User login
The author reports that he serves on the advisory boards of Amgen, Boehringer Ingelheim, Depomed, Eli Lilly, and Novo Nordisk. He is a speaker for the Alliance for Bone Health, Eli Lilly, and Warner Chilcott.
Some hormonal contraceptives may affect bone density
Berenson AB, Rahman M, Breitkopf CR, Bi LX. Effects of depot medroxyprogesterone acetate and 20-microgram oral contraceptives on bone mineral density. Obstet Gynecol. 2008;112:788–799.
American College of Obstetricians and Gynecologists. ACOG Committee Opinion. No. 415. September 2008. Depot medroxyprogesterone acetate and bone effects. Obstet Gynecol. 2008;112:727–730.
A woman’s contraceptive choice may affect her bone mineral density (BMD)—particularly if she chooses depot medroxyprogesterone acetate (DMPA) or a very-low-dose oral contraceptive (OC) as her method.
In the case of DMPA, studies have shown that its use for 2 years significantly impairs BMD at the hip and spine, regardless of the patient’s age, although BMD usually rebounds after discontinuation of the drug.1
In the case of very-low-dose OCs, there is evidence that young women who use a pill that contains only 20 μg of ethinyl estradiol have a lower increase in BMD than do women the same age who do not use hormonal contraception. (OCs that contain a higher dosage of ethinyl estradiol have not been shown to hamper BMD.) A study by Polatti and colleagues reported a 7.8% increase in BMD over 5 years among women 19 to 22 years old who did not use OCs, compared with no change in BMD among women who used OCs containing 20 μg of ethinyl estradiol.2
DMPA, BMD, and the FDA
The deleterious effect of DMPA on BMD is particularly relevant in perimenopausal women, who have already begun to experience the age-related decline in BMD that starts around the age of 30. The effect is also troubling in adolescents, who normally experience a large accretion of bone during the teen and early adult years.
In 2004, the US Food and Drug Administration (FDA) added a boxed warning to DMPA labeling advising women to limit their use of the drug to 2 years. Since that time, other studies have found that BMD increases to a greater degree among past users of DMPA than among never users—suggesting that DMPA-related bone loss is reversible.
ACOG: DMPA can be used longer than 2 years in some
In September 2008, the American College of Obstetricians and Gynecologists (ACOG) released a committee opinion acknowledging the association between DMPA and BMD loss. The committee pointed out, however, that “current evidence suggests that partial or full recovery of BMD occurs at the spine and at least partial recovery occurs at the hip after discontinuation of DMPA” ( FIGURE ).
The ACOG opinion also noted that, “given the efficacy of DMPA, particularly for populations such as adolescents, for whom contraceptive adherence can be challenging, or for those who feel they could not comply with a daily contraceptive method or a method that must be used with each act of intercourse, the possible adverse effects of DMPA must be balanced against the significant personal and public health impact of unintended pregnancy.”
The committee recommended that, despite concerns about bone loss, practitioners should not hesitate to prescribe DMPA. Nor should they limit its use to 2 consecutive years or perform BMD monitoring solely in response to DMPA use. “Any observed short-term loss in BMD associated with DMPA use may be recovered and is unlikely to place a woman at risk of fracture during use or in later years,” the committee opinion noted.
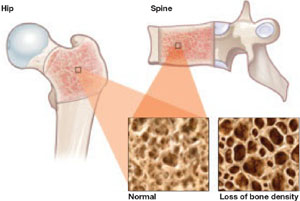
FIGURE DMPA-related bone loss is largely reversible
Depot medroxyprogesterone acetate (DMPA) is associated with a loss of bone mineral density at the hip and spine. Once the drug is discontinued, however, bone density appears to recover at least partially at both sites.
Study explores bone gains after DMPA
Berenson and associates measured BMD every 6 months for as long as 3 years in 703 white, African-American, and Hispanic women who used an OC, DMPA, or nonhormonal contraception. They also measured BMD for up to 2 additional years in 68 women who discontinued DMPA. They found no differences between races—although they did find the expected DMPA-associated bone loss.
They also found a small amount of bone loss (0.5% at the lumbar spine and 1.3% at the femoral neck) in users of very-low-dose OCs (20 μg ethinyl estradiol), compared with a gain of 1.9% at the lumbar spine and 0.6% at the femoral neck in nonusers of hormonal contraception.
Women who made a transition from DMPA to a very-low-dose OC recovered bone mass slowly. After DMPA was discontinued, women who selected nonhormonal contraception recovered BMD (4.9% at the spine, 3.2% at the femoral neck)—unlike those who chose a very-low-dose OC, who regained BMD at the spine (2.3%) but not the femoral neck (–0.7%).
Use of very-low-dose oral contraception (20 μg ethinyl estradiol) may lead to a small amount of bone loss or failure of bone accretion. Use of depot medroxyprogesterone acetate (DMPA) is associated with a greater degree of bone loss, but this loss is largely reversible at the spine. Use of a 20-μg oral contraceptive (OC) after discontinuation of DMPA may slow bone recovery.
As ACOG has indicated, concerns about skeletal health should not influence the decision to initiate or continue DMPA. Likewise, such concerns should not lead to restrictions on the use of OCs in teens or adult women. However, clinicians may wish to take bone effects into consideration when choosing the estrogen dosage of OCs for women younger than 30 who have yet to attain peak bone mass.
Denosumab nears FDA approval for treatment of osteoporosis
Cummings SR, San Martin J, McClung MR, et al; FREEDOM trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–765.
Kendler DL, Roux C, Benhamou CL, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res. 2009 [Epub ahead of print].
Miller PD, Bolognese MA, Lewiecki EM, et al, for the Amg Bone Loss Study Group. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone. 2008;43:222–229.
An FDA panel advising the Division of Reproductive and Urologic Products voted to approve denosumab (proposed brand name: Prolia) as a treatment for osteoporosis. The drug is a fully human monoclonal antibody to the receptor activator of nuclear factor-B ligand (RANKL), a cytokine that is essential for the formation, function, and survival of osteoclasts. By binding RANKL, denosumab prevents interaction between RANKL and its receptor, RANK, on osteoclasts and osteoclast precursors and thereby inhibits osteoclast-mediated bone resorption. Its effects are reversible.
Trial: Denosumab versus placebo
Cummings and colleagues reported the findings of the Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) trial, which involved 7,868 women 60 to 90 years old who had a BMD T-score between –2.5 and –4.0 at the lumbar spine or total hip. Participants were randomly assigned to receive 60 mg of denosumab or placebo subcutaneously every 6 months for 36 months. The primary endpoint was new vertebral fracture. Secondary endpoints included nonvertebral and hip fractures.
Findings included:
- Denosumab reduced the risk of new, radiographically detected vertebral fracture, with a cumulative incidence of 2.3%, versus 7.2% in the placebo group (risk ratio, 0.32; P<.001).
- Denosumab reduced the risk of hip fracture, with a cumulative incidence of 0.7% in the denosumab group, versus 1.2% in the placebo group (hazard ratio, 0.60; P=.04).
- Denosumab reduced the risk of nonvertebral fracture, with a cumulative incidence of 6.5% in the denosumab group, versus 8.0% in the placebo group (hazard ratio, 0.80; P=.01).
- There was no increase in the risk of cancer, infection, cardiovascular disease, delayed fracture healing, or hypocalcemia, and no cases of osteonecrosis of the jaw or adverse reaction to injection of the drug.
How does denosumab compare with alendronate?
Kendler and associates explored a clinically relevant question: What are the effects of switching from a bisphosphonate (in this case, alendronate) to denosumab?
They studied 504 postmenopausal women who were at least 55 years old, had a T-score between –2 and –4, and had been taking alendronate for at least 6 months. These women were randomized in double-blinded, double-dummy fashion to 60 mg of subcutaneous denosumab or a continuation of 70 mg of oral alendronate. Follow-up was 12 months.
Findings included:
- Among the women making a transition to denosumab, total hip BMD increased by 1.9% at 12 months, versus 1.05% in women continuing on alendronate (P<.0001).
- Women making a transition to denosumab also gained significantly more BMD at 12 months at the lumbar spine, femoral neck, and the distal third of the radius (all P<.0125).
- The transition to denosumab reduced bone turnover to a greater degree than did continuing alendronate.
What happens when denosumab is discontinued?
Miller and colleagues randomized postmenopausal women who had a lumbar spine T-score of –1.8 to –4.0 or a proximal femur T-score of –1.8 to –3.5 to one of the following arms:
- denosumab every 3 months (6, 14, or 30 mg)
- denosumab every 6 months (14, 60, 100, or 210 mg)
- open-label oral alendronate every week (70 mg)
- placebo.
After 24 months, women taking denosumab either:
- continued treatment at 60 mg every 6 months for an additional 24 months
- discontinued therapy
- discontinued treatment for 12 months and then reinitiated denosumab at 60 mg every 6 months for an additional 12 months.
The placebo cohort was maintained throughout this period.
Continuous, long-term denosumab increased BMD at the lumbar spine (9.4% to 11.8%) and total hip (4.0% to 6.1%). In contrast, discontinuation of denosumab was associated with a decrease in BMD of 6.6% at the lumbar spine and 5.3% at the total hip within the first 12 months. Retreatment with denosumab increased lumbar spine BMD by 9% from the original baseline values. Levels of bone turnover markers increased upon discontinuation of denosumab and decreased with retreatment. Adverse events occurred at similar rates in all treatment groups.
Among postmenopausal women who have low bone mineral density (BMD), long-term treatment with denosumab leads to gains in BMD and a reduction of markers of bone turnover. These effects are fully reversible upon discontinuation of the drug, but reoccur when treatment is restored.
In addition, switching from alendronate to denosumab produces a greater reduction in bone turnover than does continuation on the bisphosphonate.
Denosumab is a safe, extremely potent agent that will undoubtedly find a place in the ObGyn armamentarium, where it will join bisphosphonates, selective estrogen receptor modulators, and hormone therapy. The long dosing interval (every 6 months) should help increase compliance.
TSEC, a novel compound, enters development
Lindsay R, Gallagher JC, Kagan R, Pickar JH, Constantine G. Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045–1052.
Although raloxifene is the only selective estrogen receptor modulator (SERM) approved by the FDA for prevention and treatment of osteoporosis, numerous other SERMs have been explored for this application. One new category of drug is the tissue selective estrogen complex (TSEC), which pairs a SERM with an estrogen. This was previously attempted unsuccessfully with raloxifene and 17β-estradiol. The ideal SERM–estrogen combination would have the positive attributes of both classes of drugs with fewer, or none, of the undesired effects. An appropriate TSEC would therefore alleviate hot flushes, treat vulvar and vaginal atrophy, and protect against bone loss without stimulating the endometrium and increasing the risk of breast cancer.
One third-generation SERM that has been investigated for its utility in this regard is bazedoxifene (BZA), which produced endometrial thickness and amenorrhea rates comparable to those of placebo. It also produced greater gains in BMD at the lumbar spine in a 2-year, randomized, double-blind, placebo-controlled trial.3 The incidence of new vertebral fracture was significantly lower with BZA than with placebo in a study of postmenopausal women with osteoporosis.4
Given these favorable data, a TSEC containing BZA and conjugated equine estrogen was designed as a potential new comprehensive menopausal therapy.
Substudies suggest bazedoxifene has promise
Lindsay and associates reported on two osteoporosis substudies of the Selective estrogen Menopause and Response to Therapy (SMART) Trial. The main study, which evaluated the incidence of endometrial hyperplasia at 12 months, will be reported elsewhere; it was a multicenter, double-blind, randomized, placebo-controlled phase 3 trial that enrolled 3,397 women.
Substudy I involved women who were more than 5 years postmenopausal, and Substudy II included women who were between 1 and 5 years postmenopausal. Eligible screened subjects were randomly assigned to one of the following treatment groups:
- bazedoxifene (10, 20, or 40 mg), each with conjugated equine estrogen (0.625 or 0.45 mg)
- raloxifene (60 mg)
- placebo.
To maintain blinding, the combination of BZA and conjugated equine estrogen was provided as a single, encapsulated tablet to match the placebo, as was raloxifene. Subjects were directed to take one tablet orally at approximately the same time each day for 2 years. The primary outcome for both substudies was a change in BMD at the lumbar spine, as measured by dual-energy x-ray absorptiometry.
In both substudies, BMD increased to a greater degree at the lumbar spine and total hip at all BZA-estrogen dosages than with placebo, and it increased to a greater degree at the lumbar spine at most BZA-estrogen dosages, compared with raloxifene.
Osteocalcin and N-telopeptide significantly decreased at all BZA-estrogen dosages, compared with placebo, and at most BZA-estrogen dosages, compared with raloxifene.
Bazedoxifene is a potential therapeutic agent for menopausal women that may protect the endometrium while preventing bone loss in a population at higher risk of osteoporosis. It is not yet approved for this indication; further investigation is needed.
The combination of an estrogen and a selective estrogen receptor modulator to potentially relieve vasomotor symptoms, prevent vulvovaginal atrophy, and preserve bone mass without stimulating the endometrium or increasing the risk of breast cancer or venous thromboembolism is very exciting—and just might revolutionize treatment of menopausal women
Bolognese M, Krege JH, Utian WH, et al. Effects of arzoxifene on bone mineral density and endometrium in postmenopausal women with normal or low bone mass. J Clin Endocrinol Metab. 2009;94:2284–2289.
The benzothiophene derivative arzoxifene, which has been in development for the prevention and treatment of osteoporosis, as well as for reduction of the risk of invasive breast cancer in postmenopausal women, has been pulled from the regulatory approval process by its manufacturer, Eli Lilly.
This move is somewhat surprising because, in a phase 3 trial, arzoxifene significantly increased bone mineral density at the lumbar spine (2.9%) and total hip (2.2%), compared with placebo. It also decreased levels of biochemical markers of bone metabolism.
In the trial, Bolognese and associates randomly assigned 331 postmenopausal women who had normal or low bone mass to receive 20 mg of arzoxifene or placebo daily for 2 years.
The trial also found that changes in breast density were neutral or slightly decreased in the arzoxifene group, and there was no evidence of endometrial hyperplasia or carcinoma, based on central review of baseline and follow-up endometrial biopsies. Nor were there any significant differences between groups in endometrial thickness, as assessed by transvaginal ultrasonography, or in the incidence of uterine polyps, vaginal bleeding, and hot flushes.
Nevertheless, Lilly issued a press release in late August announcing that arzoxifene would not be submitted to the FDA for regulatory review.5 It appears that, although initial results from the “pivotal” 5-year, phase 3 GJAD “Generations” trial indicated that the drug had met the primary endpoints of significantly reducing the risk of vertebral fracture and invasive breast cancer in postmenopausal women, “the study failed to demonstrate a statistically significant difference in key secondary efficacy endpoints, such as nonvertebral fractures, clinical vertebral fractures, cardiovascular events, and cognitive function, compared with placebo.”
The release went on to say: “In addition, certain adverse events, including venous thromboembolic events, hot flushes, and gynecological-related events, were reported more frequently in the arzoxifene group, compared with placebo.”5
Arzoxifene therefore joins an expanding list of selective estrogen receptor modulators (SERMs) that did not make it out of clinical trials: idoxifene, droloxifene, levormeloxifene, and lasofoxifene (approved in Europe, however), to name a few. The fall of arzoxifene again underscores the notion that “not all SERMs are created equal.”6
1. Scholes D, LaCroix AZ, Ichikawa LE, Barlow WE, Ott SM. Change in bone mineral density among adolescent women using and discontinuing depot medroxyprogesterone acetate contraception. Arch Pediatr Adolesc Med. 2005;159:139-144.
2. Polatti F, Perotti F, Filippa N, Gallina D, Nappi RE. Bone mass and long-term monophasic oral contraceptive treatment in young women. Contraception. 1995;51:221-224.
3. Miller PD, Chines AA, Christiansen C, et al. Effects of bazedoxifene on BMD and bone turnover in postmenopausal women: 2-year results of a randomized, double-blind, placebo-, and active-controlled study. J Bone Miner Res. 2008;23:525-535.
4. Silverman SL, Christiansen C, Genant HK, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo- and active-controlled clinical trial. J Bone Miner Res. 2008;23:1923-1934.
5. Based on preliminary phase III GHAD study results, Lilly concludes arzoxifene’s clinical profile does not support regulatory submission [press release]. Available at http://newsroom.lilly.com/releasedetail.cfm?ReleaseID=403905. Accessed October 2, 2009.
6. Goldstein SR. Not all SERMs are created equal. Menopause. 2006;13:325-327.
The author reports that he serves on the advisory boards of Amgen, Boehringer Ingelheim, Depomed, Eli Lilly, and Novo Nordisk. He is a speaker for the Alliance for Bone Health, Eli Lilly, and Warner Chilcott.
Some hormonal contraceptives may affect bone density
Berenson AB, Rahman M, Breitkopf CR, Bi LX. Effects of depot medroxyprogesterone acetate and 20-microgram oral contraceptives on bone mineral density. Obstet Gynecol. 2008;112:788–799.
American College of Obstetricians and Gynecologists. ACOG Committee Opinion. No. 415. September 2008. Depot medroxyprogesterone acetate and bone effects. Obstet Gynecol. 2008;112:727–730.
A woman’s contraceptive choice may affect her bone mineral density (BMD)—particularly if she chooses depot medroxyprogesterone acetate (DMPA) or a very-low-dose oral contraceptive (OC) as her method.
In the case of DMPA, studies have shown that its use for 2 years significantly impairs BMD at the hip and spine, regardless of the patient’s age, although BMD usually rebounds after discontinuation of the drug.1
In the case of very-low-dose OCs, there is evidence that young women who use a pill that contains only 20 μg of ethinyl estradiol have a lower increase in BMD than do women the same age who do not use hormonal contraception. (OCs that contain a higher dosage of ethinyl estradiol have not been shown to hamper BMD.) A study by Polatti and colleagues reported a 7.8% increase in BMD over 5 years among women 19 to 22 years old who did not use OCs, compared with no change in BMD among women who used OCs containing 20 μg of ethinyl estradiol.2
DMPA, BMD, and the FDA
The deleterious effect of DMPA on BMD is particularly relevant in perimenopausal women, who have already begun to experience the age-related decline in BMD that starts around the age of 30. The effect is also troubling in adolescents, who normally experience a large accretion of bone during the teen and early adult years.
In 2004, the US Food and Drug Administration (FDA) added a boxed warning to DMPA labeling advising women to limit their use of the drug to 2 years. Since that time, other studies have found that BMD increases to a greater degree among past users of DMPA than among never users—suggesting that DMPA-related bone loss is reversible.
ACOG: DMPA can be used longer than 2 years in some
In September 2008, the American College of Obstetricians and Gynecologists (ACOG) released a committee opinion acknowledging the association between DMPA and BMD loss. The committee pointed out, however, that “current evidence suggests that partial or full recovery of BMD occurs at the spine and at least partial recovery occurs at the hip after discontinuation of DMPA” ( FIGURE ).
The ACOG opinion also noted that, “given the efficacy of DMPA, particularly for populations such as adolescents, for whom contraceptive adherence can be challenging, or for those who feel they could not comply with a daily contraceptive method or a method that must be used with each act of intercourse, the possible adverse effects of DMPA must be balanced against the significant personal and public health impact of unintended pregnancy.”
The committee recommended that, despite concerns about bone loss, practitioners should not hesitate to prescribe DMPA. Nor should they limit its use to 2 consecutive years or perform BMD monitoring solely in response to DMPA use. “Any observed short-term loss in BMD associated with DMPA use may be recovered and is unlikely to place a woman at risk of fracture during use or in later years,” the committee opinion noted.

FIGURE DMPA-related bone loss is largely reversible
Depot medroxyprogesterone acetate (DMPA) is associated with a loss of bone mineral density at the hip and spine. Once the drug is discontinued, however, bone density appears to recover at least partially at both sites.
Study explores bone gains after DMPA
Berenson and associates measured BMD every 6 months for as long as 3 years in 703 white, African-American, and Hispanic women who used an OC, DMPA, or nonhormonal contraception. They also measured BMD for up to 2 additional years in 68 women who discontinued DMPA. They found no differences between races—although they did find the expected DMPA-associated bone loss.
They also found a small amount of bone loss (0.5% at the lumbar spine and 1.3% at the femoral neck) in users of very-low-dose OCs (20 μg ethinyl estradiol), compared with a gain of 1.9% at the lumbar spine and 0.6% at the femoral neck in nonusers of hormonal contraception.
Women who made a transition from DMPA to a very-low-dose OC recovered bone mass slowly. After DMPA was discontinued, women who selected nonhormonal contraception recovered BMD (4.9% at the spine, 3.2% at the femoral neck)—unlike those who chose a very-low-dose OC, who regained BMD at the spine (2.3%) but not the femoral neck (–0.7%).
Use of very-low-dose oral contraception (20 μg ethinyl estradiol) may lead to a small amount of bone loss or failure of bone accretion. Use of depot medroxyprogesterone acetate (DMPA) is associated with a greater degree of bone loss, but this loss is largely reversible at the spine. Use of a 20-μg oral contraceptive (OC) after discontinuation of DMPA may slow bone recovery.
As ACOG has indicated, concerns about skeletal health should not influence the decision to initiate or continue DMPA. Likewise, such concerns should not lead to restrictions on the use of OCs in teens or adult women. However, clinicians may wish to take bone effects into consideration when choosing the estrogen dosage of OCs for women younger than 30 who have yet to attain peak bone mass.
Denosumab nears FDA approval for treatment of osteoporosis
Cummings SR, San Martin J, McClung MR, et al; FREEDOM trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–765.
Kendler DL, Roux C, Benhamou CL, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res. 2009 [Epub ahead of print].
Miller PD, Bolognese MA, Lewiecki EM, et al, for the Amg Bone Loss Study Group. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone. 2008;43:222–229.
An FDA panel advising the Division of Reproductive and Urologic Products voted to approve denosumab (proposed brand name: Prolia) as a treatment for osteoporosis. The drug is a fully human monoclonal antibody to the receptor activator of nuclear factor-B ligand (RANKL), a cytokine that is essential for the formation, function, and survival of osteoclasts. By binding RANKL, denosumab prevents interaction between RANKL and its receptor, RANK, on osteoclasts and osteoclast precursors and thereby inhibits osteoclast-mediated bone resorption. Its effects are reversible.
Trial: Denosumab versus placebo
Cummings and colleagues reported the findings of the Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) trial, which involved 7,868 women 60 to 90 years old who had a BMD T-score between –2.5 and –4.0 at the lumbar spine or total hip. Participants were randomly assigned to receive 60 mg of denosumab or placebo subcutaneously every 6 months for 36 months. The primary endpoint was new vertebral fracture. Secondary endpoints included nonvertebral and hip fractures.
Findings included:
- Denosumab reduced the risk of new, radiographically detected vertebral fracture, with a cumulative incidence of 2.3%, versus 7.2% in the placebo group (risk ratio, 0.32; P<.001).
- Denosumab reduced the risk of hip fracture, with a cumulative incidence of 0.7% in the denosumab group, versus 1.2% in the placebo group (hazard ratio, 0.60; P=.04).
- Denosumab reduced the risk of nonvertebral fracture, with a cumulative incidence of 6.5% in the denosumab group, versus 8.0% in the placebo group (hazard ratio, 0.80; P=.01).
- There was no increase in the risk of cancer, infection, cardiovascular disease, delayed fracture healing, or hypocalcemia, and no cases of osteonecrosis of the jaw or adverse reaction to injection of the drug.
How does denosumab compare with alendronate?
Kendler and associates explored a clinically relevant question: What are the effects of switching from a bisphosphonate (in this case, alendronate) to denosumab?
They studied 504 postmenopausal women who were at least 55 years old, had a T-score between –2 and –4, and had been taking alendronate for at least 6 months. These women were randomized in double-blinded, double-dummy fashion to 60 mg of subcutaneous denosumab or a continuation of 70 mg of oral alendronate. Follow-up was 12 months.
Findings included:
- Among the women making a transition to denosumab, total hip BMD increased by 1.9% at 12 months, versus 1.05% in women continuing on alendronate (P<.0001).
- Women making a transition to denosumab also gained significantly more BMD at 12 months at the lumbar spine, femoral neck, and the distal third of the radius (all P<.0125).
- The transition to denosumab reduced bone turnover to a greater degree than did continuing alendronate.
What happens when denosumab is discontinued?
Miller and colleagues randomized postmenopausal women who had a lumbar spine T-score of –1.8 to –4.0 or a proximal femur T-score of –1.8 to –3.5 to one of the following arms:
- denosumab every 3 months (6, 14, or 30 mg)
- denosumab every 6 months (14, 60, 100, or 210 mg)
- open-label oral alendronate every week (70 mg)
- placebo.
After 24 months, women taking denosumab either:
- continued treatment at 60 mg every 6 months for an additional 24 months
- discontinued therapy
- discontinued treatment for 12 months and then reinitiated denosumab at 60 mg every 6 months for an additional 12 months.
The placebo cohort was maintained throughout this period.
Continuous, long-term denosumab increased BMD at the lumbar spine (9.4% to 11.8%) and total hip (4.0% to 6.1%). In contrast, discontinuation of denosumab was associated with a decrease in BMD of 6.6% at the lumbar spine and 5.3% at the total hip within the first 12 months. Retreatment with denosumab increased lumbar spine BMD by 9% from the original baseline values. Levels of bone turnover markers increased upon discontinuation of denosumab and decreased with retreatment. Adverse events occurred at similar rates in all treatment groups.
Among postmenopausal women who have low bone mineral density (BMD), long-term treatment with denosumab leads to gains in BMD and a reduction of markers of bone turnover. These effects are fully reversible upon discontinuation of the drug, but reoccur when treatment is restored.
In addition, switching from alendronate to denosumab produces a greater reduction in bone turnover than does continuation on the bisphosphonate.
Denosumab is a safe, extremely potent agent that will undoubtedly find a place in the ObGyn armamentarium, where it will join bisphosphonates, selective estrogen receptor modulators, and hormone therapy. The long dosing interval (every 6 months) should help increase compliance.
TSEC, a novel compound, enters development
Lindsay R, Gallagher JC, Kagan R, Pickar JH, Constantine G. Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045–1052.
Although raloxifene is the only selective estrogen receptor modulator (SERM) approved by the FDA for prevention and treatment of osteoporosis, numerous other SERMs have been explored for this application. One new category of drug is the tissue selective estrogen complex (TSEC), which pairs a SERM with an estrogen. This was previously attempted unsuccessfully with raloxifene and 17β-estradiol. The ideal SERM–estrogen combination would have the positive attributes of both classes of drugs with fewer, or none, of the undesired effects. An appropriate TSEC would therefore alleviate hot flushes, treat vulvar and vaginal atrophy, and protect against bone loss without stimulating the endometrium and increasing the risk of breast cancer.
One third-generation SERM that has been investigated for its utility in this regard is bazedoxifene (BZA), which produced endometrial thickness and amenorrhea rates comparable to those of placebo. It also produced greater gains in BMD at the lumbar spine in a 2-year, randomized, double-blind, placebo-controlled trial.3 The incidence of new vertebral fracture was significantly lower with BZA than with placebo in a study of postmenopausal women with osteoporosis.4
Given these favorable data, a TSEC containing BZA and conjugated equine estrogen was designed as a potential new comprehensive menopausal therapy.
Substudies suggest bazedoxifene has promise
Lindsay and associates reported on two osteoporosis substudies of the Selective estrogen Menopause and Response to Therapy (SMART) Trial. The main study, which evaluated the incidence of endometrial hyperplasia at 12 months, will be reported elsewhere; it was a multicenter, double-blind, randomized, placebo-controlled phase 3 trial that enrolled 3,397 women.
Substudy I involved women who were more than 5 years postmenopausal, and Substudy II included women who were between 1 and 5 years postmenopausal. Eligible screened subjects were randomly assigned to one of the following treatment groups:
- bazedoxifene (10, 20, or 40 mg), each with conjugated equine estrogen (0.625 or 0.45 mg)
- raloxifene (60 mg)
- placebo.
To maintain blinding, the combination of BZA and conjugated equine estrogen was provided as a single, encapsulated tablet to match the placebo, as was raloxifene. Subjects were directed to take one tablet orally at approximately the same time each day for 2 years. The primary outcome for both substudies was a change in BMD at the lumbar spine, as measured by dual-energy x-ray absorptiometry.
In both substudies, BMD increased to a greater degree at the lumbar spine and total hip at all BZA-estrogen dosages than with placebo, and it increased to a greater degree at the lumbar spine at most BZA-estrogen dosages, compared with raloxifene.
Osteocalcin and N-telopeptide significantly decreased at all BZA-estrogen dosages, compared with placebo, and at most BZA-estrogen dosages, compared with raloxifene.
Bazedoxifene is a potential therapeutic agent for menopausal women that may protect the endometrium while preventing bone loss in a population at higher risk of osteoporosis. It is not yet approved for this indication; further investigation is needed.
The combination of an estrogen and a selective estrogen receptor modulator to potentially relieve vasomotor symptoms, prevent vulvovaginal atrophy, and preserve bone mass without stimulating the endometrium or increasing the risk of breast cancer or venous thromboembolism is very exciting—and just might revolutionize treatment of menopausal women
Bolognese M, Krege JH, Utian WH, et al. Effects of arzoxifene on bone mineral density and endometrium in postmenopausal women with normal or low bone mass. J Clin Endocrinol Metab. 2009;94:2284–2289.
The benzothiophene derivative arzoxifene, which has been in development for the prevention and treatment of osteoporosis, as well as for reduction of the risk of invasive breast cancer in postmenopausal women, has been pulled from the regulatory approval process by its manufacturer, Eli Lilly.
This move is somewhat surprising because, in a phase 3 trial, arzoxifene significantly increased bone mineral density at the lumbar spine (2.9%) and total hip (2.2%), compared with placebo. It also decreased levels of biochemical markers of bone metabolism.
In the trial, Bolognese and associates randomly assigned 331 postmenopausal women who had normal or low bone mass to receive 20 mg of arzoxifene or placebo daily for 2 years.
The trial also found that changes in breast density were neutral or slightly decreased in the arzoxifene group, and there was no evidence of endometrial hyperplasia or carcinoma, based on central review of baseline and follow-up endometrial biopsies. Nor were there any significant differences between groups in endometrial thickness, as assessed by transvaginal ultrasonography, or in the incidence of uterine polyps, vaginal bleeding, and hot flushes.
Nevertheless, Lilly issued a press release in late August announcing that arzoxifene would not be submitted to the FDA for regulatory review.5 It appears that, although initial results from the “pivotal” 5-year, phase 3 GJAD “Generations” trial indicated that the drug had met the primary endpoints of significantly reducing the risk of vertebral fracture and invasive breast cancer in postmenopausal women, “the study failed to demonstrate a statistically significant difference in key secondary efficacy endpoints, such as nonvertebral fractures, clinical vertebral fractures, cardiovascular events, and cognitive function, compared with placebo.”
The release went on to say: “In addition, certain adverse events, including venous thromboembolic events, hot flushes, and gynecological-related events, were reported more frequently in the arzoxifene group, compared with placebo.”5
Arzoxifene therefore joins an expanding list of selective estrogen receptor modulators (SERMs) that did not make it out of clinical trials: idoxifene, droloxifene, levormeloxifene, and lasofoxifene (approved in Europe, however), to name a few. The fall of arzoxifene again underscores the notion that “not all SERMs are created equal.”6
The author reports that he serves on the advisory boards of Amgen, Boehringer Ingelheim, Depomed, Eli Lilly, and Novo Nordisk. He is a speaker for the Alliance for Bone Health, Eli Lilly, and Warner Chilcott.
Some hormonal contraceptives may affect bone density
Berenson AB, Rahman M, Breitkopf CR, Bi LX. Effects of depot medroxyprogesterone acetate and 20-microgram oral contraceptives on bone mineral density. Obstet Gynecol. 2008;112:788–799.
American College of Obstetricians and Gynecologists. ACOG Committee Opinion. No. 415. September 2008. Depot medroxyprogesterone acetate and bone effects. Obstet Gynecol. 2008;112:727–730.
A woman’s contraceptive choice may affect her bone mineral density (BMD)—particularly if she chooses depot medroxyprogesterone acetate (DMPA) or a very-low-dose oral contraceptive (OC) as her method.
In the case of DMPA, studies have shown that its use for 2 years significantly impairs BMD at the hip and spine, regardless of the patient’s age, although BMD usually rebounds after discontinuation of the drug.1
In the case of very-low-dose OCs, there is evidence that young women who use a pill that contains only 20 μg of ethinyl estradiol have a lower increase in BMD than do women the same age who do not use hormonal contraception. (OCs that contain a higher dosage of ethinyl estradiol have not been shown to hamper BMD.) A study by Polatti and colleagues reported a 7.8% increase in BMD over 5 years among women 19 to 22 years old who did not use OCs, compared with no change in BMD among women who used OCs containing 20 μg of ethinyl estradiol.2
DMPA, BMD, and the FDA
The deleterious effect of DMPA on BMD is particularly relevant in perimenopausal women, who have already begun to experience the age-related decline in BMD that starts around the age of 30. The effect is also troubling in adolescents, who normally experience a large accretion of bone during the teen and early adult years.
In 2004, the US Food and Drug Administration (FDA) added a boxed warning to DMPA labeling advising women to limit their use of the drug to 2 years. Since that time, other studies have found that BMD increases to a greater degree among past users of DMPA than among never users—suggesting that DMPA-related bone loss is reversible.
ACOG: DMPA can be used longer than 2 years in some
In September 2008, the American College of Obstetricians and Gynecologists (ACOG) released a committee opinion acknowledging the association between DMPA and BMD loss. The committee pointed out, however, that “current evidence suggests that partial or full recovery of BMD occurs at the spine and at least partial recovery occurs at the hip after discontinuation of DMPA” ( FIGURE ).
The ACOG opinion also noted that, “given the efficacy of DMPA, particularly for populations such as adolescents, for whom contraceptive adherence can be challenging, or for those who feel they could not comply with a daily contraceptive method or a method that must be used with each act of intercourse, the possible adverse effects of DMPA must be balanced against the significant personal and public health impact of unintended pregnancy.”
The committee recommended that, despite concerns about bone loss, practitioners should not hesitate to prescribe DMPA. Nor should they limit its use to 2 consecutive years or perform BMD monitoring solely in response to DMPA use. “Any observed short-term loss in BMD associated with DMPA use may be recovered and is unlikely to place a woman at risk of fracture during use or in later years,” the committee opinion noted.

FIGURE DMPA-related bone loss is largely reversible
Depot medroxyprogesterone acetate (DMPA) is associated with a loss of bone mineral density at the hip and spine. Once the drug is discontinued, however, bone density appears to recover at least partially at both sites.
Study explores bone gains after DMPA
Berenson and associates measured BMD every 6 months for as long as 3 years in 703 white, African-American, and Hispanic women who used an OC, DMPA, or nonhormonal contraception. They also measured BMD for up to 2 additional years in 68 women who discontinued DMPA. They found no differences between races—although they did find the expected DMPA-associated bone loss.
They also found a small amount of bone loss (0.5% at the lumbar spine and 1.3% at the femoral neck) in users of very-low-dose OCs (20 μg ethinyl estradiol), compared with a gain of 1.9% at the lumbar spine and 0.6% at the femoral neck in nonusers of hormonal contraception.
Women who made a transition from DMPA to a very-low-dose OC recovered bone mass slowly. After DMPA was discontinued, women who selected nonhormonal contraception recovered BMD (4.9% at the spine, 3.2% at the femoral neck)—unlike those who chose a very-low-dose OC, who regained BMD at the spine (2.3%) but not the femoral neck (–0.7%).
Use of very-low-dose oral contraception (20 μg ethinyl estradiol) may lead to a small amount of bone loss or failure of bone accretion. Use of depot medroxyprogesterone acetate (DMPA) is associated with a greater degree of bone loss, but this loss is largely reversible at the spine. Use of a 20-μg oral contraceptive (OC) after discontinuation of DMPA may slow bone recovery.
As ACOG has indicated, concerns about skeletal health should not influence the decision to initiate or continue DMPA. Likewise, such concerns should not lead to restrictions on the use of OCs in teens or adult women. However, clinicians may wish to take bone effects into consideration when choosing the estrogen dosage of OCs for women younger than 30 who have yet to attain peak bone mass.
Denosumab nears FDA approval for treatment of osteoporosis
Cummings SR, San Martin J, McClung MR, et al; FREEDOM trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–765.
Kendler DL, Roux C, Benhamou CL, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res. 2009 [Epub ahead of print].
Miller PD, Bolognese MA, Lewiecki EM, et al, for the Amg Bone Loss Study Group. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone. 2008;43:222–229.
An FDA panel advising the Division of Reproductive and Urologic Products voted to approve denosumab (proposed brand name: Prolia) as a treatment for osteoporosis. The drug is a fully human monoclonal antibody to the receptor activator of nuclear factor-B ligand (RANKL), a cytokine that is essential for the formation, function, and survival of osteoclasts. By binding RANKL, denosumab prevents interaction between RANKL and its receptor, RANK, on osteoclasts and osteoclast precursors and thereby inhibits osteoclast-mediated bone resorption. Its effects are reversible.
Trial: Denosumab versus placebo
Cummings and colleagues reported the findings of the Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) trial, which involved 7,868 women 60 to 90 years old who had a BMD T-score between –2.5 and –4.0 at the lumbar spine or total hip. Participants were randomly assigned to receive 60 mg of denosumab or placebo subcutaneously every 6 months for 36 months. The primary endpoint was new vertebral fracture. Secondary endpoints included nonvertebral and hip fractures.
Findings included:
- Denosumab reduced the risk of new, radiographically detected vertebral fracture, with a cumulative incidence of 2.3%, versus 7.2% in the placebo group (risk ratio, 0.32; P<.001).
- Denosumab reduced the risk of hip fracture, with a cumulative incidence of 0.7% in the denosumab group, versus 1.2% in the placebo group (hazard ratio, 0.60; P=.04).
- Denosumab reduced the risk of nonvertebral fracture, with a cumulative incidence of 6.5% in the denosumab group, versus 8.0% in the placebo group (hazard ratio, 0.80; P=.01).
- There was no increase in the risk of cancer, infection, cardiovascular disease, delayed fracture healing, or hypocalcemia, and no cases of osteonecrosis of the jaw or adverse reaction to injection of the drug.
How does denosumab compare with alendronate?
Kendler and associates explored a clinically relevant question: What are the effects of switching from a bisphosphonate (in this case, alendronate) to denosumab?
They studied 504 postmenopausal women who were at least 55 years old, had a T-score between –2 and –4, and had been taking alendronate for at least 6 months. These women were randomized in double-blinded, double-dummy fashion to 60 mg of subcutaneous denosumab or a continuation of 70 mg of oral alendronate. Follow-up was 12 months.
Findings included:
- Among the women making a transition to denosumab, total hip BMD increased by 1.9% at 12 months, versus 1.05% in women continuing on alendronate (P<.0001).
- Women making a transition to denosumab also gained significantly more BMD at 12 months at the lumbar spine, femoral neck, and the distal third of the radius (all P<.0125).
- The transition to denosumab reduced bone turnover to a greater degree than did continuing alendronate.
What happens when denosumab is discontinued?
Miller and colleagues randomized postmenopausal women who had a lumbar spine T-score of –1.8 to –4.0 or a proximal femur T-score of –1.8 to –3.5 to one of the following arms:
- denosumab every 3 months (6, 14, or 30 mg)
- denosumab every 6 months (14, 60, 100, or 210 mg)
- open-label oral alendronate every week (70 mg)
- placebo.
After 24 months, women taking denosumab either:
- continued treatment at 60 mg every 6 months for an additional 24 months
- discontinued therapy
- discontinued treatment for 12 months and then reinitiated denosumab at 60 mg every 6 months for an additional 12 months.
The placebo cohort was maintained throughout this period.
Continuous, long-term denosumab increased BMD at the lumbar spine (9.4% to 11.8%) and total hip (4.0% to 6.1%). In contrast, discontinuation of denosumab was associated with a decrease in BMD of 6.6% at the lumbar spine and 5.3% at the total hip within the first 12 months. Retreatment with denosumab increased lumbar spine BMD by 9% from the original baseline values. Levels of bone turnover markers increased upon discontinuation of denosumab and decreased with retreatment. Adverse events occurred at similar rates in all treatment groups.
Among postmenopausal women who have low bone mineral density (BMD), long-term treatment with denosumab leads to gains in BMD and a reduction of markers of bone turnover. These effects are fully reversible upon discontinuation of the drug, but reoccur when treatment is restored.
In addition, switching from alendronate to denosumab produces a greater reduction in bone turnover than does continuation on the bisphosphonate.
Denosumab is a safe, extremely potent agent that will undoubtedly find a place in the ObGyn armamentarium, where it will join bisphosphonates, selective estrogen receptor modulators, and hormone therapy. The long dosing interval (every 6 months) should help increase compliance.
TSEC, a novel compound, enters development
Lindsay R, Gallagher JC, Kagan R, Pickar JH, Constantine G. Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045–1052.
Although raloxifene is the only selective estrogen receptor modulator (SERM) approved by the FDA for prevention and treatment of osteoporosis, numerous other SERMs have been explored for this application. One new category of drug is the tissue selective estrogen complex (TSEC), which pairs a SERM with an estrogen. This was previously attempted unsuccessfully with raloxifene and 17β-estradiol. The ideal SERM–estrogen combination would have the positive attributes of both classes of drugs with fewer, or none, of the undesired effects. An appropriate TSEC would therefore alleviate hot flushes, treat vulvar and vaginal atrophy, and protect against bone loss without stimulating the endometrium and increasing the risk of breast cancer.
One third-generation SERM that has been investigated for its utility in this regard is bazedoxifene (BZA), which produced endometrial thickness and amenorrhea rates comparable to those of placebo. It also produced greater gains in BMD at the lumbar spine in a 2-year, randomized, double-blind, placebo-controlled trial.3 The incidence of new vertebral fracture was significantly lower with BZA than with placebo in a study of postmenopausal women with osteoporosis.4
Given these favorable data, a TSEC containing BZA and conjugated equine estrogen was designed as a potential new comprehensive menopausal therapy.
Substudies suggest bazedoxifene has promise
Lindsay and associates reported on two osteoporosis substudies of the Selective estrogen Menopause and Response to Therapy (SMART) Trial. The main study, which evaluated the incidence of endometrial hyperplasia at 12 months, will be reported elsewhere; it was a multicenter, double-blind, randomized, placebo-controlled phase 3 trial that enrolled 3,397 women.
Substudy I involved women who were more than 5 years postmenopausal, and Substudy II included women who were between 1 and 5 years postmenopausal. Eligible screened subjects were randomly assigned to one of the following treatment groups:
- bazedoxifene (10, 20, or 40 mg), each with conjugated equine estrogen (0.625 or 0.45 mg)
- raloxifene (60 mg)
- placebo.
To maintain blinding, the combination of BZA and conjugated equine estrogen was provided as a single, encapsulated tablet to match the placebo, as was raloxifene. Subjects were directed to take one tablet orally at approximately the same time each day for 2 years. The primary outcome for both substudies was a change in BMD at the lumbar spine, as measured by dual-energy x-ray absorptiometry.
In both substudies, BMD increased to a greater degree at the lumbar spine and total hip at all BZA-estrogen dosages than with placebo, and it increased to a greater degree at the lumbar spine at most BZA-estrogen dosages, compared with raloxifene.
Osteocalcin and N-telopeptide significantly decreased at all BZA-estrogen dosages, compared with placebo, and at most BZA-estrogen dosages, compared with raloxifene.
Bazedoxifene is a potential therapeutic agent for menopausal women that may protect the endometrium while preventing bone loss in a population at higher risk of osteoporosis. It is not yet approved for this indication; further investigation is needed.
The combination of an estrogen and a selective estrogen receptor modulator to potentially relieve vasomotor symptoms, prevent vulvovaginal atrophy, and preserve bone mass without stimulating the endometrium or increasing the risk of breast cancer or venous thromboembolism is very exciting—and just might revolutionize treatment of menopausal women
Bolognese M, Krege JH, Utian WH, et al. Effects of arzoxifene on bone mineral density and endometrium in postmenopausal women with normal or low bone mass. J Clin Endocrinol Metab. 2009;94:2284–2289.
The benzothiophene derivative arzoxifene, which has been in development for the prevention and treatment of osteoporosis, as well as for reduction of the risk of invasive breast cancer in postmenopausal women, has been pulled from the regulatory approval process by its manufacturer, Eli Lilly.
This move is somewhat surprising because, in a phase 3 trial, arzoxifene significantly increased bone mineral density at the lumbar spine (2.9%) and total hip (2.2%), compared with placebo. It also decreased levels of biochemical markers of bone metabolism.
In the trial, Bolognese and associates randomly assigned 331 postmenopausal women who had normal or low bone mass to receive 20 mg of arzoxifene or placebo daily for 2 years.
The trial also found that changes in breast density were neutral or slightly decreased in the arzoxifene group, and there was no evidence of endometrial hyperplasia or carcinoma, based on central review of baseline and follow-up endometrial biopsies. Nor were there any significant differences between groups in endometrial thickness, as assessed by transvaginal ultrasonography, or in the incidence of uterine polyps, vaginal bleeding, and hot flushes.
Nevertheless, Lilly issued a press release in late August announcing that arzoxifene would not be submitted to the FDA for regulatory review.5 It appears that, although initial results from the “pivotal” 5-year, phase 3 GJAD “Generations” trial indicated that the drug had met the primary endpoints of significantly reducing the risk of vertebral fracture and invasive breast cancer in postmenopausal women, “the study failed to demonstrate a statistically significant difference in key secondary efficacy endpoints, such as nonvertebral fractures, clinical vertebral fractures, cardiovascular events, and cognitive function, compared with placebo.”
The release went on to say: “In addition, certain adverse events, including venous thromboembolic events, hot flushes, and gynecological-related events, were reported more frequently in the arzoxifene group, compared with placebo.”5
Arzoxifene therefore joins an expanding list of selective estrogen receptor modulators (SERMs) that did not make it out of clinical trials: idoxifene, droloxifene, levormeloxifene, and lasofoxifene (approved in Europe, however), to name a few. The fall of arzoxifene again underscores the notion that “not all SERMs are created equal.”6
1. Scholes D, LaCroix AZ, Ichikawa LE, Barlow WE, Ott SM. Change in bone mineral density among adolescent women using and discontinuing depot medroxyprogesterone acetate contraception. Arch Pediatr Adolesc Med. 2005;159:139-144.
2. Polatti F, Perotti F, Filippa N, Gallina D, Nappi RE. Bone mass and long-term monophasic oral contraceptive treatment in young women. Contraception. 1995;51:221-224.
3. Miller PD, Chines AA, Christiansen C, et al. Effects of bazedoxifene on BMD and bone turnover in postmenopausal women: 2-year results of a randomized, double-blind, placebo-, and active-controlled study. J Bone Miner Res. 2008;23:525-535.
4. Silverman SL, Christiansen C, Genant HK, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo- and active-controlled clinical trial. J Bone Miner Res. 2008;23:1923-1934.
5. Based on preliminary phase III GHAD study results, Lilly concludes arzoxifene’s clinical profile does not support regulatory submission [press release]. Available at http://newsroom.lilly.com/releasedetail.cfm?ReleaseID=403905. Accessed October 2, 2009.
6. Goldstein SR. Not all SERMs are created equal. Menopause. 2006;13:325-327.
1. Scholes D, LaCroix AZ, Ichikawa LE, Barlow WE, Ott SM. Change in bone mineral density among adolescent women using and discontinuing depot medroxyprogesterone acetate contraception. Arch Pediatr Adolesc Med. 2005;159:139-144.
2. Polatti F, Perotti F, Filippa N, Gallina D, Nappi RE. Bone mass and long-term monophasic oral contraceptive treatment in young women. Contraception. 1995;51:221-224.
3. Miller PD, Chines AA, Christiansen C, et al. Effects of bazedoxifene on BMD and bone turnover in postmenopausal women: 2-year results of a randomized, double-blind, placebo-, and active-controlled study. J Bone Miner Res. 2008;23:525-535.
4. Silverman SL, Christiansen C, Genant HK, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo- and active-controlled clinical trial. J Bone Miner Res. 2008;23:1923-1934.
5. Based on preliminary phase III GHAD study results, Lilly concludes arzoxifene’s clinical profile does not support regulatory submission [press release]. Available at http://newsroom.lilly.com/releasedetail.cfm?ReleaseID=403905. Accessed October 2, 2009.
6. Goldstein SR. Not all SERMs are created equal. Menopause. 2006;13:325-327.