User login
ILLUSTRATIVE CASE
A 43-year-old woman presents to the clinic with diffuse, intermittent abdominal discomfort, bloating, and diarrhea that has slowly but steadily worsened over the past few years to now-daily symptoms. She states her overall health is otherwise good. Her review of systems is pertinent only for 8 lbs of unintentional weight loss over the past year and increased fatigue. She takes no supplements or routine over-the-counter or prescription medications, except for low-dose combination oral contraceptives, and is unaware of any family history of gastrointestinal (GI) diseases. She does not drink or smoke. She is up to date with immunizations and with cervical and breast cancer screening. Her body mass index is 23, her vital signs are within normal limits, and her physical exam is normal except for mild, diffuse abdominal tenderness without any masses, organomegaly, or peritoneal signs.
Her diagnostic work-up includes a complete metabolic panel, magnesium level, complete blood count, thyroid-stimulating hormone measurement, cytomegalovirus IgG and IgM serology, and stool studies for fecal leukocytes, ova and parasites, and fecal fat, in addition to a kidney, ureter, and bladder noncontrast computed tomography scan. All diagnostic testing is negative except for slightly elevated fecal fat, thereby decreasing the likelihood of infection, thyroid disorder, electrolyte abnormalities, or malignancy as a source of her symptoms.
She says that based on her online searches, her symptoms seem consistent with CD—with which you concur. However, she is fearful of an endoscopic procedure and asks if there is any other way to diagnose CD.
CD is an immune-mediated disorder in genetically susceptible people that is triggered by dietary gluten, causing damage to the small intestine.1-6 The estimated worldwide prevalence of CD is approximately 1%, with greater prevalence in females.1-6 A strong genetic predisposition also has been noted: prevalence among first-degree relatives is 10% to 44%.2,3,6 Although CD can be diagnosed at any age, in the United States the mean age at diagnosis is in the fifth decade of life.6
The incidence of CD is on the rise due to true increases in disease incidence and prevalence, increased detection through better diagnostic tools, and increased screening of at-risk populations (eg, first-degree relatives, those with specific human leukocyte antigen variant genotypes, and those with certain chromosomal disorders, such as Down syndrome and Turner syndrome).2-6 However, despite the increasing prevalence of CD, most patients remain undiagnosed.1
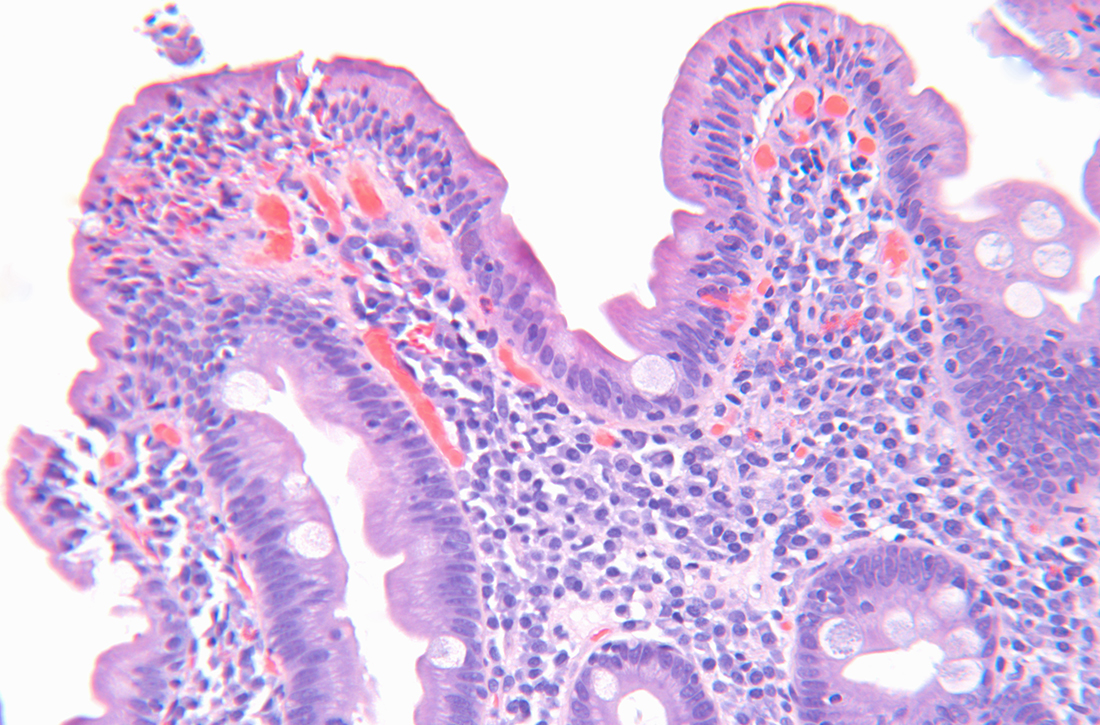
The diagnosis of CD in adults is typically made with elevated serum tTG-IgA and
STUDY SUMMARY
tTG-IgA titers were highly predictive of CD in 3 distinct cohorts
This 2021 hybrid prospective/retrospective study with 3 distinct cohorts aimed to assess the utility of serum tTG-IgA titers compared to traditional EGD with duodenal biopsy for the diagnosis of CD in adult participants (defined as ≥ 16 years of age). A serum tTG-IgA titer ≥ 10 times the ULN was set as the minimal cutoff value, and standardized duodenal biopsy sampling and evaluation for histologic mucosal changes consistent with
Continue to: Cohort 1 was a...
Cohort 1 was a prospective analysis of adults (N = 740) considered to have a high suspicion for CD, recruited from a single CD subspecialty clinic in the United Kingdom. Patients with a previous diagnosis of CD, those adhering to a gluten-free diet, and those with IgA deficiency were excluded. Study patients had tTG-IgA titers drawn and, within 6 weeks, underwent endoscopy with ≥ 1 biopsy from the duodenal bulb and/or the second part of the duodenum. The PPV of tTG-IgA titers ≥ 10 times the ULN in patients with biopsy-proven CD was 98.7% (95% CI, 97%-99.4%).
Cohort 2 was a retrospective analysis of adult patients (N = 532) considered to have low suspicion for CD. These patients were referred for endoscopy for generalized GI complaints in the same hospital as Cohort 1, but not the subspecialty clinic. Exclusion criteria and timing of IgA titers and endoscopy were identical to those of Cohort 1. The PPV of tTG-IgA titers ≥ 10 times the ULN in patients with biopsy-proven CD was 100%.
Cohort 3 (which included patients in 8 countries) was a retrospective analysis of the performance of multiple assays to enhance the validity of this approach in a wide range of settings. Adult patients (N = 145) with tTG-IgA serology positive for celiac who then underwent endoscopy with 4 to 6 duodenal biopsy samples were included in this analysis. Eleven distinct laboratories performed the tTG-IgA assay. The PPV of tTG-IgA titers ≥ 10 times the ULN in patients with biopsy-proven CD was 95.2% (95% CI, 84.6%-98.6%).
In total, this study included 1417 adult patients; 431 (30%) had tTG-IgA titers ≥ 10 times the ULN. Of those patients, 424 (98%) had histopathologic findings on duodenal biopsy consistent with CD.
Of note, there was no standardization as to the assays used for the tTG-IgA titers: Cohort 1 used 2 different manufacturers’ assays, Cohort 2 used 1 assay, and Cohort 3 used 5 assays. Regardless, the “≥ 10 times the ULN” calculation was based on each manufacturer’s published assay ranges. The lack of assay standardization did create variance in false-positive rates, however: Across all 3 cohorts, the false-positive rate for trusting the “≥ 10 times the ULN” threshold as the sole marker for CD in adults increased from 1% (Cohorts 1 and 2) to 5% (all 3 cohorts).
Continue to: WHAT'S NEW
WHAT’S NEW
Less invasive, less costly diagnosis of celiac disease in adults
In adults with symptoms suggestive of CD, the diagnosis can be made with a high level of certainty if a serum tTG-IgA titer is ≥ 10 times the ULN. Through informed, shared decision making in the presence of such a finding, patients may accept a serologic diagnosis and forgo an invasive EGD with biopsy and its inherent costs and risks. Indeed, if the majority of patients with CD are undiagnosed or underdiagnosed, and there exists a minimally invasive blood test that is highly cost effective in the absence of “red flags,” the overall benefit of this path could be substantial.
CAVEATS
“No biopsy” does not mean no risk/benefit discussion
While the PPVs are quite high, the negative predictive value varied greatly: 13%, 98%, and 10% for Cohorts 1, 2, and 3, respectively. Therefore, although serum tTG-IgA titers ≥ 10 times the ULN are useful for diagnosis, a negative result (serum tTG-IgA titers < 10 times the ULN) should not be used to rule out CD, and other testing should be pursued.
Additionally (although rare), patients with CD who have IgA deficiency may obtain false-negative results using the tTG-IgA ≥ 10 times the ULN diagnostic criterion.7,8
Also, both Cohorts 1 and 2 took place in general or subspecialty GI clinics (Cohort 3’s site types were not specified). However, the objective interpretation of tTG-IgA serology means it could be considered as an additional diagnostic tool for primary care physicians, as well.
Finally, if a primary care physician and their patient decide to go the “no-biopsy” route, it should be with a full discussion of the possible risks and benefits of not pursuing EGD. If there are any potential “red flag” symptoms suggesting the possibility of a more concerning differential diagnosis, EGD evaluation should still be pursued. Such symptoms might include (but not be limited to) chronic dyspepsia, dysphagia, weight loss, and unexplained anemia.7
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Diagnostic guidelines still favor EGD with biopsy for adults
The 2013 American College of Gastroenterology guidelines support the use of EGD and duodenal biopsy to diagnose CD in both low- and high-risk patients, regardless of serologic findings.7 In a 2019 Clinical Practice Update, the American Gastrointestinal Association (AGA) stated that when tTG-IgA titers are ≥ 10 times the ULN and EMAs are positive, the PPV is “virtually 100%” for CD. Yet they still state that in this scenario “EGD and duodenal biopsies may then be performed for purposes of differential diagnosis.”8 Furthermore, the AGA does not discuss informed and shared decision making with patients for the option of a “no-biopsy” diagnosis.8
Additionally, there may be challenges in finding commercial laboratories that report reference ranges with a clear ULN. Although costs for the serum tTG-IgA assay vary, they are less expensive than endoscopy with biopsy and histopathologic examination, and therefore may present less of a financial barrier.
1. Penny HA, Raju SA, Lau MS, et al. Accuracy of a no-biopsy approach for the diagnosis of coeliac disease across different adult cohorts. Gut. 2021;70:876-883. doi: 10.1136/gutjnl-2020-320913
2. Al-Toma A, Volta U, Auricchio R, et al. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United European Gastroenterol J. 2019;7:583-613. doi: 10.1177/2050640619844125
3. Caio G, Volta U, Sapone A, et al. Celiac disease: a comprehensive current review. BMC Med. 2019;17:142. doi: 10.1186/s12916-019-1380-z
4. Lebwohl B, Rubio-Tapia A. Epidemiology, presentation, and diagnosis of celiac disease. Gastroenterology. 2021;160:63-75. doi: 10.1053/j.gastro.2020.06.098
5. Lebwohl B, Sanders DS, Green PHR. Coeliac disease. Lancet. 2018;391:70-81. doi: 10.1016/S0140-6736(17)31796-8
6. Rubin JE, Crowe SE. Celiac disease. Ann Intern Med. 2020;172:ITC1-ITC16. doi: 10.7326/AITC202001070
7. Rubio-Tapia A, Hill ID, Kelly CP, et al; American College of Gastroenterology. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108:656-676; quiz 677. doi: 10.1038/ajg.2013.79
8. Husby S, Murray JA, Katzka DA. AGA clinical practice update on diagnosis and monitoring of celiac disease—changing utility of serology and histologic measures: expert review. Gastroenterology. 2019;156:885-889. doi: 10.1053/j.gastro.2018.12.010
9. Husby S, Koletzko S, Korponay-Szabó I, et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition guidelines for diagnosing coeliac disease 2020. J Pediatr Gastroenterol Nutr. 2020;70:141-156. doi: 10.1097/MPG.0000000000002497
ILLUSTRATIVE CASE
A 43-year-old woman presents to the clinic with diffuse, intermittent abdominal discomfort, bloating, and diarrhea that has slowly but steadily worsened over the past few years to now-daily symptoms. She states her overall health is otherwise good. Her review of systems is pertinent only for 8 lbs of unintentional weight loss over the past year and increased fatigue. She takes no supplements or routine over-the-counter or prescription medications, except for low-dose combination oral contraceptives, and is unaware of any family history of gastrointestinal (GI) diseases. She does not drink or smoke. She is up to date with immunizations and with cervical and breast cancer screening. Her body mass index is 23, her vital signs are within normal limits, and her physical exam is normal except for mild, diffuse abdominal tenderness without any masses, organomegaly, or peritoneal signs.
Her diagnostic work-up includes a complete metabolic panel, magnesium level, complete blood count, thyroid-stimulating hormone measurement, cytomegalovirus IgG and IgM serology, and stool studies for fecal leukocytes, ova and parasites, and fecal fat, in addition to a kidney, ureter, and bladder noncontrast computed tomography scan. All diagnostic testing is negative except for slightly elevated fecal fat, thereby decreasing the likelihood of infection, thyroid disorder, electrolyte abnormalities, or malignancy as a source of her symptoms.
She says that based on her online searches, her symptoms seem consistent with CD—with which you concur. However, she is fearful of an endoscopic procedure and asks if there is any other way to diagnose CD.
CD is an immune-mediated disorder in genetically susceptible people that is triggered by dietary gluten, causing damage to the small intestine.1-6 The estimated worldwide prevalence of CD is approximately 1%, with greater prevalence in females.1-6 A strong genetic predisposition also has been noted: prevalence among first-degree relatives is 10% to 44%.2,3,6 Although CD can be diagnosed at any age, in the United States the mean age at diagnosis is in the fifth decade of life.6
The incidence of CD is on the rise due to true increases in disease incidence and prevalence, increased detection through better diagnostic tools, and increased screening of at-risk populations (eg, first-degree relatives, those with specific human leukocyte antigen variant genotypes, and those with certain chromosomal disorders, such as Down syndrome and Turner syndrome).2-6 However, despite the increasing prevalence of CD, most patients remain undiagnosed.1
The diagnosis of CD in adults is typically made with elevated serum tTG-IgA and
STUDY SUMMARY
tTG-IgA titers were highly predictive of CD in 3 distinct cohorts
This 2021 hybrid prospective/retrospective study with 3 distinct cohorts aimed to assess the utility of serum tTG-IgA titers compared to traditional EGD with duodenal biopsy for the diagnosis of CD in adult participants (defined as ≥ 16 years of age). A serum tTG-IgA titer ≥ 10 times the ULN was set as the minimal cutoff value, and standardized duodenal biopsy sampling and evaluation for histologic mucosal changes consistent with
Continue to: Cohort 1 was a...
Cohort 1 was a prospective analysis of adults (N = 740) considered to have a high suspicion for CD, recruited from a single CD subspecialty clinic in the United Kingdom. Patients with a previous diagnosis of CD, those adhering to a gluten-free diet, and those with IgA deficiency were excluded. Study patients had tTG-IgA titers drawn and, within 6 weeks, underwent endoscopy with ≥ 1 biopsy from the duodenal bulb and/or the second part of the duodenum. The PPV of tTG-IgA titers ≥ 10 times the ULN in patients with biopsy-proven CD was 98.7% (95% CI, 97%-99.4%).
Cohort 2 was a retrospective analysis of adult patients (N = 532) considered to have low suspicion for CD. These patients were referred for endoscopy for generalized GI complaints in the same hospital as Cohort 1, but not the subspecialty clinic. Exclusion criteria and timing of IgA titers and endoscopy were identical to those of Cohort 1. The PPV of tTG-IgA titers ≥ 10 times the ULN in patients with biopsy-proven CD was 100%.
Cohort 3 (which included patients in 8 countries) was a retrospective analysis of the performance of multiple assays to enhance the validity of this approach in a wide range of settings. Adult patients (N = 145) with tTG-IgA serology positive for celiac who then underwent endoscopy with 4 to 6 duodenal biopsy samples were included in this analysis. Eleven distinct laboratories performed the tTG-IgA assay. The PPV of tTG-IgA titers ≥ 10 times the ULN in patients with biopsy-proven CD was 95.2% (95% CI, 84.6%-98.6%).
In total, this study included 1417 adult patients; 431 (30%) had tTG-IgA titers ≥ 10 times the ULN. Of those patients, 424 (98%) had histopathologic findings on duodenal biopsy consistent with CD.
Of note, there was no standardization as to the assays used for the tTG-IgA titers: Cohort 1 used 2 different manufacturers’ assays, Cohort 2 used 1 assay, and Cohort 3 used 5 assays. Regardless, the “≥ 10 times the ULN” calculation was based on each manufacturer’s published assay ranges. The lack of assay standardization did create variance in false-positive rates, however: Across all 3 cohorts, the false-positive rate for trusting the “≥ 10 times the ULN” threshold as the sole marker for CD in adults increased from 1% (Cohorts 1 and 2) to 5% (all 3 cohorts).
Continue to: WHAT'S NEW
WHAT’S NEW
Less invasive, less costly diagnosis of celiac disease in adults
In adults with symptoms suggestive of CD, the diagnosis can be made with a high level of certainty if a serum tTG-IgA titer is ≥ 10 times the ULN. Through informed, shared decision making in the presence of such a finding, patients may accept a serologic diagnosis and forgo an invasive EGD with biopsy and its inherent costs and risks. Indeed, if the majority of patients with CD are undiagnosed or underdiagnosed, and there exists a minimally invasive blood test that is highly cost effective in the absence of “red flags,” the overall benefit of this path could be substantial.
CAVEATS
“No biopsy” does not mean no risk/benefit discussion
While the PPVs are quite high, the negative predictive value varied greatly: 13%, 98%, and 10% for Cohorts 1, 2, and 3, respectively. Therefore, although serum tTG-IgA titers ≥ 10 times the ULN are useful for diagnosis, a negative result (serum tTG-IgA titers < 10 times the ULN) should not be used to rule out CD, and other testing should be pursued.
Additionally (although rare), patients with CD who have IgA deficiency may obtain false-negative results using the tTG-IgA ≥ 10 times the ULN diagnostic criterion.7,8
Also, both Cohorts 1 and 2 took place in general or subspecialty GI clinics (Cohort 3’s site types were not specified). However, the objective interpretation of tTG-IgA serology means it could be considered as an additional diagnostic tool for primary care physicians, as well.
Finally, if a primary care physician and their patient decide to go the “no-biopsy” route, it should be with a full discussion of the possible risks and benefits of not pursuing EGD. If there are any potential “red flag” symptoms suggesting the possibility of a more concerning differential diagnosis, EGD evaluation should still be pursued. Such symptoms might include (but not be limited to) chronic dyspepsia, dysphagia, weight loss, and unexplained anemia.7
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Diagnostic guidelines still favor EGD with biopsy for adults
The 2013 American College of Gastroenterology guidelines support the use of EGD and duodenal biopsy to diagnose CD in both low- and high-risk patients, regardless of serologic findings.7 In a 2019 Clinical Practice Update, the American Gastrointestinal Association (AGA) stated that when tTG-IgA titers are ≥ 10 times the ULN and EMAs are positive, the PPV is “virtually 100%” for CD. Yet they still state that in this scenario “EGD and duodenal biopsies may then be performed for purposes of differential diagnosis.”8 Furthermore, the AGA does not discuss informed and shared decision making with patients for the option of a “no-biopsy” diagnosis.8
Additionally, there may be challenges in finding commercial laboratories that report reference ranges with a clear ULN. Although costs for the serum tTG-IgA assay vary, they are less expensive than endoscopy with biopsy and histopathologic examination, and therefore may present less of a financial barrier.
ILLUSTRATIVE CASE
A 43-year-old woman presents to the clinic with diffuse, intermittent abdominal discomfort, bloating, and diarrhea that has slowly but steadily worsened over the past few years to now-daily symptoms. She states her overall health is otherwise good. Her review of systems is pertinent only for 8 lbs of unintentional weight loss over the past year and increased fatigue. She takes no supplements or routine over-the-counter or prescription medications, except for low-dose combination oral contraceptives, and is unaware of any family history of gastrointestinal (GI) diseases. She does not drink or smoke. She is up to date with immunizations and with cervical and breast cancer screening. Her body mass index is 23, her vital signs are within normal limits, and her physical exam is normal except for mild, diffuse abdominal tenderness without any masses, organomegaly, or peritoneal signs.
Her diagnostic work-up includes a complete metabolic panel, magnesium level, complete blood count, thyroid-stimulating hormone measurement, cytomegalovirus IgG and IgM serology, and stool studies for fecal leukocytes, ova and parasites, and fecal fat, in addition to a kidney, ureter, and bladder noncontrast computed tomography scan. All diagnostic testing is negative except for slightly elevated fecal fat, thereby decreasing the likelihood of infection, thyroid disorder, electrolyte abnormalities, or malignancy as a source of her symptoms.
She says that based on her online searches, her symptoms seem consistent with CD—with which you concur. However, she is fearful of an endoscopic procedure and asks if there is any other way to diagnose CD.
CD is an immune-mediated disorder in genetically susceptible people that is triggered by dietary gluten, causing damage to the small intestine.1-6 The estimated worldwide prevalence of CD is approximately 1%, with greater prevalence in females.1-6 A strong genetic predisposition also has been noted: prevalence among first-degree relatives is 10% to 44%.2,3,6 Although CD can be diagnosed at any age, in the United States the mean age at diagnosis is in the fifth decade of life.6
The incidence of CD is on the rise due to true increases in disease incidence and prevalence, increased detection through better diagnostic tools, and increased screening of at-risk populations (eg, first-degree relatives, those with specific human leukocyte antigen variant genotypes, and those with certain chromosomal disorders, such as Down syndrome and Turner syndrome).2-6 However, despite the increasing prevalence of CD, most patients remain undiagnosed.1
The diagnosis of CD in adults is typically made with elevated serum tTG-IgA and
STUDY SUMMARY
tTG-IgA titers were highly predictive of CD in 3 distinct cohorts
This 2021 hybrid prospective/retrospective study with 3 distinct cohorts aimed to assess the utility of serum tTG-IgA titers compared to traditional EGD with duodenal biopsy for the diagnosis of CD in adult participants (defined as ≥ 16 years of age). A serum tTG-IgA titer ≥ 10 times the ULN was set as the minimal cutoff value, and standardized duodenal biopsy sampling and evaluation for histologic mucosal changes consistent with
Continue to: Cohort 1 was a...
Cohort 1 was a prospective analysis of adults (N = 740) considered to have a high suspicion for CD, recruited from a single CD subspecialty clinic in the United Kingdom. Patients with a previous diagnosis of CD, those adhering to a gluten-free diet, and those with IgA deficiency were excluded. Study patients had tTG-IgA titers drawn and, within 6 weeks, underwent endoscopy with ≥ 1 biopsy from the duodenal bulb and/or the second part of the duodenum. The PPV of tTG-IgA titers ≥ 10 times the ULN in patients with biopsy-proven CD was 98.7% (95% CI, 97%-99.4%).
Cohort 2 was a retrospective analysis of adult patients (N = 532) considered to have low suspicion for CD. These patients were referred for endoscopy for generalized GI complaints in the same hospital as Cohort 1, but not the subspecialty clinic. Exclusion criteria and timing of IgA titers and endoscopy were identical to those of Cohort 1. The PPV of tTG-IgA titers ≥ 10 times the ULN in patients with biopsy-proven CD was 100%.
Cohort 3 (which included patients in 8 countries) was a retrospective analysis of the performance of multiple assays to enhance the validity of this approach in a wide range of settings. Adult patients (N = 145) with tTG-IgA serology positive for celiac who then underwent endoscopy with 4 to 6 duodenal biopsy samples were included in this analysis. Eleven distinct laboratories performed the tTG-IgA assay. The PPV of tTG-IgA titers ≥ 10 times the ULN in patients with biopsy-proven CD was 95.2% (95% CI, 84.6%-98.6%).
In total, this study included 1417 adult patients; 431 (30%) had tTG-IgA titers ≥ 10 times the ULN. Of those patients, 424 (98%) had histopathologic findings on duodenal biopsy consistent with CD.
Of note, there was no standardization as to the assays used for the tTG-IgA titers: Cohort 1 used 2 different manufacturers’ assays, Cohort 2 used 1 assay, and Cohort 3 used 5 assays. Regardless, the “≥ 10 times the ULN” calculation was based on each manufacturer’s published assay ranges. The lack of assay standardization did create variance in false-positive rates, however: Across all 3 cohorts, the false-positive rate for trusting the “≥ 10 times the ULN” threshold as the sole marker for CD in adults increased from 1% (Cohorts 1 and 2) to 5% (all 3 cohorts).
Continue to: WHAT'S NEW
WHAT’S NEW
Less invasive, less costly diagnosis of celiac disease in adults
In adults with symptoms suggestive of CD, the diagnosis can be made with a high level of certainty if a serum tTG-IgA titer is ≥ 10 times the ULN. Through informed, shared decision making in the presence of such a finding, patients may accept a serologic diagnosis and forgo an invasive EGD with biopsy and its inherent costs and risks. Indeed, if the majority of patients with CD are undiagnosed or underdiagnosed, and there exists a minimally invasive blood test that is highly cost effective in the absence of “red flags,” the overall benefit of this path could be substantial.
CAVEATS
“No biopsy” does not mean no risk/benefit discussion
While the PPVs are quite high, the negative predictive value varied greatly: 13%, 98%, and 10% for Cohorts 1, 2, and 3, respectively. Therefore, although serum tTG-IgA titers ≥ 10 times the ULN are useful for diagnosis, a negative result (serum tTG-IgA titers < 10 times the ULN) should not be used to rule out CD, and other testing should be pursued.
Additionally (although rare), patients with CD who have IgA deficiency may obtain false-negative results using the tTG-IgA ≥ 10 times the ULN diagnostic criterion.7,8
Also, both Cohorts 1 and 2 took place in general or subspecialty GI clinics (Cohort 3’s site types were not specified). However, the objective interpretation of tTG-IgA serology means it could be considered as an additional diagnostic tool for primary care physicians, as well.
Finally, if a primary care physician and their patient decide to go the “no-biopsy” route, it should be with a full discussion of the possible risks and benefits of not pursuing EGD. If there are any potential “red flag” symptoms suggesting the possibility of a more concerning differential diagnosis, EGD evaluation should still be pursued. Such symptoms might include (but not be limited to) chronic dyspepsia, dysphagia, weight loss, and unexplained anemia.7
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Diagnostic guidelines still favor EGD with biopsy for adults
The 2013 American College of Gastroenterology guidelines support the use of EGD and duodenal biopsy to diagnose CD in both low- and high-risk patients, regardless of serologic findings.7 In a 2019 Clinical Practice Update, the American Gastrointestinal Association (AGA) stated that when tTG-IgA titers are ≥ 10 times the ULN and EMAs are positive, the PPV is “virtually 100%” for CD. Yet they still state that in this scenario “EGD and duodenal biopsies may then be performed for purposes of differential diagnosis.”8 Furthermore, the AGA does not discuss informed and shared decision making with patients for the option of a “no-biopsy” diagnosis.8
Additionally, there may be challenges in finding commercial laboratories that report reference ranges with a clear ULN. Although costs for the serum tTG-IgA assay vary, they are less expensive than endoscopy with biopsy and histopathologic examination, and therefore may present less of a financial barrier.
1. Penny HA, Raju SA, Lau MS, et al. Accuracy of a no-biopsy approach for the diagnosis of coeliac disease across different adult cohorts. Gut. 2021;70:876-883. doi: 10.1136/gutjnl-2020-320913
2. Al-Toma A, Volta U, Auricchio R, et al. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United European Gastroenterol J. 2019;7:583-613. doi: 10.1177/2050640619844125
3. Caio G, Volta U, Sapone A, et al. Celiac disease: a comprehensive current review. BMC Med. 2019;17:142. doi: 10.1186/s12916-019-1380-z
4. Lebwohl B, Rubio-Tapia A. Epidemiology, presentation, and diagnosis of celiac disease. Gastroenterology. 2021;160:63-75. doi: 10.1053/j.gastro.2020.06.098
5. Lebwohl B, Sanders DS, Green PHR. Coeliac disease. Lancet. 2018;391:70-81. doi: 10.1016/S0140-6736(17)31796-8
6. Rubin JE, Crowe SE. Celiac disease. Ann Intern Med. 2020;172:ITC1-ITC16. doi: 10.7326/AITC202001070
7. Rubio-Tapia A, Hill ID, Kelly CP, et al; American College of Gastroenterology. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108:656-676; quiz 677. doi: 10.1038/ajg.2013.79
8. Husby S, Murray JA, Katzka DA. AGA clinical practice update on diagnosis and monitoring of celiac disease—changing utility of serology and histologic measures: expert review. Gastroenterology. 2019;156:885-889. doi: 10.1053/j.gastro.2018.12.010
9. Husby S, Koletzko S, Korponay-Szabó I, et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition guidelines for diagnosing coeliac disease 2020. J Pediatr Gastroenterol Nutr. 2020;70:141-156. doi: 10.1097/MPG.0000000000002497
1. Penny HA, Raju SA, Lau MS, et al. Accuracy of a no-biopsy approach for the diagnosis of coeliac disease across different adult cohorts. Gut. 2021;70:876-883. doi: 10.1136/gutjnl-2020-320913
2. Al-Toma A, Volta U, Auricchio R, et al. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United European Gastroenterol J. 2019;7:583-613. doi: 10.1177/2050640619844125
3. Caio G, Volta U, Sapone A, et al. Celiac disease: a comprehensive current review. BMC Med. 2019;17:142. doi: 10.1186/s12916-019-1380-z
4. Lebwohl B, Rubio-Tapia A. Epidemiology, presentation, and diagnosis of celiac disease. Gastroenterology. 2021;160:63-75. doi: 10.1053/j.gastro.2020.06.098
5. Lebwohl B, Sanders DS, Green PHR. Coeliac disease. Lancet. 2018;391:70-81. doi: 10.1016/S0140-6736(17)31796-8
6. Rubin JE, Crowe SE. Celiac disease. Ann Intern Med. 2020;172:ITC1-ITC16. doi: 10.7326/AITC202001070
7. Rubio-Tapia A, Hill ID, Kelly CP, et al; American College of Gastroenterology. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108:656-676; quiz 677. doi: 10.1038/ajg.2013.79
8. Husby S, Murray JA, Katzka DA. AGA clinical practice update on diagnosis and monitoring of celiac disease—changing utility of serology and histologic measures: expert review. Gastroenterology. 2019;156:885-889. doi: 10.1053/j.gastro.2018.12.010
9. Husby S, Koletzko S, Korponay-Szabó I, et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition guidelines for diagnosing coeliac disease 2020. J Pediatr Gastroenterol Nutr. 2020;70:141-156. doi: 10.1097/MPG.0000000000002497
PRACTICE CHANGER
Consider a “no-biopsy” approach by evaluating serum immunoglobulin (Ig) A anti-tissue transglutaminase (tTG-IgA) antibody titers in adult patients who present with symptoms concerning for celiac disease (CD). An increase of ≥ 10 times the upper limit of normal (ULN) for tTG-IgA has a positive predictive value (PPV) of ≥ 95% for diagnosing CD when compared with esophagogastroduodenoscopy (EGD) with duodenal biopsy—the current gold standard.
STRENGTH OF RECOMMENDATION
A: Consistent findings from 3 good-quality diagnostic cohorts presented in a single study.1
Penny HA, Raju SA, Lau MS, et al. Accuracy of a no-biopsy approach for the diagnosis of coeliac disease across different adult cohorts. Gut. 2021;70:876-883. doi: 10.1136/gutjnl-2020-320913