User login
Dr. Goldstein serves on the advisory boards of Eli Lilly, Pfizer, GlaxoSmithKline, Novo Nordisk, Novartis, Procter & Gamble, Upsher Smith, and Wyeth; is a consultant for Cook ObGyn and Ackrad Labs (a Cooper Co.); and is a speaker for Eli Lilly, Novo Nordisk, Procter & Gamble, and Wyeth.
- release of the long-awaited fracture risk-assessment tool, FRAX, from the World Health Organization
- release of updated guidelines on osteoporosis treatment from the National Osteoporosis Foundation—the first revision since 2003
- investigations of a possible association between atrial fibrillation and oral bisphosphonates
- release of guidelines on diagnosis, risk identification, prevention, and management of bisphosphonate-associated osteonecrosis of the jaw
- reports of low-energy femoral-shaft fractures associated with long-term use of alendronate
- report of data from a comparison of alendronate and denosumab, a new antiresorptive agent.
Each of these is explored in detail in this review.
FRAX tool makes it possible to direct therapy to women who need it most
The World Health Organization (WHO) has finally released the FRAX risk-assessment tool, which enables clinicians to calculate a woman’s 10-year risk of developing a hip fracture or any major osteoporotic fracture. The tool (at www.shef.ac.uk/FRAX) should, ultimately, be available as part of all dual-energy x-ray absorptiometry (DXA) software so that, when bone mass is measured, the patient’s 10-year risk of hip fracture and overall osteoporotic fracture is reported along with bone density.
FRAX has different thresholds for treatment from country to country, depending on resources available. The tool uses age, weight, height, fracture history, parental fracture history, smoking status, glucocorticoid use, history of rheumatoid arthritis, alcohol consumption, and bone mineral density (BMD) of the femoral neck to determine a woman’s risk of fracture.
In many respects, this tool is a welcome change from the use of BMD measurements alone. I have long been concerned that many clinicians base treatment decisions solely on T-scores. Compare, for example, a 51-year-old newly menopausal woman who has a T-score of -2.0 at the hip with a 67-year-old woman who has the same T-score but who entered menopause at age 48 with a T-score of 0. These women have the same bone mass but very different degrees of bone quality and fracture risk.
Nevertheless, use of an arbitrary threshold (i.e., 3% risk of hip fracture and 20% risk of any osteoporotic fracture over the next 10 years) to determine who gets treatment has limitations. Virtually all bone experts would agree that a pharmacotherapeutic agent that reduces hip fracture by 50% is a “home run.” However, if we deny treatment until a woman’s 10-year risk of hip fracture reaches 3%, that is the same as saying that, for every 100 women who are treated, only 1.5 will fracture a hip instead of three. The health establishment may call that cost-effective, but it will not be acceptable to all patients.
Moreover, patients do not always understand the difference between risk reduction and prevention. It pays to remember these facts when counseling women.
NOF uses new risk-assessment tool to refine treatment guidelines
National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Available at: www.nof.org/professionals/clinicians_guide_landing_pg.htm. Accessed October 8, 2008.
Dawson-Hughes B, Tosteson ANA, Melton LJ 3rd, et al, for the National Osteoporosis Foundation Guide Committee. Implications of absolute fracture risk assessment for osteoporosis practice guidelines in the USA. Osteoporos Int. 2008;19:449–458.
Siris E, Delmas PD. Assessment of 10-year absolute fracture risk: a new paradigm with worldwide application [editorial]. Osteoporos Int. 2008;19:383–384.
In February, the National Osteoporosis Foundation (NOF) updated its Clinician’s Guide to Prevention and Treatment of Osteoporosis, first published in 1999 and last revised (with minor changes) in 2003. The guidelines are available at www.nof.org/professionals/clinicians_guide_landing_pg.htm, along with a link to the WHO fracture risk-assessment tool, FRAX (www.shef.ac.uk/FRAX).
The previous NOF guidelines applied only to postmenopausal white women and based recommendations for intervention entirely on a patient’s T-score, with some modification of the level of intervention with the presence of clinical risk factors. The new guidelines make use of FRAX to focus recommendations on those at highest risk of fracture.
When to begin treatment
The new NOF guidelines advise the practitioner to:
- check for secondary causes of osteoporosis
- recommend BMD testing for women 65 years and older, for younger postmenopausal women when the risk-factor profile raises concern, and when there is a history of fracture
- initiate treatment in women who have had hip or vertebral fracture
- initiate treatment in women who have a DXA-based T-score ≤-2.5 at the femoral neck, total hip, or spine
- initiate treatment in postmenopausal women who have low bone mass (T-score >-2.5 but <-1.0) and a 10-year risk of hip fracture ≥3% or a 10-year probability of any major osteoporosis-related fracture >20%, based on the US-adopted WHO absolute fracture risk model
- measure BMD in DXA centers that use accepted quality assurance measures appropriate for monitoring bone loss every 2 years. For patients on pharmacotherapy, DXA BMD testing is typically performed 2 years after initiating therapy and at 2-year intervals thereafter.
New determinants of treatment
These guidelines replace earlier ones in which all postmenopausal women who had a T-score <-2.0 and those who had a T-score <-1.5 “with risk factors” were candidates for therapy.
Treatment shifts to older population
The new guidelines will probably shift some treatment from younger patients who have a modestly reduced BMD to an older population more likely to have a higher risk of fracture.
For example, consider the following patient—a 52-year-old Caucasian woman who:
- is 5 ft 4 in tall and weighs 130 lb
- has no family or personal history of fracture
- doesn’t smoke or use alcohol excessively
- doesn’t use glucocorticoids
- has no rheumatoid arthritis
- has a femoral-neck T-score of -2.1.
She has a 10-year risk of hip fracture of 1.5% and an 8.5% risk of any major osteoporotic fracture. Therefore, she is no longer a candidate for pharmacotherapy. (Under the previous guidelines, she was.)
Conversely, a 77-year-old woman who has the same height, weight, and history and a T-score of the femoral neck of -1.4, has a 10-year risk of hip fracture of 2.7% and a 23% risk of any major osteoporotic fracture. She is now a candidate for pharmacotherapy. (Under the previous guidelines, she was not a candidate.)
How to counsel the patient
The updated guidelines also include a range of recommendations on what information to include in patient counseling:
- the risk of osteoporosis and related fracture
- the need to get adequate calcium (1,200 mg/day) and vitamin D (800 to 1,000 IU/day)
- the importance of regular weight-bearing and muscle-strengthening exercise to reduce the risk of fall and fracture
- the need to avoid smoking and excess alcohol intake.
Oral bisphosphonates and atrial fibrillation—is there a link?
Heckbert SR, Li G, Cummings SR, Smith NL, Psaty BM. Use of alendronate and risk of incident atrial fibrillation in women. Arch Intern Med. 2008;168:826–831.
Black DM, Delmas PD, Eastell R, et al, for the HORIZON Pivotal Fracture Trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822.
Sørensen HT, Christensen S, Mehnert F, et al. Use of bisphosphonates among women and risk of atrial fibrillation and flutter: population-based case-control study. BMJ. 2008;336:813–816.
Postmenopausal women who have osteoporosis and are treated with once-yearly IV zoledronic acid have a higher risk of serious atrial fibrillation than nonusers do, according to a recent publication from the Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly (HORIZON) trial. This finding was unexpected and had not been recognized previously. But does it indicate elevated risk with oral bisphosphonate use?
In the Fracture Intervention Trial (FIT) of alendronate for patients who have osteoporosis, the risk of serious atrial fibrillation was higher in alendronate recipients (1.5%, n=47) than in nonusers (1.0%, n=31).1 However, this difference did not quite reach statistical significance (p=.07).
One case-control study points to 3% risk
The findings in regard to annual infusion of zoledronic acid prompted further evaluation of oral bisphosphonates. Heckbert and colleagues conducted a population-based case-control study at Group Health, an integrated health-care delivery system in Washington state, and estimated that 3% of incident atrial fibrillation might be explained by alendronate use.
Over 3 years, they identified 719 women who had a confirmed history of atrial fibrillation and 966 controls who did not, selected at random from the Group Health enrollment but matched for age and presence or absence of treated hypertension. More atrial fibrillation case patients than controls had ever used alendronate (6.5% [n=47] vs 4.1% [n=40]; p=.03).
Compared with never users of any bisphosphonate, those who had used alendronate had a higher risk of incident atrial fibrillation (odds ratio, 1.86; 95% confidence interval [CI], 1.09–3.15) after adjustment for matching variables, a diagnosis of osteoporosis, and history of cardiovascular disease.
Second case-control study finds no elevated risk
Sørensen and associates conducted a case-control study using medical databases in Denmark and concluded that there is no increased risk of atrial fibrillation and flutter with use of an oral bisphosphonate. They identified 13,586 patients who had atrial fibrillation and flutter and 65,054 patients who did not. Of these, 435 cases (3.2%) and 1,958 controls (2.9%) were current users of a bisphosphonate for osteoporosis. Etidronate and alendronate were used with almost the same frequency among cases and controls. The adjusted relative risk of atrial fibrillation with current use of a bisphosphonate, compared with nonuse, was 0.95 (95% CI, 0.84–1.07). New users had a relative risk of 0.75 (95% CI, 0.49–1.16), broadly similar to the estimate for continuing users (relative risk, 0.96; 95% CI, 0.85–1.09).
Bottom line? There is no compelling evidence that oral bisphosphonates cause an increase in atrial fibrillation. Even in the smaller case-control study that found a suggestion of elevated risk, the authors think that, at most, 3% of cases of atrial fibrillation might be attributable to oral alendronate.
An approach to osteonecrosis of the jaw among bisphosphonate users
Khan AA, Sándor GK, Dore E, et al. Canadian consensus practice guidelines for bisphosphonate-associated osteonecrosis of the jaw. J Rheumatol. 2008;35:1391–1397.
Since 2003, when the first reports of osteonecrosis of the jaw (ONJ) in patients receiving bisphosphonates were published, there has been widespread uncertainty among patients, physicians, and oral surgeons about diagnosis, identification of individuals at risk, prevention, and management of this troubling disorder (FIGURE 1).
To address these concerns, a multidisciplinary task force was convened by the Canadian Association of Oral and Maxillofacial Surgeons to systematically review the data. The task force included representatives from national and international societies representing the disciplines of oral surgery, dentistry, oral pathology, oral medicine, endocrinology, rheumatology, and oncology.
After reviewing the data, the task force made the following recommendations:
- In all oncology patients, a thorough dental examination, including radiographs, should be completed before IV bisphosphonate therapy is initiated. In this population, any invasive dental procedure is ideally completed before the start of high-dose bisphosphonate therapy. For nonurgent procedures in current users of bisphosphonate therapy, the drug should be discontinued 3 to 6 months before the dental treatment.
- Nononcology patients who are starting oral or IV bisphosphonate therapy do not require a dental examination beforehand, provided dental care is appropriate and oral hygiene is good.
- All patients taking a bisphosponate should be encouraged to stop smoking, limit alcohol use, and maintain good oral hygiene.
- Patients who have already been diagnosed with ONJ are best managed with supportive care, including pain control, treatment of secondary infection, and removal of necrotic debris. Aggressive debridement is contraindicated.
These recommendations are extremely helpful, especially because they make it clear that the average patient who has osteoporosis does not need to discontinue therapy before undergoing a dental procedure. Nor do patients who are about to embark on therapy—oral or IV—need any special dental examination as long as they maintain good oral hygiene and dental self-care.
Task force members were identified on the basis of their knowledge and expertise in the diagnosis and management of ONJ.
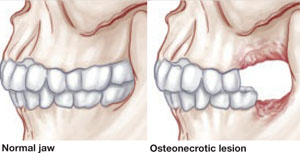
FIGURE 1 Osteonecrosis of the jaw
Blood flow to bone tissue is decreased in osteonecrosis of the jaw, leading to death of that tissue and the eventual collapse of bone.
ILLUSTRATIONS BY ROB FLEWELL FOR OBG MANAGEMENT
Distinctive fracture pattern linked to long-term alendronate
Neviaser AS, Lane JM, Lenart BA, Edobor-Osula F, Lorich DG. Low-energy femoral shaft fractures associated with alendronate use. J Orthop Trauma. 2008;22:346–350.
Patients who sustain a fracture of the proximal femoral shaft after minimal or no trauma are likely to be long-term users of alendronate, according to a recent study. These fractures are characterized by a simple transverse pattern, “beaking” of the cortex on one side, and hypertrophy of the diaphyseal cortex (FIGURE 2).
In a retrospective study, Neviaser and colleagues blindly reviewed both radiographs and medical records of 59 patients who had femoral-shaft fractures. Among the 25 users of alendronate, 19 had experienced low- or no-trauma fractures with this distinctive pattern; only one nonuser had (odds ratio, 139.33; 95% CI, 19.0–939.4; p<.0001). This fracture pattern was 98% specific to alendronate use.
The average duration of alendronate use in patients who had this fracture pattern was significantly longer than in those who did not (6.9 years vs 2.5 years, respectively; p=.002). Only one patient with this fracture pattern had been taking alendronate for less than 4 years.
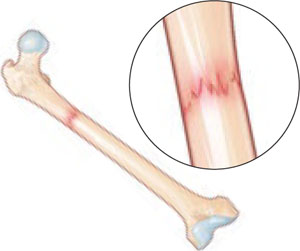
FIGURE 2 Low-impact femoral fracture
Simple transverse fractures of the proximal femur after low or no trauma have been linked to long-term alendronate use.
First reports came in 2005
Neviaser and associates mention case reports from 2005 that described nine patients who sustained spontaneous nontraumatic, nonpathologic fractures while on prolonged alendronate therapy (>3 years).2 In 2007, Goh and colleagues reported 13 subtrochanteric fractures, nine of which occurred in patients treated with alendronate. Of the nine, eight had a pattern associated with cortical hypertrophy.3
Cause-and-effect relationship remains unproven
The proximal femoral shaft is normally subjected to high stress, Neviaser and colleagues observe, and would not be expected to fracture from minimal trauma without underlying bone pathology.
In their study, 11 patients who had untreated osteoporosis had femoral-shaft fractures, but none had this specific pattern (unicortical beak, hypertrophied diaphyseal cortex). The authors hypothesize that adynamic metabolism from impaired resorption may be the underlying pathophysiology that leads to these fractures. They also point out that, although the pattern was 98% specific to alendronate users, this does not necessarily prove cause and effect—only an association. Clearly, further study is necessary.
Denosumab outperforms alendronate in phase 3 trial
Brown JP, Prince RL, Deal C, et al. Comparison of the effect of denosumab and alendronate on bone mineral density and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res. 2008; Sep 3 [Epub ahead of print].
In the first head-to-head comparison of a nonbisphosphonate with alendronate, Brown and colleagues found significantly increased BMD at the total hip with denosumab after 12 months of use (3.5% vs 2.6%; p<.0001). This finding was reported at the American Society of Bone and Mineral Research annual meeting in Montreal in September.
Denosumab is an antiresorptive agent that inhibits osteoclast-mediated bone resorption and works through a different pathway than bisphosphonates. It is a fully human monoclonal antibody that neutralizes RANKL, a key mediator of osteoclast function, formation, and survival. Denosumab is injectable (subcutaneous) and is given every 6 months.
All sites showed improvement in BMD
In the phase 3 trial, 1,189 postmenopausal women who had a T-score at the total hip or lumbar spine ≤-2.0 were randomized to receive a subcutaneous injection of denosumab (60 mg every 6 months plus an oral placebo weekly) or oral alendronate (70 mg weekly plus a subcutaneous placebo injection every 6 months). Bone mineral density was monitored at various sites to detect any changes, as were bone-turnover markers at various times during the study.
In addition to BMD at the total hip, denosumab increased BMD at the following sites at 12 months, compared with alendronate:
- femoral neck, 0.6%
- trochanter, 1.0%
- lumbar spine, 1.1%
- distal radius, 0.6% (p≤.0002 at all sites).
Denosumab also was associated with a significantly greater reduction of bone-turnover markers than alendronate. The two groups had similar laboratory values and adverse events.
Although these preliminary results are extremely encouraging, we await data on fracture reduction from a study under way in postmenopausal women who have osteoporosis before definitive recommendations can be made about this agent.
1. Cummings SR, Schwartz AV, Black DM. Alendronate and atrial fibrillation [letter]. N Engl J Med. 2007;356:1895-1896.
2. Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005;90:1294-1301.
3. Goh SK, Yang KY, Koh JS, et al. Subtrochanteric insufficiency fractures in patients on alendronate therapy: a caution. J Bone Joint Surg Br. 2007;89:349-353.
Dr. Goldstein serves on the advisory boards of Eli Lilly, Pfizer, GlaxoSmithKline, Novo Nordisk, Novartis, Procter & Gamble, Upsher Smith, and Wyeth; is a consultant for Cook ObGyn and Ackrad Labs (a Cooper Co.); and is a speaker for Eli Lilly, Novo Nordisk, Procter & Gamble, and Wyeth.
- release of the long-awaited fracture risk-assessment tool, FRAX, from the World Health Organization
- release of updated guidelines on osteoporosis treatment from the National Osteoporosis Foundation—the first revision since 2003
- investigations of a possible association between atrial fibrillation and oral bisphosphonates
- release of guidelines on diagnosis, risk identification, prevention, and management of bisphosphonate-associated osteonecrosis of the jaw
- reports of low-energy femoral-shaft fractures associated with long-term use of alendronate
- report of data from a comparison of alendronate and denosumab, a new antiresorptive agent.
Each of these is explored in detail in this review.
FRAX tool makes it possible to direct therapy to women who need it most
The World Health Organization (WHO) has finally released the FRAX risk-assessment tool, which enables clinicians to calculate a woman’s 10-year risk of developing a hip fracture or any major osteoporotic fracture. The tool (at www.shef.ac.uk/FRAX) should, ultimately, be available as part of all dual-energy x-ray absorptiometry (DXA) software so that, when bone mass is measured, the patient’s 10-year risk of hip fracture and overall osteoporotic fracture is reported along with bone density.
FRAX has different thresholds for treatment from country to country, depending on resources available. The tool uses age, weight, height, fracture history, parental fracture history, smoking status, glucocorticoid use, history of rheumatoid arthritis, alcohol consumption, and bone mineral density (BMD) of the femoral neck to determine a woman’s risk of fracture.
In many respects, this tool is a welcome change from the use of BMD measurements alone. I have long been concerned that many clinicians base treatment decisions solely on T-scores. Compare, for example, a 51-year-old newly menopausal woman who has a T-score of -2.0 at the hip with a 67-year-old woman who has the same T-score but who entered menopause at age 48 with a T-score of 0. These women have the same bone mass but very different degrees of bone quality and fracture risk.
Nevertheless, use of an arbitrary threshold (i.e., 3% risk of hip fracture and 20% risk of any osteoporotic fracture over the next 10 years) to determine who gets treatment has limitations. Virtually all bone experts would agree that a pharmacotherapeutic agent that reduces hip fracture by 50% is a “home run.” However, if we deny treatment until a woman’s 10-year risk of hip fracture reaches 3%, that is the same as saying that, for every 100 women who are treated, only 1.5 will fracture a hip instead of three. The health establishment may call that cost-effective, but it will not be acceptable to all patients.
Moreover, patients do not always understand the difference between risk reduction and prevention. It pays to remember these facts when counseling women.
NOF uses new risk-assessment tool to refine treatment guidelines
National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Available at: www.nof.org/professionals/clinicians_guide_landing_pg.htm. Accessed October 8, 2008.
Dawson-Hughes B, Tosteson ANA, Melton LJ 3rd, et al, for the National Osteoporosis Foundation Guide Committee. Implications of absolute fracture risk assessment for osteoporosis practice guidelines in the USA. Osteoporos Int. 2008;19:449–458.
Siris E, Delmas PD. Assessment of 10-year absolute fracture risk: a new paradigm with worldwide application [editorial]. Osteoporos Int. 2008;19:383–384.
In February, the National Osteoporosis Foundation (NOF) updated its Clinician’s Guide to Prevention and Treatment of Osteoporosis, first published in 1999 and last revised (with minor changes) in 2003. The guidelines are available at www.nof.org/professionals/clinicians_guide_landing_pg.htm, along with a link to the WHO fracture risk-assessment tool, FRAX (www.shef.ac.uk/FRAX).
The previous NOF guidelines applied only to postmenopausal white women and based recommendations for intervention entirely on a patient’s T-score, with some modification of the level of intervention with the presence of clinical risk factors. The new guidelines make use of FRAX to focus recommendations on those at highest risk of fracture.
When to begin treatment
The new NOF guidelines advise the practitioner to:
- check for secondary causes of osteoporosis
- recommend BMD testing for women 65 years and older, for younger postmenopausal women when the risk-factor profile raises concern, and when there is a history of fracture
- initiate treatment in women who have had hip or vertebral fracture
- initiate treatment in women who have a DXA-based T-score ≤-2.5 at the femoral neck, total hip, or spine
- initiate treatment in postmenopausal women who have low bone mass (T-score >-2.5 but <-1.0) and a 10-year risk of hip fracture ≥3% or a 10-year probability of any major osteoporosis-related fracture >20%, based on the US-adopted WHO absolute fracture risk model
- measure BMD in DXA centers that use accepted quality assurance measures appropriate for monitoring bone loss every 2 years. For patients on pharmacotherapy, DXA BMD testing is typically performed 2 years after initiating therapy and at 2-year intervals thereafter.
New determinants of treatment
These guidelines replace earlier ones in which all postmenopausal women who had a T-score <-2.0 and those who had a T-score <-1.5 “with risk factors” were candidates for therapy.
Treatment shifts to older population
The new guidelines will probably shift some treatment from younger patients who have a modestly reduced BMD to an older population more likely to have a higher risk of fracture.
For example, consider the following patient—a 52-year-old Caucasian woman who:
- is 5 ft 4 in tall and weighs 130 lb
- has no family or personal history of fracture
- doesn’t smoke or use alcohol excessively
- doesn’t use glucocorticoids
- has no rheumatoid arthritis
- has a femoral-neck T-score of -2.1.
She has a 10-year risk of hip fracture of 1.5% and an 8.5% risk of any major osteoporotic fracture. Therefore, she is no longer a candidate for pharmacotherapy. (Under the previous guidelines, she was.)
Conversely, a 77-year-old woman who has the same height, weight, and history and a T-score of the femoral neck of -1.4, has a 10-year risk of hip fracture of 2.7% and a 23% risk of any major osteoporotic fracture. She is now a candidate for pharmacotherapy. (Under the previous guidelines, she was not a candidate.)
How to counsel the patient
The updated guidelines also include a range of recommendations on what information to include in patient counseling:
- the risk of osteoporosis and related fracture
- the need to get adequate calcium (1,200 mg/day) and vitamin D (800 to 1,000 IU/day)
- the importance of regular weight-bearing and muscle-strengthening exercise to reduce the risk of fall and fracture
- the need to avoid smoking and excess alcohol intake.
Oral bisphosphonates and atrial fibrillation—is there a link?
Heckbert SR, Li G, Cummings SR, Smith NL, Psaty BM. Use of alendronate and risk of incident atrial fibrillation in women. Arch Intern Med. 2008;168:826–831.
Black DM, Delmas PD, Eastell R, et al, for the HORIZON Pivotal Fracture Trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822.
Sørensen HT, Christensen S, Mehnert F, et al. Use of bisphosphonates among women and risk of atrial fibrillation and flutter: population-based case-control study. BMJ. 2008;336:813–816.
Postmenopausal women who have osteoporosis and are treated with once-yearly IV zoledronic acid have a higher risk of serious atrial fibrillation than nonusers do, according to a recent publication from the Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly (HORIZON) trial. This finding was unexpected and had not been recognized previously. But does it indicate elevated risk with oral bisphosphonate use?
In the Fracture Intervention Trial (FIT) of alendronate for patients who have osteoporosis, the risk of serious atrial fibrillation was higher in alendronate recipients (1.5%, n=47) than in nonusers (1.0%, n=31).1 However, this difference did not quite reach statistical significance (p=.07).
One case-control study points to 3% risk
The findings in regard to annual infusion of zoledronic acid prompted further evaluation of oral bisphosphonates. Heckbert and colleagues conducted a population-based case-control study at Group Health, an integrated health-care delivery system in Washington state, and estimated that 3% of incident atrial fibrillation might be explained by alendronate use.
Over 3 years, they identified 719 women who had a confirmed history of atrial fibrillation and 966 controls who did not, selected at random from the Group Health enrollment but matched for age and presence or absence of treated hypertension. More atrial fibrillation case patients than controls had ever used alendronate (6.5% [n=47] vs 4.1% [n=40]; p=.03).
Compared with never users of any bisphosphonate, those who had used alendronate had a higher risk of incident atrial fibrillation (odds ratio, 1.86; 95% confidence interval [CI], 1.09–3.15) after adjustment for matching variables, a diagnosis of osteoporosis, and history of cardiovascular disease.
Second case-control study finds no elevated risk
Sørensen and associates conducted a case-control study using medical databases in Denmark and concluded that there is no increased risk of atrial fibrillation and flutter with use of an oral bisphosphonate. They identified 13,586 patients who had atrial fibrillation and flutter and 65,054 patients who did not. Of these, 435 cases (3.2%) and 1,958 controls (2.9%) were current users of a bisphosphonate for osteoporosis. Etidronate and alendronate were used with almost the same frequency among cases and controls. The adjusted relative risk of atrial fibrillation with current use of a bisphosphonate, compared with nonuse, was 0.95 (95% CI, 0.84–1.07). New users had a relative risk of 0.75 (95% CI, 0.49–1.16), broadly similar to the estimate for continuing users (relative risk, 0.96; 95% CI, 0.85–1.09).
Bottom line? There is no compelling evidence that oral bisphosphonates cause an increase in atrial fibrillation. Even in the smaller case-control study that found a suggestion of elevated risk, the authors think that, at most, 3% of cases of atrial fibrillation might be attributable to oral alendronate.
An approach to osteonecrosis of the jaw among bisphosphonate users
Khan AA, Sándor GK, Dore E, et al. Canadian consensus practice guidelines for bisphosphonate-associated osteonecrosis of the jaw. J Rheumatol. 2008;35:1391–1397.
Since 2003, when the first reports of osteonecrosis of the jaw (ONJ) in patients receiving bisphosphonates were published, there has been widespread uncertainty among patients, physicians, and oral surgeons about diagnosis, identification of individuals at risk, prevention, and management of this troubling disorder (FIGURE 1).
To address these concerns, a multidisciplinary task force was convened by the Canadian Association of Oral and Maxillofacial Surgeons to systematically review the data. The task force included representatives from national and international societies representing the disciplines of oral surgery, dentistry, oral pathology, oral medicine, endocrinology, rheumatology, and oncology.
After reviewing the data, the task force made the following recommendations:
- In all oncology patients, a thorough dental examination, including radiographs, should be completed before IV bisphosphonate therapy is initiated. In this population, any invasive dental procedure is ideally completed before the start of high-dose bisphosphonate therapy. For nonurgent procedures in current users of bisphosphonate therapy, the drug should be discontinued 3 to 6 months before the dental treatment.
- Nononcology patients who are starting oral or IV bisphosphonate therapy do not require a dental examination beforehand, provided dental care is appropriate and oral hygiene is good.
- All patients taking a bisphosponate should be encouraged to stop smoking, limit alcohol use, and maintain good oral hygiene.
- Patients who have already been diagnosed with ONJ are best managed with supportive care, including pain control, treatment of secondary infection, and removal of necrotic debris. Aggressive debridement is contraindicated.
These recommendations are extremely helpful, especially because they make it clear that the average patient who has osteoporosis does not need to discontinue therapy before undergoing a dental procedure. Nor do patients who are about to embark on therapy—oral or IV—need any special dental examination as long as they maintain good oral hygiene and dental self-care.
Task force members were identified on the basis of their knowledge and expertise in the diagnosis and management of ONJ.

FIGURE 1 Osteonecrosis of the jaw
Blood flow to bone tissue is decreased in osteonecrosis of the jaw, leading to death of that tissue and the eventual collapse of bone.
ILLUSTRATIONS BY ROB FLEWELL FOR OBG MANAGEMENT
Distinctive fracture pattern linked to long-term alendronate
Neviaser AS, Lane JM, Lenart BA, Edobor-Osula F, Lorich DG. Low-energy femoral shaft fractures associated with alendronate use. J Orthop Trauma. 2008;22:346–350.
Patients who sustain a fracture of the proximal femoral shaft after minimal or no trauma are likely to be long-term users of alendronate, according to a recent study. These fractures are characterized by a simple transverse pattern, “beaking” of the cortex on one side, and hypertrophy of the diaphyseal cortex (FIGURE 2).
In a retrospective study, Neviaser and colleagues blindly reviewed both radiographs and medical records of 59 patients who had femoral-shaft fractures. Among the 25 users of alendronate, 19 had experienced low- or no-trauma fractures with this distinctive pattern; only one nonuser had (odds ratio, 139.33; 95% CI, 19.0–939.4; p<.0001). This fracture pattern was 98% specific to alendronate use.
The average duration of alendronate use in patients who had this fracture pattern was significantly longer than in those who did not (6.9 years vs 2.5 years, respectively; p=.002). Only one patient with this fracture pattern had been taking alendronate for less than 4 years.

FIGURE 2 Low-impact femoral fracture
Simple transverse fractures of the proximal femur after low or no trauma have been linked to long-term alendronate use.
First reports came in 2005
Neviaser and associates mention case reports from 2005 that described nine patients who sustained spontaneous nontraumatic, nonpathologic fractures while on prolonged alendronate therapy (>3 years).2 In 2007, Goh and colleagues reported 13 subtrochanteric fractures, nine of which occurred in patients treated with alendronate. Of the nine, eight had a pattern associated with cortical hypertrophy.3
Cause-and-effect relationship remains unproven
The proximal femoral shaft is normally subjected to high stress, Neviaser and colleagues observe, and would not be expected to fracture from minimal trauma without underlying bone pathology.
In their study, 11 patients who had untreated osteoporosis had femoral-shaft fractures, but none had this specific pattern (unicortical beak, hypertrophied diaphyseal cortex). The authors hypothesize that adynamic metabolism from impaired resorption may be the underlying pathophysiology that leads to these fractures. They also point out that, although the pattern was 98% specific to alendronate users, this does not necessarily prove cause and effect—only an association. Clearly, further study is necessary.
Denosumab outperforms alendronate in phase 3 trial
Brown JP, Prince RL, Deal C, et al. Comparison of the effect of denosumab and alendronate on bone mineral density and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res. 2008; Sep 3 [Epub ahead of print].
In the first head-to-head comparison of a nonbisphosphonate with alendronate, Brown and colleagues found significantly increased BMD at the total hip with denosumab after 12 months of use (3.5% vs 2.6%; p<.0001). This finding was reported at the American Society of Bone and Mineral Research annual meeting in Montreal in September.
Denosumab is an antiresorptive agent that inhibits osteoclast-mediated bone resorption and works through a different pathway than bisphosphonates. It is a fully human monoclonal antibody that neutralizes RANKL, a key mediator of osteoclast function, formation, and survival. Denosumab is injectable (subcutaneous) and is given every 6 months.
All sites showed improvement in BMD
In the phase 3 trial, 1,189 postmenopausal women who had a T-score at the total hip or lumbar spine ≤-2.0 were randomized to receive a subcutaneous injection of denosumab (60 mg every 6 months plus an oral placebo weekly) or oral alendronate (70 mg weekly plus a subcutaneous placebo injection every 6 months). Bone mineral density was monitored at various sites to detect any changes, as were bone-turnover markers at various times during the study.
In addition to BMD at the total hip, denosumab increased BMD at the following sites at 12 months, compared with alendronate:
- femoral neck, 0.6%
- trochanter, 1.0%
- lumbar spine, 1.1%
- distal radius, 0.6% (p≤.0002 at all sites).
Denosumab also was associated with a significantly greater reduction of bone-turnover markers than alendronate. The two groups had similar laboratory values and adverse events.
Although these preliminary results are extremely encouraging, we await data on fracture reduction from a study under way in postmenopausal women who have osteoporosis before definitive recommendations can be made about this agent.
Dr. Goldstein serves on the advisory boards of Eli Lilly, Pfizer, GlaxoSmithKline, Novo Nordisk, Novartis, Procter & Gamble, Upsher Smith, and Wyeth; is a consultant for Cook ObGyn and Ackrad Labs (a Cooper Co.); and is a speaker for Eli Lilly, Novo Nordisk, Procter & Gamble, and Wyeth.
- release of the long-awaited fracture risk-assessment tool, FRAX, from the World Health Organization
- release of updated guidelines on osteoporosis treatment from the National Osteoporosis Foundation—the first revision since 2003
- investigations of a possible association between atrial fibrillation and oral bisphosphonates
- release of guidelines on diagnosis, risk identification, prevention, and management of bisphosphonate-associated osteonecrosis of the jaw
- reports of low-energy femoral-shaft fractures associated with long-term use of alendronate
- report of data from a comparison of alendronate and denosumab, a new antiresorptive agent.
Each of these is explored in detail in this review.
FRAX tool makes it possible to direct therapy to women who need it most
The World Health Organization (WHO) has finally released the FRAX risk-assessment tool, which enables clinicians to calculate a woman’s 10-year risk of developing a hip fracture or any major osteoporotic fracture. The tool (at www.shef.ac.uk/FRAX) should, ultimately, be available as part of all dual-energy x-ray absorptiometry (DXA) software so that, when bone mass is measured, the patient’s 10-year risk of hip fracture and overall osteoporotic fracture is reported along with bone density.
FRAX has different thresholds for treatment from country to country, depending on resources available. The tool uses age, weight, height, fracture history, parental fracture history, smoking status, glucocorticoid use, history of rheumatoid arthritis, alcohol consumption, and bone mineral density (BMD) of the femoral neck to determine a woman’s risk of fracture.
In many respects, this tool is a welcome change from the use of BMD measurements alone. I have long been concerned that many clinicians base treatment decisions solely on T-scores. Compare, for example, a 51-year-old newly menopausal woman who has a T-score of -2.0 at the hip with a 67-year-old woman who has the same T-score but who entered menopause at age 48 with a T-score of 0. These women have the same bone mass but very different degrees of bone quality and fracture risk.
Nevertheless, use of an arbitrary threshold (i.e., 3% risk of hip fracture and 20% risk of any osteoporotic fracture over the next 10 years) to determine who gets treatment has limitations. Virtually all bone experts would agree that a pharmacotherapeutic agent that reduces hip fracture by 50% is a “home run.” However, if we deny treatment until a woman’s 10-year risk of hip fracture reaches 3%, that is the same as saying that, for every 100 women who are treated, only 1.5 will fracture a hip instead of three. The health establishment may call that cost-effective, but it will not be acceptable to all patients.
Moreover, patients do not always understand the difference between risk reduction and prevention. It pays to remember these facts when counseling women.
NOF uses new risk-assessment tool to refine treatment guidelines
National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Available at: www.nof.org/professionals/clinicians_guide_landing_pg.htm. Accessed October 8, 2008.
Dawson-Hughes B, Tosteson ANA, Melton LJ 3rd, et al, for the National Osteoporosis Foundation Guide Committee. Implications of absolute fracture risk assessment for osteoporosis practice guidelines in the USA. Osteoporos Int. 2008;19:449–458.
Siris E, Delmas PD. Assessment of 10-year absolute fracture risk: a new paradigm with worldwide application [editorial]. Osteoporos Int. 2008;19:383–384.
In February, the National Osteoporosis Foundation (NOF) updated its Clinician’s Guide to Prevention and Treatment of Osteoporosis, first published in 1999 and last revised (with minor changes) in 2003. The guidelines are available at www.nof.org/professionals/clinicians_guide_landing_pg.htm, along with a link to the WHO fracture risk-assessment tool, FRAX (www.shef.ac.uk/FRAX).
The previous NOF guidelines applied only to postmenopausal white women and based recommendations for intervention entirely on a patient’s T-score, with some modification of the level of intervention with the presence of clinical risk factors. The new guidelines make use of FRAX to focus recommendations on those at highest risk of fracture.
When to begin treatment
The new NOF guidelines advise the practitioner to:
- check for secondary causes of osteoporosis
- recommend BMD testing for women 65 years and older, for younger postmenopausal women when the risk-factor profile raises concern, and when there is a history of fracture
- initiate treatment in women who have had hip or vertebral fracture
- initiate treatment in women who have a DXA-based T-score ≤-2.5 at the femoral neck, total hip, or spine
- initiate treatment in postmenopausal women who have low bone mass (T-score >-2.5 but <-1.0) and a 10-year risk of hip fracture ≥3% or a 10-year probability of any major osteoporosis-related fracture >20%, based on the US-adopted WHO absolute fracture risk model
- measure BMD in DXA centers that use accepted quality assurance measures appropriate for monitoring bone loss every 2 years. For patients on pharmacotherapy, DXA BMD testing is typically performed 2 years after initiating therapy and at 2-year intervals thereafter.
New determinants of treatment
These guidelines replace earlier ones in which all postmenopausal women who had a T-score <-2.0 and those who had a T-score <-1.5 “with risk factors” were candidates for therapy.
Treatment shifts to older population
The new guidelines will probably shift some treatment from younger patients who have a modestly reduced BMD to an older population more likely to have a higher risk of fracture.
For example, consider the following patient—a 52-year-old Caucasian woman who:
- is 5 ft 4 in tall and weighs 130 lb
- has no family or personal history of fracture
- doesn’t smoke or use alcohol excessively
- doesn’t use glucocorticoids
- has no rheumatoid arthritis
- has a femoral-neck T-score of -2.1.
She has a 10-year risk of hip fracture of 1.5% and an 8.5% risk of any major osteoporotic fracture. Therefore, she is no longer a candidate for pharmacotherapy. (Under the previous guidelines, she was.)
Conversely, a 77-year-old woman who has the same height, weight, and history and a T-score of the femoral neck of -1.4, has a 10-year risk of hip fracture of 2.7% and a 23% risk of any major osteoporotic fracture. She is now a candidate for pharmacotherapy. (Under the previous guidelines, she was not a candidate.)
How to counsel the patient
The updated guidelines also include a range of recommendations on what information to include in patient counseling:
- the risk of osteoporosis and related fracture
- the need to get adequate calcium (1,200 mg/day) and vitamin D (800 to 1,000 IU/day)
- the importance of regular weight-bearing and muscle-strengthening exercise to reduce the risk of fall and fracture
- the need to avoid smoking and excess alcohol intake.
Oral bisphosphonates and atrial fibrillation—is there a link?
Heckbert SR, Li G, Cummings SR, Smith NL, Psaty BM. Use of alendronate and risk of incident atrial fibrillation in women. Arch Intern Med. 2008;168:826–831.
Black DM, Delmas PD, Eastell R, et al, for the HORIZON Pivotal Fracture Trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822.
Sørensen HT, Christensen S, Mehnert F, et al. Use of bisphosphonates among women and risk of atrial fibrillation and flutter: population-based case-control study. BMJ. 2008;336:813–816.
Postmenopausal women who have osteoporosis and are treated with once-yearly IV zoledronic acid have a higher risk of serious atrial fibrillation than nonusers do, according to a recent publication from the Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly (HORIZON) trial. This finding was unexpected and had not been recognized previously. But does it indicate elevated risk with oral bisphosphonate use?
In the Fracture Intervention Trial (FIT) of alendronate for patients who have osteoporosis, the risk of serious atrial fibrillation was higher in alendronate recipients (1.5%, n=47) than in nonusers (1.0%, n=31).1 However, this difference did not quite reach statistical significance (p=.07).
One case-control study points to 3% risk
The findings in regard to annual infusion of zoledronic acid prompted further evaluation of oral bisphosphonates. Heckbert and colleagues conducted a population-based case-control study at Group Health, an integrated health-care delivery system in Washington state, and estimated that 3% of incident atrial fibrillation might be explained by alendronate use.
Over 3 years, they identified 719 women who had a confirmed history of atrial fibrillation and 966 controls who did not, selected at random from the Group Health enrollment but matched for age and presence or absence of treated hypertension. More atrial fibrillation case patients than controls had ever used alendronate (6.5% [n=47] vs 4.1% [n=40]; p=.03).
Compared with never users of any bisphosphonate, those who had used alendronate had a higher risk of incident atrial fibrillation (odds ratio, 1.86; 95% confidence interval [CI], 1.09–3.15) after adjustment for matching variables, a diagnosis of osteoporosis, and history of cardiovascular disease.
Second case-control study finds no elevated risk
Sørensen and associates conducted a case-control study using medical databases in Denmark and concluded that there is no increased risk of atrial fibrillation and flutter with use of an oral bisphosphonate. They identified 13,586 patients who had atrial fibrillation and flutter and 65,054 patients who did not. Of these, 435 cases (3.2%) and 1,958 controls (2.9%) were current users of a bisphosphonate for osteoporosis. Etidronate and alendronate were used with almost the same frequency among cases and controls. The adjusted relative risk of atrial fibrillation with current use of a bisphosphonate, compared with nonuse, was 0.95 (95% CI, 0.84–1.07). New users had a relative risk of 0.75 (95% CI, 0.49–1.16), broadly similar to the estimate for continuing users (relative risk, 0.96; 95% CI, 0.85–1.09).
Bottom line? There is no compelling evidence that oral bisphosphonates cause an increase in atrial fibrillation. Even in the smaller case-control study that found a suggestion of elevated risk, the authors think that, at most, 3% of cases of atrial fibrillation might be attributable to oral alendronate.
An approach to osteonecrosis of the jaw among bisphosphonate users
Khan AA, Sándor GK, Dore E, et al. Canadian consensus practice guidelines for bisphosphonate-associated osteonecrosis of the jaw. J Rheumatol. 2008;35:1391–1397.
Since 2003, when the first reports of osteonecrosis of the jaw (ONJ) in patients receiving bisphosphonates were published, there has been widespread uncertainty among patients, physicians, and oral surgeons about diagnosis, identification of individuals at risk, prevention, and management of this troubling disorder (FIGURE 1).
To address these concerns, a multidisciplinary task force was convened by the Canadian Association of Oral and Maxillofacial Surgeons to systematically review the data. The task force included representatives from national and international societies representing the disciplines of oral surgery, dentistry, oral pathology, oral medicine, endocrinology, rheumatology, and oncology.
After reviewing the data, the task force made the following recommendations:
- In all oncology patients, a thorough dental examination, including radiographs, should be completed before IV bisphosphonate therapy is initiated. In this population, any invasive dental procedure is ideally completed before the start of high-dose bisphosphonate therapy. For nonurgent procedures in current users of bisphosphonate therapy, the drug should be discontinued 3 to 6 months before the dental treatment.
- Nononcology patients who are starting oral or IV bisphosphonate therapy do not require a dental examination beforehand, provided dental care is appropriate and oral hygiene is good.
- All patients taking a bisphosponate should be encouraged to stop smoking, limit alcohol use, and maintain good oral hygiene.
- Patients who have already been diagnosed with ONJ are best managed with supportive care, including pain control, treatment of secondary infection, and removal of necrotic debris. Aggressive debridement is contraindicated.
These recommendations are extremely helpful, especially because they make it clear that the average patient who has osteoporosis does not need to discontinue therapy before undergoing a dental procedure. Nor do patients who are about to embark on therapy—oral or IV—need any special dental examination as long as they maintain good oral hygiene and dental self-care.
Task force members were identified on the basis of their knowledge and expertise in the diagnosis and management of ONJ.

FIGURE 1 Osteonecrosis of the jaw
Blood flow to bone tissue is decreased in osteonecrosis of the jaw, leading to death of that tissue and the eventual collapse of bone.
ILLUSTRATIONS BY ROB FLEWELL FOR OBG MANAGEMENT
Distinctive fracture pattern linked to long-term alendronate
Neviaser AS, Lane JM, Lenart BA, Edobor-Osula F, Lorich DG. Low-energy femoral shaft fractures associated with alendronate use. J Orthop Trauma. 2008;22:346–350.
Patients who sustain a fracture of the proximal femoral shaft after minimal or no trauma are likely to be long-term users of alendronate, according to a recent study. These fractures are characterized by a simple transverse pattern, “beaking” of the cortex on one side, and hypertrophy of the diaphyseal cortex (FIGURE 2).
In a retrospective study, Neviaser and colleagues blindly reviewed both radiographs and medical records of 59 patients who had femoral-shaft fractures. Among the 25 users of alendronate, 19 had experienced low- or no-trauma fractures with this distinctive pattern; only one nonuser had (odds ratio, 139.33; 95% CI, 19.0–939.4; p<.0001). This fracture pattern was 98% specific to alendronate use.
The average duration of alendronate use in patients who had this fracture pattern was significantly longer than in those who did not (6.9 years vs 2.5 years, respectively; p=.002). Only one patient with this fracture pattern had been taking alendronate for less than 4 years.

FIGURE 2 Low-impact femoral fracture
Simple transverse fractures of the proximal femur after low or no trauma have been linked to long-term alendronate use.
First reports came in 2005
Neviaser and associates mention case reports from 2005 that described nine patients who sustained spontaneous nontraumatic, nonpathologic fractures while on prolonged alendronate therapy (>3 years).2 In 2007, Goh and colleagues reported 13 subtrochanteric fractures, nine of which occurred in patients treated with alendronate. Of the nine, eight had a pattern associated with cortical hypertrophy.3
Cause-and-effect relationship remains unproven
The proximal femoral shaft is normally subjected to high stress, Neviaser and colleagues observe, and would not be expected to fracture from minimal trauma without underlying bone pathology.
In their study, 11 patients who had untreated osteoporosis had femoral-shaft fractures, but none had this specific pattern (unicortical beak, hypertrophied diaphyseal cortex). The authors hypothesize that adynamic metabolism from impaired resorption may be the underlying pathophysiology that leads to these fractures. They also point out that, although the pattern was 98% specific to alendronate users, this does not necessarily prove cause and effect—only an association. Clearly, further study is necessary.
Denosumab outperforms alendronate in phase 3 trial
Brown JP, Prince RL, Deal C, et al. Comparison of the effect of denosumab and alendronate on bone mineral density and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res. 2008; Sep 3 [Epub ahead of print].
In the first head-to-head comparison of a nonbisphosphonate with alendronate, Brown and colleagues found significantly increased BMD at the total hip with denosumab after 12 months of use (3.5% vs 2.6%; p<.0001). This finding was reported at the American Society of Bone and Mineral Research annual meeting in Montreal in September.
Denosumab is an antiresorptive agent that inhibits osteoclast-mediated bone resorption and works through a different pathway than bisphosphonates. It is a fully human monoclonal antibody that neutralizes RANKL, a key mediator of osteoclast function, formation, and survival. Denosumab is injectable (subcutaneous) and is given every 6 months.
All sites showed improvement in BMD
In the phase 3 trial, 1,189 postmenopausal women who had a T-score at the total hip or lumbar spine ≤-2.0 were randomized to receive a subcutaneous injection of denosumab (60 mg every 6 months plus an oral placebo weekly) or oral alendronate (70 mg weekly plus a subcutaneous placebo injection every 6 months). Bone mineral density was monitored at various sites to detect any changes, as were bone-turnover markers at various times during the study.
In addition to BMD at the total hip, denosumab increased BMD at the following sites at 12 months, compared with alendronate:
- femoral neck, 0.6%
- trochanter, 1.0%
- lumbar spine, 1.1%
- distal radius, 0.6% (p≤.0002 at all sites).
Denosumab also was associated with a significantly greater reduction of bone-turnover markers than alendronate. The two groups had similar laboratory values and adverse events.
Although these preliminary results are extremely encouraging, we await data on fracture reduction from a study under way in postmenopausal women who have osteoporosis before definitive recommendations can be made about this agent.
1. Cummings SR, Schwartz AV, Black DM. Alendronate and atrial fibrillation [letter]. N Engl J Med. 2007;356:1895-1896.
2. Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005;90:1294-1301.
3. Goh SK, Yang KY, Koh JS, et al. Subtrochanteric insufficiency fractures in patients on alendronate therapy: a caution. J Bone Joint Surg Br. 2007;89:349-353.
1. Cummings SR, Schwartz AV, Black DM. Alendronate and atrial fibrillation [letter]. N Engl J Med. 2007;356:1895-1896.
2. Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005;90:1294-1301.
3. Goh SK, Yang KY, Koh JS, et al. Subtrochanteric insufficiency fractures in patients on alendronate therapy: a caution. J Bone Joint Surg Br. 2007;89:349-353.