User login
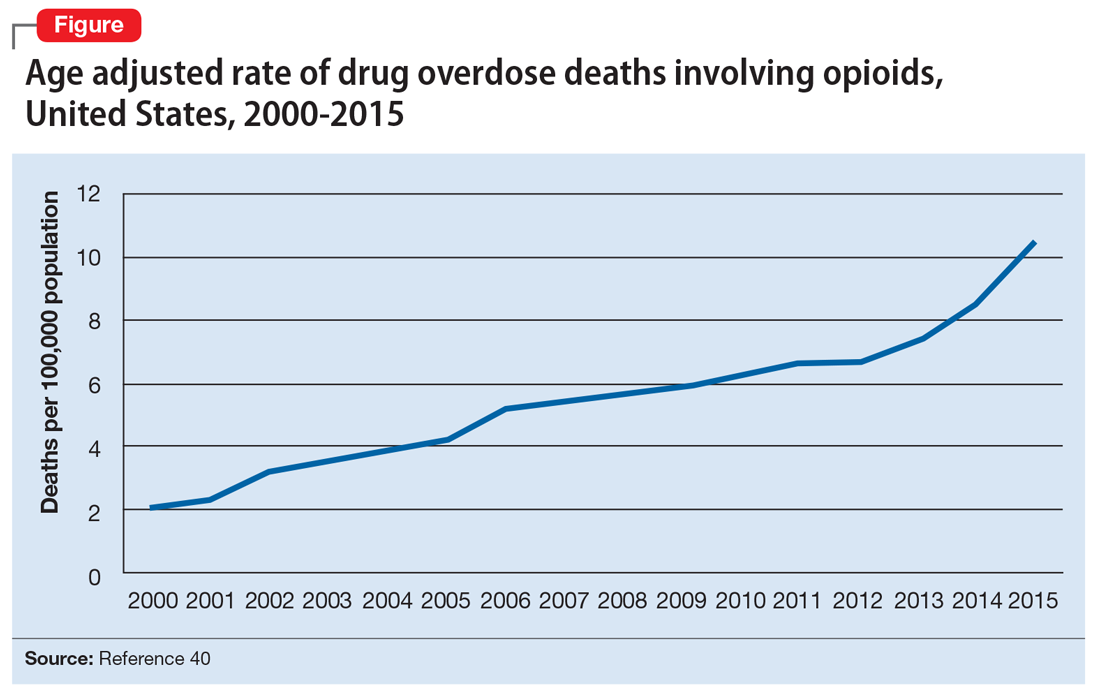
Opioid abuse and overdose are large and growing problems, and in recent years the numbers have been staggering. Overdose deaths related to opioids increased from 28,647 in 2014 to 33,091 in 2015 (Figure).1 More than 2 million individuals in the United States had opioid use disorder in 2015,2 and approximately 80% of them received no treatment,3 even though effective treatment could reduce the scope of abuse.4,5
Although psychiatrists typically are not the primary prescribers of opioid medications, they often treat psychiatric disorders in patients with chronic pain who take prescription opioids. A recent study found that, despite representing only 16% of the adult population, adults with mental health disorders receive more than one-half of all opioid prescriptions distributed each year in the United States.6 Therefore, psychiatrists must be aware of risk assessment strategies for patients receiving opioids.
In this article, we provide recommendations for managing individuals with opioid use disorder, including:
- how to identify risk factors for opioid use disorder and use screening tools
- how to evaluate a patient with suspected opioid use disorder and make the diagnosis
- how to treat a patient with opioid use disorder, including a review of approved pharmaceutical agents.
Risk factors for opioid abuse and overdose
Patients with a history of mental health and/or substance use disorders or at least 3 months of prescribed opioid treatment are at risk for opioid abuse. Those taking a high daily dose of opioids or who have a history of overdose are at risk for overdose from opioid abuse (Table 1).7-12 Standardized tools, such as the Opioid Risk Tool, can be used to screen to assess risk for opioid abuse among individuals prescribed opioids for treatment of chronic pain.12 However, clinicians must be aware that even patients without characteristic risk factors can become dependent on opioids and/or be at risk for an accidental or intentional overdose. For example, opioid therapy following surgical procedures, even in patients who do not have a history of opioid use, increases risk of developing opioid use disorder.13

Evaluation and diagnosis
DSM-5 criteria define 3 degrees of opioid use disorder, depending on how many of the following traits a patient exhibits (mild, 2 to 3; moderate, 4 to 5; and severe, ≥6 )14:
- taking more than the initially intended quantities of opioids or for a longer period of time than intended
- continuous attempts to reduce or otherwise manage opioid use or desires to do so
- a great deal of time using, recovering from, or acquiring opioids
- reports of strong cravings to use opioids
- failing to meet personal objectives at home, work, or school
- continued opioid use even though it causes recurrent social problems
- reduction or elimination of activities the patient once considered important due to opioid use
- opioid use in situations where it is physically dangerous
- continued opioid use despite persistent psychological or physiologic problems despite knowing that continued use is causing or worsening those problems
- tolerance to opioids (not consequential for the diagnosis if the patient is taking opioids under appropriate medical supervision)
- withdrawal or use of opioids (or related substances) to prevent withdrawal (not consequential for the diagnosis if the patient is taking opioids under appropriate medical supervision).
Clinicians should be vigilant for symptoms of opioid use or withdrawal, such as needle marks and weight loss, during the interview (Table 2). High-risk populations that require regular screening include individuals with a history of opioid use disorder, patients taking chronic pain medication, and psychiatric patients.15 During the interview, clinicians should take an nonjudgmental approach and avoid “shame and blame.”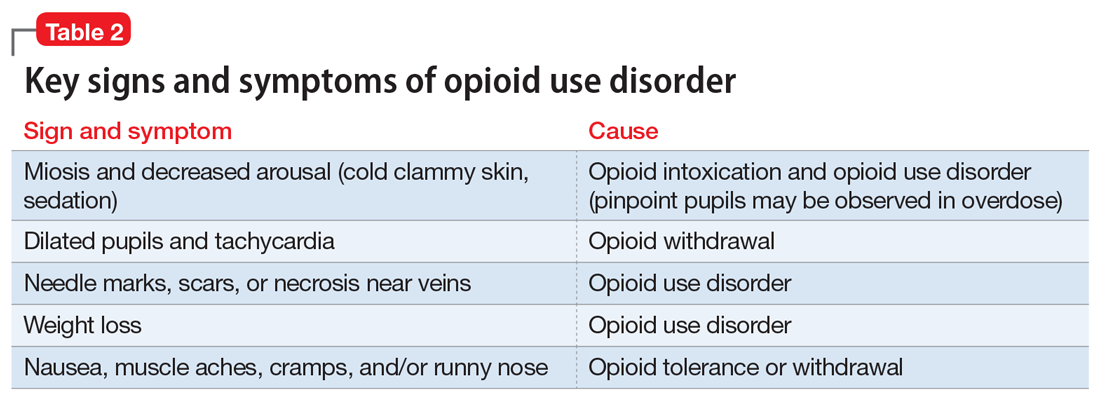
Patients often will withhold information about drug use for various reasons.16 Therefore, collateral information from the patient’s family, close friends, or a referral source is important.
Standardized scales. Various standardized scales can be used to evaluate patients for opioid withdrawal and risk for substance use disorder. Scales for assessing opioid withdrawal include:
- Clinical Opiate Withdrawal Scale
- Subjective Opiate Withdrawal Scale.
Substance use disorder screening tools include:
- Drug Abuse Screen Test-10
- Alcohol Use Disorders Identification Test
- National Institute on Drug Abuse (NIDA) Drug Screening Tool.17
Examination findings. A brief physical examination is necessary to document key findings (Table 2). Patients should undergo a urine drug screen; gas chromatography/mass spectroscopy can confirm positive results. During the examination, clinicians should look for signs and symptoms of co-occurring substance use (eg, benzodiazepines, marijuana, alcohol, cocaine) or mental disorders (mood, anxiety, attention-deficit).18-21 Because nonprescription opioid use is associated with increased risk of suicide attempts and ideation,22 a suicide risk assessment is necessary.
Managing opioid use disorder
Detoxification is a 3-tiered approach that requires judicious prescription of medication, psychosocial support, and supervision to relieve opioid withdrawal symptoms. In both inpatient and outpatient settings, medications used for opioid detoxification include buprenorphine, clonidine, and methadone administered in doses tapered over 5 to 7 days. Appropriate detoxification increases treatment retention for continuing care.23,24
Buprenorphine or buprenorphine/naloxone is the first-line option for outpatient and inpatient detoxification. Short-term detoxification schedules include starting doses between 4 and 16 mg/d, tapered over 5 to 7 days. Compared with methadone, buprenorphine has a lower risk of overdose25 and abuse potential and can be given in an office-based setting. Clonidine, 0.3 to 1.2 mg/d in divided doses, is an alternative to buprenorphine and can be used in inpatient settings.26
Clonidine is not as effective as buprenorphine for detoxification, but it may be used when buprenorphine is contraindicated. Clonidine may require adjuvant symptomatic treatment for insomnia (eg, trazodone, 100 mg at bedtime), anxiety (eg, hydroxyzine, 25 mg, twice a day), or diarrhea (loperamide, 2 mg/d). If a patient needs more structure and monitoring, he (she) should be referred for inpatient detoxification or to a methadone program.27
Medication-assisted therapies
Detoxification alone often is not sufficient treatment. Medication-assisted therapy (MAT) is typically recommended by federal guidelines provided by the Substance Abuse and Mental Health Administration (SAMHSA) for patients with opioid use disorders.3 Patients can be directly transitioned from currently abused opioids to MAT on an outpatient basis. FDA-approved medications for MAT for opioid use disorder include buprenorphine, naltrexone (oral and long-acting injectable), and methadone (Table 3). Choice of MAT depends on several factors, including cost, patient preference, and availability of methadone programs and buprenorphine providers.28

MAT should include psychosocial support29-33 and active monitoring with urine drug screens. Maintenance therapy with medications is usually long-term and has been shown to have better outcomes than detoxification alone or short-term treatment.34 Relapse during MAT should not be cause to discontinue treatment; instead, the patient should be referred to a higher level of care.
Some patients require individualized treatment approaches. For example, the SAMHSA has developed specific treatment improvement protocols to tailor treatments to address specific needs of adolescents.32 The American Academy of Pediatrics recommends MAT with buprenorphine in adolescents with opioid use disorder.33 Although methadone has been approved for pregnant, opioid-dependent patients, recent data indicate buprenorphine is as effective with lower intensity of neonatal abstinence syndrome.34
Buprenorphine. This long-acting (half-life of 24 to 42 hours) opioid partial agonist is approved for treating opioid use disorder in office-based settings according to the Drug Abuse Treatment Act of 2000. Buprenorphine is administered in doses of 8 to 16 mg/d in film or tablet form (sublingual or buccal) and is available in various formulations (Table 4). It is well tolerated; constipation and unpleasant taste are the most common adverse effects. Physicians are required to have a federal waiver to obtain the Drug Enforcement Administration license to prescribe buprenorphine for opioid use disorder in an office setting.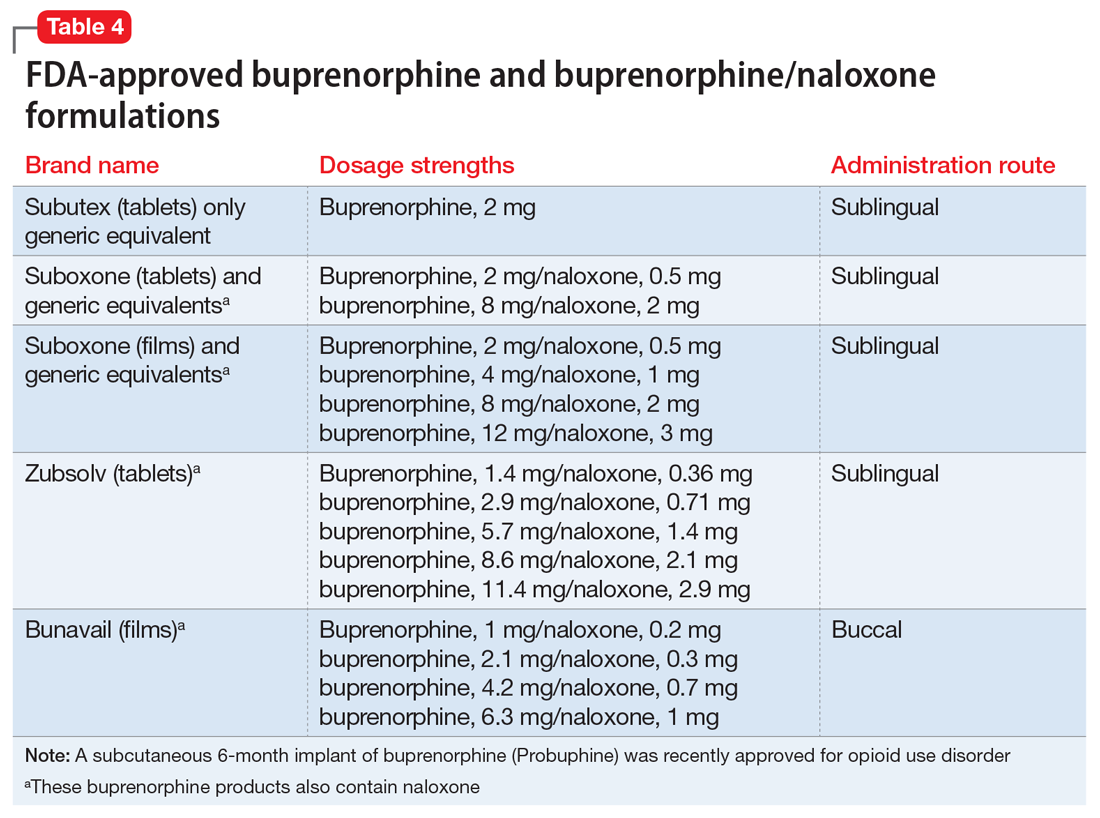
Buprenorphine reduces or eliminates cravings and withdrawal symptoms and helps improve outcomes of abstinence from opioids and retention in treatment.31 Formulations of naloxone combined with buprenorphine reduce the risk of abuse via injection.35 Buprenorphine is safe; however, overdoses can occur when it is combined with benzodiazepines and/or other opiates.
Methadone. This long-acting (half-life 8 to 59 hours), full opioid agonist is approved to treat opioid addiction in federal- and state-regulated opioid treatment programs, also known as methadone maintenance programs. These programs are highly structured and include intensive counseling, monitoring, and dispensing to reduce relapse. Methadone is administered orally either via powder, liquid concentrate, tablet, or solution of diskette. Typically, methadone is dispensed daily in doses of 60 to 100 mg, although higher doses are sometimes necessary. Patients who meet certain criteria for stability may be allowed to take home supplies of methadone.
Methadone has a “black-box” warning for overdose, QT prolongation, and risk for respiratory depression when used in combination with benzodiazepines. Because of its long and unpredictable half-life and tissue accumulation, methadone carries a high overdose risk, particularly with rapidly titrated doses during therapy initiation.35 However, most overdose deaths have occurred with methadone prescribed for pain management. When prescribed and monitored in an opioid treatment program, methadone has shown a high safety profile with respect to overdoses.36
Injectable and oral naltrexone. Used for prevention of relapse to opioid dependence, naltrexone is a pure opioid antagonist that is available as an oral or IM form. Naltrexone has high affinity for the opioid receptors and in therapeutic doses provides an effective blockade for heroin or opioids. Compliance with oral naltrexone has been poor, leading to development of an IM form of naltrexone that can be administered as a single 380-mg dose once every 4 weeks for 6 months or sometimes longer. Naltrexone is also approved for alcohol dependence.
To avoid precipitated withdrawal, patients should be detoxified from opioids for 7 to 10 days before they begin naltrexone, which has no potential for abuse. Common adverse effects include fatigue, nausea, headache, and, for the IM formulation, injection site reactions. There is a “black-box” warning for liver toxicity; therefore, baseline and periodic liver function tests are necessary.
A NIDA review reported poor compliance with oral naltrexone compared with methadone.35 However, naltrexone has been shown to be effective in highly motivated patients (eg, impaired physicians) and the criminal justice population and for preventing relapse following taper from buprenorphine or methadone.37,38
Treatment for opioid overdose
Naloxone is a highly effective treatment to reverse opioid overdose that is delivered via IM or IV injection or by nasal application. Naloxone has no abuse potential. In doses of 0.4 to 2 mg, naloxone reverses overdose within 2 minutes and is effective for 30 to 90 minutes.39 One should call 911 as soon as possible after naloxone is administered. In several states, naloxone is available without a prescription for patients and family members to combat opioid overdoses. The CDC recommends offering naloxone to patients who have risk factors for opioid overdose.40
1. Centers for Disease Control and Prevention. Opioid data analysis. http://www.cdc.gov/drugoverdose/data/analysis.html. Updated February 9, 2017. Accessed June 27, 2017.
2. Substance Abuse and Mental Health Services Administration. Results from the 2015 National Survey on Drug Use and Health: detailed tables. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.pdf.
3. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment of opioid use disorder pocket guide. https://store.samhsa.gov/shin/content//SMA16-4892PG/SMA16-4892PG.pdf. Accessed June 29, 2017.
4. Mutlu C, Demirci AC, Yalcin O, et al. One-year follow-up of heroin-dependent adolescents treated with buprenorphine/naloxone for the first time in a substance treatment unit. J Subst Abuse Treat. 2016;67:1-8.
5. Sharma B, Bruner A, Barnett G, et al. Opioid use disorders. Child Adolesc Psychiatr Clin N Am. 2016;25(3):473-487.
6. Davis MA, Lin LA, Liu H, Sites BD. Prescription Opioid Use among Adults with mental health disorders in the United States. J Am Board Fam Med. 2017;30:42-47.
7. Icahn School of Medicine at Mount Sinai. Substance use: prescription drugs. http://www.mountsinai.org/patient-care/health-library/diseases-and-conditions/opioid-abuse#risk. Accessed June 27, 2017.
8. Boscarino JA, Rukstalis M, Hoffman SN, et al. Risk factors for drug dependence among out-patients on opioid therapy in a large US health-care system. Addiction. 2010;105(10):1776-1782.
9. Edlund M, Steffick D, Hudson T, et al. Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non-cancer pain. Pain. 2007;129(3):355-362.
10. Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81(2):103-107.
11. Bohnert AS, Valenstein M, Bair M, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321.
12. Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6(6):432.
13. Sun EC, Darnall BD, Baker LC, et al. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-1293.
14. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
15. Starrels JL, Becker WC, Alford DP, et al. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Internal Med. 2010;152(11):712-720.
16. Substance Abuse and Mental Health Services Administration. Clinical guidelines for the use of buprenorphine in the treatment of opioid addiction: a treatment improvement protocol: TIP 40. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2004.
17. NIDA drug screening tool: clinician’s screening tool for drug use in general medical settings. National Institutes of Health. https://www.drugabuse.gov/nmassist. Accessed June 27, 2017.
18. Fareed A, Eilender P, Haber M, et al. Comorbid posttraumatic stress disorder and opiate addiction: a literature review. J Addict Dis. 2013;32(2):168-179.
19. Rosen D, Smith ML, Reynolds CF 3rd. The prevalence of mental and physical health disorders among older methadone patients. Am J Geriatr Psychiatry. 2008;16(6):488-497.
20. Goldner EM, Lusted A, Roerecke M, et al. Prevalence of Axis-1 psychiatric (with focus on depression and anxiety) disorder and symptomatology among non-medical prescription opioid users in substance use treatment: systematic review and meta-analyses. Addict Behav. 2014;39(3):520-531.
21. Barry DT, Cutter CJ, Beitel M, et al. Psychiatric disorders among patients seeking treatment for co-occurring chronic pain and opioid use disorder. J Clin Psychiatry. 2016;77(10):1413-1419.
22. Kuramoto SJ, Chilcoat HD, Ko J, et al. Suicidal ideation and suicide attempt across stages of nonmedical prescription opioid use and presence of prescription opioid disorders among U.S. adults. J Stud Alcohol Drugs. 2012;73(2):178-184.
23. Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;(2):CD002207. doi: 10.1002/14651858.CD002207.pub4.
24. Evans E, Li L, Min J, et al. Mortality among individuals accessing pharmacological treatment for opioid dependence in California, 2006-10. Addiction. 2015;110(6):996-1005.
25. Marteu D, McDonald R, Patel K. The relative risk of fatal poisoning by methadone or buprenorphine within the wider population of England and Wales. BMJ Open. 2015;5(5):e007629. doi: 10.1136/bmjopen-2015-007629.
26. Jasinski DR, Johnson RE, Kocher TR. Clonidine in morphine withdrawal. Differential effects on signs and symptoms. Arch Gen Psychiatry. 1985;42(11):1063-1066.
27. Whelan PJ, Remski K. Buprenorphine vs methadone treatment: a review of evidence in both developed and developing worlds. J Neurosci Rural Pract. 2012;3(1):45-50.
28. Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375(4):357-368.
29. Dutra L, Stathopoulou G, Basden SL, et al. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. 2008;165(2):179-187.
30. Brown HL, Britton KA, Mahaffey D, et al. Methadone maintenance in pregnancy: a reappraisal. Am J Obstet Gynecol. 1998;179(2):459-463.
31. Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Treatment Improvement Protocol (TIP) 43.
32. Zimlich R. AAP recommends on medication assisted therapy for adolescent opioid addiction. Contemporary Pediatrics. http://contemporarypediatrics.modernmedicine.com/contemporary-pediatrics/news/aap-recommends-medication-assisted-therapy-adolescent-opioid-addiction. Published September 15, 2016. Accessed June 29, 2017.
33. Patkar A, Lee J, Burgess D. Opioid use disorder. BMJ Publishing Group. http://bestpractice.bmj.com/best-practice/monograph/200.html. Published 2015. Accessed July 6, 2017.
34. Alho H, Sinclair D, Vuori E, et al. Abuse liability of buprenorphine-naloxone tablets in untreated IV drug users. Drug Alcohol Depend. 2007;88(1):75-78.
35. Centers for Disease Control and Prevention (CDC). Vital signs: risk for overdose from methadone used for pain relief - United States, 1999-2010. MMWR Morb Mortal Wkly Rep. 2012;61(26):493-497.
36. Soyka M. New developments in the management of opioid dependence: focus on sublingual buprenorphine-naloxone. Subst Abuse Rehabil. 2015;6:1-14.
37. Lee JD, Friedmann PD, Kinlock TW, et al. Extended-Release Naltrexone to Prevent Opioid Relapse in Criminal Justice Offenders N Engl J Med. 2016;374(13):1232-1242.
38. Vo HT, Robbins E, Westwood M, et al. Relapse prevention medications in community treatment for young adults with opioid addiction. Subst Abus. 2016;37(3):392-397.
39. McDonald R, Campbell ND, Strang J. Twenty years of take-home naloxone for the prevention of overdose deaths from heroin and other opioids-conception and maturation. Drug Alcohol Depend. 2017;178:176-187.
40. Centers for Disease Control and Prevention. Overdose prevention. https://www.cdc.gov/drugoverdose/opioids/odprevention.html. Updated February 9, 2017. Accessed July 6, 2017.
Opioid abuse and overdose are large and growing problems, and in recent years the numbers have been staggering. Overdose deaths related to opioids increased from 28,647 in 2014 to 33,091 in 2015 (Figure).1 More than 2 million individuals in the United States had opioid use disorder in 2015,2 and approximately 80% of them received no treatment,3 even though effective treatment could reduce the scope of abuse.4,5

Although psychiatrists typically are not the primary prescribers of opioid medications, they often treat psychiatric disorders in patients with chronic pain who take prescription opioids. A recent study found that, despite representing only 16% of the adult population, adults with mental health disorders receive more than one-half of all opioid prescriptions distributed each year in the United States.6 Therefore, psychiatrists must be aware of risk assessment strategies for patients receiving opioids.
In this article, we provide recommendations for managing individuals with opioid use disorder, including:
- how to identify risk factors for opioid use disorder and use screening tools
- how to evaluate a patient with suspected opioid use disorder and make the diagnosis
- how to treat a patient with opioid use disorder, including a review of approved pharmaceutical agents.
Risk factors for opioid abuse and overdose
Patients with a history of mental health and/or substance use disorders or at least 3 months of prescribed opioid treatment are at risk for opioid abuse. Those taking a high daily dose of opioids or who have a history of overdose are at risk for overdose from opioid abuse (Table 1).7-12 Standardized tools, such as the Opioid Risk Tool, can be used to screen to assess risk for opioid abuse among individuals prescribed opioids for treatment of chronic pain.12 However, clinicians must be aware that even patients without characteristic risk factors can become dependent on opioids and/or be at risk for an accidental or intentional overdose. For example, opioid therapy following surgical procedures, even in patients who do not have a history of opioid use, increases risk of developing opioid use disorder.13

Evaluation and diagnosis
DSM-5 criteria define 3 degrees of opioid use disorder, depending on how many of the following traits a patient exhibits (mild, 2 to 3; moderate, 4 to 5; and severe, ≥6 )14:
- taking more than the initially intended quantities of opioids or for a longer period of time than intended
- continuous attempts to reduce or otherwise manage opioid use or desires to do so
- a great deal of time using, recovering from, or acquiring opioids
- reports of strong cravings to use opioids
- failing to meet personal objectives at home, work, or school
- continued opioid use even though it causes recurrent social problems
- reduction or elimination of activities the patient once considered important due to opioid use
- opioid use in situations where it is physically dangerous
- continued opioid use despite persistent psychological or physiologic problems despite knowing that continued use is causing or worsening those problems
- tolerance to opioids (not consequential for the diagnosis if the patient is taking opioids under appropriate medical supervision)
- withdrawal or use of opioids (or related substances) to prevent withdrawal (not consequential for the diagnosis if the patient is taking opioids under appropriate medical supervision).
Clinicians should be vigilant for symptoms of opioid use or withdrawal, such as needle marks and weight loss, during the interview (Table 2). High-risk populations that require regular screening include individuals with a history of opioid use disorder, patients taking chronic pain medication, and psychiatric patients.15 During the interview, clinicians should take an nonjudgmental approach and avoid “shame and blame.”
Patients often will withhold information about drug use for various reasons.16 Therefore, collateral information from the patient’s family, close friends, or a referral source is important.
Standardized scales. Various standardized scales can be used to evaluate patients for opioid withdrawal and risk for substance use disorder. Scales for assessing opioid withdrawal include:
- Clinical Opiate Withdrawal Scale
- Subjective Opiate Withdrawal Scale.
Substance use disorder screening tools include:
- Drug Abuse Screen Test-10
- Alcohol Use Disorders Identification Test
- National Institute on Drug Abuse (NIDA) Drug Screening Tool.17
Examination findings. A brief physical examination is necessary to document key findings (Table 2). Patients should undergo a urine drug screen; gas chromatography/mass spectroscopy can confirm positive results. During the examination, clinicians should look for signs and symptoms of co-occurring substance use (eg, benzodiazepines, marijuana, alcohol, cocaine) or mental disorders (mood, anxiety, attention-deficit).18-21 Because nonprescription opioid use is associated with increased risk of suicide attempts and ideation,22 a suicide risk assessment is necessary.
Managing opioid use disorder
Detoxification is a 3-tiered approach that requires judicious prescription of medication, psychosocial support, and supervision to relieve opioid withdrawal symptoms. In both inpatient and outpatient settings, medications used for opioid detoxification include buprenorphine, clonidine, and methadone administered in doses tapered over 5 to 7 days. Appropriate detoxification increases treatment retention for continuing care.23,24
Buprenorphine or buprenorphine/naloxone is the first-line option for outpatient and inpatient detoxification. Short-term detoxification schedules include starting doses between 4 and 16 mg/d, tapered over 5 to 7 days. Compared with methadone, buprenorphine has a lower risk of overdose25 and abuse potential and can be given in an office-based setting. Clonidine, 0.3 to 1.2 mg/d in divided doses, is an alternative to buprenorphine and can be used in inpatient settings.26
Clonidine is not as effective as buprenorphine for detoxification, but it may be used when buprenorphine is contraindicated. Clonidine may require adjuvant symptomatic treatment for insomnia (eg, trazodone, 100 mg at bedtime), anxiety (eg, hydroxyzine, 25 mg, twice a day), or diarrhea (loperamide, 2 mg/d). If a patient needs more structure and monitoring, he (she) should be referred for inpatient detoxification or to a methadone program.27
Medication-assisted therapies
Detoxification alone often is not sufficient treatment. Medication-assisted therapy (MAT) is typically recommended by federal guidelines provided by the Substance Abuse and Mental Health Administration (SAMHSA) for patients with opioid use disorders.3 Patients can be directly transitioned from currently abused opioids to MAT on an outpatient basis. FDA-approved medications for MAT for opioid use disorder include buprenorphine, naltrexone (oral and long-acting injectable), and methadone (Table 3). Choice of MAT depends on several factors, including cost, patient preference, and availability of methadone programs and buprenorphine providers.28

MAT should include psychosocial support29-33 and active monitoring with urine drug screens. Maintenance therapy with medications is usually long-term and has been shown to have better outcomes than detoxification alone or short-term treatment.34 Relapse during MAT should not be cause to discontinue treatment; instead, the patient should be referred to a higher level of care.
Some patients require individualized treatment approaches. For example, the SAMHSA has developed specific treatment improvement protocols to tailor treatments to address specific needs of adolescents.32 The American Academy of Pediatrics recommends MAT with buprenorphine in adolescents with opioid use disorder.33 Although methadone has been approved for pregnant, opioid-dependent patients, recent data indicate buprenorphine is as effective with lower intensity of neonatal abstinence syndrome.34
Buprenorphine. This long-acting (half-life of 24 to 42 hours) opioid partial agonist is approved for treating opioid use disorder in office-based settings according to the Drug Abuse Treatment Act of 2000. Buprenorphine is administered in doses of 8 to 16 mg/d in film or tablet form (sublingual or buccal) and is available in various formulations (Table 4). It is well tolerated; constipation and unpleasant taste are the most common adverse effects. Physicians are required to have a federal waiver to obtain the Drug Enforcement Administration license to prescribe buprenorphine for opioid use disorder in an office setting.
Buprenorphine reduces or eliminates cravings and withdrawal symptoms and helps improve outcomes of abstinence from opioids and retention in treatment.31 Formulations of naloxone combined with buprenorphine reduce the risk of abuse via injection.35 Buprenorphine is safe; however, overdoses can occur when it is combined with benzodiazepines and/or other opiates.
Methadone. This long-acting (half-life 8 to 59 hours), full opioid agonist is approved to treat opioid addiction in federal- and state-regulated opioid treatment programs, also known as methadone maintenance programs. These programs are highly structured and include intensive counseling, monitoring, and dispensing to reduce relapse. Methadone is administered orally either via powder, liquid concentrate, tablet, or solution of diskette. Typically, methadone is dispensed daily in doses of 60 to 100 mg, although higher doses are sometimes necessary. Patients who meet certain criteria for stability may be allowed to take home supplies of methadone.
Methadone has a “black-box” warning for overdose, QT prolongation, and risk for respiratory depression when used in combination with benzodiazepines. Because of its long and unpredictable half-life and tissue accumulation, methadone carries a high overdose risk, particularly with rapidly titrated doses during therapy initiation.35 However, most overdose deaths have occurred with methadone prescribed for pain management. When prescribed and monitored in an opioid treatment program, methadone has shown a high safety profile with respect to overdoses.36
Injectable and oral naltrexone. Used for prevention of relapse to opioid dependence, naltrexone is a pure opioid antagonist that is available as an oral or IM form. Naltrexone has high affinity for the opioid receptors and in therapeutic doses provides an effective blockade for heroin or opioids. Compliance with oral naltrexone has been poor, leading to development of an IM form of naltrexone that can be administered as a single 380-mg dose once every 4 weeks for 6 months or sometimes longer. Naltrexone is also approved for alcohol dependence.
To avoid precipitated withdrawal, patients should be detoxified from opioids for 7 to 10 days before they begin naltrexone, which has no potential for abuse. Common adverse effects include fatigue, nausea, headache, and, for the IM formulation, injection site reactions. There is a “black-box” warning for liver toxicity; therefore, baseline and periodic liver function tests are necessary.
A NIDA review reported poor compliance with oral naltrexone compared with methadone.35 However, naltrexone has been shown to be effective in highly motivated patients (eg, impaired physicians) and the criminal justice population and for preventing relapse following taper from buprenorphine or methadone.37,38
Treatment for opioid overdose
Naloxone is a highly effective treatment to reverse opioid overdose that is delivered via IM or IV injection or by nasal application. Naloxone has no abuse potential. In doses of 0.4 to 2 mg, naloxone reverses overdose within 2 minutes and is effective for 30 to 90 minutes.39 One should call 911 as soon as possible after naloxone is administered. In several states, naloxone is available without a prescription for patients and family members to combat opioid overdoses. The CDC recommends offering naloxone to patients who have risk factors for opioid overdose.40
Opioid abuse and overdose are large and growing problems, and in recent years the numbers have been staggering. Overdose deaths related to opioids increased from 28,647 in 2014 to 33,091 in 2015 (Figure).1 More than 2 million individuals in the United States had opioid use disorder in 2015,2 and approximately 80% of them received no treatment,3 even though effective treatment could reduce the scope of abuse.4,5

Although psychiatrists typically are not the primary prescribers of opioid medications, they often treat psychiatric disorders in patients with chronic pain who take prescription opioids. A recent study found that, despite representing only 16% of the adult population, adults with mental health disorders receive more than one-half of all opioid prescriptions distributed each year in the United States.6 Therefore, psychiatrists must be aware of risk assessment strategies for patients receiving opioids.
In this article, we provide recommendations for managing individuals with opioid use disorder, including:
- how to identify risk factors for opioid use disorder and use screening tools
- how to evaluate a patient with suspected opioid use disorder and make the diagnosis
- how to treat a patient with opioid use disorder, including a review of approved pharmaceutical agents.
Risk factors for opioid abuse and overdose
Patients with a history of mental health and/or substance use disorders or at least 3 months of prescribed opioid treatment are at risk for opioid abuse. Those taking a high daily dose of opioids or who have a history of overdose are at risk for overdose from opioid abuse (Table 1).7-12 Standardized tools, such as the Opioid Risk Tool, can be used to screen to assess risk for opioid abuse among individuals prescribed opioids for treatment of chronic pain.12 However, clinicians must be aware that even patients without characteristic risk factors can become dependent on opioids and/or be at risk for an accidental or intentional overdose. For example, opioid therapy following surgical procedures, even in patients who do not have a history of opioid use, increases risk of developing opioid use disorder.13

Evaluation and diagnosis
DSM-5 criteria define 3 degrees of opioid use disorder, depending on how many of the following traits a patient exhibits (mild, 2 to 3; moderate, 4 to 5; and severe, ≥6 )14:
- taking more than the initially intended quantities of opioids or for a longer period of time than intended
- continuous attempts to reduce or otherwise manage opioid use or desires to do so
- a great deal of time using, recovering from, or acquiring opioids
- reports of strong cravings to use opioids
- failing to meet personal objectives at home, work, or school
- continued opioid use even though it causes recurrent social problems
- reduction or elimination of activities the patient once considered important due to opioid use
- opioid use in situations where it is physically dangerous
- continued opioid use despite persistent psychological or physiologic problems despite knowing that continued use is causing or worsening those problems
- tolerance to opioids (not consequential for the diagnosis if the patient is taking opioids under appropriate medical supervision)
- withdrawal or use of opioids (or related substances) to prevent withdrawal (not consequential for the diagnosis if the patient is taking opioids under appropriate medical supervision).
Clinicians should be vigilant for symptoms of opioid use or withdrawal, such as needle marks and weight loss, during the interview (Table 2). High-risk populations that require regular screening include individuals with a history of opioid use disorder, patients taking chronic pain medication, and psychiatric patients.15 During the interview, clinicians should take an nonjudgmental approach and avoid “shame and blame.”
Patients often will withhold information about drug use for various reasons.16 Therefore, collateral information from the patient’s family, close friends, or a referral source is important.
Standardized scales. Various standardized scales can be used to evaluate patients for opioid withdrawal and risk for substance use disorder. Scales for assessing opioid withdrawal include:
- Clinical Opiate Withdrawal Scale
- Subjective Opiate Withdrawal Scale.
Substance use disorder screening tools include:
- Drug Abuse Screen Test-10
- Alcohol Use Disorders Identification Test
- National Institute on Drug Abuse (NIDA) Drug Screening Tool.17
Examination findings. A brief physical examination is necessary to document key findings (Table 2). Patients should undergo a urine drug screen; gas chromatography/mass spectroscopy can confirm positive results. During the examination, clinicians should look for signs and symptoms of co-occurring substance use (eg, benzodiazepines, marijuana, alcohol, cocaine) or mental disorders (mood, anxiety, attention-deficit).18-21 Because nonprescription opioid use is associated with increased risk of suicide attempts and ideation,22 a suicide risk assessment is necessary.
Managing opioid use disorder
Detoxification is a 3-tiered approach that requires judicious prescription of medication, psychosocial support, and supervision to relieve opioid withdrawal symptoms. In both inpatient and outpatient settings, medications used for opioid detoxification include buprenorphine, clonidine, and methadone administered in doses tapered over 5 to 7 days. Appropriate detoxification increases treatment retention for continuing care.23,24
Buprenorphine or buprenorphine/naloxone is the first-line option for outpatient and inpatient detoxification. Short-term detoxification schedules include starting doses between 4 and 16 mg/d, tapered over 5 to 7 days. Compared with methadone, buprenorphine has a lower risk of overdose25 and abuse potential and can be given in an office-based setting. Clonidine, 0.3 to 1.2 mg/d in divided doses, is an alternative to buprenorphine and can be used in inpatient settings.26
Clonidine is not as effective as buprenorphine for detoxification, but it may be used when buprenorphine is contraindicated. Clonidine may require adjuvant symptomatic treatment for insomnia (eg, trazodone, 100 mg at bedtime), anxiety (eg, hydroxyzine, 25 mg, twice a day), or diarrhea (loperamide, 2 mg/d). If a patient needs more structure and monitoring, he (she) should be referred for inpatient detoxification or to a methadone program.27
Medication-assisted therapies
Detoxification alone often is not sufficient treatment. Medication-assisted therapy (MAT) is typically recommended by federal guidelines provided by the Substance Abuse and Mental Health Administration (SAMHSA) for patients with opioid use disorders.3 Patients can be directly transitioned from currently abused opioids to MAT on an outpatient basis. FDA-approved medications for MAT for opioid use disorder include buprenorphine, naltrexone (oral and long-acting injectable), and methadone (Table 3). Choice of MAT depends on several factors, including cost, patient preference, and availability of methadone programs and buprenorphine providers.28

MAT should include psychosocial support29-33 and active monitoring with urine drug screens. Maintenance therapy with medications is usually long-term and has been shown to have better outcomes than detoxification alone or short-term treatment.34 Relapse during MAT should not be cause to discontinue treatment; instead, the patient should be referred to a higher level of care.
Some patients require individualized treatment approaches. For example, the SAMHSA has developed specific treatment improvement protocols to tailor treatments to address specific needs of adolescents.32 The American Academy of Pediatrics recommends MAT with buprenorphine in adolescents with opioid use disorder.33 Although methadone has been approved for pregnant, opioid-dependent patients, recent data indicate buprenorphine is as effective with lower intensity of neonatal abstinence syndrome.34
Buprenorphine. This long-acting (half-life of 24 to 42 hours) opioid partial agonist is approved for treating opioid use disorder in office-based settings according to the Drug Abuse Treatment Act of 2000. Buprenorphine is administered in doses of 8 to 16 mg/d in film or tablet form (sublingual or buccal) and is available in various formulations (Table 4). It is well tolerated; constipation and unpleasant taste are the most common adverse effects. Physicians are required to have a federal waiver to obtain the Drug Enforcement Administration license to prescribe buprenorphine for opioid use disorder in an office setting.
Buprenorphine reduces or eliminates cravings and withdrawal symptoms and helps improve outcomes of abstinence from opioids and retention in treatment.31 Formulations of naloxone combined with buprenorphine reduce the risk of abuse via injection.35 Buprenorphine is safe; however, overdoses can occur when it is combined with benzodiazepines and/or other opiates.
Methadone. This long-acting (half-life 8 to 59 hours), full opioid agonist is approved to treat opioid addiction in federal- and state-regulated opioid treatment programs, also known as methadone maintenance programs. These programs are highly structured and include intensive counseling, monitoring, and dispensing to reduce relapse. Methadone is administered orally either via powder, liquid concentrate, tablet, or solution of diskette. Typically, methadone is dispensed daily in doses of 60 to 100 mg, although higher doses are sometimes necessary. Patients who meet certain criteria for stability may be allowed to take home supplies of methadone.
Methadone has a “black-box” warning for overdose, QT prolongation, and risk for respiratory depression when used in combination with benzodiazepines. Because of its long and unpredictable half-life and tissue accumulation, methadone carries a high overdose risk, particularly with rapidly titrated doses during therapy initiation.35 However, most overdose deaths have occurred with methadone prescribed for pain management. When prescribed and monitored in an opioid treatment program, methadone has shown a high safety profile with respect to overdoses.36
Injectable and oral naltrexone. Used for prevention of relapse to opioid dependence, naltrexone is a pure opioid antagonist that is available as an oral or IM form. Naltrexone has high affinity for the opioid receptors and in therapeutic doses provides an effective blockade for heroin or opioids. Compliance with oral naltrexone has been poor, leading to development of an IM form of naltrexone that can be administered as a single 380-mg dose once every 4 weeks for 6 months or sometimes longer. Naltrexone is also approved for alcohol dependence.
To avoid precipitated withdrawal, patients should be detoxified from opioids for 7 to 10 days before they begin naltrexone, which has no potential for abuse. Common adverse effects include fatigue, nausea, headache, and, for the IM formulation, injection site reactions. There is a “black-box” warning for liver toxicity; therefore, baseline and periodic liver function tests are necessary.
A NIDA review reported poor compliance with oral naltrexone compared with methadone.35 However, naltrexone has been shown to be effective in highly motivated patients (eg, impaired physicians) and the criminal justice population and for preventing relapse following taper from buprenorphine or methadone.37,38
Treatment for opioid overdose
Naloxone is a highly effective treatment to reverse opioid overdose that is delivered via IM or IV injection or by nasal application. Naloxone has no abuse potential. In doses of 0.4 to 2 mg, naloxone reverses overdose within 2 minutes and is effective for 30 to 90 minutes.39 One should call 911 as soon as possible after naloxone is administered. In several states, naloxone is available without a prescription for patients and family members to combat opioid overdoses. The CDC recommends offering naloxone to patients who have risk factors for opioid overdose.40
1. Centers for Disease Control and Prevention. Opioid data analysis. http://www.cdc.gov/drugoverdose/data/analysis.html. Updated February 9, 2017. Accessed June 27, 2017.
2. Substance Abuse and Mental Health Services Administration. Results from the 2015 National Survey on Drug Use and Health: detailed tables. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.pdf.
3. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment of opioid use disorder pocket guide. https://store.samhsa.gov/shin/content//SMA16-4892PG/SMA16-4892PG.pdf. Accessed June 29, 2017.
4. Mutlu C, Demirci AC, Yalcin O, et al. One-year follow-up of heroin-dependent adolescents treated with buprenorphine/naloxone for the first time in a substance treatment unit. J Subst Abuse Treat. 2016;67:1-8.
5. Sharma B, Bruner A, Barnett G, et al. Opioid use disorders. Child Adolesc Psychiatr Clin N Am. 2016;25(3):473-487.
6. Davis MA, Lin LA, Liu H, Sites BD. Prescription Opioid Use among Adults with mental health disorders in the United States. J Am Board Fam Med. 2017;30:42-47.
7. Icahn School of Medicine at Mount Sinai. Substance use: prescription drugs. http://www.mountsinai.org/patient-care/health-library/diseases-and-conditions/opioid-abuse#risk. Accessed June 27, 2017.
8. Boscarino JA, Rukstalis M, Hoffman SN, et al. Risk factors for drug dependence among out-patients on opioid therapy in a large US health-care system. Addiction. 2010;105(10):1776-1782.
9. Edlund M, Steffick D, Hudson T, et al. Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non-cancer pain. Pain. 2007;129(3):355-362.
10. Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81(2):103-107.
11. Bohnert AS, Valenstein M, Bair M, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321.
12. Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6(6):432.
13. Sun EC, Darnall BD, Baker LC, et al. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-1293.
14. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
15. Starrels JL, Becker WC, Alford DP, et al. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Internal Med. 2010;152(11):712-720.
16. Substance Abuse and Mental Health Services Administration. Clinical guidelines for the use of buprenorphine in the treatment of opioid addiction: a treatment improvement protocol: TIP 40. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2004.
17. NIDA drug screening tool: clinician’s screening tool for drug use in general medical settings. National Institutes of Health. https://www.drugabuse.gov/nmassist. Accessed June 27, 2017.
18. Fareed A, Eilender P, Haber M, et al. Comorbid posttraumatic stress disorder and opiate addiction: a literature review. J Addict Dis. 2013;32(2):168-179.
19. Rosen D, Smith ML, Reynolds CF 3rd. The prevalence of mental and physical health disorders among older methadone patients. Am J Geriatr Psychiatry. 2008;16(6):488-497.
20. Goldner EM, Lusted A, Roerecke M, et al. Prevalence of Axis-1 psychiatric (with focus on depression and anxiety) disorder and symptomatology among non-medical prescription opioid users in substance use treatment: systematic review and meta-analyses. Addict Behav. 2014;39(3):520-531.
21. Barry DT, Cutter CJ, Beitel M, et al. Psychiatric disorders among patients seeking treatment for co-occurring chronic pain and opioid use disorder. J Clin Psychiatry. 2016;77(10):1413-1419.
22. Kuramoto SJ, Chilcoat HD, Ko J, et al. Suicidal ideation and suicide attempt across stages of nonmedical prescription opioid use and presence of prescription opioid disorders among U.S. adults. J Stud Alcohol Drugs. 2012;73(2):178-184.
23. Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;(2):CD002207. doi: 10.1002/14651858.CD002207.pub4.
24. Evans E, Li L, Min J, et al. Mortality among individuals accessing pharmacological treatment for opioid dependence in California, 2006-10. Addiction. 2015;110(6):996-1005.
25. Marteu D, McDonald R, Patel K. The relative risk of fatal poisoning by methadone or buprenorphine within the wider population of England and Wales. BMJ Open. 2015;5(5):e007629. doi: 10.1136/bmjopen-2015-007629.
26. Jasinski DR, Johnson RE, Kocher TR. Clonidine in morphine withdrawal. Differential effects on signs and symptoms. Arch Gen Psychiatry. 1985;42(11):1063-1066.
27. Whelan PJ, Remski K. Buprenorphine vs methadone treatment: a review of evidence in both developed and developing worlds. J Neurosci Rural Pract. 2012;3(1):45-50.
28. Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375(4):357-368.
29. Dutra L, Stathopoulou G, Basden SL, et al. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. 2008;165(2):179-187.
30. Brown HL, Britton KA, Mahaffey D, et al. Methadone maintenance in pregnancy: a reappraisal. Am J Obstet Gynecol. 1998;179(2):459-463.
31. Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Treatment Improvement Protocol (TIP) 43.
32. Zimlich R. AAP recommends on medication assisted therapy for adolescent opioid addiction. Contemporary Pediatrics. http://contemporarypediatrics.modernmedicine.com/contemporary-pediatrics/news/aap-recommends-medication-assisted-therapy-adolescent-opioid-addiction. Published September 15, 2016. Accessed June 29, 2017.
33. Patkar A, Lee J, Burgess D. Opioid use disorder. BMJ Publishing Group. http://bestpractice.bmj.com/best-practice/monograph/200.html. Published 2015. Accessed July 6, 2017.
34. Alho H, Sinclair D, Vuori E, et al. Abuse liability of buprenorphine-naloxone tablets in untreated IV drug users. Drug Alcohol Depend. 2007;88(1):75-78.
35. Centers for Disease Control and Prevention (CDC). Vital signs: risk for overdose from methadone used for pain relief - United States, 1999-2010. MMWR Morb Mortal Wkly Rep. 2012;61(26):493-497.
36. Soyka M. New developments in the management of opioid dependence: focus on sublingual buprenorphine-naloxone. Subst Abuse Rehabil. 2015;6:1-14.
37. Lee JD, Friedmann PD, Kinlock TW, et al. Extended-Release Naltrexone to Prevent Opioid Relapse in Criminal Justice Offenders N Engl J Med. 2016;374(13):1232-1242.
38. Vo HT, Robbins E, Westwood M, et al. Relapse prevention medications in community treatment for young adults with opioid addiction. Subst Abus. 2016;37(3):392-397.
39. McDonald R, Campbell ND, Strang J. Twenty years of take-home naloxone for the prevention of overdose deaths from heroin and other opioids-conception and maturation. Drug Alcohol Depend. 2017;178:176-187.
40. Centers for Disease Control and Prevention. Overdose prevention. https://www.cdc.gov/drugoverdose/opioids/odprevention.html. Updated February 9, 2017. Accessed July 6, 2017.
1. Centers for Disease Control and Prevention. Opioid data analysis. http://www.cdc.gov/drugoverdose/data/analysis.html. Updated February 9, 2017. Accessed June 27, 2017.
2. Substance Abuse and Mental Health Services Administration. Results from the 2015 National Survey on Drug Use and Health: detailed tables. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.pdf.
3. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment of opioid use disorder pocket guide. https://store.samhsa.gov/shin/content//SMA16-4892PG/SMA16-4892PG.pdf. Accessed June 29, 2017.
4. Mutlu C, Demirci AC, Yalcin O, et al. One-year follow-up of heroin-dependent adolescents treated with buprenorphine/naloxone for the first time in a substance treatment unit. J Subst Abuse Treat. 2016;67:1-8.
5. Sharma B, Bruner A, Barnett G, et al. Opioid use disorders. Child Adolesc Psychiatr Clin N Am. 2016;25(3):473-487.
6. Davis MA, Lin LA, Liu H, Sites BD. Prescription Opioid Use among Adults with mental health disorders in the United States. J Am Board Fam Med. 2017;30:42-47.
7. Icahn School of Medicine at Mount Sinai. Substance use: prescription drugs. http://www.mountsinai.org/patient-care/health-library/diseases-and-conditions/opioid-abuse#risk. Accessed June 27, 2017.
8. Boscarino JA, Rukstalis M, Hoffman SN, et al. Risk factors for drug dependence among out-patients on opioid therapy in a large US health-care system. Addiction. 2010;105(10):1776-1782.
9. Edlund M, Steffick D, Hudson T, et al. Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non-cancer pain. Pain. 2007;129(3):355-362.
10. Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81(2):103-107.
11. Bohnert AS, Valenstein M, Bair M, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321.
12. Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6(6):432.
13. Sun EC, Darnall BD, Baker LC, et al. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-1293.
14. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
15. Starrels JL, Becker WC, Alford DP, et al. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Internal Med. 2010;152(11):712-720.
16. Substance Abuse and Mental Health Services Administration. Clinical guidelines for the use of buprenorphine in the treatment of opioid addiction: a treatment improvement protocol: TIP 40. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2004.
17. NIDA drug screening tool: clinician’s screening tool for drug use in general medical settings. National Institutes of Health. https://www.drugabuse.gov/nmassist. Accessed June 27, 2017.
18. Fareed A, Eilender P, Haber M, et al. Comorbid posttraumatic stress disorder and opiate addiction: a literature review. J Addict Dis. 2013;32(2):168-179.
19. Rosen D, Smith ML, Reynolds CF 3rd. The prevalence of mental and physical health disorders among older methadone patients. Am J Geriatr Psychiatry. 2008;16(6):488-497.
20. Goldner EM, Lusted A, Roerecke M, et al. Prevalence of Axis-1 psychiatric (with focus on depression and anxiety) disorder and symptomatology among non-medical prescription opioid users in substance use treatment: systematic review and meta-analyses. Addict Behav. 2014;39(3):520-531.
21. Barry DT, Cutter CJ, Beitel M, et al. Psychiatric disorders among patients seeking treatment for co-occurring chronic pain and opioid use disorder. J Clin Psychiatry. 2016;77(10):1413-1419.
22. Kuramoto SJ, Chilcoat HD, Ko J, et al. Suicidal ideation and suicide attempt across stages of nonmedical prescription opioid use and presence of prescription opioid disorders among U.S. adults. J Stud Alcohol Drugs. 2012;73(2):178-184.
23. Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;(2):CD002207. doi: 10.1002/14651858.CD002207.pub4.
24. Evans E, Li L, Min J, et al. Mortality among individuals accessing pharmacological treatment for opioid dependence in California, 2006-10. Addiction. 2015;110(6):996-1005.
25. Marteu D, McDonald R, Patel K. The relative risk of fatal poisoning by methadone or buprenorphine within the wider population of England and Wales. BMJ Open. 2015;5(5):e007629. doi: 10.1136/bmjopen-2015-007629.
26. Jasinski DR, Johnson RE, Kocher TR. Clonidine in morphine withdrawal. Differential effects on signs and symptoms. Arch Gen Psychiatry. 1985;42(11):1063-1066.
27. Whelan PJ, Remski K. Buprenorphine vs methadone treatment: a review of evidence in both developed and developing worlds. J Neurosci Rural Pract. 2012;3(1):45-50.
28. Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375(4):357-368.
29. Dutra L, Stathopoulou G, Basden SL, et al. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. 2008;165(2):179-187.
30. Brown HL, Britton KA, Mahaffey D, et al. Methadone maintenance in pregnancy: a reappraisal. Am J Obstet Gynecol. 1998;179(2):459-463.
31. Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Treatment Improvement Protocol (TIP) 43.
32. Zimlich R. AAP recommends on medication assisted therapy for adolescent opioid addiction. Contemporary Pediatrics. http://contemporarypediatrics.modernmedicine.com/contemporary-pediatrics/news/aap-recommends-medication-assisted-therapy-adolescent-opioid-addiction. Published September 15, 2016. Accessed June 29, 2017.
33. Patkar A, Lee J, Burgess D. Opioid use disorder. BMJ Publishing Group. http://bestpractice.bmj.com/best-practice/monograph/200.html. Published 2015. Accessed July 6, 2017.
34. Alho H, Sinclair D, Vuori E, et al. Abuse liability of buprenorphine-naloxone tablets in untreated IV drug users. Drug Alcohol Depend. 2007;88(1):75-78.
35. Centers for Disease Control and Prevention (CDC). Vital signs: risk for overdose from methadone used for pain relief - United States, 1999-2010. MMWR Morb Mortal Wkly Rep. 2012;61(26):493-497.
36. Soyka M. New developments in the management of opioid dependence: focus on sublingual buprenorphine-naloxone. Subst Abuse Rehabil. 2015;6:1-14.
37. Lee JD, Friedmann PD, Kinlock TW, et al. Extended-Release Naltrexone to Prevent Opioid Relapse in Criminal Justice Offenders N Engl J Med. 2016;374(13):1232-1242.
38. Vo HT, Robbins E, Westwood M, et al. Relapse prevention medications in community treatment for young adults with opioid addiction. Subst Abus. 2016;37(3):392-397.
39. McDonald R, Campbell ND, Strang J. Twenty years of take-home naloxone for the prevention of overdose deaths from heroin and other opioids-conception and maturation. Drug Alcohol Depend. 2017;178:176-187.
40. Centers for Disease Control and Prevention. Overdose prevention. https://www.cdc.gov/drugoverdose/opioids/odprevention.html. Updated February 9, 2017. Accessed July 6, 2017.
