User login
As 2007 draws to a close, we are still awaiting the World Health Organization’s fracture risk-assessment tool. The much-anticipated instrument will calculate 5- and 10-year fracture risks using an individual’s femoral neck T-score, age, history of low-trauma fracture, body mass index, steroid exposure, family history of hip fracture, smoking status, and alcohol intake. Once it is implemented, the tool will eliminate much of the confusion that arises when the T-score is the only variable used to determine the need for pharmacotherapy.
Why is the ability to stratify risk important? Although the incidence of fragility fractures is highest in osteoporotic women (as defined by the T-score), the absolute number of fractures is greater in those who have osteopenia. All clinicians should realize that the current definitions of normal bone density, osteopenia, and osteoporosis apply to the postmenopausal population only:
- normal – T-score above -1.0
- osteopenia – T-score below -1.0 but above -2.5
- osteoporosis – T-score below -2.5.
Indications added for raloxifene
The year did bring new indications for raloxifene, based on data from the RUTH and STAR trials,1,2 which were mentioned in this Update 1 year ago. On September 14, the Food and Drug Administration approved two new indications:
- reduction of risk of invasive breast cancer in postmenopausal women with osteoporosis
- reduction of risk of invasive breast cancer in postmenopausal women at high risk of breast cancer.
These new indications are very important for clinicians who prescribe agents to prevent fragility fractures. Raloxifene should be considered for breast cancer risk reduction when deciding which agent to prescribe.
REAL study finds real advantage with risedronate
Silverman SL, Watts NB, Delmas PD, et al. Effectiveness of bisphosphonates on nonvertebral and hip fractures in the first year of therapy: the risedronate and alendronate (REAL) cohort study. Osteoporos Int. 2007;18(1):25–34.
Patients who take risedronate have lower rates of hip and nonvertebral fracture during their first year of therapy than do those who take alendronate. That is the finding of the RisedronatE and ALendronate (REAL) cohort study, a retrospective observation of the records of health-care utilization among women in the United States. Silverman and colleagues analyzed data sets for women older than age 65 who had ever used once-weekly dosing of risedronate or alendronate. The risedronate cohort included 12,215 women who were followed for a mean of 226 days of therapy. The alendronate cohort included 21,615 women followed for a mean of 238 days of therapy.
Risedronate group had more risk factors for fracture
At baseline, women taking risedronate had a statistically greater incidence of:
- advanced age
- use of concomitant medications
- glucocorticoid use
- rheumatoid arthritis.
Each of these characteristics might have been expected to increase the risk of fragility fracture. However, through 1 year of therapy, women using risedronate had an incidence of nonvertebral fractures 18% lower than those using alendronate (2.0% versus 2.3%; 95% confidence interval [CI], 0.02–0.32). They also had an incidence of hip fracture 43% lower than those using alendronate (0.4% versus 0.6%; 95% CI, 0.13–0.63). Overall, there were 507 nonvertebral fractures and 109 hip fractures.
Footnote: Large database analyses complement randomized trials
Randomized clinical trials (RCTs) are, of course, the gold standard for determining drug safety and efficacy and the key requisite for regulatory approval of new drugs. By design, RCTs have strict inclusion and exclusion criteria to meet the regulatory standard for evaluating drug efficacy and safety and to exclude internal bias. Often, the majority of patients are deemed ineligible for entry into an RCT because of comorbidity, concomitant medication use, age, or severity of disease. Therefore, database analyses are often more “real world” than RCTs.
As clinical practice data accumulate over time, observational, or outcomes, studies can be conducted to complement the data that have been generated by RCTs. For example, over the past decade, large databases of health-care utilization claims have become available in the United States and are being tapped to conduct effectiveness studies. These observational studies can supplement the efficacy measures obtained from the carefully controlled environment of a placebo-controlled RCT. They also provide a measure of effectiveness over a wide range of patients and health-care practices. Data generated from the REAL study are just another piece of the huge puzzle we must grapple with as we seek good information about agents to treat osteoporosis and prevent fragility fractures.
Annual infusion of zoledronic acid reduces risk of fracture
Black DM, Delmas PD, Eastell R, et al, for the HORIZON Pivotal Fracture Trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822.
Lyles KW, Colon-Emeris C, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357. DOI: 10.1056/NEJMoa074941.
Zoledronic acid (Zometa) is indicated for the treatment of high levels of serum calcium associated with Paget’s disease and various malignancies (multiple myeloma, breast, prostate, and lung). These two studies explore use of this agent to prevent fracture in postmenopausal women with osteoporosis—a use for which it proved effective. Other benefits may include improved compliance and ease of administration in some women.
Black and colleagues conducted their randomized, double-blind, placebo-controlled trial to assess the effect of annual infusion of zoledronic acid on the risk of fracture over a 3-year period. A total of 3,889 postmenopausal women with osteoporosis (mean age, 73 years; range, 65–89 years) were assigned to receive a single 15-minute, 5-mg infusion of the drug at baseline, 12 months, and 24 months, and a total of 3,876 women received placebo. Approximately half the women were from Europe, and the other half were from North and South America and Asia. All women received oral daily calcium (1,000–1,500 mg) and vitamin D (400–1,200 U), and all were monitored for 36 months.
The risk of vertebral fracture was reduced in the treatment group by 70% over 3 years, compared with the placebo group (3.3% or 92 women in the treatment group versus 10.9% or 310 women receiving placebo). The risk of hip fracture was reduced by 41% in the treatment group (1.4% or 52 women receiving zoledronic acid versus 2.5% or 88 women in the placebo group). (For all comparisons, P<.001.)
The most common postdose symptoms, seen within 3 days of infusion, included fever, flu-like symptoms, myalgia, headache, and arthralgias. There were more serious adverse events related to atrial fibrillation in the women receiving zoledronic acid (50 women receiving zoledronic acid versus 20 in the placebo group; P<.001).
Treatment is a valuable option for carefully selected populations
The trial by Black and colleagues holds great promise for some patients, especially those who have (or appear to have) upper gastrointestinal intolerance of oral bisphosphonates or who may have difficulty adhering to the positional requirements (i.e., remaining upright) of oral therapy. This may be especially true of patients in nursing homes. Once-yearly intravenous (IV) infusion may also make compliance easier for these patients.
One important detail of this trial: Of almost 8,000 women studied, the youngest was age 65. The implication: Don’t automatically assume this regimen is an appropriate alternative for younger osteopenic women who perceive themselves to have acid reflux.
Women at extremely high risk of fracture also benefit
Fracture is most likely to occur in women who have already experienced it (FIGURE 1). Lyles and colleagues chose this population for their study of once-yearly infusion of zoledronic acid. The study involved 2,127 patients within 90 days after repair of low-trauma hip fracture. Subjects were randomized to receive 5-mg IV zoledronic acid annually or placebo in a blinded fashion. All patients also received vitamin D and calcium supplements. Mean age was 74.5 years, as might be expected in a hip-fracture cohort, and median follow-up was 1.9 years.
New clinical fractures occurred in 8.6% of women in the zoledronic acid group and 13.9% of the placebo group—a 35% risk reduction with IV zoledronic acid (P=.001). Deaths occurred in 101 of 1,054 patients (9.6%) in the zoledronic acid group and 141 of 1,057 patients (13.3%) in the placebo group. This was a reduction of 28% in death from any cause in the zoledronic acid group (P=.01).
The most frequent adverse events in patients receiving zoledronic acid were pyrexia, myalgia, and bone and musculoskeletal pain. No cases of osteonecrosis of the jaw were reported. Rates of atrial fibrillation and stroke were similar in both the zoledronic acid and placebo groups.
This study provides further evidence that, for extremely high-risk women (those who have already suffered hip fracture), yearly zoledronic acid may be an extremely useful tool, especially for elderly women, in whom compliance with any medication—weekly or monthly—may be difficult.
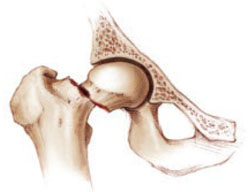
FIGURE 1 Once a fracture occurs, another is likely
Although the risk of new fractures is heightened in women who have already experienced one, Lyles and colleagues found a 35% risk reduction with zoledronic acid.
Parathyroid hormone reduces vertebral fractures in osteoporotic women
Greenspan SL, Bone HG, Ettinger MP, et al, for the Treatment of Osteoporosis with Parathyroid Hormone Study Group. Effect of recombinant human parathyroid hormone (1–84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trial. Ann Intern Med. 2007;146:326–339.
Teriparatide (Forteo) is a synthetic portion (1-34) of the parathyroid hormone (PTH) molecule that is identical in sequence to the biologically active segment of the 84-amino acid human PTH.
It has been shown to prevent fracture in women with low bone mineral density (BMD). Teriparatide is the only anabolic bone agent approved for clinical use; all other pharmacotherapies are antiresorptive.
The study by Greenspan and associates—the Treatment of Osteoporosis with Parathyroid Hormone, or TOP trial—involved the full-length PTH molecule (1–84) and provides evidence that it, too, can prevent vertebral fracture in women who have low BMD (FIGURE 2). This agent is used in Europe but not yet available in the United States.
TOP was an 18-month, randomized, double-blind, placebo-controlled, parallel-group study of 2,532 women with low BMD at the hip or lumbar spine. It was conducted at 168 centers in nine countries.
The primary outcome measure was new or worsened vertebral fracture; secondary outcomes were changes in BMD and safety. The trial investigated the safety of recombinant PTH and its effect on the incidence of vertebral fractures in postmenopausal women with osteoporosis.
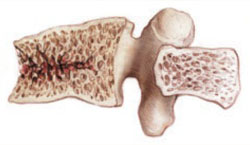
FIGURE 2 Fracture-prone bone responds to PTH
PTH prevented vertebral fracture in women with low BMD, existing fractures, or both.
Women had very low BMD, or low BMD and existing fractures
Participants were postmenopausal women aged 45 to 54 years. They had a BMD 3 standard deviations or more (T-score ≤ -3.0) below the mean peak bone mass of young adult women at the lumbar spine, femoral neck, or total hip, with no vertebral fractures, or they had a BMD T-score of -2.5 and 1 to 4 vertebral fractures. Postmenopausal women aged 55 years or older were included if their BMD T-score was -2.5 and they had no vertebral fractures, or if their BMD T-score was -2.0 and they had 1 to 4 vertebral fractures before enrollment. The women were given 100 μg of recombinant human PTH or placebo daily, as well as calcium (700 mg/day) and vitamin D3 (400 U/day).
PTH reduced the risk of new fractures or prevented worsening of existing fractures. The reduction in relative risk (RR) for vertebral fracture was 0.42 (95% CI, 0.24–0.72; P=.001). Women who received PTH had an increase in mean BMD of 6.9% at the spine (95% CI, 6.4–7.4) and 2.1% at the hip (95% CI, 1.7–2.5). Adverse events included a higher incidence of hypercalciuria, hypercalcemia, and nausea.
Although it is unlikely that many gynecologists will be ordering or monitoring injectable PTH therapy, we should be aware of the data. All too often such therapy is not even considered for women with severe osteoporosis (T-score < -2.5 and preexisting fracture), who may be excellent candidates.
Long-term alendronate users can sometimes take a “drug holiday”
Black DM, Schwartz AV, Ensrud KE, et al, for the FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment. The Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927–2938.
As early as 2002, Greenspan and colleagues3 demonstrated that women on alendronate for 21 months maintained femoral-neck BMD through 15 months of crossover to placebo. This study by Black and associates, known as the FLEX trial, is a long-term extension of the Fracture Intervention Trial (FIT). A total of 1,099 women who had participated in FIT and taken alendronate for 5 years were then randomized to one of two doses of alendronate or placebo for an additional 5 years. To qualify for the FLEX trial, all women had to have low bone mass at the beginning of FIT. The average age of women in the FLEX trial was 73 years. The primary outcome was total hip BMD; secondary outcomes were BMD at other sites and biochemical markers.
Women who remained on alendronate maintained a higher BMD of the hip and spine than women on placebo, but all patients’ levels remained at or above pretreatment levels of 10 years earlier. The same was true for markers of bone remodeling. The cumulative risk of nonvertebral fractures did not differ between the two groups (19% for placebo, 18.9% for alendronate; RR, 1.0; 95% CI, 0.76–1.32). The risk of clinically recognized vertebral fractures was lower in the women who continued alendronate (5.3% for placebo and 2.4% for alendronate; RR, 0.45; 95% CI, 0.24–0.85), but there was no significant reduction in morphometric vertebral fractures.
Data can aid in determining duration of therapy
These data are extremely helpful, especially for clinicians who are trying to determine how long to continue bisphosphonate therapy and which patients may be candidates for a “drug holiday.” We have all had women whose response to a bisphosphonate has been so robust that follow-up BMD measurements have climbed to a range in which therapy would not have been initiated. This study clearly shows that the cumulative effect of 5 years of alendronate followed by 5 years of placebo is positive, compared with the bone loss one would expect in untreated women.
The answer isn’t clear, but women with a previous fracture, and those at high risk for spine fracture, are likely to benefit from continued treatment with alendronate. Patients with a lower risk of fracture are better candidates for the holiday.
As for when the drug holiday should end, that isn’t clear, either. Continued close monitoring of these lower-risk women using bone-density measurements may help identify that minority of patients who do not maintain bone mass off the medication.
1. Barrett-Connor E, Mosca L, Collins P, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125-137.
2. Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes. The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 Trial. JAMA. 2006;295:2727-2741.
3. Greenspan SL, Emkey RD, Bone HG, et al. Significant differential effects of alendronate, estrogen, or combination therapy on the rate of bone loss after discontinuation of treatment of postmenopausal osteoporosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2002;137:875-883.
As 2007 draws to a close, we are still awaiting the World Health Organization’s fracture risk-assessment tool. The much-anticipated instrument will calculate 5- and 10-year fracture risks using an individual’s femoral neck T-score, age, history of low-trauma fracture, body mass index, steroid exposure, family history of hip fracture, smoking status, and alcohol intake. Once it is implemented, the tool will eliminate much of the confusion that arises when the T-score is the only variable used to determine the need for pharmacotherapy.
Why is the ability to stratify risk important? Although the incidence of fragility fractures is highest in osteoporotic women (as defined by the T-score), the absolute number of fractures is greater in those who have osteopenia. All clinicians should realize that the current definitions of normal bone density, osteopenia, and osteoporosis apply to the postmenopausal population only:
- normal – T-score above -1.0
- osteopenia – T-score below -1.0 but above -2.5
- osteoporosis – T-score below -2.5.
Indications added for raloxifene
The year did bring new indications for raloxifene, based on data from the RUTH and STAR trials,1,2 which were mentioned in this Update 1 year ago. On September 14, the Food and Drug Administration approved two new indications:
- reduction of risk of invasive breast cancer in postmenopausal women with osteoporosis
- reduction of risk of invasive breast cancer in postmenopausal women at high risk of breast cancer.
These new indications are very important for clinicians who prescribe agents to prevent fragility fractures. Raloxifene should be considered for breast cancer risk reduction when deciding which agent to prescribe.
REAL study finds real advantage with risedronate
Silverman SL, Watts NB, Delmas PD, et al. Effectiveness of bisphosphonates on nonvertebral and hip fractures in the first year of therapy: the risedronate and alendronate (REAL) cohort study. Osteoporos Int. 2007;18(1):25–34.
Patients who take risedronate have lower rates of hip and nonvertebral fracture during their first year of therapy than do those who take alendronate. That is the finding of the RisedronatE and ALendronate (REAL) cohort study, a retrospective observation of the records of health-care utilization among women in the United States. Silverman and colleagues analyzed data sets for women older than age 65 who had ever used once-weekly dosing of risedronate or alendronate. The risedronate cohort included 12,215 women who were followed for a mean of 226 days of therapy. The alendronate cohort included 21,615 women followed for a mean of 238 days of therapy.
Risedronate group had more risk factors for fracture
At baseline, women taking risedronate had a statistically greater incidence of:
- advanced age
- use of concomitant medications
- glucocorticoid use
- rheumatoid arthritis.
Each of these characteristics might have been expected to increase the risk of fragility fracture. However, through 1 year of therapy, women using risedronate had an incidence of nonvertebral fractures 18% lower than those using alendronate (2.0% versus 2.3%; 95% confidence interval [CI], 0.02–0.32). They also had an incidence of hip fracture 43% lower than those using alendronate (0.4% versus 0.6%; 95% CI, 0.13–0.63). Overall, there were 507 nonvertebral fractures and 109 hip fractures.
Footnote: Large database analyses complement randomized trials
Randomized clinical trials (RCTs) are, of course, the gold standard for determining drug safety and efficacy and the key requisite for regulatory approval of new drugs. By design, RCTs have strict inclusion and exclusion criteria to meet the regulatory standard for evaluating drug efficacy and safety and to exclude internal bias. Often, the majority of patients are deemed ineligible for entry into an RCT because of comorbidity, concomitant medication use, age, or severity of disease. Therefore, database analyses are often more “real world” than RCTs.
As clinical practice data accumulate over time, observational, or outcomes, studies can be conducted to complement the data that have been generated by RCTs. For example, over the past decade, large databases of health-care utilization claims have become available in the United States and are being tapped to conduct effectiveness studies. These observational studies can supplement the efficacy measures obtained from the carefully controlled environment of a placebo-controlled RCT. They also provide a measure of effectiveness over a wide range of patients and health-care practices. Data generated from the REAL study are just another piece of the huge puzzle we must grapple with as we seek good information about agents to treat osteoporosis and prevent fragility fractures.
Annual infusion of zoledronic acid reduces risk of fracture
Black DM, Delmas PD, Eastell R, et al, for the HORIZON Pivotal Fracture Trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822.
Lyles KW, Colon-Emeris C, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357. DOI: 10.1056/NEJMoa074941.
Zoledronic acid (Zometa) is indicated for the treatment of high levels of serum calcium associated with Paget’s disease and various malignancies (multiple myeloma, breast, prostate, and lung). These two studies explore use of this agent to prevent fracture in postmenopausal women with osteoporosis—a use for which it proved effective. Other benefits may include improved compliance and ease of administration in some women.
Black and colleagues conducted their randomized, double-blind, placebo-controlled trial to assess the effect of annual infusion of zoledronic acid on the risk of fracture over a 3-year period. A total of 3,889 postmenopausal women with osteoporosis (mean age, 73 years; range, 65–89 years) were assigned to receive a single 15-minute, 5-mg infusion of the drug at baseline, 12 months, and 24 months, and a total of 3,876 women received placebo. Approximately half the women were from Europe, and the other half were from North and South America and Asia. All women received oral daily calcium (1,000–1,500 mg) and vitamin D (400–1,200 U), and all were monitored for 36 months.
The risk of vertebral fracture was reduced in the treatment group by 70% over 3 years, compared with the placebo group (3.3% or 92 women in the treatment group versus 10.9% or 310 women receiving placebo). The risk of hip fracture was reduced by 41% in the treatment group (1.4% or 52 women receiving zoledronic acid versus 2.5% or 88 women in the placebo group). (For all comparisons, P<.001.)
The most common postdose symptoms, seen within 3 days of infusion, included fever, flu-like symptoms, myalgia, headache, and arthralgias. There were more serious adverse events related to atrial fibrillation in the women receiving zoledronic acid (50 women receiving zoledronic acid versus 20 in the placebo group; P<.001).
Treatment is a valuable option for carefully selected populations
The trial by Black and colleagues holds great promise for some patients, especially those who have (or appear to have) upper gastrointestinal intolerance of oral bisphosphonates or who may have difficulty adhering to the positional requirements (i.e., remaining upright) of oral therapy. This may be especially true of patients in nursing homes. Once-yearly intravenous (IV) infusion may also make compliance easier for these patients.
One important detail of this trial: Of almost 8,000 women studied, the youngest was age 65. The implication: Don’t automatically assume this regimen is an appropriate alternative for younger osteopenic women who perceive themselves to have acid reflux.
Women at extremely high risk of fracture also benefit
Fracture is most likely to occur in women who have already experienced it (FIGURE 1). Lyles and colleagues chose this population for their study of once-yearly infusion of zoledronic acid. The study involved 2,127 patients within 90 days after repair of low-trauma hip fracture. Subjects were randomized to receive 5-mg IV zoledronic acid annually or placebo in a blinded fashion. All patients also received vitamin D and calcium supplements. Mean age was 74.5 years, as might be expected in a hip-fracture cohort, and median follow-up was 1.9 years.
New clinical fractures occurred in 8.6% of women in the zoledronic acid group and 13.9% of the placebo group—a 35% risk reduction with IV zoledronic acid (P=.001). Deaths occurred in 101 of 1,054 patients (9.6%) in the zoledronic acid group and 141 of 1,057 patients (13.3%) in the placebo group. This was a reduction of 28% in death from any cause in the zoledronic acid group (P=.01).
The most frequent adverse events in patients receiving zoledronic acid were pyrexia, myalgia, and bone and musculoskeletal pain. No cases of osteonecrosis of the jaw were reported. Rates of atrial fibrillation and stroke were similar in both the zoledronic acid and placebo groups.
This study provides further evidence that, for extremely high-risk women (those who have already suffered hip fracture), yearly zoledronic acid may be an extremely useful tool, especially for elderly women, in whom compliance with any medication—weekly or monthly—may be difficult.

FIGURE 1 Once a fracture occurs, another is likely
Although the risk of new fractures is heightened in women who have already experienced one, Lyles and colleagues found a 35% risk reduction with zoledronic acid.
Parathyroid hormone reduces vertebral fractures in osteoporotic women
Greenspan SL, Bone HG, Ettinger MP, et al, for the Treatment of Osteoporosis with Parathyroid Hormone Study Group. Effect of recombinant human parathyroid hormone (1–84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trial. Ann Intern Med. 2007;146:326–339.
Teriparatide (Forteo) is a synthetic portion (1-34) of the parathyroid hormone (PTH) molecule that is identical in sequence to the biologically active segment of the 84-amino acid human PTH.
It has been shown to prevent fracture in women with low bone mineral density (BMD). Teriparatide is the only anabolic bone agent approved for clinical use; all other pharmacotherapies are antiresorptive.
The study by Greenspan and associates—the Treatment of Osteoporosis with Parathyroid Hormone, or TOP trial—involved the full-length PTH molecule (1–84) and provides evidence that it, too, can prevent vertebral fracture in women who have low BMD (FIGURE 2). This agent is used in Europe but not yet available in the United States.
TOP was an 18-month, randomized, double-blind, placebo-controlled, parallel-group study of 2,532 women with low BMD at the hip or lumbar spine. It was conducted at 168 centers in nine countries.
The primary outcome measure was new or worsened vertebral fracture; secondary outcomes were changes in BMD and safety. The trial investigated the safety of recombinant PTH and its effect on the incidence of vertebral fractures in postmenopausal women with osteoporosis.

FIGURE 2 Fracture-prone bone responds to PTH
PTH prevented vertebral fracture in women with low BMD, existing fractures, or both.
Women had very low BMD, or low BMD and existing fractures
Participants were postmenopausal women aged 45 to 54 years. They had a BMD 3 standard deviations or more (T-score ≤ -3.0) below the mean peak bone mass of young adult women at the lumbar spine, femoral neck, or total hip, with no vertebral fractures, or they had a BMD T-score of -2.5 and 1 to 4 vertebral fractures. Postmenopausal women aged 55 years or older were included if their BMD T-score was -2.5 and they had no vertebral fractures, or if their BMD T-score was -2.0 and they had 1 to 4 vertebral fractures before enrollment. The women were given 100 μg of recombinant human PTH or placebo daily, as well as calcium (700 mg/day) and vitamin D3 (400 U/day).
PTH reduced the risk of new fractures or prevented worsening of existing fractures. The reduction in relative risk (RR) for vertebral fracture was 0.42 (95% CI, 0.24–0.72; P=.001). Women who received PTH had an increase in mean BMD of 6.9% at the spine (95% CI, 6.4–7.4) and 2.1% at the hip (95% CI, 1.7–2.5). Adverse events included a higher incidence of hypercalciuria, hypercalcemia, and nausea.
Although it is unlikely that many gynecologists will be ordering or monitoring injectable PTH therapy, we should be aware of the data. All too often such therapy is not even considered for women with severe osteoporosis (T-score < -2.5 and preexisting fracture), who may be excellent candidates.
Long-term alendronate users can sometimes take a “drug holiday”
Black DM, Schwartz AV, Ensrud KE, et al, for the FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment. The Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927–2938.
As early as 2002, Greenspan and colleagues3 demonstrated that women on alendronate for 21 months maintained femoral-neck BMD through 15 months of crossover to placebo. This study by Black and associates, known as the FLEX trial, is a long-term extension of the Fracture Intervention Trial (FIT). A total of 1,099 women who had participated in FIT and taken alendronate for 5 years were then randomized to one of two doses of alendronate or placebo for an additional 5 years. To qualify for the FLEX trial, all women had to have low bone mass at the beginning of FIT. The average age of women in the FLEX trial was 73 years. The primary outcome was total hip BMD; secondary outcomes were BMD at other sites and biochemical markers.
Women who remained on alendronate maintained a higher BMD of the hip and spine than women on placebo, but all patients’ levels remained at or above pretreatment levels of 10 years earlier. The same was true for markers of bone remodeling. The cumulative risk of nonvertebral fractures did not differ between the two groups (19% for placebo, 18.9% for alendronate; RR, 1.0; 95% CI, 0.76–1.32). The risk of clinically recognized vertebral fractures was lower in the women who continued alendronate (5.3% for placebo and 2.4% for alendronate; RR, 0.45; 95% CI, 0.24–0.85), but there was no significant reduction in morphometric vertebral fractures.
Data can aid in determining duration of therapy
These data are extremely helpful, especially for clinicians who are trying to determine how long to continue bisphosphonate therapy and which patients may be candidates for a “drug holiday.” We have all had women whose response to a bisphosphonate has been so robust that follow-up BMD measurements have climbed to a range in which therapy would not have been initiated. This study clearly shows that the cumulative effect of 5 years of alendronate followed by 5 years of placebo is positive, compared with the bone loss one would expect in untreated women.
The answer isn’t clear, but women with a previous fracture, and those at high risk for spine fracture, are likely to benefit from continued treatment with alendronate. Patients with a lower risk of fracture are better candidates for the holiday.
As for when the drug holiday should end, that isn’t clear, either. Continued close monitoring of these lower-risk women using bone-density measurements may help identify that minority of patients who do not maintain bone mass off the medication.
As 2007 draws to a close, we are still awaiting the World Health Organization’s fracture risk-assessment tool. The much-anticipated instrument will calculate 5- and 10-year fracture risks using an individual’s femoral neck T-score, age, history of low-trauma fracture, body mass index, steroid exposure, family history of hip fracture, smoking status, and alcohol intake. Once it is implemented, the tool will eliminate much of the confusion that arises when the T-score is the only variable used to determine the need for pharmacotherapy.
Why is the ability to stratify risk important? Although the incidence of fragility fractures is highest in osteoporotic women (as defined by the T-score), the absolute number of fractures is greater in those who have osteopenia. All clinicians should realize that the current definitions of normal bone density, osteopenia, and osteoporosis apply to the postmenopausal population only:
- normal – T-score above -1.0
- osteopenia – T-score below -1.0 but above -2.5
- osteoporosis – T-score below -2.5.
Indications added for raloxifene
The year did bring new indications for raloxifene, based on data from the RUTH and STAR trials,1,2 which were mentioned in this Update 1 year ago. On September 14, the Food and Drug Administration approved two new indications:
- reduction of risk of invasive breast cancer in postmenopausal women with osteoporosis
- reduction of risk of invasive breast cancer in postmenopausal women at high risk of breast cancer.
These new indications are very important for clinicians who prescribe agents to prevent fragility fractures. Raloxifene should be considered for breast cancer risk reduction when deciding which agent to prescribe.
REAL study finds real advantage with risedronate
Silverman SL, Watts NB, Delmas PD, et al. Effectiveness of bisphosphonates on nonvertebral and hip fractures in the first year of therapy: the risedronate and alendronate (REAL) cohort study. Osteoporos Int. 2007;18(1):25–34.
Patients who take risedronate have lower rates of hip and nonvertebral fracture during their first year of therapy than do those who take alendronate. That is the finding of the RisedronatE and ALendronate (REAL) cohort study, a retrospective observation of the records of health-care utilization among women in the United States. Silverman and colleagues analyzed data sets for women older than age 65 who had ever used once-weekly dosing of risedronate or alendronate. The risedronate cohort included 12,215 women who were followed for a mean of 226 days of therapy. The alendronate cohort included 21,615 women followed for a mean of 238 days of therapy.
Risedronate group had more risk factors for fracture
At baseline, women taking risedronate had a statistically greater incidence of:
- advanced age
- use of concomitant medications
- glucocorticoid use
- rheumatoid arthritis.
Each of these characteristics might have been expected to increase the risk of fragility fracture. However, through 1 year of therapy, women using risedronate had an incidence of nonvertebral fractures 18% lower than those using alendronate (2.0% versus 2.3%; 95% confidence interval [CI], 0.02–0.32). They also had an incidence of hip fracture 43% lower than those using alendronate (0.4% versus 0.6%; 95% CI, 0.13–0.63). Overall, there were 507 nonvertebral fractures and 109 hip fractures.
Footnote: Large database analyses complement randomized trials
Randomized clinical trials (RCTs) are, of course, the gold standard for determining drug safety and efficacy and the key requisite for regulatory approval of new drugs. By design, RCTs have strict inclusion and exclusion criteria to meet the regulatory standard for evaluating drug efficacy and safety and to exclude internal bias. Often, the majority of patients are deemed ineligible for entry into an RCT because of comorbidity, concomitant medication use, age, or severity of disease. Therefore, database analyses are often more “real world” than RCTs.
As clinical practice data accumulate over time, observational, or outcomes, studies can be conducted to complement the data that have been generated by RCTs. For example, over the past decade, large databases of health-care utilization claims have become available in the United States and are being tapped to conduct effectiveness studies. These observational studies can supplement the efficacy measures obtained from the carefully controlled environment of a placebo-controlled RCT. They also provide a measure of effectiveness over a wide range of patients and health-care practices. Data generated from the REAL study are just another piece of the huge puzzle we must grapple with as we seek good information about agents to treat osteoporosis and prevent fragility fractures.
Annual infusion of zoledronic acid reduces risk of fracture
Black DM, Delmas PD, Eastell R, et al, for the HORIZON Pivotal Fracture Trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822.
Lyles KW, Colon-Emeris C, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357. DOI: 10.1056/NEJMoa074941.
Zoledronic acid (Zometa) is indicated for the treatment of high levels of serum calcium associated with Paget’s disease and various malignancies (multiple myeloma, breast, prostate, and lung). These two studies explore use of this agent to prevent fracture in postmenopausal women with osteoporosis—a use for which it proved effective. Other benefits may include improved compliance and ease of administration in some women.
Black and colleagues conducted their randomized, double-blind, placebo-controlled trial to assess the effect of annual infusion of zoledronic acid on the risk of fracture over a 3-year period. A total of 3,889 postmenopausal women with osteoporosis (mean age, 73 years; range, 65–89 years) were assigned to receive a single 15-minute, 5-mg infusion of the drug at baseline, 12 months, and 24 months, and a total of 3,876 women received placebo. Approximately half the women were from Europe, and the other half were from North and South America and Asia. All women received oral daily calcium (1,000–1,500 mg) and vitamin D (400–1,200 U), and all were monitored for 36 months.
The risk of vertebral fracture was reduced in the treatment group by 70% over 3 years, compared with the placebo group (3.3% or 92 women in the treatment group versus 10.9% or 310 women receiving placebo). The risk of hip fracture was reduced by 41% in the treatment group (1.4% or 52 women receiving zoledronic acid versus 2.5% or 88 women in the placebo group). (For all comparisons, P<.001.)
The most common postdose symptoms, seen within 3 days of infusion, included fever, flu-like symptoms, myalgia, headache, and arthralgias. There were more serious adverse events related to atrial fibrillation in the women receiving zoledronic acid (50 women receiving zoledronic acid versus 20 in the placebo group; P<.001).
Treatment is a valuable option for carefully selected populations
The trial by Black and colleagues holds great promise for some patients, especially those who have (or appear to have) upper gastrointestinal intolerance of oral bisphosphonates or who may have difficulty adhering to the positional requirements (i.e., remaining upright) of oral therapy. This may be especially true of patients in nursing homes. Once-yearly intravenous (IV) infusion may also make compliance easier for these patients.
One important detail of this trial: Of almost 8,000 women studied, the youngest was age 65. The implication: Don’t automatically assume this regimen is an appropriate alternative for younger osteopenic women who perceive themselves to have acid reflux.
Women at extremely high risk of fracture also benefit
Fracture is most likely to occur in women who have already experienced it (FIGURE 1). Lyles and colleagues chose this population for their study of once-yearly infusion of zoledronic acid. The study involved 2,127 patients within 90 days after repair of low-trauma hip fracture. Subjects were randomized to receive 5-mg IV zoledronic acid annually or placebo in a blinded fashion. All patients also received vitamin D and calcium supplements. Mean age was 74.5 years, as might be expected in a hip-fracture cohort, and median follow-up was 1.9 years.
New clinical fractures occurred in 8.6% of women in the zoledronic acid group and 13.9% of the placebo group—a 35% risk reduction with IV zoledronic acid (P=.001). Deaths occurred in 101 of 1,054 patients (9.6%) in the zoledronic acid group and 141 of 1,057 patients (13.3%) in the placebo group. This was a reduction of 28% in death from any cause in the zoledronic acid group (P=.01).
The most frequent adverse events in patients receiving zoledronic acid were pyrexia, myalgia, and bone and musculoskeletal pain. No cases of osteonecrosis of the jaw were reported. Rates of atrial fibrillation and stroke were similar in both the zoledronic acid and placebo groups.
This study provides further evidence that, for extremely high-risk women (those who have already suffered hip fracture), yearly zoledronic acid may be an extremely useful tool, especially for elderly women, in whom compliance with any medication—weekly or monthly—may be difficult.

FIGURE 1 Once a fracture occurs, another is likely
Although the risk of new fractures is heightened in women who have already experienced one, Lyles and colleagues found a 35% risk reduction with zoledronic acid.
Parathyroid hormone reduces vertebral fractures in osteoporotic women
Greenspan SL, Bone HG, Ettinger MP, et al, for the Treatment of Osteoporosis with Parathyroid Hormone Study Group. Effect of recombinant human parathyroid hormone (1–84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trial. Ann Intern Med. 2007;146:326–339.
Teriparatide (Forteo) is a synthetic portion (1-34) of the parathyroid hormone (PTH) molecule that is identical in sequence to the biologically active segment of the 84-amino acid human PTH.
It has been shown to prevent fracture in women with low bone mineral density (BMD). Teriparatide is the only anabolic bone agent approved for clinical use; all other pharmacotherapies are antiresorptive.
The study by Greenspan and associates—the Treatment of Osteoporosis with Parathyroid Hormone, or TOP trial—involved the full-length PTH molecule (1–84) and provides evidence that it, too, can prevent vertebral fracture in women who have low BMD (FIGURE 2). This agent is used in Europe but not yet available in the United States.
TOP was an 18-month, randomized, double-blind, placebo-controlled, parallel-group study of 2,532 women with low BMD at the hip or lumbar spine. It was conducted at 168 centers in nine countries.
The primary outcome measure was new or worsened vertebral fracture; secondary outcomes were changes in BMD and safety. The trial investigated the safety of recombinant PTH and its effect on the incidence of vertebral fractures in postmenopausal women with osteoporosis.

FIGURE 2 Fracture-prone bone responds to PTH
PTH prevented vertebral fracture in women with low BMD, existing fractures, or both.
Women had very low BMD, or low BMD and existing fractures
Participants were postmenopausal women aged 45 to 54 years. They had a BMD 3 standard deviations or more (T-score ≤ -3.0) below the mean peak bone mass of young adult women at the lumbar spine, femoral neck, or total hip, with no vertebral fractures, or they had a BMD T-score of -2.5 and 1 to 4 vertebral fractures. Postmenopausal women aged 55 years or older were included if their BMD T-score was -2.5 and they had no vertebral fractures, or if their BMD T-score was -2.0 and they had 1 to 4 vertebral fractures before enrollment. The women were given 100 μg of recombinant human PTH or placebo daily, as well as calcium (700 mg/day) and vitamin D3 (400 U/day).
PTH reduced the risk of new fractures or prevented worsening of existing fractures. The reduction in relative risk (RR) for vertebral fracture was 0.42 (95% CI, 0.24–0.72; P=.001). Women who received PTH had an increase in mean BMD of 6.9% at the spine (95% CI, 6.4–7.4) and 2.1% at the hip (95% CI, 1.7–2.5). Adverse events included a higher incidence of hypercalciuria, hypercalcemia, and nausea.
Although it is unlikely that many gynecologists will be ordering or monitoring injectable PTH therapy, we should be aware of the data. All too often such therapy is not even considered for women with severe osteoporosis (T-score < -2.5 and preexisting fracture), who may be excellent candidates.
Long-term alendronate users can sometimes take a “drug holiday”
Black DM, Schwartz AV, Ensrud KE, et al, for the FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment. The Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927–2938.
As early as 2002, Greenspan and colleagues3 demonstrated that women on alendronate for 21 months maintained femoral-neck BMD through 15 months of crossover to placebo. This study by Black and associates, known as the FLEX trial, is a long-term extension of the Fracture Intervention Trial (FIT). A total of 1,099 women who had participated in FIT and taken alendronate for 5 years were then randomized to one of two doses of alendronate or placebo for an additional 5 years. To qualify for the FLEX trial, all women had to have low bone mass at the beginning of FIT. The average age of women in the FLEX trial was 73 years. The primary outcome was total hip BMD; secondary outcomes were BMD at other sites and biochemical markers.
Women who remained on alendronate maintained a higher BMD of the hip and spine than women on placebo, but all patients’ levels remained at or above pretreatment levels of 10 years earlier. The same was true for markers of bone remodeling. The cumulative risk of nonvertebral fractures did not differ between the two groups (19% for placebo, 18.9% for alendronate; RR, 1.0; 95% CI, 0.76–1.32). The risk of clinically recognized vertebral fractures was lower in the women who continued alendronate (5.3% for placebo and 2.4% for alendronate; RR, 0.45; 95% CI, 0.24–0.85), but there was no significant reduction in morphometric vertebral fractures.
Data can aid in determining duration of therapy
These data are extremely helpful, especially for clinicians who are trying to determine how long to continue bisphosphonate therapy and which patients may be candidates for a “drug holiday.” We have all had women whose response to a bisphosphonate has been so robust that follow-up BMD measurements have climbed to a range in which therapy would not have been initiated. This study clearly shows that the cumulative effect of 5 years of alendronate followed by 5 years of placebo is positive, compared with the bone loss one would expect in untreated women.
The answer isn’t clear, but women with a previous fracture, and those at high risk for spine fracture, are likely to benefit from continued treatment with alendronate. Patients with a lower risk of fracture are better candidates for the holiday.
As for when the drug holiday should end, that isn’t clear, either. Continued close monitoring of these lower-risk women using bone-density measurements may help identify that minority of patients who do not maintain bone mass off the medication.
1. Barrett-Connor E, Mosca L, Collins P, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125-137.
2. Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes. The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 Trial. JAMA. 2006;295:2727-2741.
3. Greenspan SL, Emkey RD, Bone HG, et al. Significant differential effects of alendronate, estrogen, or combination therapy on the rate of bone loss after discontinuation of treatment of postmenopausal osteoporosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2002;137:875-883.
1. Barrett-Connor E, Mosca L, Collins P, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125-137.
2. Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes. The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 Trial. JAMA. 2006;295:2727-2741.
3. Greenspan SL, Emkey RD, Bone HG, et al. Significant differential effects of alendronate, estrogen, or combination therapy on the rate of bone loss after discontinuation of treatment of postmenopausal osteoporosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2002;137:875-883.