Pearce CL, Meza R, Jeon J. Trends of ovarian cancer incidence by histotype and race/ethnicity in the United States 1992–2019. Cancer Res Commun. 2023;3(1):1-8. doi:10.1158/2767-9764.CRC-22-0410
- Coburn SB, Bray F, Sherman ME, Trabert B. International patterns and trends in ovarian cancer incidence, overall and by histologic subtype. Int J Cancer. 2017;140(11):2451-2460. doi:10.1002/ijc.30676
- Kroeger PT Jr, Drapkin R. Pathogenesis and heterogeneity of ovarian cancer. Curr Obstet Gynecol. 2017;29(1):26-34. doi:10.1097/GCO.0000000000000340
- Shih lM, Wang Y, Wang TL. The origin of ovarian cancer species and precancerous landscape. Am J Pathol. 2021;191(1):26-39. doi:10.1016/j.ajpath.2020.09.006
- Meserve EEK, Brouwer J, Crum CP. Serous tubal intraepithelial neoplasia: the concept and its application. Mod Pathol. 2017;30(5):710-721. doi:10.1038/modpathol.2016.23
- Crum CP, Drapkin R, Kindelberger D, Medeiros F, Miron A, Lee Y. Lessons from BRCA: the tubal fimbria emerges as an origin for pelvic serous cancer. Clin Med Res. 2007;5(1):35-44. doi:10.3121/cmr.2007.702
- Wu RC, Wang P, Lin SF, et al. Genomic landscape and evolutionary trajectories of ovarian cancer precursor lesions. J Pathol. 2019;248(1):41-50. doi:10.1002/path.5219
- Eckert MA, Pan S, Hernandez KM, et al. Genomics of ovarian cancer progression reveals diverse metastatic trajectories including intraepithelial metastasis to the fallopian tube. Cancer Discov. 2016;6(12):1342-1351. doi:10.1158/2159-8290.CD-16-0607
- Labidi-Galy SI, Papp E, Hallberg D, et al. High grade serous ovarian carcinomas originate in the fallopian tube. Nat Comm. 2017;8(1):1093. doi:10.1038/s41467-017-00962-1
- Centers for Disease Control and Prevention; National Comprehensive Cancer Control Program (NCCP). Promoting early detection and treatment of cancer. Reviewed July 30, 2021. Accessed May 8, 2023. https://www.cdc.gov/cancer/ncccp/priorities/early-detection-treatment.htm
- Menon U, Gentry-Maharaj A, Burnell M, et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2021;397(10290):2182-2193. doi:10.1016/S0140-6736(21)00731-5
- Žilovic D, Ciurliene R, Sabaliauskaite R, Jarmalaitė S. Future screening prospects for ovarian cancer. Cancers (Basel). 2021;13(15):3840. doi:10.3390/cancers1315384
- Michels KA, Pfeiffer RM, Brinton LA, Trabert B. Modification of the associations between duration of oral contraceptive use and ovarian, endometrial, breast, and colorectal cancers. JAMA Oncol. 2018;4(4):516-521. doi:10.1001/jamaoncol.2017.4942
- Havrilesky LJ, Moorman PG, Lowery WJ, et al. Oral contraceptive pills as primary prevention for ovarian cancer: a systematic review and meta-analysis. Obstet Gynecol. 2013;122(1):139-147. doi:10.1097/AOG.0b013e318291c235
- Kotsopoulos J, Narod SA. Prophylactic salpingectomy for the prevention of ovarian cancer: who should we target? Int J Cancer. 2020;147(5):1245-1251. doi:10.1002/ijc.32916
- Steenbeek MP, Harmsen MG, Hoogerbrugge N, et al. Association of salpingectomy with delayed oophorectomy versus salpingo-oophorectomy with quality of life in BRCA 1/2 pathogenic variant carriers. A nonrandomized controlled trial. JAMA Oncol. 2021;7(8):1203-1212. doi:10.1001/jamaoncol.2021.1590
- Hanley GE, Pearce CL, Talhouk A, et al. Outcomes from opportunistic salpingectomy for ovarian cancer prevention. JAMA Netw Open. 2022;5(2):e2147343. doi:10.1001/jamanetworkopen.2021.47343
- Society of Gynecologic Oncology (SGO). SGO clinical practice statement: salpingectomy for ovarian cancer prevention (SGO, November 2013). Published November 1, 2013. Accessed May 8, 2023. https://www.sgo.org/resources/sgo-clinical-practice-statement-salpingectomy-for-ovarian-cancer-prevention/
- Steenbeek MP, van Bommel MHD, intHout J, et al. TUBectomy with delayed oophorectomy as an alternative to risk-reducing salpingo-oophorectomy in high-risk women to assess the safety of prevention: the TUBA-WISP II study protocol [published online ahead of print, 2023 Apr 12]. Int J Gynecol Cancer. 2023;ijgc-2023-004377. doi:10.1136/ijgc-2023-004377
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: Ovarian cancer including fallopian tube cancer and primary peritoneal cancer. Version 1.2023. December 22, 2022. Accessed May 8, 2023. https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf
- Chawla A, Hunt KK, Mittendorf EA. Surgical considerations in patients receiving neoadjuvant systemic therapy. Future Oncol. 2012;8(3):239-250. doi:10.2217/fon.12.12
- Coleridge SL, Bryant A, Kehoe S, Morrison J. Neoadjuvant chemotherapy before surgery versus surgery followed by chemotherapy for initial treatment in advanced ovarian epithelial cancer. Cochrane Database Syst Rev. 2021;7(7):CD005343. doi:10.1002/14651858.CD005343.pub6
- Lim MC, Chang SJ, Park B, et al; HIPEC for Ovarian Cancer Collaborators. Survival after hyperthermic intraperitoneal chemotherapy and primary or interval cytoreductive surgery in ovarian cancer: a randomized clinical trial. JAMA Surg. 2022;157(5):374-383. doi:10.1001/jamasurg.2022.0143
- Walker JL, Brady MF, Wenzel L, et al. Randomized trial of intravenous versus intraperitoneal chemotherapy plus bevacizumab in advanced ovarian carcinoma: an NRG Oncology/Gynecologic Oncology Group study. J Clin Oncol. 2019;37(16):1380-1390. doi:10.1200/JCO.18.01568
- Konstantinopoulos PA, Lheureux S, Moore KN. PARP inhibitors for ovarian cancer: current indications, future combinations, and novel assets in development to target DNA damage repair. Am Soc Clin Oncol Educ Book. 2020;40:1-16. doi:10.1200/EDBK_288015
- Markman M, Bookman MA. Second-line treatment of ovarian cancer. Oncologist. 2000;5(1):26-35. doi:10.1634/theoncologist.5-1-26
- Markman M. Pharmaceutical management of ovarian cancer: current status. Drugs. 2019;79(11):1231-1239. doi:10.1007/s40265-019-01158-1
- Bristow RE, Puri I, Chi DS. Cytoreductive surgery for recurrent ovarian cancer: a meta-analysis. Gynecol Oncol. 2009;112(1):265-274. doi:10.1016/j.ygyno.2008.08.033
- de Bree E, Michelakis D, Anagnostopoulou E. The current role of secondary cytoreductive surgery for recurrent ovarian cancer. Front Oncol. 2022;12:1029976. doi:10.3389/fonc.2022.1029976
- Shah PD, Wethington SL, Pagan C, et al. Combination ATR and PARP inhibitor (CAPRI): a phase 2 study of ceralasertib plus olaparib in patients with recurrent, platinum-resistant epithelial ovarian cancer. Gynecol Oncol. 2021;163(2): 246-253. doi:10.1016/j.ygyno.2021.08.024
- Borella F, Ghisoni E, Giannone G, et al. Immune checkpoint inhibitors in epithelial ovarian cancer: an overview on efficacy and future perspectives. Diagnostics (Basel). 2020;10(3):146. doi:10.3390/diagnostics10030146
- Wu JWY, Dand S, Doig L, et al. T-cell receptor therapy in the treatment of ovarian cancer: a mini review. Front Immunol. 2021;12:672502. doi:10.3389/fimmu.2021.672502
- Benard E, Casey NP, Inderberg EM, Wälchli S. SJI 2020 special issue: a catalogue of ovarian cancer targets for CAR therapy. Scand J Immunol. 2020;92(4):e12917 doi:10.1111/sji.12917
- Martín-Sabroso C, Lozza I, Torres-Suárez AI, Fraguas-Sánchez AI. Antibodyantineoplastic conjugates in gynecological malignancies: current status and future perspectives. Pharmaceutics. 2021;13(10):1705. doi:10.3390/pharmaceutics13101705
- US Food and Drug Administration. FDA grants accelerated approval to mirvetuximab soravtansine-gynx for FRα positive, platinum-resistant epithelial ovarian, fallopian tube, or peritoneal cancer. Published November 14, 2022. Accessed May 8, 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-mirvetuximab-soravtansine-gynx-fra-positive-platinum-resistant
- Tolcher A, Hamilton E, Coleman RL. The evolving landscape of antibody-drug conjugates in gynecologic cancers. Cancer Treat Rev. 2023;116:102546. doi:10.1016/j.ctrv.2023.102546
- Banerjee S, Drapkin R, Richarson DL, Birrer M. Targeting NaPi2b in ovarian cancer. Cancer Treat Rev. 2023;112:102489. doi:10.1016/j.ctrv.2022.102489
- Karnezis AN, Cho KR, Gilks CB, Pearce CL, Huntsman DG. The disparate origins of ovarian cancers: pathogenesis and prevention strategies. Nat Rev Cancer. 2017;17(1):65-74. doi:10.1038/nrc.2016.113
- Cabasag CJ, Fagan PJ, Ferlay J, et al. Ovarian cancer today and tomorrow: A global assessment by world region and Human Development Index using GLOBOCAN 2020. Int J Cancer. 2022;151(9):1535-1541. doi:10.1002/ijc.34002
- Huang J, Chan WC, Ngai CH, et al. Worldwide burden, risk factors, and temporal trends of ovarian cancer: a global study. Cancers (Basel). 2022;14(9):2230. doi:10.3390/cancers14092230
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17-48. doi:10.3322/caac.21763
- World Ovarian Cancer Coalition. Ovarian cancer key stats. Accessed May 8, 2023. https://worldovariancancercoalition.org/about-ovarian-cancer/key-stats/
- American Cancer Society. Survival rates for breast cancer. Updated March 1, 2023. Accessed May 8, 2023. https://www.cancer.org/cancer/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-survival-rates.html
- Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: a review. Cancer Biol Med. 2017;14(1):9-32. doi:10.20892/j.issn.2095-3941.2016.0084
- Phung MT, Pearce CL, Meza R, Jeon J. Trends of ovarian cancer incidence by histotype and race/ethnicity in the United States 1992–2019. Cancer Res Commun. 2023;3(1):1-8. doi:10.1158/2767-9764.CRC-22-0410
- Coburn SB, Bray F, Sherman ME, Trabert B. International patterns and trends in ovarian cancer incidence, overall and by histologic subtype. Int J Cancer. 2017;140(11):2451-2460. doi:10.1002/ijc.30676
- Kroeger PT Jr, Drapkin R. Pathogenesis and heterogeneity of ovarian cancer. Curr Obstet Gynecol. 2017;29(1):26-34. doi:10.1097/GCO.0000000000000340
- Shih lM, Wang Y, Wang TL. The origin of ovarian cancer species and precancerous landscape. Am J Pathol. 2021;191(1):26-39. doi:10.1016/j.ajpath.2020.09.006
- Meserve EEK, Brouwer J, Crum CP. Serous tubal intraepithelial neoplasia: the concept and its application. Mod Pathol. 2017;30(5):710-721. doi:10.1038/modpathol.2016.23
- Crum CP, Drapkin R, Kindelberger D, Medeiros F, Miron A, Lee Y. Lessons from BRCA: the tubal fimbria emerges as an origin for pelvic serous cancer. Clin Med Res. 2007;5(1):35-44. doi:10.3121/cmr.2007.702
- Wu RC, Wang P, Lin SF, et al. Genomic landscape and evolutionary trajectories of ovarian cancer precursor lesions. J Pathol. 2019;248(1):41-50. doi:10.1002/path.5219
- Eckert MA, Pan S, Hernandez KM, et al. Genomics of ovarian cancer progression reveals diverse metastatic trajectories including intraepithelial metastasis to the fallopian tube. Cancer Discov. 2016;6(12):1342-1351. doi:10.1158/2159-8290.CD-16-0607
- Labidi-Galy SI, Papp E, Hallberg D, et al. High grade serous ovarian carcinomas originate in the fallopian tube. Nat Comm. 2017;8(1):1093. doi:10.1038/s41467-017-00962-1
- Centers for Disease Control and Prevention; National Comprehensive Cancer Control Program (NCCP). Promoting early detection and treatment of cancer. Reviewed July 30, 2021. Accessed May 8, 2023. https://www.cdc.gov/cancer/ncccp/priorities/early-detection-treatment.htm
- Menon U, Gentry-Maharaj A, Burnell M, et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2021;397(10290):2182-2193. doi:10.1016/S0140-6736(21)00731-5
- Žilovic D, Ciurliene R, Sabaliauskaite R, Jarmalaitė S. Future screening prospects for ovarian cancer. Cancers (Basel). 2021;13(15):3840. doi:10.3390/cancers1315384
- Michels KA, Pfeiffer RM, Brinton LA, Trabert B. Modification of the associations between duration of oral contraceptive use and ovarian, endometrial, breast, and colorectal cancers. JAMA Oncol. 2018;4(4):516-521. doi:10.1001/jamaoncol.2017.4942
- Havrilesky LJ, Moorman PG, Lowery WJ, et al. Oral contraceptive pills as primary prevention for ovarian cancer: a systematic review and meta-analysis. Obstet Gynecol. 2013;122(1):139-147. doi:10.1097/AOG.0b013e318291c235
- Kotsopoulos J, Narod SA. Prophylactic salpingectomy for the prevention of ovarian cancer: who should we target? Int J Cancer. 2020;147(5):1245-1251. doi:10.1002/ijc.32916
- Steenbeek MP, Harmsen MG, Hoogerbrugge N, et al. Association of salpingectomy with delayed oophorectomy versus salpingo-oophorectomy with quality of life in BRCA 1/2 pathogenic variant carriers. A nonrandomized controlled trial. JAMA Oncol. 2021;7(8):1203-1212. doi:10.1001/jamaoncol.2021.1590
- Hanley GE, Pearce CL, Talhouk A, et al. Outcomes from opportunistic salpingectomy for ovarian cancer prevention. JAMA Netw Open. 2022;5(2):e2147343. doi:10.1001/jamanetworkopen.2021.47343
- Society of Gynecologic Oncology (SGO). SGO clinical practice statement: salpingectomy for ovarian cancer prevention (SGO, November 2013). Published November 1, 2013. Accessed May 8, 2023. https://www.sgo.org/resources/sgo-clinical-practice-statement-salpingectomy-for-ovarian-cancer-prevention/
- Steenbeek MP, van Bommel MHD, intHout J, et al. TUBectomy with delayed oophorectomy as an alternative to risk-reducing salpingo-oophorectomy in high-risk women to assess the safety of prevention: the TUBA-WISP II study protocol [published online ahead of print, 2023 Apr 12]. Int J Gynecol Cancer. 2023;ijgc-2023-004377. doi:10.1136/ijgc-2023-004377
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: Ovarian cancer including fallopian tube cancer and primary peritoneal cancer. Version 1.2023. December 22, 2022. Accessed May 8, 2023. https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf
- Chawla A, Hunt KK, Mittendorf EA. Surgical considerations in patients receiving neoadjuvant systemic therapy. Future Oncol. 2012;8(3):239-250. doi:10.2217/fon.12.12
- Coleridge SL, Bryant A, Kehoe S, Morrison J. Neoadjuvant chemotherapy before surgery versus surgery followed by chemotherapy for initial treatment in advanced ovarian epithelial cancer. Cochrane Database Syst Rev. 2021;7(7):CD005343. doi:10.1002/14651858.CD005343.pub6
- Lim MC, Chang SJ, Park B, et al; HIPEC for Ovarian Cancer Collaborators. Survival after hyperthermic intraperitoneal chemotherapy and primary or interval cytoreductive surgery in ovarian cancer: a randomized clinical trial. JAMA Surg. 2022;157(5):374-383. doi:10.1001/jamasurg.2022.0143
- Walker JL, Brady MF, Wenzel L, et al. Randomized trial of intravenous versus intraperitoneal chemotherapy plus bevacizumab in advanced ovarian carcinoma: an NRG Oncology/Gynecologic Oncology Group study. J Clin Oncol. 2019;37(16):1380-1390. doi:10.1200/JCO.18.01568
- Konstantinopoulos PA, Lheureux S, Moore KN. PARP inhibitors for ovarian cancer: current indications, future combinations, and novel assets in development to target DNA damage repair. Am Soc Clin Oncol Educ Book. 2020;40:1-16. doi:10.1200/EDBK_288015
- Markman M, Bookman MA. Second-line treatment of ovarian cancer. Oncologist. 2000;5(1):26-35. doi:10.1634/theoncologist.5-1-26
- Markman M. Pharmaceutical management of ovarian cancer: current status. Drugs. 2019;79(11):1231-1239. doi:10.1007/s40265-019-01158-1
- Bristow RE, Puri I, Chi DS. Cytoreductive surgery for recurrent ovarian cancer: a meta-analysis. Gynecol Oncol. 2009;112(1):265-274. doi:10.1016/j.ygyno.2008.08.033
- de Bree E, Michelakis D, Anagnostopoulou E. The current role of secondary cytoreductive surgery for recurrent ovarian cancer. Front Oncol. 2022;12:1029976. doi:10.3389/fonc.2022.1029976
- Shah PD, Wethington SL, Pagan C, et al. Combination ATR and PARP inhibitor (CAPRI): a phase 2 study of ceralasertib plus olaparib in patients with recurrent, platinum-resistant epithelial ovarian cancer. Gynecol Oncol. 2021;163(2): 246-253. doi:10.1016/j.ygyno.2021.08.024
- Borella F, Ghisoni E, Giannone G, et al. Immune checkpoint inhibitors in epithelial ovarian cancer: an overview on efficacy and future perspectives. Diagnostics (Basel). 2020;10(3):146. doi:10.3390/diagnostics10030146
- Wu JWY, Dand S, Doig L, et al. T-cell receptor therapy in the treatment of ovarian cancer: a mini review. Front Immunol. 2021;12:672502. doi:10.3389/fimmu.2021.672502
- Benard E, Casey NP, Inderberg EM, Wälchli S. SJI 2020 special issue: a catalogue of ovarian cancer targets for CAR therapy. Scand J Immunol. 2020;92(4):e12917 doi:10.1111/sji.12917
- Martín-Sabroso C, Lozza I, Torres-Suárez AI, Fraguas-Sánchez AI. Antibodyantineoplastic conjugates in gynecological malignancies: current status and future perspectives. Pharmaceutics. 2021;13(10):1705. doi:10.3390/pharmaceutics13101705
- US Food and Drug Administration. FDA grants accelerated approval to mirvetuximab soravtansine-gynx for FRα positive, platinum-resistant epithelial ovarian, fallopian tube, or peritoneal cancer. Published November 14, 2022. Accessed May 8, 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-mirvetuximab-soravtansine-gynx-fra-positive-platinum-resistant
- Tolcher A, Hamilton E, Coleman RL. The evolving landscape of antibody-drug conjugates in gynecologic cancers. Cancer Treat Rev. 2023;116:102546. doi:10.1016/j.ctrv.2023.102546
- Banerjee S, Drapkin R, Richarson DL, Birrer M. Targeting NaPi2b in ovarian cancer. Cancer Treat Rev. 2023;112:102489. doi:10.1016/j.ctrv.2022.102489