User login
Photoallergic contact dermatitis (PACD), a subtype of allergic contact dermatitis that occurs because of the specific combination of exposure to an exogenous chemical applied topically to the skin and UV radiation, may be more common than was once thought.1 Although the incidence in the general population is unknown, current research points to approximately 20% to 40% of patients with suspected photosensitivity having a PACD diagnosis.2 Recently, the North American Contact Dermatitis Group (NACDG) reported that 21% of 373 patients undergoing photopatch testing (PPT) were diagnosed with PACD2; however, PPT is not routinely performed, which may contribute to underdiagnosis.
Mechanism of Disease
Similar to allergic contact dermatitis, PACD is a delayed type IV hypersensitivity reaction; however, it only occurs when an exogenous chemical is applied topically to the skin with concomitant exposure to UV radiation, usually in the UVA range (315–400 nm).3,4 When exposed to UV radiation, it is thought that the exogenous chemical combines with a protein in the skin and transforms into a photoantigen. In the sensitization phase, the photoantigen is taken up by antigen-presenting cells in the epidermis and transported to local lymph nodes where antigen-specific T cells are generated.5 In the elicitation phase, the inflammatory reaction of PACD occurs upon subsequent exposure to the same chemical plus UV radiation.4 Development of PACD does not necessarily depend on the dose of the chemical or the amount of UV radiation.6 Why certain individuals may be more susceptible is unknown, though major histocompatibility complex haplotypes could be influential.7,8
Clinical Manifestations
Photoallergic contact dermatitis primarily presents in sun-exposed areas of the skin (eg, face, neck, V area of the chest, dorsal upper extremities) with sparing of naturally photoprotected sites, such as the upper eyelids and nasolabial and retroauricular folds. Other than its characteristic photodistribution, PACD often is clinically indistinguishable from routine allergic contact dermatitis. It manifests as a pruritic, poorly demarcated, eczematous or sometimes vesiculobullous eruption that develops in a delayed fashion—24 to 72 hours after sun exposure. The dermatitis may extend to other parts of the body either through spread of the chemical agent by the hands or clothing or due to the systemic nature of the immune response. The severity of the presentation can vary depending on multiple factors, such as concentration and absorption of the agent, length of exposure, intensity and duration of UV radiation exposure, and individual susceptibility.4 Chronic PACD may become lichenified. Generally, rashes resolve after discontinuation of the causative agent; however, long-term exposure may lead to development of chronic actinic dermatitis, with persistent photodistributed eczema regardless of contact with the initial inciting agent.9
Differential Diagnosis
The differential diagnosis for patients presenting with photodistributed dermatitis is broad; therefore, taking a thorough history is important. Considerations include age of onset, timing and persistence of reactions, use of topical and systemic medications (both prescription and over-the-counter [OTC]), personal care products, occupation, and hobbies, as well as a thorough review of systems.
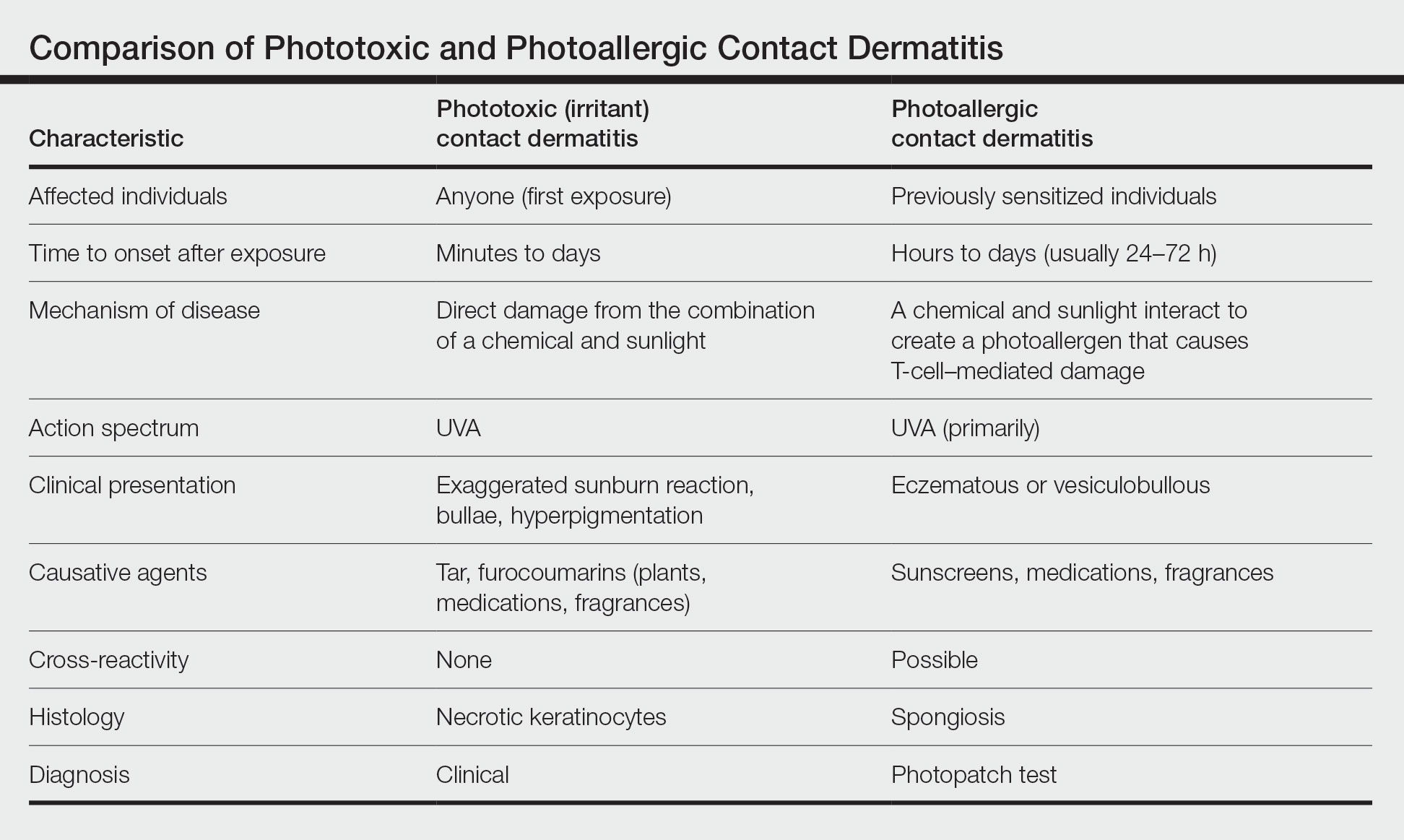
It is important to distinguish PACD from phototoxic contact dermatitis (PTCD)(also known as photoirritant contact dermatitis)(Table). Asking about the onset and timing of the eruption may be critical for distinction, as PTCD can occur within minutes to hours of the first exposure to a chemical and UV radiation, while there is a sensitization delay in PACD.6 Phytophotodermatitis is a well-known type of PTCD caused by exposure to furocoumarin-containing plants, most commonly limes.10 Other causes of PTCD include tar products and certain medications.11 Importantly, PPT to a known phototoxic chemical should never be performed because it will cause a strong reaction in anyone tested, regardless of exposure history.
Other diagnoses to consider include photoaggravated dermatoses (eg, atopic dermatitis, lupus erythematosus, dermatomyositis) and idiopathic photodermatoses (eg, chronic actinic dermatitis, actinic prurigo, polymorphous light eruption). Although atopic dermatitis usually improves with UV light exposure, photoaggravated atopic dermatitis is suggested in eczema patients who flare with sun exposure, in a seasonal pattern, or after phototherapy; this condition is challenging to differentiate from PACD if PPT is not performed.12 The diagnosis of idiopathic photodermatoses is nuanced; however, asking about the timeline of the reaction including onset, duration, and persistence, as well as characterization of unique clinical features, can help in differentiation.13 In certain scenarios, a biopsy may be helpful. A thorough review of systems will help to assess for autoimmune connective tissue disorders, and relevant serologies should be checked as indicated.
Diagnosis
Histologically, PACD presents similarly to allergic contact dermatitis with spongiotic dermatitis; therefore, biopsy cannot be relied upon to make the diagnosis.6 Photopatch testing is required for definitive diagnosis. It is reasonable to perform PPT in any patient with chronic dermatitis primarily affecting sun-exposed areas without a clear alternative diagnosis.14,15 Of note, at present there are no North American consensus guidelines for PPT, but typically duplicate sets of photoallergens are applied to both sides of the patient’s back and one side is exposed to UVA radiation. The reactions are compared after 48 to 96 hours.15 A positive reaction only at the irradiated site is consistent with photoallergy, while a reaction of equal strength at both the irradiated and nonirradiated sites indicates regular contact allergy. The case of a reaction occurring at both sites with a stronger response at the irradiated site is known as photoaggravated contact allergy, which can be thought of as allergic contact dermatitis that worsens but does not solely occur with exposure to sunlight.
Although PPT is necessary for the accurate diagnosis of PACD, it is infrequently used. Two surveys of 112 and 117 American Contact Dermatitis Society members, respectively, have revealed that only around half performed PPT, most of them testing fewer than 20 times per year.16,17 Additionally, there was variability in the test methodology and allergens employed. Nevertheless, most respondents tested sunscreens, nonsteroidal anti-inflammatory drugs (NSAIDs), fragrances, and their patients’ own products.16,17 The most common reasons for not performing PPT were lack of equipment, insufficient skills, rare clinical suspicion, and cost. Dermatologists at academic centers performed more PPT than those in other practice settings, including multispecialty group practices and private offices.16 These findings highlight multiple factors that may contribute to reduced patient access to PPT and thus potential underdiagnosis of PACD.
Common Photoallergens
The most common photoallergens change over time in response to market trends; for example, fragrance was once a top photoallergen in the United States in the 1970s and 1980s but declined in prominence after musk ambrette—the primary allergen associated with PACD at the time—was removed as an ingredient in fragrances.18
In the largest and most recent PPT series from North America (1999-2009),2 sunscreens comprised 7 of the top 10 most common photoallergens, which is consistent with other studies showing sunscreens to be the most common North American photoallergens.19-22 The frequency of PACD due to sunscreens likely relates to their increasing use worldwide as awareness of photocarcinogenesis and photoaging grows, as well as the common use of UV filters in nonsunscreen personal care products, ranging from lip balms to perfumes and bodywashes. Chemical (organic) UV filters—in particular oxybenzone (benzophenone-3) and avobenzone (butyl methoxydibenzoylmethane)—are the most common sunscreen photoallergens.2,23 Para-aminobenzoic acid was once a common photoallergen, but it is no longer used in US sunscreens due to safety concerns.19,20 The physical (inorganic) UV filters zinc oxide and titanium dioxide are not known photosensitizers.
Methylisothiazolinone (MI) is a highly allergenic preservative commonly used in a wide array of personal care products, including sunscreens.24 In the most recent NACDG patch test data, MI was the second most common contact allergen.25 Allergic contact dermatitis caused by MI in sunscreen can mimic PACD.26 In addition, MI can cause photoaggravated contact dermatitis, with some affected patients experiencing ongoing photosensitivity even after avoiding this allergen.26-30 The European Union and Canada have introduced restrictions on the use of MI in personal care products, but no such regulatory measures have been taken in the United States to date.25,31,32
After sunscreens, another common cause of PACD are topical NSAIDs, which are frequently used for musculoskeletal pain relief. These are of particular concern in Europe, where a variety of formulations are widely available OTC.33 Ketoprofen and etofenamate are responsible for the largest number of PACD reactions in Europe.2,34,35 Meanwhile, the only OTC topical NSAID available in the United States is diclofenac gel, which was approved in 2020. Cases of PACD due to use of diclofenac gel have been reported in the literature, but testing in larger populations is needed.36-39
Notably, ketoprofen may co- or cross-react with certain UV filters—oxybenzone and octocrylene—and the lipid-lowering agent fenofibrate due to chemical similarities.40-43 Despite the relatively high number of photoallergic reactions to ketoprofen in the NACDG photopatch series, only 25% (5/20) were considered clinically relevant (ie, the allergen could not be verified as present in the known skin contactants of the patient, and the patient was not exposed to circumstances in which contact with materials known to contain the allergen would likely occur), which suggests that they likely represented cross-reactions in patients sensitized to sunscreens.2
Other agents that may cause PACD include antimicrobials, plants and plant derivatives, and pesticides.2,4,18 The antimicrobial fentichlor is a common cause of positive PPT reactions, but it rarely is clinically relevant.44
Treatment
The primary management of PACD centers on identification of the causative photoallergen to avoid future exposure. Patients should be educated on the various names by which the causative allergen can be identified on product labels and should be given a list of safe products that are free from relevant allergens and cross-reacting chemicals.45 Additionally, sun protection education should be provided. Exposure to UVA radiation can occur through windows, making the use of broad-spectrum sunscreens and protective clothing crucial. In cases of sunscreen-induced PACD, the responsible chemical UV filter(s) should be avoided, or alternatively, patients may use physical sunscreens containing only zinc oxide and/or titanium dioxide as active ingredients, as these are not known to cause PACD.4
When avoidance alone is insufficient, topical corticosteroids are the usual first-line treatment for localized PACD. When steroid-sparing treatments are preferred, topical calcineurin inhibitors such as tacrolimus and pimecrolimus may be used. If PACD is more widespread and severe, systemic therapy using steroids or steroid-sparing agents may be necessary to provide symptomatic relief.4
Final Interpretation
Photoallergic contact dermatitis is not uncommon, particularly among photosensitive patients. Most cases are due to sunscreens or topical NSAIDs. Consideration of PPT should be given in any patient with a chronic photodistributed dermatitis to evaluate for the possibility of PACD.
- Darvay A, White IR, Rycroft RJ, et al. Photoallergic contact dermatitis is uncommon. Br J Dermatol. 2001;145:597-601.
- DeLeo VA, Adler BL, Warshaw EM, et al. Photopatch test results of the North American contact dermatitis group, 1999-2009. Photodermatol Photoimmunol Photomed. 2022;38:288-291.
- Kerr A, Ferguson J. Photoallergic contact dermatitis. Photodermatol Photoimmunol Photomed. 2010;26:56-65.
- As¸kın Ö, Cesur SK, Engin B, et al. Photoallergic contact dermatitis. Curr Derm Rep. 2019;8:157-163.
- Wilm A, Berneburg M. Photoallergy. J Dtsch Dermatol Ges. 2015;13:7-13.
- DeLeo VA. Photocontact dermatitis. Dermatol Ther. 2004;17:279-288.
- Imai S, Atarashi K, Ikesue K, et al. Establishment of murine model of allergic photocontact dermatitis to ketoprofen and characterization of pathogenic T cells. J Dermatol Sci. 2006;41:127-136.
- Tokura Y, Yagi H, Satoh T, et al. Inhibitory effect of melanin pigment on sensitization and elicitation of murine contact photosensitivity: mechanism of low responsiveness in C57BL/10 background mice. J Invest Dermatol. 1993;101:673-678.
- Stein KR, Scheinfeld NS. Drug-induced photoallergic and phototoxic reactions. Expert Opin Drug Saf. 2007;6:431-443.
- Janusz SC, Schwartz RA. Botanical briefs: phytophotodermatitis is an occupational and recreational dermatosis in the limelight. Cutis. 2021;107:187-189.
- Atwal SK, Chen A, Adler BL. Phototoxic contact dermatitis from over-the-counter 8-methoxypsoralen. Cutis. 2022;109:E2-E3.
- Rutter KJ, Farrar MD, Marjanovic EJ, et al. Clinicophotobiological characterization of photoaggravated atopic dermatitis [published online July 27, 2022]. JAMA Dermatol. doi:10.1001/jamadermatol.2022.2823
- Lecha M. Idiopathic photodermatoses: clinical, diagnostic and therapeutic aspects. J Eur Acad Dermatol Venereol. 2001;15:499-505.
- Marks JG Jr, Anderson BE, DeLeo VA. Contact & Occupational Dermatology. 4th ed. Jaypee Brothers; 2016.
- Bruynzeel DP, Ferguson J, Andersen K, et al. Photopatch testing: a consensus methodology for Europe. J Eur Acad Dermatol Venereol. 2004;18:679-682.
- Kim T, Taylor JS, Maibach HI, et al. Photopatch testing among members of the American Contact Dermatitis Society. Dermatitis. 2020;31:59-67.
- Asemota E, Crawford G, Kovarik C, et al. A survey examining photopatch test and phototest methodologies of contact dermatologists in the United States: platform for developing a consensus. Dermatitis. 2017;28:265-269.
- Scalf LA, Davis MD, Rohlinger AL, et al. Photopatch testing of 182 patients: a 6-year experience at the Mayo Clinic. Dermatitis. 2009;20:44-52.
- Greenspoon J, Ahluwalia R, Juma N, et al. Allergic and photoallergic contact dermatitis: a 10-year experience. Dermatitis. 2013;24:29-32.
- Victor FC, Cohen DE, Soter NA. A 20-year analysis of previous and emerging allergens that elicit photoallergic contact dermatitis. J Am Acad Dermatol. 2010;62:605-610.
- Schauder S, Ippen H. Contact and photocontact sensitivity to sunscreens. review of a 15-year experience and of the literature. Contact Dermatitis. 1997;37:221-232.
- Collaris EJ, Frank J. Photoallergic contact dermatitis caused by ultraviolet filters in different sunscreens. Int J Dermatol. 2008;47(suppl 1):35-37.
- Heurung AR, Raju SI, Warshaw EM. Adverse reactions to sunscreen agents: epidemiology, responsible irritants and allergens, clinical characteristics, and management. Dermatitis. 2014;25:289-326.
- Reeder M, Atwater AR. Methylisothiazolinone and isothiazolinone allergy. Cutis. 2019;104:94-96.
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group Patch Test Results: 2017-2018. Dermatitis. 2021;32:111-123.
- Kullberg SA, Voller LM, Warshaw EM. Methylisothiazolinone in “dermatology-recommended” sunscreens: an important mimicker of photoallergic contact dermatitis. Photodermatol Photoimmunol Photomed. 2021;37:366-370.
- Herman A, Aerts O, de Montjoye L, et al. Isothiazolinone derivatives and allergic contact dermatitis: a review and update. J Eur Acad Dermatol Venereol. 2019;33:267-276.
- Adler BL, Houle MC, Pratt M. Photoaggravated contact dermatitis to methylisothiazolinone and associated photosensitivity: a case series [published online January 25, 2022]. Dermatitis. doi:10.1097/DER.0000000000000833
- Aerts O, Goossens A, Marguery MC, et al. Photoaggravated allergic contact dermatitis and transient photosensitivity caused by methylisothiazolinone. Contact Dermatitis. 2018;78:241-245.
- Pirmez R, Fernandes AL, Melo MG. Photoaggravated contact dermatitis to Kathon CG (methylchloroisothiazolinone/methylisothiazolinone): a novel pattern of involvement in a growing epidemic?. Br J Dermatol. 2015;173:1343-1344.
- Uter W, Aalto-Korte K, Agner T, et al. The epidemic of methylisothiazolinone contact allergy in Europe: follow-up on changing exposures.J Eur Acad Dermatol Venereol. 2020;34:333-339.
- Government of Canada. Changes to the cosmetic ingredient hotlist. December 3, 2019. Updated August 26, 2022. Accessed October 20, 2022. https://www.canada.ca/en/health-canada/services/consumer-product-safety/cosmetics/cosmetic-ingredient-hotlist-prohibited-restricted-ingredients/changes.html
- Barkin RL. Topical nonsteroidal anti-inflammatory drugs: the importance of drug, delivery, and therapeutic outcome. Am J Ther. 2015;22:388-407.
- European Multicentre Photopatch Test Study (EMCPPTS) Taskforce. A European multicentre photopatch test study. Br J Dermatol. 2012;166:1002-1009.
- Ophaswongse S, Maibach H. Topical nonsteroidal antiinflammatory drugs: allergic and photoallergic contact dermatitis and phototoxicity. Contact Dermatitis. 1993;29:57-64.
- Kowalzick L, Ziegler H. Photoallergic contact dermatitis from topical diclofenac in Solaraze gel. Contact Dermatitis. 2006;54:348-349.
- Montoro J, Rodríguez M, Díaz M, et al. Photoallergic contact dermatitis due to diclofenac. Contact Dermatitis. 2003;48:115.
- Fernández-Jorge B, Goday-Buján JJ, Murga M, et al. Photoallergic contact dermatitis due to diclofenac with cross-reaction to aceclofenac: two case reports. Contact Dermatitis. 2009;61:236-237.
- Akat PB. Severe photosensitivity reaction induced by topical diclofenac. Indian J Pharmacol. 2013;45:408-409.
- Leroy D, Dompmartin A, Szczurko C, et al. Photodermatitis from ketoprofen with cross-reactivity to fenofibrate and benzophenones. Photodermatol Photoimmunol Photomed. 1997;13:93-97.
- Devleeschouwer V, Roelandts R, Garmyn M, et al. Allergic and photoallergic contact dermatitis from ketoprofen: results of (photo) patch testing and follow-up of 42 patients. Contact Dermatitis. 2008;58:159-166.
- Matsushita T, Kamide R. Five cases of photocontact dermatitisdue to topical ketoprofen: photopatch testing and cross-reaction study. Photodermatol Photoimmunol Photomed. 2001;17:26-31.
- de Groot AC, Roberts DW. Contact and photocontact allergy to octocrylene: a review. Contact Dermatitis. 2014;70:193-204.
- Wolverton JE, Soter NA, Cohen DE. Fentichlor photocontact dermatitis: a persistent enigma. Dermatitis. 2013;24:77-81.
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient management and education. J Am Acad Dermatol. 2016;74:1043-1054.
Photoallergic contact dermatitis (PACD), a subtype of allergic contact dermatitis that occurs because of the specific combination of exposure to an exogenous chemical applied topically to the skin and UV radiation, may be more common than was once thought.1 Although the incidence in the general population is unknown, current research points to approximately 20% to 40% of patients with suspected photosensitivity having a PACD diagnosis.2 Recently, the North American Contact Dermatitis Group (NACDG) reported that 21% of 373 patients undergoing photopatch testing (PPT) were diagnosed with PACD2; however, PPT is not routinely performed, which may contribute to underdiagnosis.
Mechanism of Disease
Similar to allergic contact dermatitis, PACD is a delayed type IV hypersensitivity reaction; however, it only occurs when an exogenous chemical is applied topically to the skin with concomitant exposure to UV radiation, usually in the UVA range (315–400 nm).3,4 When exposed to UV radiation, it is thought that the exogenous chemical combines with a protein in the skin and transforms into a photoantigen. In the sensitization phase, the photoantigen is taken up by antigen-presenting cells in the epidermis and transported to local lymph nodes where antigen-specific T cells are generated.5 In the elicitation phase, the inflammatory reaction of PACD occurs upon subsequent exposure to the same chemical plus UV radiation.4 Development of PACD does not necessarily depend on the dose of the chemical or the amount of UV radiation.6 Why certain individuals may be more susceptible is unknown, though major histocompatibility complex haplotypes could be influential.7,8
Clinical Manifestations
Photoallergic contact dermatitis primarily presents in sun-exposed areas of the skin (eg, face, neck, V area of the chest, dorsal upper extremities) with sparing of naturally photoprotected sites, such as the upper eyelids and nasolabial and retroauricular folds. Other than its characteristic photodistribution, PACD often is clinically indistinguishable from routine allergic contact dermatitis. It manifests as a pruritic, poorly demarcated, eczematous or sometimes vesiculobullous eruption that develops in a delayed fashion—24 to 72 hours after sun exposure. The dermatitis may extend to other parts of the body either through spread of the chemical agent by the hands or clothing or due to the systemic nature of the immune response. The severity of the presentation can vary depending on multiple factors, such as concentration and absorption of the agent, length of exposure, intensity and duration of UV radiation exposure, and individual susceptibility.4 Chronic PACD may become lichenified. Generally, rashes resolve after discontinuation of the causative agent; however, long-term exposure may lead to development of chronic actinic dermatitis, with persistent photodistributed eczema regardless of contact with the initial inciting agent.9
Differential Diagnosis
The differential diagnosis for patients presenting with photodistributed dermatitis is broad; therefore, taking a thorough history is important. Considerations include age of onset, timing and persistence of reactions, use of topical and systemic medications (both prescription and over-the-counter [OTC]), personal care products, occupation, and hobbies, as well as a thorough review of systems.
It is important to distinguish PACD from phototoxic contact dermatitis (PTCD)(also known as photoirritant contact dermatitis)(Table). Asking about the onset and timing of the eruption may be critical for distinction, as PTCD can occur within minutes to hours of the first exposure to a chemical and UV radiation, while there is a sensitization delay in PACD.6 Phytophotodermatitis is a well-known type of PTCD caused by exposure to furocoumarin-containing plants, most commonly limes.10 Other causes of PTCD include tar products and certain medications.11 Importantly, PPT to a known phototoxic chemical should never be performed because it will cause a strong reaction in anyone tested, regardless of exposure history.

Other diagnoses to consider include photoaggravated dermatoses (eg, atopic dermatitis, lupus erythematosus, dermatomyositis) and idiopathic photodermatoses (eg, chronic actinic dermatitis, actinic prurigo, polymorphous light eruption). Although atopic dermatitis usually improves with UV light exposure, photoaggravated atopic dermatitis is suggested in eczema patients who flare with sun exposure, in a seasonal pattern, or after phototherapy; this condition is challenging to differentiate from PACD if PPT is not performed.12 The diagnosis of idiopathic photodermatoses is nuanced; however, asking about the timeline of the reaction including onset, duration, and persistence, as well as characterization of unique clinical features, can help in differentiation.13 In certain scenarios, a biopsy may be helpful. A thorough review of systems will help to assess for autoimmune connective tissue disorders, and relevant serologies should be checked as indicated.
Diagnosis
Histologically, PACD presents similarly to allergic contact dermatitis with spongiotic dermatitis; therefore, biopsy cannot be relied upon to make the diagnosis.6 Photopatch testing is required for definitive diagnosis. It is reasonable to perform PPT in any patient with chronic dermatitis primarily affecting sun-exposed areas without a clear alternative diagnosis.14,15 Of note, at present there are no North American consensus guidelines for PPT, but typically duplicate sets of photoallergens are applied to both sides of the patient’s back and one side is exposed to UVA radiation. The reactions are compared after 48 to 96 hours.15 A positive reaction only at the irradiated site is consistent with photoallergy, while a reaction of equal strength at both the irradiated and nonirradiated sites indicates regular contact allergy. The case of a reaction occurring at both sites with a stronger response at the irradiated site is known as photoaggravated contact allergy, which can be thought of as allergic contact dermatitis that worsens but does not solely occur with exposure to sunlight.
Although PPT is necessary for the accurate diagnosis of PACD, it is infrequently used. Two surveys of 112 and 117 American Contact Dermatitis Society members, respectively, have revealed that only around half performed PPT, most of them testing fewer than 20 times per year.16,17 Additionally, there was variability in the test methodology and allergens employed. Nevertheless, most respondents tested sunscreens, nonsteroidal anti-inflammatory drugs (NSAIDs), fragrances, and their patients’ own products.16,17 The most common reasons for not performing PPT were lack of equipment, insufficient skills, rare clinical suspicion, and cost. Dermatologists at academic centers performed more PPT than those in other practice settings, including multispecialty group practices and private offices.16 These findings highlight multiple factors that may contribute to reduced patient access to PPT and thus potential underdiagnosis of PACD.
Common Photoallergens
The most common photoallergens change over time in response to market trends; for example, fragrance was once a top photoallergen in the United States in the 1970s and 1980s but declined in prominence after musk ambrette—the primary allergen associated with PACD at the time—was removed as an ingredient in fragrances.18
In the largest and most recent PPT series from North America (1999-2009),2 sunscreens comprised 7 of the top 10 most common photoallergens, which is consistent with other studies showing sunscreens to be the most common North American photoallergens.19-22 The frequency of PACD due to sunscreens likely relates to their increasing use worldwide as awareness of photocarcinogenesis and photoaging grows, as well as the common use of UV filters in nonsunscreen personal care products, ranging from lip balms to perfumes and bodywashes. Chemical (organic) UV filters—in particular oxybenzone (benzophenone-3) and avobenzone (butyl methoxydibenzoylmethane)—are the most common sunscreen photoallergens.2,23 Para-aminobenzoic acid was once a common photoallergen, but it is no longer used in US sunscreens due to safety concerns.19,20 The physical (inorganic) UV filters zinc oxide and titanium dioxide are not known photosensitizers.
Methylisothiazolinone (MI) is a highly allergenic preservative commonly used in a wide array of personal care products, including sunscreens.24 In the most recent NACDG patch test data, MI was the second most common contact allergen.25 Allergic contact dermatitis caused by MI in sunscreen can mimic PACD.26 In addition, MI can cause photoaggravated contact dermatitis, with some affected patients experiencing ongoing photosensitivity even after avoiding this allergen.26-30 The European Union and Canada have introduced restrictions on the use of MI in personal care products, but no such regulatory measures have been taken in the United States to date.25,31,32
After sunscreens, another common cause of PACD are topical NSAIDs, which are frequently used for musculoskeletal pain relief. These are of particular concern in Europe, where a variety of formulations are widely available OTC.33 Ketoprofen and etofenamate are responsible for the largest number of PACD reactions in Europe.2,34,35 Meanwhile, the only OTC topical NSAID available in the United States is diclofenac gel, which was approved in 2020. Cases of PACD due to use of diclofenac gel have been reported in the literature, but testing in larger populations is needed.36-39
Notably, ketoprofen may co- or cross-react with certain UV filters—oxybenzone and octocrylene—and the lipid-lowering agent fenofibrate due to chemical similarities.40-43 Despite the relatively high number of photoallergic reactions to ketoprofen in the NACDG photopatch series, only 25% (5/20) were considered clinically relevant (ie, the allergen could not be verified as present in the known skin contactants of the patient, and the patient was not exposed to circumstances in which contact with materials known to contain the allergen would likely occur), which suggests that they likely represented cross-reactions in patients sensitized to sunscreens.2
Other agents that may cause PACD include antimicrobials, plants and plant derivatives, and pesticides.2,4,18 The antimicrobial fentichlor is a common cause of positive PPT reactions, but it rarely is clinically relevant.44
Treatment
The primary management of PACD centers on identification of the causative photoallergen to avoid future exposure. Patients should be educated on the various names by which the causative allergen can be identified on product labels and should be given a list of safe products that are free from relevant allergens and cross-reacting chemicals.45 Additionally, sun protection education should be provided. Exposure to UVA radiation can occur through windows, making the use of broad-spectrum sunscreens and protective clothing crucial. In cases of sunscreen-induced PACD, the responsible chemical UV filter(s) should be avoided, or alternatively, patients may use physical sunscreens containing only zinc oxide and/or titanium dioxide as active ingredients, as these are not known to cause PACD.4
When avoidance alone is insufficient, topical corticosteroids are the usual first-line treatment for localized PACD. When steroid-sparing treatments are preferred, topical calcineurin inhibitors such as tacrolimus and pimecrolimus may be used. If PACD is more widespread and severe, systemic therapy using steroids or steroid-sparing agents may be necessary to provide symptomatic relief.4
Final Interpretation
Photoallergic contact dermatitis is not uncommon, particularly among photosensitive patients. Most cases are due to sunscreens or topical NSAIDs. Consideration of PPT should be given in any patient with a chronic photodistributed dermatitis to evaluate for the possibility of PACD.
Photoallergic contact dermatitis (PACD), a subtype of allergic contact dermatitis that occurs because of the specific combination of exposure to an exogenous chemical applied topically to the skin and UV radiation, may be more common than was once thought.1 Although the incidence in the general population is unknown, current research points to approximately 20% to 40% of patients with suspected photosensitivity having a PACD diagnosis.2 Recently, the North American Contact Dermatitis Group (NACDG) reported that 21% of 373 patients undergoing photopatch testing (PPT) were diagnosed with PACD2; however, PPT is not routinely performed, which may contribute to underdiagnosis.
Mechanism of Disease
Similar to allergic contact dermatitis, PACD is a delayed type IV hypersensitivity reaction; however, it only occurs when an exogenous chemical is applied topically to the skin with concomitant exposure to UV radiation, usually in the UVA range (315–400 nm).3,4 When exposed to UV radiation, it is thought that the exogenous chemical combines with a protein in the skin and transforms into a photoantigen. In the sensitization phase, the photoantigen is taken up by antigen-presenting cells in the epidermis and transported to local lymph nodes where antigen-specific T cells are generated.5 In the elicitation phase, the inflammatory reaction of PACD occurs upon subsequent exposure to the same chemical plus UV radiation.4 Development of PACD does not necessarily depend on the dose of the chemical or the amount of UV radiation.6 Why certain individuals may be more susceptible is unknown, though major histocompatibility complex haplotypes could be influential.7,8
Clinical Manifestations
Photoallergic contact dermatitis primarily presents in sun-exposed areas of the skin (eg, face, neck, V area of the chest, dorsal upper extremities) with sparing of naturally photoprotected sites, such as the upper eyelids and nasolabial and retroauricular folds. Other than its characteristic photodistribution, PACD often is clinically indistinguishable from routine allergic contact dermatitis. It manifests as a pruritic, poorly demarcated, eczematous or sometimes vesiculobullous eruption that develops in a delayed fashion—24 to 72 hours after sun exposure. The dermatitis may extend to other parts of the body either through spread of the chemical agent by the hands or clothing or due to the systemic nature of the immune response. The severity of the presentation can vary depending on multiple factors, such as concentration and absorption of the agent, length of exposure, intensity and duration of UV radiation exposure, and individual susceptibility.4 Chronic PACD may become lichenified. Generally, rashes resolve after discontinuation of the causative agent; however, long-term exposure may lead to development of chronic actinic dermatitis, with persistent photodistributed eczema regardless of contact with the initial inciting agent.9
Differential Diagnosis
The differential diagnosis for patients presenting with photodistributed dermatitis is broad; therefore, taking a thorough history is important. Considerations include age of onset, timing and persistence of reactions, use of topical and systemic medications (both prescription and over-the-counter [OTC]), personal care products, occupation, and hobbies, as well as a thorough review of systems.
It is important to distinguish PACD from phototoxic contact dermatitis (PTCD)(also known as photoirritant contact dermatitis)(Table). Asking about the onset and timing of the eruption may be critical for distinction, as PTCD can occur within minutes to hours of the first exposure to a chemical and UV radiation, while there is a sensitization delay in PACD.6 Phytophotodermatitis is a well-known type of PTCD caused by exposure to furocoumarin-containing plants, most commonly limes.10 Other causes of PTCD include tar products and certain medications.11 Importantly, PPT to a known phototoxic chemical should never be performed because it will cause a strong reaction in anyone tested, regardless of exposure history.

Other diagnoses to consider include photoaggravated dermatoses (eg, atopic dermatitis, lupus erythematosus, dermatomyositis) and idiopathic photodermatoses (eg, chronic actinic dermatitis, actinic prurigo, polymorphous light eruption). Although atopic dermatitis usually improves with UV light exposure, photoaggravated atopic dermatitis is suggested in eczema patients who flare with sun exposure, in a seasonal pattern, or after phototherapy; this condition is challenging to differentiate from PACD if PPT is not performed.12 The diagnosis of idiopathic photodermatoses is nuanced; however, asking about the timeline of the reaction including onset, duration, and persistence, as well as characterization of unique clinical features, can help in differentiation.13 In certain scenarios, a biopsy may be helpful. A thorough review of systems will help to assess for autoimmune connective tissue disorders, and relevant serologies should be checked as indicated.
Diagnosis
Histologically, PACD presents similarly to allergic contact dermatitis with spongiotic dermatitis; therefore, biopsy cannot be relied upon to make the diagnosis.6 Photopatch testing is required for definitive diagnosis. It is reasonable to perform PPT in any patient with chronic dermatitis primarily affecting sun-exposed areas without a clear alternative diagnosis.14,15 Of note, at present there are no North American consensus guidelines for PPT, but typically duplicate sets of photoallergens are applied to both sides of the patient’s back and one side is exposed to UVA radiation. The reactions are compared after 48 to 96 hours.15 A positive reaction only at the irradiated site is consistent with photoallergy, while a reaction of equal strength at both the irradiated and nonirradiated sites indicates regular contact allergy. The case of a reaction occurring at both sites with a stronger response at the irradiated site is known as photoaggravated contact allergy, which can be thought of as allergic contact dermatitis that worsens but does not solely occur with exposure to sunlight.
Although PPT is necessary for the accurate diagnosis of PACD, it is infrequently used. Two surveys of 112 and 117 American Contact Dermatitis Society members, respectively, have revealed that only around half performed PPT, most of them testing fewer than 20 times per year.16,17 Additionally, there was variability in the test methodology and allergens employed. Nevertheless, most respondents tested sunscreens, nonsteroidal anti-inflammatory drugs (NSAIDs), fragrances, and their patients’ own products.16,17 The most common reasons for not performing PPT were lack of equipment, insufficient skills, rare clinical suspicion, and cost. Dermatologists at academic centers performed more PPT than those in other practice settings, including multispecialty group practices and private offices.16 These findings highlight multiple factors that may contribute to reduced patient access to PPT and thus potential underdiagnosis of PACD.
Common Photoallergens
The most common photoallergens change over time in response to market trends; for example, fragrance was once a top photoallergen in the United States in the 1970s and 1980s but declined in prominence after musk ambrette—the primary allergen associated with PACD at the time—was removed as an ingredient in fragrances.18
In the largest and most recent PPT series from North America (1999-2009),2 sunscreens comprised 7 of the top 10 most common photoallergens, which is consistent with other studies showing sunscreens to be the most common North American photoallergens.19-22 The frequency of PACD due to sunscreens likely relates to their increasing use worldwide as awareness of photocarcinogenesis and photoaging grows, as well as the common use of UV filters in nonsunscreen personal care products, ranging from lip balms to perfumes and bodywashes. Chemical (organic) UV filters—in particular oxybenzone (benzophenone-3) and avobenzone (butyl methoxydibenzoylmethane)—are the most common sunscreen photoallergens.2,23 Para-aminobenzoic acid was once a common photoallergen, but it is no longer used in US sunscreens due to safety concerns.19,20 The physical (inorganic) UV filters zinc oxide and titanium dioxide are not known photosensitizers.
Methylisothiazolinone (MI) is a highly allergenic preservative commonly used in a wide array of personal care products, including sunscreens.24 In the most recent NACDG patch test data, MI was the second most common contact allergen.25 Allergic contact dermatitis caused by MI in sunscreen can mimic PACD.26 In addition, MI can cause photoaggravated contact dermatitis, with some affected patients experiencing ongoing photosensitivity even after avoiding this allergen.26-30 The European Union and Canada have introduced restrictions on the use of MI in personal care products, but no such regulatory measures have been taken in the United States to date.25,31,32
After sunscreens, another common cause of PACD are topical NSAIDs, which are frequently used for musculoskeletal pain relief. These are of particular concern in Europe, where a variety of formulations are widely available OTC.33 Ketoprofen and etofenamate are responsible for the largest number of PACD reactions in Europe.2,34,35 Meanwhile, the only OTC topical NSAID available in the United States is diclofenac gel, which was approved in 2020. Cases of PACD due to use of diclofenac gel have been reported in the literature, but testing in larger populations is needed.36-39
Notably, ketoprofen may co- or cross-react with certain UV filters—oxybenzone and octocrylene—and the lipid-lowering agent fenofibrate due to chemical similarities.40-43 Despite the relatively high number of photoallergic reactions to ketoprofen in the NACDG photopatch series, only 25% (5/20) were considered clinically relevant (ie, the allergen could not be verified as present in the known skin contactants of the patient, and the patient was not exposed to circumstances in which contact with materials known to contain the allergen would likely occur), which suggests that they likely represented cross-reactions in patients sensitized to sunscreens.2
Other agents that may cause PACD include antimicrobials, plants and plant derivatives, and pesticides.2,4,18 The antimicrobial fentichlor is a common cause of positive PPT reactions, but it rarely is clinically relevant.44
Treatment
The primary management of PACD centers on identification of the causative photoallergen to avoid future exposure. Patients should be educated on the various names by which the causative allergen can be identified on product labels and should be given a list of safe products that are free from relevant allergens and cross-reacting chemicals.45 Additionally, sun protection education should be provided. Exposure to UVA radiation can occur through windows, making the use of broad-spectrum sunscreens and protective clothing crucial. In cases of sunscreen-induced PACD, the responsible chemical UV filter(s) should be avoided, or alternatively, patients may use physical sunscreens containing only zinc oxide and/or titanium dioxide as active ingredients, as these are not known to cause PACD.4
When avoidance alone is insufficient, topical corticosteroids are the usual first-line treatment for localized PACD. When steroid-sparing treatments are preferred, topical calcineurin inhibitors such as tacrolimus and pimecrolimus may be used. If PACD is more widespread and severe, systemic therapy using steroids or steroid-sparing agents may be necessary to provide symptomatic relief.4
Final Interpretation
Photoallergic contact dermatitis is not uncommon, particularly among photosensitive patients. Most cases are due to sunscreens or topical NSAIDs. Consideration of PPT should be given in any patient with a chronic photodistributed dermatitis to evaluate for the possibility of PACD.
- Darvay A, White IR, Rycroft RJ, et al. Photoallergic contact dermatitis is uncommon. Br J Dermatol. 2001;145:597-601.
- DeLeo VA, Adler BL, Warshaw EM, et al. Photopatch test results of the North American contact dermatitis group, 1999-2009. Photodermatol Photoimmunol Photomed. 2022;38:288-291.
- Kerr A, Ferguson J. Photoallergic contact dermatitis. Photodermatol Photoimmunol Photomed. 2010;26:56-65.
- As¸kın Ö, Cesur SK, Engin B, et al. Photoallergic contact dermatitis. Curr Derm Rep. 2019;8:157-163.
- Wilm A, Berneburg M. Photoallergy. J Dtsch Dermatol Ges. 2015;13:7-13.
- DeLeo VA. Photocontact dermatitis. Dermatol Ther. 2004;17:279-288.
- Imai S, Atarashi K, Ikesue K, et al. Establishment of murine model of allergic photocontact dermatitis to ketoprofen and characterization of pathogenic T cells. J Dermatol Sci. 2006;41:127-136.
- Tokura Y, Yagi H, Satoh T, et al. Inhibitory effect of melanin pigment on sensitization and elicitation of murine contact photosensitivity: mechanism of low responsiveness in C57BL/10 background mice. J Invest Dermatol. 1993;101:673-678.
- Stein KR, Scheinfeld NS. Drug-induced photoallergic and phototoxic reactions. Expert Opin Drug Saf. 2007;6:431-443.
- Janusz SC, Schwartz RA. Botanical briefs: phytophotodermatitis is an occupational and recreational dermatosis in the limelight. Cutis. 2021;107:187-189.
- Atwal SK, Chen A, Adler BL. Phototoxic contact dermatitis from over-the-counter 8-methoxypsoralen. Cutis. 2022;109:E2-E3.
- Rutter KJ, Farrar MD, Marjanovic EJ, et al. Clinicophotobiological characterization of photoaggravated atopic dermatitis [published online July 27, 2022]. JAMA Dermatol. doi:10.1001/jamadermatol.2022.2823
- Lecha M. Idiopathic photodermatoses: clinical, diagnostic and therapeutic aspects. J Eur Acad Dermatol Venereol. 2001;15:499-505.
- Marks JG Jr, Anderson BE, DeLeo VA. Contact & Occupational Dermatology. 4th ed. Jaypee Brothers; 2016.
- Bruynzeel DP, Ferguson J, Andersen K, et al. Photopatch testing: a consensus methodology for Europe. J Eur Acad Dermatol Venereol. 2004;18:679-682.
- Kim T, Taylor JS, Maibach HI, et al. Photopatch testing among members of the American Contact Dermatitis Society. Dermatitis. 2020;31:59-67.
- Asemota E, Crawford G, Kovarik C, et al. A survey examining photopatch test and phototest methodologies of contact dermatologists in the United States: platform for developing a consensus. Dermatitis. 2017;28:265-269.
- Scalf LA, Davis MD, Rohlinger AL, et al. Photopatch testing of 182 patients: a 6-year experience at the Mayo Clinic. Dermatitis. 2009;20:44-52.
- Greenspoon J, Ahluwalia R, Juma N, et al. Allergic and photoallergic contact dermatitis: a 10-year experience. Dermatitis. 2013;24:29-32.
- Victor FC, Cohen DE, Soter NA. A 20-year analysis of previous and emerging allergens that elicit photoallergic contact dermatitis. J Am Acad Dermatol. 2010;62:605-610.
- Schauder S, Ippen H. Contact and photocontact sensitivity to sunscreens. review of a 15-year experience and of the literature. Contact Dermatitis. 1997;37:221-232.
- Collaris EJ, Frank J. Photoallergic contact dermatitis caused by ultraviolet filters in different sunscreens. Int J Dermatol. 2008;47(suppl 1):35-37.
- Heurung AR, Raju SI, Warshaw EM. Adverse reactions to sunscreen agents: epidemiology, responsible irritants and allergens, clinical characteristics, and management. Dermatitis. 2014;25:289-326.
- Reeder M, Atwater AR. Methylisothiazolinone and isothiazolinone allergy. Cutis. 2019;104:94-96.
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group Patch Test Results: 2017-2018. Dermatitis. 2021;32:111-123.
- Kullberg SA, Voller LM, Warshaw EM. Methylisothiazolinone in “dermatology-recommended” sunscreens: an important mimicker of photoallergic contact dermatitis. Photodermatol Photoimmunol Photomed. 2021;37:366-370.
- Herman A, Aerts O, de Montjoye L, et al. Isothiazolinone derivatives and allergic contact dermatitis: a review and update. J Eur Acad Dermatol Venereol. 2019;33:267-276.
- Adler BL, Houle MC, Pratt M. Photoaggravated contact dermatitis to methylisothiazolinone and associated photosensitivity: a case series [published online January 25, 2022]. Dermatitis. doi:10.1097/DER.0000000000000833
- Aerts O, Goossens A, Marguery MC, et al. Photoaggravated allergic contact dermatitis and transient photosensitivity caused by methylisothiazolinone. Contact Dermatitis. 2018;78:241-245.
- Pirmez R, Fernandes AL, Melo MG. Photoaggravated contact dermatitis to Kathon CG (methylchloroisothiazolinone/methylisothiazolinone): a novel pattern of involvement in a growing epidemic?. Br J Dermatol. 2015;173:1343-1344.
- Uter W, Aalto-Korte K, Agner T, et al. The epidemic of methylisothiazolinone contact allergy in Europe: follow-up on changing exposures.J Eur Acad Dermatol Venereol. 2020;34:333-339.
- Government of Canada. Changes to the cosmetic ingredient hotlist. December 3, 2019. Updated August 26, 2022. Accessed October 20, 2022. https://www.canada.ca/en/health-canada/services/consumer-product-safety/cosmetics/cosmetic-ingredient-hotlist-prohibited-restricted-ingredients/changes.html
- Barkin RL. Topical nonsteroidal anti-inflammatory drugs: the importance of drug, delivery, and therapeutic outcome. Am J Ther. 2015;22:388-407.
- European Multicentre Photopatch Test Study (EMCPPTS) Taskforce. A European multicentre photopatch test study. Br J Dermatol. 2012;166:1002-1009.
- Ophaswongse S, Maibach H. Topical nonsteroidal antiinflammatory drugs: allergic and photoallergic contact dermatitis and phototoxicity. Contact Dermatitis. 1993;29:57-64.
- Kowalzick L, Ziegler H. Photoallergic contact dermatitis from topical diclofenac in Solaraze gel. Contact Dermatitis. 2006;54:348-349.
- Montoro J, Rodríguez M, Díaz M, et al. Photoallergic contact dermatitis due to diclofenac. Contact Dermatitis. 2003;48:115.
- Fernández-Jorge B, Goday-Buján JJ, Murga M, et al. Photoallergic contact dermatitis due to diclofenac with cross-reaction to aceclofenac: two case reports. Contact Dermatitis. 2009;61:236-237.
- Akat PB. Severe photosensitivity reaction induced by topical diclofenac. Indian J Pharmacol. 2013;45:408-409.
- Leroy D, Dompmartin A, Szczurko C, et al. Photodermatitis from ketoprofen with cross-reactivity to fenofibrate and benzophenones. Photodermatol Photoimmunol Photomed. 1997;13:93-97.
- Devleeschouwer V, Roelandts R, Garmyn M, et al. Allergic and photoallergic contact dermatitis from ketoprofen: results of (photo) patch testing and follow-up of 42 patients. Contact Dermatitis. 2008;58:159-166.
- Matsushita T, Kamide R. Five cases of photocontact dermatitisdue to topical ketoprofen: photopatch testing and cross-reaction study. Photodermatol Photoimmunol Photomed. 2001;17:26-31.
- de Groot AC, Roberts DW. Contact and photocontact allergy to octocrylene: a review. Contact Dermatitis. 2014;70:193-204.
- Wolverton JE, Soter NA, Cohen DE. Fentichlor photocontact dermatitis: a persistent enigma. Dermatitis. 2013;24:77-81.
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient management and education. J Am Acad Dermatol. 2016;74:1043-1054.
- Darvay A, White IR, Rycroft RJ, et al. Photoallergic contact dermatitis is uncommon. Br J Dermatol. 2001;145:597-601.
- DeLeo VA, Adler BL, Warshaw EM, et al. Photopatch test results of the North American contact dermatitis group, 1999-2009. Photodermatol Photoimmunol Photomed. 2022;38:288-291.
- Kerr A, Ferguson J. Photoallergic contact dermatitis. Photodermatol Photoimmunol Photomed. 2010;26:56-65.
- As¸kın Ö, Cesur SK, Engin B, et al. Photoallergic contact dermatitis. Curr Derm Rep. 2019;8:157-163.
- Wilm A, Berneburg M. Photoallergy. J Dtsch Dermatol Ges. 2015;13:7-13.
- DeLeo VA. Photocontact dermatitis. Dermatol Ther. 2004;17:279-288.
- Imai S, Atarashi K, Ikesue K, et al. Establishment of murine model of allergic photocontact dermatitis to ketoprofen and characterization of pathogenic T cells. J Dermatol Sci. 2006;41:127-136.
- Tokura Y, Yagi H, Satoh T, et al. Inhibitory effect of melanin pigment on sensitization and elicitation of murine contact photosensitivity: mechanism of low responsiveness in C57BL/10 background mice. J Invest Dermatol. 1993;101:673-678.
- Stein KR, Scheinfeld NS. Drug-induced photoallergic and phototoxic reactions. Expert Opin Drug Saf. 2007;6:431-443.
- Janusz SC, Schwartz RA. Botanical briefs: phytophotodermatitis is an occupational and recreational dermatosis in the limelight. Cutis. 2021;107:187-189.
- Atwal SK, Chen A, Adler BL. Phototoxic contact dermatitis from over-the-counter 8-methoxypsoralen. Cutis. 2022;109:E2-E3.
- Rutter KJ, Farrar MD, Marjanovic EJ, et al. Clinicophotobiological characterization of photoaggravated atopic dermatitis [published online July 27, 2022]. JAMA Dermatol. doi:10.1001/jamadermatol.2022.2823
- Lecha M. Idiopathic photodermatoses: clinical, diagnostic and therapeutic aspects. J Eur Acad Dermatol Venereol. 2001;15:499-505.
- Marks JG Jr, Anderson BE, DeLeo VA. Contact & Occupational Dermatology. 4th ed. Jaypee Brothers; 2016.
- Bruynzeel DP, Ferguson J, Andersen K, et al. Photopatch testing: a consensus methodology for Europe. J Eur Acad Dermatol Venereol. 2004;18:679-682.
- Kim T, Taylor JS, Maibach HI, et al. Photopatch testing among members of the American Contact Dermatitis Society. Dermatitis. 2020;31:59-67.
- Asemota E, Crawford G, Kovarik C, et al. A survey examining photopatch test and phototest methodologies of contact dermatologists in the United States: platform for developing a consensus. Dermatitis. 2017;28:265-269.
- Scalf LA, Davis MD, Rohlinger AL, et al. Photopatch testing of 182 patients: a 6-year experience at the Mayo Clinic. Dermatitis. 2009;20:44-52.
- Greenspoon J, Ahluwalia R, Juma N, et al. Allergic and photoallergic contact dermatitis: a 10-year experience. Dermatitis. 2013;24:29-32.
- Victor FC, Cohen DE, Soter NA. A 20-year analysis of previous and emerging allergens that elicit photoallergic contact dermatitis. J Am Acad Dermatol. 2010;62:605-610.
- Schauder S, Ippen H. Contact and photocontact sensitivity to sunscreens. review of a 15-year experience and of the literature. Contact Dermatitis. 1997;37:221-232.
- Collaris EJ, Frank J. Photoallergic contact dermatitis caused by ultraviolet filters in different sunscreens. Int J Dermatol. 2008;47(suppl 1):35-37.
- Heurung AR, Raju SI, Warshaw EM. Adverse reactions to sunscreen agents: epidemiology, responsible irritants and allergens, clinical characteristics, and management. Dermatitis. 2014;25:289-326.
- Reeder M, Atwater AR. Methylisothiazolinone and isothiazolinone allergy. Cutis. 2019;104:94-96.
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group Patch Test Results: 2017-2018. Dermatitis. 2021;32:111-123.
- Kullberg SA, Voller LM, Warshaw EM. Methylisothiazolinone in “dermatology-recommended” sunscreens: an important mimicker of photoallergic contact dermatitis. Photodermatol Photoimmunol Photomed. 2021;37:366-370.
- Herman A, Aerts O, de Montjoye L, et al. Isothiazolinone derivatives and allergic contact dermatitis: a review and update. J Eur Acad Dermatol Venereol. 2019;33:267-276.
- Adler BL, Houle MC, Pratt M. Photoaggravated contact dermatitis to methylisothiazolinone and associated photosensitivity: a case series [published online January 25, 2022]. Dermatitis. doi:10.1097/DER.0000000000000833
- Aerts O, Goossens A, Marguery MC, et al. Photoaggravated allergic contact dermatitis and transient photosensitivity caused by methylisothiazolinone. Contact Dermatitis. 2018;78:241-245.
- Pirmez R, Fernandes AL, Melo MG. Photoaggravated contact dermatitis to Kathon CG (methylchloroisothiazolinone/methylisothiazolinone): a novel pattern of involvement in a growing epidemic?. Br J Dermatol. 2015;173:1343-1344.
- Uter W, Aalto-Korte K, Agner T, et al. The epidemic of methylisothiazolinone contact allergy in Europe: follow-up on changing exposures.J Eur Acad Dermatol Venereol. 2020;34:333-339.
- Government of Canada. Changes to the cosmetic ingredient hotlist. December 3, 2019. Updated August 26, 2022. Accessed October 20, 2022. https://www.canada.ca/en/health-canada/services/consumer-product-safety/cosmetics/cosmetic-ingredient-hotlist-prohibited-restricted-ingredients/changes.html
- Barkin RL. Topical nonsteroidal anti-inflammatory drugs: the importance of drug, delivery, and therapeutic outcome. Am J Ther. 2015;22:388-407.
- European Multicentre Photopatch Test Study (EMCPPTS) Taskforce. A European multicentre photopatch test study. Br J Dermatol. 2012;166:1002-1009.
- Ophaswongse S, Maibach H. Topical nonsteroidal antiinflammatory drugs: allergic and photoallergic contact dermatitis and phototoxicity. Contact Dermatitis. 1993;29:57-64.
- Kowalzick L, Ziegler H. Photoallergic contact dermatitis from topical diclofenac in Solaraze gel. Contact Dermatitis. 2006;54:348-349.
- Montoro J, Rodríguez M, Díaz M, et al. Photoallergic contact dermatitis due to diclofenac. Contact Dermatitis. 2003;48:115.
- Fernández-Jorge B, Goday-Buján JJ, Murga M, et al. Photoallergic contact dermatitis due to diclofenac with cross-reaction to aceclofenac: two case reports. Contact Dermatitis. 2009;61:236-237.
- Akat PB. Severe photosensitivity reaction induced by topical diclofenac. Indian J Pharmacol. 2013;45:408-409.
- Leroy D, Dompmartin A, Szczurko C, et al. Photodermatitis from ketoprofen with cross-reactivity to fenofibrate and benzophenones. Photodermatol Photoimmunol Photomed. 1997;13:93-97.
- Devleeschouwer V, Roelandts R, Garmyn M, et al. Allergic and photoallergic contact dermatitis from ketoprofen: results of (photo) patch testing and follow-up of 42 patients. Contact Dermatitis. 2008;58:159-166.
- Matsushita T, Kamide R. Five cases of photocontact dermatitisdue to topical ketoprofen: photopatch testing and cross-reaction study. Photodermatol Photoimmunol Photomed. 2001;17:26-31.
- de Groot AC, Roberts DW. Contact and photocontact allergy to octocrylene: a review. Contact Dermatitis. 2014;70:193-204.
- Wolverton JE, Soter NA, Cohen DE. Fentichlor photocontact dermatitis: a persistent enigma. Dermatitis. 2013;24:77-81.
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient management and education. J Am Acad Dermatol. 2016;74:1043-1054.
Practice Points
- Photoallergic contact dermatitis (PACD) presents clinically and histologically similar to allergic contact dermatitis but is concentrated in sun-exposed body sites.
- Sunscreens currently are the most common photoallergens in North America, whereas topical nonsteroidal anti-inflammatory drugs are more common culprits in Europe.
- Photopatch testing is required to diagnose PACD; however, it is infrequently performed, and there currently are no North American consensus guidelines.